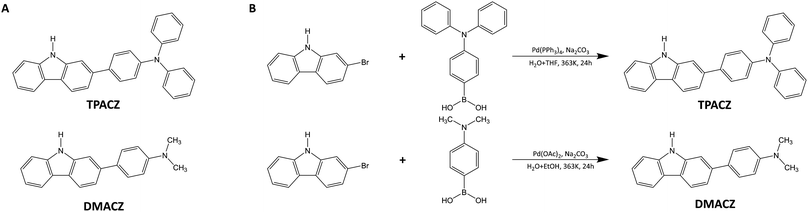
Vitrimer enhanced carbazole-based organic room-temperature phosphorescent materials†
Tianqi
Xu
,
Peng
Wu
,
Lingyun
Lou
,
Yuzhan
Li
,
Dong
Wang
 ,
Hui
Cao
,
Hui
Cao
 ,
Wanli
He
,
Wanli
He
 and
Zhou
Yang
and
Zhou
Yang
 *
*
School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: yangz@ustb.edu.cn
First published on 15th November 2021
Abstract
Organic room-temperature phosphorescent (RTP) materials have promising applications, but due to the lack of inter-system scaling and susceptibility to non-radiative transition, they typically have low quantum yields (<2%) and short lifetimes (<1 s), and their performance is not comparable to that of traditional inorganic phosphorescent materials. In this paper, we designed two phosphorescent molecules (TPACZ and DMACZ) based on carbazole, incorporated them into a dynamic epoxy crosslinking network, and successfully obtained pure organic RTP vitrimers with high quantum yields (7.28%) and long phosphorescent lifetimes (1.689 s). Our study confirms the potential phosphorescent properties of both molecules and the contribution of the vitrimer's rigid cross-linked network to phosphorescent emission. We demonstrate the ability to reprocess the RTP vitrimer at high temperatures and retain phosphorescence emission properties after reprocessing. Furthermore, by adjusting the dopant molecules and the type of curing agent, the phosphorescence lifetime of the RTP vitrimers can be tailored, allowing for anti-counterfeiting applications. Our research offers a new strategy for the design of pure organic RTP materials for anti-counterfeiting and information applications.
Introduction
In recent years, room-temperature phosphorescence (RTP) materials have attracted great interest due to their long lifetime and abundant excited state properties.1–4 They are suitable for a variety of technological applications, ranging from decorative and emergency displays to information anti-counterfeiting and biological applications.5–9 In recent decades, RTP has been considered as an exclusive property of inorganic materials because organic materials typically exhibit inefficient intersystem crossing (ISC) and strong non-radiative transition. However, conventional inorganic phosphorescent materials require the consumption of precious metals, such as iridium and palladium. In addition, the harsh preparation conditions and high toxicity of inorganic RTP materials severely limit their use in biological applications,10,11 which has prompted the search for metal-free organic alternatives.12 A variety of molecular design rules and enhancement strategies, such as crystal engineering,13 host–guest doping,14,15 MOFs,16 and H-aggregates,17,18 have been proposed to improve ISC and suppress the non-radiative transition of triplet state excitons to realize organic RTP materials with ultra-long lifetimes. Carbazole is one of the well-known phosphorescent groups because it possesses a nitrogen atom that can promote the n–π leap to facilitate the generation of triplet state excitons. The construction of a donor–acceptor system based on the carbazole group is expected to provide a large HOMO–LUMO energy level difference, thereby enabling a smaller energy difference (ΔEst) between the singlet and triplet states and promoting ISC.17,19–22Polymer-based RTP materials are well-suited for the fabrication of organic phosphorescent devices with complex shapes because of their unique properties, such as tunable elasticity and flexibility, outstanding processability, and excellent transparency.23 For example, the rigid structure of polymers can inhibit the vibrations of the phosphorescent molecules and the entanglement of the polymer chains can allow for the isolation of water and oxygen, thus reducing the consumption of the triplet state excitons and facilitating the emission of phosphorescence.24–27 Cross-linked polymers have shown greater potential to enhance the phosphorescence properties because of their rigidity and network structures.28,29 Wu et al. reported that the presence of a densely cross-linked network can effectively inhibit the non-radiative relaxation process of organic phosphorescent molecules. They have succeeded in obtaining RTP materials with a record phosphorescence lifetime of 2.28 s and a phosphorescence quantum yield of 8.35% by simply doping tetramethylbenzidine into an epoxy resin based on ethylenediamine and bisphenol A diglycidyl ether. While this strategy is effective in protecting triplet state excitons by suppressing molecular vibrations with oxygen isolation and enhancing the phosphorescence emission of the material,29 thermosets are insoluble and non-fusible, limiting reprocessing, recycling, and remolding of these organic RTP materials. In 2011, Leibler et al. developed dynamically cross-linked epoxy networks, known as vitrimers, by adding a transesterification catalyst to an epoxy/acid or epoxy/anhydride polyester network.30,31 The network contains dynamic covalent bonds that can undergo an exchange reaction while maintaining a constant cross-link density. At the service temperatures, the vitrimer exhibits similar properties to conventional thermosetting resins, with good thermal and mechanical properties.30,31 When heated above the topological freezing transition temperature, the exchange reactions proceed rapidly (e.g. transesterification reactions).30,32,33 Under external forces, the ester exchange reaction can lead to topological rearrangement and rapid stress relaxation, so that the vitrimer can “flow” like a viscoelastic fluid. This allows the vitrimer to be repaired, welded, reprocessed, and recycled at high temperatures.31,34
Herein, we designed and modified carbazole to obtain two molecules with an intramolecular charge transfer structure that promotes phosphorescence performance. By doping the RTP molecules into an epoxy-based vitrimer, reprocessable organic RTP films with a phosphorescence lifetime of 1.6 s and phosphorescence quantum yield of 7.28% were obtained. The RTP films exhibited good phosphorescence emission after reshaping at high temperatures.
Experimental
Synthesis of molecules
Two phosphorescent molecules, TPACZ and DMACZ (Fig. 1A), were synthesized using Suzuki coupling reaction.15,35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The mixture was sonicated for 30 min under an argon atmosphere to remove oxygen from the solution, and then 0.05 g of Pd(PPh3)4 was added. The solution was heated to 363 K under an argon atmosphere and was refluxed for 24 h. After the reaction, the solution was cooled to room temperature and extracted three times with dichloromethane (DCM). The organic phase was dried over anhydrous Na2SO4 and filtered to remove solid impurities. The crude product was purified by column chromatography (DCM/petroleum ether v/v = 1
1) solution. The mixture was sonicated for 30 min under an argon atmosphere to remove oxygen from the solution, and then 0.05 g of Pd(PPh3)4 was added. The solution was heated to 363 K under an argon atmosphere and was refluxed for 24 h. After the reaction, the solution was cooled to room temperature and extracted three times with dichloromethane (DCM). The organic phase was dried over anhydrous Na2SO4 and filtered to remove solid impurities. The crude product was purified by column chromatography (DCM/petroleum ether v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The yield was 74%.
3). The yield was 74%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The mixture was sonicated for 30 min under an argon atmosphere to remove oxygen from the solution, and then 0.05 g of Pd(OAc)2 was added. The solution was heated to 363 K under an argon atmosphere and was refluxed for 24 h. After the reaction, the solution was cooled to room temperature and was extracted three times with dichloromethane. The organic phase was dried over anhydrous Na2SO4 and filtered to remove solid impurities. The crude product was purified by column chromatography (EA/petroleum ether v/v = 1
1) solution. The mixture was sonicated for 30 min under an argon atmosphere to remove oxygen from the solution, and then 0.05 g of Pd(OAc)2 was added. The solution was heated to 363 K under an argon atmosphere and was refluxed for 24 h. After the reaction, the solution was cooled to room temperature and was extracted three times with dichloromethane. The organic phase was dried over anhydrous Na2SO4 and filtered to remove solid impurities. The crude product was purified by column chromatography (EA/petroleum ether v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The yield was 45%.
3). The yield was 45%.
Both molecules were characterized using 1H-NMR, 13C-NMR, high-resolution mass spectrometry and CHN elemental analysis (Fig. S1–S6, ESI†).
Preparation of vitrimer films
The phosphorescent molecule was dissolved into Bisphenol A diglycidyl ether (DGEBA) at different molar ratios (x mol% to the DGEBA, e.g., 0.2 mol%: 0.002 mol TMB to 1 mol DGEBA). Then, an equal amount of adipic acid and 5 mol% 1,5,7-triazabicyclo[4.4.0]dec-5-ene(TBD) were added. The mixture was heated to 150 °C and was stirred until the liquid was clarified. Then, the mixture was transferred to a mould and cured at 150 °C for 4 h. FTIR spectroscopy was used to characterize the vitrimer samples after polymerisation. Compared to the uncured DEGBA, the characteristic peak of the epoxy group at 916 cm−1 disappeared from the vitrimer film, proving that the epoxy vitrimer was fully cured (Fig. S7, ESI†).Results and discussion
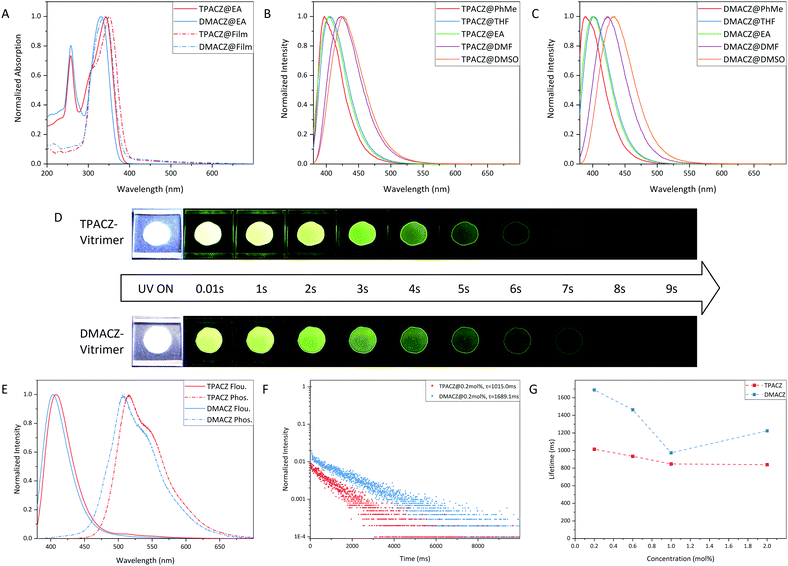
The photophysical properties of the two molecules dissolved in organic solvents and doped in vitrimer films were studied separately at room temperature. When dissolved in ethyl acetate, TPACZ and DMACZ exhibited absorption peaks at 342 nm and 330 nm, respectively (Fig. 2A). In the vitrimer films, however, the absorption peaks of TPACZ and DMACZ were slightly red-shifted and were moved to 350 nm and 342 nm, respectively. This indicates the existence of a similar intramolecular charge transfer structure for both molecules. TPACZ and DMACZ emitted bright blue fluorescence with maximum emission wavelengths of 403.9 nm and 398.8 nm, respectively (Fig. 2B and C) when excited with a 365 nm UV light in ethyl acetate, while the maximum emission wavelengths of the vitrimer films doped with TPACZ and DMACZ were slightly red-shifted to 408.0 nm and 403.6 nm, respectively (Fig. 2E). The fluorescence wavelengths of the two molecules in solvents with different polarities were further investigated. Solutions of both molecules were dissolved in toluene (PhMe), THF, DMF, and DMSO to equal concentrations, excited with 365 nm UV light, and the wavelengths of fluorescence were measured. The results (Fig. 2B and C) showed that the fluorescence wavelengths of both molecules shifted to red with increasing solvent polarity, further confirming that both molecules possess an intramolecular charge transfer structure. The small difference between the absorption and fluorescence spectra of the two molecules was caused by the difference in the electron-donating ability of the diphenylamine group and the dimethylamine group.In solution, neither TPACZ nor DMACZ produced phosphorescent emission at room temperature. However, the vitrimer films, each doped with 0.2 mol% of TPACZ and DMACZ, produced green phosphorescence visible to the naked eye for more than 10 s after being excited by a 365 nm UV light (Fig. 2D). The phosphorescent properties of films were further systematically investigated. The maximum phosphorescence emission wavelength of the TPACZ-doped vitrimer film was observed at 515.4 nm, with an inflexion point at 546 nm (Fig. 2E). Similarly, the DAMCZ doped vitrimer film showed a maximum emission wavelength of 505.8 nm with an inflexion point at 536 nm (Fig. 2E). Transient fluorescence spectroscopy tests were used to characterize the phosphorescence performance of the vitrimer films. The fluorescence lifetimes of both TPACZ- and DMACZ-doped films were determined to be in the scale of nanoseconds, corresponding to phosphorescence lifetimes up to 1 s. By measuring the time-resolved spectra of the materials and fitting the results using a quadratic polynomial, we obtained the phosphorescence lifetimes of the vitrimer films. Taking into account the possible aggregation-caused quenching effect of the luminescent molecules, the phosphorescence lifetimes of the two molecules were tested at different doping concentrations in the films. The phosphor lifetime of both TPACZ- and DMACZ-doped vitrimers decreased with increasing doping concentrations. At a doping concentration of 0.2 mol%, the TPACZ-vitrimer film exhibited a phosphorescence lifetime of 1015 ms, while the DMACZ-vitrimer film showed a phosphorescence lifetime of 1689.1 ms (Fig. 2F). When the doping concentration was increased to 1 mol%, the phosphorescence lifetime dropped to 839.2 ms for TPACZ-vitrimer and 973.8 ms for DMACZ -vitrimer (Fig. 2G and Fig S8, S9, ESI†). Therefore, we decided to use 0.2 mol% as the optimum doping concentration for all the subsequent experiments if not mentioned. At this concentration, the TPACZ-vitrimer exhibited a phosphorescent quantum yield of up to 7.28% and the DMACZ-vitrimer exhibited a phosphorescent quantum yield of 2.32%, both are excellent values for a pure organic RTP polymer material (Table 1).
| Doped Film | λ F (nm) | λ P (nm) | τ F (ns) | Φ F (%) | τ P (ms) | Φ P (%) | k F (s−1) | k P (s−1) | k ISC(s−1) |
|---|---|---|---|---|---|---|---|---|---|
| TPACZ | 408.0 | 515.4 | 1.26 | 58.02 | 1015.0 | 7.28 | 4.6 × 108 | 7.2 × 10−2 | 5.8 × 107 |
| DMACZ | 403.6 | 505.8 | 1.47 | 79.82 | 1689.1 | 2.32 | 5.4 × 108 | 1.4 × 10−2 | 1.6 × 107 |
As shown in Fig. 3A, a simple Jablonski diagram allows for a better investigation of the phosphorescence emission mechanism of the two phosphorescent films.
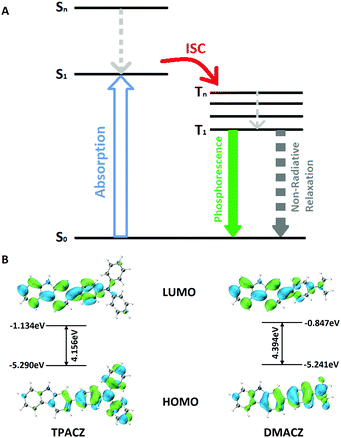 | ||
| Fig. 3 (A) A general Jablonski diagram illustrating the phosphorescence emission process. (B) Frontier orbitals of TPACZ and DMACZ. | ||
According to the equation for the quantum yield of phosphorescence: ΦP = ΦISC × kP/(kP + kNR), to achieve higher quantum yields of phosphorescence, it is necessary to improve ISC efficiency (ΦISC) and reduce non-radiative transition (kNR). Theoretical calculations based on density functional theory and time-dependent density functional theory for both molecules were carried out to obtain more information on the RTP mechanism. Fig. 3B shows the frontier molecular orbitals of TPACZ and DMACZ. The results of the frontline molecular orbital calculations similarly demonstrate the strong intramolecular charge transfer that exists within TPACZ and DMACZ. For both molecules, HOMO is present on the outer substituents (diphenylaniline and dimethylaniline), while LUMO is transferred to the main body of the carbazole. This results in a large energy difference of 4.156 eV versus 4.394 eV for both molecules HOMO and LUMO, respectively, which is crucial to achieving a smaller energy gap between the singlet and triplet states. The detailed triplet and singlet energies of the molecules were calculated and are listed in Table 2. TPACZ exhibited an S1 state energy of 3.545 eV, T5 state energy of 3.413 eV, and ΔEst of 0.132 eV. DMACZ exhibited an S1 state energy of 3.838 eV, T4 state energy of 3.611 eV and ΔEst of 0.227 eV. A small ΔEst contributes to the onset of ISC and increasing kISC, which promotes the emission of molecular phosphorescence. TPACZ showed a higher quantum yield of phosphorescence than that of DMACZ, which was consistent with the theoretical calculations.
To achieve better phosphorescent performance, it is important to reduce non-radiative transition in addition to promoting ISC. As mentioned previously, only the doping of both molecules into the vitrimer network resulted in a phosphorescent emission visible to the naked eye. It is generally accepted that rigid vitrimers with a cross-linked structure can effectively suppress the vibration and free rotation of the phosphorescent molecules, thus reducing the non-radiative transition rate (kNR) of molecules and achieving better phosphorescence emission performance. To confirm this, the matrix of the RTP material was modified and the phosphorescent lifetime was measured. Firstly, the vitimrer matrix was replaced by a more commonly used PMMA,26,27 a thermoplastic polymer without a cross-linked structure, and was doped with TPACZ. PMMA-based phosphorescent samples were prepared by drop-casting anisole solution of PMMA/TPACZ onto a glass substrate and heating to 150 °C for 15 minutes to remove the anisole residue. As shown in Fig. S11 (ESI†), the phosphorescence lifetime was significantly lower for the samples with PMMA as the matrix, suggesting that the rigid network structure of the vitrimer was more effective in restricting the vibration of the phosphorescence molecules. To further confirm the role of the rigid cross-linked network, the curing agent for vitrimer was changed from adipic acid to tetradecanedioic acid, which is characterized by a longer carbon chain and is expected to result in a less rigid network structure. The difference in the network rigidity was confirmed by the glass transition temperatures of the two vitrimer films measured using differential scanning calorimetry (Fig. S12, ESI†). Phosphorescence lifetime tests showed that the use of tetradecanedioic acid resulted in a significant reduction in the phosphorescence lifetime of the vitrimer films compared to adipic acid, demonstrating the effect of network rigidity on phosphorescence performance (Fig. S11, ESI†).
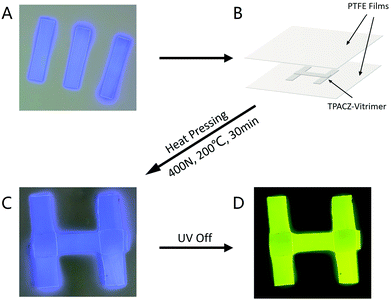
Unlike conventional epoxy resins, vitrimers have a distinct advantage in that they can be reprocessed, reshaped, and remoulded when the processing temperature exceeds the topological transition temperature. Here, we demonstrate this advantage by welding vitrimer RTP material at high temperatures. As shown in Fig. 4, a piece of vitrimer film doped with TPACZ was cut into three pieces and then reassembled into the shape of the letter “H”. The assembled film was placed between two sheets of PTFE film and placed in a hot press with a force of 400 N at 200 °C for 30 minutes. After cooling to room temperature, the vitrimer was successfully bonded together while maintaining its RTP emission properties.
The material's phosphorescent lifetime can be significantly altered by changing the curing agent in the vitrimer preparation process and the phosphorescent molecules doped in it, which promises a time-dependent luminescence technology for anti-counterfeiting applications. Fig. 5 shows an example of this application. The number “1” is a tetradecanedioic acid cured, TPACZ-doped vitrimer film, the number “2” is an adipic acid cured, TPACZ-doped film, and the number “3” is an adipic acid cured, DMACZ-doped film. Under normal conditions, all three figures are colourless and transparent. When exposed to ultraviolet light, all three figures fluoresced blue and immediately emitted green phosphorescence when the ultraviolet light was removed. Due to the different lifetimes of the phosphorescence of the three samples, the number “1” was the first to become invisible after 2 s. Then, the number “2” went out, leaving only the number “3” still emitting green phosphorescence visible to the naked eye.
 | ||
| Fig. 5 Vitrimer enhanced phosphorescent material for anti-counterfeiting applications. Photographs taken after different times with UV light removed. | ||
Conclusions
In summary, we have designed two exceptional carbazole-based phosphorescent molecules and incorporated them with a vitrimer dynamic epoxy network to suppress the non-radiative transition process. Pure organic RTP materials with excellent performance were obtained and their phosphorescent emission mechanisms were investigated. Importantly, the vitrimer dynamic network was reprocessable at high temperatures while maintaining its excellent non-radiative transition suppression properties and RTP properties. The tunable RTP properties of the vitrimer components allowed for the adjustment of the phosphorescence lifetime, which can be used in lifetime coding applications. Taking advantage of these unique properties, we demonstrated the material's great potential for anti-counterfeiting applications. Our research results provide new design concepts for organic RTP materials and promote the development of a new generation of pure organic optical materials with a wide range of applications in data security, displays, information storage, etc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51973017 and 51773017), the State Key Laboratory for Advanced Metals and Materials (2018Z-06), and the Fundamental Research Funds for the Central Universities (FRF-DF-19-001).Notes and references
- L. Gu, H. Shi, L. Bian, M. Gu, K. Ling, X. Wang, H. Ma, S. Cai, W. Ning, L. Fu, H. Wang, S. Wang, Y. Gao, W. Yao, F. Huo, Y. Tao, Z. An, X. Liu and W. Huang, Nat. Photonics, 2019, 13, 406–411 CrossRef CAS.
- T. Maldiney, A. Bessière, J. Seguin, E. Teston, S. K. Sharma, B. Viana, A. J. J. Bos, P. Dorenbos, M. Bessodes, D. Gourier, D. Scherman and C. Richard, Nat. Mater., 2014, 13, 418–426 CrossRef CAS.
- X. Liu, Y. Wang, X. Li, Z. Yi, R. Deng, L. Liang, X. Xie, D. T. B. Loong, S. Song, D. Fan, A. H. All, H. Zhang, L. Huang and X. Liu, Nat. Commun., 2017, 8, 899 CrossRef.
- Z. Pan, Y.-Y. Lu and F. Liu, Nat. Mater., 2012, 11, 58–63 CrossRef CAS.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS.
- S. Xu, R. Chen, C. Zheng and W. Huang, Adv. Mater., 2016, 28, 9920–9940 CrossRef CAS.
- L. Gu, H. Shi, M. Gu, K. Ling, H. Ma, S. Cai, L. Song, C. Ma, H. Li, G. Xing, X. Hang, J. Li, Y. Gao, W. Yao, Z. Shuai, Z. An, X. Liu and W. Huang, Angew. Chem., Int. Ed., 2018, 57, 8425–8431 CrossRef CAS.
- M. S. Kwon, D. Lee, S. Seo, J. Jung and J. Kim, Angew. Chem., 2014, 126, 11359–11363 CrossRef.
- L. Bian, H. Shi, X. Wang, K. Ling, H. Ma, M. Li, Z. Cheng, C. Ma, S. Cai, Q. Wu, N. Gan, X. Xu, Z. An and W. Huang, J. Am. Chem. Soc., 2018, 140, 10734–10739 CrossRef CAS PubMed.
- A. Fermi, G. Bergamini, M. Roy, M. Gingras and P. Ceroni, J. Am. Chem. Soc., 2014, 136, 6395–6400 CrossRef CAS PubMed.
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259–3302 RSC.
- J. Xu and S. Tanabe, J. Lumin., 2019, 205, 581–620 CrossRef CAS.
- Y. Xie, Y. Ge, Q. Peng, C. Li, Q. Li and Z. Li, Adv. Mater., 2017, 29, 1606829 CrossRef.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- Y. Lei, W. Dai, J. Guan, S. Guo, F. Ren, Y. Zhou, J. Shi, B. Tong, Z. Cai, J. Zheng and Y. Dong, Angew. Chem., Int. Ed., 2020, 59, 16054–16060 CrossRef CAS.
- X. Yang and D. Yan, Adv. Opt. Mater., 2016, 4, 897–905 CrossRef CAS.
- B. Fang, L. Lai, M. Fan and M. Yin, J. Mater. Chem. C, 2021, 9, 11172–11179 RSC.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS.
- M.-S. Zhou, P.-F. Gao, Y.-Y. Jiang, Y. Zhou, J. Wu, X.-L. Zhu and H.-R. Fu, Dyes Pigm., 2021, 195, 109715 CrossRef CAS.
- Y. Gong, G. Chen, Q. Peng, W. Z. Yuan, Y. Xie, S. Li, Y. Zhang and B. Z. Tang, Adv. Mater., 2015, 27, 6195–6201 CrossRef CAS PubMed.
- Q. Huang, X. Mei, Z. Xie, D. Wu, S. Yang, W. Gong, Z. Chi, Z. Lin and Q. Ling, J. Mater. Chem. C, 2019, 7, 2530–2534 RSC.
- S. K. B. Mane, Y. Mu, E. Ubba, Z. Yang, J. Zhao and Z. Chi, J. Mater. Chem. C, 2019, 7, 15219–15224 RSC.
- M.-M. Fang, J. Yang and Z. Li, Chin. J. Polym. Sci., 2019, 37, 383–393 CrossRef CAS.
- W. Wu, R. Tang, Q. Li and Z. Li, Chem. Soc. Rev., 2015, 44, 3997–4022 RSC.
- N. Gan, H. Shi, Z. An and W. Huang, Adv. Funct. Mater., 2018, 28, 1802657 CrossRef.
- M. Louis, H. Thomas, M. Gmelch, A. Haft, F. Fries and S. Reineke, Adv. Mater., 2019, 31, 1807887 CrossRef.
- M. Louis, H. Thomas, M. Gmelch, F. Fries, A. Haft, J. Lindenthal and S. Reineke, Adv. Opt. Mater., 2020, 8, 2000427 CrossRef CAS.
- L. Gu, H. Wu, H. Ma, W. Ye, W. Jia, H. Wang, H. Chen, N. Zhang, D. Wang, C. Qian, Z. An, W. Huang and Y. Zhao, Nat. Commun., 2020, 11, 944 CrossRef CAS.
- B. Wu, N. Guo, X. Xu, Y. Xing, K. Shi, W. Fang and G. Wang, Adv. Opt. Mater., 2020, 8, 2001192 CrossRef CAS.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS.
- Y. Yang, Y. Xu, Y. Ji and Y. Wei, Prog. Mater. Sci., 2021, 120, 100710 CrossRef CAS.
- M. Chen, L. Zhou, Y. Wu, X. Zhao and Y. Zhang, ACS Macro Lett., 2019, 8, 255–260 CrossRef CAS.
- A. Ruiz de Luzuriaga, R. Martin, N. Markaide, A. Rekondo, G. Cabañero, J. Rodríguez and I. Odriozola, Mater. Horiz., 2016, 3, 241–247 RSC.
- T. Liu, C. Hao, L. Wang, Y. Li, W. Liu, J. Xin and J. Zhang, Macromolecules, 2017, 50, 8588–8597 CrossRef CAS.
- J. Wang, C.-J. Yang, H.-N. Peng, Y.-S. Deng and X.-C. Gao, Synth. Commun., 2011, 41, 832–840 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj04546f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |