C2 weakens the turnover frequency during the melting of FexCy: insights from reactive MD simulations†
Yubing
Liu
abc,
Kuan
Lu
 *bc,
Xingchen
Liu
*bc,
Xingchen
Liu
 bc,
Jinjia
Liu
bc,
Jinjia
Liu
 bcd,
Wen-Ping
Guo
c,
Wei
Chen
a,
Qing
Peng
e,
Yu-Fei
Song
bcd,
Wen-Ping
Guo
c,
Wei
Chen
a,
Qing
Peng
e,
Yu-Fei
Song
 *a,
Yong
Yang
bc,
Yong-Wang
Li
bc and
Xiao-Dong
Wen
*a,
Yong
Yang
bc,
Yong-Wang
Li
bc and
Xiao-Dong
Wen
 *bc
*bc
aState Key Laboratory of Chemical Resource Engineering, School of Chemistry, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: songyf@mail.buct.edu.cn
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi 030001, P. R. China. E-mail: wxd@sxicc.ac.cn; lukuan@sxicc.ac.cn
cNational Energy Center for Coal to Clean Fuels, Synfuels China Co., Ltd, Huairou District, Beijing 101400, P. R. China
dUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, P. R. China
ePhysics Department, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia
First published on 18th November 2021
Abstract
The first-order phase transition plays a pivotal role in material behaviors, yet that of carbides, a type of important material, has not been systematically studied. Herein, the melting process and structural properties of binary iron carbide (FexCy) nanoparticles are characterized by reactive molecular dynamics simulation. It was found that the melting point of FexCy nanoparticles decreased with the decreasing size and increased with the increasing Fe/C ratio, which were consistent with the experimental results. The melting process starts at the surface and proceeds inwards. The carbon atoms are fully activated before reaching the melting point and the iron core melts last. At high temperatures, carbon atoms exhibit significant outward diffusion behavior and form carbon deposition on the surface. When the temperature exceeds the pre-melting point, although the high temperature gives the nanoparticles more atomic active sites with low coordination, the surface carbon accumulation, such as C2, blocks the active sites leading to a lower turnover frequency of FexCy for CO dissociation. These findings provide an atomistic comprehension of the melting mechanisms and behaviors of binary FexCy nanoparticles, as well as a theoretical foundation for understanding their structural transformation as a catalyst, which is caused by the heat released from catalytic exothermic reactions.
1. Introduction
Melting is a first-order phase transition and pivotal material behavior happening in daily life. Compared with bulk materials, nanomaterials possess unique properties and more complex melting behaviors due to the large surface/volume ratio of their nanoparticles.1–5 For example, the melting point of nanomaterials depends on the particle size.6–8 Nanometer clusters and nanoparticles have been extensively studied in recent years.9–12 Precisely, probing and predicting their variations during melting has become possible due to the rapid development of experimental and computational techniques.13–16 Iron is an essential metal in the industry due to its excellent properties, low cost, and wide availability. Nano-iron is widely used as a highly active catalyst in industrial-scale catalysis for Fischer–Tropsch synthesis (FTS),17,18 bioimaging,19 and energetic materials.20 Melting point is one of the most basic properties of nano-iron and the systematic investigation of its melting behaviors can help in better understanding its catalytic and mechanical properties.The thermodynamic properties of nanoparticles, such as melting and atomic diffusion, are critical for their synthesis, characterization, and application. However, it is difficult to measure their melting points experimentally. However, the rapid development of molecular dynamics simulation methods in the past decade has allowed researchers to conduct large-scale studies on the melting process of nano iron. Sun et al.21 investigated the effects of the size of Fe nanoparticles on the melting temperature using the ReaxFF force field and found that the defects provide additional energy storage to the Fe nanoparticles for the first time. Shu et al.22 characterized the melting process of Fe nanoparticles by replica-exchange molecular dynamics (REMD) and demonstrated that REMD could effectively overcome the superheating and undercooling problems. They accurately predicted the melting temperature of Fe nanoparticles and described the size dependence of the melting temperature with a revised liquid skin melting (LSM) model. Ding et al.23 studied the surface melting behaviors of Fe clusters by constant temperature molecular dynamics simulation based on many-body interaction potential. The study revealed that the melting surface layer gradually became thicker during heating and the cluster lattice suddenly collapsed and a phase transition occurred at the critical temperature. Joshi et al.24 found by an embedded atom method (EAM) potential that the melting points of Fe and Ni nanoparticles were particle size-dependent, and the critical temperatures were linearly related to the inverse of the particle size.
In addition to the discoveries mentioned above, these studies also suggest that the melting initially happens on the surface of a nanoparticle and then extends to the whole nanoparticle. The surface pre-melting at the initial stage changes the nanoparticle surface structure and significantly affects its properties, especially the catalytic performance, which has been reported by extensive studies on the evolution of surface morphologies of various catalysts during reactions.25–29 In particular, many typical industrial catalytic reactions, such as the activation of methane and the oxidation of ammonia over transition metal nanocatalysts, occur at high temperatures (over 1000 K),30–32 which may have reconstructed the surface of the catalyst by the pre-melting process under reaction conditions. Therefore, understanding the dynamic evolution of the surface structure of nano-catalyst particles under real reaction conditions is essential for catalyst design. In the previous work, we systematically studied the surface pre-melting activities of 11 transition metal nanoparticles by molecular dynamics simulation using the EAM potential.33 Our study revealed that the surface pre-melting of 3 nm Fe nanoparticles occurred at 1050 K. The activated atoms can further activate other atoms using excess kinetic energy, and the resulting activated surface provides more active sites with different atomic coordination numbers, which illustrates the potential impacts of surface pre-melting on heterogeneous catalysis.
Currently, Fe-based compounds instead of pure iron are more widely used in the industry.19,34–37 The increased interaction and coupling between atoms may complicate the thermodynamic processes, such as melting and atomic diffusion. For instance, the Fe-based catalyst for the FTS reaction is generally exposed to the syngas containing CO, H2, etc.,17,18,38–40 which results in complex carbide phases under different reaction conditions, especially accompanied by the release of a large amount of heat during the reaction.41 The heat dissipation of nanoparticles is relatively slow under the cover of gas, and the resultant local high temperature can change the surface morphology of the nanoparticles and further affect the catalytic performance.42 An important example is that high temperatures can significantly affect the existence of carbon atoms on the catalyst surface, such as carbon-rich surface, carbon-free surface, carbon vacancy, etc., which in turn affects processes such as methanation, hydrogenation, water gas shift, and FTS.17,43 Niemantsverdriet et al.44 found that in the carbon-rich (001) surface of Fe5C2, it is difficult for CO to dissociate, while the hydrogenation of surface carbon to CH is a relatively easy reaction process. Carbon-free surface facilitates the direct dissociation of CO. On surfaces containing some carbon vacancies, hydrogen-assisted CO dissociation is more favorable than the direct dissociation of CO. In addition, carbon-containing species undergo decomposition and polymerization to generate carbon deposition on the catalyst surface. It could clog the surface active site and lead to deactivation of the catalyst.45,46 Also, carbon atoms can strengthen the mechanical properties of Fe-based materials, but inevitably cause chemical inertness.47,48 Therefore, iron carbide (FexCy) is also usually used as a constituent in metal alloys and cemented carbide coatings.49–52 Understanding the melting behavior of FexCy nanoparticles is important for revealing the interaction between Fe and C in practical industrial productions.
Due to the complex phase composition of the FexCy catalyst, it is very difficult to characterize and confirm the real active phase with experimental methods.41 Moreover, the phase changes with the environment, which leads to misinterpretation and misunderstanding of the experimental results.53 Therefore, the present work was aimed to provide an atomistic understanding of the structural evolution of the FexCy nanoparticle catalyst during its melting process by the reactive molecular dynamics (RMD) simulation. The models of FexCy nanoparticles with sizes ranging from 2 nm to 8 nm and different Fe/C ratios including ε-Fe2C, χ-Fe5C2, θ-Fe3C, and Fe4C were constructed. Its effects of size and carbon content on the melting temperature and the corresponding structural changes and atomic behaviors during melting were analyzed. CO dissociation, a key step in FTS, was also studied to understand the effect of melting on the catalytic activity of FexCy nanoparticles.
2. Method
The Lindemann index54 measures the vibration of atoms by calculating the function of the interatomic distance in the first neighbor shell. It is widely used to study the melting behavior.55–58 In general, a high Lindemann index indicates high vibrational motion which shows that active atoms are prone to leave their equilibrium sites. It could be seen as an indicator of the melting point when the Lindemann index abruptly ascends. The index is defined as eqn (1) and (2):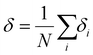 | (1) |
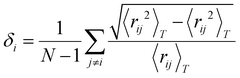 | (2) |
Information about the melting properties of FexCy nanoparticles can be derived from RMD simulations.61,62 Iron carbides can be formed by the reduction of Fe2O3, with α-Fe as an intermediate phase. They have different crystal structures at different temperatures and carbon chemical potentials (μC).41 In iron carbide, the hexagonal close-packed structure of Fe is distorted with carbon atoms occupying its vacancies. In χ-Fe5C2 and θ-Fe3C, carbon atoms are interspersed into the trigonal voids composed of iron atoms, while carbon atoms occupy the octahedral vacancies of ε-Fe2C and Fe4C (Fig. S2a, ESI†). The nanoparticles with diameters of 2 nm (∼300 atoms), 3 nm (∼1100 atoms), 5 nm (∼5000 atoms) and 8 nm (∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 atoms) were constructed for each of the four structured FexCy phases (Fe2C, Fe5C2, Fe3C, and Fe4C). The nanoparticles are created through the Wulff construction63 and some relatively stabled surfaces under adiabatic conditions are selected from Zhao et al.64,65 (Fig. S2b, ESI†). All the simulations were implemented by RMD using large-scale an atomic/molecular massively parallel simulator (LAMMPS)66 and the corresponding structural changes were observed with the Open Visualization Tool (OVITO).67 The reactive force field (ReaxFF) parameters are derived from our previous work and have been proven to be suitable for describing the Fe–C–O interactions.40,68,69 The periodic simulation box size was 12 nm × 12 nm × 12 nm (Fig. S3a, ESI†). For melting simulations, all the nanoparticles were initially pre-equilibrated at 300 K for 100 ps with a canonical (NVT) ensemble. These well-equilibrated structures were then heated up from 300 K to 2000 K for further investigation. Each system lasted for 300 ps with a 0.25 fs time step. For CO activation simulations, the nanoparticle structures simulated at different temperatures were quickly heated or quenched to 800 K and then equilibrated for 10 ps before CO activation reactions. The periodic simulation box has a size of 10.7 nm × 10.7 nm × 10.7 nm, including a Fe5C2 nanoparticle and 500 CO (Fig. S3b, ESI†). Its initial pressure is about 4.6 MPa. The CO activation simulations were run at 800 K for 200 ps. These conditions inhibit the swift evolution of elected temperature structures but accelerate the collision of CO with the nanoparticles, allowing us to accurately examine the effect of melting on the catalytic performance of FexCy nanoparticles. The velocity Verlet integrator has been employed.
000 atoms) were constructed for each of the four structured FexCy phases (Fe2C, Fe5C2, Fe3C, and Fe4C). The nanoparticles are created through the Wulff construction63 and some relatively stabled surfaces under adiabatic conditions are selected from Zhao et al.64,65 (Fig. S2b, ESI†). All the simulations were implemented by RMD using large-scale an atomic/molecular massively parallel simulator (LAMMPS)66 and the corresponding structural changes were observed with the Open Visualization Tool (OVITO).67 The reactive force field (ReaxFF) parameters are derived from our previous work and have been proven to be suitable for describing the Fe–C–O interactions.40,68,69 The periodic simulation box size was 12 nm × 12 nm × 12 nm (Fig. S3a, ESI†). For melting simulations, all the nanoparticles were initially pre-equilibrated at 300 K for 100 ps with a canonical (NVT) ensemble. These well-equilibrated structures were then heated up from 300 K to 2000 K for further investigation. Each system lasted for 300 ps with a 0.25 fs time step. For CO activation simulations, the nanoparticle structures simulated at different temperatures were quickly heated or quenched to 800 K and then equilibrated for 10 ps before CO activation reactions. The periodic simulation box has a size of 10.7 nm × 10.7 nm × 10.7 nm, including a Fe5C2 nanoparticle and 500 CO (Fig. S3b, ESI†). Its initial pressure is about 4.6 MPa. The CO activation simulations were run at 800 K for 200 ps. These conditions inhibit the swift evolution of elected temperature structures but accelerate the collision of CO with the nanoparticles, allowing us to accurately examine the effect of melting on the catalytic performance of FexCy nanoparticles. The velocity Verlet integrator has been employed.
3. Results
3.1. Melting and surface pre-melting
Fig. S4 (ESI†) shows the curves of temperature, total energy and potential energy of bulk Fe5C2 with time. From these curves, it can be deduced that the melting point is 1910 K. However, the Lindemann index is a more definite melting criterion, and more information can be obtained. To explore the melting mechanism of FexCy nanoparticles, the Lindemann index is introduced to characterize the surface pre-melting behaviors and the structural evolution during melting. The Lindemann indices of the nanoparticles with different structures and sizes from 300 K to 2000 K are calculated.Fig. 1 shows the temperature dependence of the overall Lindemann index of FexCy nanoparticles from 2 nm to 8 nm. Similar to pure iron clusters, the melting process of most FexCy nanoparticles can be divided into three stages.23 In the first stage, the Lindemann index increases linearly and slowly with the increasing temperature. The nanoparticles maintain a solid-state, and all atoms vibrate only near their equilibrium positions. In the early second stage, the rising trend of the Lindemann index increases (pre-melting process). Some surface atoms can obtain enough kinetic energy to overcome the binding energy at the initial position and diffuse to other positions on the surface due to its low coordination number when a certain temperature is reached. As the temperature further increases, the kinetic energy of the surface atoms is transferred inward, causing the internal atoms to gradually leave their equilibrium positions and begin to migrate. When the Lindemann index reaches around 0.1, the gradient of the Lindemann line suddenly increases, which indicates the occurrence of the melting process and is also used to judge the melting point of the nanoparticles. It is noted that the Lindemann indices of some nanoparticles undergoing melting range from 0.1 to 0.15, which is because there are still some internal solid atoms with the Lindemann index less than 0.1 when the overall Lindemann index reaches 0.1. After exceeding the threshold of its critical temperature, the Lindemann line continuously increases which resembles its initial increasing form until the nanoparticles are completely melted. At this time, the third stage has also been reached, the Lindemann line flattens again, and the nanoparticles are totally in the liquid form.
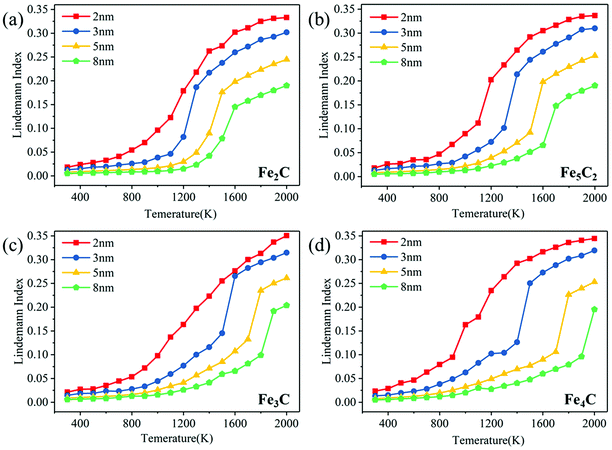 | ||
| Fig. 1 The overall Lindemann index of different sizes of FexCy nanoparticles: (a) Fe2C; (b) Fe5C2; (c) Fe3C; (d) Fe4C. | ||
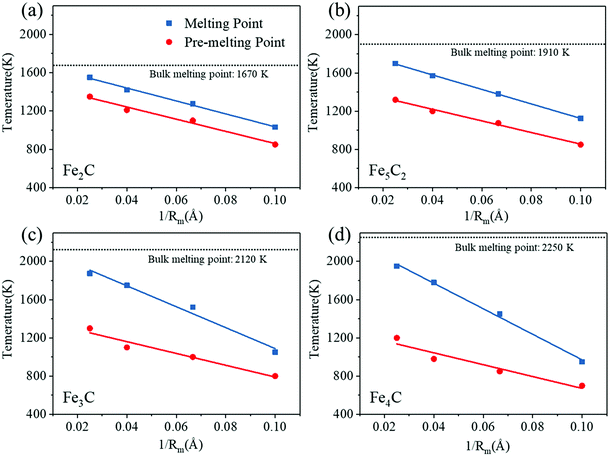
The melting point of nanoparticles exhibits a strong size effect. Compared with large-sized nanoparticles, the Lindemann indices of small-sized particles increase faster with temperature. Due to the larger surface area to volume ratio, they are easier to be fully activated. As the particle size increased, the surface atomic percentage decreases, and higher temperatures are required for the melting of nanoparticles.70,71 Taking Fe5C2 as an example, the critical temperatures of the nanoparticles of size 2 nm, 3 nm, 5 nm, and 8 nm are 1125 K, 1380 K, 1570 K, and 1700 K, respectively. It can be predicted that the critical temperature will become higher as the particle size further increases. In addition, the melting points obtained by the potential energy are consistent with the results of the Lindemann index (Fig. S5a, ESI†). The density of nanoparticles decreases gradually with the increasing temperature, increases in magnitude above the pre-melting point, and decreases sharply near the melting point (Fig. S5b, ESI†). This is consistent with the pattern of density change of nanoparticles during melting in the previous studies.72,73 The density of Fe3C calculated with ReaxFF at a low temperature (7.775 g cm−3) is in agreement with the report in the experimental work (∼ 7.68 g cm−3).74,75
Here, we summarize the dependence of the melting and surface pre-melting point of FexCy nanoparticles on their size. Liu et al.33 reported that the melting point and pre-melting point of pure metal nanoparticles are linearly related to the inverse of the nanoparticle radius (1/Rm). A similar relationship is found in the two-component iron carbide nanoparticles in our work, as shown in Fig. 2. The differences between the melting point and the surface pre-melting point of the Fe2C nanoparticles with different particle sizes are all ∼200 K. As the Fe/C ratio increases, that is, the carbon content declines, the difference becomes size-dependent. The pre-melting point of the 8 nm Fe5C2 nanoparticles is 380 K lower than their melting point and the difference between the pre-melting and melting points of the 2 nm nanoparticles is only ∼270 K. As the Fe/C ratio further increased, the size dependence of the difference between the pre-melting and melting points becomes more obvious. The differences for 8 nm Fe3C and Fe4C nanoparticles reach 575 K and 750 K, respectively. Therefore, it can be concluded that carbon content plays a very important role in the melting process of FexCy nanoparticles. The detailed discussion is presented in Section 3.2. The linear relationship may be used to estimate the surface activation temperature of FexCy nanoparticles based on their melting temperature. In addition, it is found that relatively large (5 nm and 8 nm) nanoparticles with low carbon contents exhibit wider temperature ranges where the solid and liquid phases coexist. The lower the Fe/C ratio (higher the carbon content), the lower the melting point, which is applicable to larger nanoparticles, but not to smaller ones due to their low atomic numbers (Table S1, ESI†). The bulk melting temperatures of Fe2C, Fe5C2, Fe3C and Fe4C are 1670 K, 1910 K, 2120 K, and 2250 K, respectively. In the experiment, the bulk melting temperature of Fe3C is 2055 K.76 Our conclusion is consistent with the work reported by Xi et al.77 that increasing carbon content accelerates the melting of steel and lowers the melting point. Moreover, the order of melting points was found to be consistent with that of the stability of FexCy, that is, the stability gradually increases as the carbon concentration decreases.78,79
3.2. Melting mechanism and structural evolution
The melting process of monometallic nanoparticles starts on the particle shell or surface and then extends to the entire particle.21–24 The melting behaviors of bimetallic and multimetallic heterogeneous nanoparticles are more complicated due to their different element compositions and structural constructions.58,80–82 The melting behaviors of the binary metal-nonmetal FexCy nanoparticles have not been systematically investigated yet. As the important active phases in Fischer–Tropsch synthesis, FexCy nanoparticles are under the influence of large amounts of reaction heat released from the catalytic reaction and are subject to rapid temperature increases. It will lead to partial or even total melting of the catalyst, thereby affecting the catalytic properties. Therefore, the melting mechanism and structural evolution of FexCy nanoparticles during heating are explored in this section.Fig. 3 shows the radial distribution of the Lindemann indices of all atoms in a 5 nm Fe5C2 nanoparticle at different temperatures, averaged over the trajectories of MD simulations. At low temperatures, such as 500 K, the Lindemann indices of all atoms are quite low, indicating that they only vibrate around their initial positions. The Lindemann indices increase with the increase of temperature. Some surface atoms are activated and migrate to other positions when heated to 1200 K and show a Lindemann index of greater than 0.1 (Fig. 3d and Fig. S1, ESI†). The nanoparticles undergo surface pre-melting and are in the solid–liquid coexistence state. As the temperature further increases, the number of migrating atoms gradually increases (Fig. 3e–h). C atoms are more sensitive to temperature than Fe atoms.83 At 1500 K, almost all C atoms are activated, while the internal Fe atoms remain inactivated, suggesting that the C atoms can be fully activated before the melting point is reached, and the internal Fe atoms maintain the crystal structure. As the temperature increased to the critical point (Fig. 3h), the Lindemann index of the inner Fe atoms suddenly increases, rising above 0.1, and the nanoparticles are completely melted. In addition, both 3 nm and 8 nm nanoparticles exhibit well-defined melting processes, while the processing of 2 nm nanoparticles is indeed less clear (Fig. S6, ESI†). This is due to the smaller atomic number and larger surface-to-volume ratio of small-sized particles, which make it difficult to form a stable solid core inside to resist high temperatures. After surface pre-melting occurs, they are completely melted in a shorter temperature range. The narrow solid–liquid coexistence range of small nanoparticles leads to a lower melting temperature. These deductions indicate that the high temperature causes the phase transition of FexCy nanoparticles from the solid to the amorphous state.
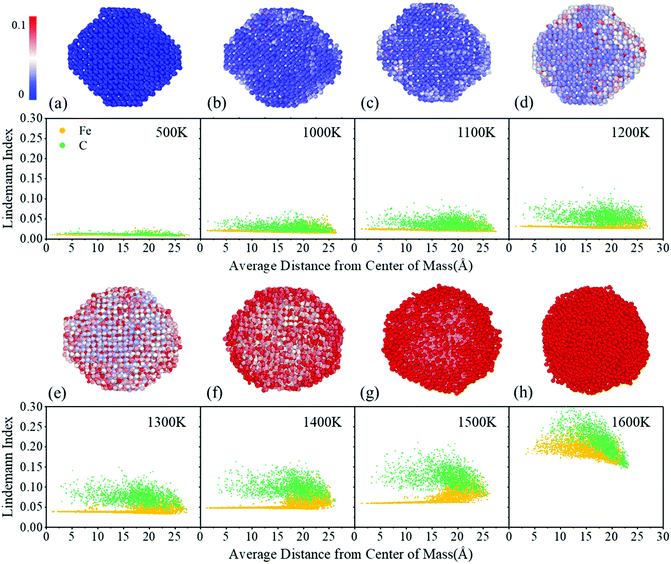 | ||
| Fig. 3 Individual atom Lindemann index variation from a lower temperature to higher of 5 nm Fe5C2 nanoparticles. Large spheres and small spheres represent the Fe atoms and C atoms, respectively. | ||
The Lindemann index variations of the individual atom of other FexCy nanoparticles were also investigated. These nanoparticles show similar melting behaviors in that the C atoms are more easily activated than the Fe atoms during the heating process (Fig. S7–S9, ESI†). As the carbon content decreases, the solid–liquid coexistence phenomenon is more obvious and the coexistence temperature range is larger, which is reflected in the increase of the melting point. We speculate that the carbon content affects the melting temperature of FexCy nanoparticles from two aspects. First, at the activation temperature, the activated C atoms transfer excess kinetic energy to the Fe atoms to accelerate the melting of Fe.33 Second, the melting point of structurally ordered nanoparticles is higher and thus their thermal stability is better than the disordered one.84,85 High carbon contents increase the degree of disorder of the FexCy lattice, and thus lower the melting point to facilitate the melting process.
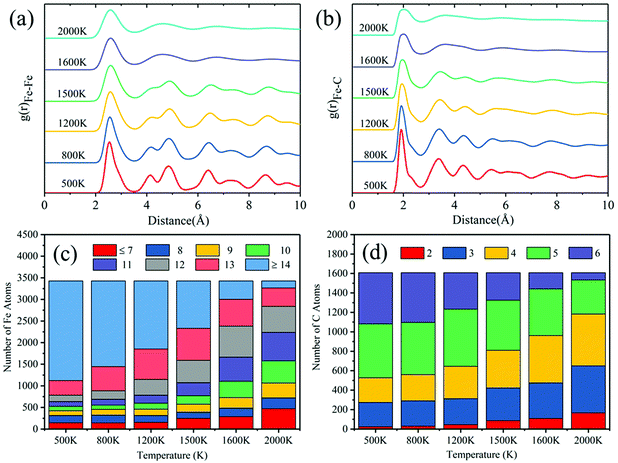
For the specific melting process of binary FexCy nanoparticles characterized by the radial distribution function (RDF) and coordination numbers (CNs) to determine the influences of temperature on their structure, 5 nm Fe5C2 nanoparticles are taken as an example for analyses. The surface atoms of the 5 nm Fe5C2 equilibrium structure at 300 K are obtained by the method reported by Barron et al.26 RDF is an important tool used to determine the structural properties of a material. It can provide more information for better understanding the melting process than the Lindemann index. The RDFs of three pairs of atoms are calculated. As shown in Fig. 4a, the pair interactions between the surface atoms gradually become weaker with the increase of temperature. It should be noted that there are no neighboring C atoms in the Fe5C2 crystal at low temperatures. However, a tiny peak appears around 1.15 Å at 1200 K, indicating the possible formation of C–C bonds after surface pre-melting. The peak becomes significantly stronger at 1600 K, which suggests the occurrence of the aggregation of C atoms on the surface. Fig. 4b shows the distributions of the CN of Fe and C atoms at different temperatures. At 500 K, the surface Fe atoms are mostly in the 8-fold coordination form, and the high-fold coordination (CN > 12) Fe atoms are relatively rare. As the temperature is increased to over the pre-melting point, the coordination number of the surface atoms changes dramatically. Owing to the complete melting of the entire nanoparticles at 1600 K, the internal and surface atoms undergo violent atomic exchange. The surface atoms at low temperatures may become internal atoms at high temperatures.33 Therefore, the overall coordination number of the surface Fe atoms is prone to increase with the increase of temperature, showing a shift of uniform distribution centered around 8 at 500 K to 12 at 1600 K. Carbon atoms exhibit a similar pattern. But this does not mean that the overall activity of the catalyst is reduced, as shown in Fig. 5c. The atomic stress distribution becomes obviously broader with the increase of temperature (Fig. 4c). The kinetic energy of atoms increases with the increase of temperature, which causes structural expansion and greater internal stresses.86 The increased kinetic energy drives atomic exchange to form internal higher-fold coordination of atoms and generate superior internal stresses, which further weakens the RDF distribution of atomic pairs.
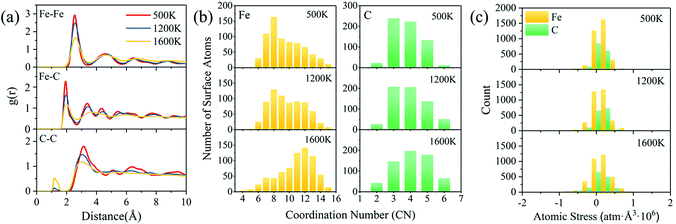 | ||
| Fig. 4 Radial distribution functions of surface atoms (a), coordination numbers of surface atoms (b), and atomic stress distributions (c) of 5 nm Fe5C2 nanoparticles. | ||
Fig. 5 shows the RDF of all atoms in 5 nm Fe5C2 nanoparticles at different temperatures. The first peak of the RDF of all Fe atoms at 500 K is observed at ∼2.55 Å, which is consistent with that reported in previous studies87 and the structural characteristics of bulk Fe5C2 (Fig. S10, ESI†). The cut-off distance of the first coordination layer of Fe atoms (∼2.55 Å) is irrelevant to the temperature and particle size, and the main peaks become wider and weaker as the temperature increased. The minor peaks become inconspicuous as the temperature increased to 1200 K, and the second and the third peaks tend to merge into one. It corresponds exactly to the RDF peak pattern of the Fe atoms on the surface and is a signal for the onset of surface melting. All peaks outside the first coordination layer disappear at 1600 K, indicating that the nanoparticles are completely melted. For the RDF between the Fe and C atoms, the first peak at 500 K is located at ∼1.95 Å, consistent with that reported in the literature87 and the structure of bulk Fe5C2. The changing trend of RDF with temperature is similar to that of Fe–Fe due to the gradual cleavage of Fe–C bonds. The broadening of the main RDF peaks and the vanishing of the minor RDF peaks are the major features of the solid–liquid phase transition process of nanoparticles.80 The melting points derived from RDF measurements are consistent with those obtained from the Lindemann index analysis. Overall, the atomic coordination number shows a decreasing trend for high-fold coordination atoms and an increasing trend for low-fold coordination atoms with the increase of temperature (Fig. 5c and d). It can be explained that more surface atoms are produced as the lattice expands under internal stress during surface melting.
3.3. Atomic diffusion behavior during heating
As mentioned above, in addition to thermal evolution during heating, the atomic diffusion properties are also important for the melting process of FexCy nanoparticles. Here, atomic diffusion analysis is used to further characterize the effect of temperature on the structure. In general, the atomic diffusion rate rapidly increases with the increase of temperature, which inevitably leads to the redistribution of components in the binary nanoparticles, and eventually changes the bulk structure and surface morphology and thereby affects all aspects of the properties of the nanoparticles. Therefore, the diffusion behaviors of atoms are crucial for studying the performances of binary nanoparticles at different temperatures.Fig. 6 shows the snapshots of the atomic configurations of 5 nm nanoparticles at different temperatures. At low-temperature intervals in the range from 300 K to 1200 K, the Wulff structure of the nanoparticles shows no significant changes. As the temperature increases to 1200 K, the arrangements of some surface C atoms become irregular. Some edges and corners disappear due to the local morphological changes. A certain number of surface C atoms leave their original equilibrium positions and migrate, and some of them even neighbor with each other to form C–C bonds (green circles in Fig. 6). A large number of internal C atoms diffuse to the surface at 1500 K. Meanwhile, the internal Fe atoms remain in their position as a core to maintain the regular crystal structure (dashed circle in Fig. 6). Further increasing the temperature to 1600 K causes the fierce transformation of the nanoparticles to a completely amorphous phase. The Fe5C2 nanoparticle surface begins to accumulate carbon atoms after surface pre-melting occurs. The di-carbon (C2) on the surface of the nanoparticles increases with the increasing temperature, especially after melting. This may be the main cause of deactivation in the high-temperature Fischer–Tropsch process.88,89
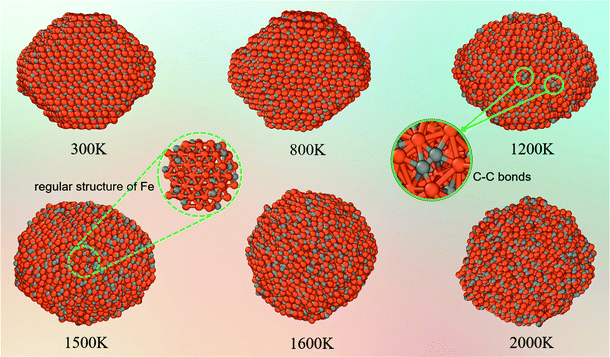 | ||
| Fig. 6 Structural evolution of 5 nm Fe5C2 nanoparticles with the increasing temperature (orange: Fe; grey: C). | ||
The detailed diffusion behaviors of the atoms in Fe5C2 nanoparticles at different temperatures are presented in Fig. 7a. To simplify the statistical analysis, the Fe and C atoms are averaged to an axis by the volume formula of a sphere. The axial distribution is defined as 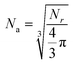 ,90 where Nr is the total number of atoms within the r distance from the center of mass and Na is the corresponding average number of the atoms distributed along the axis. As can be seen from Fig. 7a, the distribution of carbon atoms along the axis at 500 K is linear, indicating that there is no obvious diffusion behavior below the pre-melting point. The axial distribution of atoms at ∼ 20 Å from the center of mass gradually becomes flat because the nanoparticle is not a sphere and the axis partially reaches the surface at this point. As the temperature increased to 1200 K, the curve goes downward slightly, suggesting that the pre-melting point is reached. The internal atoms tend to diffuse outward because the activated surface atoms transfer their excess kinetic energy towards the internal structure of the nanoparticle. The downtrend of the curve becomes dramatic when the temperature is close to the melting point. A large number of internal carbon atoms are activated at high temperatures, and atomic exchange between the inner and outer layers becomes more frequent. The internal carbon atoms tend to diffuse outward and continuously segregate to the surface. The iron atoms maintain the framework of the nanoparticle, and thus migrate in smaller migration ranges than C atoms at high temperatures. As shown in Fig. 7a, the number of internal Fe atoms remains almost consistent near the melting point. Although the Fe atoms also display thermal diffusion at high temperatures, they do not migrate outward as much as the C atoms do. The distribution of Fe atoms increases to a certain extent at 22.5 Å away from the center of mass because the core structure is destroyed at the melting point. All of the surface edges and corners disappear, and the nanoparticles are amorphized (Fig. 6).
,90 where Nr is the total number of atoms within the r distance from the center of mass and Na is the corresponding average number of the atoms distributed along the axis. As can be seen from Fig. 7a, the distribution of carbon atoms along the axis at 500 K is linear, indicating that there is no obvious diffusion behavior below the pre-melting point. The axial distribution of atoms at ∼ 20 Å from the center of mass gradually becomes flat because the nanoparticle is not a sphere and the axis partially reaches the surface at this point. As the temperature increased to 1200 K, the curve goes downward slightly, suggesting that the pre-melting point is reached. The internal atoms tend to diffuse outward because the activated surface atoms transfer their excess kinetic energy towards the internal structure of the nanoparticle. The downtrend of the curve becomes dramatic when the temperature is close to the melting point. A large number of internal carbon atoms are activated at high temperatures, and atomic exchange between the inner and outer layers becomes more frequent. The internal carbon atoms tend to diffuse outward and continuously segregate to the surface. The iron atoms maintain the framework of the nanoparticle, and thus migrate in smaller migration ranges than C atoms at high temperatures. As shown in Fig. 7a, the number of internal Fe atoms remains almost consistent near the melting point. Although the Fe atoms also display thermal diffusion at high temperatures, they do not migrate outward as much as the C atoms do. The distribution of Fe atoms increases to a certain extent at 22.5 Å away from the center of mass because the core structure is destroyed at the melting point. All of the surface edges and corners disappear, and the nanoparticles are amorphized (Fig. 6).
 | ||
| Fig. 7 (a) The axial distribution of atoms and (b) the atomic distribution functions of Fe5C2 at five representative temperatures. The solid lines represent Fe, and the dashed lines represent C. | ||
The atomic distribution function N(r) of the Fe and C atoms in the nanoparticles was calculated to determine their distributions at different temperatures. N(r)dr is the number of atoms within a shell of thickness dr at the distance r from the center of mass.91 As shown in Fig. 7b, the N(r)dr peak of the C atoms gradually shifts to the right with the increase of temperature, which confirms the conclusion drawn above that C atoms tend to diffuse outward at high temperatures. The peak position shifts and the peak intensity increases dramatically at 1600 K, suggesting that the phase transition is completed. The N(r)dr peak of the Fe atoms shifts slightly to the right before the critical temperature is reached because of the structural expansion caused by the kinetic energy and atomic stress at high temperatures. The peak shape also changes significantly at the melting point because of the migration and rearrangement of external atoms. To explain the diffusion behaviors of atoms more clearly, the temperature dependence of the Fe/C ratio in each shell is also calculated and summarized in Table 1. As can be seen, the internal Fe/C ratio gradually increases with the increase of temperature, up to more than 3 at 2000 K, and the surface Fe/C ratio decreases. In addition, the slope of mean square displacements (MSD) and the diffusion coefficients gradually increase as the temperature increases, indicating that the diffusion rates of Fe and C atoms accelerate during heating (Fig. S11, ESI†). It should be noted that the diffusion coefficient of C atoms is always greater than that of Fe atoms, and the difference between the two becomes larger and larger after reaching the pre-melting point. This provides support for the outward diffusion behavior of C atoms. These results further suggest that the C atoms in the iron carbide nanoparticle tend to diffuse outwards at high temperatures and segregate and aggregate on the surface.
| T (K) | R (Å) | ||||
|---|---|---|---|---|---|
| Interior | Near the surface | Surface | |||
| 0–5 | 5–10 | 10–15 | 15–20 | >20 | |
| 500 | 2.5 | 2.5 | 2.5 | 2.1 | 1.8 |
| 1200 | 2.5 | 2.5 | 2.6 | 2.2 | 1.7 |
| 1500 | 2.8 | 2.5 | 2.8 | 2.2 | 1.6 |
| 1600 | 2.1 | 2.8 | 2.5 | 2.5 | 1.5 |
| 2000 | 1.5 | 3.1 | 3.3 | 2.8 | 1.3 |
3.4 Catalytic performance of melted nanoparticles
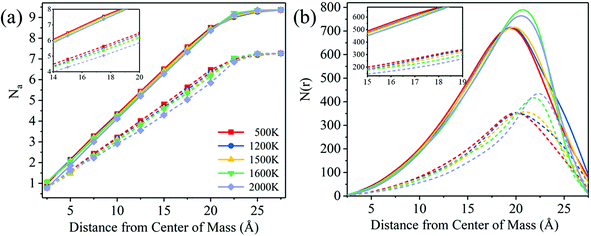
As mentioned above, the structural evolution of FexCy nanoparticles with the increasing temperature is mainly manifested in the decrease of the atomic coordination number and the accumulation of C atoms on the surface. The former provides more active sites for catalytic reactions, while the latter hinders the local adsorption of gas molecules.92 In addition, it has been reported that both C and Fe atoms exhibit catalytic activity for CO.93–95 Therefore, the activity of the melted nanoparticles needs to be further explored.CO dissociation is the critical step in the Fischer–Tropsch reaction.38,39 To understand the effect of melting on the catalytic activity of FexCy nanoparticles, their catalytic performance represented by CO dissociation behaviors before and after melting was examined. As shown in Fig. 8a, the CO dissociation number was assumed to be temperature-dependent on the nanoparticles throughout the 200 ps reaction. That is, the number of CO dissociation catalyzed by nanoparticles is more or less after higher temperature treatment. The turnover frequency (TOF) is one of the key indicators for assessing the catalytic activity, which is defined as TOF = N/M/T, where N represents the amount of CO dissociation, M represents the number of surface atoms (Fe and C atoms) approximating the number of surface sites, and t represents the reaction time.69 It was inferred from the inflection point in Fig. 8a that the gas pressure drops rapidly near 125 ps, so we used 125 ps as the reaction time in order to eliminate the influence of the pressure difference. Fig. 8b shows that the melted nanoparticles have lower TOF values compared to the unmelted ones. Moreover, the surface Fe/C ratio decreases due to the segregation of C atoms to the surface with the increasing temperature. The TOF value was positively correlated with the surface Fe/C ratio. However, it should be pointed out that there is no hydrogen added to generate hydrocarbons. In actual catalytic reactions, the formation of hydrocarbons may lead to more complex situations.39,44
 | ||
| Fig. 8 (a) Curves of CO dissociation number with time, (b) the TOF values and surface Fe/C ratios for Fe5C2 nanoparticles at different temperatures. | ||
According to Fig. 6, the aggregation of carbon on the surface of the nanoparticles mainly exists in the form of C2. To further explore the effect of the presence of surface C2 on CO dissociation, we assumed that the surface C2 were removed in the form of olefins, and the melted nanoparticles without C2 on the surface (removed 111 pairs for 1600 K and 262 pairs for 2000 K) were reacted with CO under the same conditions. As shown in Fig. S12 (ESI†), the CO dissociation curves of the nanoparticles after removal of C2 were all higher than those before removal. The TOF values of nanoparticles without C2 are also higher than those of untreated nanoparticles (1600 K: 1.10 vs. 0.838; 2000 K: 1.17 vs. 0.674), and even higher than that of unmelted nanoparticles (500 K: 0.992; 1200 K: 0.914). Therefore, melting of the surface at high temperatures can increase the catalytic activity of FexCy, but an important prerequisite is to control the aggregation of relatively weak carbon atoms in a hydrogen-deficient atmosphere. The carbon accumulation in the form of C2 on the surface at high temperatures blocks the surface catalytic active sites, reducing the activity of melted FexCy nanoparticles.
4. Conclusion
The melting behaviors and mechanisms of binary FexCy nanoparticles with different Fe/C ratios and different sizes were investigated by RMD simulations. We introduced the Lindemann index as an indicator of solid–liquid phase transition and determined the melting points and surface pre-melting points for different nanoparticles. The critical point of melting temperature increases with the increase of particle size. Both the melting temperature and the pre-melting temperature have a good linear relationship with the inverse of the nanoparticle size. For the FexCy nanoparticles of similar sizes, the higher the C content, the lower the melting point. The melting process largely occurs from the surface to the internal region, but it should be noted that the C atoms are always fully activated before reaching the melting point, while the inner core region, composed of Fe atoms, melts abruptly only when the critical temperature is reached. C atoms are more sensitive to temperature than Fe atoms and exhibit temperature-dependent thermal diffusion behaviors. They tend to diffuse outward and accelerate their structural evolution. At the same time, they can aggregate on the surface of nanoparticles at high temperatures. Such carbon accumulation in the form of C2 clogs the surface-active sites and leads to a lower TOF value of FexCy nanoparticles for CO dissociation. Our work sheds light on the melting mechanism, surface pre-melting, and atomic thermal diffusion of binary FexCy nanoparticles at the atomic level. More importantly, C2 maybe the key intermediate in the CO activation process, whose contents determine the surface activity of the catalyst. We hope that these findings could provide some enlightenment for the possible controllable structural evolution of FexCy in catalytic reactions.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 21625101, No. 21972157, and No. 21972160), the Fundamental Research Funds for the Central Universities (XK1802-6), the CAS Project for Young Scientists in Basic Research (YSBR-005), Shanxi Province Foundation for PhD (2020SHB008), and the funding support from Synfuels China, Co. Ltd and “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academic of Sciences (No. XDA 21000000). We also acknowledge the innovation foundation of the Institute of Coal Chemistry, Chinese Academy of Sciences, Hundred-Talent Program of Chinese Academy of Sciences, Shanxi Hundred-Talent Program, and National Thousand Young Talents Program of China.References
- S. U. Son, Y. Jang, J. Park, H. B. Na, H. M. Park, H. J. Yun, J. Lee and T. Hyeon, J. Am. Chem. Soc., 2004, 126, 5026–5027 CrossRef CAS PubMed.
- L. Lu, J. Liu, Y. Hu, Y. Zhang and W. Chen, Adv. Mater., 2013, 25, 1270–1274 CrossRef CAS PubMed.
- A. Kryshtal, A. Minenkov, S. Bogatyrenko and A. Gruszczyński, J. Alloys Compd., 2019, 786, 817–825 CrossRef CAS.
- C. Cui, L. Gan, M. Heggen, S. Rudi and P. Strasser, Nat. Mater., 2013, 12, 765–771 CrossRef CAS PubMed.
- M. Takagi, J. Phys. Soc. Jpn., 2007, 9, 359–363 CrossRef.
- K. Dick, T. Dhanasekaran, Z. Zhang and D. Meisel, J. Am. Chem. Soc., 2002, 124, 2312–2317 CrossRef CAS PubMed.
- K. Nanda, Pramana, 2009, 72, 617–628 CrossRef CAS.
- Y. Gao, C. Zou, B. Yang, Q. Zhai, J. Liu, E. Zhuravlev and C. Schick, J. Alloys Compd., 2009, 484, 777–781 CrossRef CAS.
- C. Gao, F. Lyu and Y. Yin, Chem. Rev., 2020, 121, 834–881 CrossRef.
- Z. Li, S. Ji, Y. Liu, X. Cao, S. Tian, Y. Chen, Z. Niu and Y. Li, Chem. Rev., 2019, 120, 623–682 CrossRef PubMed.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- M. Böyükata, E. Borges, J. P. Braga and J. C. Belchior, J. Alloys Compd., 2005, 403, 349–356 CrossRef.
- U. Domekeli, S. Sengul, M. Celtek and C. Canan, Philos. Mag., 2017, 98, 371–387 CrossRef.
- R. Essajai, A. Rachadi, E. Feddi and N. hassanain, Mater. Chem. Phys., 2018, 218, 116–121 CrossRef CAS.
- J. Liu, M. Wang and P. Liu, Mater. Res. Express, 2018, 5, 065011 CrossRef.
- S. Alavi and D. L. Thompson, J. Phys. Chem. A, 2006, 110, 1518–1523 CrossRef CAS PubMed.
- J. Xie, H. M. Torres Galvis, A. C. Koeken, A. Kirilin, A. I. Dugulan, M. Ruitenbeek and K. P. de Jong, ACS Catal., 2016, 6, 4017–4024 CrossRef CAS PubMed.
- J. Xie, J. Yang, A. I. Dugulan, A. Holmen, D. Chen, K. P. de Jong and M. J. Louwerse, ACS Catal., 2016, 6, 3147–3157 CrossRef CAS.
- Z. Schnepp, S. C. Wimbush, M. Antonietti and C. Giordano, Chem. Mater., 2010, 22, 5340–5344 CrossRef CAS.
- S. Mahadik-Khanolkar, S. Donthula, A. Bang, C. Wisner, C. Sotiriou-Leventis and N. Leventis, Chem. Mater., 2014, 26, 1318–1331 CrossRef CAS.
- J. Sun, P. Liu, M. Wang and J. Liu, Sci. Rep., 2020, 10, 1–11 CrossRef PubMed.
- Q. Shu, Y. Yang, Y. T. Zhai, D. Y. Sun, H. J. Xiang and X. G. Gong, Nanoscale, 2012, 4, 6307–6311 RSC.
- F. Ding, K. Bolton and A. Rosén, Eur. Phys. J. D, 2005, 34, 275–277 CrossRef CAS.
- N. P. Joshi, D. E. Spearot and D. Bhat, J. Nanosci. Nanotechnol., 2010, 10, 5587–5593 CrossRef CAS PubMed.
- S. K. Matam, E. V. Kondratenko, M. H. Aguirre, P. Hug, D. Rentsch, A. Winkler, A. Weidenkaff and D. Ferri, Appl. Catal., B, 2013, 129, 214–224 CrossRef CAS.
- H. Barron, G. Opletal, R. D. Tilley and A. S. Barnard, Catal. Sci. Technol., 2016, 6, 144–151 RSC.
- S. Vajda, S. Lee, K. Sell, I. Barke, A. Kleibert, V. von Oeynhausen, K. H. Meiwes-Broer, A. F. Rodriguez, J. W. Elam, M. M. Pellin, B. Lee, S. Seifert and R. E. Winans, J. Chem. Phys., 2009, 131, 121104 CrossRef PubMed.
- X.-Q. Zhang, E. Iype, S. V. Nedea, A. P. J. Jansen, B. M. Szyja, E. J. M. Hensen and R. A. van Santen, J. Phys. Chem. C, 2014, 118, 6882–6886 CrossRef CAS.
- M. N. Krstajić Pajić, S. I. Stevanović, V. V. Radmilović, A. Gavrilović-Wohlmuther, V. R. Radmilović, S. L. Gojković and V. M. Jovanović, Appl. Catal., B, 2016, 196, 174–184 CrossRef.
- M. P. Suárez and D. G. Löffler, J. Catal., 1986, 97, 240–242 CrossRef.
- P. Tang, Q. Zhu, Z. Wu and D. Ma, Energy Environ. Sci., 2014, 7, 2580–2591 RSC.
- V. Sadykov, L. Isupova, I. Zolotarskii, L. Bobrova, A. Noskov, V. Parmon, E. Brushtein, T. Telyatnikova, V. Chernyshev and V. Lunin, Appl. Catal., A, 2000, 204, 59–87 CrossRef CAS.
- X. Liu, X. Wen and R. Hoffmann, ACS Catal., 2018, 8, 3365–3375 CrossRef CAS.
- Y.-K. Dou, H. Cao, X.-F. He, J. Gao, J.-L. Cao and W. Yang, J. Alloys Compd., 2021, 857, 157556 CrossRef CAS.
- N. Yan, L. Qin, H. Hao, L. Hui, F. Zhao and H. Feng, Appl. Surf. Sci., 2017, 408, 51–59 CrossRef CAS.
- C. Ni, H. Ding and X. J. Jin, J. Alloys Compd., 2013, 546, 1–6 CrossRef CAS.
- J. Yang, W. Hu and J. Tang, RSC Adv., 2013, 4, 2155–2160 RSC.
- C. Yang, H. Zhao, Y. Hou and D. Ma, J. Am. Chem. Soc., 2012, 134, 15814–15821 CrossRef CAS PubMed.
- Y. Li, Z. Li, A. Ahsen, L. Lammich, G. J. A. Mannie, J. W. H. Niemantsverdriet and J. V. Lauritsen, ACS Catal., 2018, 9, 1264–1273 CrossRef.
- K. Lu, C.-F. Huo, Y. He, W.-P. Guo, Q. Peng, Y. Yang, Y.-W. Li and X.-D. Wen, J. Catal., 2019, 374, 150–160 CrossRef CAS.
- E. de Smit, F. Cinquini, A. M. Beale, O. V. Safonova, W. van Beek, P. Sautet and B. M. Weckhuysen, J. Am. Chem. Soc., 2010, 132, 14928–14941 CrossRef CAS PubMed.
- L. Zhu, Applied Sciences, 2005.
- Y.-g. C. Keiichi Tomishige and Kaoru Fujimoto, J. Catal., 1999, 181, 91–103 CrossRef.
- M. O. Ozbek and J. W. Niemantsverdriet, J. Catal., 2014, 317, 158–166 CrossRef CAS.
- D. C. L. Peter and E. Nolan, Andrew Hall Cutler, J. Phys. Chem. B, 1998, 102, 4165–4175 CrossRef.
- R. Haldeman and M. Botty, J. Phys. Chem., 1959, 63, 489–496 CrossRef CAS.
- P. S. Ghosh, K. Ali, A. Vineet, A. Voleti and A. Arya, J. Alloys Compd., 2017, 726, 989–1002 CrossRef CAS.
- W. Ge, W. Gao, J. Zhu and Y. Li, J. Alloys Compd., 2019, 781, 1069–1073 CrossRef CAS.
- K. Jack, Acta Crystallogr., 1950, 3, 392–394 CrossRef CAS.
- C. K. Ande and M. H. Sluiter, Metall. Mater. Trans. A, 2012, 43, 4436–4444 CrossRef CAS.
- X. Chong, Y. Jiang and J. Feng, J. Alloys Compd., 2018, 745, 196–211 CrossRef CAS.
- A. K. Biswas, Indian J. Hist. Sci., 1994, 29, 579–610 Search PubMed.
- E. de Smit, I. Swart, J. F. Creemer, G. H. Hoveling, M. K. Gilles, T. Tyliszczak, P. J. Kooyman, H. W. Zandbergen, C. Morin, B. M. Weckhuysen and F. M. de Groot, Nature, 2008, 456, 222–225 CrossRef CAS PubMed.
- H. J. Hoffmann, Materialwiss. Werkst., 2004, 35, 79–81 CrossRef CAS.
- K. Zhang, G. M. Stocks and J. Zhong, Nanotechnology, 2007, 18, 285703 CrossRef.
- Q. S. Mei and K. Lu, Prog. Mater. Sci., 2007, 52, 1175–1262 CrossRef CAS.
- Y. Engelmann, A. Bogaerts and E. C. Neyts, Nanoscale, 2014, 6, 11981–11987 RSC.
- Y. H. Wen, L. Li, Y. M. Li and R. Huang, J. Phys. Chem. Lett., 2021, 12, 2454–2462 CrossRef CAS PubMed.
- H. Löwen, Phys. Rep., 1994, 237, 249–324 CrossRef.
- K. Hansen, Statistical Physics of Nanoparticles in the Gas Phase, 2013 Search PubMed.
- A. C. T. van Duin, S. Dasgupta, F. Lorant and W. A. Goddard, J. Phys. Chem. A, 2001, 105, 9396–9409 CrossRef CAS.
- L. Liu, A. Jaramillo-Botero, W. A. Goddard and H. Sun, J. Phys. Chem. A, 2012, 116, 3918–3925 CrossRef CAS PubMed.
- G. Wulff, Z. Kristallogr., 1901, 34, 449–530 CAS.
- S. Zhao, X.-W. Liu, C.-F. Huo, Y.-W. Li, J. Wang and H. Jiao, J. Catal., 2012, 294, 47–53 CrossRef CAS.
- S. Zhao, X.-W. Liu, C.-F. Huo, Y.-W. Li, J. Wang and H. Jiao, Catal., Struct. React., 2014, 1, 44–60 Search PubMed.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- A. Stukowski, Model. Simul. Mater. Sci., 2010, 18, 2154–2162 Search PubMed.
- K. Lu, Y. He, C.-F. Huo, W.-P. Guo, Q. Peng, Y. Yang, Y.-W. Li and X.-D. Wen, J. Phys. Chem. C, 2018, 122, 27582–27589 CrossRef CAS.
- K. Lu, D. Luo, Y. He, C.-F. Huo, Y. Zhou, W.-P. Guo, Q. Peng, Y. Yang, Y.-W. Li and X.-D. Wen, Appl. Surf. Sci., 2021, 570, 151018 CrossRef CAS.
- S. L. Lai, J. Y. Guo, V. Petrova, G. Ramanath and L. H. Allen, Phys. Rev. Lett., 2010, 77, 99–102 CrossRef PubMed.
- A. P. Chernyshev, Mater. Lett., 2009, 63, 1525–1527 CrossRef CAS.
- V. S. Tsepelev, Y. N. Starodubtsev, K. M. Wu and Y. A. Kochetkova, Key Eng. Mater., 2020, 861, 107–112 Search PubMed.
- F. Font, T. G. Myers and S. L. Mitchell, Microfluid. Nanofluid., 2014, 18, 233–243 CrossRef.
- G. Fiquet, J. Badro, E. Gregoryanz, Y. Fei and F. Occelli, Phys. Earth Planet. Inter., 2009, 172, 125–129 CrossRef CAS.
- K. D. Litasov, I. S. Sharygin, P. I. Dorogokupets, A. Shatskiy, P. N. Gavryushkin, T. S. Sokolova, E. Ohtani, J. Li and K. Funakoshi, J. Geophys. Res.: Solid Earth, 2013, 118, 5274–5284 CrossRef CAS.
- O. T. Lord, M. J. Walter, R. Dasgupta, D. Walker and S. M. Clark, Earth Planet. Sci. Lett., 2009, 284, 157–167 CrossRef CAS.
- X. Xi, S. Li, S. Yang, M. Zhao and J. Li, Ironmaking Steelmaking, 2020, 47, 1087–1099 CrossRef CAS.
- G. Raupp and W. Delgass, J. Catal., 1979, 58, 337–347 CrossRef CAS.
- J. Amelse, J. Butt and L. Schwartz, J. Phys. Chem., 1978, 82, 558–563 CrossRef CAS.
- X. Zhang, B. Li, H. X. Liu, G. H. Zhao, Q. L. Yang, X. M. Cheng, C. H. Wong, Y. M. Zhang and C. W. J. Lim, Appl. Surf. Sci., 2019, 465, 871–879 CrossRef CAS.
- Y.-H. Wen, L. Li, T. Zhao and R. Huang, ACS Appl. Nano Mater., 2020, 3, 12369–12378 CrossRef CAS.
- M. Li and D. Cheng, J. Phys. Chem. C, 2013, 117, 18746–18751 CrossRef CAS.
- P. Thibaux, A. Métenier and C. Xhoffer, Metall. Mater. Trans. A, 2007, 38, 1169–1176 CrossRef.
- Y.-H. Wen, L.-H. Zhang, J.-B. Wang and R. Huang, J. Alloys Compd., 2019, 776, 629–635 CrossRef CAS.
- Y.-H. Wen and R. Huang, J. Phys. Chem. C, 2019, 123, 12007–12014 CrossRef CAS.
- X. Zhang, C. Fu, Y. Xia, Y. Duan, Y. Li, Z. Wang, Y. Jiang and H. Li, ACS Nano, 2019, 13, 3005–3014 CrossRef CAS PubMed.
- M. Ruda, D. Farkas and G. Garcia, Comput. Mater. Sci., 2009, 45, 550–560 CrossRef CAS.
- J. Li, E. Croiset and L. Ricardez-Sandoval, Phys. Chem. Chem. Phys., 2014, 16, 2954–2961 RSC.
- R. Gao, X. Liu, Z. Cao, X.-W. Liu, K. Lu, D. Ma, Y. Yang, Y.-W. Li, R. Hoffmann and X.-D. Wen, Catal. Lett., 2019, 149, 645–664 CrossRef CAS.
- A. Seidenberg, Arch. Hist. Exact. Sci, 1988, 39, 97–119 Search PubMed.
- R. Huang, Y.-H. Wen, G.-F. Shao, Z.-Z. Zhu and S.-G. Sun, J. Phys. Chem. C, 2013, 117, 6896–6903 CrossRef CAS.
- D. C. Sorescu, J. Phys. Chem. C, 2009, 113, 9256–9274 CrossRef CAS.
- D.-B. Cao, F.-Q. Zhang, Y.-W. Li and H. Jiao, J. Phys. Chem. B, 2004, 108, 9094–9104 CrossRef CAS.
- X.-Y. Liao, D.-B. Cao, S.-G. Wang, Z.-Y. Ma, Y.-W. Li, J. Wang and H. Jiao, J. Mol. Catal. A: Chem., 2007, 269, 169–178 CrossRef CAS.
- C.-M. Deng, C.-F. Huo, L.-L. Bao, G. Feng, Y.-W. Li, J. Wang and H. Jiao, J. Phys. Chem. C, 2008, 112, 19018–19029 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj05114h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |