Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in materials and fabrication of separators in supercapacitors
Sandeep
Ahankari
 *a,
Dylan
Lasrado
a and
Ramesh
Subramaniam
b
*a,
Dylan
Lasrado
a and
Ramesh
Subramaniam
b
aSchool of Mechanical Engineering, VIT University, Vellore, TN-632014, India. E-mail: asandeep.s@vit.ac.in
bDepartment of Physics, Faculty of Science, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
First published on 29th December 2021
Abstract
Supercapacitors (SCs) have been extensively used in advanced energy applications due to their superior energy storage capacity and rapid charge–discharge rate. The significant constituents of a SC include two electrodes, an electrolyte and a separator. The integrated performance of all these constituents is necessary to enhance the energy storage ability of the device. For advanced electrochemical energy systems, the search for separator materials with higher ionic conductivity, mechanical strength, thermal stability, longer life and low cost of manufacturing is necessary. Appropriate design and fabrication of a separator improves the thermal stability, specific capacity, efficiency and life of a SC. In this review, a study on various materials that are used in the fabrication of separator membranes namely polymer, polymer–ceramic, and bio-based materials and the influence of these materials on the overall performance of the SC is presented. New trends in developing devices by integrating smart separator materials with SCs are also highlighted.
1. Introduction
In the last decade, the field of portable electronic systems has witnessed tremendous growth with the development and application of numerous types of sensors and other flexible electronic devices.1,2 This has stimulated the need for the development of portable energy storage devices such as batteries, fuel cells and supercapacitors (SCs). Batteries have the ability to store significant amounts of energy and are able to provide the same for many applications. However, a major drawback in the use of batteries is their less power density and relatively lower shelf and cycle life. SCs, on the other hand, are energy storage devices that have properties such as specific power density (0.1–100 kW kg−1) and specific energy density (0.11–10 W h kg−1) which lie in between those of electrochemical batteries (0.01–1 kW kg−1; 10–100 W h kg−1) and capacitors (100–104 kW kg−1; <0.1 W h kg−1).3,4 SCs exhibit promising properties such as a fast charge–discharge rate within seconds, a high specific power density (10 kW kg−1) and a long cycle life (>105 cycles).5–7 In recent times, SCs have found tremendous applications in various fields such as consumer electronics, renewable energy, military applications, etc. The automotive industry is one of the major fields in which SCs are extensively being used, mainly in cars, buses and trains where they are primarily used for starting the engine, cold starting at low temperatures and vehicle acceleration. The possibility of using SCs in hybrid as well as electric vehicles is currently being explored. SCs are also being used in applications that we encounter in our day to day lives, such as lifts, cranes, elevators, smart phones, laptops and security systems.8,9 An important parameter that researchers are concentrating on is the volumetric energy density of SCs. It is desired that SCs have less volume so that they can be used in consumer electronics.10SCs are chiefly classified into two types namely pseudo-capacitors and electrical double layer capacitors (EDLCs).11,12 In EDLCs, the absorption and de-absorption of charges take place at the electrode/electrolyte interface within seconds leading to fast charge–discharge rates and excellent cycling stability. The surface area of the electrodes as well as their pore size, distribution and structure has a significant impact on the capacitance of EDLCs. Activated carbon materials, carbon nanofibers and mesoporous carbons are commonly used as electrode materials in EDLCs because of their high surface area and their abundant supply.13–15 In contrast, reversible faradaic redox reactions between the electrodes and the electrolyte are responsible for storing energy in pseudo-capacitors. The commonly used electrode materials for pseudo-capacitors include metal oxides such as MnO2, NiO, TiO2, polypyrrole, polyaniline and other conducting polymers.16–18 Pseudo-capacitors exhibit higher specific capacitance and poor cycling stability as compared to EDLCs.
Current research chiefly focuses on the manufacturing and characterization of flexible electrode materials such as CNTs,19 graphene,20etc. and their composites with conducting polymers,21 metal oxides,22etc. However, the electrochemical performance of a SC also depends on the material and type of electrolyte and separator used. Solid electrolytes are preferred in flexible SCs as they act as an electrolyte as well as a separator to prevent a short circuit and chemical leakage; furthermore, if an SC is present in the form of a film, it also minimizes the distance of ion transport.23,24 Quasi-solid gelled polymer electrolytes demonstrate higher ionic conductivity (10−1–100 mS cm−1) than solid electrolytes (10−5–10−3 mS cm−1).25,26
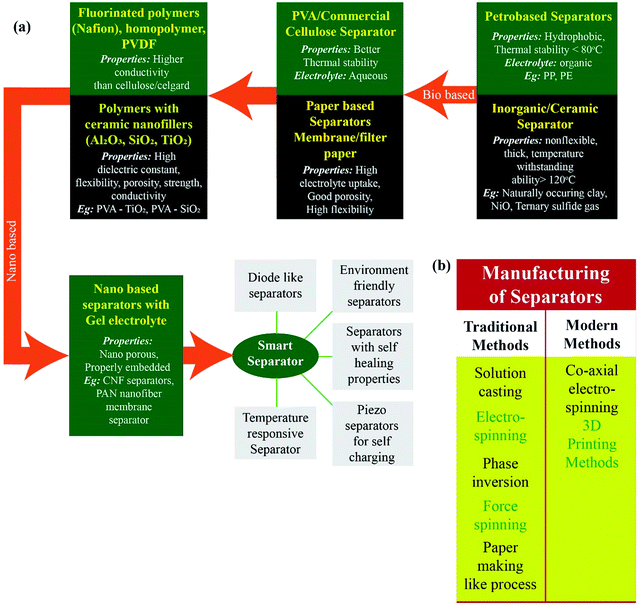
A separator, a vital component that enables free ionic flow and isolates electronic flow, greatly influences the safety and electrochemical performance of SCs. Unfortunately, very few researchers have focussed their attention towards optimising the characteristics of separator membranes i.e. their chemical composition and morphology.27,28 Separators have been developed from a variety of sources such as polyolefins, aqua gel and rubber. These separators must (i) be thin and provide electrical insulation so as to avoid a short-circuit between the electrodes,29 (ii) be good dielectric materials and exhibit electrochemical stability in the electrolytes used, and (iii) also favour high ionic mobility between the electrolyte and electrode surface. However, separators tend to show low ionic conductivity or dry out and collapse over a period of time.4,30 Macro porous separators made from poly(vinylidene fluoride) (PVDF) and poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) show good affinity with the organic liquid electrolyte, which offers high ionic conductivity and more electrolyte retention due to their porous structure.31 But the mechanical strength of such macro porous membranes is inferior to that of dense membranes.32 The use of microporous separators fabricated from polyolefins is confined due to their sluggish ion transfer kinetics and poor thermal stability. Currently, the most commonly used dense separator membranes are Nafion and sulfonated poly(ether ether ketone) (SPEEK) membranes. Separators from these membranes are fabricated by immersing them in sulfuric acid solutions. Nafion is mainly composed of a hydrophobic Teflon backbone and hydrophilic sulfonic acid groups.33–35 However a major drawback with these membranes is that they are expensive and the raw materials required for their fabrication are limited with high carbon footprint.36,37Fig. 1 illustrates the progress made in the choice of materials used to fabricate separators and the manufacturing processes that are used.
The choice of the separator depends on the application involved. Separators employed in structural members should carry mechanical load. Wearable electronics need flexible separators. For applications like oil drilling, separators should withstand high temperatures with least compromise on their performance. Sometimes they should be able to harvest as well as store energy. This review focuses on the different materials being recently used as separators, various fabrication processes involved and their combined effect on the performance of SCs.
2. Materials for separator membranes
2.1. Polymer based membranes
Polymer based materials are promising candidates for use as separators as they possess high chemical and mechanical resistance apart from being cheap, abundant and easily processible. Polypropylene (Celgard) separators are the most commonly used commercial separators in SCs. Several other polymers such as polyethylene (PE), PVDF, PVDF-HFP, poly(vinyl chloride) (PVC), poly(ethylene glycol) (PEG) and poly(ethylene oxide) (PEO) have also been used to fabricate separators for SCs.38–40 Polymer based separators can be fabricated using a variety of methods. Electrospinning is commonly used to fabricate both single component membranes (e.g. PVDF) and composite separator membranes (PVDF combined with nano sized ceramic particles or blend with other polymers). The porosity and pore size of the separator membranes can be closely controlled using this process.41,42 Electro-spun fibre membranes can be applied over electrodes as separators as they are highly porous with large specific surface area and have a wide electrochemical performance window compared to commercial separators.43 Compared with separators manufactured by solution casting or the phase inversion method, electro-spun membranes offer more electrolyte uptake due to high porosity, reduced interfacial resistance and faster ionic diffusion.44 The electrospinning process was used to fabricate two separator membranes namely TUX5 (25% PVDF solution dissolved in N,N-dimethylacetamide (DMA)) and TUX10 (22.5% PVDF solution dissolved in N,N-dimethylformamide (DMF)–acetone mixture).45 The influence of the temperature and separator material characteristics such as porosity and composition on the performance of SCs was studied. A meso–macro porous melted structure was observed in the SEM micrographs of the TUX5 separator (thickness ∼10 μm) which was mainly attributed to the high PVDF concentration and rapid polymer solution feed rate.46,47 On the other hand, a uniform structure of fibres was observed in the SEM images of TUX10 separator membranes (thickness ∼20 μm). The porosity in TUX10 (∼91%) was also found to be much higher than that in TUX5 membranes (∼40%). This resulted in higher frequency series resistance in TUX5 as compared to that in TUX10 separator membranes. The relaxation time constant was influenced by the temperature and separator properties. The specific power of SCs employing TUX10 separators was 5 times lower at −30 °C as compared to the maximum specific power which was observed at 24 °C as it also depends on the temperature and separator material parameters like porosity. Aarthi et al. fabricated separator membranes by varying the concentration of PVDF (5% to 25%) in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% of DMA and tetrahydrofuran using the electrospinning process.48 In a similar way, it was observed that the average diameter of the fibres was enhanced with the increasing concentration of PVDF. However, membranes with 20% concentration of PVDF were found to be optimum as it exhibited promising properties such as high thermal stability up to 450 °C, maximum electrolyte uptake of 200 ± 2%, a porosity of 86.8 ± 2% and a crystallinity of 42.7%. The high porosity and high electrolyte uptake in separator membranes with 20% PVDF were attributed to the lower fibre diameter (400 ± 8 nm) as compared to the other membranes.
50 wt% of DMA and tetrahydrofuran using the electrospinning process.48 In a similar way, it was observed that the average diameter of the fibres was enhanced with the increasing concentration of PVDF. However, membranes with 20% concentration of PVDF were found to be optimum as it exhibited promising properties such as high thermal stability up to 450 °C, maximum electrolyte uptake of 200 ± 2%, a porosity of 86.8 ± 2% and a crystallinity of 42.7%. The high porosity and high electrolyte uptake in separator membranes with 20% PVDF were attributed to the lower fibre diameter (400 ± 8 nm) as compared to the other membranes.
He et al. employed electrospinning followed by the phase separation method to manufacture porous PAN nanofiber separators from polyacrylonitrile/polyvinylidene fluoride (PAN/PVP) blends where PVP, a separated phase, was used as a pore forming agent (Fig. 2a).49,50 Increasing concentrations of PVP (up to PAN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVP-10
PVP-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 wt%) in the blend increased the pore size (and hence an increased specific area and meso/macropore capacity) of the electrospun PAN nanofibers after the removal of PVP (Table 3). Due to this, the electrolyte uptake of such porous nanofibers was much higher as the electrolyte was not only adsorbed on the surface but also impregnated into the meso/macropores of the separator (Fig. 2h). Faster ion diffusion corresponds to lesser electrical double layer forming time.
5 wt%) in the blend increased the pore size (and hence an increased specific area and meso/macropore capacity) of the electrospun PAN nanofibers after the removal of PVP (Table 3). Due to this, the electrolyte uptake of such porous nanofibers was much higher as the electrolyte was not only adsorbed on the surface but also impregnated into the meso/macropores of the separator (Fig. 2h). Faster ion diffusion corresponds to lesser electrical double layer forming time.
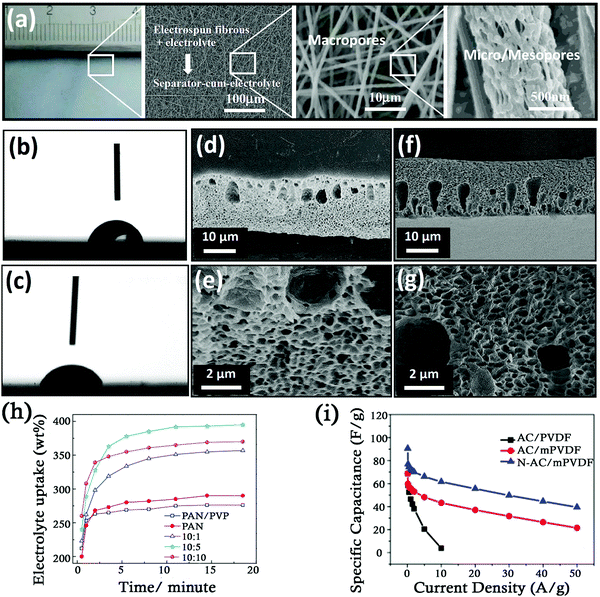 | ||
| Fig. 2 (a) Hierarchical porous structure of separators fabricated from PVDF fibres. Reproduced with permission.49 (b) Contact angle test for PVDF. (c) Contact angle test for modified PVDF. (d and e) SEM images of PVDF. (f and g) SEM images of modified PVDF. (h) Plot of the electrolyte uptake vs. time of various separators. Reproduced with permission.50 (i) Specific capacitance vs. current density. Reproduced with permission.51 | ||
The phase inversion process is widely used in the fabrication of porous SC membranes as it is cheaper and more efficient as compared to electrospinning. Karabelli et al. used the phase inversion method to fabricate microporous separator membranes from PVDF and PVDF-HFP using acetone as a solvent.32 The PVDF separators displayed good mechanical properties and had a high porosity of 80%. The authors also reported that the PVDF separators exhibited a higher value of ionic conductivity (18 mS cm−1) as compared to CelgardTM (commercial polyolefin separators with 4 mS cm−1) and cellulose separators (10 mS cm−1) when filled with a molar solution of tetraethylammonium tetrafluoroborate (TEABF4) in acetonitrile. Xie et al. fabricated symmetric SCs using two different carbon materials namely activated carbon (AC) (specific surface area of 1187 m2 g−1) and nitrogen enriched activated carbon (N-AC) (specific surface area of 1744 m2 g−1) as electrodes and a PVDF membrane as a separator.51 The PVDF membrane was fabricated through the phase inversion process and modified using poly (vinyl alcohol) (PVA) and glutaraldehyde (GA). The decreased contact angle for the modified PVDF membrane (from 73.5 to 61.0°) indicated the reduced surface free energy and increased hydrophilicity (Fig. 2b and c). These modifications did not change the thickness of the membrane significantly (∼20 μm). The membrane possessed an asymmetrical structure comprising mainly of honeycomb like nano sized pores in the skin layer and finger like large pores in the sub layer, providing a sufficient electrolyte reservoir and channels for ion diffusion. (Fig. 2d–g) The decrease in porosity of the membranes (72.4% to 64.4%) post modification was attributed to the vacant pores being occupied by cross-linked PVA and GA molecules. SCs fabricated using the unmodified PVDF separators showed less specific capacitance because of being poorly hydrophilic and was unable to store sufficient amount of electrolyte, thus hindering the movement of free electrolyte ions. The specific capacitance of AC/PVDF SCs was reported to fall dramatically from 69 F g−1 to 4 F g−1 as the current density increased from 0.1 A g−1 to 10 A g−1 while in the case of AC/modified PVDF SCs, the specific capacitance decreased from 43 F g−1 to 22 F g−1 as the current density increased from 10 A g−1 to 50 A g−1 (Fig. 2i). The better retention of capacitance in AC/modified PVDF SCs was due to low internal resistance as compared to AC/PVDF SCs.
Apart from the phase inversion process and electrospinning, other novel methods have also been used to fabricate polymer based separators. Hashim et al. demonstrated a cost-effective method of fabricating SCs.33 The authors prepared a separator from a mixture of hybrid polymer electrolyte PVA (70%) and phosphoric acid (30%) which was immersed in a mixture of lauroyl chitosan and poly(methyl methacrylate) (PMMA). PVA and lauroyl chitosan were chosen as they possess good mechanical properties and the latter also has the ability to retain high levels of ionic liquids.52,53 The separator was sprinkled with 0.15 g of commercially prepared multi-walled CNTs on either side following which it was assembled into an innovative SC tester. The authors also observed a very high ionic conductivity of 64.2 and 1.84 mS cm−1 when electrochemical impedance spectroscopy (EIS) was carried out in an interval of 7 days. Coaxial wet spinning is one such process which was used to fabricate PVDF nanofiber separators whose thickness was controlled by regulating the PVDF content (5 wt% to 30 wt%).54 10 wt% of PVDF was found to be the ideal concentration in the separator. Though the separator displayed a highly wrinkled network architecture, it favoured a large contact between the electrode/electrolyte and rapid diffusion of electrolyte ions with a minimum risk of a short circuit. The separator also exhibited exceptional flexibility as no change was observed on bending the separator at angles up to 180°. Even after 100 such bending cycles (0° to 180°), only a 3% drop in the specific capacitance was reported. The SC (graphene/PVDF nanofiber/graphene) fabricated using this separator has potential to be used in flexible and foldable devices as it can undergo twisting, coiling, knotting, etc. It displayed promising electrochemical properties (Table 3).
The use of polymer based membranes in SCs is highly restricted due to their limited electrolyte wettability owing to their inherent hydrophobicity, low ionic conductivity and low porosity.60 In order to address this limitation, radio frequency (RF) air plasma treatment was used by Vargun et al. to improve the wettability of a porous polylactic acid (PLA) based biodegradable separator, commercially available Celgard 2400 and NKK-MPF30AC-100 separators.56 The water contact angles were decreased in all the three separators post plasma treatment. The plasma treatment led to the formation of super hydrophilic surfaces (water contact angle = 0°) in PLA and NKK-MPF30AC-100 separators. The separators also showed improved water uptake values as well as higher ionic conductivities in 1 M H2SO4 and 1 M Na2SO4 electrolytes post plasma treatment (Table 3). The higher ionic conductivities were attributed to the improved hydrophilicity of the separator membranes. The improved wettability of Celgard 2400 and the PLA separators were attributed to two major factors namely the formation of an oxidized nano layer and the introduction of functional groups on the separator surface. The mechanical property analysis of the membranes revealed that the RF-PLA membranes exhibited good tensile properties despite their highly porous nature (Table 1). Surface modifications of polyamide and polypropylene non-woven fabrics based separators using low energy plasma also resulted in an improved wettability of the separators which was attributed to the surface oxidation of polymers.61 The improved wettability led to a reduction in the total wetting time of the separator as well as influenced an increase in the conductivity of the electrolyte present inside the separator.
| Separator material | Young's modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) | Ref. |
|---|---|---|---|---|
| SPAES/PEO-10 wt% | — | 39.1 | 15.4 | 55 |
| SPAES/PEO-50 wt% | — | 19.0 | 41.5 | |
| SPAES/PEO-60 wt% | — | 7.0 | 4.7 | |
| RF-PLA | 327.1 ± 64.2 | 15.2 ± 9 | 10.9 ± 2.4 | 56 |
| RF-Celgard 2400 | 481.6 ± 56.6 | 20.5 ± 2.1 | 6.7 ± 1.6 | |
| RF-NKK-MPF30AC-100 | 62.7 ± 15.4 | 3.3 ± 0.8 | 44.2 ± 6.5 | |
| Porous cellulose (ACR-7) | 5430 | 71.71 | — | 57 |
| Porous cellulose (8 wt%) | 2010 | 29.22 | — | 58 |
| Mesoporous cellulose membrane | 8930 | 171.5 | — | 59 |
The electrolyte separator gel polymer films used in FTSCs are prone to getting compressed when the SCs are subjected to compression and bending. The change in the thickness of these films results in a change in the electrode spacing leading to unstable capacitance performance. In order to control the electrode spacing, spacers in the form of monodispersed polystyrene (PS) microspheres were mixed with PVA–LiCl gel which performed the role of an electrolyte separator.68 The diameter of the PS microspheres (20, 40 and 80 μm) determined the spacing between the indium tin oxide-polyethylene terephthalate (ITO-PET) glass electrodes. The rectangular like shape of the CV curves of the ITO-PET/PVA-LiCl-PS/ITO-PET SC at a scan rate of 100 mV s−1 suggested an ideal behaviour of the SC (Fig. 3a). The SCs with 20, 40 and 80 μm PS microspheres exhibited a specific capacitance of 27.3, 28.2 and 23.5 μF cm−2, respectively. A comparison of the properties of FTSCs with and without PS microspheres showed that both FTSCs exhibited similar current densities and capacitance performance under normal operating conditions However, under bending conditions, the FTSC with PS microspheres showed a nearly unchanged CV curve when bent from 0 to 180° while the FTSC without PS microspheres exhibited CV curves which changed from rectangular to a shuttle like shape as displayed in Fig. 3b and c. The change in the shape of the CV curve was attributed to extra sheet resistance and internal resistance caused due to uneven electrode spacing.69 The SC demonstrated the best performance with a specific capacitance of 147.1 μF cm−2 when electrode spacing was controlled using 40 μm microspheres.
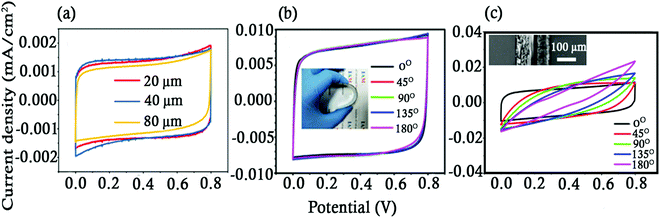 | ||
| Fig. 3 CV curves of the (a) FTSC and the FTSC with (b) 40 μm spacers and (c) without PS microspheres at different bending angles at a scan rate of 100 mV s−1. Reproduced with permission.68 | ||
The challenge with the flexible SCs developed for wearable electronics is to offer consistent electrochemical performance during stretching, twisting and bending. Li et al. developed vertically aligned PVA–H2SO4 (APH) hydrogel films (employed as an electrolyte separator); manufactured by freeze–thawing and directional-freezing methods as shown in Fig. 4a.70In situ growth of poly(aniline) (PANI) electrodes on both sides of this film was achieved to form an integrated SC (APH-PANI). It exhibited 4.65 times higher specific capacitance (25.85 mF cm−2 at 0.05 mA cm−2) compared to the SC with a random network (PH-PANI). This SC demonstrated a consistent and stable electrochemical performance during various deformations (Fig. 4b and c). The APH-PANI film also displayed self-healing ability by reorganizing its dynamic physical hydrogen bonds (Fig. 4a and d–f).71,72 In a similar way, Hsu et al. developed hydrogels (separator/electrolyte) based on inexpensive natural polymers of gelatin and cellulose nano-crystals (CNCs).72 Mussel inspired tannic acid (TA) was used to treat this hydrogel and endow it with self-healable properties.73 This separator exhibited autonomous self-healing within 6 h at room temperature and did not require any external stimuli for the same. A flexible, nontoxic, biodegradable, and biocompatible SC was manufactured by coating PANI and RGO on either sides of this hydrogel. It displayed 84, 69 and 82% retention of specific capacitance, energy density, and power density respectively compared to its original values after five cuts and healing cycles. Such self-healable SCs have potential uses in wearable energy storage devices, sensors, e-skins, etc.
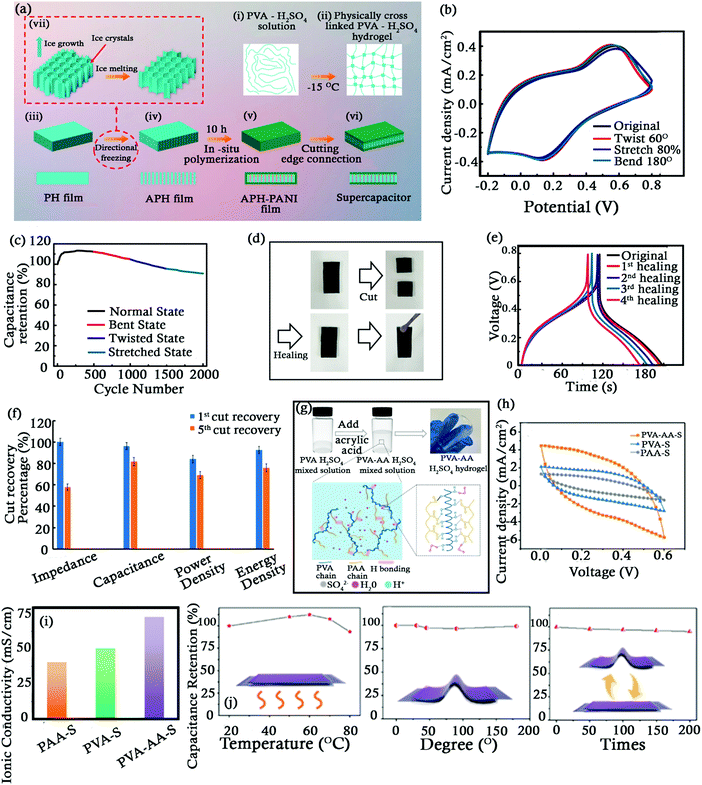 | ||
| Fig. 4 (a) Schematic diagram showing the fabrication of an APH-PANI SC. (b) CV curves of the APH-PANI SC showing its capacitance retention ability under different conditions. (c) Capacitance retention vs. number of cycles. (d) The process of self-healing in the APH-PANI SC. (e) Voltage vs. time curves with different self-healing times. Reproduced with permission.70 (f) Recovery percentage vs. electrochemical performance of a self-healing SC. Reproduced with permission.72 (g) Schematic showing the preparation of the PVA–AA–S hydrogel. (h) CV curves of 3 different hydrogels. (i) Ionic conductivity of 3 different hydrogels. (j) Capacitance retention of the SC under heating, bending and bending – releasing cycles. Reproduced with permission.74 | ||
To impart mechanical strength and low temperature tolerance to flexible energy storage devices, Yu et al. developed an all-in-one SC with a novel, hydrogen-bonding reinforced, dual-crosslinked hydrogel (separator/electrolyte) made of poly(vinyl alcohol), acrylic acid, and H2SO4 (PVA–AA–S) as shown in Fig. 4g and h.74 This hydrogel did not only tolerate high compressive stress (0.53 MPa) and demonstrated high stretchability (up to 500%) but also displayed high ionic conductivity (75 mS cm−1) (Fig. 3i). Strong and abundant hydrogen bonding formed between PVA, PAA chains and water molecules was responsible for this superior performance.75 The SC so formed was resilient to various thermo-mechanical stimuli and also exhibited very high capacitance retention for repeated processes as shown in Fig. 4j. This SC was also able to retain 80% of capacitance even after working at −35 °C for 23 days and hence made themselves capable of serving at high altitudes, thanks to the excellent low temperature resistance of this hydrogel. Hydrogen bonding also stimulates the self-healing properties, permitting the self-assembly of all-in-one SCs.76 It was surprisingly found that the hydrogel when cut into two halves self-healed and reformed within one hour, displaying the excellent self-healing properties of this hydrogel. Li et al. obtained a cellulose based, flexible hydrogel employing polydopamine (PDA) as a crosslinking agent between polyacrylamide (PMA) and cellulose.77 At a ratio of 0.4 (DA/PM), the double cross-linking (Π–Π stacking in PDA and hydrogen bonds formed in the gel network) ensured superior mechanical (high flexibility) and self-healing properties. After Fe3+ functionalization, this hydrogel was converted to an aerogel separator. The EDLC using a KOH saturated aerogel membrane displayed 127% higher capacitance (172 F g−1) than the commercial polypropylene (PP) separator membrane (75.8 F g−1) at 1 A g−1. It also displayed high capacitance retention (84.7%) with longer life (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), especially due to the high electrolyte retention (549%). The integrated micro-SC fabricated employing this hydrogel displayed remarkable areal and volumetric capacitances of 275.8 mF cm−2 and 394.1 F cm−3 at 10 mV s−1 respectively.
000 cycles), especially due to the high electrolyte retention (549%). The integrated micro-SC fabricated employing this hydrogel displayed remarkable areal and volumetric capacitances of 275.8 mF cm−2 and 394.1 F cm−3 at 10 mV s−1 respectively.
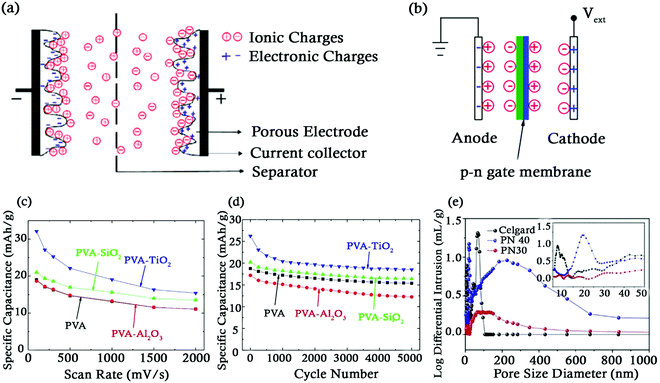 | ||
| Fig. 5 (a) Schematic of a SC. (b) Mid cell gate structure similar to a diode. Reproduced with permission.80 (c) Variation of specific capacitance as a function of scan rates at a potential window of 0–1.5 V, (d) Cycle life of various separators. Reproduced with permission.83 (e) Plot of porosity distribution. Reproduced with permission.84 | ||
2.2. Polymer–ceramic based membranes
Dielectric materials comprising polymers and ceramics are commonly used as separators to achieve high power and energy densities. Polymers exhibit excellent mechanical and chemical stability but have a low dielectric constant. On the other hand, ceramics have a high dielectric constant, high ionic conductivity and high thermal stability but lack mechanical strength.85,86 Alvarez et al. combined the desired properties of both ceramics and polymers by fabricating polymer/ceramic composite (PCC) separators comprising of PVDF, polypropylene (PPy) and a mixture of earth based metal titanates such as SrTiO3, CaTiO3, and BaTiO3 using the phase inversion process.87 The porosity of the separator was improved by the addition of lithium chloride. The PCC separators also offered a lower ohmic resistance (∼45 Ω) and had an improved dielectric constant (4.52) as compared to commercially available separators (CelgardEz 2090 grade- ohmic resistance 47 Ω, dielectric constant 2.2). The PCC separators were heat treated to allow thermodynamic arrangements of the various components of the separator. The Young's modulus of the PCC separator improved by 71.1% on average post heat treatment. The improvement in the dielectric constant of PCC separators as compared to that of commercial separators was attributed to the addition of the ceramic into the separator. The specific capacitance of the separator was found to reduce at elevated temperatures. This was attributed to an increase in the density as the pores tend to close at higher temperatures enabling the presence of a higher mass in the same volume.The control of pore's size and its distribution in the separator membrane is very important as it has an influence on the internal resistance and ionic conductivity of SCs. Ceramic particles can be used to increase the porosity of separators having a polymer matrix. PVA–ceramic composite separators were fabricated by adding ceramic particles of Al2O3, SiO2 and TiO2 to an aqueous solution of PVA.83 This addition of ceramic particles creates amorphous regions in the crystalline polymer matrix, thus creating a more porous morphology leading to an increase in the ionic conductivity (maximum by 100% in the PVA–TiO2 separator). The increase in porosity did not appreciably affect the structural integrity of the separator making them safe for use in flexible energy storage devices. A 68.6% increase in the specific capacitance of PVA–TiO2 composite separators (32 F g−1) was observed when compared with bare PVA separators (19 F g−1) at a scan rate of 100 mV s−1 (Fig. 5c). But the % capacitance retention for PVA–TiO2 was nearly the same as that of the bare PVA separator (∼70%) after 5000 cycles as shown in (Fig. 5d). It is desired that SCs possess a low internal resistance during the charge/discharge process in order to minimise the energy loss in the form of heat. When a BaTiO3/(PEDOT:PSS) composite was used as a separator in a graphene SC with PVA as the electrolyte, a low internal resistance of 42 Ω was reported.88 The lower internal resistance was attributed to the pore size of the separator membrane which was smaller as compared to that of the PVA film.89 The graphene SC exhibited a high specific capacitance of 195 F g−1.
The pore size in separators can be controlled using different manufacturing processes. However, a majority of the manufacturing processes that control the pores like electrospinning and solvent exchange are expensive and time consuming. Liu et al. employed a low-cost casting (stir-pour-dry) technique to develop a highly porous network of Al2O3 nanowires (NW)/polyvinyl butyral (PVB) composite membrane.84 At Al2O3 NW concentrations between 30 and 40 wt% (denoted as) PN30 and PN40 in Fig. 5e, this amazing non-woven membrane exhibited high porosity (42–75%), high flexibility, high strength (>30 MPa), high temperature withstanding ability (up to 200 °C), high electrolyte uptake (>200 wt%), little to no swelling behaviour even at 200 °C, and higher ionic conductivity of up to 13.5 mS cm−1; a performance way better than those of the commercial Celgard separators. Mercury porosimetry measurements revealed higher porosity (42 and 75%) and broad pore size distributions (5 to 1000 nm) for PN30 and PN40 compared to their commercial counterpart (pore size <20% for its distribution) (Fig. 5e).
2.3. Ceramic membranes
The use of separators fabricated from cellulose, polymers, etc. in SCs employed in high temperature applications such as oil drilling, under hood automotive components, etc. is not feasible as they undergo unreliable shrinkage and deformation at elevated temperatures. For such high temperature application-specific SCs, a combination of solid organic electrolytes/ionic liquids90,91 as electrolytes along with natural clay/ternary sulphide glass/combinations of ceramic materials is used.92,93 SCs were fabricated for such high temperature applications having ceramic based separators, carbon electrodes and a 2 M KOH–glycerine solution (boiling point of 290.9 °C) as an electrolyte.94 The ceramic separators mainly comprised of nickel oxide (NiO) and zirconium dioxide (ZrO2). NiO was added to the separator membrane as it has a negative temperature coefficient of resistance and thus offers lower resistance at elevated temperatures. This actually enhanced the capacitance at higher temperatures (Table 3). Graphite powder was added as a pore forming agent with its content being varied from 10 to 30%. The addition of graphite also led to an increase in the operating temperature and the pore size in the separator resulting in rapid ion diffusion and enhanced capacitance. The specific capacitance increased with an increase in the graphite concentration. However, at graphite concentrations of 40%, the separator membrane exhibited poor mechanical behaviour. SCs with separator membranes having 30% graphite composition exhibited good electrochemical properties at a temperature of 140 °C (Table 3).Apart from high temperature applications, ceramic based separators have also been employed in SCs used for wearable electronic devices. SCs to be used in such devices are characterized by their compactness, flexibility and high energy storage ability. Scalable printing technology has been used to fabricate planar asymmetric SCs for such applications.95 Monolithic films were layered one over the other and integrated on a single substrate. The stacked layers were made of MnO2/poly 3,4-ehtylene dioxythiophene: polystyrene sulfonate (MnO2/PEDOT:PSS) as the positive electrode, ionically conducting boron nitride as a separator (∼2.2 μm), and the graphene nano-sheet as a negative electrode; all were integrated on a single substrate without additives, binders or metal based current collectors. This flexible SC delivered a volumetric energy density of 8.6 mW h cm−3 (much higher than any conventional asymmetric SC with two substrates reported); demonstrated ∼99% of the initial capacitance even after bending it by 180° and a capacitance retention of 92% after 5000 cycles.
Structural energy storage applications demand that the SC behaves as a structural member and has the ability to carry loads. The separators used in such SCs must contribute to the load carrying capacity without inducing structural defects. This has led to a demand for mechanically robust separators.96,97 To reduce the weight of the system,98 Acauan et al. proposed the concept of development of multifunctional energy storage devices that can carry load like composites. They fabricated structural separators by covering vertically aligned carbon nano tubes (VACNTs) with Al2O3 using the atomic layer deposition technique and sintering followed by the removal of CNTs (Fig. 6).99 The main function of the CNTs was to control the porosity and align the alumina nanotubes (ANTs). These ANT arrays along with ionic polymer electrolytes formed vertically aligned nano fibre separators (VANS). These VANS were placed between the stackings of uni-directional carbon fibres (electrodes of the SC) to fabricate the multifunctional composite laminate/SC. The presence of ANTs in the polymer electrolyte reduces its crystallinity, thus resulting in high ionic conductivity of VANS. The major advantage of VANS over other separators is its resistance to delamination (de-bonding of adjacent plies due to the weak interface) which is a very common mode of failure in laminated composites. The authors found the strength of the VANS to be similar to that of the structural separator and improved by 47% as compared to that of a commercial separator. The authors reported that the VANS based laminate exhibited nearly similar mechanical performance to that of the structural separator, whereas it displayed a 47% increase in tensile strength, 131% increase in effective stiffness, 51% increase in inter laminar shear strength compared to commercial separators. However, a 6% decrease was reported in the inter-laminar shear strength of VANS as compared to that of the structural separator. This decrease was attributed to the presence of interlaminar voids present in VANS.
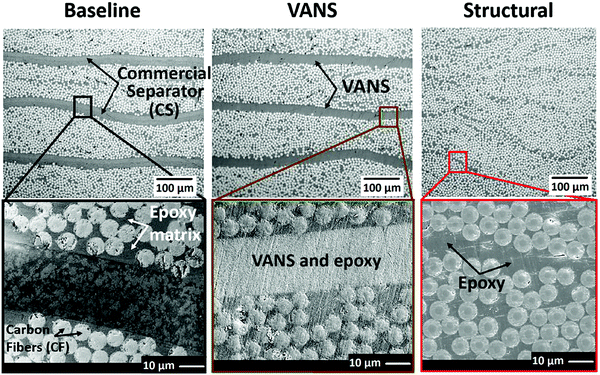 | ||
| Fig. 6 Morphological comparison of commercial separator, VANS, and traditional monofunctional structural composite interfaces via optical (top) and SEM (bottom) images of interior (4 plies) of the composites. Reproduced with permission.99 | ||
2.4. Bio based separator membranes
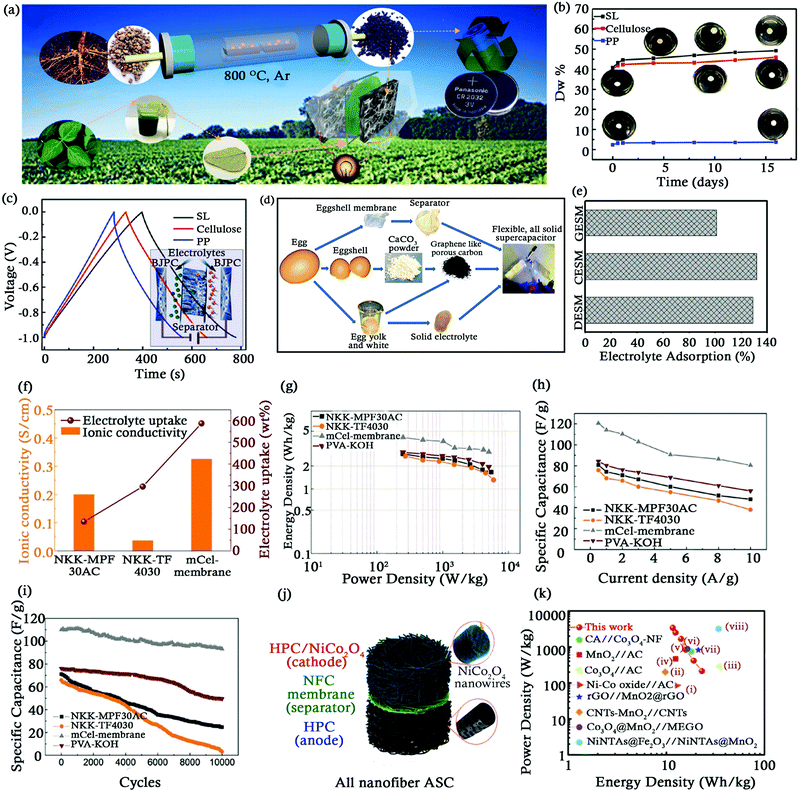 | ||
| Fig. 7 (a) Preparation of BJPCs, (b) the electrolyte uptake of the separators, (c) GCD curves obtained on using BJPC – 1.5 as the electrode and the three different materials as separators. Reproduced with permission.100 (d) An illustration of the various components of eggs used for the fabrication of all solid SCs. Reproduced with permission.110 (e) The electrolyte absorption capacity of different membranes. Reproduced with permission.107 (f) The electrolyte uptake of the three separators. (g) The ionic conductivity of the three separators. (h) Specific capacitance of the separators at different current densities. (i) Specific capacitance vs. number of cycles. Reproduced with permission.59 (j) Schematic diagram of an ASC fabricated using nanofibers. (k) Comparison of electrochemical performance of ASCs with other SCs fabricated in previous studies namely (i) ref. 122, (ii) ref. 123, (iii) ref. 124,(iv) ref. 125, (v) ref. 126, (vi) ref. 127, (vii) ref. 128, and (viii) ref. 129. Reproduced with permission.119 | ||
However, the low porosity of tree leaves serves as a major limitation for its use as separators. Chemical modification using alkaline solutions is one of the methods by which the porosity of tree leaves can be improved. This method was used by Jin et al. in their experiments where they carried out the NaOH activation process on the surface of four different kinds of tree leaves namely cinnamomum camphora (CC), magnolia grandiflora (MG), platanus orientalis (PO) and osmanthus fragrans (OF).103 These leaves had different chemical compositions and hence different pore morphologies were observed. A 3-D porous network structure comprising nano-sized and macropores was observed in CC leaves while irregular porous network structures were observed in MG and PO leaves. Lignin and hemicellulose present in the CC leaf were partially removed by the NaOH solution leading to an increased number of pores resulting in higher capacitance performance (Table 3). The activation time with NaOH significantly impacted the porous structure of the CC leaf. Higher the activation time, greater the size of the pores formed and lower the capacitance performance as the ion concentration in the separator was reduced.104 CC leaves demonstrated to be a good candidate for separators in SCs.
Poli et al. fabricated easy-to-dispose SCs employing green raw materials; a step towards sustainable manufacturing and easy recycling.105 Electrospinning was performed at room temperature to produce a biodegradable pullulan separator. Carbon electrodes were prepared from pepper seed-waste and 1-ethyl-3-methyl-imidazoliumbis(trifluoro-methyl-sulfonyl)imide was used as an electrolyte. Such a SC delivered up to 27.8 W h kg−1 energy density and 5 kW kg−1 power density at 3.2 V. Combining a hydrophilic separator with a hydrophobic ionic liquid electrolyte eases the task of separation of the components after the end-of-life.
| Egg shell membrane | Thickness (mm) | Average fibre diameter (nm) | Current (mA) | Voltage (V) | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|---|
| DESM + 1 wt% TiO2 | — | — | 0.02 | 0.002 | 0.7 | 108 |
| DESM + 5 wt% TiO2 | — | — | 0.007 | 0.002 | 2.3 | 108 |
| DESM + 10 wt% TiO2 | — | — | −0.0021 | −0.004 | 0.035 | 108 |
| DESM | 0.10 | 1.314 | — | 0.5 | 156.14 | 107 |
| CESM | 0.03 | 1.177 | — | 0.5 | 159.22 | 107 |
| GESM | 0.14 | 937 | — | 0.5 | 153.06 | 107 |
ESM based separators exhibit excellent thermal stability, mechanical strength (tensile strength = 6.59 MPa) and electrochemical performance. ESMs of 0.8 mm thickness can sustain a maximum stress of 6.6 ± 0.5 MPa and a strain of 7 ± 0.3% which is slightly lower when compared to commercially used polypropylene (PP) separators.109 They can also retain their properties at temperatures below 100 °C and degrade at temperatures above 220 °C thus indicating their excellent thermal stability. A comparison of the degree of water uptake and swelling revealed that it was higher in ESMs (10 and 8%, respectively) as compared with that in PP based commercial membranes (3%). SCs fabricated using ESM based separators display improvement due to low resistance, lesser relaxation time (t ∼ 4.76 s), and splendid cyclic stability (more than 90% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).
000 cycles).
Apart from fabricating separators from egg shells, attempts have also been made to fabricate SCs entirely from materials derived from eggs. All solid-state, flexible SCs were successfully fabricated as shown in Fig. 7d.110 The electrodes were developed using 1.25 nm thick 2D graphene like egg derived carbon sheets as they exhibited exceptional power and energy densities owing to their high surface area of 1527 m2 g−1 and naturally doped functional groups. Egg white/yolk reacting with KOH was used as a gel like solid electrolyte while ESMs were used as separator membranes. The ESMs had an interwoven, macroporous network of fibres whose diameter ranged between 0.5 and 1 μm. The SC thus developed showed excellent flexibility as its specific capacitance remains unchanged on being subjected to bending and twisting. When two such SCs were connected in series, they were able to light up an LED for hundreds of seconds thus highlighting their potential for employment in practical applications. In another such attempt, bio-waste ESMs were used for the development of electrodes and separators to be employed in asymmetric SCs.111 Biomaterials when carbonized, oxygen and nitrogen can firmly be doped in the carbon structure. Such bio-derived carbon does not only offer an optimum pore size (size bigger than the microporous structure) for ion absorption/transport but also the additional pseudo-capacitance due to the presence of O and N.112,113 The combination of air-activated ESM carbon as the cathode, MnO2 nanoparticle-chemically activated ESM carbon as the anode, and a natural ESM bio-separator displayed good electrochemical performance (Table 3) and showed a path for making renewable energy devices by utilizing biowaste materials.
| Separator material || Electrolyte | Separator membrane thickness (μm) | Porosity of the separator | Ionic Conductivity of the separator (mS cm−1) | Electrolyte uptake | Electrode material | Specific capacitance (F g−1) | Current density (A g−1) | Scan rate (mV s−1) | Energy density (W h kg−1) | Power density (W kg−1) | No. of cycles | Capacitance retention | Refe. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrolyte separators | ||||||||||||
| PVA-KOH-K3[Fe(CN)6] | — | — | 45.56 | — | Activated carbon | 430.95 | 0.5 | — | 57.94 | 59840 | 1000 cycles | 89.3% | 67 |
| — | — | — | 322.8 | 3 | — | |||||||
| PVA, H2SO4 and BAAS | 1500 | — | 21.40 | — | Activated carbon | 390 | 0.8 | — | 30.5 | 600 | 1000 cycles | 90% | 66 |
| — | — | 358 | 1.5 | — | ||||||||
| — | — | 305 | 3 | — | ||||||||
| PVA, H2SO4 and AQQS | 1500 | — | 28.50 | — | Activated carbon | 448 | 0.5 | — | 30.5 | 350 | 1000 cycles | 91% | 62 |
| — | — | — | 23.2 | 2037 | ||||||||
| PVA, H2SO4 | 1500 | — | — | — | Activated carbon | 148 | 0.5 | — | 10 | 350 | — | 62 |
| Polymer based membranes | ||||||||||||
| PVA, methacrylate and lauroyl chitosan | — | — | — | — | Commercially prepared multiwalled CNT | 33 | — | — | 4.64 | 8.3 J g−1 s−1 | > 1000 (cycle life) | 33 |
| PVDF ultrafine porous fibres | — | — | 1.8 | 360 wt% | Activated carbon | 39.5 | 0.1 | — | — | — | — | 49 |
| PVDF || 6 M KOH | 20 | 72.4% | — | — | Activated carbon | 69 | 0.1 | 100 | — | — | — | 51 |
| — | — | 4 | 10 | 100 | — | — | — | |||||
| 20 | 72.4% | — | — | Nitrogen enriched activated carbon | 91 | 0.1 | 100 | — | — | — | ||
| — | — | 40 | 50 | 100 | — | — | — | |||||
| PVDF modified with PVA and GA || 6 M KOH | 20 | 64.4% | — | — | Activated carbon | 43 | 10 | 100 | 8.3 | 50 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles | 92% 000 cycles | 92% |
51 |
| — | — | 22 | 50 | 100 | 51 | |||||||
| PVDF/LiTFS || PEO/LiClO4 (NaI/I2 as mediator) | — | — | 17.4 | — | Porous carbon | 209 | — | 10 | 49.1 | 1600 | — | 37 |
| — | — | — | 107.5 | — | 50 | — | 37 | |||||
| — | — | — | 52.9 | — | 200 | — | 37 | |||||
| PVDF/LiTFS || PEO/LiClO4 (K3Fe(CN)6/K4Fe(CN)6 as mediator) | — | — | 17.4 | — | Porous carbon | 138.8 | — | 10 | 33.6 | 1300 | — | 37 |
| — | — | — | 82.5 | — | 50 | — | 37 | |||||
| — | — | — | 36.5 | — | 200 | — | 37 | |||||
| PVDF nanofiber || H3PO4 | — | — | — | — | Graphene | 246.5 | 0.6 mA cm−2 | — | 30.8 mW h cm−2 | 0.24 mW cm−2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles | 94% 000 cycles | 94% |
54 |
| SPAES/PEO composite membrane || 1 M Li2SO4 | 80 | — | — | 161% | Activated carbon | 142.5 | 0.1 | — | 19.04 | 50.74 | 5000 cycles | ∼100% | 55 |
| — | — | 119.7 | 1 | — | 11.98 | 503.63 | 55 | |||||
| Ceramic membranes | ||||||||||||
| NiO/ZrO2 (30 wt% of graphite) || 2 M KOH glycerin solution | 550 | — | — | — | Activated carbon | 342 (Temperature = 140 °C) | 2 | — | — | — | 2000 cycles | 97% | 94 |
| — | — | — | 279 (Temperature = 120 °C) | — | — | — | 94 | |||||
| Bio based separators | ||||||||||||
| CC leaf (NaOH activation treatment) | — | 15% | — | — | Reduced Graphene oxide | 251 F g−1 after 12 h activation time | 0.5 | — | — | — | 1000 cycles | 80% | 103 |
| — | 10% | — | — | 225 F g−1 after 24 h activation time | — | — | — | 103 | ||||
| — | 11% | — | — | 136 F g−1 after 36 h activation time | — | — | — | — | 103 | |||
| Soya bean leaf || 6 M KOH | 129 | 85% | 1940 | 52% | Activated carbon | 358 | 1 | — | — | — | 8000 cycles | 91% | 100 |
| Porous cellulose (ACR-7) || 6 M KOH | — | 74.90% | 298.6 | 323.68% | Self-made electrode | 130 | 0.5 | — | 25.94 | 360 | 4000 cycles | 81.99% | 57 |
| Porous cellulose (8 wt%) || 6 M KOH | — | 58.43% | — | 329.30% | Activated carbon, acetylene carbon black, polytetrafluoroethylene | 123 | 0.5 | — | 25.94 | 360 | 4000 cycles | 92.09% | 58 |
| Mesoporous cellulose membrane || KOH | 22 | 71.78% | 325 | 587.5 wt% | Activated carbon | 81.4 | 10 | — | 2.94 | 5031 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles | 84.7% 000 cycles | 84.7% |
59 |
| Mesoporous NC || KOH | — | 59% | 265 | 770% | HPC, NiCo2O4 | 64.83 | 0.25 | — | 23.05 | 213 | 1000 cycles | 93% | 119 |
| Bio waste ESM || 1 M Na2SO4 | — | — | — | — | Air and chemically activated carbons | 478.5 | 0.5 | — | 14 | 150 | 1000 cycles | 79% | 111 |
| Graphene oxide films | ||||||||||||
| Graphene oxide paper || 1 M H2SO4 | — | 87.8% | — | — | Graphene oxide | 210 | — | — | 80 | — | 1500 cycles | ∼95% | 142 |
| Smart separators | ||||||||||||
| Braided spandex yarns || PLA and H3PO4 | — | — | 6520 | Modified PLA yarns | 31.02 | — | — | 2.8 | 184 | 500 cycles | 88.5% | 148 | |
| P(VDF-TrFE) || PVA/H2SO4 | — | — | — | — | PDMS –rGO/C | 44.6 μF cm−2 | 25 μA cm−2 | — | 0.078 μW h cm−2 | 0.025 mW cm−2 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles | 98% 000 cycles | 98% |
149 |
| Miscellaneous | ||||||||||||
| PAN@SDBS membrane || 2 M KOH | 82 | — | 14.14 | Activated carbon | 42.8 ± 2.1 | 0.5 | — | — | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles | 84.6% 000 cycles | 84.6% |
28 | |
| Glass Wool || H2SO4 | — | — | — | Activated carbon | 90.3 | — | 5 | — | — | 300 cycles | 97.6% | 150 | |
The use of nanocellulose (NC) in energy storage applications has gained increasing importance in recent years. NC has a high mechanical strength (∼2–3 GPa) and Young's modulus (∼110–140 GPa);117,118 and a potential to fabricate separators as tightly controlled porous structures can be developed.11 Separators fabricated using pure NC get peeled off from the glass substrate upon drying due their poor adhesion with the glass. One can observe the bubble formation when chitosan is used as a separator over a glass substrate which offers an increased possibility of a short-circuit. In contrast, separators comprising 50% NC and 50% chitosan exhibited both good adhesion and no bubble formation. Chitosan plays a key role in such separators as a binder and an adhesion promoter for NC, thus eliminating the peeling off issue observed in separators fabricated from pure NC. The ionic conductivity of this separator film (0.9 S m−1) too is similar to that of pure NC separators (1 S m−1) and hence is advantageous from the manufacturing point of view.
NC was used in the fabrication of all nanofiber asymmetric SCs as shown in Fig. 7j.119 Hierarchical porous carbon (HPC) and HPC/NiCo2O4 derived from forest based NC were used as electrodes and were found to contain 3D fibrilar network with an ultrahigh surface area of 2046 m2 g−1. The mesoporous NC membrane used as a separator facilitated faster ion as well as electron transfer, even with a thicker design of an ASC. The electrochemical functioning of the all nanofiber ASCs outperformed other NC based ASCs reported so far (Fig. 7k). The SC also reported promising electrochemical properties (Table 3).
Bacterial cellulose (BC), a type of NC, has also shown tremendous potential in the fabrication of separators.120 This was observed by Lv et al., who fabricated a flexible, ASC (thickness ∼15 μm) having an integrated electrode–separator.121 The in situ deposition of layers of PANI-BC//BC//KPBC(KOH activated pyrolysis PANI/BC)-CNT formed the cell. As the 3D interconnected network of BC was used as a matrix of integral electrode–separator, the solution resistance was only 2.48 Ω at 1 mol PVA/H2SO4 electrolyte. Promising electrochemical properties such as a maximum volumetric capacitance of 28.3 F cm−3 with 100% capacitance retention even after 2500 cycles at a current density of 0.1 A g−1 were exhibited by this ASC.
2.5. Graphene oxide films
Graphene oxide (GO) films have a morphology distinct from that of macro-porous materials which are commonly used to fabricate separators. Dry GO films are not electrically conducting and instead need a water or a water-based electrolyte to become electrically conducting. The level of oxidation in individual GO films also has a major influence on its electrical conductivity.138 Highly oxidized GO films are the most suitable to be used as separators and were successfully employed by Shulga et al. in SCs having a layered PANi/GO/PANi structure. The SC developed reported a capacity of 150 F g−1 and was capable of retaining its capacity by 90% after 1500 cycles. On replacing the wetting medium around the membrane from H2O to D2O (deuterium oxide), a 1.46 fold increase in the current flowing through the GO membrane was observed, indicating the effect of proton type on the conductivity of the GO separator.139,140 GO membranes were also successfully used as separators (∼15 μm thick) along with GO reduced by microwave exfoliation (MEGO) as electrodes in the fabrication of metal free SCs.141Graphene oxide papers (GOP) developed by separating thicker GO films from substrates are found to have unique membrane properties such as excellent mechanical strength, etc. The properties of GOP as a separator were further explored by using them in SCs with MEGO as electrodes.142 The porous structure of the separator was studied using the standard contact porosimetry (SCP) method with octane and water as the testing media. The separator swelled up significantly to about three times its original width in the presence of water but did not swell up in the presence of octane. The swelling up of the dry membrane when immersed in water resulted in the increase in porosity of the membrane from 1.47 cm3 g−1 to 2.64 cm3 g−1. An increase in the volume of micro pores (r < 1 nm), middle sized pores (1 nm < r < 1000 nm) and the specific surface area (648 cm2 g−1 to 2170 cm2 g−1) and a decrease in the volume of macropores (r > 1000 nm) were also observed. The value of porosity in the GOP was found to be 87.8 vol%. The SCs developed had excellent electrochemical properties such as a capacity of 200 F g−1 and an energy density of 80 W h kg−1. GOP when impregnated with water or water solutions of acids had similar properties such as high porosity, surface area and protonic conductivity to that of commercially used Nafion membranes.
2.6. Metal organic frameworks
Metal organic frameworks (MOFs) have attracted the attention of researchers due to their possible application in next generation energy storage technologies. They mainly comprise metal nodes and organic linkers and exhibit unusual yet exceptional properties such as high porosity and surface area, good thermostability and uniform cavities. These properties along with conductivity can be tuned according to the application the MOF is used in. Hence, a majority of researchers employed MOFs in making electrodes for batteries and supercapacitors.143 The properties such as pore size and topology can be varied based on the selection of appropriate linkers and nodes. Some studies have been reported in the recent past wherein MOF separators were used in batteries are but hardly in supercapacitors.144–146 In one such work of separator membranes in SCs, two thermally stable MOFs namely CoL(1,4-bdc)·2DMF as MOF1 and CdL(4,4′-bpc)·3DMF as MOF2 were fabricated.147 The MOF1 displayed the formation of large channels with a non-interpenetrated network while MOF 2 exhibited a highly interpenetrating network structure with a small pore size. Both MOFs when employed as separators in SCs and charged/discharged at high current density, displayed much smaller specific capacitance in comparison with the blank SCs. This was due to large impedance drawn by these materials for not being electro-conductive. But at a small current density (∼0.2 A g−1), separator MOF1 showed nearly three times larger specific capacitance (66.7 F g−1) than the blank SC (23.2 F g−1) due to promotion of ionic diffusion (probably due to the faradaic redox current produced by cobalt oxide in the separator) and the charge transfer process (due to more porous morphology). Even though a clear mechanism is not understood, it displayed that the right choice of an MOF with suitable functional and structural tunability changes the electrochemical performance of the SC positively.3. Separators for micro-supercapacitors
Planar (2D) micro-supercapacitors (MSCs) can be fabricated either with a sandwich or an inter-digitated structure. In a sandwich structure MSC, a separator is placed between the two electrodes with the electrolyte before assembling and packaging.151 Separators must be chemically resistant to corrosion induced by electrolytes and electrode degradation by-products. The commonly used separator materials are cellulose-based or polyolefin-based polymers such as polypropylene (PP), polyethylene (PE), Teflon, PVDF, and PVC.152,153 However, sandwiched MSCs have limitations due to the possibility of a short circuit or position dislocation of electrode films. Additionally, the thick separator layer invariably increases ion transport resistance, resulting in rapid specific capacitance degradation and low power density.154,155 On the other hand, interdigitated planar MSCs comprise interdigital electrode fingers separated by nano/micrometer size interspaces eliminating the need for a separator. It allows high active loading on each electrode and exposes more area at the electrolyte interface. The absence of a separator allows fast ion diffusion in the electrode and electrolyte surfaces, enhancing the specific capacitance of the MSC.156Cellulose-based membranes as separators have been extensively investigated for MSCs. Furthermore, membranes having self-healing properties may extend the life of the electrolyte separator.157 Li et al.158 developed a flexible double-cross-linked cellulose-based hydrogel membrane as an alternative for conventional polymer separators. They used poly-dopamine (PDA) as a cross-linker between rigid cellulose and flexible poly-acrylamide (PAM) networks. The effect of cellulose and DA on the characteristics of hydrogels was investigated by producing hydrogels (Cn-DM-x) with various cellulose contents (n) and DA/AM ratios (x). The mechanical and self-healing properties of the C4-DM-40 (4% cellulose-40 wt% of DA/AM) hydrogel were found to be superior compared to those of other hydrogels. The MSC electrode fabricated by directly depositing activated carbon materials on the C4-DM-40 hydrogel membrane presented an areal capacitance of 275.8 mF cm−2 and a volumetric capacitance of 394.1 F cm−3 at a scan rate of 10 mV s−1.
For stretchable MSCs, solely the gel electrolyte as a separator fails to prevent the device from dislocating and short circuits when subjected to an external force due to the poor interaction between the internal molecules.159 The manufacturing of commercial polyolefin separators is easy but have poor ionic conductivity because of their small volume porosity and pore size. As a result, an effective strategy is needed to meet the stretchability of the device, with a simplified fabrication process considering large-scale applications. Laser direct writing (LDW) with arbitrary pattern cutting can precisely edit the electrodes and separator, allowing the negative Poisson's ratio (NPR) structure to be transferred into the MSC.160 Yan et al.161 used electrospinning and LDW methods to develop asymmetric MSCs with NPR structures and polyacrylonitrile (PAN) nanofiber separators. The separator was then coated with the gel electrolyte and assembled with two composite electrodes composed of carbon nanofibers and nanoarrays (CNF@Fe2O3 nanorods and CNF@MnO2 nanosheets).
The conductivity of GO depends on the environment and it ranges between 5 × 10−6 S cm−1 and 4 × 10−3 S cm−1,162 indicating that GO is almost electrically insulating. When a significant amount of water is entrapped in the layered GO structure, it transforms into a strong anisotropic ionic conductor as well as an electrical insulator, allowing it to be used as an electrolyte and electrode separator.163 Gao et al.164 utilized direct laser reduction for patterning of hydrated GO films to develop a new type of all-carbon MSC. The electrodes were made from the laser-patterned part of the GO film (rGO). When high-intensity light was absorbed by GO, it was transformed to graphene, and the hydrated GO between the electrodes functioned as a MSC separator.
4. Smart separators
The biggest challenge with the energy storage devices used for electronic applications is the continuous supply of energy for longer time-period, leading to its frequent recharging. To fulfil the requirements of electronic devices for next generation, portable and lightweight self-powered systems such as self-charging SCs are developed which are capable of converting mechanical energy into electrochemical energy through piezoelectric activity. One such flexible piezoelectric self-charging SC (PSCS) stuck to a human finger was fabricated using a piezoelectric material P(VDF-TrFE) film and PVA/H2SO4 as a gel electrolyte (Fig. 8a and b).149 The film performed the dual role of a separator and a potential nano-generator in the SC. It formed a network like structure with nanofibers of diameter 200 nm and lengths ranging in several tens of micrometres. An initial voltage of −0.15 V was observed when the human finger was flat which further increased to 0.05 V and 0.45 V when the human finger was bent at 30° and 90° respectively. The piezo electric field of the P(VDF-TrFE) film was responsible for driving the H+ and SO42− ions towards the positive and negative electrodes when deformation was applied. The increase in the potential window was proportional to the stress applied on the PSCS device. On relaxing the finger from 30° and 90°, the discharge time was found to be 4 and 18 seconds, respectively, thus confirming the self-charging ability of the PSCS device. Apart from promising electrochemical properties (Table 3), a stable discharge current of 6.4 μA further reinforces its potential to be used in future wearable micro electronic devices. A similar self-charging ASC was developed having a bio-piezoelectric separator (BPES).165 A porous fish bladder was used as the BPES which was soaked in a PVA–KOH gel electrolyte. The SC could be charged either by imparting mechanical deformations such as finger pressing, heel pressing, etc. or by electrical charging. In a period of 80 seconds, the SC could be charged up to 281.3 mV just by human finger imparting at a frequency of 1.65 Hz. The specific capacitance of the SC was found to be 165 F g−1 at a current density of 1 A g−1 which slightly decreased with an increase in the current density as seen in Fig. 8f. Fig. 8c and d represent the CV curves of the SC under different conditions including varied applied pressures, sole shoe pressing and elbow bending at different angles. The SC was able to retain 82.2%, 80.8% and 77% of its initial specific capacitance under heel pressing, finger imparting and twisted mode at 20 A g−1. A plot of specific capacitance vs. current density of the SC under various bending and twisting modes can be seen in Fig. 8e. It was also reported that eight such SCs connected in series can power portable electronic devices too. Apart from self-charging SCs, Kirigami paper based flexible SCs were designed for powering wearable electronic devices due to their high stretch-ability (∼210%) and mechanical durability (∼2000 loading/unloading cycles).166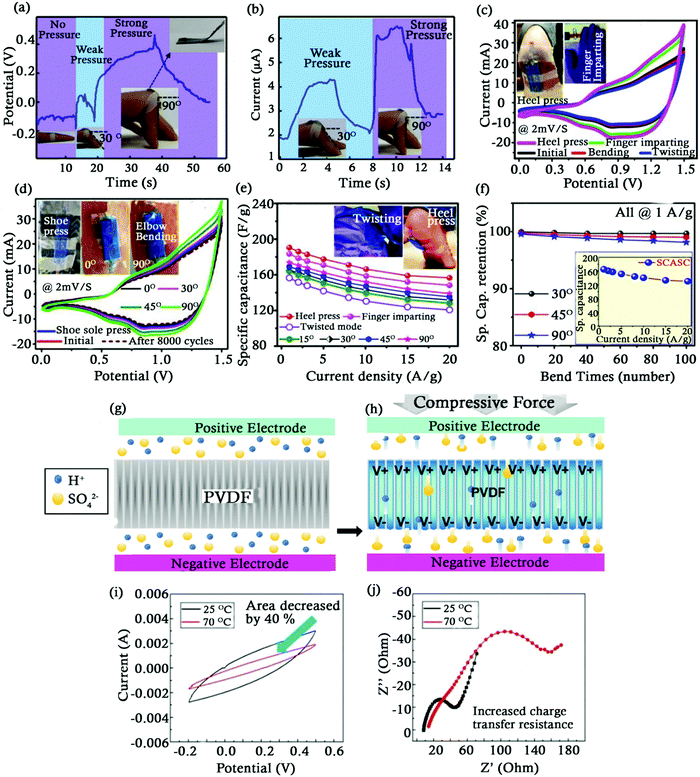 | ||
| Fig. 8 (a) Charge discharge curve of the PSCS (b) Short circuit current curve at different bending angles of the PSCS. Reproduced with permission.149 CV profile of the device (c) under various conditions, (d) under shoe sole pressing and elbow bending. Plots of (e) specific capacitance vs. current density and (f) specific capacitance retention vs. number of bending times. Reproduced with permission.165 (g) Hybrid piezo-SC's working mechanism. (h) Electrochemical and piezoelectric potential at equilibrium. Reproduced with permission.169 (i) Cyclic voltammetry (0.5 V s−1) and (j) Nyquist plot of trilayered-PNIPAM under 25 and 70 °C. Reproduced with permission.184 | ||
New trends have also led to the development of one-step ‘energy harvesting and storage’ devices by integrating smart materials (separators) with batteries/SCs to reduce unnecessary energy loss.167,168 A hybrid piezo-SC, integrating an energy harvesting film with an SC, was fabricated without any rectification device by Song et al.169 Herein, the flexible PVDF film, which acted as a separator as well as an energy harvester, was used to convert mechanical vibrations into a built-in electric field. This electric field served as a driving force for the migration of the ions through the PVA electrolyte towards the interface of functionalized carbon cloth electrodes (Fig. 8g and h). Such a flexible SC demonstrated a high specific capacitance of 357.6 F m−2 even during bending and stretching along with stable electrochemical performance with a power density and an energy density of 400 mW m−2 and 49.7 mW h m−2, respectively.
Fibre shaped stretchable SCs have gained attention lately as they can achieve high levels of strain and three-dimensional flexibility. This enables them to be integrated into microelectronics or even to be woven into cloth. However, there are certain limitations to the length and number of fibre electrodes that can be incorporated in a SC.170,171 Another major challenge is maintaining high stretchability of the SC while simultaneously avoiding contact between the inner and outer electrodes.172 Nie et al. developed cord shaped stretchable SCs with layer by layer braiding.148 The inner and outer electrodes are comprised of 6 modified PLA yarns each while the separator is comprised of 12 spandex monofilament yarns. Spandex monofilament yarns of 3 different diameters (0.5 mm, 0.3 mm and 0.1 mm) were used as separators in different samples in order study their influence on the mechanical and electrochemical properties of SCs. These spandex monofilament yarns were braided onto the surface of the inner electrodes (modified PLA yarns) while the outer electrodes (modified PLA yarns) were braided onto the surface of the monofilament spandex yarns. The braiding was carried out in such a way as to avoid contact between the inner and the outer electrodes. Smaller was the diameter of the spandex monofilament yarns, better was the energy storage performance observed, attributed to the increase in its porosity. An analysis of the area enclosed in the CV curves showed that the optimal length of the SC was 20 mm and the diameter of the spandex monofilament used for the braiding of separator was 0.1 mm.
Traditionally used SCs are able to withstand temperatures up to 100 °C. The commercially used separators in these SCs, fabricated using polymers and cellulose papers, undergo shrinkage and deformation at temperatures above 80 °C, making them unreliable to be used for high temperature applications.173,174 An increase of 10 °C can accelerate the ageing of the SC by a factor ranging anywhere between 1.7 and 2.5.175 Development of SCs that can be used for high temperature applications (>120 °C) such as oil drilling, military and for devices used in outer space is gearing up.176,177 A major cause of accumulation of heat within SCs is due to thermal runaway mainly due to overcharging, overheating, electrolyte decomposition, mechanical shocks, etc. This results in severe consequences such as reduction in cycle life and performance, hazardous fires, etc.178,179 An effective way to reduce the thermal runaway is by breaking the thermal loop caused by undesired electrochemical reactions at high temperatures. With an aim to control the capacitive behaviour at elevated temperatures and break the electrochemical reaction, researchers developed temperature responsive polymers which can manipulate ion migration and adsorption by changing its conformation or physical properties like viscosity with temperature.180–182 They have the ability to sense temperature and are reversible once they return to room temperature.183 Jiang et al. developed temperature responsive separators by casting poly(N-isopropylacrylamide) (PNIPAM) into thin films of varying thicknesses (20, 40 and 60 μm) which were then sandwiched between polypropylene to form a SC.184 PNIPAM possesses a lower critical solution temperature (LCST) of 32 °C, below which they are hydrophilic, and shift towards the hydrophobic state at higher temperatures. Due to this property, ions can easily migrate between the electrodes at room temperature but would face higher resistance during migration at temperatures above the LCST due to steric hindrance of solvated ions. Fig. 8j displays that the charge transfer resistance increased suddenly above room temperature as PNIPAM precipitated at high temperatures, causing blockage of pores in PP films, resulting in reduced ion migration. This led to a reduction in both the capacitance of the SCs and the redox reactions taking place at high temperatures. A 40% reduction in the capacitance of the SC with a 60 μm thickness separator from 375 mF g−1 at 25 °C to 221 mF g−1 at 70 °C was observed (Fig. 8i). Thicker PNIPAM layers offered greater hindrance during ion migration leading to a greater capacitance reduction. This was attributed to the more tortuous path ions would have to take as well as the hindrance they would encounter in thicker polymer films. A greater capacitance reduction was also reported at higher scan rates (−20% at 0.1 V s−1 to −45% at 1 V s−1). This technique can be used to control the degree of reduction of capacitance at elevated temperatures and return back to normal functioning at room temperature according to the application.
5. Influence of the separator material, wettability and electrolyte concentration on the performance of SCs
The properties as well as concentration of the electrolyte have a major influence on the energy density and capacitance value of SCs.185 It has been observed that at higher electrolyte concentrations, SCs exhibit a higher specific capacitance and higher energy densities.185–187 However, many conventional separators (cellulose-based) are not capable of resisting highly concentrated electrolytes, such as H2SO4. Corrosion resistant materials such as fibreglass, polypropylene and glass wool have been used as a separator material in the past (Table 3).188–191 The performance of glass wool separators under various concentrations of the H2SO4 electrolyte was compared with that of conventional cellulose based separators by Yaacob et al.150 CV analysis showed that the SC employing a glass wool separator even at low concentrations (1 mol dm−3) of the H2SO4 electrolyte displayed higher specific capacitance (90.3 F g−1) compared to the SC containing a cellulose based separator (68.8 F g−1) at the same scan rate. This was attributed to the higher porosity of glass wool due to which higher amounts of liquid electrolyte can be held.47 A 40% increase in the specific capacitance (120 F g−1) of the SC with glass wool separators was observed on increasing the concentration of the H2SO4 electrolyte (from 1 to 18 mol dm−3), indicating its dominance as a separator to achieve superior performance.The influence of the wettability of the separator and the material with which it is fabricated on the SC performance was highlighted by Liivand et al.192 SCs were fabricated using four different separators (two commercial ones made from cellulose and polypropylene (Celgard 2400) and two self-made separators fabricated by electrospinning PVDF with varying surface morphologies) in three different electrolytes. At low temperatures and high scan rates, a poorly wetted separator limited the capacitive behaviour. In contrast, when the separator material was completely wet, the maximum capacitance was weakly dependent on the material of the separator membrane. Numerous other factors such as phase angle, specific power and characteristic relaxation time constant were found to depend on the characteristics of the separator material (chemical composition and surface morphology) through the impedance data.
Electrolytes also play a critical role in determining the operational voltage of SCs and have an influence on their energy density. Neutral aqueous electrolytes such as Na+, Li+, and K+ solutions are the preferred one for use in SCs over acidic and alkaline solutions because of their higher operating voltages. A di-sulfonated poly(arylene ether sulfone) (SPAES)/PEO composite separator membrane was employed along with a neutral aqueous lithium sulphate (Li2SO4) electrolyte with varying mass fractions of PEO (10 wt% to 60 wt%).55 The SC having a SPAES/PEO-50 wt% separator membrane exhibited a high energy density of 19.04 W h kg−1 at a current density of 0.1 A g−1 which was attributed to the wide operating voltage range of Li2SO4 (2.2 V) (Table 3). The electrolyte uptake of the SPAES/PEO composite membrane (161% in the SPAES/PEO-60 wt% separator membrane) increased while the mechanical properties such as tensile strength and ‘elongation at break’ decreased with the increasing concentration of PEO in the composite membrane (Table 1).
As SCs are widely being used as energy storage devices, it is essential that they have low self-discharge rates. Self-discharge in EDLCs can be attributed to a variety of reasons. Some of them are as follows: (a) redox reactions taking place in the electrodes and electrolytes due to overcharging and (b) charge redistribution in SCs taking place over a period of time which returns it to a steady state.193,194 Many authors have attempted to solve the charge–discharge problem.195,196 Peng et al. developed environment friendly separators from nanofiber membranes prepared using coaxial spinning of polyacrylonitrile (PAN) which was coated by varying concentrations of sodium dodecyl benzene sulfonate (SDBS) (0 to 15 wt%) in order to study the effect of the microstructure of the nanofibers on the self-discharge behaviour of the separator.28 (Fig. 9d) During the study of the surface morphology of the nanofibers, the authors observed that the nanofibers prepared without SDBS were rougher as compared to those prepared with SDBS (5%, 10%, 15%). The SDBS also played a key role in reducing the surface tension of the fibre. The authors also observed that the SCs with 0%, 5%, 10% and 15% SDBS were able to retain a capacitance of 60.7%, 69.0%, 75.7%, and 66.0% respectively. The authors speculated that the self-discharge in SCs with the PAN@SDBS membrane was supressed as follows. In fully charged SCs having separator membranes with 0% SDBS, the ions from the electrolyte accumulate on the surface of the electrodes. Ion migration takes place from the surface of the electrode to the electrolyte under open circuit conditions leading to a drop in the voltage. However, in SCs with the separator membrane having 10% SDBS, the Na+ on the separator enters into the KOH electrolyte solution. Due to this, the separator membrane is negatively charged and repels the anions in the diffusion layer on the positive electrode as seen in Fig. 9a. The zeta potential test also revealed the fact that the membrane with 10% SDBS was indeed negatively charged in the alkaline solution, supporting the theory as displayed in Fig. 9b. Similar suppression of cations on the positive electrode was observed which was attributed to the lack of charge compensation of the anion.27 This leads to a suppression in the migration of ions, thus supressing the self-discharge (Fig. 9c). Such membranes fabricated by the coaxial electrospinning method were costing approximately $40 m−2, much less than Nafion cation exchange membranes ($800 m−2)197 and NKK-MPF30AC ($54 m−2) membranes.
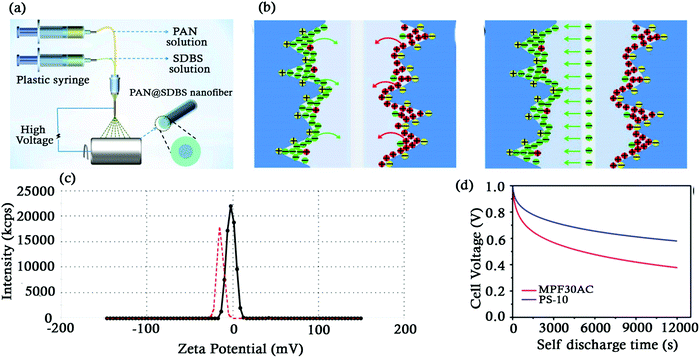 | ||
| Fig. 9 (a) Schematic diagram of the fabrication process of the separator. (b) Self-discharge process of SCs with the separator membrane having 0% SDBS and 10% SDBS. (c) Zeta potential of the separator membrane having 0% SDBS (black line) and 10% SDBS (red line). (d) Plot of cell voltage vs. current discharge. Reproduced with permission.28 | ||
6. Conclusions
Separators in SCs enable free ionic flow and isolate electronic flow. The design and fabrication decide the structure and properties of separators which in turn play a vital role in determining the performance of a SC including energy and power densities by regulating the cell kinetics, cycle life and safety. The material that exhibits high specific surface area, excellent mechanical properties and high thermal stability, serves as an electrolyte reservoir, remains electrochemically inert in a higher potential range, shows good cycling stability with high capacity retention, and allows easy and cheaper fabrication can be considered as an ideal separator material, whereas the process that can not only create nano-pores with controlled pore structures in a simple and efficient way on a large scale but also separate the electrodes and facilitate ion transport can be the ideal fabrication process. Retaining the desired mechanical properties with controlled pore structure is the key.The characteristics (its chemical composition and morphology) of separator membranes influence the equivalent series resistance (depends on porosity and thickness), wettability (phase angle) and power density (relaxation time constant) of the SCs. High porosity (high specific surface area) is demanded in the design of separators to ensure plentiful electrolyte retention and continuous, rapid movement of ions back and forth between the electrodes.
To achieve longer service life and enhance the safety and thermal stability of SCs, thermal runaway can be reduced by employing temperature responsive polymers/separators which can manipulate ion migration and adsorption by changing their conformation or physical property like viscosity with temperature. Depending on the application involved, separators are expected to carry the load as they are integrated with the structural members, withstand low/high temperature without compromising with its required characteristics, and function as a multitasking component e.g. sometimes as diodes along with separating electrodes. Research analysis shows that a search for new nano based separators, smart separators and novel processing techniques is needed to enhance the performance of SCs under odd conditions as well. Apart from the improved power performance, a step towards the use of cheaper, non-toxic, biodegradable/low carbon footprint/green materials and achieving sustainable manufacturing of separators/SCs and their recycling is needed.
Conflicts of interest
There are no conflicts to declare.References
- J. Jose, V. Thomas, V. Vinod, R. Abraham and S. Abraham, J. Sci. Adv. Mater. Dev., 2019, 4, 333–340 Search PubMed.
- K. W. Tan, S. K. Heo, M. L. Foo, I. M. L. Chew and C. K. Yoo, Sci. Total Environ., 2019, 650, 1309–1326 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nature Materials, World Scientific Publishing Co., 2008, vol. 7, pp. 138–147 Search PubMed.
- M. M. Pérez-Madrigal, M. G. Edo and C. Alemán, Green Chem., 2016, 18, 5930–5956 RSC.
- J. Cao, Y. Zhao, Y. Xu, Y. Zhang, B. Zhang and H. Peng, J. Mater. Chem. A, 2018, 6, 3355–3360 RSC.
- Y. Huang, Y. Zeng, M. Yu, P. Liu, Y. Tong, F. Cheng and X. Lu, Small Methods, 2018, 2, 1700230 CrossRef.
- W. Xu, Z. Jiang, Q. Yang, W. Huo, M. S. Javed, Y. Li, L. Huang, X. Gu and C. Hu, Nano Energy, 2018, 43, 168–176 CrossRef CAS.
- B. K. Deka, A. Hazarika, J. Kim, Y. Bin Park and H. W. Park, Int. J. Energy Res., 2017, 41, 1397–1411 CrossRef.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816 RSC.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- W. Chen, H. Yu, S. Y. Lee, T. Wei, J. Li and Z. Fan, Chem. Soc. Rev., 2018, 47, 2837–2872 RSC.
- Y. Z. Zhang, Y. Wang, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Chem. Soc. Rev., 2015, 44, 5181–5199 RSC.
- X. Lin, H. Lou, W. Lu, F. Xu, R. Fu and D. Wu, Chin. Chem. Lett., 2018, 29, 633–636 CrossRef CAS.
- Z. Song, D. Zhu, L. Li, T. Chen, H. Duan, Z. Wang, Y. Lv, W. Xiong, M. Liu and L. Gan, J. Mater. Chem. A, 2019, 7, 1177–1186 RSC.
- L.-F. Chen, Z.-H. Huang, H.-W. Liang, W.-T. Yao, Z.-Y. Yu and S.-H. Yu, Energy Environ. Sci., 2013, 6, 3331–3338 RSC.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- S. Chen, J. Zhu, X. Wu, Q. Han and X. Wang, ACS Nano, 2010, 4, 2822–2830 CrossRef CAS PubMed.
- L. Yuan, B. Yao, B. Hu, K. Huo, W. Chen and J. Zhou, Energy Environ. Sci., 2013, 6, 470–476 RSC.
- Z. Yang, J. Tian, Z. Yin, C. Cui, W. Qian and F. Wei, Carbon N. Y., 2019, 141, 467–480 CrossRef CAS.
- S. Sankar, H. Lee, H. Jung, A. Kim, A. T. A. Ahmed, A. I. Inamdar, H. Kim, S. Lee, H. Im and D. Young Kim, New J. Chem., 2017, 41, 13792–13797 RSC.
- S. B. Singh, T. Kshetri, T. I. Singh, N. H. Kim and J. H. Lee, Chem. Eng. J., 2019, 359, 197–207 CrossRef CAS.
- Y. Wang, W. Zhou, Q. Kang, J. Chen, Y. Li, X. Feng, D. Wang, Y. Ma and W. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 27001–27008 CrossRef CAS PubMed.
- R. Wang, M. Yao and Z. Niu, InfoMat, 2020, 2, 113–125 CrossRef CAS.
- T. Chen and L. Dai, J. Mater. Chem. A, 2014, 2, 10756 RSC.
- Z. Yang, J. Deng, X. Chen, J. Ren and H. Peng, Angew. Chem., Int. Ed., 2013, 52, 13453–13457 CrossRef CAS PubMed.
- T. Chen, H. Peng, M. Durstock and L. Dai, Sci. Rep., 2015, 4, 3612 CrossRef PubMed.
- H. Wang, Q. Zhou, B. Yao, H. Ma, M. Zhang, C. Li and G. Shi, Adv. Mater. Interfaces, 2018, 5, 1701547 CrossRef.
- H. Peng, L. Xiao, K. Sun, G. Ma, G. Wei and Z. Lei, J. Power Sources, 2019, 435, 226800 CrossRef CAS.
- X. Zhang, B. He, Y. Zhao and Q. Tang, J. Power Sources, 2018, 379, 60–67 CrossRef CAS.
- M. Y. Jen, Z. W. Hung and C. Y. Chun, J. Membr. Sci., 2008, 322, 74–80 CrossRef.
- J. Saunier, F. Alloin, J. Y. Sanchez and L. Maniguet, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 2308–2317 CrossRef CAS.
- D. Karabelli, J. C. Leprêtre, F. Alloin and J. Y. Sanchez, Electrochim. Acta, 2011, 57, 98–103 CrossRef CAS.
- M. A. Hashim, L. Sa’adu, M. Bin Baharuddin and K. A. Dasuki, J. Mater. Sci. Res., 2013, 3, 25–29 Search PubMed.
- Y. Yin, O. Yamada, Y. Suto, T. Mishima, K. Tanaka, H. Kita and K. Okamoto, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 1545–1553 CrossRef CAS.
- J. A. Elliott and S. J. Paddison, Phys. Chem. Chem. Phys., 2007, 9, 2602–2618 RSC.
- P. Staiti and F. Lufrano, Electrochim. Acta, 2010, 55, 7436–7442 CrossRef CAS.
- J. Zhou, J. Cai, S. Cai, X. Zhou and A. N. Mansour, J. Power Sources, 2011, 196, 10479–10483 CrossRef CAS.
- A. K. Solarajan, V. Murugadoss and S. Angaiah, J. Appl. Polym. Sci., 2017, 134, 45177 CrossRef.
- A. K. Solarajan, V. Murugadoss and S. Angaiah, Appl. Mater. Today, 2016, 5, 33–40 CrossRef.
- V. Murugadoss, S. Arunachalam, V. Elayappan and S. Angaiah, Ionics, 2018, 24, 4071–4080 CrossRef CAS.
- A. Laforgue and L. Robitaille, ECS Trans., 2011, 35, 13–20 CrossRef CAS.
- A. Laforgue and L. Robitaille, J. Electrochem. Soc., 2012, 159, 929–936 CrossRef.
- M. Kumar, A. Subramania and K. Balakrishnan, Electrochim. Acta, 2014, 149, 152–158 CrossRef CAS.
- B. T. Weili Li, Y. Xing, Y. Wu, J. Wang, L. Chen and G. Yang, Electrochim. Acta, 2016, 151, 289–296 Search PubMed.
- K. Tõnurist, T. Thomberg, A. Jänes and E. Lust, J. Electrochem. Soc., 2013, 160, 449–457 CrossRef.
- K. Tõnurist, A. Jänes, T. Thomberg, H. Kurig and E. Lust, J. Electrochem. Soc., 2009, 156, A334–A342 CrossRef.
- K. Tõnurist, T. Thomberg, A. Jänes, T. Romann, V. Sammelselg and E. Lust, J. Electroanal. Chem., 2013, 689, 8–20 CrossRef.
- R. Arthi, V. Jaikumar and P. Muralidharan, Energy Sources, Part A, 2019, 1–15 Search PubMed.
- T. He, R. Jia, X. Lang, X. Wu and Y. Wang, J. Electrochem. Soc., 2017, 164, E379–E384 CrossRef CAS.
- T. He, Y. Fu, X. Meng, X. Yu and X. Wang, Electrochim. Acta, 2018, 282, 97–104 CrossRef CAS.
- Q. Xie, X. Huang, Y. Zhang, S. Wu and P. Zhao, Appl. Surf. Sci., 2018, 443, 412–420 CrossRef CAS.
- M. Mastragostino and F. Soavi, J. Power Sources, 2007, 174, 89–93 CrossRef CAS.
- X. Liu and P. G. Pickup, J. Power Sources, 2008, 176, 410–416 CrossRef CAS.
- Z. Yang, Y. Jia, Y. Niu, Z. Yong, K. Wu, C. Zhang, M. Zhu, Y. Zhang and Q. Li, Chem. Eng. J., 2020, 400, 125835 CrossRef CAS.
- R. Na, X. Zhang, P. Huo, Y. Du, G. Huo, K. Zhu and G. Wang, High Perform. Polym., 2017, 29, 984–993 CrossRef CAS.
- E. Vargun, K. Ozaltin, H. Fei, E. Harea, J. Vilčáková, N. Kazantseva and P. Saha, J. Appl. Polym. Sci., 2020, 137, 49270 CrossRef CAS.
- D. Xu, G. Teng, Y. Heng, Z. Chen and D. Hu, Mater. Chem. Phys., 2020, 249, 122979 CrossRef CAS.
- G. Teng, S. Lin, D. Xu, Y. Heng and D. Hu, J. Mater. Sci.: Mater. Electron., 2020, 31, 7916–7926 CrossRef CAS.
- D. Zhao, C. Chen, Q. Zhang, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, Adv. Energy Mater., 2017, 7 DOI:10.1002/aenm.201700739.
- H. Zhang, C.-E. Lin, M.-Y. Zhou, A. E. John and B.-K. Zhu, Electrochim. Acta, 2016, 187, 125–133 CrossRef CAS.
- B. Szubzda, A. Szmaja, M. Ozimek and S. Mazurkiewicz, Appl. Phys. A: Mater. Sci. Process., 2014, 117, 1801–1809 CrossRef CAS.
- E. Feng, G. Ma, K. Sun, Q. Yang, H. Peng and Z. Lei, RSC Adv., 2016, 6, 75896–75904 RSC.
- Y. Zhong, X. Zhang, Y. He, H. Peng, G. Wang and G. Xin, Adv. Funct. Mater., 2018, 28, 1801998 CrossRef.
- H. Peng, Y. Zhong, X. Zhang, Y. He and G. Wang, Langmuir, 2018, 34, 15245–15252 CrossRef CAS PubMed.
- X. Liu, D. Li, X. Chen, W. Y. Lai and W. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 32536–32542 CrossRef CAS PubMed.
- E. Feng, G. Ma, K. Sun, F. Ran, H. Peng and Z. Lei, New J. Chem., 2017, 41, 1986–1992 RSC.
- G. Ma, J. Li, K. Sun, H. Peng, J. Mu and Z. Lei, J. Power Sources, 2014, 256, 281–287 CrossRef CAS.
- J. Chen, W. Xiao, T. Hu, P. Chen, T. Lan, P. Li, Y. Li, B. Mi and Y. Ma, ACS Appl. Mater. Interfaces, 2020, 12, 5885–5891 CrossRef CAS PubMed.
- D. Boonpakdee, C. F. Guajardo Yévenes, W. Surareungchai and C. La-O-Vorakiat, J. Mater. Chem. A, 2018, 6, 7162–7167 RSC.
- W. Li, X. Li, X. Zhang, J. Wu, X. Tian, M.-J. Zeng, J. Qu and Z.-Z. Yu, ACS Appl. Energy Mater., 2020, 3, 9408–9416 CrossRef CAS.
- J. Han, H. Wang, Y. Yue, C. Mei, J. Chen, C. Huang, Q. Wu and X. Xu, Carbon N. Y., 2019, 149, 1–18 CrossRef CAS.
- H. H. Hsu, Y. Liu, Y. Wang, B. Li, G. Luo, M. Xing and W. Zhong, ACS Sustainable Chem. Eng., 2020, 8, 6935–6948 CrossRef CAS.
- P. Tang, L. Han, P. Li, Z. Jia, K. Wang, H. Zhang, H. Tan, T. Guo and X. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 7703–7714 CrossRef CAS PubMed.
- H. Yu, N. Rouelle, A. Qiu, J.-A. Oh, D. M. Kempaiah, J. D. Whittle, M. Aakyiir, W. Xing and J. Ma, ACS Appl. Mater. Interfaces, 2020, 12, 37977–37985 CrossRef CAS PubMed.
- Z. Pei, Z. Yuan, C. Wang, S. Zhao, J. Fei, L. Wei, J. Chen, C. Wang, R. Qi, Z. Liu and Y. Chen, Angew. Chem., Int. Ed., 2020, 59, 4793–4799 CrossRef CAS PubMed.
- J. W. Kim, H. Park, G. Lee, Y. R. Jeong, S. Y. Hong, K. Keum, J. Yoon, M. S. Kim and J. S. Ha, Adv. Funct. Mater., 2019, 29, 1905968 CrossRef CAS.
- L. Li, F. Lu, C. Wang, F. Zhang, W. Liang, S. Kuga, Z. Dong, Y. Zhao, Y. Huang and M. Wu, J. Mater. Chem. A, 2018, 6, 24468–24478 RSC.
- H. Grebel and A. Patel, Chem. Phys. Lett., 2015, 640, 36–39 CrossRef CAS.
- J. Grebel, A. Banerjee and H. Grebel, Electrochim. Acta, 2013, 95, 308–312 CrossRef CAS.
- T. S. Chowdhury and H. Grebel, Electrochim. Acta, 2019, 307, 459–464 CrossRef CAS.
- W. G. Pell and B. E. Conway, J. Power Sources, 2004, 136, 334–345 CrossRef CAS.
- T. S. Chowdhury and H. Grebel, ChemEngineering, 2019, 3, 39 CrossRef CAS.
- C. Y. Bon, L. Mohammed, S. Kim, M. Manasi, P. Isheunesu, K. S. Lee and J. M. Ko, J. Ind. Eng. Chem., 2018, 68, 173–179 CrossRef CAS.
- M. Liu, K. Turcheniuk, W. Fu, Y. Yang, M. Liu and G. Yushin, Nano Energy, 2020, 71, 104627 CrossRef CAS.
- S. H. Ko, J. Chung, H. Pan, C. P. Grigoropoulos and D. Poulikakos, Sens. Actuators, A, 2007, 134, 161–168 CrossRef CAS.
- J. Hao, W. Si, X. X. Xi, R. Guo, A. S. Bhalla and L. E. Cross, Appl. Phys. Lett., 2000, 76, 3100–3102 CrossRef CAS.
- C. O. Alvarez-sanchez, R. Massó-ferret and E. Nicolau, Energy Sci. Eng., 2019, 7, 730–740 CrossRef CAS.
- M. M. Ghobad Behzadi Pour and L. Fekri Aval, J. Mater. Sci. Mater. Electron., 2018, 29, 17432–17437 CrossRef.
- H. H. Y. Jen Yu Shieh, S.-Y. Tsai and B.-Y. Li, Mater. Chem. Phys., 2017, 195, 114–122 CrossRef.
- P. Sivaraman, S. P. Mishra, D. D. Potphode, A. P. Thakur, K. Shashidhara, A. B. Samui and A. R. Bhattacharyya, RSC Adv., 2015, 5, 83546–83557 RSC.
- C.-H. Chang, B. Hsia, J. P. Alper, S. Wang, L. E. Luna, C. Carraro, S.-Y. Lu and R. Maboudian, ACS Appl. Mater. Interfaces, 2015, 7, 26658–26665 CrossRef CAS PubMed.
- R. Na, P. Huo, X. Zhang, S. Zhang, Y. Du, K. Zhu, Y. Lu, M. Zhang, J. Luan and G. Wang, RSC Adv., 2016, 6, 65186–65195 RSC.
- S. Mo, P. Lu, F. Ding, Z. Xu, J. Liu, X. Liu and Q. Xu, Solid State Ionics, 2016, 296, 37–41 CrossRef CAS.
- Z. Pang, J. Duan, Y. Zhao, Q. Tang, B. He and L. Yu, J. Power Sources, 2018, 400, 126–134 CrossRef CAS.
- S. Zheng, W. Lei, J. Qin, Z.-S. Wu, F. Zhou, S. Wang, X. Shi, C. Sun, Y. Chen and X. Bao, Energy Storage Mater., 2018, 10, 24–31 CrossRef.
- H. Lee, M. Yanilmaz, O. Toprakci, K. Fu and X. Zhang, Energy Environ. Sci., 2014, 7, 3857–3886 RSC.
- C. González, J. J. Vilatela, J. M. Molina-Aldareguía, C. S. Lopes and J. LLorca, Prog. Mater. Sci., 2017, 89, 194–251 CrossRef.
- D. J. O’Brien, D. M. Baechle and E. D. Wetzel, J. Compos. Mater., 2011, 45, 2797–2809 CrossRef.
- L. H. Acauan, Y. Zhou, E. Kalfon-Cohen, N. K. Fritz and B. L. Wardle, Nanoscale, 2019, 11, 21964–21973 RSC.
- Q. Yao, H. Wang, C. Wang, C. Jin and Q. Sun, ACS Sustainable Chem. Eng., 2018, 6, 4695–4704 CrossRef CAS.
- N. Guo, M. Li, Y. Wang, X. Sun, F. Wang and R. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 33626–33634 CrossRef CAS PubMed.
- J. Chen, X. Zhou, C. Mei, J. Xu, S. Zhou and C. P. Wong, J. Power Sources, 2017, 342, 48–55 CrossRef CAS.
- L. Jin, K. Wei, Y. Xia, B. Liu, K. Zhang, H. Gao, X. Chu, M. Ye, L. He and P. Lin, Mater. Today Energy, 2019, 14, 100348 CrossRef.
- N. Liang, Y. Ji, D. Zuo, H. Zhang and J. Xu, Polym. Int., 2019, 68, 120–124 CrossRef CAS.
- F. Poli, D. Momodu, G. E. Spina, A. Terella, B. K. Mutuma, M. L. Focarete, N. Manyala and F. Soavi, Electrochim. Acta, 2020, 338, 135872 CrossRef CAS.
- D. Yang, L. Qi and J. Ma, Adv. Mater., 2002, 14, 1543–1546 CrossRef CAS.
- E. Taer, Sugianto, M. A. Sumantre, R. Taslim, Iwantono, D. Dahlan and M. Deraman, Adv. Mater. Res., 2014, 896, 66–69 Search PubMed.
- D. Dahlan, N. Sartika, Astuti, E. L. Namigo and E. Taer, Mater. Sci. Forum, 2015, 827, 151–155 Search PubMed.
- H. Yu, Q. Tang, J. Wu, Y. Lin, L. Fan, M. Huang, J. Lin, Y. Li and F. Yu, J. Power Sources, 2012, 206, 463–468 CrossRef CAS.
- Y. Zhang, J. He, Z. Gao and X. Li, Nano Energy, 2019, 65, 104045 CrossRef CAS.
- P. Yang, J. Xie and C. Zhong, ACS Appl. Energy Mater., 2018, 1, 616–622 CrossRef CAS.
- Y. Zhao, S. Huang, M. Xia, S. Rehman, S. Mu, Z. Kou, Z. Zhang, Z. Chen, F. Gao and Y. Hou, Nano Energy, 2016, 28, 346–355 CrossRef CAS.
- D. Hulicova, J. Yamashita, Y. Soneda, H. Hatori and M. Kodama, Chem. Mater., 2005, 17, 1241–1247 CrossRef CAS.
- K. Torvinen, S. Lehtimäki, J. T. Keränen, J. Sievänen, J. Vartiainen, E. Hellén, D. Lupo and S. Tuukkanen, Electron. Mater. Lett., 2015, 11, 1040–1047 CrossRef CAS.
- Y. S. Yang, I.-K. You, S.-H. Hong and H.-G. Yun, Electrochem. Soc., 2014, 64, 135–137 CAS.
- M. Arvani, J. Keskinen, A. Railanmaa, S. Siljander, T. Björkqvist, S. Tuukkanen and D. Lupo, J. Appl. Electrochem., 2020, 50, 689–697 CrossRef CAS.
- I. Sakurada, Y. Nukushina and T. Ito, J. Polym. Sci., 1962, 57, 651–660 CrossRef CAS.
- D. Lasrado, S. Ahankari and K. Kar, J. Appl. Polym. Sci., 2020, 137, 48959 CrossRef CAS.
- Q. Zhang, C. Chen, W. Chen, G. Pastel, X. Guo, S. Liu, Q. Wang, Y. Liu, J. Li, H. Yu and L. Hu, ACS Appl. Mater. Interfaces, 2019, 11, 5919–5927 CrossRef CAS PubMed.
- K. K. Liu, Q. Jiang, C. Kacica, H. G. Derami, P. Biswas and S. Singamaneni, RSC Adv., 2018, 8, 31296–31302 RSC.
- X. Lv, G. Li, D. Li, F. Huang, W. Liu and Q. Wei, J. Phys. Chem. Solids, 2017, 110, 202–210 CrossRef CAS.
- C. Tang, Z. Tang and H. Gong, J. Electrochem. Soc., 2012, 159, A651–A656 CrossRef CAS.
- C. X. Guo, A. A. Chitre and X. Lu, Phys. Chem. Chem. Phys., 2014, 16, 4672 RSC.
- R. R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J. H. Kim and Y. Yamauchi, ACS Nano, 2015, 9, 6288–6296 CrossRef CAS PubMed.
- Y.-T. Wang, A.-H. Lu, H.-L. Zhang and W.-C. Li, J. Phys. Chem. C, 2011, 115, 5413–5421 CrossRef CAS.
- M. Huang, Y. Zhang, F. Li, L. Zhang, Z. Wen and Q. Liu, J. Power Sources, 2014, 252, 98–106 CrossRef CAS.
- W. Liu, X. Li, M. Zhu and X. He, J. Power Sources, 2015, 282, 179–186 CrossRef CAS.
- S. Wu, W. Chen and L. Yan, J. Mater. Chem. A, 2014, 2, 2765 RSC.
- Y. Li, J. Xu, T. Feng, Q. Yao, J. Xie and H. Xia, Adv. Funct. Mater., 2017, 27, 1606728 CrossRef.
- C. G. Cameron and S. M. Fitzsimmons, Supercapacitor separators and polypyrrole composites, defence R&D Canada-Atlantic, 2008 Search PubMed.
- J. Cai, S. Liu, J. Feng, S. Kimura, M. Wada, S. Kuga and L. Zhang, Angew. Chem., Int. Ed., 2012, 51, 2076–2079 CrossRef CAS PubMed.
- L. Hu, W. Chen, X. Xie, N. Liu, Y. Yang, H. Wu, Y. Yao, M. Pasta, H. N. Alshareef and Y. Cui, ACS Nano, 2011, 5, 8904–8913 CrossRef CAS PubMed.
- H. Koga, H. Tonomura, M. Nogi, K. Suganuma and Y. Nishina, Green Chem., 2016, 18, 1117–1124 RSC.
- P. Tammela, H. Olson, M. Strømme and L. Nyholm, J. Power Sources, 2014, 272, 468–475 CrossRef CAS.
- L. Hu, H. Wu and Y. Cui, Appl. Phys. Lett., 2010, 96, 183502 CrossRef.
- I. Bispo-Fonseca, J. Aggar, C. Sarrazin, P. Simon and J. F. Fauvarque, J. Power Sources, 1999, 79, 238–241 CrossRef CAS.
- A. M. Bittner, M. Zhu, Y. Yang, H. F. Waibel, M. Konuma, U. Starke and C. J. Weber, J. Power Sources, 2012, 203, 262–273 CrossRef CAS.
- I. Jung, D. A. Dikin, R. D. Piner and R. S. Ruoff, Nano Lett., 2008, 8, 4283–4287 CrossRef CAS PubMed.
- J. H. Sluyters and M. Sluyters-Rehbach, J. Phys. Chem. B, 2010, 114, 15582–15589 CrossRef CAS PubMed.
- Y. M. Shulga, S. A. Baskakov, V. A. Smirnov, N. Y. Shulga, K. G. Belay and G. L. Gutsev, J. Power Sources, 2014, 245, 33–36 CrossRef CAS.
- S. Baskakov, Y. V. Baskakova, N. V. Lyskov, N. N. Dremova, A. V. Irzhak, Y. Kumar, A. Michtchenok and Y. Shulga, Electrochim. Acta, 2018, 260, 557–563 CrossRef CAS.
- Y. M. Shulga, S. A. Baskakov, Y. V. Baskakova, Y. M. Volfkovich, N. Y. Shulga, E. A. Skryleva, Y. N. Parkhomenko, K. G. Belay, G. L. Gutsev, A. Y. Rychagov, V. E. Sosenkin and I. D. Kovalev, J. Power Sources, 2015, 279, 722–730 CrossRef CAS.
- H. Gao, H. Shen, H. Wu, H. Jing, Y. Sun, B. Liu, Z. Chen, J. Song, L. Lu, Z. Wu and Q. Hao, Energy Fuels, 2021, 35, 12884–12901 CrossRef CAS.
- J. Cai, Y. Song, X. Chen, Z. Sun, Y. Yi, J. Sun and Q. Zhang, J. Mater. Chem. A, 2020, 8, 1757–1766 RSC.
- A. E. Baumann, D. A. Burns, B. Liu and V. S. Thoi, Chem. Commun., 2019, 2, 86 CrossRef.
- S. Suriyakumar, A. M. Stephan, N. Angulakshmi, M. H. Hassan and M. H. Alkordi, J. Mater. Chem. A, 2018, 6, 14623–14632 RSC.
- J.-P. Meng, Y. Gong, Q. Lin, M.-M. Zhang, P. Zhang, H.-F. Shi and J.-H. Lin, Dalton Trans., 2015, 44, 5407–5416 RSC.
- W. Nie, S. Zhang, X. Ding, J. Hu, X. Yang and Q. Li, Text. Res. J., 2019, 89, 4265–4271 CrossRef CAS.
- Y. Lu, Y. Jiang, Z. Lou, R. Shi, D. Chen and G. Shen, Prog. Nat. Sci. Mater. Int., 2020, 30, 174–179 CrossRef CAS.
- M. F. M. Yaacob, Z. A. Noorden and J. J. Jamian, PECON 2016–2016 IEEE 6th Int. Conf. Power Energy, Conf. Proceeding, 2017, pp. 718–722.
- R. Kötz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef.
- P. Arora and Z. Zhang, Chem. Rev., 2004, 104, 4419–4462 CrossRef CAS PubMed.
- A. Yu, V. Chabot and J. Zhang, Electrochemical Supercapacitors for Energy Storage and Delivery: Fundamentals and Applications, CRC Press, 1st edn, 2013 Search PubMed.
- L. Dong, C. Xu, Y. Li, Z. H. Huang, F. Kang, Q. H. Yang and X. Zhao, J. Mater. Chem. A, 2016, 4, 4659–4685 RSC.
- L. Zhang, D. Liu, Z. S. Wu and W. Lei, Energy Storage Mater., 2020, 32, 402–417 CrossRef.
- J. Qin, P. Das, S. Zheng and Z. S. Wu, APL Mater., 2019, 7, 090902 CrossRef.
- Q. Zhang, L. Liu, C. Pan and D. Li, J. Mater. Sci., 2018, 53, 27–46 CrossRef CAS.
- L. Li, F. Lu, C. Wang, F. Zhang, W. Liang, S. Kuga, Z. Dong, Y. Zhao, Y. Huang and M. Wu, J. Mater. Chem. A, 2018, 6, 24468–24478 RSC.
- B. Xie, C. Yang, Z. Zhang, P. Zou, Z. Lin, G. Shi, Q. Yang, F. Kang and C. P. Wong, ACS Nano, 2015, 9, 5636–5645 CrossRef CAS PubMed.
- J. Gao, C. Shao, S. Shao, C. Bai, U. R. Khalil, Y. Zhao, L. Jiang and L. Qu, ACS Nano, 2019, 13, 7463–7470 CrossRef CAS PubMed.
- Y. Yan, J. Yan, X. Gong, X. Tang, X. Xu, T. Meng, F. Bu, D. Cai, Z. Zhang, G. Nie and H. Zhang, Chem. Eng. J., 2021, 133580 CrossRef.
- W. Gao, L. B. Alemany, L. Ci and P. M. Ajayan, Nat. Chem., 2009, 1, 403–408 CrossRef CAS PubMed.
- S. Cerveny, F. Barroso-Bujans, Á. Alegría and J. Colmenero, J. Phys. Chem. C, 2010, 114, 2604–2612 CrossRef CAS.
- W. Gao, N. Singh, L. Song, Z. Liu, A. L. M. Reddy, L. Ci, R. Vajtai, Q. Zhang, B. Wei and P. M. Ajayan, Nat. Nanotechnol., 2011, 6, 496–500 CrossRef CAS PubMed.
- A. Maitra, S. K. Karan, S. Paria, A. K. Das, R. Bera, L. Halder, S. K. Si, A. Bera and B. B. Khatua, Nano Energy, 2017, 40, 633–645 CrossRef CAS.
- H. Guo, M.-H. Yeh, Y.-C. Lai, Y. Zi, C. Wu, Z. Wen, C. Hu and Z. L. Wang, ACS Nano, 2016, 10, 10580–10588 CrossRef CAS PubMed.
- A. Ramadoss, B. Saravanakumar, S. W. Lee, Y. S. Kim, S. J. Kim and Z. L. Wang, ACS Nano, 2015, 9, 4337–4345 CrossRef CAS PubMed.
- L. Xing, Y. Nie, X. Xue and Y. Zhang, Nano Energy, 2014, 10, 44–52 CrossRef CAS.
- R. Song, H. Jin, X. Li, L. Fei, Y. Zhao, H. Huang, H. Lai-Wa Chan, Y. Wang and Y. Chai, J. Mater. Chem. A, 2015, 3, 14963–14970 RSC.
- T. Chen, R. Hao, H. Peng and L. Dai, Angew. Chem., Int. Ed., 2015, 54, 618–622 CAS.
- L. Liu, Y. Yu, C. Yan, K. Li and Z. Zheng, Nat. Commun., 2015, 6, 7260 CrossRef CAS PubMed.
- Q. Huang, D. Wang and Z. Zheng, Adv. Energy Mater., 2016, 6, 1600783 CrossRef.
- Y. Kang, H. J. Kim, E. Kim, B. Oh and J. H. Cho, J. Power Sources, 2001, 92, 255–259 CrossRef CAS.
- Z. Gui, H. Zhu, E. Gillette, X. Han, G. W. Rubloff, L. Hu and S. B. Lee, ACS Nano, 2013, 7, 6037–6046 CrossRef CAS PubMed.
- P. Azaïs, L. Duclaux, P. Florian, D. Massiot, M. A. Lillo-Rodenas, A. Linares-Solano, J. P. Peres, C. Jehoulet and F. Béguin, J. Power Sources, 2007, 171, 1046–1053 CrossRef.
- T. Hibino, K. Kobayashi, M. Nagao and S. Kawasaki, Sci. Rep., 2015, 5, 7903 CrossRef CAS PubMed.
- B. Asbani, C. Douard, T. Brousse and J. Le Bideau, Energy Storage Mater., 2019, 21, 439–445 CrossRef.
- M. Ouyang, D. Ren, L. Lu, J. Li, X. Feng, X. Han and G. Liu, J. Power Sources, 2015, 279, 626–635 CrossRef CAS.
- R. Kötz, P. W. Ruch and D. Cericola, J. Power Sources, 2010, 195, 923–928 CrossRef.
- Y. Shi, H. Ha, A. Al-Sudani, C. J. Ellison and G. Yu, Adv. Mater., 2016, 28, 7921–7928 CrossRef CAS PubMed.
- H. Yang, Z. Liu, B. K. Chandran, J. Deng, J. Yu, D. Qi, W. Li, Y. Tang, C. Zhang and X. Chen, Adv. Mater., 2015, 27, 5593–5598 CrossRef CAS PubMed.
- P. Zhang, J. Wang, W. Sheng, F. Wang, J. Zhang, F. Zhu, X. Zhuang, R. Jordan, O. G. Schmidt and X. Feng, Energy Environ. Sci., 2018, 11, 1717–1722 RSC.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- H. Jiang, R. K. Emmett and M. E. Roberts, J. Appl. Electrochem., 2019, 49, 271–280 CrossRef CAS.
- M. Inagaki, H. Konno and O. Tanaike, J. Power Sources, 2010, 195, 7880–7903 CrossRef CAS.
- Y. Soneda, M. Toyoda, Y. Tani, J. Yamashita, M. Kodama, H. Hatori and M. Inagaki, J. Phys. Chem. Solids, 2004, 65, 219–222 CrossRef CAS.
- T. W. Weng, W. Huang and K. Y. Lee, Vacuum, 2008, 83, 629–632 CrossRef CAS.
- R. Kötz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef.
- Z. A. Noorden, S. Sugawara and S. Matsumoto, IEEJ Trans. Electr. Electron. Eng., 2014, 9, 235–240 CrossRef CAS.
- Z. A. Noorden, S. Sugawara and S. Matsumoto, ECS Trans., 2013, 53, 43–51 CrossRef.
- A. Lewandowski, A. Olejniczak, M. Galinski and I. Stepniak, J. Power Sources, 2010, 195, 5814–5819 CrossRef CAS.
- K. Liivand, T. Thomberg, A. Jänes and E. Lust, ECS Trans., 2015, 64, 41–49 CrossRef CAS.
- M. Kaus, J. Kowal and D. U. Sauer, Electrochim. Acta, 2010, 55, 7516–7523 CrossRef CAS.
- A. Lewandowski, P. Jakobczyk, M. Galinski and M. Biegun, Phys. Chem. Chem. Phys., 2013, 15, 8692–8699 RSC.
- T. Tevi and A. Takshi, J. Power Sources, 2015, 273, 857–862 CrossRef CAS.
- T. Xiong, Z. G. Yu, W. S. V. Lee and J. Xue, ChemSusChem, 2018, 11, 3307–3314 CrossRef CAS PubMed.
- H.-S. Dang, E. A. Weiber and P. Jannasch, J. Mater. Chem. A, 2015, 3, 5280–5284 RSC.
| This journal is © The Royal Society of Chemistry 2022 |