Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli†
Mauro A.
Rinaldi‡
 ,
Clara A.
Ferraz‡
,
Clara A.
Ferraz‡
 and
Nigel S.
Scrutton
and
Nigel S.
Scrutton
 *
*
Manchester Institute of Biotechnology, Department of Chemistry, School of Natural Sciences, The University of Manchester, 131 Princess Street, Manchester, M1 7DN, UK. E-mail: nigel.scrutton@manchester.ac.uk
First published on 7th July 2021
Abstract
Covering: up to 2021
Terpenoids are a diverse group of chemicals used in a wide range of industries. Microbial terpenoid production has the potential to displace traditional manufacturing of these compounds with renewable processes, but further titre improvements are needed to reach cost competitiveness. This review discusses strategies to increase terpenoid titres in Escherichia coli with a focus on alternative metabolic pathways. Alternative pathways can lead to improved titres by providing higher orthogonality to native metabolism that redirects carbon flux, by avoiding toxic intermediates, by bypassing highly-regulated or bottleneck steps, or by being shorter and thus more efficient and easier to manipulate. The canonical 2-C-methyl-D-erythritol 4-phosphate (MEP) and mevalonate (MVA) pathways are engineered to increase titres, sometimes using homologs from different species to address bottlenecks. Further, alternative terpenoid pathways, including additional entry points into the MEP and MVA pathways, archaeal MVA pathways, and new artificial pathways provide new tools to increase titres. Prenyl diphosphate synthases elongate terpenoid chains, and alternative homologs create orthogonal pathways and increase product diversity. Alternative sources of terpenoid synthases and modifying enzymes can also be better suited for E. coli expression. Mining the growing number of bacterial genomes for new bacterial terpenoid synthases and modifying enzymes identifies enzymes that outperform eukaryotic ones and expand microbial terpenoid production diversity. Terpenoid removal from cells is also crucial in production, and so terpenoid recovery and approaches to handle end-product toxicity increase titres. Combined, these strategies are contributing to current efforts to increase microbial terpenoid production towards commercial feasibility.
1 Introduction
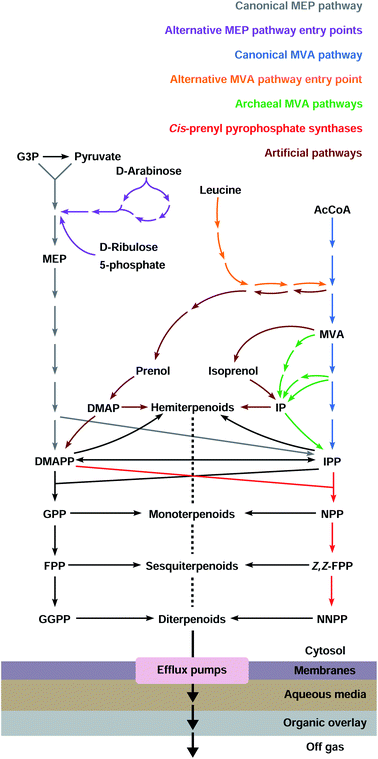
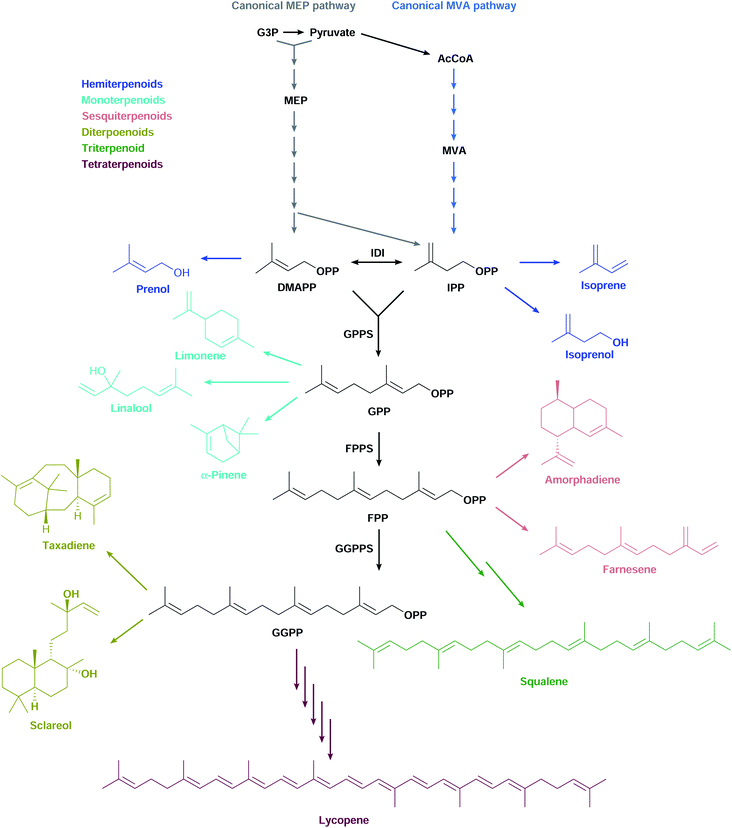
Terpenoids form one of the largest and most diverse classes of chemicals comprising thousands of structures.1 They have a wide range of applications in multiple industries, ranging from pharmaceuticals such as anti-cancer drugs and antimalarials, to flavours and fragrances, and to agricultural products such as pesticides and insect repellents.2 These valuable chemicals are mostly made synthetically from petroleum or extracted from plants grown at agricultural scales. As the world economy turns away from fossil fuels and non-food uses for arable land, the need arises to develop microbial cell factories to produce terpenoids sustainably through fermentation of renewable feedstocks.Most terpenoids are made in nature from the interconvertible C5 precursors isopentenyl diphosphate (IPP)§ and dimethylallyl diphosphate (DMAPP), produced from central metabolism via the 2-C-methyl-D-erythritol 4-phosphate (MEP) or the mevalonate (MVA) pathways (Fig. 1). IPP/DMAPP is used directly to make C5 hemiterpenoids or is combined with additional IPP/DMAPP molecules by prenyl diphosphate synthases (PPPS) to build longer prenyl diphosphates, such as C10 geranyl diphosphate (GPP), C15 farnesyl diphosphate (FPP), and C20 geranylgeranyl diphosphate (GGPP). Terpenoid synthases (TPSs) convert prenyl diphosphates into terpenoid backbones for C10 monoterpenoids, C15 sesquiterpenoids, C20 diterpenoids, C30 triterpenoids, or C40 tetraterpenoids, among others. These backbones are functionalised by additional enzymes that further expand the structural and chemical diversity of this class of compounds.
 | ||
| Fig. 1 The canonical MEP and MVA pathways are the most commonly used to make diverse terpenoid structures. | ||
Escherichia coli is one of the main model organisms for terpenoid production, with vast physiological, biochemical and metabolic knowledge available as well as diverse engineering tools. E. coli is advantageous from an industrial perspective because it accepts a wide range of substrates and grows efficiently under low-cost and large-scale conditions.3 In general, engineered E. coli produces higher levels of hemi-, mono- and tetraterpenoids than other organisms, whereas engineered yeast strains produce higher levels of sesqui-, di- and triterpenoid compounds.4–6 Because E. coli is easy to manipulate, insights gained into the benefits of using this host and discussed in this review, can also be applied to more industrially relevant host bacterial organisms.
Advances in the last few decades have dramatically improved microbial terpenoid production. Only a few selected processes however are considered as having the potential to be marginally cost competitive. For example, microbial β-farnesene and semi-synthetic artemisinin production were demonstrated at industrial scales,7,8 but have not yet displaced traditional manufacturing routes that remain less expensive. Techno-economic analyses of microbial terpenoid production identify productivity or yield as a main cost driver and conclude titre improvements could enable commercialisation.9,10 Other cost intensive aspects are also a concern (e.g. capital infrastructure, feedstock provision, downstream processing amongst others).
Titres are the most commonly reported measure in metabolic engineering programs. Titres, however, can vary based on fermentation time and conditions, expression levels of genetic constructs, enzymatic activity of limiting steps, regulation points in the pathway, genetic background, carbon source and other factors.11 Varied production conditions have led to some of the highest reported titres for different terpenoids (Table S1†). Reported titres measured under different production conditions are not necessarily the best metric of the effectiveness of a pathway tested. However, a comparison of titres measured under different fermentation conditions available across the extensive terpenoid literature can still be useful to compare strategies for terpenoid production. In this review, we use titres to make direct comparisons (i.e. identical production conditions and within a single publication) whenever available. We review also titres obtained across different publications to gain insight into conditions that lead to the highest titres for a specific terpenoid. Our rationale is that different strategies that produce high titres are of interest from a learning perspective. Combinatorial approaches, for example, could potentially increase these titres further. In those cases where reported titres are low (i.e. lower than the highest reported titres for a specific terpenoid), the interpretation is more challenging but higher titres may become achievable pending further optimisation. In these cases, these lower titres provide an indication of how much optimisation remains to be done to approach the highest reported values in the literature.
Engineering the native MEP pathway or the heterologous MVA pathway in E. coli has enabled terpenoid production at the g L−1 scale (Table 1). Bottlenecks and regulation points in these canonical pathways have been overcome by overexpressing native genes or by using homologous enzymes from other organisms that are resistant to regulation or are more active. Using alternative pathways with higher orthogonality to native metabolism, and that avoid intrinsic regulation,12 potentially could lead to higher production titres. The use of alternative pathways can also avoid toxic intermediate build-up, bypass highly-regulated or bottleneck steps, and present more energetically efficient or shorter routes to the desired product. Such pathways are likely to be easier targets for optimisation through pathway engineering.
| Terpenoids and derivatives | Highest reported titre (g L−1 culture)a | Engineered precursor pathway | Reference |
|---|---|---|---|
| a Where titres were reported only for the organic phase, the organic phase titre was divided by the aqueous-to-organic-phase ratio. b For reference, the next highest titre reported in g L−1 is 3.52 g L−1 or 50.6 mg g−1 DCW lycopene.300 Dashes indicate that this terpenoid is a derivative of the terpenoid in the row above. | |||
| Hemiterpenoids | |||
| Isoprene | 60 | MVA | 76 |
| Isoprenol | 10.8 | IPP bypass | 127 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Monoterpenoids | |||
| Limonene | 3.65a | MVA | 160 |
| -Perillyl alcohol | 0.105 | MVA | 138 |
| Sabinene | 2.65 | MVA | 161 |
| Geraniol | 2.124 | MVA | 170 |
| -Geranyl acetate | 4.8 | MVA | 163 |
| Linalool | 1.523 | MVA | 307 |
| α-Pinene | 0.97 | MVA | 164 |
| 1,8-Cineole | 0.653 | MVA | 165 |
| -Hydroxycineole | 0.056 | MVA | 308 |
| Myrcene | 0.058 | MVA | 166 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| C11 | |||
| 2-Methylenebornane | 0.014 | MVA | 146 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Sesquiterpenoids | |||
| Amorphadiene | 30 | MVA | 172 |
| -Artemisinic-11S,12-epoxide | 0.25 | MVA | 173 |
| -Artemisinic acid | 0.105 | MVA | 174 |
| Viridiflorol | 25.7 | MVA | 172 |
| β-Farnesene | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67 0.67 |
MVA | 175 |
| (−)-α-Bisabolol | 9.1 | MVA | 309 |
| (+)-Isodauc-8-en-11-ol | 1.16 | MVA | 184 |
| α-Farnesene | 1.1 | MVA | 176 |
| α-Bisabolene | 1.15 | MVA | 58 |
| Farnesol | 1.419 | MVA | 310 |
| Protoilludene | 1.119 | MVA | 311 |
| Pentalenene | 0.78 | MVA | 231 |
| Epi-isozizaene | 0.728 | MVA | 231 |
| -Albaflavenol | 0.013 | MVA | 308 |
| -Albaflavenone | 0.003 | MVA | 308 |
| Cubebol | 0.497 | MEP | 44 |
| Guaia-6,10(14)-diene | 0.468 | MVA | 312 |
| Longifolene | 0.382 | MVA | 182 |
| Nerolidol | 0.323 | MVA | 178 |
| β-Copaene | 0.215 | MEP | 44 |
| (+)-Zizaene | 0.211 | MVA | 179 |
| Caryophyllene | 0.1 | MVA | 229 |
| -Caryolan-1-ol | 0.01 | MVA | 229 |
| α-Isocomene | 0.0775 | MVA | 231 |
| (−)-5-Epieremophilene | 0.076 | MVA | 180 |
| Valerenadiene | 0.062 | MVA | 88 |
| α-Humulene | 0.06 | MVA | 313 |
| (−)-Patchoulol | 0.04 | MVA | 314 |
| α-Copaene | 0.007 | MVA | 181 |
| Cadinene | 0.0035 | MVA | 181 |
| -8-Hydroxycadinene | 0.06 | MVA | 174 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Diterpenoids | |||
| Sclareol | 1.46 | MVA | 185 |
| Taxadiene | 1.02 | MEP | 40 |
| -Taxadien-5α-ol | 0.058 | MEP | 40 |
| Levopimaradiene | 0.7 | MEP | 186 |
| cis-Abienol | 0.22 | MVA | 183 |
| Cembratriene-ol | 0.079 | MEP | 45 |
| Kaurene | 0.032 | MEP | 187 |
| Abietadiene | 0.03 | MVA | 89 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Triterpenoids | |||
| Squalene | 0.612 | MVA | 277 |
| Dammarenediol-II | 0.0086 | MEP | 315 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Tetraterpenoids | |||
| Lycopene | 448 mg g−1 DCWb | MEP | 41 |
| -β-Carotene | 3.6 | MEP and MVA | 316 |
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -Astaxanthin -Astaxanthin |
1.18 | MEP and MVA | 316 |
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -Zeaxanthin -Zeaxanthin |
0.722 | MVA | 317 |
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -β-Ionone (C13) -β-Ionone (C13) |
0.5 | MVA | 318 |
-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) -Retinol (C15) -Retinol (C15) |
0.076 | MEP and MVA | 319 |
| α-Ionone (C13) | 0.48 | MVA | 318 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Other | |||
| Coenzyme Q10 | 0.00564 | MEP | 320 |
In this review, we focus on both canonical and alternative pathways for terpenoid production in E. coli and discuss strategies to increase production titres to levels that might become commercially attractive. Also, we review ways to expand terpenoid diversity and to create new-to-nature industrially relevant molecules. Finally, we discuss methods used to increase the robustness of the production host and physical separation methods during fermentation to address problems associated with terpenoid end-product toxicity.
2 Canonical pathways to IPP and DMAPP
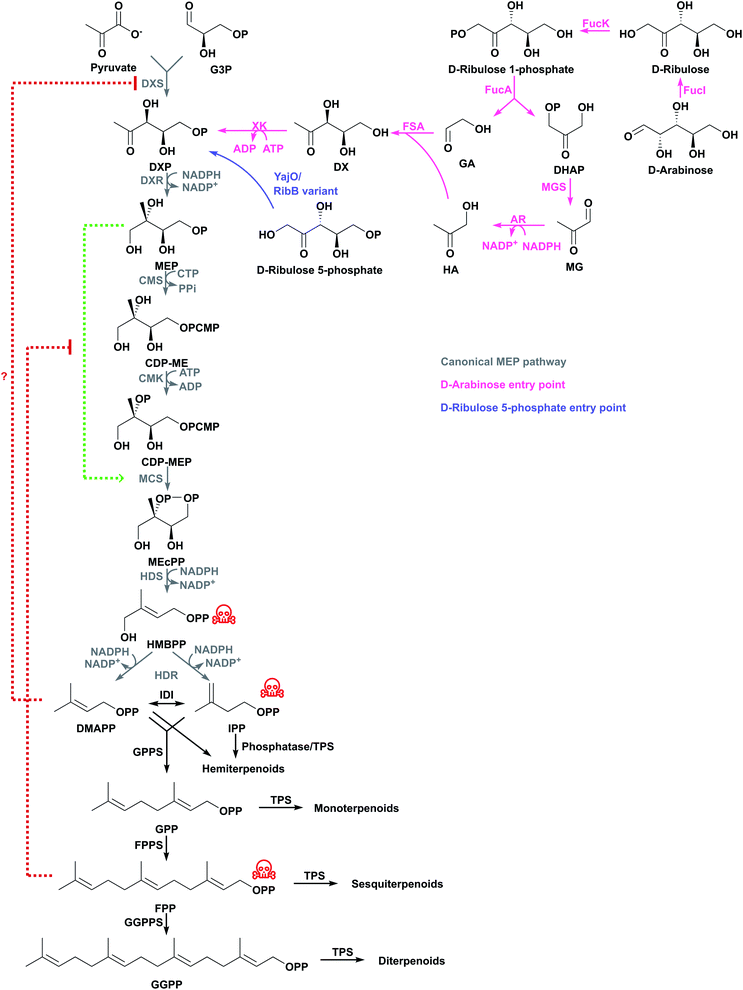
Most organisms use the classical MEP or MVA pathways to make terpenoids (Fig. 1). Bacteria use the MEP pathway, with the exception of Actinobacteria, Bacteroidetes, Chloroflexi, Firmicutes and Proteobacteria which have the classical MVA pathway or a variation thereof. Eukaryotes use the MVA pathway.13,14 In addition, plants use the MEP pathway in plastids, which is also essential for survival. Most archaea use alternative MVA pathways, except Sulfolobus, Metallosphaera and Acidianus which use the classical MVA pathway.14 Both the canonical MEP and MVA pathways have been engineered and opitimised to make terpenoids in E. coli.2.1 The native MEP pathway
The MEP pathway starts with the condensation of pyruvate and D-glyceraldehyde 3-phosphate (G3P) from central metabolism to form 1-deoxy-D-xylulose 5-phosphate (DXP) catalysed by DXP synthase (DXS) in a thiamine diphosphate-dependent reaction (Fig. 2).15,16 DXP is converted to MEP by DXP reductoisomerase (DXR) using NADPH,17 and MEP is activated with cytidine 5′-triphosphate (CTP) to produce 4-diphosphocytidyl-2-C-methyl-D-erythritol (CDP-ME) by CPD-ME synthase (CMS).18 CDP-ME is phosphorylated by CDP-ME kinase (CMK), and cyclised to 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) by MEcPP synthase (MCS).19,20 The cyclic diphosphate ring is opened and a reductive dehydration is catalysed by 4-hydroxy-3-methyl-butenyl diphosphate synthase (HDS) to produce 4-hydroxy-3-methyl-butenyl diphosphate (HMBPP).21–23 Finally, a reductive dehydration of HMBPP by HMBPP reductase (HDR) produces both IPP and DMAPP at a ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in E. coli,23–25 and IPP and DMAPP are interconverted by IPP delta-isomerase (IDI). Because both IPP and DMAPP are produced by the MEP pathway, IDI is not essential in organisms containing the MEP pathway, and many bacteria do not have a gene encoding this enzyme.26
1 in E. coli,23–25 and IPP and DMAPP are interconverted by IPP delta-isomerase (IDI). Because both IPP and DMAPP are produced by the MEP pathway, IDI is not essential in organisms containing the MEP pathway, and many bacteria do not have a gene encoding this enzyme.26
NADPH is consumed in the DXR, HDS and HDR steps.27 CTP is consumed at the CDP-ME step and ATP is consumed at the CMK step. Because CTP loses a diphosphate moiety, it needs 2 ATP molecules for regeneration. Therefore, the MEP pathway consumes 3 NADPH and 3 ATP for each IPP/DMAPP from pyruvate and G3P.28
DXS catalyses the most tightly regulated step. Populus trichocarpa DXS is feedback inhibited by IPP and DMAPP, which compete in the thiamine diphosphate binding pocket.29 This regulation is thought to occur in other species as well. Overexpressing dxs stimulates the MEP pathway and it leads to up to 10-fold titre increases;30–33 this has contributed to some of the highest reported terpenoid titres using the MEP pathway (Tables 1 and S1†).
Along with DXS, IDI is another important regulation point of the MEP pathway.34 As under normal growth conditions idi is expressed at low levels,35idi overexpression leads to higher terpenoid titres. For example, idi overexpression doubled isoprene production to 1 mg g−1 h−1 (ref. 36) and, together with dxs overexpression, increased limonene titres from 4.9 mg L−1 to 17.4 mg L−1.37
Optimisation of other steps in the MEP pathway might also be necessary to achieve higher terpenoid titres. Overexpressing dxs, dxr, and idi increases isoprene production 4.8-fold to 2.7 mg g−1 h−1.36 The insertion of a strong bacteriophage T5 chromosomal promoter controlling the genes encoding CMS and MCS, or CMK in E. coli increased β-carotene production 1.4- or 1.2-fold, respectively,35 suggesting these enzymes are also partially limiting. A potential reason why MCS is limiting might be that MEP activates MCS, and this feed-forward mechanism is inhibited by FPP (Fig. 2).38 Additional MCS might help to overcome this negative feedback mechanism.
Balancing expression of the genes that encode HDS and HDR increased β-carotene production by preventing accumulation of the toxic intermediate HMBPP and increasing MEcPP consumption before it effluxes from the cell.34,39 In one of the most successful attempts to engineer the MEP pathway in E. coli, a multivariate-modular pathway engineering approach was used to balance expression levels of DXS, CMS, CMK and IDI. This leads to the production of 1 g L−1 taxadiene in fed-batch fermentation.40 Similarly, overexpressing these genes and balancing the three remaining genes in the lycopene biosynthesis pathway leads to the highest reported lycopene titres.41
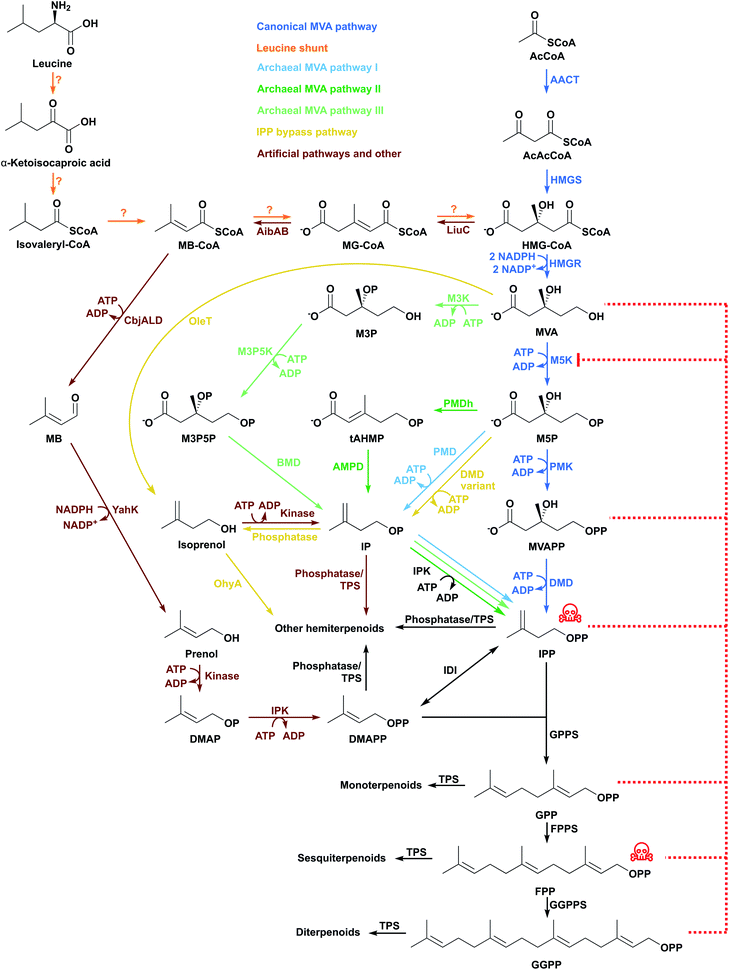
2.2 The MVA pathway
The Saccharomyces cerevisiae MVA pathway was the first alternative pathway to IPP/DMAPP to be introduced into E. coli and led to major improvements in reported titres.46 In part, these increases in reported titres are likely attributable to the MVA pathway improving IPP and DMAPP supply.47 This follows because the MVA pathway is mostly orthogonal to native E. coli metabolism.The MVA pathway starts with a Claisen condensation of two acetyl-CoA (AcCoA) molecules catalysed by an acetoacetyl-CoA thiolase (AACT), producing acetoacetyl-CoA (Fig. 3). The addition of a third AcCoA produces 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) in an aldol reaction catalysed by HMG-CoA synthase (HMGS).48 The reduction of HMG-CoA by HMG-CoA reductase (HMGR) with two equivalents of NADPH yields the 6-carbon intermediate MVA. MVA is phosphorylated twice in the 5-OH position by ATP, first by mevalonate 5-kinase (M5K) producing mevalonate 5-phosphate (M5P), and then by phosphomevalonate kinase (PMK), resulting in mevalonate diphosphate (MVAPP).49–51 This molecule is further decarboxylated in an ATP-mediated reaction catalysed by mevalonate diphosphate decarboxylase (DMD), producing only IPP.50 DMAPP is obtained from IPP by the action of IDI. The MVA pathway is conceptually divided into an upper pathway, from AcCoA to MVA, and a lower pathway, from MVA to IPP/DMAPP.
HMGR consumes 2 molecules of NADPH and M5K, PMK and DMD each consume ATP. In total, the MVA pathway therefore uses 2 NADPH and 3 ATP molecules to produce one molecule of IPP/DMAPP from AcCoA.
The upper MVA pathway (from AcCoA to MVA) can be limiting in E. coli terpenoid production. MVA supplementation into the culture broth of cells harbouring the entire MVA pathway increases amorphadiene titres, suggesting that provision of mevalonate by the expressed pathway is a bottleneck.52 Fine-tuning HMGS expression is important as high levels of this enzyme can accumulate the cytotoxic product HMG-CoA,52 which inhibits fatty acid biosynthesis, membrane formation and cell growth.55 HMGS allocates carbon flux to the MVA pathway and low HMGS levels can cause metabolic carbon to be redirected to acetate production instead.56 Furthermore, HMGR catalyses the only enzymatic reaction of the mevalonate pathway that uses a redox cofactor. Overexpression of HMGR can therefore disrupt the cellular redox balance. Fine-tuning HMGR levels and/or supply of redox cofactors are potentially important strategies to optimise pathway performance.57,58
M5K is one of the most regulated enzymes of the MVA pathway and its activity can be inhibited by high levels of substrate and product (Fig. 3).57,59–62 In most organisms, downstream diphosphate intermediates such as IPP, DMAPP, GPP, FPP and GGPP inhibit M5K by binding competitively to the ATP-binding site of M5K.60,63–65Streptococcus pneumoniae M5K is inhibited by MVAPP,66 but Staphylococcus aureus M5K is not.60S. aureus and S. cerevisiae M5K are also inhibited by substrate at millimolar MVA concentrations.57,60 This might explain why MVA can be produced at 30 g L−1,67 whereas high level accumulation of metabolites downstream of this step in the MVA pathway is challenging. PMK was also identified as a bottleneck in an amorphadiene producing E. coli strain. Targeted proteomics indicated poor production of this enzyme in the cell. Codon-optimisation and use of stronger promoters to express the gene increased both enzyme and mRNA transcript levels, with concomitant improvements in amorphadiene titres.68
E. coli has basal levels of IDI activity. However, overexpression of idi leads to higher terpenoid production when using the MVA pathway. Basal IDI levels are relatively low and the MVA pathway requires IDI to balance IPP and DMAPP. Overexpression of IDI in a strain harbouring the MVA pathway was found to increase lycopene titres 10-fold to 22.2 mg L−1.54 Similarly, idi overexpression increases DMAPP supply and leads to increases in β-farnesene titres of almost 200-fold (8.74 g L−1).69
Protein engineering has also been used to generate a catalytically superior S. cerevisiae IDI variant (L141H/Y195F/W256C), which improves lycopene production 1.8-fold, reaching 1.2 g L−1.78 Moreover, type II IDIs recently identified in some archaea,79–81 Gram-positive bacteria82,83 and cyanobacteria84 might also be beneficial in terpenoid production using the MVA pathway when compared to type I IDIs. These type II enzymes differ in amino acid sequence, structure, and reaction mechanism compared to the type I IDIs.84 Use of B. licheniformis type II idi in place of E. coli idi increased lycopene production (from 132 mg g−1 DCW to 181 mg g−1 DCW) in E. coli strains containing the MVA pathway.43 Use of S. aureus type II IDI also increased isoprene levels by 1.57-fold relative to E. coli strains containing the S. cerevisiae idi.74
Use of an Enterococcus faecalis HMGS variant (HMGSA110G) increases isoprene production 1.5-fold relative to strains containing the wild-type homolog, achieving production titres for isoprene of 6.3 g L−1.85 Furthermore, use of five different HMGR homologs has been investigated for amorphadiene production in E. coli.57In vitro assays showed that this enzyme can readily catalyse the reverse reaction. HMGR homologs with a high forward and low reverse reaction rate produced higher mevalonate levels in vivo. Nevertheless, when the MVA pathway harbouring these HMGR homologs was coupled to an amorphadiene producing cassette, the strains that accumulated more mevalonate did not produce the highest amorphadiene titres. The low titre obtained with the high MVA producing strains was attributed to substrate inhibition of M5K.57
In a more integrated approach, Liu et al. (2019) built an MVA pathway using E. faecalis AACT and HMGR, M. mazei M5K, and S. pneumoniae PMK, DMD and IDI for isoprene production.53 This pathway initially produced only 55.4 mg L−1 isoprene, but optimising promoter, plasmid copy number and the gene expression cassette, increased isoprene titres to almost 600 mg L−1.53 Replacing S. cerevisiae enzymes of the upper MVA pathway with E. faecalis homologs increased MVA accumulation 50-fold and isoprene production reached 500 mg L−1.85 Comparing the lower MVA pathways from S. aureus, Streptococcus pyogenes, S. pneumoniae, E. faecalis, and S. cerevisiae in a Coenzyme Q10 producing E. coli strain showed that S. pneumoniae genes led to a higher Coenzyme Q10 content under almost all conditions.47 In a similar study, the S. pneumoniae, S. pyogenes, S. aureus, E. faecalis and S. cerevisiae lower MVA pathways were compared to produce β-carotene supplying MVA as substrate. In this case, the S. pneumoniae and S. cerevisiae pathways were shown to be the best performers.86
The highest titres reported in E. coli for the production of most terpenoids – especially the hemi-, mono- and sesquiterpenoids – are obtained through heterologous expression and engineering of the MVA pathway. That said, the highest reported titres for some diterpenoids and lycopene have been obtained using optimised MEP pathways (Section 2.1 and Table 1). Higher titres are generally obtained using optimised MVA pathways rather than MEP pathways for isoprene,87 amorphadiene,46 valerenadiene,88 abietadiene,89 β-carotene,42 and retinoids90 production. These pathways can also be combined. Simultaneous engineering of both pathways leads to higher titres of the tetraterpenoid β-carotene than through the engineering of either pathway alone, leading to the highest reported β-carotene titres.42 Consistently, for lycopene production, inserting MVA pathways leads to further improvements in product titres compared to the use of MEP pathways alone.91 Furthermore, use of both the MEP and MVA pathways leads to a synergistic increase in isoprene titres to 24 g L−1, with flux increasing through both pathways, possibly because under oxygen-limiting conditions the MVA pathway produces reducing power from glucose that can be used by the MEP pathway.92 In contrast, inserting the MEP pathway into S. cerevisiae did not lead to major improvements in terpenoid titres, likely because E. coli HDS and HDR are expressed mainly as insoluble proteins in S. cerevisiae.93
3 Alternative precursor pathways
Canonical terpenoid pathways have been extensively engineered and optimised, but only a small number of terpenoids have been obtained at levels that might be considered as being attractive for commercial production. Variations of existing canonical pathways, or the development of fundamentally new precursor pathways, are alternative engineering approaches of potential benefit. Such strategies can give rise to higher degrees of orthogonality, or by replacing specific steps in existing pathways with metabolite shunts, bypass regulation points and/or avoid the accumulation of potentially toxic intermediates. Inclusion of alternative substrates and biocatalysts in biosynthetic pathways can also increase terpenoid diversity and lead to the production of compounds that are of potential commercial value (e.g. pharmaceutically active compounds, flavours, fragrances, and fuels) and that are readily accessed through modified terpenoid biosynthetic pathways.943.1 Alternative entry points to the MEP and MVA pathways
More recently, another pathway to DXP from pentoses was developed to produce lycopene in E. coli. In this pathway, a first module of genes degrades exogenous D-arabinose to glycolaldehyde and hydroxyacetone, which are subsequently condensed by a second module consisting of fructose-6-phosphate aldolase to produce 1-deoxyxylulose. This latter compound is then promiscuously phosphorylated by xylulose kinase to DXP (Fig. 2). The engineered strains were reported to have a 4-fold increase in lycopene content with exogenous hydroxyacetatone, reaching almost 900 μg g−1 DCW lycopene.97
A natural MEP pathway shunt was discovered in Rhodospirillum rubrum as part of S-adenosylmethionine-dependent polyamine metabolism98,99 and later found in several other bacteria.100 In this route, DXP is produced in four enzymatic steps from 5′-methylthioadenosine, the dead-end product of universal polyamine metabolism. This shunt increased levels of native carotenoid-based pigments in R. rubrum98 possibly by increasing DXP levels, although DXP was not quantified. A similar pathway in pathogenic E. coli produces DXP from 5′-deoxyadenosine, a radical S-adenosylmethionine biproduct.101 These pathways could be exploited in non-pathogenic E. coli in attempts to increase terpenoid titres.
3.2 Archaeal MVA pathways
The use of two different specialised enzymes (M3K and BMD) to catalyse a decarboxylation step performed by a single enzyme (DMD in the canonical MVA pathway and PMD in the archaeal MVA pathway I) could have arisen from an adaptation to extremely acid environments that did not favour the canonical pathway.113 DMD and PMD phosphorylate the hydroxyl in the 3-C position of MVAPP and M5P to produce IPP and IP, respectively.113,115,116 PMD loses kinase activity at low pH, but the decarboxylase activity remains intact, becoming a BMD.113 Therefore, there was potentially a need to evolve two separate enzymes that are homologous to PMD (M3K and BMD) to give rise to this alternative pathway.113 Of interest is that the phosphorylation in the 3-OH position and the decarboxylation of M3P are not sequential steps catalysed by the same enzyme (as is the case in the archaeal MVA pathway I) but rather are catalysed by two enzymes. There is also an additional step, a phosphorylation at the 5-OH position that needs a third enzyme from a different family.117
This pathway requires one more catalytic step compared to the canonical pathway, but has the same ATP requirement. As discussed above, the phosphorylation of MVA by M5K in the classical MVA pathway is one of the most heavily regulated enzymatic steps in the pathway.63 Consequently, an alternative pathway that by-passes this enzyme is of potential interest for terpenoid production. M3P accumulates in T. acidophilum and could be used to increase terpenoid production by heterologous expression of this alternative MVA pathway in E. coli.
Picrophilus torridus M3K was also used among other homologs to decarboxylate MVA and produce isoprenol in vivo and in vitro without needing to pass through IPP as an intermediate.118 In a different strategy, M3K was converted into a mevalonate 3-phosphate 5-kinase via a single amino acid substitution, and used with the archaeal MVA pathway I to produce approximately 0.347 μg lycopene per mg wet cells. This value is comparable to 0.491 μg lycopene/mg wet cells using the S. cerevisiae classical MVA pathway.119
A complete archaeal MVA pathway II has not been tested in E. coli. This would be of interest as it lacks one of the most regulated steps found in the canonical MVA pathway. Moreover, it would also be of interest to express both the archaeal MVA pathway II and the canonical MVA pathway together to explore any synergistic effects. M3K consumes MVA, and could thus potentially relieve substrate inhibition of M5K.
This pathway comprises enzymes which are not homologous to either mevalonate monophosphate or diphosphate decarboxylases, and seems to be widely conserved among archaea, including the taxonomically distant archaeon M. mazei.14,121 These enzymes do not require ATP. This might therefore create a more energy efficient route to terpenoids. M. mazei and A. pernix genes for the lower part of the archaeal MVA pathway III were introduced in E. coli to produce lycopene from exogenous mevalonolactone.121 Cells were cultivated semi-anaerobically because mevalonate 5-phosphate dehydratase is sensitive to oxidation, and produced up to 6 mg/OD600 lycopene.
There are very few examples of heterologous expression of any of the archaeal MVA pathways in E. coli. A lack of genetic tools for archaea may have limited studies of these pathways to in vitro assays.14 Nevertheless, these pathways may offer important advantages in terpenoid production compared to the classical MVA pathway. IPK is also present in plants and regulates carbon flux between the MEP and MVA pathways, balancing IP/IPP and DMAP/DMAPP ratios and helping to increase terpenoid production.13,122 Because IPP is toxic and inhibitory123 – as might also be the case with these other related compounds – it is possible that IPK could help reduce the effects caused by their accumulation. Other benefits of using archaeal variations compared to the canonical MVA pathway include bypassing highly regulated enzymes (archaeal MVA pathway II) and reducing ATP consumption (archaeal MVA pathway III).
3.3 IPP-bypass pathways
IPP accumulation is undesirable46 because it is converted to a toxic prenyl-ATP analog,123 and therefore strategies that bypass IPP can be beneficial by preventing IPP accumulation. Additionally, IPP-bypass pathways can reduce the number of metabolic steps and avoid competition for IPP with native metabolism and negative-feedback regulation. For example, combining the upper MVA pathway with a bacterial Jeotgalicoccus sp. ATCC 8456 fatty acid decarboxylase and a bacterial Elizabethkingia meningoseptica oleate hydratase led to isoprene production in E. coli.124 In this shorter pathway, MVA is decarboxylated directly to isoprenol (Fig. 3), which is further dehydrated to isoprene, thus allowing production of up to 620 mg L−1 isoprene using only two enzymatic steps from MVA (instead of five). This approach led to improved terpenoid levels, exceeding the highest titre achieved using the MEP pathway (314 mg L−1),31 but substantially lower than the highest titre achieved using a canonical MVA pathway (60 g L−1).76 Nonetheless, a shorter pathway could be beneficial when subjected to further titre optimisation (e.g. flux balancing and engineering).Similarly, new pathways that utilise decarboxylation of M5P by a variant DMD (to produce IP) and endogenous phosphatases (to dephosphorylate IP into isoprenol) have been reported (Fig. 3).125–127 Isoprenol production at up to 10.8 g L−1 has been achieved in fed-batch cultures, the highest titre reported to date for this compound.126,127
Although promising for hemiterpenoids, these reduced MVA pathways have not been exploited for longer terpenoid products because they bypass the common building blocks IPP and DMAPP. Because TPSs require IPP/DMAPP and their derivatives, these are the only orthogonal pathways to terpenoids that branch before IPP/DMAPP.128
3.4 Isoprenol/prenol to IPP/DMAPP pathways
A novel and simpler three-step pathway which uses isoprenol or prenol as feedstocks to produce IPP and DMAPP was recently exploited by several groups.129–134 This pathway decouples terpenoid biosynthesis from central metabolism and therefore has the potential to increase metabolic flux towards the production of terpenoids.135 The pathway also reduces any negative impacts on cell growth, since glucose (or other carbon source used for bacterial growth) is reserved for primary metabolism.136 The first of the three steps in this pathway involves phosphorylation of exogenously added isoprenol or prenol to produce IP or DMAP. These are then converted by IPK to IPP or DMAPP, respectively (Fig. 3). Finally, IDI can be used to balance IPP and DMAPP levels.Because the first reaction has not been found in existing metabolic pathways, several enzymes were tested to identify promiscuous kinase activity towards isoprenol and prenol. This new pathway was developed using S. cerevisiae choline kinase to catalyse the first phosphorylation reaction, Arabidopsis thaliana IPK and E. coli IDI.129 The pathway has been coupled to different downstream operons to produce several mono-, sesqui- and diterpenoids and lycopene, achieving a maximum titre of 9 mg g−1 DCW lycopene and 4.5 mg L−1 taxadiene,129 and 33.4 mg L−1 linalool.137 In a different study, Shigella flexneri non-specific acid phosphatase was used to catalyse the initial phosphorylation, and combined with T. acidophilum IPK and E. coli IDI to produce 190 mg L−1 lycopene.133 The highest reported terpenoid titres with this shorter 3-step pathway were obtained using E. coli hydroxyethylthiazole kinase, archaeal Methanothermobacter thermautotrophicus IPK and Streptomyces sp. IDI producing 2 g L−1 of geranoids and 0.5 g L−1 of limonene.130 The same authors also developed an alternative pathway comprising 8 enzymatic steps and requiring 2 ATP and 2 NAD(P)H (a lower cofactor requirement than for the canonical MVA or the MEP pathways) to obtain DMAPP from AcCoA, with isoprenol and prenol as intermediates. This new pathway produces 0.6 g L−1 of geranoids.
Titres obtained with isoprenol and prenol-derived pathways are generally lower compared to canonical pathways. However, in selected cases titres are promising and higher than those reported using canonical pathways.37,138 Shorter and orthogonal pathways are less regulated. This makes them easier to optimise compared to the canonical MEP and MVA pathways, which have been iteratively optimised over the past two decades. Consequently, the use of fed-batch processes and relatively simple pathway improvements may lead to competitive production strains after only a few rounds of pathway/strain optimisation.
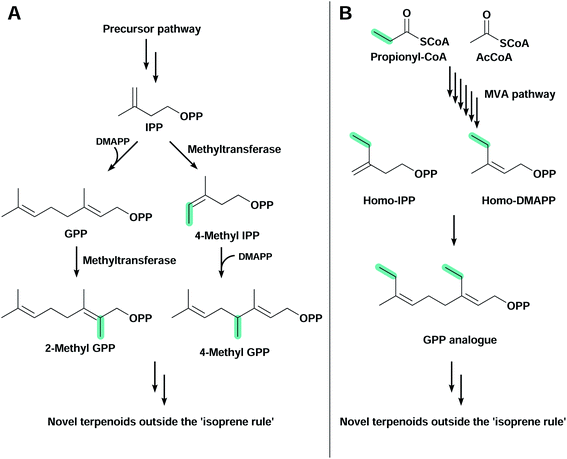
3.5 Terpenoids outside the ‘isoprene rule’
The large majority of terpenoids comprise C5 modules and follow the so-called ‘isoprene rule’,139 but recently discovered modifications in canonical pathways have allowed the biosynthesis of terpenoids outside this rule (Fig. 4). These orthogonal pathways allow the introduction of a flexible number of carbon atoms in terpenoid structures, further expanding chemical diversity. Simple modifications (e.g. methyl group addition) can improve biological activity,140 or generate novel organic structures that are otherwise difficult to obtain.The C11 terpenoids 2-methylisoborneol and 2-methylenebornane, are responsible for the muddy smell in contaminated drinking water, and are derived from the 2-methylgeranyl diphosphate intermediate, a product of the electrophilic methylation of GPP141 catalysed by a S-adenosyl-L-methionine-dependent methyl transferase (Fig. 4A).142,143 Other methyl transferases capable of methylating IPP144 and FPP145 have also been found in nature and play an important role in the synthesis of non-conventional terpenoids. In E. coli, the expression of wild-type and variant 2-methylisoborneol and 2-methylenebornane synthases with a GPP methyl transferase and the MVA pathway have allowed the production of several known and unknown flavours and fragrances compromising mono-, C11-, sesqui- and C16 terpenoids.146,147 Another study used an IPP methyl transferase from Streptomyces monomycini to produce a variety of C6 and C7 IPP analogues in E. coli containing the MVA pathway. This has led to the production of several C11, C12, C16 and C17 terpenoids, and new-to-nature methylated carotenoids when co-expressed with β-carotene or zeaxanthin biosynthetic pathways.148
The second alternative pathway found in nature that is capable of producing terpenoids outside the isoprene rule is the homomevalonate pathway, used by insects to produce C16, C17 or C18 terpenoid hormones (Fig. 4B).149–151 Instead of starting the MVA pathway with the condensation of two AcCoA molecules, in this orthogonal pathway propionyl-CoA is condensed with AcCoA, producing an unusual ethyl branch in terpenoid skeletons instead of the conventional methyl branch.152 This homomevalonate pathway was recently expressed in E. coli to produce C16 terpenoids.153 Although C16 terpenoids were produced at low titre, this approach has the advantage of not requiring additional ATP or the regeneration of S-adenosyl-L-methionine, which is a feature when using methyl transferases.153
The recently developed pathways from isoprenol and prenol have also been used to produce non-conventional terpenoids by using similar alcohols as substrates. An in vitro study explored the substrate promiscuity of E. coli hydroxyethylthiazole kinase and M. jannaschii IPK on 15 isoprenol and prenol analogues.134 These enzymes are able to produce diphosphates from alcohols with up to seven carbons, and the addition of a PPPS and TPSs produce a number of known and new terpenoids.134
4 Alternative prenyl diphosphate synthases
PPPSs elongate terpenoid chains by catalysing sequential condensations of allylic diphosphates (DMAPP, GPP, FPP and GGPP) with IPP. In E. coli, the same PPPS, IspA, catalyses GPP and FPP synthesis consecutively and thus favours production of FPP over GPP.154,155 FPP might be favoured because several native E. coli terpenoids are sesquiterpenoids involved in vital cellular processes. For example, FPP is used in the biosynthesis of heme O of cytochrome bo, part of the respiratory chain.156 Also, FPP is elongated with IPP to make the essential C40 octaprenyl diphosphate, used in the biosynthesis of the side chains of ubiquinone and menaquinone, which are components of electron transfer systems in the respiratory chain.157 Furthermore, FPP is elongated with IPP to make the C55 undecaprenyl diphosphate, used in the biosynthesis of peptidoglycans, which are essential cell wall constituents.158Heterologous PPPSs are used to increase monoterpenoid production in E. coli. Plant Abies grandis GPP synthase (AgGPPS) is the PPPS that leads to the highest monoterpenoid titres in E. coli (Table S1†).138,159–166 Unlike IspA, AgGPPS makes only GPP,167 and therefore likely increases the GPP pool available to monoterpenoid synthases. Thus, expressing AgGPPS leads to higher sabinene, pinene, limonene, or linalool titres compared to titres obtained by overexpression of ispA.37,159,161,164,168 Consistently, using a variant of IspA to favour GPP accumulation leads to higher titres for 1,8-cineole and linalool.165 Also, expressing this mutant ispA leads to higher pinene or linalool levels than overexpressing wild-type ispA, and to similar pinene levels to expressing AgGPPS in direct comparisons.159,168 AgGPPS also outperforms other GPPSs in direct comparisons159,169,170 and performs similarly to bacterial Streptomyces sp. strain KO-3988 GPPS.171 In one of the highest limonene titres reported, soluble AgGPPS levels did not correlate with titres, suggesting GPPS was not limiting.171
IspA is the most common PPPS used in those studies reporting the highest obtained sesquiterpenoid titres58,172–181 (Table S1†). IspA outperformed Blakeslea trispora and S. cerevisiae FPP synthase (FPPS) in direct comparisons.182 However, in two instances, S. cerevisiae FPPS outperformed IspA.183,184 ScFPPS is less catalytically efficient than IspA and this might be a reason for why IspA is often selected over ScFPPS.183 Several plant GGPP synthases (GGPPS) have also been used in some of the highest reported diterpenoids titres40,45,89,185–187 (Table S1†).
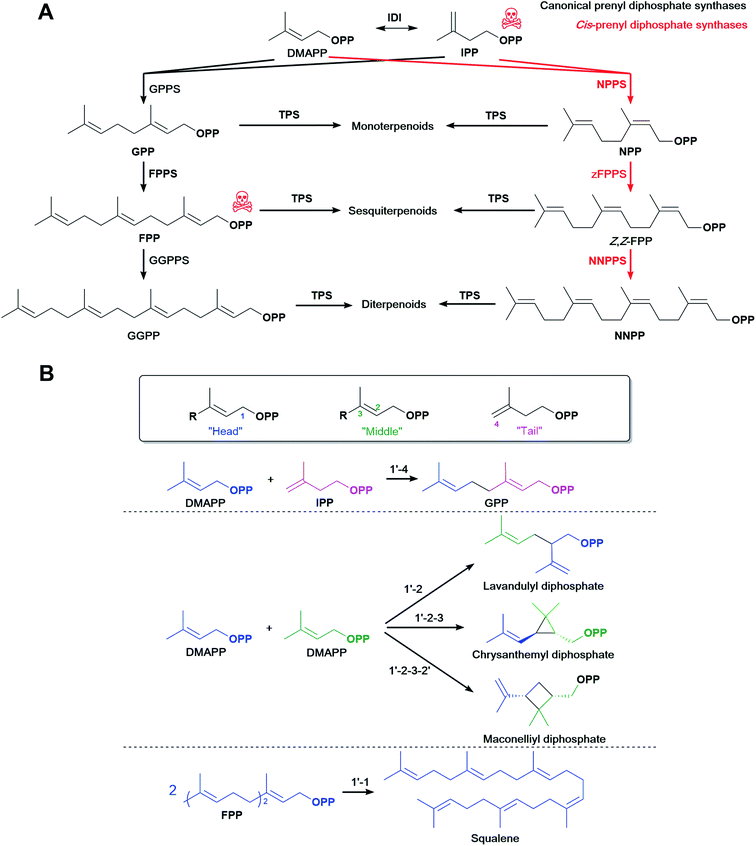
4.1 cis-Prenyl diphosphate synthases
Most commonly, terpenoid biosynthesis uses trans-prenyl diphosphates produced by trans-PPPSs. Alternative cis-prenyl diphosphates are products of cis-PPPSs which were discovered in plants. These were recently used to create orthogonal pathways for microbial terpenoid production. In tomato, IPP/DMAPP is also converted to neryl diphosphate (NPP), the cis-isomer of GPP, then to Z,Z-FPP, the all-cis-isomer of the more common all-trans E,E-FPP, and then to nerylneryl diphosphate (NNPP), the all-cis-isomer of GGPP (Fig. 5A). Likewise, cis-specific TPSs convert these precursors to several terpenoids.188–195Other cis-PPPSs and cis-specific TPSs were later characterised in other plant species,196,197 expanding the genes available for a combinational approach. That said, most canonical TPSs tested also accept cis-prenyl diphosphates,75,198–203 suggesting that canonical TPSs and their wide range of products are still available for use with cis-PPPSs. Variants of plant Citrus limon limonene synthase have higher affinity for NPP than the wild type,198 which suggests that canonical TPSs could be engineered to increase NPP affinity and further increase titres. cis-PPPSs can help divert metabolic flux towards the end product of interest, especially when the TPS might be the limiting factor owing to low catalytic activity and ineffective competition for trans-prenyl diphosphates.
This alternative pathway using cis-PPPSs was explored in yeast to increase monoterpenoid production. In S. cerevisiae, terpenoid biosynthesis might also favour production of essential FPP-derived terpenoids such as squalene, a yeast membrane component and major terpenoid carbon sink.198 Thus, monoterpenoid synthases compete with FPP production for GPP, and indeed monoterpenoid production is lower in S. cerevisiae than in some other organisms.4 Because the native S. cerevisiae FPPS cannot use NPP as a substrate,198 expressing Solanum lycopersicum NPP synthase (SlNPPS) diverts the metabolic flux from IPP/DMAPP to monoterpenoids instead of to FPP (Fig. 5A). Although SlNPPS does not accept IPP/DMAPP as efficiently as S. cerevisiae GPPS/FPPS and canonical TPSs have lower affinity for NPP than for GPP, monoterpenoid production is higher with SlNPPS. This suggests that making NPP increases monoterpenoid titres by creating an orthogonal pathway that effectively diverts metabolic flux. Indeed, assays using 13C-labelled substrates confirmed that most of the limonene, the intended monoterpenoid product in this case, is derived from NPP, whereas most of the FPP is derived from GPP.198 Consistent with this, a similar study produced higher limonene titres and lower squalene levels with NPPS than with a GPPS/FPPS variant that favours GPP in a direct comparison.199 Expressing NPPS also leads to high limonene titres in the yeast Yarrowia lipolytica.204
Competition with FPP in E. coli might not be as strong as in S. cerevisiae because generally higher monoterpenoid titres are achieved in E. coli.4 However, expressing SlNPPS in E. coli led to 694 mg L−1 limonene, 2.9-fold more than when expressing AgGPPS (181 mg L−1).75 This suggests that either the NPP pathway enzymes are more active, which is not the case in yeast,198 or alternatively that NPP bypasses competition with FPP for GPP in E. coli. Further optimisation showed that SlNPPS is efficient enough to sustain limonene production in E. coli to as much as 1.29 g L−1 limonene,75 an amount comparable to the highest reports obtained with other strategies.58,138,160,171 Thus, NPPS can sustain at least as much production as other approaches, and could produce higher titres under different conditions.
A Mycobacterium tuberculosis Z,E-FPPS was used in E. coli to produce Z,E-farnesol from exogenous MVA. Z,E-FPP is likely not incorporated into native E. coli metabolism, therefore creating an orthogonal pathway.205 Also, S. lycopersicum NNPP synthase (NNPPS) and lycosantalonol synthase were used in E. coli to demonstrate biosynthesis of the diterpenoid lycosantalene.188 Beyond these examples, cis-prenyl diphosphates longer than NPP have not yet been explored in E. coli or compared to trans-prenyl diphosphate pathways. However, they could be useful for the production of longer terpenoids if the native PPPSs and TPSs are trans-specific; the cis-prenyl diphosphates can then redirect carbon flux to the terpenoid of interest (Fig. 5A).
4.2 Non-head-to-tail prenyl diphosphate synthases, terpenoid cyclases and other prenyltransferases
The most common way of elongating prenyl diphosphate chains is in a 1′-4 head-to-tail fashion, referred to as ‘regular elongation’, where the allylic diphosphate precursor (such as DMAPP) is first ionised to form a carbocation and then undergoes a nucleophilic attack by the double bond of IPP followed by proton elimination to form the elongated allylic diphosphate.206Some PPPSs catalyse alternative reactions to form non-head-to-tail connected terpenoid skeletons (Fig. 5B). These reactions, termed ‘irregular’, include head-to-head (1′-1) elongation, head-to-middle (1′-2) branching, cyclopropanation (c1′-2-3) or cyclobutanation (c1′-2-3-2′) reactions and are most commonly involved in the synthesis of some irregular longer terpenoids such as the triterpenoid squalene and C40 phytoene, the precursor of carotenoids.207,208 Shorter terpenoids formed in a non-head-to-tail fashion are very rare, but mono- and sesquiterpenoids with these irregular structures are found for example in plants209 and marine bacteria.210 A plant Lavandula x intermedia PPPS catalyses a head-to-middle condensation of two DMAPP molecules to generate lavandulyl diphosphate, the precursor of (R)-lavandulol and (R)-lavandulyl acetate.209,211 Also, a plant Chrysanthemum cinerariaefolium PPPS catalyses the cyclopropanation of two DMAPP molecules to form the irregular diphosphate chrysanthemyl diphosphate.209,212 Other irregular short diphosphates include cyclolavandulyl, maconellyl, planococcyl and isosesquilavandulyl diphosphates and are converted to short irregular terpenoids.213 To the best of our knowledge, E. coli has not been used as a platform to produce short terpenoids from non-head-to-tail condensations. Expressing and engineering these alternative PPPS could potentially create orthogonal terpenoid biosynthetic pathways similar to the examples listed above with cis-PPPS, and further diversify terpenoid molecules.
Terpenoid cyclases promote changes in terpenoid structure by catalysing reactions which generate multiple rings and stereocentres.214 Recently, several class I terpenoid cyclases were also found to be bifunctional and able to work as aromatic prenyltransferases. These enzymes catalyse the condensation of DMAPP or GPP and indole to produce prenylated indole compounds in E. coli. This activity might be more widely spread among terpenoid cyclases, opening up a strategy to decrease the accumulation of toxic phosphorylated intermediates such as DMAPP.214 A distant relationship between prenyltransferases and terpenoid cyclases was identified recently that revealed a new family of prenyltransferases, which uses a repurposed terpenoid cyclase structural fold to prenylate glutamic acid.215
Other prenyltransferases generated different diphosphate molecules, including chlorinated analogues, molecules having a hydrophilic moiety in their alkyl chain, or cyclic diphosphate molecules in previous in vitro studies,216–219 and were reviewed recently.220 Because these irregular and modified prenyl diphosphates differ structurally from their native counterparts, they could be used to create orthogonal pathways to terpenoids in E. coli, or expand the end-product range.
5 Bacterial terpenoid synthases and modifying enzymes
Prenyl diphosphates are used by TPSs to make terpenoid backbones, which subsequently can be modified by additional enzymes to make functionalised structures. TPSs and modifying enzymes were first mostly elucidated in plants and therefore most studies in E. coli and most of the highest titre reports use genes of plant origin (Table S1†). However, eukaryotic proteins expressed in E. coli can fold incorrectly and form inclusion bodies, and in general are not successfully expressed in bacteria,221 even after codon optimisation and removal of eukaryotic elements such as N-terminal signal peptides. A lack of eukaryote-specific post-translational modifications can also lead to less than optimal catalytic activities222 or different product profiles, which might impact terpenoid titres. Expressing soluble protein is particularly important for TPSs because they often catalyse the bottleneck step in terpenoid production, especially after precursor supply optimisation.46,172,223 Also, toxic intermediates like FPP can accumulate without efficient uptake by TPSs.46 Indeed, few of the tested plant TPSs are sufficiently active to support high-titre production. For example, only 20 of a group of 37 plant monoterpenoid synthases tested together produced the expected monoterpenoid in E. coli and only approximately a quarter of these had sufficiently high titres to enable subsequent optimisation.224In contrast, bacterial enzymes are more similar to E. coli proteins, and therefore more likely to be highly expressed, soluble, and catalytically active. In fact, the highest titres obtained in E. coli with bacterial TPSs are comparable or higher than those obtained with the corresponding plant TPSs. For example, bacterial Streptomyces clavuligerus 1,8-cineole synthase (CinS) produces 23.4 mg L−1 1,8-cineole, comparable to 23.6 mg L−1 1,8-cineole produced with plant Salvia fruticosa CinS, and higher than 9.4 mg L−1 1,8-cineole produced with plant A. thaliana CinS and 3.6 mg L−1 1,8-cineole produced with plant Citrus unshiu CinS in a direct comparison.225,226 In this case, the bacterial enzyme also produced almost exclusively 1,8-cineole which is not the case with plant enzymes (96% vs. 42–67%). In an independent study, the same S. clavuligerus CinS produces 228 mg L−1 1,8-cineole, consistently higher but comparable to 200 mg L−1 using the fungal Hypoxylon sp. CinS. Further optimisation allows production of 653 mg L−1 1,8-cineole using this bacterial CinS,165 the highest 1,8-cineole titre in E. coli reported so far. Additionally, bacterial S. clavuligerus linalool synthase (LinS) allowed accumulation to 72.7 mg L−1 linalool, 300 times the 0.26 mg L−1 linalool titre produced by plant Artemisia annua LinS.225,226 When comparing the highest reported titres, bacterial LinS led to more linalool accumulation than plant LinS: 1.03 g L−1 linalool was produced with bacterial S. clavuligerus LinS,159 whereas 601.2 mg L−1 linalool was produced with a fungal LinS,227 505 mg L−1 linalool with plant Mentha citrata LinS165 and approximately 85 mg L−1 linalool with plant Clarkia breweri LinS.228 Thus, whereas several eukaryotic monoterpenoid synthases were screened to find high producers in E. coli, the only two identified bacterial monoterpenoid synthases produced higher or comparable titres to the best eukaryotic synthases.
Most of the TPS diversity comes from plant enzymes, but for a few terpenoids, only bacterial TPSs have been found, which highlights the value of bacterial TPSs despite the difficulty in identifying them. For example, Streptomyces griseus (+)-caryolan-1-ol synthase makes up to 406 mg L−1 sesquiterpenoids in E. coli including 100 mg L−1 caryophyllene and 10 mg L−1 caryolan-1-ol (449 mg L−1 total terpenoid).229 A bacterium such as E. coli might be the preferred chassis for production of these and other terpenoids made by bacterial TPSs. For example, the bacterial S. clavuligerus LinS that produced one of the highest linalool titres in E. coli produces none in S. cerevisiae, even though linalool can be produced in S. cerevisiae with plant M. citrata LinS.230 Also, bacterial Streptomyces coelicor epi-isozizane synthase and Streptomyces UC5319 pentalenene synthase produced 728 mg L−1epi-isozizaene and 780 mg L−1 pentalenene in E. coli, respectively, whereas 344 mg L−1 pentalenene was obtained in S. cerevisiae, although less titre optimisation was applied to the latter.231
Subsequent enzymes functionalise the terpenoid backbones made by TPSs and increase terpenoid diversity, creating additional molecules with biological activities of interest. Oxidations are usually catalysed by cytochromes P450 (CYPs) and eukaryotic CYPs are more abundant. CYPs are membrane proteins and so to enhance expression in E. coli and obtain high terpenoid titres, the N-terminal domain that contains a signal peptide for membrane insertion is usually modified to direct the protein to the plasma membrane, or truncated to direct to the cytosol.232,233 Thus, eukaryotic CYPs are successfully used for high-titre terpenoid production in E. coli. For example, plant Artemisia annua CYP71AV1 was used to make 105 mg L−1 artemisinic acid, which can be converted to the antimalarial artemisinin,174 and the plant Taxus brevifolia CYP taxadiene 5α-hydroxylase was used to make 58 mg L−1 taxadien-5α-ol.40 However, even following N-terminal domain engineering, eukaryotic CYPs are poorly expressed and have low activity in E. coli as eukaryotic CYPs are usually bound to the ER.174 The recent focus on bacterial terpenoid pathways is also identifying bacterial CYPs that have been used for terpenoid production in E. coli.234 Notable examples include using Bacillus megaterium CYP102A1 (BM3) engineered to change substrate specificity to convert amorphadiene to 250 mg L−1 artemisinic-11S,12-epoxide, which can in turn be converted to artemisinin,173 and Mycobacterium HXN-1500 CYP153A6 to convert limonene to 105 mg L−1 perillyl alcohol.138 To reach high terpenoid titres, CYPs require reducing partners, which work well at a ratio lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It is not a trivial exercise to determine which partners will work well with specific CYPs so several are often tested.235,236
1. It is not a trivial exercise to determine which partners will work well with specific CYPs so several are often tested.235,236
There are few other examples of attempts to optimise titres using bacterial TPSs and modifying enzymes in E. coli (Table S1†). However, several bacterial TPSs, CYPs and reducing partners have been expressed in E. coli to characterise product profiles,236–241 which further suggests that bacterial terpenoid biosynthesis genes express well in E. coli. Titres might be improved further by optimising precursor pathways and performing protein engineering studies.
One of the challenges of identifying bacterial TPSs and modifying enzymes is that pathways for natural products biosynthesis are often silent under standard laboratory culture conditions. However, the advent of inexpensive whole-genome sequencing has made thousands of bacterial genomes available for mining of terpenoid pathways and new TPSs. Bacterial TPSs have been elusive because of low whole sequence similarity with eukaryotic TPSs. Recently, Hidden Markov Models, which are trained with known sequences to create profiles that might encode functionality, were used to identify candidate bacterial TPS.242–244 After several iterations, this approach suggested as many as 600 candidates (in addition to the almost ubiquitous geosmin or 2-methylisoborneol synthases) in the thousands of bacterial genomic sequences currently available, mostly in the Streptomyces genus.245 Experimental validation has confirmed the activity of over 70 bacterial TPSs for two monoterpenoids (1,8-cineole and linalool), over 40 sesquiterpenoids and over 30 diterpenoids.240,245–247 Therefore, there are a growing number of bacterial TPS candidates that could be used for terpenoid production.
With the demonstrated benefits of using bacterial TPSs and modifying enzymes, identification of new bacterial terpenoids and the characterisation of associated terpenoid pathways among the thousands of available genomic sequences is attractive. As pathways are often silent, different culturing conditions might need to be used to induce genes and identify compounds.248 Genes that encode natural product biosynthetic pathways are often clustered in genomes,249 and this is also true for the terpenoids, where TPS genes are physically close to genes for CYPs and other modifying enzymes. Therefore, identification of biosynthetic gene clusters is useful in genome mining.250 The bacterial genomes database “Antibiotics and Secondary Metabolites Analysis Shell” counts over 5000 putative terpenoid biosynthetic gene clusters.251 So far, over 70 clusters have been characterised and deposited in the Minimum Information about a Biosynthetic Gene cluster database with nucleotide sequences and product descriptions.252
The challenge is to characterise the large and growing number of remaining cryptic clusters. Natural product pathways can be characterised by laboriously expressing single genes or assembling putative pathways, but recent methodologies allow expression of whole gene clusters in E. coli, Streptomyces or other bacterial strains to identify products,253 and manipulation of native genomes to activate silent clusters.254 Additionally, omics approaches are also being used. For example, genomics are correlated with metabolomics in groups of closely related species with different terpenoid profiles to match metabolites to conserved gene clusters.255 Many of these approaches can be accelerated using high-throughput automated assembly, screening and MS analysis.256,257 Consequently, new bacterial terpenoid biosynthetic pathways will be identified and utilised in future high-titre production in E. coli or other bacterial hosts.
6 Handling toxicity
6.1 Mechanisms of toxicity
Production of several terpenoids seems to reach a maximum limit despite additional attempts to increase titres through optimising biosynthetic pathways. This limitation might be imposed by end-product toxicity. Terpenoid toxicity varies between reports perhaps due to the different conditions used, and few reports analyse multiple terpenoids under the same conditions. That said, in general, several terpenoids, such as isoprenol, limonene, linalool, sabinene, bisabolol, farnesene, and sclareol inhibit E. coli growth at g L−1 levels (Table 2), which are levels similar to the highest titres produced (Table 1).| Terpenoids and derivatives | Minimum concentration that causes growth reduction but not complete inhibition (g L−1) | MIC (>indicates that total inhibition was not reached at that concentration) (g L−1) | T (°C) | Scale (mL) | Reference |
|---|---|---|---|---|---|
| a Where concentrations were expressed as “%”, “% v/v” was assumed for liquid terpenoids and g L−1 were calculated using density. Dashes indicate that this terpenoid is a derivative of the terpenoid in the row above. | |||||
| Hemiterpenoids | |||||
| Isoprenol | 1.5 | 2.4 | 37 | ∼1? | 274 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Monoterpenoids | |||||
| α−Pinene | 4.29 | >42.9 | 37 | 0.8 | 275 |
| α−Pinene | 0.429 | 1.28 | 37 | 5 | 281 |
| 1,8-Cineole | 37 | 0.1 | 321 | ||
| Geraniol | 1.78 | 8.89 | 30 | 5 | 273 |
| Geraniol | 0.222 | 0.444 | 37 | 0.8 | 275 |
| Geraniol | 0.075 | 0.300 | 37 | 1 | 163 |
| -Geranyl acetate | 4.58 | >45.8 | 37 | 0.8 | 275 |
| -Geranyl acetate | 0.5 | 37 | 1 | 163 | |
| Myrcene | >7.94 | >7.94 | 30 | 5 | 273 |
| Sabinene | 1 | >5 | 31 | 50 | 161 |
| Linalool | 1 | 37 | 0.2 | 159 | |
| Limonene | 1.05 | 42.05 | 30 | 0.1? | 264 |
| Limonene | >25.23 | >25.23 | 30 | 0.15 | 263 |
| Limonene | 3.36 | 8.41 | 37 | 5 | 281 |
| Limonene | 0.05 | 0.21 | 37 | 0.8 | 275 |
| -Perillyl alcohol | 4.79 | 30 | 0.15 | 263 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Sesquiterpenoids | |||||
| α−Bisabolene | >180 | >180 | 37 | 1 | 322 |
| Bisabolol | >27.6 | >27.6 | 30 | 0.15 | 263 |
| (−)-α−Bisabolol | 5 | >5 | 309 | ||
| γ-Bisabolene | >27 | >27 | 30 | 0.15 | 263 |
| Farnesol | >26.61 | >26.61 | 30 | 0.15 | 263 |
| α−Farnesene | >24.39 | >24.39 | 30 | 0.15 | 263 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Diterpenoids | |||||
| Cis-Abienol | >2 | >2 | 37 | 10 | 183 |
| Cembratriene-ol | >2 | >2 | 37 | 2 | 45 |
| Sclareol | >2 | >2 | 37 | 10 | 185 |
In general, terpenoids are hydrophobic hydrocarbons and therefore can accumulate in biological membranes and increase membrane permeability, thus disrupting vital biological processes (e.g. by allowing proton leakage and dissipating the proton motive force needed for energy production; Fig. 6).258,259 A commonly used metric for hydrophobicity is the logarithm of the partition coefficient between octanol and water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P).260 Hydrocarbons with log
P).260 Hydrocarbons with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P between 1 and 4 partition easily into membranes and therefore can be toxic;258 above log
P between 1 and 4 partition easily into membranes and therefore can be toxic;258 above log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4 water solubility also determines toxicity.260
P 4 water solubility also determines toxicity.260
Hydrophobicity might also alter secretion times. For example, even when efflux pumps are induced to export terpenoids, zeaxanthin accumulates in the broth at 72 h post-induction, whereas the more hydrophobic canthaxantin peaks at 96 h, and the more hydrophobic β-carotene takes 120–144 h to accumulate extracellularly; lycopene requires outer membrane removal through spheroplast formation to detect extracellular levels.261 Consistently, carotenoids accumulate in the inner membrane instead of in the media because of low water solubility in E. coli.261
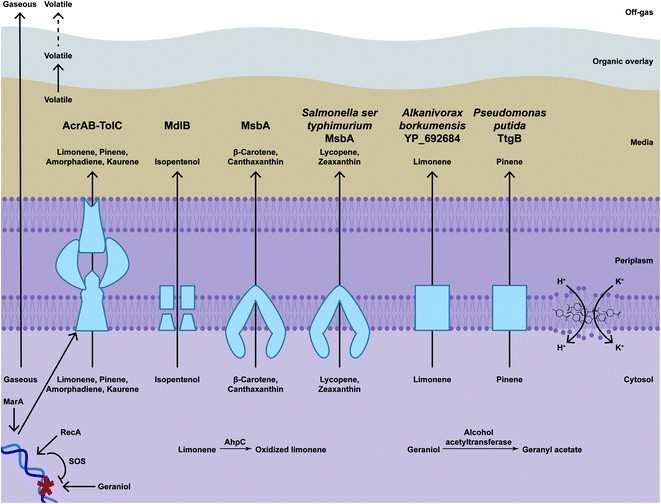
Some structural features can also determine toxicity. In general, hydroxyl groups and double bonds can increase toxicity.262 Thus, some terpenoids can be toxic at lower concentrations than other similarly hydrophobic terpenoids. For example, E. coli grows as well with 25.23 g L−1 exogenously added limonene as a non-supplemented control, so limonene itself might not be toxic. But oxidised forms of limonene such as terpineol and perillyl alcohol inhibit growth almost completely at concentrations of 4.67 g L−1 and 4.79 g L−1, respectively.263 Also, limonene is oxidised in vivo to a hydroperoxide that might cause oxidative damage, as evidenced by the finding that an endogenous alkyl hydroperoxidase AhpC (L177Q) variant alleviates toxicity.264 Another example is that of geraniol; in one report, 75 mg L−1 exogenous geraniol reduces growth rate and 300 mg L−1 inhibits growth entirely,163 whereas similar terpenoids are toxic in the g L−1 range (Table 2). This additional toxicity might be due to DNA damage; recA, which encodes a protein that activates SOS box genes for DNA repair, confers resistance to geraniol and, consistently, ΔrecA strains are more geraniol sensitive than wild-type counterparts.265 Therefore, toxicity can be hard to predict based on terpenoid structure and rather is assessed empirically.
Terpenoid production in E. coli therefore needs to address arguably the last step in the biosynthetic pathway i.e. removal of the end-product from the cell, and even from the media, to handle end-product toxicity, as well as to achieve efficient recovery and purification from the production broth. Of note, the concentration of exogenously added terpenoids that can be tolerated might be higher than the maximum titres that cells can produce, because the intracellular concentration is expected to be higher when the cell produces the terpenoid than when it is added externally.
6.2 Physical extraction
Physically removing terpenoids from the media reduces the exposure of the cells to the end product. Some terpenoids with high volatility simply evaporate. For example, isoprene is gaseous and volatilises from the cultivation medium, which enables production to 60 g L−1 isoprene in E. coli.76 Other terpenoids might form salts that precipitate out of solution. For example, artemisinic acid is known to accumulate on the walls of cultivation flasks and therefore is produced to high titres in S. cerevisiae.266While volatility prevents toxicity, it also leads to product loss and titre underestimation. Therefore, condensers and gas-stripping are often used for product recovery from the culture off-gas. Also, a hydrophobic overlay of an organic solvent chosen to maximise terpenoid solubilisation can be directly applied to the culture medium to create a two-phase fermentation system (Fig. 6). For example, titres of the volatile amorphadiene increase from 24 mg L−1 to 281 mg L−1 when using a condenser to trap volatiles in the off-gas and dodecane as an organic overlay; by further improving culturing conditions, titres reach 0.5 g L−1 amorphadiene with this recovery method.267
Organic overlays also help handle toxicity of less volatile terpenoids, perhaps by displacing the equilibrium from the media and therefore reducing exposure of cells to the end product. Many examples of the highest titres achieved so far report using overlays (Tables 1 and S1†). Solid adsorbents similarly sequester terpenoids in culture media and increase titres (Tables 1 and S1†).
6.3 Secretion
Microbes can be engineered to enhance hydrocarbon resistance, and thus also increase production titres. One of the most common approaches is to increase secretion by inducing or overexpressing efflux pumps, which have broad substrate specificity and export metabolites that might be toxic (Fig. 6).268The multiprotein AcrAB-TolC is the main E. coli pump that increases terpenoid tolerance and production titres. AcrAB-TolC is composed of AcrB, an ATP-binding cassette (ABC) transporter in the inner membrane, TolC, an outer membrane channel, and AcrA, an accessory protein in the periplasmic space that mediates interaction between AcrB and TolC to export the substrate through the two membranes.269 Overexpressing tolC increases amorphadiene titres from approximately 45 mg L−1 to approximately 160 mg L−1.270 However, overexpressing acrAB has modest, if any, effect on pinene titres, which range from 7.3 to 8.1 mg L−1.271 Also, overexpressing tolC is reported to increase amorphadiene titres from 250 mg L−1 to approximately 350 mg L−1 but kaurene production is perhaps not significantly affected (from approximately 17 mg L−1 to approximately 21 mg L−1). Combining efflux pump components can also further increase titres. Overexpressing acrB and two copies of tolC increases titres to 404.8 mg L−1 amorphadiene and overexpressing acrA, acrB and tolC increases titres to 31.76 mg L−1 kaurene.187 AcrAB-TolC is positively regulated by MarA, a global transcriptional factor that induces efflux pumps.272 Overexpression of marA allows growth on 8.9 g L−1 geraniol, which otherwise completely inhibits growth, and reduces intracellular geraniol levels from 12.9 μg mg−1 to 6.3 μg mg−1, suggesting that efflux pumps might act by reducing intracellular terpenoid concentrations.273
Other E. coli pumps also increase terpenoid titres. For example, overexpressing mdlB, which encodes a putative ABC transporter, increases isopentenol titres by 12% to 931 mg L−1.274 Also, overexpressing msbA, which encodes an ABC transporter that transports the lipid A-core moiety of lipopolysaccharide from the inner leaflet to the outer leaflet of the inner membrane, increases canthaxantin 4.4-fold from approximately 39 μg L−1 to approximately 170 μg L−1 and β-carotene also 4.4-fold to approximately 250 μg L−1.261
Expressing pumps from other organisms also improves tolerance and production. For example, expressing Alcanivorax borkumensis YP_692684 modestly increases limonene titres from approximately 35 mg L−1 to approximately 55 mg L−1.275 In addition, expressing Salmonella enterica serovar typhimurium msbA with a mutation that results in a I89T change increases zeaxanthin 2.4-fold from approximately 100 μg L−1 to approximately 249 μg L−1 and secreted lycopene 4.3-fold from approximately 48 μg L−1 to approximately 210 μg L−1.261 Expressing Pseudomomas putida ttgB however has very modest (if any) effects on titres (from 8.1 to 9.1 mg L−1 pinene).271
Efflux pumps differ in terpenoid specificity. For example, a combination of efflux pumps that is optimal for increasing amorphadiene titres is different than the optimal combination for increasing kaurene titres.187 Also, when testing a group of pumps, StMbsA increases zeaxanthin and lycopene titres the most, whereas EcMbsA increases canthaxanthin the most.261 Similarly, several pumps that increase amorphadiene production do not increase lycopene production.270
Therefore, the use of efflux pumps seems to provide some advantage, although the effects in titre can be modest. This suggests that terpenoid production is affected by terpenoid toxicity or intracellular accumulation, but the effects so far are limited, providing about 4-fold titre increase at most. The limitation might be because overexpressing pumps also inhibit bacterial growth.276 Export could be improved through protein engineering to increase specificity and/or activity, particularly with AcrB, which recognises substrates in the cytosol and the inner membrane. Importantly, use of inducing pumps have not yet been reported in most of the higher titre producing systems where they would be expected to be the most beneficial.
6.4 Membrane engineering
Because terpenoids accumulate in membranes, extending membranes can increase the storage capacity for terpenoids and lead to higher terpenoid production while still allowing cellular functions. There are several E. coli genes that ‘bend’ membranes when overexpressed, causing invaginations and increasing levels of membrane constituents. For example, overexpressing tsr, which encodes a methyl-accepting chemotaxis protein, promotes the formation of membrane invaginations and extensions, and increases squalene titres 2.3-fold from 272 to 612 mg L−1, the highest squalene titre reported to date.277 Similarly, overexpressing almgs, which encodes monoglucosyldiacylglycerol synthase, causes membrane extension and improves β-carotene titres 1.5-fold from 24.7 mg L−1 to 36.9 mg L−1. Also, overexpressing two genes involved in diglyceride-3-phosphate biosynthesis increases glycerophospholipids (membrane components) and β-carotene titres 1.3-fold (to 31.3 mg L−1). Overexpressing these last three genes together leads to a further synergistic improvement, increasing titres 4.2-fold relative to the parental strain to 103.5 mg L−1 β-carotene. In a separate β-carotene hyperproducing strain, this membrane engineering leads to a 1.4-fold titre increase from 196.3 mg L−1 to 268.1 mg L−1 β-carotene.278 These early results show promise for further rounds of membrane engineering, combining membrane-altering factors to increase storage capacity and terpenoid titres while preserving cell integrity and growth rates.6.5 Excretion
An alternative way of removing terpenoids from cells is to stimulate excretion. E. coli excretes outer membrane vesicles when deleting tolA or tolR, which encode components of the Tol complex that maintains outer membrane integrity, or nlpI, which encodes an outer membrane-bound protein that might contribute to protein complex formation. Thus, deleting nlpI together with either tolA or tolR increases β-carotene excretion and total production. Additionally, overexpressing genes in the biosynthetic pathway of phosphatidylethanolamine, the main phospholipid in E. coli membranes, to supply additional membrane for the excretion further increases β-carotene production 71-fold relative to the parent strain (final production of 10.7 mg g−1 DCW β-carotene). Carrying out similar changes in a β-carotene hyperproducing strain leads to a similar 24-fold production increase relative to the parent strain (reaching 44.8 mg g−1 DCW β-carotene279).6.6 Adaptive laboratory evolution
Because toxicity is multi-causal and can be specific to each terpenoid, adaptive laboratory evolution experiments which address toxicity in a more general fashion are a good strategy to increase terpenoid titres. Adaptive laboratory evolution on E. coli grown on media supplemented with pinene increases tolerance from 5 to 20 g L−1, and increases titres by 31% to 7.3 mg L−1 pinene.271 Also, evolving E. coli to grow on increasing sabinene concentrations from 3 g L−1, below the initial point of no growth at 3.5 g L−1, to 12 g L−1 generates a strain with 8-fold increased production (from 22.8 mg L−1 to 191.8 mg L−1). In this strain, a large number of genes have mutations or altered expression levels. Three genes in particular contribute to sabinene tolerance: ycbK, scpA, and ygiZ. ycbK is a gene of unknown function that belongs to the defective Lambda prophage 12 family with roles in biofilm formation, stress response and cell wall maintenance. scpA encodes methylmalonyl-CoA mutase and affects central metabolism. ygiZ encodes an internal membrane protein of unknown function and is induced by the BglJ-RcsB transcriptional activator that regulates motility, biofilm formation and stress responses. Overexpressing ycbK or scpA increases sabinene titres approximately 2-fold, but overexpressing ygiZ decreases sabinene titres.280 This work illustrates the multi-causality of toxicity and how engineering tolerance might require a multi-pronged approach involving several relevant cellular processes simultaneously and mechanisms we still do not fully understand.Similarly, other genes increase terpenoid resistance and production titres but by poorly defined mechanisms. For example, several genes are upregulated in response to E. coli cultivation with isopentenol. Six of these genes involved in oxidative stress response (fpr), general stress response (metR, yqhD, and gidB), heat shock-related response (ibpA) and transport (mdlB) increase isopentenol titres when overexpressed. Overexpressing metR, which encodes a DNA-binding transcriptional activator, leads to the highest improvement (55%, from 838 mg L−1 to 1290 mg L−1 isopentenol274). Like efflux pumps, genes granting tolerance and titre increases might be terpenoid specific. For example, overexpressing Marinobacter aquaeolei VT8 yceI improves resistance to pinene and terpinolene, but not to limonene or terpinene.281
6.7 Bioderivatisation
Bioderivatisation consists of converting the target terpenoid to a less toxic derivative inside the cell to enable accumulation to high titres and subsequently reconverting the derivative to the original target terpenoid.282 This approach was partially applied to the particularly toxic geraniol, which reduces growth rate at 75 mg L−1 and fully inhibits growth at 300 mg L−1.163 In contrast, geranyl acetate, an esterified derivative of geraniol, reduces final optical density (OD) by 40% at 4.6 g L−1 but then no further reduces OD up to 45.8 g L−1 (probably because it forms a second phase at concentrations higher than its aqueous solubility,275 which is lower than that of geraniol163). Therefore, expressing the gene encoding Rosa hybrida alcohol acetyltransferase in E. coli to catalyse intracellular esterification of geraniol increases titres from 35 mg L−1 geraniol to 375 mg L−1 geranyl acetate, which represents a more than 8-fold molar increase. By further optimising conditions, 4.8 g L−1 geranyl acetate was produced,163 over 2-fold higher than the previously reported maximum of 2 g L−1 geraniol.162,170 Esterification also prevents spontaneous conversion to toxic geraniol derivatives. Conversion of geranyl acetate back to geraniol however was not discussed in this report. To that end, an endogenous E. coli acetylesterase hydrolyses geranyl acetate to geraniol.162 So far it has been used to increase geraniol levels by preventing spontaneous conversion to geranyl acetate, but has not yet been utilised in a bioderivatisation strategy of the type described above. Expressing this acetylesterase with a secretion tag that allows reconversion outside the cell could potentially enable recovery of geraniol when using bioderivatisation approaches.In summary, several approaches have been used separately in attempts to mitigate product toxicity. In many cases, the benefits are modest but a combination of approaches could in principle give rise to synergistic effects. Available evidence suggests that further combinatorial analysis of these types of approaches is warranted.
7 Conclusions and future perspectives
The use of alternative and artificial biosynthetic pathways is now demonstrated for the production of terpenoids in metabolic engineering programs (Fig. 7). The ever-increasing efforts to understand the rich diversity of terpenoid biosynthetic chemistry in nature, particularly ongoing searches for new bacterial TPSs and modifying enzymes, and pathways to their synthesis, will provide new opportunities for the future microbial production of terpenoids. Where natural pathways are not currently available, retrosynthesis tools will be used to suggest artificial biosynthetic pathway designs and enable searches for suitable candidate enzymes.283,284 The studies described in this review have helped to validate the use of enzymes within a pathway context and provide useful frameworks for further optimisation to reach high-titre terpenoid production. It goes without saying that enzyme engineering, including both rational design and directed evolution, will continue to contribute to this field. This might involve the engineering of enzymes that are more resistant to feedback control, or enzymes with improved catalytic efficiency, or new specificities towards desired target activities/products.77,137,168,173,186,198,257,285,286 The use of alternative and artificial pathways with this ever-expanding enzyme resource and pathway engineering tools should extend current capabilities, thereby allowing high-titre production of a wide range of terpenoids in E. coli and other microbial species.Extensive studies of the MEP and the MVA pathways have demonstrated that balancing is required to (i) avoid the accumulation of toxic intermediates, (ii) overcome the limiting effects of regulation and (iii) optimise metabolic flux. Complete understanding of canonical pathway regulation and how accumulation of pathway intermediates limits flux remain to be elucidated. This is necessary to inform pathway balancing realised through engineering and modelling, and will likely involve combinatorial approaches that address a range of issues of the type identified in this review.
High-throughput methodologies that harness automation and miniaturisation to construct and assess the capabilities of newly engineered strains will become increasingly important to assemble new genetic constructs and test terpenoid production. Automated Design-Build-Test-Learn platforms that are embedded in biofoundry approaches for microbial strain engineering will provide the basis for such work. This will enable rapid exploration of DNA parts (e.g. promoters, ribosome binding sites)287–289 to identify improved pathways and target compound production,41,256,290 with an increased emphasis on the use of data-driven predictive engineering.289,291,292 Establishing high-throughput screening protocols for terpenoids beyond available low sensitivity and non-specific methods will be important.293 Currently, GC-MS is widely used in the field but suffers from low throughput. Amongst others, developments in high-throughput GC-QTOF, which measures terpenoids from the small volumes of multi-well plates, is potentially an attractive approach.257
Balancing central metabolism precursors, replacing the carbon source, modifying the energy and reducing power balance in the cell, and other approaches will also need to be explored alongside the engineering of new biosynthetic pathways. Redirecting carbon flux and balancing precursor levels are known to increase terpenoid levels,294–298 as is enhanced availability of essential cofactors such as ATP and NADPH.299,300 Other compounds of value are also produced by pathways that require the same metabolic precursors used for terpenoid production,301 with opportunities for learning across different product types. End-product toxicity remains as a major challenge for high-titre terpenoid production. This will require new solutions from microbial strain engineering and also biochemical engineering (e.g. in situ product removal in continuous culture). Ultimately, production from renewable feedstocks is required. Limited examples are available, such as the production of pinene from pre-treated switchgrass302 and hydrolysed macroalgae,303 and isopentenol produced from pre-treated and hydrolysed lignocellulose.304 Production may need also to migrate into industrial production hosts to take advantage of agricultural or industrial feedstock waste streams, or carbon dioxide.305,306 There is clearly much to do. This review will hopefully define some of the key challenges and strategies to achieve high level production of terpenoids in E. coli. The hope is that through this expanding knowledge base large-scale production of terpenoids using microbial cell factories will become a reality.
8 Abbreviations
| AACT | Acetoacetyl-CoA thiolase |
| ABC | ATP binding cassette |
| AcAcCoA | Acetoacetyl-CoA |
| AcCoA | Acetyl-CoA |
| AgGPPS | Abies grandis geranyl diphosphate synthase |
| AMPD | trans-Anhydromevalonate 5-phosphate decarboxylase |
| AR | Aldehyde reductase |
| BMD | Mevalonate biphosphate decarboxylase |
| CPD-ME | 4-Diphophocytidyl-2-C-methyl-D-erythritol |
| CDP-MEP | 4-Diphophocytidyl-2-C-methyl-D-erythritol 2-phosphate |
| CinS | 1,8-Cineole synthase |
| CMK | 4-Diphophocytidyl-2-C-methyl-D-erythritol kinase |
| CMS | 4-Diphophocytidyl-2-C-methyl-D-erythritol synthase |
| CTP | Cytidine 5′-triphosphate |
| CYP | Cytochrome P450 |
| DHAP | Dihydroxyacetone phosphate |
| DMAP | Dimethylallyl phosphate |
| DMAPP | Dimethylallyl diphosphate |
| DMD | Mevalonate diphosphate decarboxylase |
| DX | 1-Deoxy-D-xylulose |
| DXP | 1-Deoxy-D-xylulose 5-phosphate |
| DXR | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase |
| DXS | 1-deoxy-D-xylulose 5-phosphate synthase |
| FPP | Farnesyl diphosphate |
| FPPS | Farnesyl diphosphate synthase |
| FSA | Fructose-6-phosphate aldolase |
| G3P | D-Glyceraldehyde 3-phosphate |
| GA | Glycolaldehyde |
| GGPP | Geranylgeranyl diphosphate |
| GGPPS | Geranylgeranyl diphosphate synthase |
| GPP | Geranyl diphosphate |
| GPPS | Geranyl diphosphate synthase |
| HA | Hydroxyacetone |
| HDR | 4-Hydroxy-3-methyl-butenyl diphosphate reductase |
| HDS | 4-Hydroxy-3-methyl-butenyl diphosphate synthase |
| HMBPP | 4-Hydroxy-3-methyl-butenyl diphosphate |
| HMG-CoA | 3-Hydroxy-3-methylglutaryl-CoA |
| HMGR | 3-Hydroxy-3-methylglutaryl-CoA reductase |
| HMGS | 3-Hydroxy-3-methylglutaryl-CoA synthase |
| IDI | Isopentenyl diphosphate delta-isomerase |
| IP | Isopentenyl phosphate |
| IPK | Isopentenyl phosphate kinase |
| IPP | Isopentenyl diphosphate |
| LinS | Linalool synthase |
| M3K | Mevalonate 3-kinase |
| M3P | Mevalonate 3-phosphate |
| M3P5K | Mevalonate 3-phosphate 5-kinase |
| M3P5P | Mevalonate 3,5-biphosphate |
| M5K | Mevalonate 5-kinase |
| M5P | Mevalonate 5-phosphate |
| MB | 3-Methyl-2-butenal |
| MB-CoA | 3-Methyl-2-butenoyl-CoA |
| MCS | 2-C-Methyl-D-erythritol 2,4-cyclodiphosphate synthase |
| MEcPP | 2-C-Methyl-D-erythritol 2,4-cyclodiphosphate |
| MEP | 2-C-Methyl-D-erythritol 4-phosphate |
| MG | Methylglyoxal |
| MG-CoA | 3-Methylglutaconyl-CoA |
| MGS | Methylglyoxal synthase |
| MVA | Mevalonate |
| MVAPP | Mevalonate diphosphate |
| NNPP | Nerylneryl diphosphate |
| NNPPS | Nerylneryl diphosphate synthase |
| NPP | Neryl diphosphate |
| NPPS | Neryl diphosphate synthase |
| OD | Optical density |
| PMD | Mevalonate 5-phosphate decarboxylase |
| PMDh | Phosphomevalonate dehydratase |
| PMK | Phosphomevalonate kinase |
| PPPS | Prenyl diphosphate synthase |
| SlNPPS | Solanum lycopersicum neryl diphosphate synthase |
| tAHMP | trans-Anhydromevalonate 5-phosphate |
| TPS | Terpenoid synthase |
| XK | D-Xylulose kinase |
| zFPPS | Z,Z-Farnesyl diphosphate synthase |
9 Author contributions
MAR: conceptualisation, visualisation, writing – original draft, writing – review & editing. CAF: conceptualisation, writing – original draft, writing – review & editing. NSS: conceptualisation, supervision, funding acquisition, writing – review & editing.10 Conflicts of interest
There are no conflicts to declare.11 Acknowledgements
MAR and NSS are supported by the UK Biotechnology and Biological Sciences Research Council (BB/S011684/1). The authors also acknowledge funding from the Office of Naval Research Global. We thank Brenda F. Narice for critical comments on the manuscript.12 Notes and references
- J. Buckingham, Dictionary of natural products, supplement 4, CRC press, 1997 Search PubMed.
- S. D. Tetali, Planta, 2019, 249, 1–8 CrossRef CAS.
- J. M. Clomburg and R. Gonzalez, Appl. Microbiol. Biotechnol., 2010, 86, 419–434 CrossRef CAS PubMed.
- Z. Zebec, J. Wilkes, A. J. Jervis, N. S. Scrutton, E. Takano and R. Breitling, Curr. Opin. Chem. Biol., 2016, 34, 37–43 CrossRef CAS PubMed.
- F.-X. Niu, Q. Lu, Y.-F. Bu and J.-Z. Liu, Synthetic and Systems Biotechnology, 2017, 2, 167–175 CrossRef.
- S. Moser and H. Pichler, Appl. Microbiol. Biotechnol., 2019, 103, 5501–5516 CrossRef CAS PubMed.
- S. H. Kung, S. Lund, A. Murarka, D. McPhee and C. J. Paddon, Front. Plant Sci., 2018, 9, 87 CrossRef PubMed.
- A. L. Meadows, K. M. Hawkins, Y. Tsegaye, E. Antipov, Y. Kim, L. Raetz, R. H. Dahl, A. Tai, T. Mahatdejkul-Meadows, L. Xu, L. Zhao, M. S. Dasika, A. Murarka, J. Lenihan, D. Eng, J. S. Leng, C. L. Liu, J. W. Wenger, H. Jiang, L. Chao, P. Westfall, J. Lai, S. Ganesan, P. Jackson, R. Mans, D. Platt, C. D. Reeves, P. R. Saija, G. Wichmann, V. F. Holmes, K. Benjamin, P. W. Hill, T. S. Gardner and A. E. Tsong, Nature, 2016, 537, 694–697 CrossRef CAS.
- W. Wu and C. T. Maravelias, Biotechnol. Biofuels, 2018, 11, 294 CrossRef CAS PubMed.
- C. Sun, C. Theodoropoulos and N. S. Scrutton, Bioresour. Technol., 2020, 300, 122666 CrossRef CAS PubMed.
- V. Shukla and S. C. Phulara, Appl. Environ. Microbiol., 2021, 87, e02369-20 CrossRef.
- A. V. Pandit, S. Srinivasan and R. Mahadevan, Nat. Commun., 2017, 8, 1–11 CrossRef.
- L. K. Henry, M. Gutensohn, S. T. Thomas, J. P. Noel and N. Dudareva, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10050–10055 CrossRef CAS.
- S. T. Thomas, G. V. Louie, J. W. Lubin, V. Lundblad and J. P. Noel, ACS Chem. Biol., 2019, 14, 1767–1779 CrossRef CAS PubMed.
- L. M. Lois, N. Campos, S. R. Putra, K. Danielsen, M. Rohmer and A. Boronat, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 2105–2110 CrossRef CAS PubMed.
- M. Rohmer, M. Seemann, S. Horbach, S. Bringer-Meyer and H. Sahm, J. Am. Chem. Soc., 1996, 118, 2564–2566 CrossRef CAS.
- T. Kuzuyama, S. Takahashi, H. Watanabe and H. Seto, Tetrahedron Lett., 1998, 39, 4509–4512 CrossRef CAS.
- T. Kuzuyama, M. Takagi, K. Kaneda, T. Dairi and H. Seto, Tetrahedron Lett., 2000, 41, 703–706 CrossRef CAS.
- T. Kuzuyama, M. Takagi, K. Kaneda, H. Watanabe, T. Dairi and H. Seto, Tetrahedron Lett., 2000, 41, 2925–2928 CrossRef CAS.
- M. Takagi, T. Kuzuyama, K. Kaneda, H. Watanabe, T. Dairi and H. Seto, Tetrahedron Lett., 2000, 41, 3395–3398 CrossRef CAS.
- S. Hecht, W. Eisenreich, P. Adam, S. Amslinger, K. Kis, A. Bacher, D. Arigoni and F. Rohdich, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 14837–14842 CrossRef CAS.
- A.-K. Kollas, E. C. Duin, M. Eberl, B. Altincicek, M. Hintz, A. Reichenberg, D. Henschker, A. Henne, I. Steinbrecher and D. N. Ostrovsky, FEBS Lett., 2002, 532, 432–436 CrossRef CAS PubMed.
- F. Rohdich, F. Zepeck, P. Adam, S. Hecht, J. Kaiser, R. Laupitz, T. Gräwert, S. Amslinger, W. Eisenreich, A. Bacher and D. Arigoni, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1586 CrossRef CAS.
- F. Rohdich, S. Hecht, K. Gärtner, P. Adam, C. Krieger, S. Amslinger, D. Arigoni, A. Bacher and W. Eisenreich, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 1158–1163 CrossRef CAS.
- B. Altincicek, E. C. Duin, A. Reichenberg, R. Hedderich, A.-K. Kollas, M. Hintz, S. Wagner, J. Wiesner, E. Beck and H. Jomaa, FEBS Lett., 2002, 532, 437–440 CrossRef CAS PubMed.
- F. M. Hahn, A. P. Hurlburt and C. D. Poulter, J. Bacteriol., 1999, 181, 4499–4504 CrossRef CAS PubMed.
- L. Zhao, W. C. Chang, Y. Xiao, H. W. Liu and P. Liu, Annu. Rev. Biochem., 2013, 82, 497–530 CrossRef CAS PubMed.
- A. Banerjee and T. Sharkey, Nat. Prod. Rep., 2014, 31, 1043–1055 RSC.
- A. Banerjee, Y. Wu, R. Banerjee, Y. Li, H. Yan and T. D. Sharkey, J. Biol. Chem., 2013, 288, 16926–16936 CrossRef CAS PubMed.
- P. Matthews and d. E. T. Wurtzel, Appl. Microbiol. Biotechnol., 2000, 53, 396–400 CrossRef CAS.
- Y. Zhao, J. Yang, B. Qin, Y. Li, Y. Sun, S. Su and M. Xian, Appl. Microbiol. Biotechnol., 2011, 90, 1915–1922 CrossRef CAS PubMed.
- S. W. Kim and J. D. Keasling, Biotechnol. Bioeng., 2001, 72, 408–415 CrossRef CAS PubMed.
- Y. Li and G. Wang, J. Appl. Microbiol., 2016, 121, 932–940 CrossRef CAS PubMed.
- Q. Li, F. Fan, X. Gao, C. Yang, C. Bi, J. Tang, T. Liu and X. Zhang, Metab. Eng., 2017, 44, 13–21 CrossRef CAS PubMed.
- L. Z. Yuan, P. E. Rouviere, R. A. Larossa and W. Suh, Metab. Eng., 2006, 8, 79–90 CrossRef CAS PubMed.
- X. Lv, H. Xu and H. Yu, Appl. Microbiol. Biotechnol., 2013, 97, 2357–2365 CrossRef CAS PubMed.
- F.-L. Du, H.-L. Yu, J.-H. Xu and C.-X. Li, Bioresources and Bioprocessing, 2014, 1, 10 CrossRef.
- J. K. Bitok and C. F. Meyers, ACS Chem. Biol., 2012, 7, 1702–1710 CrossRef CAS.
- K. Zhou, R. Zou, G. Stephanopoulos and H. P. Too, PLoS One, 2012, 7, e47513 CrossRef CAS PubMed.
- P. K. Ajikumar, W.-H. Xiao, K. E. Tyo, Y. Wang, F. Simeon, E. Leonard, O. Mucha, T. H. Phon, B. Pfeifer and G. Stephanopoulos, Science, 2010, 330, 70–74 CrossRef CAS.
- P. Coussement, D. Bauwens, J. Maertens and M. De Mey, ACS Synth. Biol., 2017, 6, 224–232 CrossRef CAS PubMed.
- J. Yang and L. Guo, Microb. Cell Fact., 2014, 13, 160 CrossRef PubMed.
- S. A. Rad, H. S. Zahiri, K. A. Noghabi, S. Rajaei, R. Heidari and L. Mojallali, World J. Microbiol. Biotechnol., 2012, 28, 313–321 CrossRef CAS PubMed.
- W. Mischko, M. Hirte, M. Fuchs, N. Mehlmer and T. B. Brück, Microb. Cell Fact., 2018, 17, 164 CrossRef CAS PubMed.
- W. Mischko, M. Hirte, S. Roehrer, H. Engelhardt, N. Mehlmer, M. Minceva and T. Brück, Green Chemistry, 2018, 20, 2637–2650 RSC.
- V. J. Martin, D. J. Pitera, S. T. Withers, J. D. Newman and J. D. Keasling, Nat. Biotechnol., 2003, 21, 796 CrossRef CAS PubMed.
- H. S. Zahiri, S. H. Yoon, J. D. Keasling, S. H. Lee, S. Won Kim, S. C. Yoon and Y. C. Shin, Metab. Eng., 2006, 8, 406–416 CrossRef CAS PubMed.
- H. Rudney and J. J. Ferguson Jr, J. Am. Chem. Soc., 1957, 79, 5580–5581 CrossRef CAS.
- S. Chaykin, J. Law, A. Phillips, T. Tchen and K. Bloch, Proc. Natl. Acad. Sci. U. S. A., 1958, 44, 998 CrossRef CAS.
- U. Henning, E. Möslein and F. Lynen, Arch. Biochem. Biophys., 1959, 83, 259–267 CrossRef CAS.
- T. Tchen, J. Am. Chem. Soc., 1957, 79, 6344–6345 CrossRef CAS.
- D. J. Pitera, C. J. Paddon, J. D. Newman and J. D. Keasling, Metab. Eng., 2007, 9, 193–207 CrossRef CAS PubMed.
- C. L. Liu, H. R. Bi, Z. Bai, L. H. Fan and T. W. Tan, Appl. Microbiol. Biotechnol., 2019, 103, 239–250 CrossRef CAS.
- B. H. Choi, J. H. Kim, S. Y. Choi, S. J. Han and P. C. Lee, Bioresource Technology Reports, 2019, 7, 100291 CrossRef.
- L. Kizer, D. J. Pitera, B. F. Pfleger and J. D. Keasling, Appl. Environ. Microbiol., 2008, 74, 3229–3241 CrossRef CAS PubMed.
- K. W. George, A. Chen, A. Jain, T. S. Batth, E. E. Baidoo, G. Wang, P. D. Adams, C. J. Petzold, J. D. Keasling and T. S. Lee, Biotechnol. Bioeng., 2014, 111, 1648–1658 CrossRef CAS.
- S. M. Ma, D. E. Garcia, A. M. Redding-Johanson, G. D. Friedland, R. Chan, T. S. Batth, J. R. Haliburton, D. Chivian, J. D. Keasling and C. J. Petzold, Metab. Eng., 2011, 13, 588–597 CrossRef CAS.
- J. Alonso-Gutierrez, E.-M. Kim, T. S. Batth, N. Cho, Q. Hu, L. J. G. Chan, C. J. Petzold, N. J. Hillson, P. D. Adams and J. D. Keasling, Metab. Eng., 2015, 28, 123–133 CrossRef CAS PubMed.
- R. D. Tanaka, L. Y. Lee, B. L. Schafer, V. J. Kratunis, W. A. Mohler, G. W. Robinson and S. T. Mosley, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 2872–2876 CrossRef CAS.
- N. E. Voynova, S. E. Rios and H. M. Miziorko, J. Bacteriol., 2004, 186, 61–67 CrossRef CAS PubMed.
- S. H. Yoon, Y. M. Lee, J. E. Kim, S. H. Lee, J. H. Lee, J. Y. Kim, K. H. Jung, Y. C. Shin, J. D. Keasling and S. W. Kim, Biotechnol. Bioeng., 2006, 94, 1025–1032 CrossRef CAS PubMed.
- H. M. Woo, G. W. Murray, T. S. Batth, N. Prasad, P. D. Adams, J. D. Keasling, C. J. Petzold and T. S. Lee, Chem. Eng. Sci., 2013, 103, 21–28 CrossRef CAS.
- J. K. Dorsey and J. W. Porter, J. Biol. Chem., 1968, 243, 4667–4670 CrossRef CAS.
- J. Gray and R. Kekwick, Biochim. Biophys. Acta, Gen. Subj., 1972, 279, 290–296 CrossRef CAS.
- K.-x. Huang, A. Scott and G. N. Bennett, Protein Expression Purif., 1999, 17, 33–40 CrossRef CAS.
- J. L. Andreassi, K. Dabovic and T. S. Leyh, Biochemistry, 2004, 43, 16461–16466 CrossRef CAS PubMed.
- J. Wang, S. Niyompanich, Y.-S. Tai, J. Wang, W. Bai, P. Mahida, T. Gao and K. Zhang, Appl. Environ. Microbiol., 2016, 82, 7176–7184 CrossRef CAS PubMed.
- A. M. Redding-Johanson, T. S. Batth, R. Chan, R. Krupa, H. L. Szmidt, P. D. Adams, J. D. Keasling, T. S. Lee, A. Mukhopadhyay and C. J. Petzold, Metab. Eng., 2011, 13, 194–203 CrossRef CAS PubMed.
- S. You, Q. Yin, J. Zhang, C. Zhang, W. Qi, L. Gao, Z. Tao, R. Su and Z. He, Bioresour. Technol., 2017, 243, 228–236 CrossRef CAS.
- Y. A. Primak, M. Du, M. C. Miller, D. H. Wells, A. T. Nielsen, W. Weyler and Z. Q. Beck, Appl. Environ. Microbiol., 2011, 77, 7772–7778 CrossRef CAS PubMed.
- E. Kazieva, Y. Yamamoto, Y. Tajima, K. Yokoyama, J. Katashkina and Y. Nishio, Microbiology, 2017, 163, 1283 CrossRef CAS.
- K. W. George, M. G. Thompson, A. Kang, E. Baidoo, G. Wang, L. J. G. Chan, P. D. Adams, C. J. Petzold, J. D. Keasling and T. S. Lee, Sci. Rep., 2015, 5, 11128 CrossRef PubMed.
- S.-J. Kim, S. K. Kim, W. Seong, S.-G. Woo, H. Lee, S.-J. Yeom, H. Kim, D.-H. Lee and S.-G. Lee, Catalysts, 2019, 9, 432 CrossRef CAS.
- M. Li, H. Chen, C. Liu, J. Guo, X. Xu, H. Zhang, R. Nian and M. Xian, Microb. Cell Fact., 2019, 18, 1–12 CrossRef PubMed.
- J. Wu, S. Cheng, J. Cao, J. Qiao and G.-R. Zhao, J. Agric. Food Chem., 2019, 67, 7087–7097 CrossRef CAS PubMed.
- G. M. Whited, F. J. Feher, D. A. Benko, M. A. Cervin, G. K. Chotani, J. C. McAuliffe, R. J. LaDuca, E. A. Ben-Shoshan and K. J. Sanford, Ind. Biotechnol., 2010, 6, 152–163 CrossRef CAS.
- H. Chen, C. Liu, M. Li, H. Zhang, M. Xian and H. Liu, RSC Adv., 2018, 8, 15021–15028 RSC.
- H. Chen, M. Li, C. Liu, H. Zhang, M. Xian and H. Liu, Microb. Cell Fact., 2018, 17, 65 CrossRef PubMed.
- R. Dutoit, J. De Ruyck, V. Durisotti, C. Legrain, E. Jacobs and J. Wouters, Proteins, 2008, 71, 1699–1707 CrossRef CAS.
- M. A. Siddiqui, A. Yamanaka, K. Hirooka, T. Bamaba, A. Kobayashi, T. Imanaka, E. I. Fukusaki and S. Fujiwara, Biochem. Biophys. Res. Commun., 2005, 331, 1127–1136 CrossRef CAS PubMed.
- S. Yamashita, H. Hemmi, Y. Ikeda, T. Nakayama and T. Nishino, Eur. J. Biochem., 2004, 271, 1087–1093 CrossRef CAS PubMed.
- K. Kaneda, T. Kuzuyama, M. Takagi, Y. Hayakawa and H. Seto, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 932–937 CrossRef CAS.
- R. Laupitz, S. Hecht, S. Amslinger, F. Zepeck, J. Kaiser, G. Richter, N. Schramek, S. Steinbacher, R. Huber and D. Arigoni, Eur. J. Biochem., 2004, 271, 2658–2669 CrossRef CAS.
- S. J. Barkley, R. M. Cornish and C. D. Poulter, J. Bacteriol., 2004, 186, 1811–1817 CrossRef CAS PubMed.
- J. Yang, M. Xian, S. Su, G. Zhao, Q. Nie, X. Jiang, Y. Zheng and W. Liu, PLoS One, 2012, 7, e33509 CrossRef CAS PubMed.
- S. H. Yoon, S. H. Lee, A. Das, H. K. Ryu, H. J. Jang, J. Y. Kim, D. K. Oh, J. D. Keasling and S. W. Kim, J. Biotechnol., 2009, 140, 218–226 CrossRef CAS PubMed.
- A. Zurbriggen, H. Kirst and A. Melis, BioEnergy Res., 2012, 5, 814–828 CrossRef CAS.
- S. E. Nybo, J. Saunders and S. P. McCormick, J. Biotechnol., 2017, 262, 60–66 CrossRef CAS.
- D. Morrone, L. Lowry, M. K. Determan, D. M. Hershey, M. Xu and R. J. Peters, Appl. Microbiol. Biotechnol., 2010, 85, 1893–1906 CrossRef CAS.
- H.-J. Jang, S.-H. Yoon, H.-K. Ryu, J.-H. Kim, C.-L. Wang, J.-Y. Kim, D.-K. Oh and S.-W. Kim, Microb. Cell Fact., 2011, 10, 59 CrossRef CAS PubMed.
- Z. Li, Q. Chen, J. Tang, Q. Li and X. Zhang, Chin. J. Biotechnol., 2019, 35, 404–414 Search PubMed.
- C. Yang, X. Gao, Y. Jiang, B. Sun, F. Gao and S. Yang, Metab. Eng., 2016, 37, 79–91 CrossRef CAS PubMed.
- J. Kirby, K. L. Dietzel, G. Wichmann, R. Chan, E. Antipov, N. Moss, E. E. K. Baidoo, P. Jackson, S. P. Gaucher, S. Gottlieb, J. LaBarge, T. Mahatdejkul, K. M. Hawkins, S. Muley, J. D. Newman, P. Liu, J. D. Keasling and L. Zhao, Metab. Eng., 2016, 38, 494–503 CrossRef CAS.
- K. A. Rising, C. M. Crenshaw, H. J. Koo, T. Subramanian, K. A. H. Chehade, C. Starks, K. D. Allen, D. A. Andres, H. P. Spielmann, J. P. Noel and J. Chappell, ACS Chem. Biol., 2015, 10, 1729–1736 CrossRef CAS.
- J. Kirby, M. Nishimoto, R. W. Chow, E. E. Baidoo, G. Wang, J. Martin, W. Schackwitz, R. Chan, J. L. Fortman and J. D. Keasling, Appl. Environ. Microbiol., 2015, 81, 130–138 CrossRef PubMed.
- J. Perez-Gil, E. M. Uros, S. Sauret-Güeto, L. M. Lois, J. Kirby, M. Nishimoto, E. E. Baidoo, J. D. Keasling, A. Boronat and M. Rodriguez-Concepcion, PLoS One, 2012, 7, e43775 CrossRef CAS.
- J. R. King, B. M. Woolston and G. Stephanopoulos, ACS Synth. Biol., 2017, 6, 1416–1426 CrossRef CAS PubMed.
- T. J. Erb, B. S. Evans, K. Cho, B. P. Warlick, J. Sriram, B. M. Wood, H. J. Imker, J. V. Sweedler, F. R. Tabita and J. A. Gerlt, Nat. Chem. Biol., 2012, 8, 926 CrossRef CAS.
- B. P. Warlick, B. S. Evans, T. J. Erb, U. A. Ramagopal, J. Sriram, H. J. Imker, J. M. Sauder, J. B. Bonanno, S. K. Burley and F. R. Tabita, Biochemistry, 2012, 51, 8324–8326 CrossRef CAS PubMed.
- A. R. Miller, J. A. North, J. A. Wildenthal and F. R. Tabita, mBio, 2018, 9, e00407-18 Search PubMed.
- J. A. North, J. A. Wildenthal, T. J. Erb, B. S. Evans, K. M. Byerly, J. A. Gerlt and F. R. Tabita, Mol. Microbiol., 2020, 113, 923–937 CrossRef CAS PubMed.
- C. E. Domenech, W. Giordano, J. Ávalos and E. Cerdá-olmedo, Eur. J. Biochem., 1996, 239, 720–725 CrossRef CAS.
- T. Mahmud, S. C. Wenzel, E. Wan, K. W. Wen, H. B. Bode, N. Gaitatzis and R. Müller, ChemBioChem, 2005, 6, 322–330 CrossRef CAS.
- M. L. Ginger, M. L. Chance, I. H. Sadler and L. J. Goad, J. Biol. Chem., 2001, 276, 11674–11682 CrossRef CAS PubMed.
- N. Yamauchi, Biosci., Biotechnol., Biochem., 2010, 74, 443–446 CrossRef CAS PubMed.
- M. L. Ginger, M. L. Chance and L. J. Goad, Biochem. J., 1999, 342, 397–405 CrossRef CAS.
- L. L. Grochowski, H. Xu and R. H. White, J. Bacteriol., 2006, 188, 3192–3198 CrossRef CAS.
- N. Dellas, S. T. Thomas, G. Manning and J. P. Noel, eLife, 2013, 2, e00672 CrossRef PubMed.
- J. C. VanNice, D. A. Skaff, A. Keightley, J. K. Addo, G. J. Wyckoff and H. M. Miziorko, J. Bacteriol., 2014, 196, 1055–1063 CrossRef.
- Z. Q. Beck, J. Mampel, G. Meurer, M. C. Miller, K. J. Sanford, D. V. Vaviline, W. Weyler and G. M. Whited, United States Pat., US20160002672A1, 2016 Search PubMed.
- Y. Azami, A. Hattori, H. Nishimura, H. Kawaide, T. Yoshimura and H. Hemmi, J. Biol. Chem., 2014, 289, 15957–15967 CrossRef CAS.
- J. M. Vinokur, T. P. Korman, Z. Cao and J. U. Bowie, Biochemistry, 2014, 53, 4161–4168 CrossRef CAS PubMed.
- J. M. Vinokur, M. C. Cummins, T. P. Korman and J. U. Bowie, Sci. Rep., 2016, 6, 1–11 CrossRef.
- J. M. Vinokur, T. P. Korman, M. R. Sawaya, M. Collazo, D. Cascio and J. U. Bowie, Protein Sci., 2015, 24, 212–220 CrossRef CAS PubMed.
- A. De Waard, A. Phillips and K. Bloch, J. Am. Chem. Soc., 1959, 81, 2913–2914 CrossRef CAS.
- M. Lindberg, C. Yuan, A. Dewaard and K. Bloch, Biochemistry, 1962, 1, 182–188 CrossRef CAS PubMed.
- H. Hayakawa, F. Sobue, K. Motoyama, T. Yoshimura and H. Hemmi, Biochem. Biophys. Res. Commun., 2017, 487, 702–708 CrossRef CAS PubMed.
- P. Marliere, M. Anissimova, R. Chayot and M. Delcourt, WIPO Pat., WO2011076261, 2011 Search PubMed.
- K. Motoyama, F. Sobue, H. Kawaide, T. Yoshimura and H. Hemmi, Appl. Environ. Microbiol., 2019, 85, e00256-19 CrossRef.
- H. Hayakawa, K. Motoyama, F. Sobue, T. Ito, H. Kawaide, T. Yoshimura and H. Hemmi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10034–10039 CrossRef CAS PubMed.
- R. Yoshida, T. Yoshimura and H. Hemmi, Appl. Environ. Microbiol., 2020, 86, e02889-19 CrossRef.
- L. K. Henry, S. T. Thomas, J. R. Widhalm, J. H. Lynch, T. C. Davis, S. A. Kessler, J. Bohlmann, J. P. Noel and N. Dudareva, Nat. Plants, 2018, 4, 721–729 CrossRef CAS.
- K. W. George, M. G. Thompson, J. Kim, E. E. K. Baidoo, G. Wang, V. T. Benites, C. J. Petzold, L. J. G. Chan, S. Yilmaz, P. Turhanen, P. D. Adams, J. D. Keasling and T. S. Lee, Metab. Eng., 2018, 47, 60–72 CrossRef CAS.
- J. Yang, Q. Nie, H. Liu, M. Xian and H. Liu, BMC Biotechnol., 2016, 16, 5 CrossRef PubMed.
- A. Kang, K. W. George, G. Wang, E. Baidoo, J. D. Keasling and T. S. Lee, Metab. Eng., 2016, 34, 25–35 CrossRef CAS PubMed.
- A. Kang, C. W. Meadows, N. Canu, J. D. Keasling and T. S. Lee, Metab. Eng., 2017, 41, 125–134 CrossRef CAS.
- A. Kang, D. Mendez-Perez, E.-B. Goh, E. E. Baidoo, V. T. Benites, H. R. Beller, J. D. Keasling, P. D. Adams, A. Mukhopadhyay and T. S. Lee, Metab. Eng., 2019, 56, 85–96 CrossRef CAS PubMed.
- Y. Gao, R. B. Honzatko and R. J. Peters, Nat. Prod. Rep., 2012, 29, 1153–1175 RSC.
- A. O. Chatzivasileiou, V. Ward, S. M. Edgar and G. Stephanopoulos, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 506–511 CrossRef CAS.
- J. M. Clomburg, S. Qian, Z. Tan, S. Cheong and R. Gonzalez, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 12810–12815 CrossRef CAS PubMed.
- J. Couillaud, J. Rico, A. Rubini, T. Hamrouni, E. Courvoisier-Dezord, J.-L. Petit, A. Mariage, E. Darii, K. Duquesne and V. de Berardinis, ACS Omega, 2019, 4, 7838–7849 CrossRef CAS.
- Y. Liu, Z. Yan, X. Lu, D. Xiao and H. Jiang, Sci. Rep., 2016, 6, 24117 CrossRef CAS.
- S. Lund, R. Hall and G. J. Williams, ACS Synth. Biol., 2019, 8, 232–238 CrossRef CAS.
- L. A. Johnson, A. Dunbabin, J. C. R. Benton, R. J. Mart and R. K. Allemann, Angew. Chem., Int. Ed., 2020, 59, 1433–7851 CrossRef.
- M. Li, F. Hou, T. Wu, X. Jiang, F. Li, H. Liu, M. Xian and H. Zhang, Nat. Prod. Rep., 2020, 37, 80–99 RSC.
- J. Rico, K. Duquesne, J. L. Petit, A. Mariage, E. Darii, F. Peruch, V. de Berardinis and G. Iacazio, Microb. Cell Fact., 2019, 18, 23 CrossRef.
- C. A. Ferraz, N. G. H. Leferink, I. Kosov and N. S. Scrutton, ChemBioChem, 2021 DOI:10.1002/cbic.202100110.
- J. Alonso-Gutierrez, R. Chan, T. S. Batth, P. D. Adams, J. D. Keasling, C. J. Petzold and T. S. Lee, Metab. Eng., 2013, 19, 33–41 CrossRef CAS PubMed.
- L. Ruzicka, Experientia, 1953, 9, 357–367 CrossRef CAS.
- H. Schönherr and T. Cernak, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef.
- J. S. Dickschat, T. Nawrath, V. Thiel, B. Kunze, R. Müller and S. Schulz, Angew. Chem., Int. Ed., 2007, 46, 8287–8290 CrossRef CAS.
- M. Komatsu, M. Tsuda, S. Omura, H. Oikawa and H. Ikeda, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7422–7427 CrossRef CAS.
- C. M. Wang and D. E. Cane, J. Am. Chem. Soc., 2008, 130, 8908–8909 CrossRef CAS PubMed.
- T. Ozaki, S. S. Shinde, L. Gao, R. Okuizumi, C. Liu, Y. Ogasawara, X. Lei, T. Dairi, A. Minami and H. Oikawa, Angew. Chem., Int. Ed., 2018, 57, 6629–6632 CrossRef CAS.
- S. von Reuss, D. Domik, M. C. Lemfack, N. Magnus, M. Kai, T. Weise and B. Piechulla, J. Am. Chem. Soc., 2018, 140, 11855–11862 CrossRef CAS PubMed.
- M. J. Kschowak, H. Wortmann, J. S. Dickschat, J. Schrader and M. Buchhaupt, PLoS One, 2018, 13, e0196082 CrossRef PubMed.
- M. J. Kschowak, F. Maier, H. Wortmann and M. Buchhaupt, ACS Synth. Biol., 2020, 9, 981–986 CrossRef CAS.
- L. Drummond, M. J. Kschowak, J. Breitenbach, H. Wolff, Y. M. Shi, J. Schrader, H. B. Bode, G. Sandmann and M. Buchhaupt, ACS Synth. Biol., 2019, 8, 1303–1313 CrossRef CAS.
- M. Kobayashi, T. Koyama, K. Ogura, S. Seto, F. J. Ritter and I. E. M. Briiggemann-Rotgans, J. Am. Chem. Soc., 1980, 102, 6604–6605 CrossRef.
- T. Koyama and K. Ogura, J. Am. Chem. Soc., 1987, 109, 2853–2854 CrossRef CAS.
- A. B. Attygalle and E. D. Morgan, J. Chem. Soc., 1982, 949–951 CAS.
- D. A. Schooley, K. J. Judy, B. J. Bergot, M. S. Hall and J. B. Siddal, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 2921–2925 CrossRef CAS.
- C. B. Eiben, T. de Rond, C. Bloszies, J. Gin, J. Chiniquy, E. E. K. Baidoo, C. J. Petzold, N. J. Hillson, O. Fiehn and J. D. Keasling, ACS Synth. Biol., 2019, 8, 2238–2247 CrossRef CAS PubMed.
- K. K. Reiling, Y. Yoshikuni, V. J. Martin, J. Newman, J. Bohlmann and J. D. Keasling, Biotechnol. Bioeng., 2004, 87, 200–212 CrossRef CAS.
- S. Fujisaki, H. Hara, Y. Nishimura, K. Horiuchi and T. Nishino, J. Biochem., 1990, 108, 995–1000 CrossRef CAS PubMed.
- K. Saiki, T. Mogi and Y. Anraku, Biochem. Biophys. Res. Commun., 1992, 189, 1491–1497 CrossRef CAS.
- K. Okada, M. Minehira, X. Zhu, K. Suzuki, T. Nakagawa, H. Matsuda and M. Kawamukai, J. Bacteriol., 1997, 179, 3058–3060 CrossRef CAS PubMed.
- C. M. Apfel, B. Takács, M. Fountoulakis, M. Stieger and W. Keck, J. Bacteriol., 1999, 181, 483–492 CrossRef CAS PubMed.
- X. Wang, J. Wu, J. Chen, L. Xiao and X. Li, J. Agric. Food Chem., 2019, 68, 8381–8390 CrossRef.
- J. Rolf, M. K. Julsing, K. Rosenthal and S. Lütz, Molecules, 2020, 25, 1881 CrossRef CAS.
- H. Zhang, Q. Liu, Y. Cao, X. Feng, Y. Zheng, H. Zou, H. Liu, J. Yang and M. Xian, Microb. Cell Fact., 2014, 13, 20 CrossRef PubMed.
- W. Liu, X. Xu, R. Zhang, T. Cheng, Y. Cao, X. Li, J. Guo, H. Liu and M. Xian, Biotechnol. Biofuels, 2016, 9, 58 CrossRef PubMed.
- M. G. Chacón, A. Marriott, E. G. Kendrick, M. Q. Styles and D. J. Leak, Microb. Cell Fact., 2019, 18, 105 CrossRef.
- J. Yang, Q. Nie, M. Ren, H. Feng, X. Jiang, Y. Zheng, M. Liu, H. Zhang and M. Xian, Biotechnol. Biofuels, 2013, 6, 1–10 CrossRef.
- D. Mendez-Perez, J. Alonso-Gutierrez, Q. Hu, M. Molinas, E. E. Baidoo, G. Wang, L. J. Chan, P. D. Adams, C. J. Petzold and J. D. Keasling, Biotechnol. Bioeng., 2017, 114, 1703–1712 CrossRef CAS.
- E.-M. Kim, J.-H. Eom, Y. Um, Y. Kim and H. M. Woo, J. Agric. Food Chem., 2015, 63, 4606–4612 CrossRef CAS.
- C. Burke and R. Croteau, Arch. Biochem. Biophys., 2002, 405, 130–136 CrossRef CAS.
- M. Tashiro, H. Kiyota, S. Kawai-Noma, K. Saito, M. Ikeuchi, Y. Iijima and D. Umeno, ACS Synth. Biol., 2016, 5, 1011–1020 CrossRef CAS.
- S. Sarria, B. Wong, H. G. Martín, J. D. Keasling and P. Peralta-Yahya, ACS Synth. Biol., 2014, 3, 466–475 CrossRef CAS.
- X. Wang, J. Chen, J. Zhang, Y. Zhou, Y. Zhang, F. Wang and X. Li, Metab. Eng., 2021, 66, 60–67 CrossRef CAS PubMed.
- C. Willrodt, C. David, S. Cornelissen, B. Bühler, M. K. Julsing and A. Schmid, Biotechnol. J., 2014, 9, 1000–1012 CrossRef CAS PubMed.
- S. Shukal, X. Chen and C. Zhang, Metab. Eng., 2019, 55, 170–178 CrossRef CAS.
- J. A. Dietrich, Y. Yoshikuni, K. J. Fisher, F. X. Woolard, D. Ockey, D. J. McPhee, N. S. Renninger, M. C. Chang, D. Baker and J. D. Keasling, ACS Chem. Biol., 2009, 4, 261–267 CrossRef CAS PubMed.
- M. C. Chang, R. A. Eachus, W. Trieu, D.-K. Ro and J. D. Keasling, Nat. Chem. Biol., 2007, 3, 274–277 CrossRef CAS.
- P. Yao, S. You, W. Qi, R. Su and Z. He, Environ. Sci. Pollut. Res., 2020, 27, 1–12 CrossRef PubMed.
- F. Zhu, X. Zhong, M. Hu, L. Lu, Z. Deng and T. Liu, Biotechnol. Bioeng., 2014, 111, 1396–1405 CrossRef CAS.
- C. Wang, J.-E. Park, E.-S. Choi and S.-W. Kim, Biotechnol. J., 2016, 11, 1291–1297 CrossRef CAS.
- C. Zhang, V. Y. Seow, X. Chen and H.-P. Too, Nat. Commun., 2018, 9, 1858 CrossRef.
- F. Aguilar, T. Scheper and S. Beutel, Molecules, 2019, 24, 3356 CrossRef CAS.
- L.-Q. Luo, Y.-G. Chen, D.-S. Li, Y. Liu and S.-H. Li, Chem. Biodiversity, 2020, 17, e2000219 CAS.
- X. Xie, J. Kirby and J. D. Keasling, Phytochemistry, 2012, 78, 20–28 CrossRef CAS.
- Y. Cao, R. Zhang, W. Liu, G. Zhao, W. Niu, J. Guo, M. Xian and H. Liu, Sci. Rep., 2019, 9, 95 CrossRef.
- T. Cheng, G. Zhao, M. Xian and C. Xie, Sci. Rep., 2020, 10, 16791 CrossRef CAS.
- S.-B. Mou, W. Xiao, H.-Q. Wang, K.-Y. Chen and Z. Xiang, Org. Lett., 2020, 23, 400–404 CrossRef PubMed.
- M. Schalk, L. Pastore, M. A. Mirata, S. Khim, M. Schouwey, F. Deguerry, V. Pineda, L. Rocci and L. Daviet, J. Am. Chem. Soc., 2012, 134, 18900–18903 CrossRef CAS.
- E. Leonard, P. K. Ajikumar, K. Thayer, W.-H. Xiao, J. D. Mo, B. Tidor, G. Stephanopoulos and K. L. Prather, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 13654–13659 CrossRef CAS.
- J.-F. Wang, Z.-Q. Xiong, S.-Y. Li and Y. Wang, Appl. Microbiol. Biotechnol., 2013, 97, 8057–8067 CrossRef CAS PubMed.
- Y. Matsuba, J. Zi, A. D. Jones, R. J. Peters and E. Pichersky, PLoS One, 2015, 10, e0119302 CrossRef.
- R. S. van der Hoeven, A. J. Monforte, D. Breeden, S. D. Tanksley and J. C. Steffens, Plant Cell, 2000, 12, 2283–2294 CrossRef CAS.
- C. Sallaud, D. Rontein, S. Onillon, F. Jabès, P. Duffé, C. Giacalone, S. Thoraval, C. Escoffier, G. Herbette and N. Leonhardt, Plant Cell, 2009, 21, 301–317 CrossRef CAS PubMed.
- J. Zi, Y. Matsuba, Y. J. Hong, A. J. Jackson, D. J. Tantillo, E. Pichersky and R. J. Peters, J. Am. Chem. Soc., 2014, 136, 16951–16953 CrossRef CAS.
- T. A. Akhtar, Y. Matsuba, I. Schauvinhold, G. Yu, H. A. Lees, S. E. Klein and E. Pichersky, Plant J., 2013, 73, 640–652 CrossRef CAS.
- E. Gonzales-Vigil, D. E. Hufnagel, J. Kim, R. L. Last and C. S. Barry, Plant J., 2012, 71, 921–935 CrossRef CAS.
- V. Falara, T. A. Akhtar, T. T. Nguyen, E. A. Spyropoulou, P. M. Bleeker, I. Schauvinhold, Y. Matsuba, M. E. Bonini, A. L. Schilmiller and R. L. Last, Plant Physiol., 2011, 157, 770–789 CrossRef CAS PubMed.
- A. L. Schilmiller, I. Schauvinhold, M. Larson, R. Xu, A. L. Charbonneau, A. Schmidt, C. Wilkerson, R. L. Last and E. Pichersky, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10865–10870 CrossRef CAS.
- M. Zhang, J. Liu, K. Li and D. Yu, PLoS One, 2013, 8, e75972 CrossRef CAS.
- O. Gericke, N. L. Hansen, G. B. Pedersen, L. Kjaerulff, D. Luo, D. Staerk, B. L. Møller, I. Pateraki and A. M. Heskes, BMC Plant Biol., 2020, 20, 1–15 CrossRef.
- C. Ignea, M. H. Raadam, M. S. Motawia, A. M. Makris, C. E. Vickers and S. C. Kampranis, Nat. Commun., 2019, 10, 1–15 CrossRef CAS.
- S. Cheng, X. Liu, G. Jiang, J. Wu, J.-l. Zhang, D. Lei, Y.-J. Yuan, J. Qiao and G.-R. Zhao, ACS Synth. Biol., 2019, 8, 968–975 CrossRef CAS.
- S. R. Johnson, W. W. Bhat, R. Sadre, G. P. Miller, A. S. Garcia and B. Hamberger, New Phytol., 2019, 223, 323–335 CrossRef CAS PubMed.
- Q. Jia, G. Li, T. G. Köllner, J. Fu, X. Chen, W. Xiong, B. J. Crandall-Stotler, J. L. Bowman, D. J. Weston and Y. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12328–12333 CrossRef CAS.
- M. Jia and R. J. Peters, Org. Biomol. Chem., 2017, 15, 3158–3160 RSC.
- K. A. Pelot, D. M. Hagelthorn, Y. J. Hong, D. J. Tantillo and P. Zerbe, ChemBioChem, 2019, 20, 111–117 CrossRef CAS.
- X. Cao, Y.-B. Lv, J. Chen, T. Imanaka, L.-J. Wei and Q. Hua, Biotechnol. Biofuels, 2016, 9, 214 CrossRef PubMed.
- C. Wang, J. Zhou, H.-J. Jang, S.-H. Yoon, J.-Y. Kim, S.-G. Lee, E.-S. Choi and S.-W. Kim, Metab. Eng., 2013, 18, 53–59 CrossRef CAS PubMed.
- E. Oldfield and F. Y. Lin, Angew. Chem., Int. Ed., 2012, 51, 1124–1137 CrossRef CAS.
- J. S. Lee, J. J. Pan, G. Ramamoorthy and C. D. Poulter, J. Am. Chem. Soc., 2017, 139, 14556–14567 CrossRef CAS PubMed.
- Y. Zhang, J. Nielsen and Z. Liu, FEMS Yeast Res., 2017, 17, fox080 CrossRef.
- Z. A. Demissie, L. A. Erland, M. R. Rheault and S. S. Mahmoud, J. Biol. Chem., 2013, 288, 6333–6341 CrossRef CAS PubMed.
- R. Teufel, Methods Enzymol., 2018, 604, 425–439 CAS.
- M. Liu, C. C. Chen, L. Chen, X. Xiao, Y. Zheng, J. W. Huang, W. Liu, T. P. Ko, Y. S. Cheng, X. Feng, E. Oldfield, R. T. Guo and Y. Ma, Angew. Chem., Int. Ed., 2016, 55, 4721–4724 CrossRef CAS PubMed.
- S. B. Rivera, B. D. Swedlund, G. J. King, R. N. Bell, C. E. Hussey, D. M. Shattuck-Eidens, W. M. Wrobel, G. D. Peiser and C. D. Poulter, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4373–4378 CrossRef CAS PubMed.
- H. V. Thulasiram, H. K. Erickson and C. D. Poulter, J. Am. Chem. Soc., 2008, 130, 1966–1971 CrossRef CAS PubMed.
- H. He, G. Bian, C. J. Herbst-Gervasoni, T. Mori, S. A. Shinsky, A. Hou, X. Mu, M. Huang, S. Cheng, Z. Deng, D. W. Christianson, I. Abe and T. Liu, Nat. Commun., 2020, 11, 3958 CrossRef CAS.
- J. R. Chekan, S. M. K. McKinnie, J. P. Noel and B. S. Moore, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12799–12805 CrossRef CAS.
- T. Koyama, A. Saito, K. Ogura and S. Seto, J. Am. Chem. Soc., 1980, 102, 3614–3618 CrossRef CAS.
- M. Nagaki, H. Yamamoto, A. Takahashi, Y. Maki, J. Ishibashi, T. Nishino and T. Koyama, J. Mol. Catal. B: Enzym., 2002, 17, 81–89 CrossRef CAS.
- Y. Maki, M. Komabayashi, Y. Gotoh, N. Ohya, H. Hemmi, K. Hirooka, T. Nishino and T. Koyama, J. Mol. Catal. B: Enzym., 2002, 19–20, 431–436 CrossRef CAS.
- N. A. Heaps and C. D. Poulter, J. Org. Chem., 2011, 76, 1838–1843 CrossRef CAS.
- V. Harms, A. Kirschning and J. S. Dickschat, Nat. Prod. Rep., 2020, 37, 1080–1097 RSC.
- P. Braun and J. LaBaer, Trends Biotechnol., 2003, 21, 383–388 CrossRef CAS.
- F. Yesilirmak and Z. Sayers, Int. J. Plant Genomics, 2009, 2009, 296482 CrossRef PubMed.
- S. Soliman and Y. Tang, Biotechnol. Bioeng., 2015, 112, 229–235 CrossRef CAS PubMed.
- N. G. Leferink, A. J. Jervis, Z. Zebec, H. S. Toogood, S. Hay, E. Takano and N. S. Scrutton, ChemistrySelect, 2016, 1, 1893–1896 CrossRef CAS.
- V. Karuppiah, K. E. Ranaghan, N. G. Leferink, L. O. Johannissen, M. Shanmugam, A. Ní Cheallaigh, N. J. Bennett, L. J. Kearsey, E. Takano and J. M. Gardiner, ACS Catal., 2017, 7, 6268–6282 CrossRef CAS PubMed.
- V. Karuppiah, N. G. Leferink and N. S. Scrutton, WIPO Pat., WO2018142109A1, 2020 Search PubMed.
- C. Zhang, X. Chen, R. T. C. Lee, R. T, S. Maurer-Stroh and M. Rühl, Commun. Biol., 2021, 4, 223 CrossRef CAS PubMed.
- R. Thanasomboon, D. Waraho, S. Cheevadhanarak and A. Meechai, Procedia Computer Science, 2012, 11, 88–95 CrossRef.
- W. Wu, F. Liu and R. W. Davis, Macromol. Rapid Commun., 2018, 6, 13–21 Search PubMed.
- P. Zhou, Y. Du, N. Xu, C. Yue and L. Ye, Biochem. Eng. J., 2020, 161, 107655 CrossRef CAS.
- C.-L. Liu, T. Tian, J. Alonso-Gutierrez, B. Garabedian, S. Wang, E. E. Baidoo, V. Benites, Y. Chen, C. J. Petzold and P. D. Adams, Biotechnol. Biofuels, 2018, 11, 285 CrossRef CAS.
- E. Leonard and M. A. G. Koffas, Appl. Environ. Microbiol., 2007, 73, 7246 CrossRef CAS.
- S. Zelasko, A. Palaria and A. Das, Protein Expression Purif., 2013, 92, 77–87 CrossRef CAS.
- S. Janocha, D. Schmitz and R. Bernhardt, in Biotechnology of Isoprenoids, Springer, 2015, pp. 215–250 Search PubMed.
- B. W. Biggs, C. G. Lim, K. Sagliani, S. Shankar, G. Stephanopoulos, M. De Mey and P. K. Ajikumar, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 3209–3214 CrossRef CAS.
- H. Harada, K. Shindo, K. Iki, A. Teraoka, S. Okamoto, F. Yu, J. I. Hattan, R. Utsumi and N. Misawa, Appl. Microbiol. Biotechnol., 2011, 90, 467–476 CrossRef CAS.
- A. Schifrin, Y. Khatri, P. Kirsch, V. Thiel, S. Schulz and R. Bernhardt, Org. Biomol. Chem., 2016, 14, 3385–3393 RSC.
- P. Rabe and J. S. Dickschat, Angew. Chem., Int. Ed., 2013, 52, 1810–1812 CrossRef CAS.
- S. A. Agger, F. Lopez-Gallego, T. R. Hoye and C. Schmidt-Dannert, J. Bacteriol., 2008, 190, 6084–6096 CrossRef PubMed.
- G. K. Reddy, N. G. H. Leferink, M. Umemura, S. T. Ahmed, R. Breitling, N. S. Scrutton and E. Takano, PLoS One, 2020, 15, e0232220 CrossRef CAS PubMed.
- P. Rabe, T. Schmitz and J. S. Dickschat, Beilstein J. Org. Chem., 2016, 12, 1839–1850 CrossRef CAS PubMed.
- Y. Yamada, T. Kuzuyama, M. Komatsu, K. Shin-ya, S. Omura, D. E. Cane and H. Ikeda, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 857–862 CrossRef CAS.
- S. Giglio, W. Chou, H. Ikeda, D. Cane and P. Monis, Environ. Sci. Technol., 2011, 45, 992–998 CrossRef CAS PubMed.
- M. Komatsu, M. Tsuda, S. Ōmura, H. Oikawa and H. Ikeda, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7422–7427 CrossRef CAS.
- J. S. Dickschat, Nat. Prod. Rep., 2016, 33, 87–110 RSC.
- J. S. Dickschat, Angew. Chem., Int. Ed., 2019, 58, 15964–15976 CrossRef CAS.
- E. J. N. Helfrich, G.-M. Lin, C. A. Voigt and J. Clardy, Beilstein J. Org. Chem., 2019, 15, 2889–2906 CrossRef CAS.
- H. A. Tomm, L. Ucciferri and A. C. Ross, J. Ind. Microbiol. Biotechnol., 2019, 46, 1381–1400 CrossRef CAS.
- P. Cimermancic, M. H. Medema, J. Claesen, K. Kurita, L. C. W. Brown, K. Mavrommatis, A. Pati, P. A. Godfrey, M. Koehrsen and J. Clardy, Cell, 2014, 158, 412–421 CrossRef CAS PubMed.
- N. Ziemert, T. Weber and M. H. Medema, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2020, 6, 19–33 Search PubMed.
- K. Blin, V. Pascal Andreu, E. L. C. de los Santos, F. Del Carratore, S. Y. Lee, M. H. Medema and T. Weber, Nucleic Acids Res., 2019, 47, D625–D630 CrossRef CAS.
- M. H. Medema, R. Kottmann, P. Yilmaz, M. Cummings, J. B. Biggins, K. Blin, I. De Bruijn, Y. H. Chooi, J. Claesen and R. C. Coates, Nat. Chem. Biol., 2015, 11, 625–631 CrossRef CAS PubMed.
- L. Huo, J. J. Hug, C. Fu, X. Bian, Y. Zhang and R. Müller, Nat. Prod. Rep., 2019, 36, 1412–1436 RSC.
- X. Zhang, Hindra and M. A. Elliot, Curr. Opin. Microbiol., 2019, 51, 9–15 CrossRef CAS.
- E. Palazzotto and T. Weber, Curr. Opin. Microbiol., 2018, 45, 109–116 CrossRef CAS PubMed.
- C. J. Robinson, P. Carbonell, A. J. Jervis, C. Yan, K. A. Hollywood, M. S. Dunstan, A. Currin, N. Swainston, R. Spiess, S. Taylor, P. Mulherin, S. Parker, W. Rowe, N. E. Matthews, K. J. Malone, R. Le Feuvre, P. Shapira, P. Barran, N. J. Turner, J. Micklefield, R. Breitling, E. Takano and N. S. Scrutton, Metab. Eng., 2020, 60, 168–182 CrossRef CAS.
- N. G. Leferink, M. S. Dunstan, K. A. Hollywood, N. Swainston, A. Currin, A. J. Jervis, E. Takano and N. S. Scrutton, Sci. Rep., 2019, 9, 1–12 CAS.
- J. Sikkema, J. A. de Bont and B. Poolman, Microbiol. Mol. Biol. Rev., 1995, 59, 201–222 CrossRef CAS PubMed.
- J. Sikkema, J. A. de Bont and B. Poolman, J. Biol. Chem., 1994, 269, 8022–8028 CrossRef CAS.
- H. Heipieper and P. Martínez, in Handbook of hydrocarbon and lipid microbiology, 2010 Search PubMed.
- R. Doshi, T. Nguyen and G. Chang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7642–7647 CrossRef CAS.
- S. G. Griffin, S. G. Wyllie, J. L. Markham and D. N. Leach, Flavour Fragrance J., 1999, 14, 322–332 CrossRef CAS.
- J. V. Vermaas, G. J. Bentley, G. T. Beckham and M. F. Crowley, J. Phys. Chem. B, 2018, 122, 10349–10361 CrossRef CAS.
- V. Chubukov, F. Mingardon, W. Schackwitz, E. E. Baidoo, J. Alonso-Gutierrez, Q. Hu, T. S. Lee, J. D. Keasling and A. Mukhopadhyay, Appl. Environ. Microbiol., 2015, 81, 4690–4696 CrossRef CAS PubMed.
- A. A. Shah, C. Wang, S.-H. Yoon, J.-Y. Kim, E.-S. Choi and S.-W. Kim, J. Biotechnol., 2013, 167, 357–364 CrossRef CAS.
- C. J. Paddon, P. J. Westfall, D. J. Pitera, K. Benjamin, K. Fisher, D. McPhee, M. Leavell, A. Tai, A. Main and D. Eng, Nature, 2013, 496, 528–532 CrossRef CAS PubMed.
- J. D. Newman, J. Marshall, M. Chang, F. Nowroozi, E. Paradise, D. Pitera, K. L. Newman and J. D. Keasling, Biotechnol. Bioeng., 2006, 95, 684–691 CrossRef CAS.
- C. M. Jones, N. J. H. Lozada and B. F. Pfleger, Appl. Microbiol. Biotechnol., 2015, 99, 9381–9393 CrossRef CAS.
- L. J. V. Piddock, Clin. Microbiol. Rev., 2006, 19, 382 CrossRef CAS.
- C. Zhang, X. Chen, G. Stephanopoulos and H. P. Too, Biotechnol. Bioeng., 2016, 113, 1755–1763 CrossRef CAS PubMed.
- F.-X. Niu, X. He, Y.-Q. Wu and J.-Z. Liu, Frontiers in Microbiology, 2018, 9, 1623 CrossRef PubMed.
- N. Weston, P. Sharma, V. Ricci and L. J. V. Piddock, Res. Microbiol., 2018, 169, 425–431 CrossRef CAS.
- A. A. Shah, C. Wang, Y.-R. Chung, J.-Y. Kim, E.-S. Choi and S.-W. Kim, J. Biosci. Bioeng., 2013, 115, 253–258 CrossRef CAS.
- J. L. Foo, H. M. Jensen, R. H. Dahl, K. George, J. D. Keasling, T. S. Lee, S. Leong and A. Mukhopadhyay, mBio, 2014, 5, e01932-14 CrossRef.
- M. J. Dunlop, Z. Y. Dossani, H. L. Szmidt, H. C. Chu, T. S. Lee, J. D. Keasling, M. Z. Hadi and A. Mukhopadhyay, Mol. Syst. Biol., 2011, 7, 487 CrossRef PubMed.
- W. J. Turner and M. J. Dunlop, ACS Synth. Biol., 2015, 4, 1056–1063 CrossRef CAS.
- Y. Meng, X. Shao, Y. Wang, Y. Li, X. Zheng, G. Wei, S. W. Kim and C. Wang, Biotechnol. Bioeng., 2020, 117, 3499–3507 CrossRef CAS.
- T. Wu, L. Ye, D. Zhao, S. Li, Q. Li, B. Zhang, C. Bi and X. Zhang, Metab. Eng., 2017, 43, 85–91 CrossRef CAS PubMed.
- T. Wu, S. Li, L. Ye, D. Zhao, F. Fan, Q. Li, B. Zhang, C. Bi and X. Zhang, ACS Synth. Biol., 2019, 8, 1037–1046 CrossRef CAS PubMed.
- T. Wu, J. Liu, M. Li, G. Zhang, L. Liu, X. Li, X. Men, M. Xian and H. Zhang, Biotechnol. Biofuels, 2020, 13, 1–15 CrossRef.
- T. A. Tomko and M. J. Dunlop, Biotechnol. Biofuels, 2015, 8, 165 CrossRef PubMed.
- P. Sattayawat, I. S. Yunus and P. R. Jones, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 1404–1413 CrossRef CAS.
- P. Carbonell, P. Parutto, C. Baudier, C. Junot and J.-L. Faulon, ACS Synth. Biol., 2014, 3, 565–577 CrossRef CAS.
- P. Carbonell, J. Wong, N. Swainston, E. Takano, N. J. Turner, N. S. Scrutton, D. B. Kell, R. Breitling and J.-L. Faulon, Bioinformatics, 2018, 34, 2153–2154 CrossRef PubMed.
- S. Edgar, F.-S. Li, K. Qiao, J.-K. Weng and G. Stephanopoulos, ACS Synth. Biol., 2017, 6, 201–205 CrossRef CAS.
- X. Lv, J. Gu, F. Wang, W. Xie, M. Liu, L. Ye and H. Yu, Biotechnol. Bioeng., 2016, 113, 2661–2669 CrossRef CAS.
- N. Swainston, M. Dunstan, A. J. Jervis, C. J. Robinson, P. Carbonell, A. R. Williams, J.-L. Faulon, N. S. Scrutton and D. B. Kell, Bioinformatics, 2018, 34, 2327–2329 CrossRef CAS.
- A. J. Jervis, P. Carbonell, S. Taylor, R. Sung, M. S. Dunstan, C. J. Robinson, R. Breitling, E. Takano and N. S. Scrutton, ACS Synth. Biol., 2019, 8, 1478–1483 CrossRef CAS.
- A. J. Jervis, P. Carbonell, M. Vinaixa, M. S. Dunstan, K. A. Hollywood, C. J. Robinson, N. J. Rattray, C. Yan, N. Swainston and A. Currin, ACS Synth. Biol., 2018, 8, 127–136 CrossRef.
- P. Carbonell, A. J. Jervis, C. J. Robinson, C. Yan, M. Dunstan, N. Swainston, M. Vinaixa, K. A. Hollywood, A. Currin and N. J. Rattray, Commun. Biol., 2018, 1, 1–10 CrossRef PubMed.
- I. Otero-Muras and P. Carbonell, Metab. Eng., 2020, 63, 61–80 CrossRef PubMed.
- C. E. Lawson, J. M. Martí, T. Radivojevic, S. V. R. Jonnalagadda, R. Gentz, N. J. Hillson, S. Peisert, J. Kim, B. A. Simmons, C. J. Petzold, S. W. Singer, A. Mukhopadhyay, D. Tanjore, J. G. Dunn and H. Garcia Martin, Metab. Eng., 2020, 63, 34–60 CrossRef PubMed.
- A. Emmerstorfer-Augustin, S. Moser and H. Pichler, J. Biotechnol., 2016, 235, 112–120 CrossRef CAS.
- W. R. Farmer and J. C. Liao, Biotechnol. Prog., 2001, 17, 57–61 CrossRef CAS PubMed.
- J. Jung, J. H. Lim, S. Y. Kim, D.-K. Im, J. Y. Seok, S.-J. V. Lee, M.-K. Oh and G. Y. Jung, Metab. Eng., 2016, 38, 401–408 CrossRef CAS.
- H. Liu, Y. Sun, K. R. M. Ramos, G. M. Nisola, K. N. G. Valdehuesa, W. K. Lee, S. J. Park and W.-J. Chung, PLoS One, 2013, 8, e83290 CrossRef PubMed.
- C. Y. Ng, I. Farasat, C. D. Maranas and H. M. Salis, Metab. Eng., 2015, 29, 86–96 CrossRef CAS PubMed.
- K. R. M. Ramos, K. N. G. Valdehuesa, H. Liu, G. M. Nisola, W.-K. Lee and W.-J. Chung, Bioprocess Biosyst. Eng., 2014, 37, 2505–2513 CrossRef CAS PubMed.
- J. Zhao, Q. Li, T. Sun, X. Zhu, H. Xu, J. Tang, X. Zhang and Y. Ma, Metab. Eng., 2013, 17, 42–50 CrossRef CAS PubMed.
- T. Sun, L. Miao, Q. Li, G. Dai, F. Lu, T. Liu, X. Zhang and Y. Ma, Biotechnol. Lett., 2014, 36, 1515–1522 CrossRef CAS PubMed.
- J. Becker and C. Wittmann, Curr. Opin. Biotechnol., 2016, 42, 178–188 CrossRef CAS.
- G. Bokinsky, P. P. Peralta-Yahya, A. George, B. M. Holmes, E. J. Steen, J. Dietrich, T. Soon Lee, D. Tullman-Ercek, C. A. Voigt, B. A. Simmons and J. D. Keasling, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 19949–19954 CrossRef CAS.
- C. Scullin, V. Stavila, A. Skarstad, J. D. Keasling, B. A. Simmons and S. Singh, Bioresour. Technol., 2015, 184, 415–420 CrossRef CAS.
- J. Shi, K. W. George, N. Sun, W. He, C. Li, V. Stavila, J. D. Keasling, B. A. Simmons, T. S. Lee and S. Singh, BioEnergy Res., 2015, 8, 1004–1013 CrossRef CAS.
- F. K. Davies, R. E. Jinkerson and M. C. Posewitz, Photosynth. Res., 2015, 123, 265–284 CrossRef CAS PubMed.
- X. Gao, F. Gao, D. Liu, H. Zhang, X. Nie and C. Yang, Energy Environ. Sci., 2016, 9, 1400–1411 RSC.
- J. Wu, X. Wang, L. Xiao, F. Wang, Y. Zhang and X. Li, J. Agric. Food Chem., 2021, 69, 5663–5670 CrossRef CAS.
- X. Wang, J. H. Pereira, S. Tsutakawa, X. Fang, P. D. Adams, A. Mukhopadhyay and T. S. Lee, Metab. Eng., 2021, 64, 41–51 CrossRef CAS PubMed.
- G. H. Han, S. K. Kim, P. K.-S. Yoon, Y. Kang, B. S. Kim, Y. Fu, B. H. Sung, H. C. Jung, D.-H. Lee, S.-W. Kim and S.-G. Lee, Microb. Cell Fact., 2016, 15, 185 CrossRef PubMed.
- B. Zada, C. Wang, J.-B. Park, S.-H. Jeong, J.-E. Park, H. B. Singh and S.-W. Kim, Biotechnol. Biofuels, 2018, 11, 210 CrossRef.
- L. Yang, C. Wang, J. Zhou and S.-W. Kim, Microb. Cell Fact., 2016, 15, 14 CrossRef PubMed.
- T. Siemon, Z. Wang, G. Bian, T. Seitz, Z. Ye, Y. Lu, S. Cheng, Y. Ding, Y. Huang, Z. Deng, T. Liu and M. Christmann, J. Am. Chem. Soc., 2020, 142, 2760–2765 CrossRef CAS.
- S. Alemdar, J. C. König, S. Hartwig, T. Frister, T. Scheper and S. Beutel, Eng. Life Sci., 2017, 17, 900–907 CrossRef CAS PubMed.
- F. Aguilar, K. Ekramzadeh, T. Scheper and S. Beutel, ACS Omega, 2020, 5, 32436–32446 CrossRef CAS PubMed.
- D. Li, Q. Zhang, Z. Zhou, F. Zhao and W. Lu, Biotechnol. Lett., 2016, 38, 603–609 CrossRef CAS.
- Z. Gong, H. Wang, J. Tang, C. Bi, Q. Li and X. Zhang, J. Agric. Food Chem., 2020, 68, 14917–14927 CrossRef CAS PubMed.
- H.-J. Shen, B.-Y. Cheng, Y.-M. Zhang, L. Tang, Z. Li, Y.-F. Bu, X.-R. Li, G.-Q. Tian and J.-Z. Liu, Metab. Eng., 2016, 38, 180–190 CrossRef CAS PubMed.
- C. Zhang, X. Chen, N. D. Lindley and H. P. Too, Biotechnol. Bioeng., 2018, 115, 174–183 CrossRef CAS.
- H.-J. Jang, B.-K. Ha, J. Zhou, J. Ahn, S.-H. Yoon and S.-W. Kim, Biotechnol. Bioeng., 2015, 112, 1604–1612 CrossRef CAS.
- M. Huang, Y. Wang, J. Liu and Z. Mao, Chin. J. Chem. Eng., 2011, 19, 316–326 CrossRef CAS.
- S. v. Vuuren and A. M. Viljoen, Flavour Fragrance J., 2007, 22, 540–544 CrossRef.
- P. P. Peralta-Yahya, M. Ouellet, R. Chan, A. Mukhopadhyay, J. D. Keasling and T. S. Lee, Nat. Commun., 2011, 2, 483 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1. Selected reports of the highest terpenoid titres produced from central metabolism in E. coli, expanded table with genetic modifications and fermentation conditions. See DOI: 10.1039/d1np00025j |
| ‡ These authors contributed equally. |
| § We use the correct name “diphosphate”, but the more frequently used abbreviations IPP, DMAPP, etc. |
| This journal is © The Royal Society of Chemistry 2022 |