Open Access Article
Open Access ArticleA N-doped NbOx nanoparticle electrocatalyst deposited on carbon black for oxygen reduction and evolution reactions in alkaline media†
Jeongsuk
Seo
 *a,
Won-Jin
Moon
b,
Wan-Gil
Jung
b and
Jun-Woo
Park
c
*a,
Won-Jin
Moon
b,
Wan-Gil
Jung
b and
Jun-Woo
Park
c
aDepartment of Chemistry, College of Natural Sciences, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju 61186, Republic of Korea. E-mail: j_seo@chonnam.ac.kr
bKorea Basic Science Institute, Gwangju Center, 77 Yongbong-ro, Buk-gu, Gwangju 61186, Republic of Korea
cNext-Generation Battery Research Center, Korea Electrotechnology Research Institute, 12 Bulmosan-ro 10beon-gil, Seongsan-gu, Changwon-si, Gyeongsangnam-do 51543, Republic of Korea
First published on 11th May 2022
Abstract
There is growing interest in novel bifunctional electocatalysts for oxygen reduction (ORR) and evolution reactions (OER), for application in electrochemical energy conversion systems. While ultrafine oxides have been reported as efficient electrocatalysts for the ORR and OER, there are no reports on ultrafine oxides based on the group 4 or 5 metals as a bifunctional oxygen electrocatalyst. Herein, we present and demonstrate N-doped NbOx/CB nanoparticles as a highly active and stable, bifunctional oxygen electrocatalyst in alkaline media. The NbOx nanoparticles approximately 3 nm in size were successfully synthesized on carbon black (CB) by potentiostatic electrodeposition. The employment of a CB support with a large surface area resulted in a loading amount of 20 wt% Nb in the N-doped NbOx/CB and a uniform distribution of the oxide on CB, leading to an increase of the reaction site. The subsequent annealing in an NH3 flow decreased the concentration of oxygen and intercalated a small amount of nitrogen in the as-deposited NbOx particles, largely lowering the overpotentials of the ORR and OER. The surface Nb species on the N-doped NbOx particles were made of multivalent Nb4+ and Nb5+. As a result, the prepared N-doped NbOx/CB nanoparticles exhibited high catalytic activities for both the ORR and OER in a 0.1 M KOH solution. The potential difference, ΔE, indicating an index for the bifunctional oxygen electrocatalyst, was 1.13 V, which was comparable to those of the commercial 20 wt% Pt/CB and Ir/CB. In addition, the current densities obtained for the nanoparticles were significantly steady for both the long-term ORR and OER in alkaline media. Together these results demonstrate the N-doped NbOx/CB nanoparticles as potential bifunctional oxygen electrocatalysts.
1. Introduction
Novel electrocatalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) have attracted significant research interest for their application in various energy conversion and storage systems such as regenerative fuel cells, water electrolysis, and metal–air batteries.1–3 In addition, the environmental regulations due to global climate change, the depletion of fossil fuels, and increasing energy consumption, have accelerated the exploration of oxygen electrocatalysts that boost the efficiency of the four-electron pathway to drive the ORR or OER. An ideal electrocatalyst should be made of materials that can withstand the conditions of the energy systems, e.g., a high or low pH level and a wide potential window, for efficient and long-term performance. With this background, many potential candidates including metal oxides and carbon materials have been proposed as promising ORR and OER electrocatalysts.1–6In particular, bi-functional oxygen electrocatalysts have attracted much attention because they enable cost reduction and simplification of energy systems, and also allow metal–air batteries to operate charge–discharge cycles.2,4,5,7 RuO2 and IrO2 electrocatalysts have been shown to be very active for the OER in acidic and alkaline electrolytes,8 while Pt nanoparticles supported on carbon, as a commercial fuel cell electrocatalyst, have shown high performance for the ORR in acidic media.1 However, the typical oxygen electrocatalysts are all rare metals leading to an increase in production cost, and have limited activity for the counter oxygen reaction. Initiating from manganese oxide MnO2,9,10 transition metal oxides with special crystal structures, such as spinel oxides with a formula of AB2O4 (A = divalent metal ion; B = trivalent metal ion) or perovskite oxides with a formula of ABO3 (A = alkaline-earth metal ion; B = transition metal ion), were found to be active for both the ORR and OER in alkaline media.2,4,5,11,12 The crystal structures allow the transition metals to be tuned to variable valences separately and activated for each reaction. It therefore causes the oxygen redox electrochemistry of single catalysts.
Cobalt oxide Co3O4 in a spinel structure has two different valences of Co2+ and Co3+ ions, and Co3+ is known to act as a donor–accepter reduction site for the ORR.13,14 In this case, the electron transfer between the cations with different valences requires low activation energy. In a previous study on MnxCo3−xO4, the ORR activity was enhanced by increasing the amount of Mn4+/Mn3+ redox pairs, and the surface Co3+ was regarded as an active site for the OER.15 In perovskite oxides described by the formula 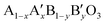 , the substitution of the A-site by another element influenced the ability to adsorb oxygen, while that of the B-site affected the activity of the adsorbed oxygen during the ORR.16,17 La0.6Ca0.4CoO3 exhibited improved ORR and OER catalytic activities simultaneously, and its electrocatalytic properties were enhanced by tuning the oxidation state of Co species.18,19 It was also revealed that the higher oxidation state of the surface Co species enhanced the oxygen electrocatalysis.20 These results indicate that the different valences of metals that co-existed on a single catalyst were a requirement to be a bi-functional oxygen electrocatalyst.
, the substitution of the A-site by another element influenced the ability to adsorb oxygen, while that of the B-site affected the activity of the adsorbed oxygen during the ORR.16,17 La0.6Ca0.4CoO3 exhibited improved ORR and OER catalytic activities simultaneously, and its electrocatalytic properties were enhanced by tuning the oxidation state of Co species.18,19 It was also revealed that the higher oxidation state of the surface Co species enhanced the oxygen electrocatalysis.20 These results indicate that the different valences of metals that co-existed on a single catalyst were a requirement to be a bi-functional oxygen electrocatalyst.
Group 4 and 5 transition metals such as Ti, Nb, and Ta are known to have multi-valence, and several Ti-, Nb-, and Ta-based compounds have been reported as ORR electrocatalysts in acidic and alkaline solutions.21–26 Although nitrides, carbides, and mixed compounds were tested for boosting ORR electrocatalytic activities by doping with additional elements and by applying heat treatments, their short-term stability in acidic electrolytes was an obstacle for polymer electrolyte fuel cell applications. In addition, it was rarely reported that Ta-based catalysts deposited on carbon black (CB) with a predominant crystal phase Na2Ta8O21, prepared by a microemulsion method, were activated as bi-functional oxygen electrocatalysts in 0.1 M NaOH aqueous solution.27 The electrocatalytic activities using the Ta-based oxides were unsatisfactory, even the anodic current for the OER did not reach 10 mA cm−2 at potentials lower than 2.0 VRHE. The metal oxides with a maximum valence, e.g., Ta2O5 and Nb2O5, were chemically the most stable under acidic and alkaline conditions.22,23,27,28 Nevertheless, these oxides are insulators in bulk, thus resulting in low electrocatalytic activity. The nanosize control of NbOx, ZrOx, and TaOx particles in 1∼2 nm diameter dispersed on CB was very effective in improving electrocatalytic activity for ORR in acid media.24,25,28 The transition metals in the oxide nanoparticles were composed of two or three different oxidation states according to XPS analyses. The ultrafine TaOx/CB electrocatalysts activated the ORR in a 0.1 M H2SO4 electrolyte via nearly a four-electron transfer pathway and also maintained long-term stability for 10,000 cycles.28 However, there are no reports on the ultrafine oxides based on the group 4 or 5 metals as bifunctional oxygen electrocatalysts in alkaline media.
Herein, for the first time, we present N-doped NbOx nanoparticles highly dispersed on CB (designated as N-doped NbOx/CB hereafter) as an active bifunctional oxygen electrocatalyst in alkaline media. The oxide nanoparticles that were approximately 3 nm were successfully synthesized at CB surfaces by electrodeposition in a nonaqueous Nb-based solution followed by annealing treatment in a NH3 flow. The N-doped NbOx particles were composed of Nb4+ and Nb5+ species. The employment of a CB support with a large surface area of 1251 m2 g−1 significantly increased the loading amount of NbOx particles, and therefore augmented the reaction sites for the oxygen reactions. These physical and surface properties resulted in high activities in both the ORR and OER over the N-doped NbOx/CB particles in a 0.1 M KOH solution. In particular, the annealing in the NH3 flow significantly lowered the overpotentials for the ORR and OER due to a decrease in the concentration of oxygen and additional doping of nitrogen in the NbOx catalyst leading to decreased charge transfer resistance. Also, the current densities over the nanoparticles were stable for both long-term ORR and OER. These results demonstrated that the N-doped NbOx/CB nanoparticles were active bifunctional oxygen electrocatalysts in alkaline media.
2. Experimental section
2.1. Preparation of N-doped NbOx/CB powder catalysts
The NbOx species were loaded on the CB powder surfaces through potentiostatic electrodeposition in a nonaqueous Nb-based solution. Ten mg of CB powder (Ketjen black EC-600JD, Akzo Nobel Functional Chemicals) was sandwiched between two carbon paper sheets (AvCarb MGL190, AvCarb Material Solutions). A home-made electrodeposition cell was used to fix the sandwiched CB assembly, presented in a previous report.28 The cell as a working electrode was mounted on a typical three-electrode system using a potentiostat (CHI700E, CH Instruments, Inc.). A carbon rod and Ag/Ag+ were used as counter and reference electrodes, respectively. The nonaqueous Nb-based solution consisted of 10 mM NbCl5 (99.9%, Sigma-Aldrich), 10 mM NH4Cl (99%, Kanto Chemical) as a supporting electrolyte, and an anhydrous ethanol solvent. In order to electrodeposit NbOx species on the CB, a constant potential of −0.4 VAg/Ag+ was applied to the working cell for 30 s in the stirred Ar-purged NbCl5–NH4Cl ethanol solution at 298 K.25 The electrodeposited CB powder was washed with anhydrous ethanol to remove the remaining Nb precursor and then completely dried at 298 K. Subsequently, the resulting powder was annealed in a pure NH3 (5N grade) or Ar (5N grade) at a flow of 200 ml min−1 at 873 K for 1 h with a ramp rate of 10 K min−1.2.2. Electrochemical measurements
A catalyst slurry was prepared for ORR and OER electrochemical measurements. Two mg of the prepared N-doped NbOx/CB powder and 12 μl of a Nafion solution (5 wt% in water/aliphatic alcohols, Sigma-Aldrich) were dispersed in 300 μl of isopropyl alcohol (99.9%, Kanto Chemical). The mixture was repeatedly placed in an ultrasonic bath and stirred with a magnetic stirrer to enhance the degree of dispersion. Two point five μl of the catalyst slurry was dropped on a glassy carbon rotating-disk electrode (RDE; 5 mm in diameter) and dried naturally. The loading amount of catalyst was approximately 82 μg cm−2. For comparison, a commercial 20 wt% Pt/CB (Premetek Co., Pt on Vulcan XC-72) and 20 wt% Ir/CB (Premetek Co., Ir on Vulcan XC-72) catalyst slurry was also cast on the RDE in the same manner.Electrochemical measurements were done using the RDE system, composed of Hg/HgO reference and carbon rod counter electrodes at 298 K. Cyclic and linear sweep voltammograms (CVs and LSVs) were scanned in the potential range of 2.0 to 0.10 VRHE at a scan rate of 10 mV s−1 in an Ar- or O2-saturated 0.1 M aqueous KOH solution. The potential scale referenced by the Hg/HgO electrode was calibrated to a reversible hydrogen electrode (RHE). For rotating ring-disk electrode (RRDE) measurements, the glassy carbon disk electrode (4.0 mm in diameter) was scanned at a sweep rate of 5 mV s−1, and a potential of 1.2 VRHE was applied at a Pt ring electrode (5.0 mm in inner diameter; 7.0 mm in outer diameter). The H2O2 yield detected at the ring electrode was estimated using the equation,
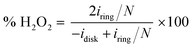 | (1) |
2.3. Surface and physical characterization
The crystal structure of the prepared N-doped NbOx/CB catalysts was investigated by X-ray diffraction (XRD; MiniFlex 600, Rigaku) using Cu Kα radiation at 40 kV and 15 mA. The surface morphology of the N-doped NbOx/CB nanoparticles was characterized by field emission transmission electron microscopy (FE-TEM; JEM-2200FS, JEOL). The loading amount of Nb in N-doped NbOx/CB was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES; PerkinElmer, Avio 500). The Brunauer–Emmett–Teller (BET) surface area of the prepared catalyst was determined using the BET (ASAP2020, Micromeritics Instrument Corp.) adsorption method based on the adsorption of N2 gas at 77 K. Its pore size distribution was also obtained by the Barrett–Joyner–Halenda (BJH) method using the desorption branch of the isotherm. The chemical states of Nb, O, and N species on the N-doped NbOx/CB surface were determined by high-performance X-ray photoelectron spectroscopy (HP-XPS; K-Alpha +, Thermo Scientific), using a monochromatic X-ray source producing Al Kα emission with a current of 6 mA, and an acceleration voltage of 12 kV.3. Results and discussion
NbOx particles were synthesized on the CB, with a large surface area, by electrodeposition at a constant potential of −0.4 VAg/Ag+ for 30 s in 10 mM Nb-based nonaqueous solution. The resulting particles were annealed in an NH3 flow at 873 K for 1 h, so as to modify their surface properties. For comparison, it was also placed in an Ar atmosphere at the same temperature. The crystal structures of the as-deposited and annealed particles were measured by XRD analysis (Fig. S1, ESI†). The XRD pattern of the as-deposited particles showed no peaks distinguishable from that of bare CB powder. After the annealing in Ar, the XRD pattern of the particles revealed some crystallinity with a weak peak at 26°, nearly assignable to β-NbO2 (220) (ICSD #35181). In contrast, the particles annealed in NH3 did not show diffraction peaks, indicating that the particles were highly fine or amorphous. Also, the peaks for the bare CB were not shifted, presenting no introduction of nitrogen in the CB support. The surface morphology of the particles was investigated by (S)TEM measurements. The particles annealed in Ar, smaller than roughly 5 nm in size, were more discrete than the as-deposited particles, however the size of particles was largely unchanged after the annealing at 873 K (Fig. S2, ESI†). Further advanced analysis using the two samples was technically difficult because the particles were made of light elements such as oxygen and amorphous (or low crystalline). Fig. 1 exhibits STEM and TEM images of the particles annealed in NH3. In the STEM image (Fig. 1A), the bright spots made of a relatively heavy element were well dispersed on ball-like supports. As cross-checked with the TEM image (Fig. 1B), the supports showed a typical surface morphology of Ketjen Black as CB, composed of primary particles smaller than 50 nm size.29 Fine particles, approximately 3 nm size, were well-dispersed on the CB as shown in Fig. 1C and D, and were regarded as Nb-based species. The nanoparticles in the inset of Fig. 1D had lattice fringes, indicating the crystallinity of the material. Although crystalline particles were observed, their crystal structures were varied in the present stage and different from β-NbO2 (220) observed in the XRD result (Fig. S3, ESI†). Therefore, the synthesis of catalysts by electrodeposition and subsequent annealing in NH3 produced well-dispersed, crystalline Nb-based nanoparticles.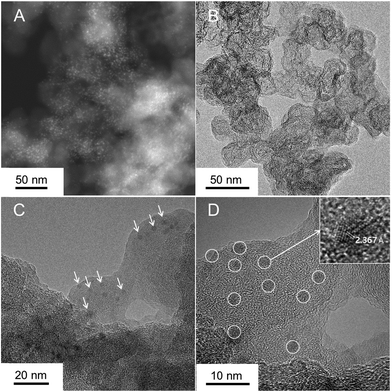 | ||
| Fig. 1 (A) STEM and (B–D) TEM images of the N-doped NbOx/CB nanoparticles, prepared by electrodeposition and subsequent annealing treatment in a NH3 flow at 873 K for 1 h. | ||
The chemical state and composition of the Nb-based species were determined by XPS analysis. Fig. 2 shows narrow-scanned Nb 3d, O 1s, and N 1s XPS spectra of the as-deposited nanoparticles and the annealed derivatives in Ar and NH3 atmospheres. The Nb 3d spectra of all the samples broadened and deviated from the double splitting peaks corresponding to the fully oxidized Nb2O5 (Nb5+), indicating the chemical shift to a lower valence state. Accordingly, the broad Nb 3d spectra were deconvoluted with different oxidation states of Nb5+ and Nb4+.30–33 The surface composition ratios of the Nb species resulting from the best-fit are summarized in Table S1 (ESI†). The convolutions clearly reveal that the electrodeposited Nb species were composed of two oxidation states indicating NbOx nanoparticles. The surface concentration of Nb5+ was increased after the annealing treatment, although the presence of multivalent Nb species was still unchanged. However, according to the O 1s spectra, the concentration of oxygen species, including an OH−/H2O group adsorbed on the surface of catalyst, decreased during the annealing in Ar. The annealing of Nb-based nanoparticles in the reducing NH3 accelerated the decrease in the oxygen level, while promoting the insertion of nitrogen with relatively low content. Besides the desorption of the OH−/H2O species on the surface of catalyst, the crystallization of the amorphous, as-deposited NbOx decreased the concentration of excess oxygen during the annealing at 873 K, probably leading to the release of oxygen gas. After the annealing treatments, the decrease in the oxygen contents of catalysts caused more concentrated Nb-based particles, as proved in Fig. 1 and Fig. S2 (ESI†) showing the sizes and distributions of the annealed particles more clearly. When CB alone was annealed in NH3 for comparison, the concentration of nitrogen was undetected, consistent with the XRD result (Fig. S4, ESI†). These results indicate that the nitrogen was intercalated exclusively in the NbOx nanoparticles by the nitridation process in NH3 exchanging oxygen with nitrogen.34,35 During the nitridation, the doped nitrogen and decreased oxygen in NbOx suppressed the crystallization of the oxide nanoparticles into single phase NbO2, consistent with the TEM result in Fig. 1D. Therefore, the prepared catalyst was identified as the NbOx nanoparticles doped with nitrogen, designated thereafter as N-doped NbOx/CB.
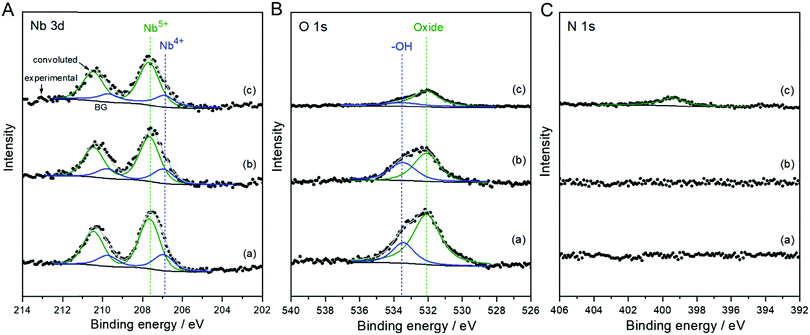 | ||
| Fig. 2 Narrow-scan (A) Nb 3d, (B) O 1s, and (C) N 1s XPS spectra of NbOx/CB nanoparticles (a) as-deposited and subsequently annealed in (b) Ar and (c) NH3 flows at 873 K for 1 h. | ||
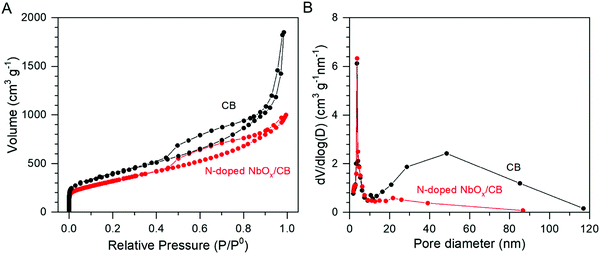
The amount of Nb in the catalyst, prepared by the electrodeposition at −0.4 VAg/Ag+ for 30 s, was determined by ICP-OES analysis. 20.1 wt% Nb was deposited on the CB surface in the electrodeposition system. In a previous report, the loading of Nb was limited to 3.6 wt% in the electrodeposition using the CP electrode mixed with Vulcan XC-72.25 Therefore, the high loading amount in this study was remarkable. Moreover, the surface area of the prepared N-doped NbOx/CB and its pore size distribution were estimated by the BET adsorption method. Fig. 3(A) exhibits a N2 adsorption-desorption isotherm for the N-doped NbOx/CB catalyst, which is compared with that for CB powder alone. In this study, Ketjen Black was used as the CB support for the NbOx nanoparticles because it is known as a conductive, mesoporous carbon with a large BET surface area.29 The hysteresis loop for the CB was typical of the type IV adsorption isotherm according to the IUPAC classification.29,36 Its pore size distribution, shown in Fig. 3(B), also exhibited the characteristic of mesoporous materials with pore diameters of 2–50 nm. The isotherm retained the mesoporous property even after the deposition of NbOx nanoparticles, although the volume trend against relative pressures became slightly lower. The BET surface areas of the N-doped NbOx/CB and CB were estimated to be 1158 and 1251 m2 g−1, respectively. The coverage of NbOx particles greater than 20 wt% significantly decreased the pore volume of CB in the range of 10–50 nm, as shown in the pore size distribution. Nevertheless, the surface area of the prepared catalyst was still large because of the loading of NbOx fine particles in ca. 3 nm. Therefore, in this study, the application of CB with a large surface area enhanced the loading amount and uniform distribution of NbOx nanoparticles, leading to an increase in the reaction site.
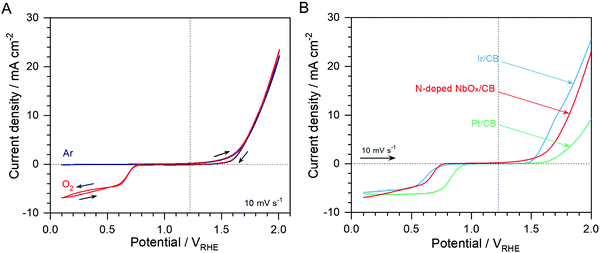
Catalytic activities of the N-doped NbOx/CB nanoparticles for the ORR and OER are shown in Fig. 4(A); it exhibits CVs in Ar- and O2-purged 0.1 M KOH electrolytes with a scan rate of 10 mV s−1. The ORR was remarkably catalyzed on the nanoparticles, judged by the high cathodic current in an O2 atmosphere as compared to nearly no current in an Ar atmosphere. It is clear that an O2 reactant was used in the ORR. The high anodic current responsive to the OER was also observed on the nanoparticles, regardless of the gas atmosphere. These results demonstrate that the N-doped NbOx/CB nanoparticles were active for both oxygen reactions in alkaline media. Noble metal Pt/CB, is a representative ORR electrocatalyst, while Ir/CB is a remarkable OER electrocatalyst.10 For comparison, commercial 20 wt% Pt/CB and Ir/CB catalysts were estimated for the ORR and OER in O2-saturated 0.1 M KOH electrolytes and the results are shown in Fig. 4(B). The N-doped NbOx/CB nanoparticles were more active for the ORR than the Ir/CB catalyst and 140 mV less active than the Pt/CB catalyst at the half-wave potential, E1/2. Also, the nanoparticles were obviously more active for the OER than Pt/CB and only 90 mV less active than Ir/CB at an anodic current density of 10 mV cm−2 indicating an OER benchmark. The potential difference, ΔE, between the ORR and OER activities for each catalyst is summarized in Table 1, showing the overall oxygen activities of the bifunctional catalysts. The potential gap for the N-doped NbOx/CB nanoparticles was 1.13 V, which was comparable to those of the noble metal catalysts. This indicates that the bifunctional activity of the nanoparticles was remarkable. Therefore, the N-doped NbOx/CB nanoparticles, synthesized by electrodeposition and subsequent annealing, are a potential ideal reversible oxygen electrocatalyst.
| Material | ORR benchmark:* E1/2(V) | OER benchmark: E(V) at 10 mA cm−2 | Oxygen electrode Δ(OER–ORR): E(V) |
|---|---|---|---|
| *E1/2(V) measured at −2.1(a), −2.5(b), and −3.1 mA cm−2(c). | |||
| N-doped NbOx/CB | 0.68a | 1.81 | 1.13 |
| 20 wt% Ir/CB | 0.62b | 1.72 | 1.10 |
| 20 wt% Pt/CB | 0.82c | 2.02 | 1.20 |
To identify the nature of the bifunctional oxygen activity on the N-doped NbOx/CB catalyst, systematic studies for the ORR and OER were carried out using the nanoparticles with different compositions. The catalytic activities of bare CB and glassy carbon were also evaluated for comparison. Fig. 5(A) shows LSVs of N-doped NbOx/CB, NbOx/CB (annealed in Ar), as-deposited NbOx/CB, CB (annealed in NH3), and glassy carbon for the ORR in the 0.1 M KOH solution. The current density for ORR, iORR, was calculated from the difference in cathodic current density between the O2- and Ar-saturated atmospheres. CB, used as a support in this study, showed some activity for the ORR as compared to that of glassy carbon. It has been reported that the ORR on the CB occurred to some degree in the alkaline solution, whereas it was almost negligible in an acidic solution.28,37,38 The as-deposited NbOx/CB displayed lower activity for the ORR than CB, although its onset potential was relatively more positive. A similar result was observed in a previous report.39 The lower activity was mainly ascribed to the presence of organic residues including oxygen species, remaining after the synthesis of the catalyst by the electrodeposition in a nonaqueous electrolyte. This was also observed in the present study, as shown in the XPS spectra. However, annealing of as-deposited catalysts in different gas atmospheres remarkably enhanced the ORR activity, and the NH3 atmosphere was particularly a superior condition. The onset potential of the N-doped NbOx/CB was 0.78 VRHE for approximately −0.01 mA cm−2, which was significantly shifted from that of the as-deposited nanoparticles. The annealed catalysts produced higher ORR currents with more positive onset-potentials, proving that the NbOx nanoparticles were activated as the ORR electrocatalyst. Also, it is remarkable that the increase in the loading amount of NbOx catalyst, resulting from the employment of CB with a large surface area, produced high current density for the ORR, as compared with previous results.28,38 The ORR activity of the NbOx nanoparticles in 0.1 M H2SO4 electrolyte was reported in a previous publication.25 Based on these findings, it is certain that the NbOx/CB nanoparticles can drive the ORR in both acidic and alkaline media.
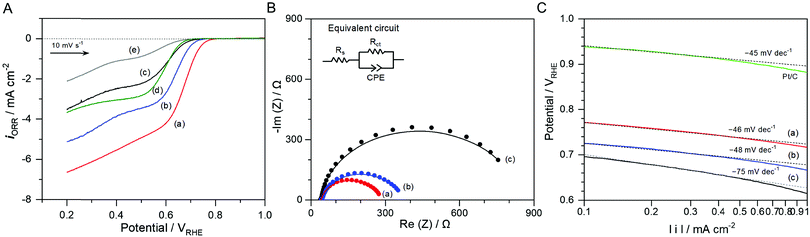 | ||
| Fig. 5 (A) LSVs for the ORR in O2-purged 0.1 M KOH aqueous solutions using (a) the N-doped NbOx/CB and (b) NbOx/CB annealed in NH3 and Ar and at 873 K for 1 h, respectively, and (c) as-deposited NbOx/CB, (d) CB, and (e) glassy carbon. (B) Nyquist plots of the prepared NbOx/CB catalyst series. The EIS measurements were performed in the O2-purged 0.1 M KOH aqueous solutions at an applied potential of 0.72 VRHE. The equivalent circuit model for the best-fits of Nyquist plots is shown in the inset of the figure. Fitting results are summarized in Table S2 (ESI†). (C) Tafel plots of the prepared NbOx/CB catalyst series corresponding to the ORR curves shown in (A). The Tafel plot of the commercial 20 wt% Pt/CB powder was also compared in the same manner. | ||
Fig. 5(B) shows Nyquist plots of the N-doped NbOx/CB, NbOx/CB (annealed in Ar), and as-deposited NbOx/CB catalysts in the 0.1 M KOH solutions at an applied potential of 0.72 VRHE. The EIS measurements were performed to determine the effect of N-doping on the ORR activity of NbOx/CB, namely, to evaluate the charge transfer behavior of the prepared catalysts during the ORR process. The fitting results of the Nyquist plots using an equivalent circuit model are summarized in Table S2 (ESI†). The electron transfer resistance, Rct, at the interface between the catalyst and solution was significantly reduced after annealing treatments. Rct was minimum in the N-doped NbOx/CB catalyst, revealing that the annealing in NH3 efficiently enhanced the electroconductivity of the catalyst. The annealing in Ar was found to modify the surface of catalyst as shown in the XPS result in Fig. 2, indicating the removal of oxygen species such as O2− in oxide and surface-adsorbed OH−/H2O. The loss of oxygen was accelerated in the reducing NH3 flow. Also, the small amount of nitrogen in the NH3 atmosphere was doped into the NbOx nanoparticles. Typically, the doping of nitrogen in the group 4 or 5 metal oxides, as an insulator inherently, mainly enhanced the electroconductivity of the oxide itself, resulting in improved electrochemical activity for the ORR.22,40 In this study, the modified surface of the NbOx catalyst, originating from the annealing, largely promoted the electrocatalysis. Therefore, the largely enhanced ORR activity was attributed to more concentrated Nb and the doping of nitrogen in NbOx nanoparticles.
Tafel plots of all the samples at low overpotentials against a thermodynamic potential of 1.23 V, obtained from Fig. 5(A), are shown in Fig. 5(C); the Tafel plot of 20 wt% Pt/CB was also compared. The Tafel slope for the as-deposited NbOx/CB was approximately −75 mV dec−1. However, it became smaller after the annealing treatment in Ar and NH3 atmospheres, indicating that ORR kinetics was faster and the rate-determining step for the ORR was changed. These are in good agreement with the Nyquist results showing the annealing effect of the NbOx catalyst. The Tafel slope was determined as −46 mV dec−1 for the N-doped NbOx/CB, which is similar to −45 mV dec−1 for Pt/CB in this study. A Tafel slope of 120 mV dec−1 typically suggests that the one-electron transfer reaction is the rate-determining step for the ORR and the surface sites of the catalyst are stable under the measurement conditions. In acidic media, the surface of metallic Pt could be oxidized to PtO so that a smaller slope of ca. 60 mV dec−1 was observed for the ORR in the low overpotential range in previous reports.41,42 The similar electrochemical behavior was also reported in alkaline media.43,44 After the annealing, the concentration of surface oxygen on the catalyst was reduced, presumably leading to electroactive NbOx surface sites for the ORR and thus the decrease in the Tafel slope at low overpotentials. A previous theoretical study on the various surface coverages such as MOO, MOO−, and MOOH (M = metal) showed different Tafel slopes during the ORR.43 Thus, the small slope for the annealed NbOx/CB nanoparticles might result from the variable surface states of the non-stoichiometric NbOx, like a metallic Pt.
The RRDE measurement of N-doped NbOx/CB nanoparticles was carried out during the ORR in O2-saturated 0.1 M KOH electrolyte (Fig. S5, ESI†). The voltammogram was recorded at a sweep rate of 5 mV s−1, although the ORR activity of the catalyst was nearly unchanged from that shown in Fig. 5(A) and scanned at 10 mV s−1. The anodic current was observed on the Pt ring electrode, indicating that a H2O2 byproduct was partly produced during the ORR via a two-electron transfer pathway. At a potential of 0.5 VRHE, the H2O2 yield originating from the ORR over the catalyst was estimated to be 32%, much lower than 67% in the ORR using bare CB particles. Moreover, the ring current began to be produced at 0.68 VRHE, close to the onset potential of CB for the ORR. These results imply that the ORR via the two-electron transfer pathway was mainly catalyzed by the bare CB support. In addition, hydrodynamic voltammetry of N-doped NbOx/CB nanoparticles was measured using a RDE at different revolution rates between 400 to 2500 rpm to establish the ORR kinetics (Fig. S6, ESI†). The ORR current at low potentials increased with increasing rotation rate, indicating that it was dominated by the diffusion-limiting current. The Koutecky–Levich plots for the nanoparticles exhibited a linear relationship between |i|−1 and ω−1/2 and the slopes at the designated potentials were nearly close to four electron transfer. This indicates that the ORR process over the N-doped NbOx/CB nanoparticles was governed by the four electron transfer pathway in the mass-transfer region.
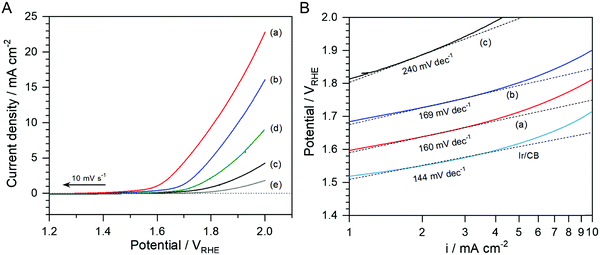
Fig. 6(A) shows LSVs of N-doped NbOx/CB, NbOx/CB (annealed in Ar), as-deposited NbOx/CB, CB (annealed in NH3), and glassy carbon for the OER in the O2-purged 0.1 M KOH solution. The LSVs were recorded in a cathodic sweep direction to avoid the self-oxidation of catalysts as shown in Fig. 4(A). The OER activity of the as-deposited NbOx/CB was significantly low, even far from the OER benchmark value of 10 mA cm−2 at an end potential of 2.0 VRHE. It was also lower than that of CB. The lower activity was mainly attributed to the presence of excess oxygen species maintained after the electrodeposition. The excess oxygen covered on the surfaces of NbOx/CB particles might suppress the electrocatalysis for the OER. The anodic current was enhanced after the annealing in Ar, and it was remarkably increased by the annealing in NH3. The activity trend in the OER was similar to that in the ORR. The modified surface of the NbOx nanoparticles, i.e., the removal of excess oxygen and the doping of nitrogen, led to the improvement in the electroconductivity of the catalysts and thus largely activated the catalysis for the OER as well as the ORR. In the Tafel plots, shown in Fig. 6(B), the slope of the N-doped NbOx/CB nanoparticles, indicating approximately 160 mV dec−1, was rather close to 120 mV dec−1 than 60 mV dec−1. This reveals the N-doped NbOx during the OER was electrochemically stable at the oxidizing potentials. The nearly one-electron transfer reaction was the rate determining step during the OER process at low overpotentials over the prepared nanoparticles. The OER kinetics on the nanoparticles was slower than that of the commercial Ir/CB catalyst, judged by the Tafel slopes. However, it was clearly distinguishable from 240 mV dec−1 for the as-deposited NbOx/CB. After the annealing, the significant decrease in the slope for the OER was consistent with that for the ORR. Therefore, the annealing in NH3 enhanced both the ORR and OER kinetics, resulting from the surface modification of the NbOx/CB nanoparticles.
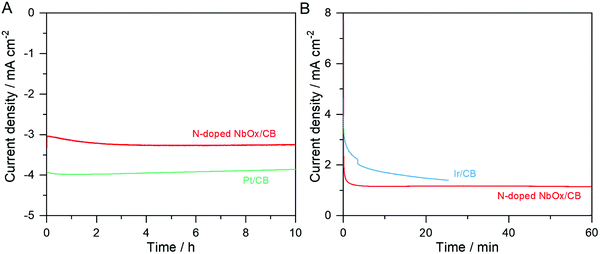
Fig. 7 shows CA curves of the N-doped NbOx/CB catalyst during the ORR and OER in the O2-purged 0.1 M KOH electrolytes. For comparison, the commercial 20 wt% Pt/CB and Ir/CB were also evaluated in the same manner. In Fig. 7(A), the ORR activity of the N-doped NbOx/CB nanoparticles applied at 0.65 VRHE was increased at the beginning point and then kept at a constant value of −3.3 mA cm−2 for the 10 h reaction, while it was gradually decreased on the Pt/CB catalyst. It is remarkable that the nanoparticles were stable for long-term ORR in alkaline media. The Ta-based oxide nanoparticles were also durable for the ORR in an acidic electrolyte in previous results.28 Therefore, we may conclude that the ORR is steadily catalyzed on the oxide nanoparticles, regardless of the pH level of the electrolyte. In addition, the long-term OER activity of the nanoparticles was measured at a benchmark potential of 1.81 VRHE for 10 mA cm−2. However, the O2 evolution was very vigorous to an extent that the catalyst was detached from a RDE and the catalytic stability could not be measured. The OER activity was thus evaluated at a potential of 1.61 VRHE, which was lower than the benchmark potential, for 1 h. As shown in Fig. 7(B), the anodic current was relatively stable as compared to that of the Ir/CB catalyst. Therefore, these results demonstrate that the catalytic activities over the N-doped NbOx/CB nanoparticles were stable in both the ORR and OER atmospheres.
4. Conclusions
In this study, for the first time, N-doped NbOx/CB nanoparticles were introduced as an active and stable bifunctional oxygen electrocatalyst in alkaline media. The NbOx nanoparticles of approximately 3 nm in size were successfully synthesized on the CB surfaces by potentiostatic electrodeposition in a nonaqueous solution and subsequent annealing treatment in a NH3 flow. The annealing in the NH3 significantly decreased the concentration of oxygen in the NbOx particles and doped additional nitrogen content. Also, the N-doped NbOx particles were made of Nb4+ and Nb5+ species, as confirmed by the XPS results. The employment of a CB support with a large surface area led to a large loading amount of 20 wt% NbOx particles and highly uniform distribution on the CB, increasing the number of reaction sites. Together, these properties remarkably enhanced the catalytic activities of the N-doped NbOx/CB nanoparticles for the ORR and OER in a 0.1 M KOH solution. The potential difference, ΔE, indicating an index for the bifunctional oxygen electrocatalyst, was 1.13 V for the N-doped NbOx/CB particles. The bifunctional oxygen electrocatalysis was comparable to those of the commercial Pt/CB and Ir/CB. According to Koutecky–Levich plots, the ORR process over the N-doped NbOx/CB nanoparticles was governed by the four electron transfer pathway in the mass-transfer region. In addition, the current densities over the nanoparticles were stable in the ORR for 10 h at a potential of 0.65 VRHE and the OER for 1 h at a potential of 1.61 VRHE. These results demonstrate the N-doped NbOx/CB nanoparticles as a novel bifunctional oxygen electrocatalyst that is active and stable for both the ORR and OER in alkaline media.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1C1C1006373).References
- M. Shao, Q. Chang, J. P. Dodelet and R. Chenitz, Recent Advances in Electrocatalysts for Oxygen Reduction Reaction, Chem. Rev., 2016, 116(6), 3594–3657 CrossRef CAS.
- Z.-F. Huang, J. Wang, Y. Peng, C.-Y. Jung, A. Fisher and X. Wang, Design of Efficient Bifunctional Oxygen Reduction/Evolution Electrocatalyst: Recent Advances and Perspectives, Adv. Energy Mater., 2017, 7(23), 1700544 CrossRef.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives, Chem. Soc. Rev., 2017, 46(2), 337–365 RSC.
- Z. L. Wang, D. Xu, J. J. Xu and X. B. Zhang, Oxygen electrocatalysts in metal-air batteries: from aqueous to nonaqueous electrolytes, Chem. Soc. Rev., 2014, 43(22), 7746–7786 RSC.
- X. Wu, C. Tang, Y. Cheng, X. Min, S. P. Jiang and S. Wang, Bifunctional Catalysts for Reversible Oxygen Evolution Reaction and Oxygen Reduction Reaction, Chem. – Eur. J., 2020, 26, 3906–3929 CrossRef CAS PubMed.
- X. Zhang, X. Xu, S. Yao, C. Hao, C. Pan, X. Xiang, Z. Q. Tian, P. K. Shen, Z. Shao and S. P. Jiang, Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering, Small, 2022, 18(10), e2105329 CrossRef PubMed.
- Z.-C. Yao, T. Tang, J.-S. Hu and L.-J. Wan, Recent Advances on Nonprecious-Metal-Based Bifunctional Oxygen Electrocatalysts for Zinc–Air Batteries, Energy Fuels, 2021, 35(8), 6380–6401 CrossRef CAS.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys, Chem. Lett., 2012, 3(3), 399–404 CAS.
- H. Y. Su, Y. Gorlin, I. C. Man, F. Calle-Vallejo, J. K. Norskov, T. F. Jaramillo and J. Rossmeisl, Identifying active surface phases for metal oxide electrocatalysts: a study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis, Phys. Chem. Chem. Phys., 2012, 14(40), 14010–14022 RSC.
- Y. Gorlin and T. F. Jaramillo, A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS.
- K. Bradley, K. Giagloglou, B. E. Hayden, H. Jungius and C. Vian, Reversible perovskite electrocatalysts for oxygen reduction/oxygen evolution, Chem. Sci., 2019, 10(17), 4609–4617 RSC.
- X. Xu, Y. Pan, Y. Zhong, C. Shi, D. Guan, L. Ge, Z. Hu, Y. Y. Chin, H. J. Lin, C. T. Chen, H. Wang, S. P. Jiang and Z. Shao, New Undisputed Evidence and Strategy for Enhanced Lattice-Oxygen Participation of Perovskite Electrocatalyst through Cation Deficiency Manipulation, Adv. Sci., 2022, e2200530 CrossRef.
- A. Restovic, E. Ríos, S. Barbato, J. Ortiz and J. L. Gautier, Oxygen reduction in alkaline medium at thin MnxCo3−xO4 (0 ≤ x ≤ 1) spinel films prepared by spray pyrolysis. Effect of oxide cation composition on the reaction kinetics, J. Electroanal. Chem., 2002, 522, 141–151 CrossRef CAS.
- A. J. Esswein, M. J. McMurdo, P. N. Ross, A. T. Bell and T. D. Tilley, Size-Dependent Activity of Co3O4 Nanoparticle Anodes for Alkaline Water Electrolysis, J. Phys. Chem. C, 2009, 113, 15068–15072 CrossRef CAS.
- E. Rios, J.-L. Gautier, G. Poillerat and P. Chartier, Mixed valency spinel oxides of transition metals and electrocatalysis: case of the MnxCo3−xO4 system, Electrochim. Acta, 1998, 44, 1491–1497 CrossRef CAS.
- H. M. Zhang, Y. Shimizu, Y. Teraoka, N. Miura and N. Yamazoe, Oxygen sorption and catalytic properties of La1−xSrxCo1−yFeyO3 perovskite-type oxides, J. Catal., 1990, 121, 432–440 CrossRef CAS.
- H. Tanaka and M. Misono, Advances in designing perovskite catalysts, Curr. Opin. Solid State Mater. Sci., 2001, 5, 381–387 CrossRef CAS.
- A. Kahoul, A. Hammouche, G. Poillerat and R. W.-D. Doncker, Electrocatalytic activity and stability of La1−xCaxCoO3 perovskite-type oxides in alkaline medium, Catal. Today, 2004, 89(3), 287–291 CrossRef CAS.
- N.-L. Wu, W.-R. Liu and S.-J. Su, Effect of oxygenation on electrocatalysis of La0.6Ca0.4CoO3−x in bifunctional air electrode, Electrochim. Acta, 2003, 48(11), 1567–1571 CrossRef CAS.
- S. Malkhandi, B. Yang, A. K. Manohar, A. Manivannan, G. K. Prakash and S. R. Narayanan, Electrocatalytic Properties of Nanocrystalline Calcium-Doped Lanthanum Cobalt Oxide for Bifunctional Oxygen Electrodes, J. Phys. Chem. Lett., 2012, 3(8), 967–972 CrossRef CAS PubMed.
- R. Ohnishi, K. Takanabe, M. Katayama, J. Kubota and K. Domen, Nano-nitride Cathode Catalysts of Ti, Ta, and Nb for Polymer Electrolyte Fuel Cells: Temperature-Programmed Desorption Investigation of Molecularly Adsorbed Oxygen at Low Temperature, J. Phys. Chem. C, 2012, 117(1), 496–502 CrossRef.
- A. Ishihara, K. Lee, S. Doi, S. Mitsushima, N. Kamiya, M. Hara, K. Domen, K. Fukuda and K.-I. Ota, Tantalum Oxynitride for a Novel Cathode of PEFC, Electrochem. Solid-State Lett., 2005, 8(4), A201 CrossRef CAS.
- Y. Ohgi, A. Ishihara, K. Matsuzawa, S. Mitsushima and K. Ota, Zirconium Oxide-Based Compound as New Cathode Without Platinum Group Metals for PEFC, J. Electrochem. Soc., 2010, 157(6), B885 CrossRef CAS.
- J. Seo, D. Cha, K. Takanabe, J. Kubota and K. Domen, Particle size dependence on oxygen reduction reaction activity of electrodeposited TaO(x) catalysts in acidic media, Phys. Chem. Chem. Phys., 2014, 16(3), 895–898 RSC.
- J. Seo, D. Cha, K. Takanabe, J. Kubota and K. Domen, Electrodeposited Ultrafine NbOx, ZrOx, and TaOx Nanoparticles on Carbon Black Supports for Oxygen Reduction Electrocatalysts in Acidic Media, ACS Catal., 2013, 3(9), 2181–2189 CrossRef CAS.
- X. Zhang, J. Guo, P. Guan, C. Liu, H. Huang, F. Xue, X. Dong, S. J. Pennycook and M. F. Chisholm, Catalytically active single-atom niobium in graphitic layers, Nat. Commun., 2013, 4, 1924 CrossRef PubMed.
- J. C. Ruiz-Cornejo, D. Sebastián, M. V. Martínez-Huerta and M. J. Lázaro, Tantalum-based electrocatalysts prepared by a microemulsion method for the oxygen reduction and evolution reactions, Electrochim. Acta, 2019, 317, 261–271 CrossRef CAS.
- J. Seo, D. H. Anjum, K. Takanabe, J. Kubota and K. Domen, Electrodeposited Ultrafine TaOx/CB Catalysts for PEFC Cathode Application: Their Oxygen Reduction Reaction Kinetics, Electrochim. Acta, 2014, 149, 76–85 CrossRef CAS.
- R. Neffati and J. M.-C. Brokken-Zijp, Structure and porosity of conductive carbon blacks, Mater. Chem. Phys., 2021, 260, 124177 CrossRef CAS.
- H. D. Asfaw, C.-W. Tai, L. Nyholm and K. Edstrçm, Over-stoichiometric NbO2 nanoparticles for a high energy and power density lithium microbattery, ChemNanoMat, 2017, 3, 646–655 CrossRef CAS.
- M. K. Hota, M. K. Bera, S. Verma and C. K. Maiti, Studies on switching mechanisms in Pd-nanodot embedded Nb2O5 memristors using scanning tunneling microscopy, Thin Solid Films, 2012, 520(21), 6648–6652 CrossRef CAS.
- M. Ziolek and I. Nowak, Characterization techniques employed in the study of niobium and tantalum-containing materials, Catal. Today, 2003, 78(1-4), 543–553 CrossRef CAS.
- S. Martínez-Méndez, Y. Henríquez, O. Domínguez, L. D’Ornelas and H. Krentzien, Catalytic properties of silica supported titanium, vanadium and niobium oxide nanoparticles towards the oxidation of saturated and unsaturated hydrocarbons, J. Mol. Catal. A: Chem., 2006, 252(1-2), 226–234 CrossRef.
- S. Ramaraj and J. Seo, Intensive-visible-light-responsive ANbO2N (A = Sr, Ba) synthesized from layered perovskite A5Nb4O15 for enhanced photoelectrochemical water splitting, J. Energy Chem., 2022, 68, 529–537 CrossRef.
- T. T.-T. Tran, S. Kim and J. Seo, Size dependence of perovskite-type BaNbO2N particles on sunlight-driven photoelectrochemical water splitting, J. Catal., 2022, 406, 157–164 CrossRef CAS.
- F. J. Sotomayor, K. A. Cychosz and M. Thommes, Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies, Acc. Mater. Surf. Res., 2018, 3(2), 34–50 Search PubMed.
- J. S. Lee, G. S. Park, H. I. Lee, S. T. Kim, R. Cao, M. Liu and J. Cho, Ketjenblack carbon supported amorphous manganese oxides nanowires as highly efficient electrocatalyst for oxygen reduction reaction in alkaline solutions, Nano Lett., 2011, 11(12), 5362–5366 CrossRef CAS PubMed.
- J.-W. Park and J. Seo, Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media, Catalysts, 2021, 12(1), 35 CrossRef.
- J. Seo, L. Zhao, D. Cha, K. Takanabe, M. Katayama, J. Kubota and K. Domen, Highly Dispersed TaOx Nanoparticles Prepared by Electrodeposition as Oxygen Reduction Electrocatalysts for Polymer Electrolyte Fuel Cells, J. Phys. Chem. C, 2013, 117(22), 11635–11646 CrossRef CAS.
- J. Chen, K. Takanabe, R. Ohnishi, D. Lu, S. Okada, H. Hatasawa, H. Morioka, M. Antonietti, J. Kubota and K. Domen, Nano-sized TiN on carbon black as an efficient electrocatalyst for the oxygen reduction reaction prepared using an mpg-C3N4 template, Chem. Commun., 2010, 46(40), 7492–7494 RSC.
- U. A. Paulus, T. J. Schmidt, H. A. Gasteiger and R. J. Behm, Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst a thin-film rotating raing-disk electrode study, J. Electroanal. Chem., 2001, 495, 134–145 CrossRef CAS.
- J. Perez, E. R. Gonzalez and E. A. Ticianelli, Oxygen electrocatalysis on thin porous coating rotating platinum electrodes, Electrochim. Acta, 1998, 44, 1329–1339 CrossRef CAS.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion, Sci. Rep., 2015, 5, 13801 CrossRef PubMed.
- L. Genies, R. Faure and R. Durand, Electrochemical reduction of oxygen on platinum nanoparticles in alkaline media, Electrochim. Acta, 1998, 44, 1317–1327 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00292b |
| This journal is © The Royal Society of Chemistry 2022 |