Dissolution-enhanced emission of 1,3,6,8-tetrakis(p-benzoic acid)pyrene for selectively detecting protamine and “on-to-on” heparin detection in water†
Hongtao
Li‡
 ,
Yuting
Zhang‡
,
Yan
Huang
*,
Dapeng
Cao
,
Yuting
Zhang‡
,
Yan
Huang
*,
Dapeng
Cao
 and
Shitao
Wang
and
Shitao
Wang
 *
*
State Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: huangyan@buct.edu.cn; stwang@buct.edu.cn
First published on 22nd November 2021
Abstract
We developed a small organic molecule, 1,3,6,8-tetrakis(p-benzoic acid)pyrene (TBAPy), as a facile turn-on fluorescence probe for selectively detecting the basic protein protamine in water with a low detection limit of 24.14 ng mL−1. The TBAPy–protamine system could be further used as an “on-to-on” probe to detect the heparin protein with great fluorescence enhancement and simultaneous color change from green to blue. It can be explained by dissolution-enhanced emission (DEE) that the insoluble TBAPy probe is deprotonated to form the anionic species and dissolve in water, which suppresses the aggregation-caused quenching by electrostatic repulsion interactions. This DEE phenomenon is different from the widely reported aggregation-induced emission (AIE). These facile fluorescence “turn-on” and “on-to-on” methods for protein detection suggest that the DEE phenomenon is a very useful strategy for biosensing and can be potentially applied in food safety, disease diagnosis and healthcare.
1. Introduction
As one of the most important and indispensable substances for cells, bio-tissues and organs, proteins play a key role in life activities and a lack of them would lead to many diseases and even death.1–3 For instance, protamine is a type of basic protein extracted from fish sperm cells and has been widely used in many fields such as the food industry and medical care.4–7 In the food industry, protamine can inhibit the growth of most microorganisms in food and has antiseptic and antibacterial properties.8–10 In the field of human health care, protamine can lower blood pressure, help breathing, promote digestion, inhibit blood coagulation, and improve the long-acting effect of insulin.11–15 By monitoring proteins, we can get useful information to determine whether the body's various functions are normal. Thus, the detection and analysis of proteins is of great significance and necessary in health-related fields.At present, many methods for detecting proteins have been developed, including the organic dye method,16–18 absorption spectroscopy,19–23 high performance liquid chromatography,24 and electrochemical assay.25 However, although these methods have certain feasibility, their shortcomings such as long reaction time, complicated operation, and low sensitivity make them not suitable for practical application.26–31 In recent years, fluorescent sensing has received increasing attention due to its low cost, simple operation, high sensitivity and intuitive display through the change of color and emission intensity of fluorescent probes.32–36 Constructing easy-to-operate fluorescence probes for sensitively and selectively detecting proteins is very desirable and meaningful. Currently, plentiful research has been devoted to fluorescence quenching (“turn-off” type) probes for detecting proteins. However, compared with fluorescence turn-off probes, the “turn-on” counterparts usually have higher credibility as they have a higher signal-to-noise ratio that can avoid misleading responses arising from objective factors. Therefore, turn-on probes are more suitable for practical applications in sensing proteins. For instance, George et al. used bovine serum albumin as a capping agent and a reducing agent to synthesize blue-emitting fluorescent copper nanoclusters (BSA-CuNCs). The fluorescence of the copper nanoclusters was found to be further enhanced after the addition of protamine.37 Song et al. developed a novel sensor based on 3-mercaptopropionic acid-capped AgInZnS quantum dots (AIZS QDs) for the detection of protamine. After the addition of protamine, the fluorescence intensity of the AIZS QDs/protamine system gradually increased, and the limit of detection (LOD) of the AIZS QDs for protamine was 0.13 μg mL−1.38 Liu et al. constructed a BODIPY-based probe encapsulated by a zeolitic imidazolate framework (BZA-BOD@ZIF-90), which can efficiently detect protamine by taking advantage of the aggregation-induced emission (AIE) effect. When protamine was incubated with BZA-BOD@ZIF-90 in phosphate-buffered saline (PBS) (pH = 7.4) and acetonitrile solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume), intensive green fluorescence of BZA-BOD@ZIF-90/protamine could be observed.39 Singh et al. employed a tetraphenylethene derivative-based anionic probe, BSPOTPE, for sensing protamine. Attributed to the AIE effect of BSPOTPE on interaction with protamine, BSPOTPE showed a turn-on emission response to protamine with a 70-fold intensity increase.40 However, these fluorescence probes either contain metal ions that are harmful to living organisms or they need to be tested in toxic organic solvents, resulting in biotoxicity and/or poor biocompatibility; therefore, they are not suitable for in vivo testing. These drawbacks greatly limit their simplicity and practical application. On the other hand, most of these probes are designed based on AIE-type molecules. So it would be of great significance to develop some other non-AIE probes to expand the diversity and broaden the potential applications of fluorescence probes in protein sensing and its related fields.
1, by volume), intensive green fluorescence of BZA-BOD@ZIF-90/protamine could be observed.39 Singh et al. employed a tetraphenylethene derivative-based anionic probe, BSPOTPE, for sensing protamine. Attributed to the AIE effect of BSPOTPE on interaction with protamine, BSPOTPE showed a turn-on emission response to protamine with a 70-fold intensity increase.40 However, these fluorescence probes either contain metal ions that are harmful to living organisms or they need to be tested in toxic organic solvents, resulting in biotoxicity and/or poor biocompatibility; therefore, they are not suitable for in vivo testing. These drawbacks greatly limit their simplicity and practical application. On the other hand, most of these probes are designed based on AIE-type molecules. So it would be of great significance to develop some other non-AIE probes to expand the diversity and broaden the potential applications of fluorescence probes in protein sensing and its related fields.
Herein, we have reported a small organic molecule 1,3,6,8-tetrakis(p-benzoic acid)pyrene (TBAPy) that could be used as a facile fluorescence “turn-on” probe for selectively detecting protamine in the aqueous phase. Interestingly, we found that the TBAPy–protamine system could be further used as an “on-to-on” fluorescence probe to detect another important protein, heparin, with obvious fluorescence enhancement and simultaneous color change from green to blue. This phenomenon was further explained by the dissolution-enhanced emission (DEE) mechanism reported by our group previously, wherein the basic proteins promote the dissolution of the TBAPy probe in aqueous solution and show stronger fluorescence emission,41 which is different from the widely reported aggregation-induced emission (AIE) phenomenon. These facile fluorescence “turn-on” and “on-to-on” methods for protein detection suggest that the DEE phenomenon could be a very useful strategy for biosensing and has great potential applications, such as in food safety, disease diagnosis and healthcare.
2. Experimental section
2.1. Materials
The proteins used in this work are: hemoglobin (Hem.), albumin (Alb.), lysozyme (Lys.), bovine serum albumin (B.S.A.), pepsin (Pep.), α-chymotrypsin (Chy.), cytochrome C (Cyt. C.), protamine (Pro.) and heparin (sodium salt) (Hep.). They were purchased from Sigma-Aldrich (Merck KGaA) and used as received. The fluorescence probe 1,3,6,8-tetrakis(p-benzoic acid)pyrene (TBAPy) was prepared according to the method in the literature.422.2. Apparatus
The fluorescent spectra were recorded by a Hitachi F-7000 fluorescence spectrophotometer. UV-Vis absorption spectra were measured using a Puxi TU-1901 spectrophotometer. 1H NMR spectra data were collected on a Bruker Advance spectrometer (600 MHz). Quantum yields were measured by a steady-state/transient fluorescence spectrometer Edinburgh FLS1000.2.3. Protein fluorescence detection experiment
Protein aqueous solutions (5 mg mL−1) were first prepared by dispersing proteins in deionized water (pH = 7) and ultra-sonicating them until completely dissolved. All the protein concentrations in this work were kept at 5 mg mL−1. TBAPy powder (2 mg) was suspended in deionized water (100 mL) and ultra-sonicated for ten minutes for thorough dispersion. 3 mL TBAPy suspended solution (0.02 mg mL−1) was taken into a cuvette and the fluorescence emission spectra were measured before and after adding 30 μL of protein aqueous solutions to the above TBAPy dispersed solution.2.4. Anti-interference experiments
First, protein aqueous solutions (30 μL) were added to 3 mL TBAPy suspended solution in a cuvette, and the fluorescence emission spectra were recorded. Then, 30 μL protamine aqueous solution was added to the above mixed solution and the fluorescence emission spectra were recorded again. The recorded fluorescence emission spectra included (A) TBAPy suspended aqueous solution; (B) TBAPy + interfering proteins except protamine; (C) TBAPy + interfering proteins + protamine.3. Results and discussion
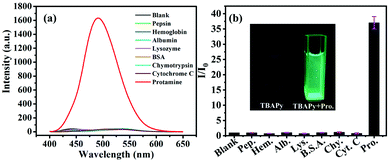
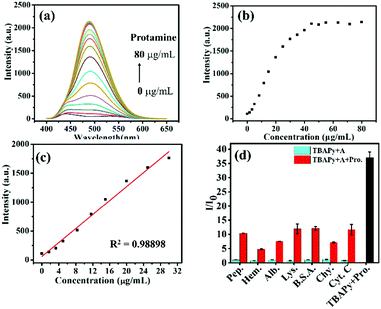
The fluorescence properties of TBAPy were studied in deionized water solution. TBAPy has poor solubility in water and has no obvious fluorescence emission. Our previous work found that TBAPy could be dissolved in an alkaline aqueous environment by deprotonation and shows strong fluorescence characteristics by suppressing the aggregation-caused quenching (ACQ) effect, which was reported as the dissolution-enhanced emission (DEE) mechanism.41 It is expected that the DEE phenomenon is also suitable for detecting basic proteins. We selected a total of eight proteins, i.e. pepsin, hemoglobin, albumin, lysozyme, bovine serum albumin, α-chymotrypsin, cytochrome C and protamine. Their corresponding isoelectric points (pI) are 2.0, 4.5, 4.7, 6.99, 7.8, 8.76, 10.7 and 12.1, respectively.43–45 The pI value is related to the strength of alkalinity. In general, the higher the pI value, the stronger the alkalinity of the protein. The fluorescence sensing properties of TBAPy (0.02 mg mL−1) for detecting these eight proteins (5 mg mL−1, all proteins are water-soluble) in neutral water (pH = 7) are shown in Fig. 1a and b. The emission intensities showed negligible change after adding the first 7 proteins, suggesting that the weak basicity of these 7 proteins could not deprotonate TBAPy and promote its dissolution. However, after adding protamine, the solution showed obvious fluorescence turn-on emission with a maximum emission peak at 490 nm, and the intensity was enhanced about 40 times, suggesting the high selectivity of the TBAPy probe for detecting protamine. The response time of TBAPy to protamine is around 3 s, and the solution showed strong green fluorescence under an ultraviolet light lamp (λex = 365 nm) (Fig. 1b inset), suggesting that TBAPy possesses a real-time fluorescent turn-on property for protamine.In order to evaluate the sensitivity of TBAPy for protamine detection, a fluorescence titration experiment was carried out. With the continuous addition of protamine, the intensity of the fluorescence spectra increased gradually (Fig. 2a) and it presents a good linear relationship with the concentration of protamine (R2 = 0.98898) (Fig. 2b and c) in the range of 0–30 μg mL−1. The limit of detection (LOD) of TBAPy for detecting protamine was then calculated to be 24.14 ng mL−1 by the formula LOD = 3σ/K, where σ is the standard deviation of blank fluorescence intensity of the TBAPy probe solution and K is the slope value of the calibration curve (Fig. S2, ESI†). The LOD value is much lower than that of other reported probes (Table S1, ESI†), suggesting the high sensitivity of the TBAPy probe for detecting protamine. In addition, anti-interference is another necessary property in the actual detection environment, as the actual environment is more complex and has a lot of uncertain interference factors. A competition experiment was also performed by measuring the fluorescence change in the presence of the other 7 proteins. As shown in Fig. 2d, when the solution contained both protamine and one of the other competitive proteins, it still showed obvious fluorescence enhanced performance (5–10 times enhancement), suggesting that the TBAPy probe has good anti-interference ability (Fig. S1, ESI†).
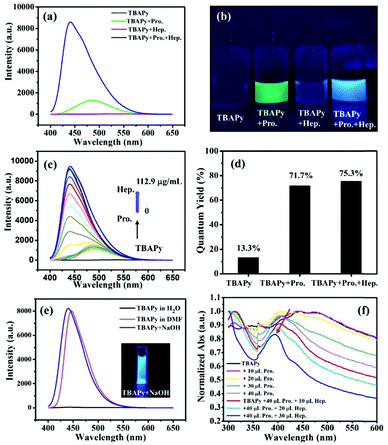
Heparin is another important protein that commonly exists in tissues such as the lungs, blood vessel walls, and intestinal mucosa. It is a natural anticoagulant substance and clinically used for cardiovascular surgery, cardiac catheterization, extracorporeal circulation, hemodialysis, etc.46,47 Heparin is negatively charged and can strongly bind to protamine to form a quite stable ion-pair complex through electrostatic interaction.48 Hence, the TABPy–protamine system is an excellent candidate for selectively detecting heparin. Interestingly, we found that as heparin was gradually added into the TBAPy–protamine aqueous solution, the fluorescence intensity first slightly increased, and then had a linear increasing relationship with the concentration of heparin in the range of 30–60 μg mL−1, and finally achieved saturation when the heparin concentration was over 60 μg mL−1 (Fig. 3c and Fig. S3, ESI†). The intensity at the saturated state was almost 7 times higher than that for the initial TBAPy–protamine, and was also more than 200 times enhanced compared with the initial TBAPy in water (Fig. 3a). Importantly, the maximum emission peak was blue-shifted from 490 nm (TBAPy–protamine solution) to 440 nm, and an obvious color change from green to blue was observed under a portable UV lamp (λex = 365 nm) (Fig. 3b). In contrast, when heparin was directly added into the TBAPy suspended aqueous solution without protamine, almost no emission enhancement happened, revealing that heparin has almost no interaction with TBAPy but has strong interaction with protamine. In addition, the quantum yields of the TBAPy suspension dramatically increased from 13.3% to 71.7% after adding protamine, and were slightly enhanced to 75.3% on further adding heparin (Fig. 3d), indicating that protamine had already deprotonated TBAPy before adding heparin, and heparin had little interaction with TBAPy.
The TBAPy probe is completely dissolved in DMF solvent and presents a strong blue fluorescence emission with a maximum peak at 440 nm. Basic analytes such as NaOH could also turn on the emission of TBAPy in aqueous solution with blue fluorescence, and the peak shape and maximum peak positions are almost the same as that in the DMF solution (Fig. 3e). According to the DEE mechanism, TBAPy was deprotonated by NaOH and dissolved in the aqueous solution. When heparin was added into the TBAPy–protamine system in aqueous solution, the fluorescence emission was recovered from green (maximum peak at 490 nm) to blue with maximum peaks at 440 nm, which is the same as that of TBAPy in DMF and the TBAPy–NaOH system in aqueous solution. Therefore, it can confirm that the fluorescence emission and change in the TBAPy–protamine–heparin system is derived from the deprotonation and dissolution of TBAPy.
To further explore the internal mechanism of the TBAPy probe for the detection of protamine and heparin, we used the proton nuclear magnetic resonance spectroscopy (1H NMR, 600 MHz, D2O) technique for further analysis. When the TBAPy probe was dispersed in the D2O solvent, no signals except the solvent peak at 4.79 ppm in the proton NMR spectrum were detected (Fig. 4a), indicating that TBAPy was almost undissolved in water. After the protamine was added, the solution was suspended (Fig. S4, ESI†), and the NMR spectrum showed that the signals in the nonaromatic regions (chemical shift δ < 7 ppm) which belong to the excess protamine were observed, but there were still no signals in the aromatic regions (δ > 7 ppm) (Fig. 4b and d), suggesting that the TBAPy–protamine complex was not dissolved but suspended in the water. Moreover, we found that the solution of the TBAPy–protamine system would become transparent after standing for several days and the insoluble precipitates were sunk on the bottom (Fig. S5, ESI†). We separated the upper clear aqueous solution and the insoluble precipitate by filtration and found that the upper solution had negligible fluorescence intensity (Fig. S6a and b, ESI†). However, the precipitate from the bottom had strong green fluorescence in the solid state under a UV lamp, consistent with the green emission of the TBAPy–protamine system in aqueous solution (Fig. S6c, ESI†). This clearly demonstrates that the fluorescence emission of the TBAPy–protamine system in the aqueous phase is ascribed to the complex dispersion but not the dissolution. Considering the fact that the TBAPy probe and protamine in their respective solids have negligible fluorescence emission, the fluorescence of the precipitates indicates that TBAPy is tightly combined with protamine to form a stable complex, and also suggests that TBAPy could be used in fluorescent labelling for protamine.
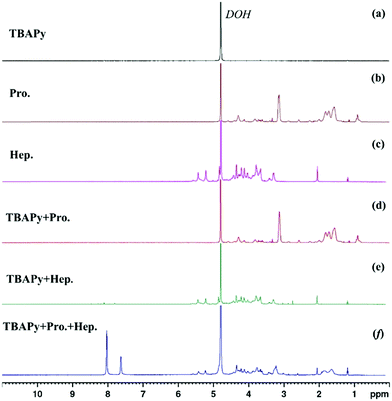 | ||
| Fig. 4 1H NMR spectra (600 MHz, 298 K, D2O) of (a) TBAPy; (b) protamine (Pro.); (c) heparin (Hep.); (d) TBAPy + Pro.; (e) TBAPy + Hep.; (f) TBAPy + Pro. + Hep. in D2O solvent. | ||
After adding heparin to the TBAPy–protamine system, heparin can strongly bind with protamine, and the heparin–protamine complex was formed quickly and precipitated to the bottom of the NMR tube after a while. The upper D2O solution became clear and transparent (Fig. S5, ESI†). The NMR spectrum showed that there were several obvious peaks in the aromatic region (7.5–8.5 ppm) which belong to the aromatic TBAPy compound, clearly suggesting that TBAPy was deprotonated and dissolved (Fig. 4f), which was further demonstrated by the fact that the upper transparent solution still showed strong fluorescence intensity with obvious blue emission under a UV lamp after the removal of the precipitate by filtration (Fig. S6a and b, ESI†). For comparison, when only heparin was added into TBAPy suspended in D2O solvent, no signal was observed for TBAPy (Fig. 4c and e), revealing that the dissolution of TBAPy in the TABPy–protamine–heparin system arose from the alkaline protamine rather than the non-alkaline heparin.
Ultraviolet-visible absorption spectra were also used here. As shown in Fig. 3f, after gradually adding protamine to TBAPy suspended in aqueous solution, the absorbance peak was blue-shifted, suggesting that the π–π stacking between TBAPy molecules is weakened by deprotonation.44 However, the NMR spectrum results showed that TBAPy was not dissolved in the aqueous solution after adding protamine. This is probable because deprotonated TBAPy is an anionic species, and it could further bind with protamine by electrostatic interaction and result in coagulation. After adding heparin, the absorbance peaks were still blue-shifted, suggesting that the π–π stacking among the TBAPy molecules was further weakened, which could be attributed to the fact that the strong combination of heparin and protamine totally released the TBAPy anionic species into the water and the electrostatic repulsion interactions reduced the molecular close packing.
Based on the above phenomenon and discussion, we can infer the possible detection mechanism (Fig. 5). The TBAPy probe has poor solubility in neutral water and shows no fluorescence emission due to the strong ACQ effect. On adding the basic protamine, TBAPy can be deprotonated to form the TBAPy− anionic species that is an electrolyte and can further quickly bind to the positively charged protamine via electrostatic interactions to form a coagulated TBAPy–protamine complex, which is insoluble in water. However, due to the repulsion between carboxyl anions, the π–π stacking effect of the deprotonated TBAPy− is weakened, and the ACQ effect is inhibited, which would contribute to the fluorescence enhancement. On the other hand, an intramolecular charge transfer in the TBAPy–protamine complex might exist, which would result in the green fluorescence emission of the TBAPy–protamine complex dispersion. After heparin is further added, since heparin has a much stronger binding affinity to protamine, protamine and heparin quickly form the protamine–heparin pair species and totally release the TBAPy− anionic species into the water. Then, the intrinsic blue emission of the free TBAPy− anionic species is displayed by the DEE mechanism, i.e., the repulsive interactions of the carboxyl anion species reduce the π–π interactions among the pyrene cores and suppress the aggregation-caused quenching effect.
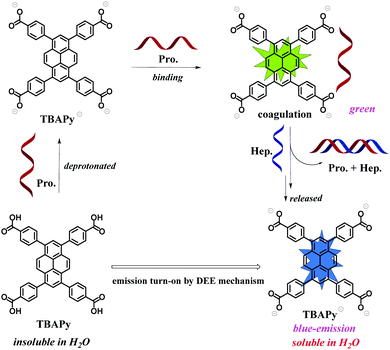 | ||
| Fig. 5 Schematic graph of the dissolution-enhanced emission (DEE) of TBAPy for detecting protamine and heparin in aqueous solution. | ||
4. Conclusions
In summary, we found that the small molecule TBAPy could be a facile and real-time fluorescence “turn-on” probe for detecting the basic bio-protein, protamine, in aqueous solution with around 40 times fluorescence intensity enhancement and a very low detection limit of 24.14 ng mL−1, as well as excellent selectivity, sensitivity and anti-interference abilities. In addition, the TBAPy–protamine system could be further used as an “on-to-on” fluorescence probe to effectively detect heparin with around a further 7 times fluorescence enhancement and an obvious color change from green to blue. The 1H NMR spectra, absorption spectra, and fluorescence emission spectra prove that the detection mechanism is the dissolution-enhanced emission (DEE) mechanism, unlike the previously reported aggregation-induced emission (AIE) fluorescence phenomena. This work can open up new ideas for the detection of bio-proteins and DEE fluorescence phenomena could yield great potential applications in the life science and healthcare fields, such as in food safety, in vivo health detection and disease diagnosis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22005017, 21978022) and the Fundamental Research Funds for the Central Universities (buctrc201918).References
- H. Mao, L. Li, Q. Fan, A. Angelini, P. K. Saha, H. Wu, C. M. Ballantyne, S. M. Hartig, L. Xie and X. Pi, Nat. Commun., 2021, 12, 1927 CrossRef PubMed.
- A. Quijano-Rubio, H. W. Yeh, J. Park, H. Lee, R. A. Langan, S. E. Boyken, M. J. Lajoie, L. Cao, C. M. Chow, M. C. Miranda, J. Wi, H. J. Hong, L. Stewart, B. H. Oh and D. Baker, Nature, 2021, 591, 482–487 CrossRef CAS PubMed.
- M. Mannes, C. Martin, S. Triest, M. Pia Dimmito, A. Mollica, T. Laeremans, C. J. Menet and S. Ballet, Angew. Chem., Int. Ed., 2021, 60, 10247–10254 CrossRef CAS PubMed.
- D. Zhou, Y. Wu, P. Liu, H. Bai, L. Tang, R. Yu and J. Jiang, Sci. China: Chem., 2011, 54, 1277–1283 CrossRef CAS.
- X. Yu, L. Gong, J. Zhang, Z. Zhao, X. Zhang and W. Tan, Sci. China: Chem., 2017, 60, 1318–1323 CrossRef CAS.
- D. A. Baker, L. B. Stewart, J. M. Large, P. W. Bowyer, K. H. Ansell, M. B. Jimenez-Diaz, M. El Bakkouri, K. Birchall, K. J. Dechering, N. S. Bouloc, P. J. Coombs, D. Whalley, D. J. Harding, E. Smiljanic-Hurley, M. C. Wheldon, E. M. Walker, J. T. Dessens, M. J. Lafuente, L. M. Sanz, F. J. Gamo, S. B. Ferrer, R. Hui, T. Bousema, I. Angulo-Barturen, A. T. Merritt, S. L. Croft, W. E. Gutteridge, C. A. Kettleborough and S. A. Osborne, Nat. Commun., 2017, 8, 430 CrossRef PubMed.
- X. Chen, Z. Hao and P. R. Chen, Sci. China: Chem., 2011, 55, 106–111 CrossRef.
- T. Hata, T. Sato, T. Ichikawa and Y. Morimitsu, J. Food Process. Pres., 2016, 40, 1180–1187 CrossRef CAS.
- D. Zhao, X. Liu, R. Zhang, X. Huang and X. Xiao, New J. Chem., 2021, 45, 1010–1019 RSC.
- Y. Y. Liu, X. F. Chen, J. W. Hu, Z. W. Chen, L. J. Zhang, M. J. Cao and G. M. Liu, J. Agric. Food Chem., 2016, 64, 1999–2011 CrossRef CAS PubMed.
- H. P. Chase, B. Dixon, J. Pearson, R. Fiallo-Scharer, P. Walravens, G. Klingensmith, M. Rewers and S. K. Garg, J. Pediatr., 2003, 143, 737–740 CrossRef CAS.
- G. Derosa, I. Franzetti, F. Querci, D. Romano, A. D'Angelo and P. Maffioli, Diabetes. Obes. Metab., 2015, 17, 554–559 CrossRef CAS PubMed.
- M. S. Thu, L. H. Bryant, T. Coppola, E. K. Jordan, M. D. Budde, B. K. Lewis, A. Chaudhry, J. Ren, N. R. Varma, A. S. Arbab and J. A. Frank, Nat. Med., 2012, 18, 463–467 CrossRef CAS PubMed.
- J. Lu, W. Ji, M. Zhao, M. Wang, W. Yan, M. Chen, S. Ren, B. Yuan, B. Wang and L. Chen, Sci. Rep., 2016, 6, 26563 CrossRef CAS PubMed.
- S. Pangeni, J. D. Prajapati, J. Bafna, M. Nilam, W. M. Nau, U. Kleinekathofer and M. Winterhalter, Angew. Chem., Int. Ed., 2021, 60, 8089–8094 CrossRef CAS PubMed.
- B. Riond, F. Steffen, O. Schmied, R. Hofmann-Lehmann and H. Lutz, Vet. Clin. Pathol., 2014, 43, 78–88 CrossRef CAS PubMed.
- F. Rashid, V. S. Raducanu, M. S. Zaher, M. Tehseen, S. Habuchi and S. M. Hamdan, Nat. Commun., 2019, 10, 2104 CrossRef PubMed.
- S. Chatterjee and G. S. Kumar, RSC. Adv., 2014, 4, 42706–42715 RSC.
- A. Cannizzo, Phys. Chem. Chem. Phys., 2012, 14, 11205–11223 RSC.
- R. Antoine and P. Dugourd, Phys. Chem. Chem. Phys., 2011, 13, 16494–16509 RSC.
- J. Woodhouse, G. Nass Kovacs, N. Coquelle, L. M. Uriarte, V. Adam, T. R. M. Barends, M. Byrdin, E. de la Mora, R. Bruce Doak, M. Feliks, M. Field, F. Fieschi, V. Guillon, S. Jakobs, Y. Joti, P. Macheboeuf, K. Motomura, K. Nass, S. Owada, C. M. Roome, C. Ruckebusch, G. Schiro, R. L. Shoeman, M. Thepaut, T. Togashi, K. Tono, M. Yabashi, M. Cammarata, L. Foucar, D. Bourgeois, M. Sliwa, J. P. Colletier, I. Schlichting and M. Weik, Nat. Commun., 2020, 11, 741 CrossRef CAS PubMed.
- T. S. Bui, T. D. Dao, L. H. Dang, L. D. Vu, A. Ohi, T. Nabatame, Y. Lee, T. Nagao and C. V. Hoang, Sci. Rep., 2016, 6, 32123 CrossRef CAS PubMed.
- V. Mittal, M. Nedeljkovic, L. G. Carpenter, A. Z. Khokhar, H. M. H. Chong, G. Z. Mashanovich, P. N. Bartlett and J. S. Wilkinson, ACS Sens., 2019, 4, 1749–1753 CrossRef CAS PubMed.
- A. Hvass and B. Skelbaek-Pedersen, J. Pharm. Biomed. Anal., 2005, 37, 551–557 CrossRef CAS PubMed.
- S. P. Pandey and P. K. Singh, J. Mol. Liq., 2020, 303, 112589 CrossRef CAS.
- P. D. Katsoulos, L. V. Athanasiou, M. A. Karatzia, N. Giadinis, H. Karatzias, C. Boscos and Z. S. Polizopoulou, Vet. Clin. Pathol., 2017, 46, 620–624 CrossRef PubMed.
- S. Prabha, D. Durgalakshmi, K. Subramani, P. Aruna and S. Ganesan, ACS Appl. Mater. Interfaces, 2020, 12, 19245–19257 CrossRef CAS PubMed.
- J. Dong, G. Chen, W. Wang, X. Huang, H. Peng, Q. Pu, F. Du, X. Cui, Y. Deng and Z. Tang, Anal. Chem., 2018, 90, 7107–7111 CrossRef CAS PubMed.
- K. Vus, U. Tarabara, A. Kurutos, O. Ryzhova, G. Gorbenko, V. Trusova, N. Gadjev and T. Deligeorgiev, Mol. Biosyst., 2017, 13, 970–980 RSC.
- O. Julien, S. Chatterjee, T. C. Bjorndahl, B. Sweeting, S. Acharya, V. Semenchenko, A. Chakrabartty, E. F. Pai, D. S. Wishart, B. D. Sykes and N. R. Cashman, Biochemistry, 2011, 50, 7536–7545 CrossRef CAS PubMed.
- S. J. Boardman, R. Lad, D. C. Green and P. D. Thornton, J. Appl. Polym. Sci., 2017, 134, 44846 CrossRef.
- Z. Qing, L. Zhu, L. Hou, Z. Zou, S. Yang and R. Yang, Sci. China: Chem., 2018, 61, 1630–1636 CrossRef CAS.
- J. Bai, Y. Luo, X. Wang, S. Li, M. Luo, M. Yin, Y. Zuo, G. Li, J. Yao, H. Yang, M. Zhang, W. Wei, M. Wang, R. Wang, C. Fan and Y. Zhao, Nat. Commun., 2020, 11, 3847 CrossRef PubMed.
- T. Kakizuka, A. Takai, K. Yoshizawa, Y. Okada and T. M. Watanabe, Chem. Commun., 2020, 56, 3625–3628 RSC.
- N. Zhao, Y. Li, W. Yang, J. Zhuang, Y. Li and N. Li, Chem. Sci., 2019, 10, 9009–9016 RSC.
- H. Narita, L. Catti and M. Yoshizawa, Angew. Chem., Int. Ed., 2021, 60, 12791–12795 CrossRef CAS PubMed.
- R. S. Aparna, J. S. Anjali Devi, R. R. Anjana, J. Nebu and S. George, Analyst, 2019, 144, 1799–1808 RSC.
- Y. B. Liu, F. M. Zhang, X. He, P. Y. Ma, Y. B. Huang, S. Tao, Y. Sun, X. H. Wang and D. Q. Song, Sens. Actuators, B, 2019, 294, 263–269 CrossRef CAS.
- L. Liu, J. Dai, Y. Ji, B. Shen, X. Zhang and R. J. Linhardt, Sens. Actuators, B, 2021, 341, 130006 CrossRef CAS.
- S. P. Pandey, P. Jha and P. K. Singh, J. Mol. Liq., 2020, 315, 113625 CrossRef CAS.
- J. S. Hao, M. Wang, S. T. Wang, Y. Huang and D. P. Cao, Dyes Pigm., 2020, 175, 108131 CrossRef CAS.
- J. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. DeMarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 10294–10297 CrossRef CAS PubMed.
- A. Y. Solovyev, S. I. Tarnovskaya, I. A. Chernova, L. K. Shataeva and Y. A. Skorik, Int. J. Biol. Macromol., 2015, 78, 39–45 CrossRef CAS PubMed.
- X. F. Wang, Q. Jiang, Y. Man, S. Feng, Y. I. Lee and H. G. Liu, Sens. Actuators, B, 2018, 261, 233–240 CrossRef CAS.
- S. Raghava, P. K. Singh, A. Ranga Rao, V. Dutta and M. N. Gupta, J. Mater. Chem., 2009, 19, 830–2834 RSC.
- Q. Chen, X. Li, R. Wang, F. Zeng, J. Zhai and X. Xie, Anal. Chem., 2019, 91, 10390–10394 CrossRef CAS PubMed.
- K. Pu, R. Zhan, J. Liang and B. Liu, Sci. China: Chem., 2011, 54, 567–574 CrossRef CAS.
- A. Vergori, P. Lorenzini, A. Cozzi-Lepri, D. R. Donno, G. Gualano, E. Nicastri, F. Iacomi, L. Marchioni, P. Campioni, V. Schinina, S. Cicalini, C. Agrati, M. R. Capobianchi, E. Girardi, G. Ippolito, F. Vaia, N. Petrosillo, A. Antinori, F. Taglietti and C. S. G. Re, Sci. Rep., 2021, 11, 11334 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj03946f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |