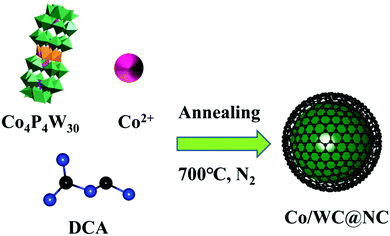
Self-assembly of bimetallic polyoxometalates and dicyandiamide to form Co/WC@NC for efficient electrochemical hydrogen generation†
Yan
Liu‡
a,
Jiao
Li‡
b,
Dan
Sun
a,
Linglan
Men
a,
Bo
Sun
a,
Xiao
Li
*a,
Qingbo
An
*a,
Fangbin
Liu
a and
Zhongmin
Su
 *a
*a
aSchool of Chemical and Environmental Engineering, Jilin Provincial Science and Technology Innovation Centre of Optical Materials and Chemistry, Changchun University of Science and Technology, International Joint Research Center for optical functional materials and chemistry, Changchun University of Science and Technology, Changchun, People's Republic of China. E-mail: lix@cust.edu.cn; zmsu@nenu.edu.cn
bSchool of Materials science and Engineering, Changchun University of Science and Technology, Changchun 130022, People's Republic of China
First published on 19th November 2021
Abstract
The design of economical and efficient electrocatalysts to replace precious platinum-based catalysts for hydrogen evolution reaction (HER) has always been an essential study direction of clean energy technology. In this work, an HER electrocatalyst (Co/WC@NC) was synthesized by pre-self assembly of polyoxometalate (Co4P4W30), cobalt ions and dicyandiamide (DCA) via a one-step calcination process. Co/WC@NC consists of cobalt and tungsten carbide nanoparticles, which are wrapped with nitrogen-doped carbon layers. Co/WC@NC shows good HER capability with relatively low overpotentials of 142 and 158 mV with small Tafel slopes of 93 and 95 mV dec−1, respectively, at a current density of 10 mA cm−2 in acidic and alkaline solutions. Moreover, Co/WC@NC provides satisfactory durability for 24 h in both 0.5 M H2SO4 and 1.0 M KOH. Founded on the above results, good catalytic property of Co/WC@NC can be primarily associated with the synergy between Co and WC nanoparticles, N element doping to make adjustments for the electronic structure of carbon layers, thus speeding up the electron transfer process, as well as graphite-coated carbon layers protecting nanoparticles from oxidation, and improving stability. This study confirms the universal idea to form transition carbides for an efficient electrocatalytic production of hydrogen.
1. Introduction
The rapid development of the economy does not merely causes fossil fuel exhaustion but has also given rise to serious environmental problems such as global warming. Therefore, there is a crying need to exploit pollution-free and sustainable energy sources that can substitute fossil fuels.1–4 Hydrogen is considered to be the most effective alternative fuel on the basis of its large energy density, renewable, environmental protection, etc.5–7 Hydrogen generated by water splitting using renewable electricity is an effective and green method, promising large-scale production.8–10 Thus far, electrocatalysts with the best catalytic activity for HER are platinum-based catalysts.11,12 However, the industrialization of platinum-based catalysts is greatly restricted due to their high cost and low output.13,14 Therefore, developing a high catalytic activity, affordable and tranquil HER electrocatalysts must be of great value for hydrogen energy economy.15Currently, non-precious electrocatalysts are mainly focused on carbides,16,17 phosphides,18–20 nitrides,21–23 sulphides,24 and a series of N-type non-metallic carbon materials.25 Thereinto, transition metal carbides (TMCs) have been widely investigated due to the special electronic configuration as well as good electrical conductivity, and similar chemical activity and catalytic performance with platinum-based catalysts in HER fields, especially in the VB/VIB family.26,27 Moreover, compared with other transition metals, the catalytic activity of W-based carbides for HER needs to be further improved.28 Recently, a few numbers of tungsten-based carbides have been reported. For instance, Zhang and co-works report dual-phased Mo2C–WC ultrathin nanosheet nanocrystals (Mo2C–WC/NCAs) through one-step pyrolysis of polymers.29 Yan and co-workers synthesized ultrafine WC by in-suit molten salt.30
Polyoxometalates (POMs), as one class of plentiful metal oxygen clusters, are composed of transition metal ions (Mo, W, V, Nb, Ta, etc.).31 POMs possess large number of negative charges with peculiar chemical compositions and structural diversification, which can be regarded as one of the ideal candidates for synthesizing uniform Mo/W-based electrocatalyst.32–35 Tungsten-based polyoxometalates are widely studied because of their unique nanostructures and inherent high conductivity,30 especially in preparing tungsten carbides. Whereas the high intensity of W–H can seriously affect H2 desorption, leading to poor HER activity. According to previous studies, the strength of the W–H bond can be reduced by adjusting the electronic structure of WC, such as by introducing a second metal source.36 Furthermore, the concurrent doping of N, S, and P heteroatoms also help to regulate the electronic structure. The abundant N elements in dicyandiamide (DCA) molecules show high electronegativity, which can accomplish the doping of N atoms to change the local electron configuration of carbon materials, promoting charge transfer, and accordingly improve the catalytic capability. Therefore, dicyandiamide (DCA) can be used as a potential carbon and nitrogen source to synthesize catalysts for electrochemical applications.37
In this work, Co2+ and POM (Co4P4W30) are selected as metal sources and DCA as nitrogen sources and carbon sources for the synthesis of hydrogen evolution catalysts (Co/WC@NC). Co/WC@NC consists of nitrogen-doped carbon-coated Co and WC nanoparticles. Thereby, under acidic conditions, Co/WC@NC only needs 142 mV to attain 10 mA cm−2, fulling of the Tafel curvature of 93 mV dec−1. Besides, the overpotential and Tafel slope of Co/WC@NC are 158 mV and 95 mV dec−1 in basic solutions. Graphitic carbon coating enhances the steadiness of the catalyst in either acid or alkaline solutions so that Co/WC@NC presents satisfactory durability for 24 h in both 0.5 M H2SO4 and 1.0 M KOH. Co and WC nanoparticles synergistically enhance charge transfer rate and improve the HER performance. Moreover, the successful doping of nitrogen will further enhance the catalytic activity Co/WC@NC. The work indicates that the development of tungsten-based materials to study electrocatalytic hydrogen evolution exhibits large research spaces for potential practical applications.
2. Results and discussion
Scheme 1 displays the synthetic strategy of Co/WC@NC. Co4P4W30, DCA and Co(NO3)2 were mixed thoroughly in water with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and then calcined at 700 °C under N2 atmosphere for 2 hours to obtain the catalyst Co/WC@NC. The solid pre-annealing process may be nanoscale carbides in a confined space to form Co/WC@NC. DCA not merely can scatter POM sections, but may also generate in situ species of graphite carbon or nitrogen. At the same time, it facilitates the deoxidation of the Co4P4W30 precursor,38 providing an adequate source of nitrogen.
2, and then calcined at 700 °C under N2 atmosphere for 2 hours to obtain the catalyst Co/WC@NC. The solid pre-annealing process may be nanoscale carbides in a confined space to form Co/WC@NC. DCA not merely can scatter POM sections, but may also generate in situ species of graphite carbon or nitrogen. At the same time, it facilitates the deoxidation of the Co4P4W30 precursor,38 providing an adequate source of nitrogen.
Fig. 1 shows the transmission electron microscope (TEM) and energy spectrum (EDS) mapping images of Co/WC@NC. These images exhibit the lattice structure, configuration of surface, and particle size of the catalyst. Co/WC@NC showcases an irregular modality with particle sizes ranging from 10 nm to 40 nm (Fig. 1a). The higher resolution transmission electron microscope (HRTEM) image (Fig. 1b) indicates that Co/WC nanoparticles are wrapped by graphitic carbon layers, and the crystal grating fringe spacing of Co and WC are 0.20 nm and 0.26 nm, respectively; correspondence with the lattice plane (111) in Co (JCPDS No. 15-0806) and the grate plane (100) in WC (JCPDS No. 51-0939). The element mapping graphs of Co/WC@NC exhibits that the elements (Co, W, C, N and P) are well-dispersed in the catalyst (Fig. 1c–h). The mass percentages of C, N, P, Co and W in EDS are 90.61, 5.23, 1.33, 1.97 and 0.87%, respectively. In addition, the atomic percentages of Co, W, C, N and P (Co/WC@NC, WC@NC and Co@NC) from XPS are shown in Table S1 (ESI†). The ICP data indicate the mass percentages of C, N, P, Co and W in Co/WC@NC as 93.152%, 4.358%, 0.350%, 0.689% and 1.451%, respectively. The presence of nitrogen attests that nitrogen is thrivingly deathniumed into Co/WC@NC.
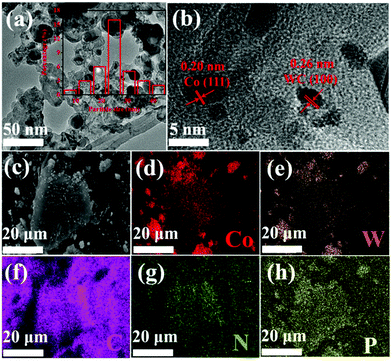 | ||
| Fig. 1 Characterization Morphology of Co/WC@NC: (a) TEM image of Co/WC@NC; (b) HRTEM image; (c–h) EDS mapping images. | ||
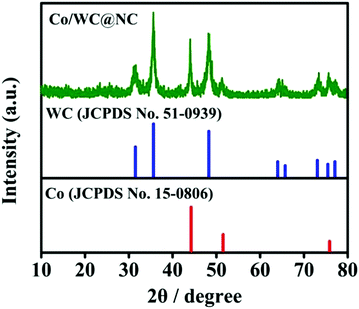
To characterize and analyze the phase composition of Co/WC@NC, powder X-ray diffraction (PXRD) patterns were recorded. It can be seen from Fig. 2 that Co/WC@NC reveals two phases: Co and WC. The main diffraction peaks of WC (JCPDS No. 51-0939) are located at 31.5°, 35.6°, 48.3°, 64.0°, 65.8°, 73.1°, 75.5° and 77.1°, corresponding to the lattice planes (001), (100), (101), (110), (002), (111), (200) and (102), respectively. The obvious peaks at 44.1°, 51.4° and 75.8° correspond to the diffraction peaks of crystal planes (111), (200) and (220) in Co (JCPDS No. 15-0806). It is highlighted that the wider peak with the scope of 20°–30° (2θ) was not evident, maybe owing to the density of the porous carbon produced by dicyandiamide being relatively low.39 Simultaneously, we also considered PXRD patterns of the comparative samples (Fig. S1, ESI†). WC/W2N@NC shows two phases, WC and W2N. The clear diffraction peaks of W2N (JCPDS No. 25-1257) are located at 37.7°, 43.8°, 63.7° and 76.5°, which correspond to (111), (200), (220) and (311) planes, respectively. Co@NC possesses the same peaks as Co/WC@NC. In addition, Raman spectra were used to investigate the degree of graphitization Co/WC@NC (Fig. S2, ESI†). The two peaks at 1326 and 1592 cm−1 represent the d-band and g-band of the disordered and graphitic carbon in Co/WC@NC, respectively. The peak-to-strength ratio of ID/IG was 1.195, indicating that the structure demonstrates defects and the degree of graphitization is equivalent, which is conducive to the absorption of H+/H2.40
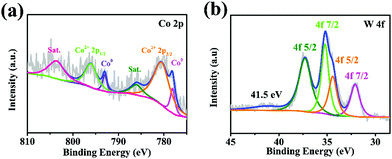
X-Ray photoelectron spectroscopy (XPS) measurements were performed on Co/WC@NC to analyze the element composition and valence state. Fig. 3a reveals the XPS spectrum of Co 2p in the Co/WC@NC catalyst. The deconvolution spectrum of Co 2p defines the existence of Co2+ 2p3/2 (780.5 eV), Co2+ 2p1/2 (796.0 eV), metallic Co (778.0 and 793.0 eV), and satellite peaks (786.0 and 802.9 eV).41,42 The peak area of cobalt metal is higher than that of other peaks. The more valence state of Co is beneficial to encourage electron shift and convenient to combine with W, and arouses the catalytic efficiency of activator. In W 4f deconvolution spectrum of Co/WC@NC (Fig. 3b), strong diffraction peaks of W 4f7/2 (32.2 eV) and W 4f5/2 (34.4 eV) may be attributed to the charged-off WC.43 Moreover, the other W 4f7/2 (35.2 eV) and W 4f5/2 (37.3 eV) peaks are all subordinated to the partial oxygenation of W. The XPS spectrum of C 1s (Fig. S3a, ESI†) can be deconvoluted into three peaks (288.8 eV, 286.5 eV and 284.6 eV), belonging to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N and C
O, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively.44 As can be seen from XPS spectra of N 1s (Fig. S3b, ESI†), the peaks at 401.5, 400.5 and 398.4 eV are in agreement with graphite N, pyrrole N and pyridine N, respectively.45 These results indicate that the N element was added into the lattice of C layers. Fig. S4 and S5 (ESI†) show the XPS spectrum of WC@NC and Co@NC, respectively. The full XPS spectrum of W 4f in the WC@NC verifies the existence of W 4f7/2 (31.7 and 36.5 eV), W 4f5/2 (34.7 and 41.0 eV) and satellite peaks (44.0 eV) (Fig. S4a, ESI†).46 The Co 2p spectrum (Fig. S4b, ESI†) of WC@NC demonstrates six peaks at 778.0, 780.2, 785.4, 793.1, 796.0 and 803.0 eV. Among them, the peaks at 785.4 and 803.0 eV are satellite peaks attributed to the vibration excitation of Co2+ ions. The peaks at 778.0, 780.2 eV and 793.1, 796.0 eV can result from tCo 2p3/2 and Co 2p1/2 of metallic Co and cobalt ions.47,48
C, respectively.44 As can be seen from XPS spectra of N 1s (Fig. S3b, ESI†), the peaks at 401.5, 400.5 and 398.4 eV are in agreement with graphite N, pyrrole N and pyridine N, respectively.45 These results indicate that the N element was added into the lattice of C layers. Fig. S4 and S5 (ESI†) show the XPS spectrum of WC@NC and Co@NC, respectively. The full XPS spectrum of W 4f in the WC@NC verifies the existence of W 4f7/2 (31.7 and 36.5 eV), W 4f5/2 (34.7 and 41.0 eV) and satellite peaks (44.0 eV) (Fig. S4a, ESI†).46 The Co 2p spectrum (Fig. S4b, ESI†) of WC@NC demonstrates six peaks at 778.0, 780.2, 785.4, 793.1, 796.0 and 803.0 eV. Among them, the peaks at 785.4 and 803.0 eV are satellite peaks attributed to the vibration excitation of Co2+ ions. The peaks at 778.0, 780.2 eV and 793.1, 796.0 eV can result from tCo 2p3/2 and Co 2p1/2 of metallic Co and cobalt ions.47,48
Fig. S4c (ESI†) reports the peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.5 eV), C–N (286.4 eV) and C
C (284.5 eV), C–N (286.4 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.6 eV) of C 1s. The peaks at 397.8, 399.6 and 401.3 eV in shown in Fig. S4d (ESI†) are due to graphitic-N, pyrrolic-N and pyridinic-N of N 1s. Fig. S5a, b and c (ESI†) show Co, N and C XPS spectra of Co@NC. The metallic Co (778.0/793.1 eV), Co2+ 2p3/2 (780.7 eV), Co2+ 2p1/2 (796.2 eV) and satellite peaks (786.7/802.5 eV) are clearly seen in the Fig. S5a (ESI†).49 C 1s exists in C
O (288.6 eV) of C 1s. The peaks at 397.8, 399.6 and 401.3 eV in shown in Fig. S4d (ESI†) are due to graphitic-N, pyrrolic-N and pyridinic-N of N 1s. Fig. S5a, b and c (ESI†) show Co, N and C XPS spectra of Co@NC. The metallic Co (778.0/793.1 eV), Co2+ 2p3/2 (780.7 eV), Co2+ 2p1/2 (796.2 eV) and satellite peaks (786.7/802.5 eV) are clearly seen in the Fig. S5a (ESI†).49 C 1s exists in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.9 eV), C–N (286.0 eV) and C
C (284.9 eV), C–N (286.0 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.6 eV) bond. The peaks at 401.8, 400.1 and 397.7 eV of N 1s can be assigned to graphitic-N, pyrrolic-N and pyridinic-N. To evaluate the porosity of Co/WC@NC, N2 adsorption–desorption measurement was conducted. As shown in Fig. S6 (ESI†), the isotherm obtained from N2 adsorption exposes an obvious hysteresis loop, indicating that the catalyst belonging to the H3 type. The BET surface area of Co/WC@NC is 72.2 m2 g−1 and the pore size is 3.97 nm, presenting mesoporous structures. The mesoporous structure facilitates more active site exposure, thereby enhancing the electrocatalytic activity.
O (288.6 eV) bond. The peaks at 401.8, 400.1 and 397.7 eV of N 1s can be assigned to graphitic-N, pyrrolic-N and pyridinic-N. To evaluate the porosity of Co/WC@NC, N2 adsorption–desorption measurement was conducted. As shown in Fig. S6 (ESI†), the isotherm obtained from N2 adsorption exposes an obvious hysteresis loop, indicating that the catalyst belonging to the H3 type. The BET surface area of Co/WC@NC is 72.2 m2 g−1 and the pore size is 3.97 nm, presenting mesoporous structures. The mesoporous structure facilitates more active site exposure, thereby enhancing the electrocatalytic activity.
In 0.5 M H2SO4 and 1.0 M KOH, a universal three-electrode system was used to preliminarily explore the HER catalytic property of Co/WC@NC at room temperature. The electrocatalysts were uniformly coated on the surface of glassy carbon electrodes with a capacity of 0.66 mg cm−2, and under the condition of a particular scan rate (5 mV s−1). After the linear sweep voltammetry (LSV) test, the polarization curves were obtained. For comparison, different calcination temperatures, distinct ratios and mass loadings were tested. Above all, we selected a proportion of Co4P4W30/DCA corresponding to the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. The LSV curves at different temperatures such as 700, 800 and 900 °C under the condition of (Co4P4W30/DCA) mass ratio of 1
7. The LSV curves at different temperatures such as 700, 800 and 900 °C under the condition of (Co4P4W30/DCA) mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were gauged (Fig. S7, ESI†). Simultaneously, XRD patterns of the catalyst with different temperatures (700, 800 and 900 °C) based on the ratio of Co4P4W30 to DCA as 1
7 were gauged (Fig. S7, ESI†). Simultaneously, XRD patterns of the catalyst with different temperatures (700, 800 and 900 °C) based on the ratio of Co4P4W30 to DCA as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 are shown in Fig. S8 (ESI†). The diffraction peak at 35.6° and 48.3° in samples heated at 700 and 800 °C are ascribed to (100) and (101) crystal planes of WC (JCPDS No. 51-0939). The sample heated at 900 °C exists two phases, WC (JCPDS No. 51-0939) and W2C (JCPDS No. 35-0776). By comparing different calcination temperatures, the catalyst at 700 °C showed better performance. In order to optimize the experiments, we then prepared electrocatalysts with different Co4P4W30/DCA mass ratios as 1
7 are shown in Fig. S8 (ESI†). The diffraction peak at 35.6° and 48.3° in samples heated at 700 and 800 °C are ascribed to (100) and (101) crystal planes of WC (JCPDS No. 51-0939). The sample heated at 900 °C exists two phases, WC (JCPDS No. 51-0939) and W2C (JCPDS No. 35-0776). By comparing different calcination temperatures, the catalyst at 700 °C showed better performance. In order to optimize the experiments, we then prepared electrocatalysts with different Co4P4W30/DCA mass ratios as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 at the optimised temperature and tested the LSV curves (Fig. S9, ESI†). The best quality ratio was 1
8 at the optimised temperature and tested the LSV curves (Fig. S9, ESI†). The best quality ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, but it still does not achieve the expectation. The ratios of Co4P4W30 to Co(NO3)2 (1
7, but it still does not achieve the expectation. The ratios of Co4P4W30 to Co(NO3)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were conducted under the condition of 700 °C and the mass ratio (Co4P4W30/DCA) of 1
1) were conducted under the condition of 700 °C and the mass ratio (Co4P4W30/DCA) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. Through the polarization curve test, the optimal mass ratio was 1
7. Through the polarization curve test, the optimal mass ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as shown in Fig. S10 (ESI†). It only needed an overpotential of 142 mV in acidic solution to reach a current density of 10 mA cm−2. After a series of sample comparison, only when the mass ratio of Co4P4W30/DCA/Co(NO3)2 was 1
2, as shown in Fig. S10 (ESI†). It only needed an overpotential of 142 mV in acidic solution to reach a current density of 10 mA cm−2. After a series of sample comparison, only when the mass ratio of Co4P4W30/DCA/Co(NO3)2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the electrocatalyst demonstrated the best electrochemical activity. Besides, loading adjustment is the last step of the catalyst condition optimization. The LSV curves of different mass loadings are shown in Fig. S11 (ESI†). 0.66 mg cm−2 was the optimal loading of the catalyst.
2, the electrocatalyst demonstrated the best electrochemical activity. Besides, loading adjustment is the last step of the catalyst condition optimization. The LSV curves of different mass loadings are shown in Fig. S11 (ESI†). 0.66 mg cm−2 was the optimal loading of the catalyst.
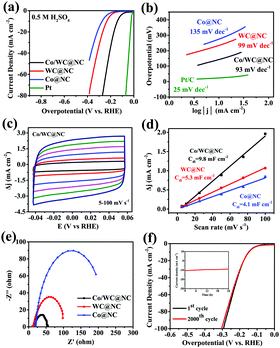
For further analyzing the HER property of Co/WC@NC, Co@NC and WC@NC were also prepared. Fig. 4a, Pt/C shows the lowest overpotential of 26 mV (10 mA cm−2) in 0.5 M H2SO4, which is consistent with previous literature reports.20Co/WC@NC exhibits a relatively good catalytic capability, requiring an overpotential of 142 mV when the current density was 10 mA cm−2. Furthermore, WC@NC and Co@NC required 233 and 273 mV, respectively, both of which exhibited under par HER performance than Co/WC@NC. The above results show the mutualism between Co and WC increases the catalytic function of Co/WC@NC. The catalysts mechanism was analysed by calculating Tafel slope values as shown in Fig. 4b. The results are 93 (Co/WC@NC) and 25 mV dec−1 (Pt/C), suggesting the Volmer–Heyrovsky mechanism, which is equivalent to the desorption as the decisive step for hydrogen production.50,51 The Tafel slopes of the comparative catalysts WC@NC and Co@NC are 99 and 135 mV dec−1, respectively.
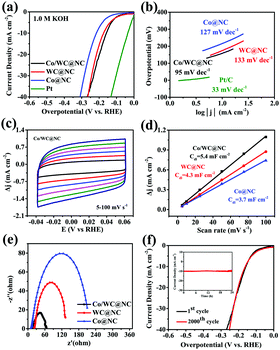
As we know, the electrochemical activity is associated with the electrochemical superficial area (ECSA) of the electrocatalyst. ECSA is usually calculated from two thicknesses electrochemical capacitance values (Cdl) using a simple cyclic voltammetry (CV) test. Here, the different scan rates of CV curves were 5, 10, 25, 50 and 100 mV s−1 in 0.5 M H2SO4 and 1.0 M KOH conditions, respectively (Fig. 4c, Fig. S12a and c, ESI†). Fig. S12a and c (ESI†) clarify the CV curves of WC@NC and Co@NC, respectively, in 0.5 M H2SO4 solution, and WC@NC is stronger than Co@NC. The values of Cdl were derived from Δj (ja–jc) at different scan rates at 0.1 V (vs. RHE), and the curve was fitted from the obtained points. The Cdl of the specimen was decided from the slope of the curve fitting. The Cdl value of Co/WC@NC under acidic conditions was 9.8 mF cm−2, more than that of Co@NC (4.1 mF cm−2) and WC@NC (5.3 mF cm−2), meaning that Co/WC@NC possesses a larger surface area and exposes numerous electrochemically-capabile sites, as shown in Fig. 4d.
Besides, electrochemical impedance spectroscopy (EIS) was used to assess the electrical conductivity, reflected by the accuse transfer resistance (Rct) value. Fig. 4e shows the Nyquist plot of the EIS of Co/WC@NC and the comparative samples, with an overpotential of 150 mV. The semicircle of Co/WC@NC is the smallest, displaying that Co/WC@NC exhibits the best electrical conductivity. Equally importantly, permanence is an essential basis for judging the suitability of electrocatalysts. The initial LSV curves before and after 2000 cycles are substantially not changing much, as shown in Fig. 4f. Furthermore, the inset of Fig. 4f is the durability test diagram of Co/WC@NC in acidic solution at 150 mV overpotential for 24 h. These results indicate that Co/WC@NC show good electrocatalytic stability.
In addition, we also tested the hydrogen evolution performance of all specimens in alkaline solutions. Undoubtedly, Pt/C was still the best catalyst for HER. The overpotential of Co/WC@NC is relatively low competed with Co@NC and WC@NC. Thereinto, the overpotentials (Tafel slopes) of Co@NC, WC@NC and Co/WC@NC are 216 mV (127 mV dec−1), 175 mV (133 mV dec−1) and 158 mV (95 mV dec−1), respectively, when the current density was 10 mA cm−2 (Fig. 5a and b). The CV curves are illustrated in Fig. 5c, and Fig. S12b and d (ESI†). The Cdl values of Co@NC, WC@NC and Co/WC@NC are 3.7, 4.3 and 5.4 mF cm−2, respectively, displaying that Co/WC@NC manifests a larger electrochemical surface area in alkaline environment (Fig. 5d). EIS spectra under the alkaline conditions with the overpotential of 0.2 V shows that the impedance worth of Co/WC@NC is smaller than that of other comparison samples, indicating that Co/WC@NC exhibits higher conductivity as shown in Fig. 5e. Fig. 5f shows that the catalyst presents good stability in alkaline media. In addition, the antithesis of HER catalysts of bimetallic carbides in recent years is encapsulated in Table S2 (ESI†). The excellent HER performance of Co/WC@NC is primarily ascribed to (1) the mutualism of Co and W in the catalyst. (2) It should be pointed out that the existence of a carbon layer facilitates porosity and conductivity of the whole catalyst. (3) What matters most is that N doping into the carbon layer raises the electron density of the graphite carbon shell and further promotes HER capability.
3. Conclusions
In conclusion, the Co/WC@NC catalyst was prepared by simple one-step synthesis and high-temperature calcination using DCA, metal ions and POM (Co4P4W30). Co/WC@NC shows superior HER property with 142 mV and 158 mV overpotential at 10 mA cm−2 in acidic and alkaline media, respectively, and electrochemical durability for up to 24 hours. The good hydrogen evolution activity can be linked to the synergistic effect of Co and WC nanoparticles, carbon layers protecting catalysts from corrosion, as well as N atoms doping enhancing the charge transfer process. This part delivers a meaningful headship for the device and preparation of HER electrocatalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Joint Fund Project of the Natural Science Foundation of Jilin Province (YDZJ202101ZYTS052), Thirteenth Five-Year Program for Science and Technology of Education Department of Jilin Province (JJKH20200738KJ, JJKH20200740KJ, JJKH20200742KJ), the Foundation of Changchun University of Science and Technology (XQNJJ-2019-12, XJJLG-2019-03). The Open Project Program of Key Laboratory of Preparation and Application of Environmental Friendly Materials (Jilin Normal University), Ministry of Education, China (No. 2020013).Notes and references
- A. H. Imani, R. Ojani and J. B. Raoof, Novel polyoxometalate-based composite as efficient electrocatalyst for alkaline water oxidation reaction, J. Iran. Chem. Soc., 2021, 18, 2079–2089 CrossRef CAS.
- Z. Huang, Z. X. Yang, Z. Hussain, B. L. Chen, Q. L. Jia, Y. Q. Zhu and Y. D. Xia, Polyoxometallates@zeolitic-imidazolate-framework derived bimetallic tungsten-cobalt sulfide/porous carbon nanocomposites as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution, Electrochim. Acta, 2020, 330(10), 135335 CrossRef CAS.
- Y. Wang, H. S. Zhao, A. D. Di, X. Yang, B. W. Cong and G. Chen, Formation of hollow MoO2@C nano-octahedrons using polyoxometalate-based metal–organic framework as a template for enhanced lithium-ion batteries, Int. J. Energy Res., 2021, 1–11 Search PubMed.
- G. P. Liu, K. K. Wang, L. Wang, B. Wang, Z. X. Lin, X. Chen, Y. J. Hua, W. S. Zhu, H. M. Li and J. X. Xia, A Janus cobalt nanoparticles and molybdenum carbide decorated N-Doped carbon for high-performance overall water splitting, J. Colloid Interface Sci., 2021, 583, 614–625 CrossRef CAS PubMed.
- M. Q. Yao, B. J. Wang, B. L. Sun, L. F. Luo, Y. J. Chen, J. W. Wang, N. Wang, S. Komarneni, X. B. Niu and W. C. Hu, Rational design of self-supported Cu@WC core-shell mesoporous nanowires for pH-universal hydrogen evolution reaction, Appl. Catal., B, 2021, 280, 119451 CrossRef CAS.
- C. L. Li, Z. J. Zhang and R. Liu, In Situ Growth of 3D NiFe LDH-POM Micro-Flowers on Nickel Foam for Overall Water Splitting, Small, 2020, 16(46), 2003777 CrossRef CAS PubMed.
- M. L. Wang, D. Yin, Y. D. Cao, G. G. Gao, T. Pang, L. L. Ma and H. Liu, Ultralow Pt0 loading on MIL-88A (Fe) derived polyoxometalate-Fe3O4@C micro-rods with highly-efficient electrocatalytic hydrogen evolution, J. Coord. Chem., 2020, 73(17-19), 658–667 Search PubMed.
- J. Zhu, L. S. Hu, P. X. Zhao, L. Lee, Y. S. Wang and K. Y. Wong, Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles, Chem. Rev., 2020, 120(2), 851–918 CrossRef CAS.
- S. J. Folkman, S. L. Joaquin, G. M. José and R. G. Finke, Electrochemically Driven Water-Oxidation Catalysis Beginning with Six Exemplary Cobalt Polyoxometalates: Is It Molecular, Homogeneous Catalysis or Electrode-Bound, Heterogeneous CoOx Catalysis?, J. Am. Chem. Soc., 2018, 140(38), 12040–12055 CrossRef CAS PubMed.
- P. W. Menezes, A. Indra, C. Das, C. Walter, C. Göbel, V. Gutkin, D. Schmeiβer and M. Driess, Uncovering the Nature of Active Species of Nickel Phosphide Catalysts in High-Performance Electrochemical Overall Water Splitting, ACS Catal., 2017, 7, 103–109 CrossRef CAS.
- P. Yu, F. M. Wang, T. A. Shifa, X. Y. Zhan, X. D. Lou, F. Xia and J. He, Earth abundant materials beyond transition metal dichalcogenides: A focus on electro-catalyzing hydrogen evolution reaction, Nano Energy, 2019, 58, 244–276 CrossRef CAS.
- L. G. Li, P. T. Wang, Q. Shao and X. Q. Huang, Metallic nanostructures with low dimensionality for electrochemical water splitting, Chem. Soc. Rev., 2020, 49, 3072–3106 RSC.
- W. H. Wang, J. Geng, L. Kuai, M. Li and B. Y. Geng, Porous Mn2O3: A Low-Cost Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media with Comparable Activity to Pt/C, Chem. – Eur. J., 2016, 26(29), 9909–9913 CrossRef.
- S. Q. Hu, X. L. Li, A. Ali, X. Y. Zhang and P. K. Shen, Large-scale Synthesis of Porous Pt Nanospheres/Three-dimensional Graphene Hybrid Materials as a Highly Active and Stable Electrocatalyst for Oxygen Reduction Reaction, ChemSystemsChem, 2021, 6(9), 2080–2084 CAS.
- L. He, S. Huang, Y. Liu, M. Wang, B. Cui, B. Wu, J. Liu, Z. Zhang and M. Du,, Multicomponent Co9S8@MoS2 nanohybrids as a novel trifunctional electrocatalyst for efficient methanol electrooxidation and overall water splitting, J. Colloid Interface Sci., 2020, 10, 119 Search PubMed.
- B. Zhang, H. Y. Qin, Y. P. Pan, W. Y. Lin, S. Xu, Q. Z. Sun, E. Z. Liu, F. He, L. C. Diao, C. N. He and L. Y. Ma, Graphite Carbon Nanosheet-Coated Cobalt-Doped Molybdenum Carbide Nanoparticles for Efficient Alkaline Hydrogen Evolution Reaction, ACS Appl. Nano Mater., 2021, 4(1), 372–380 CrossRef CAS.
- J. Y. Wang, R. L. Zhu, J. L. Cheng, Y. Y. Song, M. Mao, F. F. Chen and Y. L. Cheng, Co, Mo2C encapsulated in N-doped carbon nanofiber as self-supported electrocatalyst for hydrogen evolution reaction, Chem. Eng. J., 2020, 397, 125481 CrossRef CAS.
- J. Q. Tian, Q. Liu, A. M. Asiri and X. P. Sun, Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14, J. Am. Chem. Soc., 2014, 136(21), 7587–7590 CrossRef CAS PubMed.
- N. Coleman, M. D. Lovander, J. Leddy and E. G. Gillan, Phosphorus-Rich Metal Phosphides: Direct and Tin Flux-Assisted Synthesis and Evaluation as Hydrogen Evolution Electrocatalysts, Inorg. Chem., 2019, 58(8), 5013–5024 CrossRef CAS.
- J. Yang, F. J. Zhang, X. Wang, D. S. He, G. Wu, Q. H. Yang, X. Hong, Y. E. Wu and Y. D. Li, Porous Molybdenum Phosphide Nano-Octahedrons Derived from Confined Phosphorization in UIO-66 for Efficient Hydrogen Evolution, Angew. Chem., Int. Ed., 2016, 55, 1–6 CrossRef.
- Y. P. Zhu, G. Chen, Y. J. Zhong, W. Zhou and Z. P. Shao, Rationally Designed Hierarchically Structured Tungsten Nitride and Nitrogen-Rich Graphene-Like Carbon Nanocomposite as Efficient Hydrogen Evolution Electrocatalyst, Adv. Sci., 2018, 5(2), 1700603 CrossRef.
- Z. Z. Liu, X. M. Zhang, H. Song, Y. X. Yang, Y. Zheng, B. Gao, J. J. Fu, P. K. Chu and K. F. Huo, Electronic Modulation between Tungsten Nitride and Cobalt Dopants for Enhanced Hydrogen Evolution Reaction at a Wide Range of pH, ChemCatChem, 2020, 12(11), 2962–2966 CrossRef CAS.
- R. Tong, Y. J. Qu, Q. Zhu, X. N. Wang, Y. H. Lu, S. P. Wang and H. Pan, Combined Experimental and Theoretical Assessment of WXy (X = C, N, S, P) for Hydrogen Evolution Reaction, ACS Appl. Energy Mater., 2020, 3(1), 1082–1088 CrossRef CAS.
- S. M. Faber, R. Dziedzic, M. A. Lukowski, N. S. Kaiser, Q. Ding and S. Jin, High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures, J. Am. Chem. Soc., 2014, 136(28), 10053–10061 CrossRef PubMed.
- L. N. Zhang, S. H. Li, H. Q. Tan, S. U. Khan, Y. Y. Ma, H. Y. Zang, Y. H. Wang and Y. G. Li, MoP/Mo2C@C: a new combination of electrocatalysts for highly efficient hydrogen evolution over all pH range, ACS Appl. Mater. Interfaces, 2017, 9(19), 16270–16279 CrossRef CAS.
- J. F. Pang, J. M. Sun, M. Y. Zheng, H. Q. Li, Y. Wang and T. Zhang, Transition metal carbide catalysts for biomass conversion: A review, Appl. Catal., B, 2019, 254(5), 510–522 CrossRef CAS.
- F. Shahzad, A. Iqbal, H. Kim and C. M. Koo, 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material, Adv. Mater., 2020, 32(51), 2002159 CrossRef CAS.
- X. T. Zhang, Z. Y. Zhu, X. Y. Liang, F. X. Ma, J. Z. Zhang, Y. B. Tan, Z. C. Pan, Y. Y. Bo and C. M. Wu, Encapsulating dual-phased Mo2C-WC nanocrystals into ultrathin carbon nanosheet assemblies for efficient electrocatalytic hydrogen evolution, Chem. Eng. J., 2021, 408(15), 127270 CrossRef CAS.
- X. T. Zhang, Z. Y. Zhu, X. Y. Liang, F. X. Ma, J. H. Zhang, Y. B. Tan, Z. C. Pan, Y. Y. Bo and C. M. Wu, Encapsulating dual-phased Mo2C–WC nanocrystals into ultrathin carbon nanosheet assemblies for efficient electrocatalytic hydrogen evolution, Chem. Eng. J., 2021, 408(15), 127270 CrossRef CAS.
- P. P. Yan, Y. C. Wu, X. F. Wei, X. W. Zhu and S. Wei, Preparation of Robust Hydrogen Evolution Reaction Electrocatalyst WC/C by Molten Salt, Nanomaterials, 2020, 10, 1621 CrossRef CAS.
- C. Singh, A. Haldar, O. Basu and S. K. Das, Devising a Polyoxometalate-Based Functional Material as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction, Inorg. Chem., 2021, 10(021), 1c00734 Search PubMed.
- T. Wang, M. Xu, W. L. Chen, X. H. Li, F. R. Li, X. L. Wang, Y. W. Li, D. Liu and E. B. Wang, Polyoxometalate-derived multi-component X/W2C@X, N–C (X = Co, Si, Ge, B and P) nanoelectrocatalysts for efficient triiodide reduction in dye-sensitized solar cells, Chem. – Eur. J., 2019, 26(18), 4104–4111 Search PubMed.
- S. Zhao and X. Zhao, Polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework for photocatalytic hydrogen peroxide production under visible light, J. Catal., 2018, 366, 98–106 CrossRef CAS.
- H. J. Yan, C. G. Tian, L. Wang, A. P. Wu, M. C. Meng, L. Zhao and H. G. Fu, Phosphorus-Modified Tungsten Nitride/Reduced Graphene Oxide as a High-Performance, Non-Noble-Metal Electrocatalyst for the Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2015, 54(21), 6325–6329 CrossRef CAS.
- J. J. Chen, M. D. Symes and L. Cronin, Highly reduced and protonated aqueous solutions of [P2W18O62]6− for on-demand hydrogen generation and energy storage, Nat. Chem., 2018, 10, 1042–1047 CrossRef CAS PubMed.
- S. Emin, C. Altinkaya, A. Semerci, H. Okuyucu, A. Yildiz and P. Stefanov, Tungsten carbide electrocatalysts prepared from metallic tungsten nanoparticles for efficient hydrogen evolution, Appl. Catal., B, 2018, 236(15), 147–153 CrossRef CAS.
- Y. Zhang, J. Liu, S. L. Li, Z. M. Su and Y. Q. Lan, Polyoxometalate-based Materials for Sustainable and Clean Energy Conversion and Storage, EnergyChem, 2019, 1(3), 100021 CrossRef.
- R. G. Finke, M. W. Droege and P. J. Domaille, Trivacant Heteropolytungstate Derivatives. 3.1 Rational Syntheses, Characterization, Two-Dimensional 183W NMR, and Properties of P2W18M4(H2O)2O6810− and P4W30M4(H2O)2O11216− (M = Co, Cu, Zn), Inorg. Chem., 1987, 26, 3886–3896 CrossRef CAS.
- X. Li, X. L. Hu, X. L. Wang, Q. Q. Pan and Z. M. Su, Substrate-free Mo2C-based electrocatalyst by facile glucose-blowing for efficient hydrogen production, New J. Chem., 2017, 43, 18970–18974 RSC.
- J. Deng, P. J. Ren, D. H. Deng and X. H. Bao, Enhanced Electron Penetration through an Ultrathin Graphene Layer for Highly Efficient Catalysis of the Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2015, 54(7), 2100–2104 CrossRef CAS PubMed.
- S. Wang, H. Jang, J. Wang, Z. X. Wu, X. Liu and J. Cho, Cobalt–Tannin-Framework-Derived Amorphous Co–P/Co–N–C on N, P Co-Doped Porous Carbon with Abundant Active Moieties for Efficient Oxygen Reactions and Water Splitting, ChemSusChem, 2021, 12(4), 830–838 CrossRef.
- X. Zhang, S. W. Liu, Y. P. Zang, R. R. Liu, G. Q. Liu, G. Z. Wang, Y. X. Zhang, H. M. Zhang and H. J. Zhao, Co/Co9S8@S,N-Doped Porous Graphene Sheets Derived from S, N Dual Organic Ligands Assembled Co-MOFs as Superior Electrocatalysts for Full Water Splitting in Alkaline Media, Nano Energy, 2016, 30, 93–102 CrossRef CAS.
- W. Su, P. P. Yan, X. F. Wei, X. W. Zhu and Y. Y. Zhou, Facile one-step synthesis of nitrogen-doped carbon sheets supported tungsten carbide nanoparticles electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2020, 45, 33430–33439 CrossRef CAS.
- Y. Gao, Z. L. Lang, F. Y. Yu, H. Q. Tan, G. Yan, Y. H. Wang, Y. Y. Ma and Y. G. Li, A Co2P/WC Nano-Heterojunction Covered with N-Doped Carbon as Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction, ChemSusChem, 2017, 11(6), 1082–1091 CrossRef.
- J. Li, H. Y. Zheng, C. Y. Xu, Z. M. Su, X. Li and J. Sun, Bimetallic Phosphides as High-Efficient Electrocatalysts for Hydrogen Generation, Inorg. Chem., 2021, 60, 1624–1630 CrossRef CAS.
- S. Yeo, K. Nandi, R. Rahul, H. Kim, B. Shong, Y. Jang, J. S. Bae, J. W. Han, S. H. Kim and H. Kim, Low-temperature direct synthesis of high quality WS2 thin films by plasma-enhanced atomic layer deposition for energy related applications, Appl. Surf. Sci., 2018, 459(30), 596–605 CrossRef CAS.
- X. Zhang, S. W. Liu, Y. P. Zang, R. R. Liu, H. Q. Liu, G. Z. Wang, Y. X. Zhang, H. M. Zhang and H. J. Zhao, Co/Co9S8@S,N-doped porous graphene sheets derived from S, N dual organic ligands assembled Co-MOFs as superior electrocatalysts for full water splitting in alkaline media, Nano Energy, 2016, 30, 93–102 CrossRef CAS.
- S. A. Patil, S. Cho, Y. Jo, N. K. Shrestha, H. Kim and H. Im, Bimetallic Ni-Co@hexacyano nano-frameworks anchored on carbon nanotubes for highly efficient overall water splitting and urea decontamination, Chem. Eng. J., 2021, 426(15), 130773 CrossRef CAS.
- N. K. Shrestha, S. A. Patil, S. Cho, Y. Jo, H. Kim and H. Im, Cu–Fe–NH2 based metal-organic framework nanosheets via dropcasting for highly efficient oxygen evolution catalysts durable at ultrahigh currents, J. Mater. Chem. A, 2020, 8, 24408–24418 RSC.
- S. A. Patil, N. K. Shrestha, S. Hussain, J. W. Jung, S. W. Lee, C. Bathula, A. N. Kadam, H. Im and H. Kim, Catalytic decontamination of organic/inorganic pollutants in water and green H2 generation using nanoporous SnS2 micro-flower structured film, J. Hazard. Mater., 2021, 417(5), 126105 CrossRef CAS PubMed.
- W. J. Liu, X. L. Wang and Y. Q. Lan, Polyoxometalates Assemblies and Their Electrochemical Applications, Polyoxometalate-Based Assemblies and Functional Materials, 2017, 176, 89–119 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, characterizations, electrochemical measurements, XRD, IR spectras, Raman spectra, XPS, N2 adsorption–desorption isotherms, LSV, CV, Cdl and EIS. See DOI: 10.1039/d1nj04573c |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |