Porous carbon-based polymer monolithic template implanted with an ion-receptor molecular probe as a solid-state ocular sensor for the selective targeting and capturing of cobalt ions†
Satheesh
Kuppusamy
and
Prabhakaran
Deivasigamani
 *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology (VIT), Vellore, Tamil Nadu 632014, India. E-mail: prabhakaran.d@vit.ac.in; Tel: +91-9003137247
First published on 22nd November 2021
Abstract
In this work, we report the fabrication of a solid-state naked-eye sensor for targeting industrially relevant cobalt ions, using a tailor-made bimodal macro-/mesoporous polymer carbon-based monolithic cage-like template with a high surface area and voluminous porosity. The solid-state sensor was fabricated through the homogenous dispersion of a unique chromoionophoric ion receptor (probe), i.e., 4-((9H-purin-6-yl)diazenyl)-6-hexylbenzene-1,3-diol (PDHBD), onto a polymer monolith. The ion-receptor-anchored monolithic sensor's structural and surface morphology features were subjected to characterization by surface area analysis, electron microscopic, diffraction, and spectroscopic techniques. The monolith sensor exhibited exclusive selectivity for Co(II), with an incremental color transition from pale-orange to deep-red, for Co(II) concentrations ranging from 0–500 ppb. The influence of the investigational factors was systematically studied to enhance sensor performance. The monolithic sensor revealed a maximum signal response in the pH range of 7.0–8.0, with response kinetics of ≤3 min, at 40 °C ≤ T ≥ 20 °C, for Co(II). The multi-reusable solid-state sensor exhibited a superior detection and quantification limit of 0.3 and 1.0 ppb, respectively, for Co(II). The sensor proved its real-time monitoring efficiency in recovering Co(II) from environmental and industrial water samples with excellent data reproducibility. The binding mechanism of PDHBD molecules with Co(II) for exclusive ion-selectivity was studied using density functional theory.
1. Introduction
In recent decades, the environmental monitoring of heavy metal ions has become increasingly prevalent, considering their toxic/hazardous and non-biodegradable properties that can seriously impact life forms and ecosystems.1 Moreover, the growing utility of heavy metal ions in sectors related to mining, automobiles, electroplating, energy devices, electronics, diagnostics, and home appliances has led to their bioaccumulation in our food-chain/-web.2,3 Along this line, cobalt is one such heavy metal ion that plays a major role in many biological and industrial processes. For instance, cobalt ion in vitamin B12 complex is essential to maintain our body metabolism. However, a higher dosage of cobalt ions can result in health issues, such as cancer and genetic modifications, besides inducing serious problems to the lungs, heart, liver, and thyroid gland, as well as causing weight loss and vomiting.4–6 Cobalt-based compounds find major industrial applications, such as batteries, paints, pigments, diamond polishing, ceramics, pharmaceuticals, nuclear plants, tanneries, and mining.7 Hence, we need smart and reliable sensing methodologies to continuously monitor and remove cobalt ions from water and industrial wastewater sources.8,9Over the years, many analytical techniques, such as AAS, ICP-MS/-OES, XFS, chromatography, optical spectroscopy, and electrochemical methods, have found major utility for cation detection.10–13 Most techniques can perform multi-element determinations but are often expensive and require skilled technicians to counter the complex sampling procedures. Moreover, these sophisticated instruments are unaffordable to most underdeveloped and developing nations. In recent decades, the colorimetric recognition of heavy metal ions has been developed employing nanoparticles (NPs)-based methodologies.14,15 For instance, Mehta et al. reported an electrochemical approach using dopamine dithiocarbamate-functionalized Ag NPs to detect Co(II) ions.16 Wang et al. synthesized sulfur quantum dots-based sensor materials as a fluorescent probe for cobalt-ion detection through an aggregation-caused quenching approach.17 Mohandoss et al. developed a fluorescent sensor probe based on a photoinduced electron-transfer method to detect Co(II) ions.18 Wen et al. created a surface-enhanced Raman scattering (SERS) approach for detecting Co(II) ions using the covalent organic framework of 1,3,5-benzene tricarboxaldehyde and p-phenylenediamine loaded with Au NPs.19 Yang et al. reported a Raman scattering method for Co(II) ions using leaf-like poly(p-phenylenediamine) microcrystals and Ag NPs.20 However, NPs can be costly, besides being irrecoverable and nano-toxic. Moreover, the dispersed NPs can induce severe health issues through their undesirable interactions with various primary biological processes in various life forms. Therefore, attention is increasingly being paid toward the fabrication of efficient and straightforward sensing methodologies that are relatively inexpensive and easy to handle.21
In this aspect, colorimetric sensors are innovative sensing tools for detecting cations and anions, with color transitions derived from the charge-transfer (ligand to metal or metal to ligand) mechanism. The chromophore receptor (probe) interacts with the target species through host–guest interactions in the chemical sensing process, thus providing specific signal responses and color transformations. Moreover, optical chemosensors can bring in the possibility of faster analysis and cost-effectiveness. However, the major drawback of chemosensors is the use of supramolecular designs as ion-receptors that result in aqueous incompatibility issues due to their solubility in organic solvents. Besides, liquid-based optical chemosensors, in general, lack ion-selectivity besides exhibiting moderate sensitivity. Considering these drawbacks, the concept of solid-state optical sensing using structurally designed templates is on a steady rise.22,23
In recent years, mesoporous inorganic (silica, alumina, titania, etc.) monoliths, with unique structural and porosity features, are being increasing considered for solid-state colorimetric sensing through the direct impregnation or chemical functionalization of receptor molecules.24–27 However, it is not easy to arrive at the desired structural properties of mesoporous inorganic monoliths. Besides, these monoliths are associated with pH-based durability issues that could compromise their structural integrity.28,29 Recently, the use of porous organic polymeric carbon-based monoliths has attracted considerable attention due to their superior/tunable structural and pore properties derived through accessible synthesis routes, using a wide variety of monomers and crosslinkers. However, it is to be noted that polymer monolith columns are well-known for chromatographic applications. However, their utility in designing solid-state sensors is a fresh concept, with few literature reports on the subject.30–33 Moreover, we attempted for the first time a highly ordered bimodal meso-/macroporous combination of cage-like structured polymer monolithic templates, using monomeric acrylamide units and ethylene glycol dimethacrylate crosslinker.
Based on this aspect, we adopted a strategic approach for designing a structurally engineered porous polymer monolith template, through bulk ionic polymerization, for the homogeneous anchoring of an in-house synthesized chromo-ionophoric ion-receptor (probe) ligand through non-covalent interaction. In this work, we synthesized a new chromoionophoric probe ligand, i.e., 4-((9H-purin-6-yl) diazinyl)-6-hexylbenzene-1,3-diol (PDHBD), using the concept of a hard–soft acid–base (HSAB).34,35 The probe molecules were uniformly dispersed on a bimodal macro-/mesoporous polymer monolith template, thus resulting in a solid-state optical chemosensor for the selective sensing and recovery of ultra-trace Co(II) ions.36 The novelty/unique features of the proposed solid-state sensor are its exceptional ultra-fast sensing response (≤180 s), wide response range, exclusive ion-selectivity, and sensitivity in targeting even ultra-trace concentrations of hazardous Co(II) through concentration-proportionate visual color transitions. The proposed organic polymer-based monolithic solid-state sensor highlighted its dual functionality as an optical sensor and ion-concentrator for the simultaneous sensing and recovery of ultra-trace and macro-levels of Co(II) ions. The solid-state porous carbon-based monolithic sensor material revealed good stability, more significant probe loading, faster kinetics, and excellent ion-selectivity. Various physicochemical parameters were systematically optimized for the sensor performance to facilitate the reliable sensing of toxic Co(II) ions from environmental and industrial samples.37
2. Experimental
2.1. Materials and instrumentation
For synthesizing the probe (PDHBD), adenine (98%) and 4-hexyl resorcinol (99%) were obtained from Sigma-Aldrich. For synthesizing the porous polymer monoliths, acrylamide (99%, AM), ethylene glycol dimethacrylate (98%, EGDMA), azobisisobutyronitrile (AIBN) were obtained from TCI chemicals. The AAS grade metal standard solutions (1000 μg mL−1) used for the analytical studies were from Sigma-Aldrich. For maintaining the solution pH, we used buffers of 0.2 M, ClCH2COOH–HCl (pH 1–3), CH3COONa–CH3COOH (pH 4–6), MOPS–NaOH (pH 7–9), and CHES–NaOH (pH 10–11). Solvents such as dimethylsulphoxide (DMSO), 1-dodecanol, and other reagents were of AR grade and were obtained from Avra Chemicals.The polymer monoliths' surface morphology and structural properties were analyzed by field-emission scanning electron microscopy (FE-SEM, Thermo Fisher, FEI Quanta 250 FEG) and high-resolution transmission electron microscopy (HR-TEM, Tecnai, G2 20 S-Twin). The wide-/low-angle X-ray diffraction patterns for the polymer monolith and its probe-anchored sensor were obtained using an X-ray diffractometer (p-XRD, Bruker, D8 Advance) to understand their porosity and phase transformations. BET and BJH analysis (Quantachrome, Autosorb iQ) were carried out for the surface area and pore analysis for the porous polymer monolith and its probe-anchored sensor material. The elemental composition and oxidation state of the polymer monolith and sensor materials were studied by X-ray photoelectron spectrometry (XPS, PHI 5000 VersaProb-II). The thermal stability of the sensor materials was scrutinized by thermogravimetric analysis (TGA, Seiko, SII 7200). The probe–metal ion complexation and the resulting concentration-proportionate optical spectral patterns for the solid-state sensor were studied by UV-vis spectrophotometry interfaced with a diffused reflectance integrating sphere (UV-Vis-DRS, Jasco, V730). The structural characterization of the probe was performed by nuclear magnetic resonance spectrophotometry (NMR, Bruker, Ascend 400 MHz). Fourier transform infrared spectrophotometry (FT-IR, Thermo Scientific, Nicolet iS10) was employed to characterize the probe molecules and the polymer monolithic sensor materials. The theoretical calculations about the probe molecular electronic structure were carried through density functional theory (DFT) using Gaussian 16 software.
2.2. Synthesis of the polymer monolith, chromoionophoric probe, and solid-state sensor
The physicochemical properties of a polymer monolith depend primarily on the choice of monomer and crosslinker, apart from the nature of the porogenic solvent. For the current work, the preparation of the porous polymer monoliths was carried out by dissolving an appropriate quantity of AM (1 mmol, monomer) in a specific volume ratio of dry DMSO (13 mL; porogen) and 2-dodecanol (2 mL; porogen). To enhance the mechanical and structural properties of the porous polymer monolith, EGDMA (10 mmol, crosslinker) was slowly added to the monomer solution, followed by the addition of AIBN (0.1 mmol, initiator), under constant stirring in a N2 atmosphere. The reaction mixture was heated at 60 °C for 24 h to complete the polymerization reaction; then removing the unreacted constituents by washing with a water–acetone mixture. The resulting material was vacuum dried at 50 °C for 9 h to obtain the desired porous polymer monolith, which featured a high surface area and voluminous pore dimensions.For synthesizing the chromoionophoric and amphiphilic probe (PDHBD) ligand, an equimolar ratio of adenine (6 M HCl) and 4-hexylresorcinol (10% NaOH) was stored under ice-cold conditions. Diazonium salt formation was achieved by dropwise adding NaNO2 solution to the adenine solution, and the succeeding compound reacted with the 4-hexylresorcinol solution under ice-cold conditions. After 1 h, 10% acetic acid was added to the reaction solution for pH neutralization, and a brown-colored product was obtained and filtered and purified by recrystallization in hot methanol. A schematic diagram denoting the synthesis of the PDHBD ligand is provided in the ESI† (Fig. S1). The characterization data about the PDBHD probe molecule are discussed in the ESI† (Fig. S2(a–c)).
For the fabrication of the solid-state optical sensor, the porous polymer monolithic template (1.0 g) was equilibrated with varying concentrations (mmol) of PDBHD probe, dissolved in ethanol, in a mechanical shaker for 1 h. The resulting heterogeneous mixture was transferred to a rotary evaporator to remove the ethanol solvent; thus leading to a homogeneous dispersion of probe molecules on the polymer monolith, creating the desired solid-state optical sensor. Small doses of the resulting sensor materials were used for the solid-state colorimetric ion-sensing. The characterization details of the surface morphology and intriguing structural features of the porous polymer monolithic template and the probe-anchored sensor material are discussed in the results and discussion section.
2.3. Methodology for solid-state ion-sensing
For the optimization of solid-state ion-sensing, a specific quantity (1–5 mg) of sensor materials was discretely equilibrated with varying concentrations (0–500 ppb) of Co(II) ions, along with suitable buffers (5 mL), for maintaining the solution pH. The overall volume was maintained at 20 mL, and the solutions were equilibrated at 110 rpm in a mechanical shaker for a specific time. The equilibrated solutions were filtered through a cellulose membrane filter, and the sensor material color transitions (due to ion-complexation) were subjected to UV-Vis-DRS analysis. For color (visual/spectral) comparison, a blank solution (without Co(II) ions) was subjected to a similar analytical procedure.3. Results and discussion
3.1. Characterization of the polymer monolith and sensor material
The surface morphology and structural characteristics of the acrylamide-based polymer monoliths were analyzed by FE-SEM and HR-TEM. The SEM images (Fig. 1(a–c)) of the polymer monolith revealed a cage-like arrangement of a continuously porous framework, and the TEM images (Fig. 1(d–f)) showed a macroporous polymer network embedded with mesopore channels. The structural pattern is mainly crucial for the adsorption process, essentially for the voluminous and homogeneous dispersion of probe molecules across the monolithic template. Moreover, for the monolithic sensor, the existence of a long-range porous framework enhances the response kinetics of probe molecules during the ion-sensing process through allowing faster ion diffusion across the pore channels. The SAED pattern (Fig. S3(a), ESI†) demonstrated the absence of bright spots and dotted lines; thereby signifying the amorphous nature of the polymer monolith and sensor material, as shown in the ESI.† The SAED pattern agreed with the wide-angle p-XRD pattern (Fig. S3(b), ESI†), which showed a broad peak centered around 28° (2θ) for the polymer monolith and probe-loaded sensor material. The EDAX data (Fig. S3(c), ESI†) revealed the elemental composition of carbon, oxygen, and nitrogen atoms derived from the monomer and crosslinker units of the porous polymer monolith. The elemental mapping (Fig. S3(d–f), ESI†) confirmed the authenticity of the fabricated polymer monolith.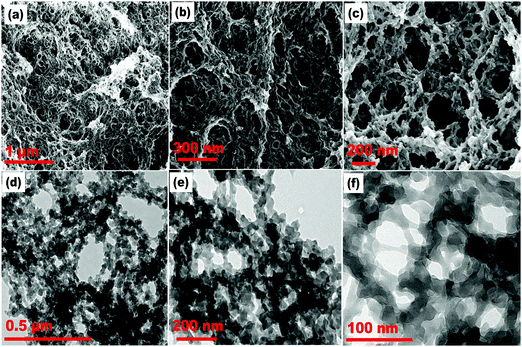 | ||
| Fig. 1 (a–c) FE-SEM images and (d–f) HR-TEM images of the AM-EGDMA-based porous polymer monolithic template at varying resolutions. | ||
Next, N2 adsorption–desorption isotherm analysis was carried to calculate the surface area and porosity features of the porous polymer monolith and the probe-loaded sensor material, as depicted in Fig. S4(a) (ESI†). The adsorption isotherm plot showed an H2 hysteresis loop with a type-IV pattern, which signified the presence of an interconnected porous network with a range of pore shapes and size distributions.38 The isotherm pattern for the PDHBD-anchored polymer monolith sensor exhibited a modest decrease in the hysteresis loop. However, it was devoid of any changes in its silhouette; thus denoting the dispersion/adsorption of the probe molecules within the porous network. The presence of probe molecules within the polymer monolith was analyzed through BET surface area analysis, which revealed a value of 131.90 m2 g−1 for the sensor material compared to a much higher value of 153.02 m2 g−1 for the polymer monolith. Likewise, the BJH pore-size distribution plot (Fig. S4(b), ESI†) for the polymer monolith revealed a value of 3.53 nm, which was reduced to 3.48 nm for the probe-anchored polymer monolith (sensor); thus confirming the occupancy of the probe molecules in the porous network. The functional groups of the PDHBD probe, empty polymer monolith, and sensor material were confirmed by FT-IR spectral analysis (Fig. S4(c), ESI†). For the polymer monolith, the presence of an acrylamide monomeric functional group was assigned to the vibrational frequency peaks at 1730 cm−1 for the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester) group, while the vibrational frequency peaks in the range of 1649–1722 cm−1 were attributed to N–C
O (ester) group, while the vibrational frequency peaks in the range of 1649–1722 cm−1 were attributed to N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (amide) stretching frequency. The presence of the crosslinker was confirmed through the vibrational peaks in the 1136–1158 cm−1 region, corresponding to C–O–C stretching frequency. However, upon probe impregnation and subsequent Co(II) complexation, the peak intensity decreased for the functional group frequencies of the polymer monolith, along with a marginal red-shift; thus confirming the loading of PDHBD molecules on the polymer monolith through physical interactions. Moreover, the spectral pattern confirmed the existence of an unchanged polymer monolithic network with no structural damage even after probe anchoring, which is in line with the data observed from the adsorption–desorption isotherm pattern.
O (amide) stretching frequency. The presence of the crosslinker was confirmed through the vibrational peaks in the 1136–1158 cm−1 region, corresponding to C–O–C stretching frequency. However, upon probe impregnation and subsequent Co(II) complexation, the peak intensity decreased for the functional group frequencies of the polymer monolith, along with a marginal red-shift; thus confirming the loading of PDHBD molecules on the polymer monolith through physical interactions. Moreover, the spectral pattern confirmed the existence of an unchanged polymer monolithic network with no structural damage even after probe anchoring, which is in line with the data observed from the adsorption–desorption isotherm pattern.
The oxidation state and elemental presence of the as-fabricated solid-state sensor were studied through XPS analysis. Fig. 2(a) shows that the survey spectra of the Co(II)-complexed sensor, revealing the elemental presence of carbon, oxygen, nitrogen, and cobalt. The high-resolution spectrum (Fig. 2(b)) showed a solid Co 2p binding energy peak at 800 eV; thus confirming the existence of cobalt ions in the divalent state during the complexation with the immobilized PDHBD probe molecules. The deconvoluted Co 2p spectrum showed two peaks at 780.9 and 789.9 eV, referring to Co 2p3/2 and Co 2p1/2 orbital states.39 The deconvoluted C 1s spectrum (Fig. 2(c)) showed three binding energy peaks at 284.6, 286.3, and 288.3 eV, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic), C–O (alcoholic hydroxyl), and COO− (carbonyl and ester) groups, respectively, that were present in the sensor material. In the case of the O 1s orbital state, two binding energy peaks appeared at 530.4 and 532.5 eV and were assigned to the polymer monolith's C
C (aromatic), C–O (alcoholic hydroxyl), and COO− (carbonyl and ester) groups, respectively, that were present in the sensor material. In the case of the O 1s orbital state, two binding energy peaks appeared at 530.4 and 532.5 eV and were assigned to the polymer monolith's C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O functional groups, respectively, as shown in Fig. 2(d). The high-resolution spectrum (Fig. 2(e)) for the N 1s orbital state showed a strong peak at 399 eV, corresponding to the C–N unit of the monomer and probe functional moieties of the sensor material. The XPS observations related to the functional groups of the polymer monolith concorded with the data obtained from the FT-IR spectral analysis.
O and C–O functional groups, respectively, as shown in Fig. 2(d). The high-resolution spectrum (Fig. 2(e)) for the N 1s orbital state showed a strong peak at 399 eV, corresponding to the C–N unit of the monomer and probe functional moieties of the sensor material. The XPS observations related to the functional groups of the polymer monolith concorded with the data obtained from the FT-IR spectral analysis.
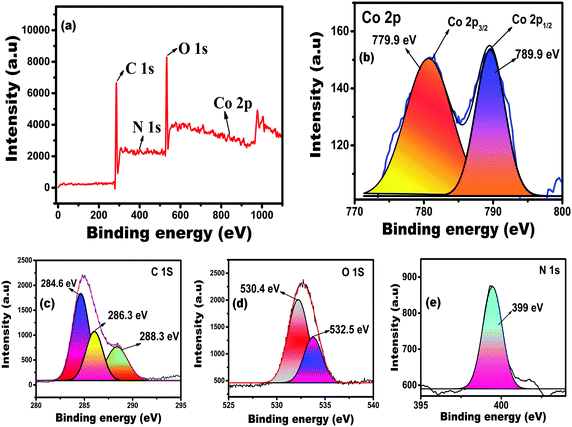 | ||
| Fig. 2 (a) XPS broad range survey spectra, and (b–e) deconvoluted high-resolution XPS spectra for the Co 2p, C 1s, O 1s, and N 1s orbital states of the PDHBD–Co2+-complexed polymer monolithic sensor. | ||
3.2. Optimization of the analytical parameters for the sensor performance
The solution pH plays a crucial role in the ion-sensing process through protonation/deprotonation of the functional groups present in the PDHBD probe. To determine the optimum pH for ion-sensing and its recovery from aqueous media, solid-state ion-sensing experiments were conducted at varying pH ranges (1.0–10.0), as shown in Fig. S5(a) (ESI†). The results revealed that the protonation of adenine and azo moieties was prominent under acidic pH conditions, along with the non-dissociated hydroxy groups of the resorcinol moiety in PDHBD molecules. Moreover, at pH ≤ 6.0, the presence of H+ ions ensured that the PDHBD molecules existed in their non-dissociated and protonated states; thereby inhibiting the ion-chelation/sensing and decreasing the sensor signal/spectral intensity and the ion-complexation based color transitions. However, at pH ≥ 9.0, most of the metal ions tended to form stable metal hydroxide [M(OH)n]n+ complexes rather than complexing with the PDHBD probe actives sites, which accounted for the decreases in the spectral intensity and color transitions. A maximum ion-sensing/signal response for the proposed sensory system was achieved in the solution pH range of 7.0–8.0.Along this line, the optimal probe content required for sensor fabrication to achieve its maximum sensing performance in terms of spectral and visual color transitions fell in the concentration range of 0.15–0.20 mmol of PDHBD per gram of the polymer monolith, as depicted in Fig. S5(b) (ESI†). Similarly, the optimal sensor dosage per experiment (Fig. S5(c), ESI†) was found to be 4.0–5.0 mg to achieve a reliable/reproducible ion-sensing performance. Subsequently, the time and temperature-dependent ion-sensing studies (Fig. S5(d), ESI†) revealed a time frame of ≤180 s was adequate, in the temperature range of 10–20 °C, for the quantitative sensing of Co(II) ions. However, at higher solution temperatures, i.e., 30–40 °C, due to the increase in the internal energy of the solution medium, a timeframe of 90 s was sufficient for ion-sensing. The optimized probe concentration, sensor dosage, response kinetics, and temperature factor significantly enhanced the ion-sensing properties of the probe-anchored monolithic sensor. Likewise, the durability of the solid-state sensor was studied in terms of its reusability characteristics, as depicted in Fig. S5(e) (ESI†). In this study, 1.5 mL of 0.2 M HCl solution was equilibrated with Co(II)-complexed PDHBD-impregnated monolithic sensor material to desorb the Co(II) ions. This process was followed by water washing to regenerate the probe molecules anchored on the polymer monolith for subsequent ion-sensing. The reused sensor's Co(II) sensing signal response revealed a recovery and reusability efficiency of ≥99.2%, for up to ten repeated cycles, without any noticeable changes in the UV-Vis-DRS data and naked-eye detection. The reusability data revealed that the sensor efficiently achieved replicable outcomes for up to ten repeated cycles, without any noticeable loss in its sensing performance.
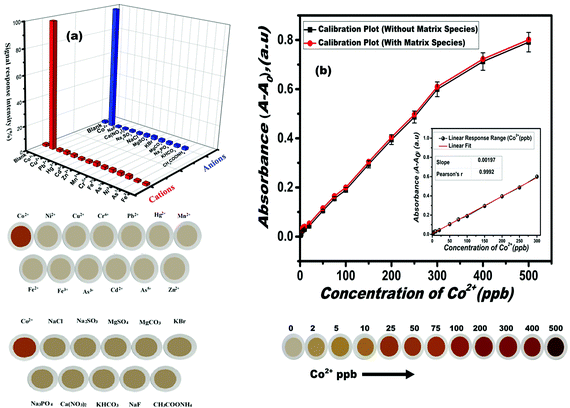
The ion-selectivity features of the proposed sensor material in targeting ultra-trace levels of Co(II) concentrations amid various cation and anion species and their tolerance limits are shown in Table S1 (ESI†). The signal response and visual color transitions of these foreign cationic and anionic species using the proposed sensor are depicted in Fig. 3(a). Interestingly, the proposed solid-state sensor exhibited exclusive selectivity in sensing Co(II) ions, which could probably be associated with the constrained geometrical orientation of the PDHBD probe molecules within the well-defined porous cage-like network structures of the polymer monolith, which favored exclusive complexation for Co(II) ions, based on the HSAB concept. However, a mild inference was observed from Cu(II) ions when exceeding its concentration of ≥10.0 ppm, which was masked by using 0.50 mM of thiosulphate, for the quantitative detection of Co(II) ions, in the concentration range of 0–500 ppb. The sensor performance toward Co(II) ion-sensing in the presence of thiosulphate solution is shown in Fig. S6 (ESI†). The results revealed no changes in the signal and colorimetric response for Co(II) ions in the presence of (0.05 mM) thiosulphate when using the proposed sensor material.
To define the ion-sensitivity parameter as a function of the color transition and spectral intensity, the influence of Co(II)-ions’ concentration was investigated. The results revealed a direct correlation between the Co(II)-ions’ concentration with the sensor signal intensity and its visual color transitions due to the PDHBD–Co(II) complex formation. The calibration plot as a function of incremental Co(II) concentration (in the presence and absence of matrix constituents) and its subsequent visual color transitions with the sensor material are depicted in Fig. 3(b). The plot revealed a linear response range in the Co(II) concentration range of 0.5–300 ppb, which was reciprocated through a brilliant color transition from pale orangish-yellow to deep-red. Calculations for the limit of detection (LOD) and quantification (LOQ) were performed using the following equation, LOD = Kσ/m, and LOQ = Kσ/m, where K (constant) is 3 and 10, for the LOD and LOQ, respectively; ‘σ’ denotes the standard deviation for the blank sensor (1.97 × 10−4) for the triplicate measurements; and 'm' represents the slope value obtained from the linear portion of the calibration plot. The LOD and LOQ data for the Co(II)-ions sensing using the fabricated solid-state monolith sensors were calculated as 0.3 and 1.0 ppb, respectively The observed LOD and LOQ values using the proposed sensor material were far superior in comparison to the existing literature reports of solid-state and liquid-based sensory systems,40–47 as tabulated in Table 1.
| Co(II)-sensing materials | Linear range (ppb) | LOD (ppb) | Ref. |
|---|---|---|---|
| 4-Phenylazo-m-phenylenediamine monohydrochloride (organic solvent-mediated liquid sensor) | 400–1000 | 100 | 40 |
| Coumarin derivative of azomethine (organic solvent-mediated liquid sensor) | 0–5300 | 418 | 41 |
| Coumarin derivative of (E)-benzyl-2-((7-(diethyl amino)-2-oxo-2H-chromen-3-yl) methylene) hydrazine carbodithioate (organic solvent-mediated liquid sensor) | 0–590 | 100 | 42 |
| Carboxyl-functionalized CdS quantum dots (aqueous colorimetric sensors) | 500–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
230 | 43 |
| Porous silica monolith physically modified with 8-(4-n-dodecyl-phenylazo)-2,4-quinolinediol | 0–90 | 0.9 | 44 |
| Mesoporous silica monolith immobilized with N,N-di-(3-carboxysalicylidene)-3,4-diamino-5-hydroxypyrazole | 2–100 | 0.3 | 45 |
| Mesoporous silica materials loaded with N,N-disalicylidene-4,5-dimethylphenylenedene | 3–100 | 0.3 | 46 |
| Mesoporous silica monolith modified with, | 1–50 | 0.5 | 47 |
| (i) 2-Nitroso-1-naphthol, | 0.2 | ||
| (ii) Bis[bis(carboxy methyl)aminomethyl]fluorescein, | 0.3 | ||
| (iii) Pyrogallol red loaded | |||
| Porous polymer monolith impregnated with 4-((9H-purin-6-yl)diazenyl)-6-hexylbenzene-1,3-diol | 0.5–300 | 0.3 | This work |
3.3. DFT calculations
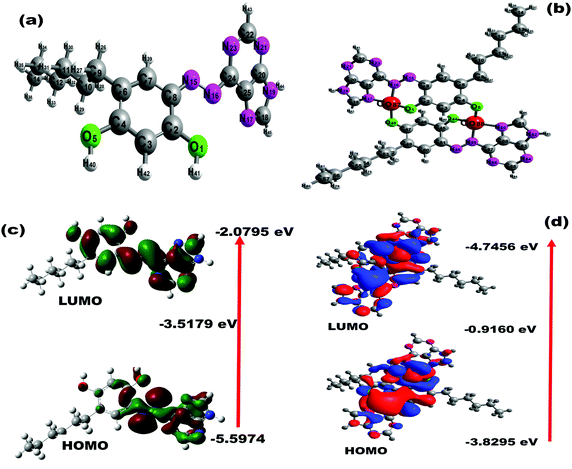
To analyze the binding mechanism of the PDHBD probe with Co(II) ions, theoretical calculations using the DFT method were performed in the gas phase. The electronic structures of the probe molecules and PDHBD–Co(II) complexes were calculated at the B3LYP/6-31G(d,p) level and B3LYP/LANL2DZ level, respectively.48 The optimized structures are shown in Fig. 4(a and b), while the bond length and Mulliken population analysis used to characterize the electronic charge distribution of the probe molecules before and after complexation are depicted in Fig. S7(a–d) (ESI†). It was calculated that two equivalences of PDHBD molecules were bound with two equivalence of Co(II) ions, which agreed with the data obtained from both the Job's plot and 1H NMR titrations, as discussed in the ESI† (Fig. S8(a and b)). In the PDHBD probe, two –OH (one each from neighboring PDHBD molecule of 4-hexylresorcinol moiety), –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (azo moiety), and –N
N– (azo moiety), and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (adenine moiety) units were involved in Co(II)-ion complexation. Thus, the theoretical bond length of the PDHBD molecule (before Co(II) ion-complexation) for O1 (O–H), O5 (O–H, adjacent molecule), N15, N16 (–N
C (adenine moiety) units were involved in Co(II)-ion complexation. Thus, the theoretical bond length of the PDHBD molecule (before Co(II) ion-complexation) for O1 (O–H), O5 (O–H, adjacent molecule), N15, N16 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), and N17 (–N
N–), and N17 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) atoms were calculated as 1.35, 1.36, 1.27, 1.41, and 1.34, respectively. Subsequently, the Mulliken charges of the PDHBD probe for O1, O5, N15, N16, and N17 atoms were calculated as −0.51, −0.55, −0.29, −0.29, and −0.49, respectively. However, for the PDHBD–Co2+ complex, the resulting bond lengths of O1, O5, N15, N16, and N17 atoms were calculated as 1.329, 1.337, 1.354, 1.345, and 1.339, respectively. Subsequently, the Mulliken charges for the PDHBD–Co2+ complex were calculated as −0.44, −0.43, −0.02, −0.31, and −0.25 for O1, O5, N15, N16, and N17, respectively. The electronic property of the probe and its Co(II) complexes were studied by frontier molecular orbital (FMO) analysis, as depicted in Fig. 4(c and d). For the PDHBD ligand, the HOMO and LUMO levels revealed an energy gap of 3.52 eV, representing the π–π* electron transfer of the ligand molecules. For the PDHBD–Co2+ complexes, the HOMO and LUMO level energy band gap was 0.92 eV. Thus, the calculated HOMO and LUMO energy gap of the PDHBD–Co2+ complexes was lower than the PDHBD energy level, thus providing strong support for the charge-transfer complexation process.
C) atoms were calculated as 1.35, 1.36, 1.27, 1.41, and 1.34, respectively. Subsequently, the Mulliken charges of the PDHBD probe for O1, O5, N15, N16, and N17 atoms were calculated as −0.51, −0.55, −0.29, −0.29, and −0.49, respectively. However, for the PDHBD–Co2+ complex, the resulting bond lengths of O1, O5, N15, N16, and N17 atoms were calculated as 1.329, 1.337, 1.354, 1.345, and 1.339, respectively. Subsequently, the Mulliken charges for the PDHBD–Co2+ complex were calculated as −0.44, −0.43, −0.02, −0.31, and −0.25 for O1, O5, N15, N16, and N17, respectively. The electronic property of the probe and its Co(II) complexes were studied by frontier molecular orbital (FMO) analysis, as depicted in Fig. 4(c and d). For the PDHBD ligand, the HOMO and LUMO levels revealed an energy gap of 3.52 eV, representing the π–π* electron transfer of the ligand molecules. For the PDHBD–Co2+ complexes, the HOMO and LUMO level energy band gap was 0.92 eV. Thus, the calculated HOMO and LUMO energy gap of the PDHBD–Co2+ complexes was lower than the PDHBD energy level, thus providing strong support for the charge-transfer complexation process.
3.4. Testing of the sensor material with synthetic and real environmental samples
To understand the ion-sensing and recovery efficiency of the proposed sensor, 1.0 g of certified material, i.e., LiCoO2 (Sigma Aldrich), was dissolved in 15 mL of aqua regia (HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl, 1
HCl, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and heated to dryness, followed by the addition of H2O2 in the presence of H2SO4 to reduce Co(III) to Co(II), thereby improving its aqueous solubility. To obtain the desired stock solution, the solution was diluted with deionized water to an overall volume of 1.0 L. Using the as-fabricated sensor, a 10 μL of aliquot (from the stock solution) was pH adjusted and subjected to cobalt-ion sensing (as per the proposed ion-sensing methodology) in an overall solution volume of 20 mL. To ensure the reliability of the obtained analytical data, the cobalt concentrations were verified by ICP-MS analysis. The recovery data (Table 2) using the proposed solid-state sensor revealed the proposed colorimetric ion-sensing methodology's data reliability and sensing robustness.
3) and heated to dryness, followed by the addition of H2O2 in the presence of H2SO4 to reduce Co(III) to Co(II), thereby improving its aqueous solubility. To obtain the desired stock solution, the solution was diluted with deionized water to an overall volume of 1.0 L. Using the as-fabricated sensor, a 10 μL of aliquot (from the stock solution) was pH adjusted and subjected to cobalt-ion sensing (as per the proposed ion-sensing methodology) in an overall solution volume of 20 mL. To ensure the reliability of the obtained analytical data, the cobalt concentrations were verified by ICP-MS analysis. The recovery data (Table 2) using the proposed solid-state sensor revealed the proposed colorimetric ion-sensing methodology's data reliability and sensing robustness.
| Source | Sample composition | Spiked Co(II) content,c (ppb) | Recovery of Co(II),d (ppb) |
|---|---|---|---|
| a Triplicate measurement. b Standard deviation. c Data based on ICP-MS analysis. d Data obtained using the proposed sensor. | |||
| Certified materials | LiCoO2 (≥99.9% purity, Sigma-Aldrich) (Co2+: 301.31 ppb, by ICP-MS, after dilution of stock dilution, 10 μL in 1000 mL) | — | 300.9 ± 1.5b |
| Ground water, Tiruvallur | Fe2+/3+, Zn2+, Mn2+, Ni2+, Cu2+: ≤0.11 ppm | 0.0 | 0.0 |
| Na+, K+, Mg2+, Ca2+: ≤415 ppm | 10.0 | 09.9a ± 0.3b | |
| F−, NO32−: ≤28 ppm | 50.1 | 49.8a ± 0.6b | |
| Cl−, HCO3−, SO42−: ≤265 ppm | 100.2 | 100.8a ± 1.2b | |
| Industrial wastewater, Tiruvallur | Fe2+/3+, Mn2+, Zn2+, Ni2+, Cu2+: ≤0.17 ppm | 0.0 | 0.0 |
| Na+, K+, Mg2+, Ca2+: ≤135 ppm | 20.2 | 20.8a ± 0.8b | |
| F−, Cl−, NO32−, PO43−: ≤16 ppm | 40.2 | 40.6a ± 0.3b | |
| SO42−, SO32−, HCO3−: ≤105 ppm | 100.9 | 99.8a ± 0.6b | |
| Lab-simulated wastewater | Hg2+, Cd2+, Pb2+, Mn2+, Ni2+, Cu2+: 2.0 ppm | 0 | 0 |
| Cr6+, As3+; Fe2+, Zn2+: 2.5 ppm | 20.3 | 20.1a ± 0.8b | |
| La3+, Al3+, Sn4+: 1.0 ppm | 75.2 | 76.0a ± 0.7b | |
| Na+, K+, Mg2+, Ca2+: 650 ppm | |||
| Cl−, NO32−, HCO3−, F−: 650 ppm | |||
To analyze the detection efficiency of the fabricated sensor material for the detection of ultra-trace levels of Co(II) ions, water samples, namely, groundwater and industrial wastewater, were collected from the industrial zones of Tiruvallur district Tamil Nadu, as indicated in Table 2. The collected water samples were tested for quantitative estimation by spiking a known concentration of Co(II) ions in the water samples, and the ion-sensing efficiency using the proposed sensor was ascertained through the extent of Co(II)-ion recovery. Preceding this analysis, the presence of matrix constituents in the collected water samples was quantified by ICP-MS analysis to understand the matrix environment and the harsh experimental conditions associated with the ion-sensing process. In addition, the amount of spiked Co(II) ions in the natural, industrial, and synthetic water samples were analyzed by ICP-MS to cross-check the results derived using the proposed sensor material. The analytical data (Table 2) obtained using the proposed sensor revealed an excellent Co(II) recovery of ≥99.81%. The obtained results demonstrated the excellent data reliability and reproducibility of the proposed ion-sensing methodology, with a relative standard deviation value of ≤1.67%, from triplicate analysis. The analytical data proves that the proposed sensor can be an efficient tool for continuous environmental and industrial water sample surveillance.
4. Conclusions
A bimodal macro-/mesoporous polymer monolith was used as a probe-anchoring template. The PDHBD probe was uniformly impregnated to create a solid-state opto-sensor for the selective and sensitive detection of industrially relevant cobalt ions. The polymer monolith and the probe-anchored monolithic optical ion-sensor were characterized by p-XRD, HR-TEM, FE-SEM, SAED, EDAX, XPS, FT-IR, and BET/BJH analysis. The solid-state sensor exhibited a series of visual color transitions from pale-orange to deep-red, with the incremental addition of Co(II) ions. The proposed sensor exhibited a near-specific detection, even for ultra-trace concentrations of Co(II) ions, with a wide linear response range of 0–300 ppb and LOD and LOQ values of 0.3 and 1.0 ppb, respectively. The solid-state sensor material exhibited fast response kinetics of ≤180 s (≤20 °C) and 90 s (≥30 °C), in the pH range of 7.0–8.0, for Co(II) ions. The developed solid-state colorimetric sensor demonstrated outstanding property in successfully detecting/recovering Co(II) ions from real/synthetic samples and industrial effluents, with consistent data reproducibility. The salient features of the developed sensor include easy handling, excellent selectivity, a broad linear response profile, and high ion-selectivity/sensitivity. The proposed sensor could effectively achieve the selective sensing of Co(II) ions, with potential applications in the field of e-waste recovery and environmental decontamination. In addition, the proposed solid-state sensor is eco-/user-friendly, cost-effective, multi-reusable, and durable, with perfect suitability for on-site real-time monitoring without the necessity for skilled labor or expensive analytical techniques.Author contributions
Prabhakaran Deivasigamani: Conceptualization, visualization, investigation, methodology, data curation, funding acquisition, project administration, resources, supervision, validation, writing – review and editing. Satheesh Kuppusamy: formal analysis, visualization, investigation, methodology, software, data curation, writing – original draft.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.Acknowledgements
The authors of this manuscript acknowledge the financial assistance from VIT-Vellore in the form of the Institute Seed Grant 2020-21. The authors thank the instrumentation support from VIT (Vellore), SRMIST (Chennai), and BIT-Bengaluru.References
- F. R. Adolfo, P. C. Do Nascimento, D. Bohrer, L. M. De Carvalho, C. Viana, A. Guarda, A. Nunes Colim and P. Mattiazzi, Talanta, 2016, 147, 241–245 CrossRef CAS PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- E. J. Song, J. Kang, G. R. You, G. J. Park, Y. Kim, S. J. Kim, C. Kim and R. G. Harrison, Dalton Trans., 2013, 42, 15514–15520 RSC.
- Z. Liu, X. Jia, P. Bian and Z. Ma, Analyst, 2014, 139, 585–588 RSC.
- L. Leyssens, B. Vinck, C. Van Der Straeten, F. Wuyts and L. Maes, Toxicology, 2017, 387, 43–56 CrossRef CAS PubMed.
- H. Y. Au-Yeung, E. J. New and C. J. Chang, Chem. Commun., 2012, 48, 5268–5270 RSC.
- N. Ullah, M. Mansha, I. Khan and A. Qurashi, TrAC, Trends Anal. Chem., 2018, 100, 155–166 CrossRef CAS.
- C. H. Min, S. Na, J. E. Shin, J. K. Kim, T. G. Jo and C. Kim, New J. Chem., 2017, 41, 3991–3999 RSC.
- D. Maity and T. Govindaraju, Inorg. Chem., 2011, 50, 11282–11284 CrossRef CAS PubMed.
- A. Pramanik and G. Das, Cryst. Growth Des., 2008, 8, 3107–3113 CrossRef CAS.
- A. U. Karatepe, M. Soylak and L. Elci, Anal. Lett., 2002, 35, 2363–2374 CrossRef CAS.
- Q. Wang, W. Qin, L. Chai and Q. Li, Environ. Sci. Pollut. Res., 2014, 21, 3866–3872 CrossRef CAS PubMed.
- S. R. Yousefi and S. J. Ahmadi, Microchim. Acta, 2011, 172, 75–82 CrossRef CAS.
- T. S. Anirudhan, J. R. Deepa and J. Christa, J. Colloid Interface Sci., 2016, 467, 307–320 CrossRef CAS PubMed.
- F. Fang, L. Kong, J. Huang, S. Wu, K. Zhang, X. Wang, B. Sun, Z. Jin, J. Wang, X. J. Huang and J. Liu, J. Hazmat., 2014, 270, 1–10 CrossRef CAS PubMed.
- V. N. Mehta, A. K. Mungara and S. K. Kailasa, Anal. Methods, 2013, 5, 1818–1822 RSC.
- S. Wang, X. Bao, B. Gao and M. Li, Dalton Trans., 2019, 48, 8288–8296 RSC.
- S. Mohandoss and T. Stalin, RSC Adv., 2017, 7, 16581–16593 RSC.
- G. Wen, Y. Xiao, S. Chen, X. Zhang and Z. Jiang, Nanoscale Adv., 2021, 3, 3846–3859 RSC.
- L. Yang, S. J. Zhen, Z. De Liu and C. Z. Huang, Anal. Methods, 2014, 6, 5054–5058 RSC.
- L. Zhang and M. Fang, Nano Today, 2010, 5, 128–142 CrossRef CAS.
- M. M. Rahman, J. Ahmed and A. M. Asiri, New J. Chem., 2019, 43, 19298–19307 RSC.
- M. Li, H. Gou, I. Al-Ogaidi and N. Wu, ACS Sustainable Chem. Eng., 2013, 1, 713–723 CrossRef CAS.
- M. R. Awual, M. M. Hasan, T. Ihara and T. Yaita, Microporous Mesoporous Mater., 2014, 197, 331–338 CrossRef.
- M. R. Awual, I. M. M. Rahman, T. Yaita, M. A. Khaleque and M. Ferdows, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2017, 307, 85–94 CrossRef CAS.
- M. R. Awual, Composites, Part B, 2019, 172, 387–396 CrossRef CAS.
- R. Rubio, J. Santander, L. Fonseca, N. Sabaté, I. Gràcia, C. Cané, S. Udina and S. Marco, Sens. Actuators, B, 2007, 127, 69–73 CrossRef CAS.
- T. Madhesan and A. M. Mohan, Anal. Bioanal. Chem., 2020, 412, 7357–7370 CrossRef CAS PubMed.
- S. Xie, F. Svec and J. M. J. Fréchet, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 1013–1021 CrossRef CAS.
- W. Luo, Z. Bai and Y. Zhu, RSC Adv., 2018, 8, 13370–13387 RSC.
- A. Kongasseri, N. K. Sompalli, V. A. Modak, A. Mohanty, S. Nagarajan, C. V. S. B. Rao, P. Deivasigamani and A. M. Mohan, Microchim. Acta, 2020, 187, 1–13 CrossRef PubMed.
- T. Zhang, R. A. Sanguramath, S. Israel and M. S. Silverstein, Macromolecules, 2019, 52, 5445–5479 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1964, 85, 3533 CrossRef.
- A. Kongasseri, N. K. Sompalli, B. C. V. S. Rao, S. Nagarajan, A. M. Mohan and P. Deivasigamani, Sens. Actuators, B, 2020, 321, 128558 CrossRef CAS.
- I. Nischang and T. J. Causon, TrAC, Trends Anal. Chem., 2016, 75, 108–117 CrossRef CAS.
- N. K. Sompalli and P. Deivasigamani, Nanotechnology, 2020, 31, 414004 CrossRef CAS PubMed.
- K. A. Cychosz and M. Thommes, Engineering, 2018, 4, 559–566 CrossRef CAS.
- R. A. Sacramento, O. M. S. Cysneiros, B. J. B. Silva and A. O. S. Silva, Ceramica, 2019, 65, 585–591 CrossRef CAS.
- D. Vashisht, K. Kaur, R. Jukaria, A. Vashisht, S. Sharma and S. K. Mehta, Sens. Actuators, B, 2019, 280, 219–226 CrossRef CAS.
- J. J. Lv, A. J. Wang, X. Ma, R. Y. Xiang, J. R. Chen and J. J. Feng, J. Mater. Chem. A, 2015, 3, 290–296 RSC.
- S. M. Kang, S. C. Jang, G. Y. Kim, C. S. Lee, Y. S. Huh and C. Roh, Sensors, 2016, 16, 1–10 Search PubMed.
- M. R. Awual, N. H. Alharthi, M. M. Hasan, M. R. Karim, A. Islam, H. Znad, M. A. Hossain, M. E. Halim, M. M. Rahman and M. A. Khaleque, Chem. Eng. J., 2017, 324, 130–139 CrossRef CAS.
- A. H. Gore, D. B. Gunjal, M. R. Kokate, V. Sudarsan, P. V. Anbhule, S. R. Patil and G. B. Kolekar, ACS Appl. Mater. Interfaces, 2012, 4, 5217–5226 CrossRef CAS PubMed.
- W. N. El-Sayed, K. Z. Elwakeel, A. Shahat and M. R. Awual, RSC Adv., 2019, 9, 14167–14175 RSC.
- S. A. El-Safty, M. R. Awual, M. A. Shenashen and A. Shahat, Sens. Actuators, B, 2013, 176, 1015–1025 CrossRef CAS.
- S. A. El-Safty, Adsorption, 2009, 15, 227–239 CrossRef CAS.
- R. Kalarani, M. Sankarganesh, G. G. V. Kumar and M. Kalanithi, J. Mol. Struct., 2020, 1206, 127725 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/D1NJ04793K |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |