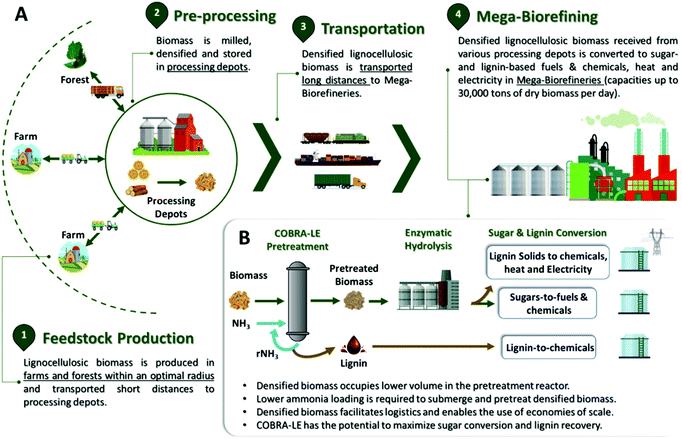
Development of an ammonia pretreatment that creates synergies between biorefineries and advanced biomass logistics models†
Ana Rita C.
Morais
 abc,
Jian
Zhang
d,
Hui
Dong
e,
William G.
Otto
f,
Thapelo
Mokomele
g,
David
Hodge
abc,
Jian
Zhang
d,
Hui
Dong
e,
William G.
Otto
f,
Thapelo
Mokomele
g,
David
Hodge
 f,
Venkatesh
Balan
f,
Venkatesh
Balan
 eh,
Bruce E.
Dale
eh,
Bruce E.
Dale
 ei,
Rafal M.
Lukasik
ei,
Rafal M.
Lukasik
 a and
Leonardo
da Costa Sousa
a and
Leonardo
da Costa Sousa
 *e
*e
aUnidade de Bioenergia e Biorrefinerias, Laboratório Nacional de Energia e Geologia, I.P., Estrada do Paco do Lumiar 22, 1649-038 Lisbon, Portugal
bLAQV-REQUIMTE, Department of Chemistry, Faculty of Science and Technology, Universidade NOVA de Lisboa, Lisbon, Portugal
cDepartment of Chemical and Petroleum Engineering, University of Kansas, Lawrence, KS 66045, USA
dState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
eBiomass Conversion Research Laboratory, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI 48824, USA. E-mail: sousaleo.eng@gmail.com
fDepartment of Chemical & Biological Engineering, Montana State University, Bozeman, MT 59717, USA
gDepartment of Process Engineering, Stellenbosch University, Private Bag X1 Matieland, Stellenbosch, South Africa
hDepartment of Engineering Technology, College of Technology, University of Houston-Sugarland campus, TX 77479, USA
iGreat Lakes Bioenergy Research Center, Michigan State University, East Lansing, MI 48824, USA
First published on 21st April 2022
Abstract
A novel ammonia-based pretreatment for densified lignocellulosic biomass was developed to reduce ammonia usage and integrate with viable biomass logistics scenarios. The COmpacted Biomass with Recycled Ammonia (COBRA) pretreatment performed at 100 °C allows >95% conversion of sugarcane bagasse (SCB) carbohydrates into soluble monomeric and oligomeric sugars (glucose and xylose) using industrially relevant 6% glucan loading (∼21% solids loading) enzymatic hydrolysis conditions at reduced enzyme loadings. Pretreatment via COBRA with simultaneous lignin extraction (COBRA-LE) improved Saccharomyces cerevisiae 424A(LNH-ST) metabolic yield from 89% to 97.5% relative to COBRA without delignification, allowing a process ethanol yield of 71.6%. A technoeconomic analysis on SCB biorefining to ethanol in the state of São Paulo, Brazil, compared COBRA to other mature technologies, such as AFEX and steam-explosion. Amongst all scenarios studied, biorefineries based on COBRA-LE pretreatment offered the lowest average minimum ethanol selling price of US$1.45 per gallon ethanol. COBRA pretreatment was subsequently tested on perennial grasses and hardwoods, and >80% total sugar yields were achieved for all cases.
1. Introduction
The future of the bioeconomy depends on the development and implementation of a feasible biorefinery concept. In the context of a biochemical refinery, it hinges on (1) robust and effective pretreatment and fractionation technologies that maximize lignocellulosic biomass conversion to usable sugars during enzyme hydrolysis and (2) technologies that enable the upgrading of lignin to fuels and chemicals. This and similar statements are often emphasized in the literature,1 but there are other key factors that are also universal to biomass processing and should be considered when developing viable biorefinery systems. For example, viable biorefinery systems must integrate within a feedstock logistics platform that provides stable, year-round biomass storage and handling, achieves essential economies of scale, and minimizes the biofuel carbon footprint. The work presented herein reports on the development of a robust ammonia-based pretreatment system for lignocellulosic biomass with those required attributes. The proposed system consists of a scalable feedstock supply chain integrated with sustainable year-round biofuel and bio-based chemical production from lignocellulosic biomass at high process yields and low chemical requirements.1.1. Pretreatment in the biorefinery context
Recent trends in lignocellulosic biomass pretreatment development focus on the fractionation of plant cell wall components, notably lignin and carbohydrates, so that they are processed separately to yield liquid biofuels and biobased chemicals.2–6 This approach is designed to use all biomass components to generate high-value products that cannot be generated by any other renewable resource, in contrast to the traditional approach in which lignin is converted to electrical power.7–10 In practice, lignin valorization to bioproducts requires the use of pretreatment/fractionation methods that preserve lignin functionalities and prevent lignin condensation to facilitate its controlled depolymerization to value-added aromatic monomers.10The biomass fractionation approach requires selective lignin removal from carbohydrates to maximize product yields from sugar and lignin streams, while simplifying product separations downstream. In addition to the value increment that lignin conversion can provide to the biorefinery, maximizing the removal of lignin prior to enzymatic hydrolysis also helps to reduce biomass recalcitrance and increases enzymatic activity on the pretreated carbohydrate-rich stream.11–13 Reducing enzyme loading during enzymatic hydrolysis has been a primary objective in biomass conversion, not only because it positively impacts the final biofuel price, but it also serves to mitigate risk due to uncertain bulk enzyme prices.14 An effective approach to significantly reduce enzyme loading is the use of pretreatment/fractionation technologies that delignify lignocellulosic biomass while manipulating the native cellulose I (CI) crystalline structure to either amorphous or other more digestible crystalline cellulose allomorphs, such as cellulose III (CIII).15–18 Pretreatments with ionic liquids (ILs), ammonium salts (ammonium thiocyanate in liquid ammonia) and liquid ammonia (Extractive Ammonia (EA) pretreatment) are approaches that can both selectively isolate usable lignin from carbohydrates and improve the enzymatic digestibility of cellulose by altering its crystalline structure.3,19–21 However, these methods still need to improve their economic and environmental sustainability. For example, although ionic liquids are highly effective in reducing biomass recalcitrance, they are nonetheless expensive and difficult to recycle.22,23 Recent research efforts to develop low-cost ILs and new IL recycling strategies reduced the effective IL cost to ∼US$5 per kg, which is still a relatively expensive proposition for biomass pretreatment.23
EA has proven to be a very effective ammonia-based pretreatment technology, generating biofuel yields comparable to those obtained for 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) pretreatment, and significantly higher than those obtained for AFEX‡ and Dilute Acid (DA) pretreatments at relatively low enzyme loadings.3 EA pretreatment uses liquid ammonia-to-biomass at ratios greater than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w to completely submerge the biomass in liquid ammonia, thereby forming highly digestible CIII and extracting nearly 50% of the lignin in corn stover (CS) without significant carbohydrate losses.3 The lignin extracted during EA pretreatment and recovered after enzymatic hydrolysis of EA-pretreated biomass is relatively intact, maintaining most of the aryl–ether crosslinks and minimal condensation levels. Such lignin materials are viable substrates for conversion to an array of valuable aromatic platform chemicals.3,5,24
1 w/w to completely submerge the biomass in liquid ammonia, thereby forming highly digestible CIII and extracting nearly 50% of the lignin in corn stover (CS) without significant carbohydrate losses.3 The lignin extracted during EA pretreatment and recovered after enzymatic hydrolysis of EA-pretreated biomass is relatively intact, maintaining most of the aryl–ether crosslinks and minimal condensation levels. Such lignin materials are viable substrates for conversion to an array of valuable aromatic platform chemicals.3,5,24
Although ammonia is a less expensive chemical (∼US$0.5 per kg) for pretreating lignocellulosic biomass and is easier to recycle than ILs, a recent analysis on EA pretreatment showed that the key factor determining its economic sustainability is the liquid ammonia-to-biomass ratio required to effectively generate CIII and extract lignin.3 Ammonia evaporation and re-condensation during recycling requires considerable energy for EA pretreatment relative to AFEX. For example, EA pretreatment with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ammonia-to-biomass ratio requires about 60% of the high heating value (HHV) of the ethanol produced in order to recycle the ammonia, while AFEX only requires about 36%.3 Reducing the energy requirements during ammonia pretreatment, while converting CI to CIII, cleaving LCC linkages and achieving biomass delignification remain as major challenges for ammonia-based pretreatments.
1 w/w ammonia-to-biomass ratio requires about 60% of the high heating value (HHV) of the ethanol produced in order to recycle the ammonia, while AFEX only requires about 36%.3 Reducing the energy requirements during ammonia pretreatment, while converting CI to CIII, cleaving LCC linkages and achieving biomass delignification remain as major challenges for ammonia-based pretreatments.
Furthermore, although ammonia pretreatments perform well on herbaceous monocots and generate highly fermentable hydrolysates, they have not yet shown comparable performance on herbaceous dicots, hardwoods and softwoods under low severity processing conditions.25–28 Thus, ammonia pretreatments are still seen as less versatile for pretreating mixed lignocellulosic feedstocks, relative to IL-based, organosolv, steam explosion (StEx), deep eutectic solvents (DES) pretreatments and Reductive Catalytic Fractionation (RCF), among others. The ability to successfully pretreat a wide range of lignocellulosic feedstocks is particularly important if the objective is to create very large scale biorefineries to benefit from economies of scale and reduce the Minimum Biofuel Selling Price (MBSP). The larger the biorefinery capacity, the wider the biomass collection radius is likely to be, which probably also increases the available feedstock diversity, except for those relatively rare geographic locations where monocultures are available in large, contiguous areas of land (e.g., corn in the US Midwest and sugarcane in Sao Paulo, Brazil, among others).
In a field that wishes to sustainably valorize lignocellulosic biomass and compete in a market dominated by petroleum-derived commodities, the scale of biorefineries is a critical topic that has been largely overlooked in the literature. For example, the US has been reducing the number of active petroleum refineries while increasing their production capacity (i.e., increasing the refinery scale), with the intention of reducing operating costs and depreciation.29 In 2014, the US average capacity for crude oil processing per refinery was 128.7 thousand barrels per day, which corresponds to about 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 Mg crude oil per refinery, per day.29 In contrast, most lignocellulosic biorefinery models in the literature assume capacities of around 2000 Mg dry biomass per day,30,31 which is very far from the scale at which petroleum is refined. There are major challenges associated with the scalability of systems that depend on highly variable, low-density solid substrates such as lignocellulosic biomass. The larger the biorefinery, the greater the biomass collection radius and transportation distances from the field to the biorefinery. As consequence, delivering low-density biomass to those large biorefineries (hereafter called ‘mega-biorefineries’) becomes more complex and expensive, resulting in the need for biomass milling, densification and drying in regional processing depots (RPDs) located closer to the biomass production fields. As such, biomass storage and long-haul shipment to mega-biorefineries can be simplified.32,33 We note also that paying farmers more for biomass induces them to grow much more biomass, leading to shorter supply chains with reduced transportation costs and much larger biorefineries (potentially with capacities greater than 20
800 Mg crude oil per refinery, per day.29 In contrast, most lignocellulosic biorefinery models in the literature assume capacities of around 2000 Mg dry biomass per day,30,31 which is very far from the scale at which petroleum is refined. There are major challenges associated with the scalability of systems that depend on highly variable, low-density solid substrates such as lignocellulosic biomass. The larger the biorefinery, the greater the biomass collection radius and transportation distances from the field to the biorefinery. As consequence, delivering low-density biomass to those large biorefineries (hereafter called ‘mega-biorefineries’) becomes more complex and expensive, resulting in the need for biomass milling, densification and drying in regional processing depots (RPDs) located closer to the biomass production fields. As such, biomass storage and long-haul shipment to mega-biorefineries can be simplified.32,33 We note also that paying farmers more for biomass induces them to grow much more biomass, leading to shorter supply chains with reduced transportation costs and much larger biorefineries (potentially with capacities greater than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mg per day), with the attendant economies of scale and only small effects on biofuel selling price.34 Overall, the issue of biorefinery scale and the associated logistics needs much more study.
000 Mg per day), with the attendant economies of scale and only small effects on biofuel selling price.34 Overall, the issue of biorefinery scale and the associated logistics needs much more study.
Based on these considerations, this work describes a highly efficient pretreatment technology using low chemical and energy inputs that is effective on a wide variety of lignocellulosic feedstocks, extracts usable lignin with preserved chemical functionalities and that fits within a scalable bioeconomy concept. The overall system accounts for feedstock availability and diversity, feedstock logistics and the need for very large scale biorefineries with their greater economic sustainability (Fig. 1A). Here, a new pretreatment technology called ‘COmpacted Biomass pretreatment with Recycled Ammonia’ (COBRA) is studied for the first time. COBRA pretreats low moisture, densified feedstocks at temperatures under 100 °C, thus allowing liquid ammonia-to-biomass ratios below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to fully submerge the densified solids and convert CI to CIII, maximizing both carbohydrate conversion and usable lignin recovery (Fig. 1B). The economic and environmental sustainability of the COBRA-based bioeconomy is elaborated. Also, a case study on a COBRA-based bioeconomy is evaluated for sugarcane bagasse in the State of São Paulo, Brazil, relative to other competing technologies that use loose feedstocks, such as AFEX, EA and StEx pretreatments.
1 to fully submerge the densified solids and convert CI to CIII, maximizing both carbohydrate conversion and usable lignin recovery (Fig. 1B). The economic and environmental sustainability of the COBRA-based bioeconomy is elaborated. Also, a case study on a COBRA-based bioeconomy is evaluated for sugarcane bagasse in the State of São Paulo, Brazil, relative to other competing technologies that use loose feedstocks, such as AFEX, EA and StEx pretreatments.
1.2. COmpacted biomass pretreatment with recycled ammonia (COBRA)
COBRA pretreatment has been tailored to synergize with decentralized depots for densification and storage of biomass. Such depots support the viability of mega-biorefineries (Fig. 1). In previous research we examined the EA pretreatment, in which ammonia acts both as a reagent and a lignin extraction solvent.3,35,36 During EA, ammonia cleaves ester bonds that link acetyl groups to the xylan backbone in hemicellulose and lignin polymers in the so-called lignin-carbohydrate complexes (LCC) found in both grasses and hardwoods.3,37 Chundawat et al. has shown that lignin and hemicellulose oligomers are removed from the plant cell wall by liquid ammonia, exposing the complex carbohydrate fibers to cellulases and hemicellulases during enzymatic hydrolysis.35,36 The EA pretreatment process takes advantage of lignin solubility in liquid ammonia to extract up to 47% of the original lignin from the biomass while retaining >95% of the carbohydrates.3 As expected, since lignin is known to inhibit hydrolytic enzymes, its removal also improves enzyme activity on the pretreated/delignified substrate.3,38 More importantly, the native CI crystal present in plant cell walls can be converted to CIII by submerging the biomass in liquid anhydrous ammonia. CIII is up to 5 times more digestible by commercial cellulases than the native CI crystal.15,16,18 All these chemical and ultra-structural modifications of the plant cell wall occur during EA pretreatment but not with AFEX pretreatment because AFEX uses higher moisture levels (typically 60% on a biomass dry weight basis) that prevent CIII formation.17,35 Therefore, EA-pretreated biomass achieves sugar yields comparable to those obtained by ionic liquid pretreatment, and significantly higher than those obtained via conventional AFEX or DA pretreatments.3 However, the biomass must be fully submerged in liquid ammonia for CIII to be formed, and a minimum of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia-to-biomass ratio was reported to maximize sugar yields after EA pretreatment.3,17 These high ammonia loading requirements proceed from the fact that bulk biomass density is extremely low, resulting in a large dead volume in the pretreatment reactor occupied by liquid ammonia that is not acting on biomass.
1 ammonia-to-biomass ratio was reported to maximize sugar yields after EA pretreatment.3,17 These high ammonia loading requirements proceed from the fact that bulk biomass density is extremely low, resulting in a large dead volume in the pretreatment reactor occupied by liquid ammonia that is not acting on biomass.
Alternatively, if we assume a feedstock logistics model based on densified biomass, as shown in Fig. 1, much larger quantities of densified biomass can be loaded per unit volume of a pretreatment reactor relative to loose or baled biomass. This can result in several potential advantages relative to pretreatment of loose biomass, as the volume of liquid ammonia required to fully submerge densified biomass is considerably lower than that required to submerge loose or baled biomass. Thus, submerging densified biomass in liquid ammonia enables conversion of CI to CIII, the cleavage of LCC linkages and selective extraction of lignin from biomass, as has been described for EA pretreatment (e.g., 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia to biomass ratio), but also using low ammonia levels as described for AFEX pretreatment (e.g., 1
1 ammonia to biomass ratio), but also using low ammonia levels as described for AFEX pretreatment (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia to densified biomass mass ratio). The lower ammonia loading requirement greatly reduces energy costs for ammonia recycling, while potentially producing highly digestible feedstocks. Also, greater biomass density allows for smaller pretreatment reactor volumes for a fixed pretreatment time, which reduces capital costs. Alternatively, it allows longer pretreatment residence times for the same reactor size relative to conventional AFEX, EA and StEx, all of which operate with loose biomass. In this article, the COBRA pretreatment has been developed, optimized, and evaluated for various pretreatment conditions (with and without lignin extraction), enzyme loadings and feedstocks.
1 ammonia to densified biomass mass ratio). The lower ammonia loading requirement greatly reduces energy costs for ammonia recycling, while potentially producing highly digestible feedstocks. Also, greater biomass density allows for smaller pretreatment reactor volumes for a fixed pretreatment time, which reduces capital costs. Alternatively, it allows longer pretreatment residence times for the same reactor size relative to conventional AFEX, EA and StEx, all of which operate with loose biomass. In this article, the COBRA pretreatment has been developed, optimized, and evaluated for various pretreatment conditions (with and without lignin extraction), enzyme loadings and feedstocks.
2. Results and discussion
2.1. COBRA pretreatment effects on saccharification of biomass at high-solid loadings
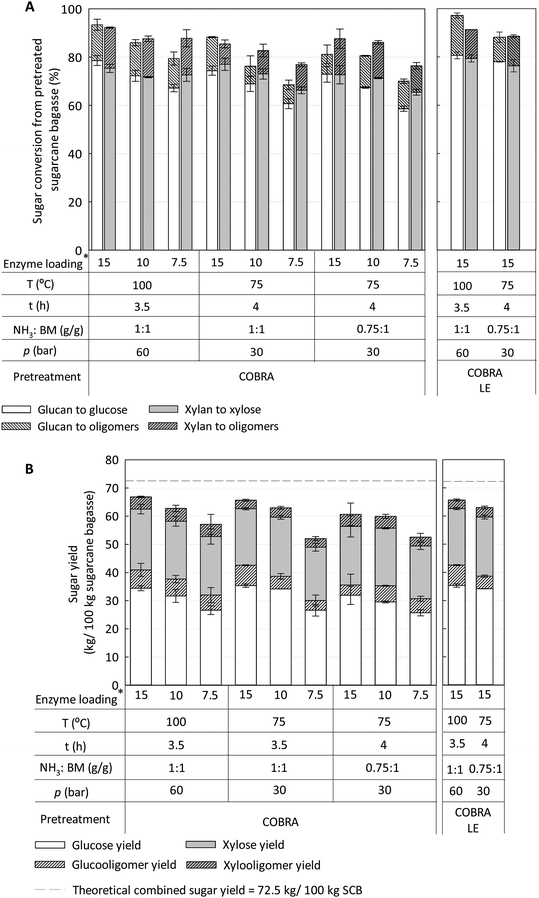
To better understand the effects of COBRA pretreatment conditions on the enzymatic digestibility of plant cell walls, sugarcane bagasse (SCB) was selected as a model substrate. SCB is a widely available substrate, an agricultural residue produced in sugar mills in many countries. These mills could serve as regional depots for biomass densification and storage. Also, ammonia-pretreated SCB tends to be more recalcitrant than other herbaceous feedstocks such as corn stover (CS), and therefore, SCB can better highlight the relative robustness toward biomass recalcitrance of the pretreatment technologies tested in this work for comparative reasons.39–41 Enzymatic hydrolysis of the pretreated feedstocks was performed under industrially-relevant conditions, i.e., 6% glucan loading (∼21% solids loading), with enzyme loadings between 7.5 and 15 mg g−1 glucan for 96 h of hydrolysis. Three COBRA conditions were selected based on pretreatment optimization models that are described in ESI (Fig. S1–S4†). The selected conditions were: (1) the least severe condition that maximized fermentable sugar yields, i.e., glucose + xylose (100 °C, 3.5 h with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g), (2) the condition that maximized fermentable sugar yields at the lowest effective temperature and at a pressure similar to that observed for AFEX pretreatment (75 °C, 4 h with 1
BM g/g), (2) the condition that maximized fermentable sugar yields at the lowest effective temperature and at a pressure similar to that observed for AFEX pretreatment (75 °C, 4 h with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g), and (3) the condition that maximized sugar yields at lower temperature, pressures similar to those observed for optimal AFEX pretreatment and lower ammonia loadings than typically used for AFEX pretreatment (75 °C, 4 h with 0.75
BM g/g), and (3) the condition that maximized sugar yields at lower temperature, pressures similar to those observed for optimal AFEX pretreatment and lower ammonia loadings than typically used for AFEX pretreatment (75 °C, 4 h with 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g).
BM g/g).
Furthermore, the enzyme cocktail for optimal conversion of COBRA-pretreated SCB to fermentable glucose + xylose sugars was determined (71 wt% CTec3: 23 wt% HTec3: 6 wt% Multifect Pectinase) (Fig. S5 and S6, ESI†) and used to fully explore the enzymatic hydrolysis potential of COBRA-pretreated SCB. Fig. 2A and B show that COBRA pretreatment performed under the mildest condition scrutinized here, i.e., 75 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM loading g/g for 4 h of residence time, and 15 mg of enzyme loading converted >88% glucan and >85% xylan, for a combined sugar yield of 63 kg per 100 kg of sugarcane bagasse (Fig. 2B).
BM loading g/g for 4 h of residence time, and 15 mg of enzyme loading converted >88% glucan and >85% xylan, for a combined sugar yield of 63 kg per 100 kg of sugarcane bagasse (Fig. 2B).
As expected, combined sugar yields were greater for the highest temperature studied (e.g., 100 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM loading g/g for 3.5 h and 15 mg of enzyme loading), resulting in an increase of 6% glucan and 8% xylan yields relative to COBRA-pretreated SCB at 75 °C, when hydrolyzed with 15 mg g−1 glucan enzyme loading. As observed with EA, COBRA pretreatment temperature does not affect the formation of CIII, as it is formed at both 75 °C and 100 °C using 1
BM loading g/g for 3.5 h and 15 mg of enzyme loading), resulting in an increase of 6% glucan and 8% xylan yields relative to COBRA-pretreated SCB at 75 °C, when hydrolyzed with 15 mg g−1 glucan enzyme loading. As observed with EA, COBRA pretreatment temperature does not affect the formation of CIII, as it is formed at both 75 °C and 100 °C using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM (g/g) (Fig. S4, ESI†), but it does promote a more effective cleavage of ester bonds and greater lignin solubilization, as previously reported by da Costa Sousa et al., making the CIII more accessible to cellulases.3 However, higher temperatures generate higher operating pressures (60 bar at 100 °C). For example, a pressure of 30 bar was observed when COBRA was performed at 75 °C, which is similar to the pressure found for AFEX pretreatment at 120 °C.42 AFEX uses higher moisture levels (approximately 60% of biomass dry weight)43 than does COBRA, reducing the pressure for a given temperature relative to using solely anhydrous ammonia to pretreat dried biomass. However, those moisture levels suitable for AFEX prevent CIII formation during ammonia pretreatment, thus reducing pretreatment effectiveness.3,16,17 COBRA pretreatment is performed on storage-grade, densified biomass with about 10 wt% moisture to prevent microbial decomposition of the biomass. Thus, when the temperature is raised to 100 °C, the pressure increases to about 60 bar, approaching the pressures observed for EA pretreatment. Note that EA pretreatment is also performed using anhydrous ammonia, but on dried loose biomass instead of densified biomass.3 Reducing ammonia loading from 1
BM (g/g) (Fig. S4, ESI†), but it does promote a more effective cleavage of ester bonds and greater lignin solubilization, as previously reported by da Costa Sousa et al., making the CIII more accessible to cellulases.3 However, higher temperatures generate higher operating pressures (60 bar at 100 °C). For example, a pressure of 30 bar was observed when COBRA was performed at 75 °C, which is similar to the pressure found for AFEX pretreatment at 120 °C.42 AFEX uses higher moisture levels (approximately 60% of biomass dry weight)43 than does COBRA, reducing the pressure for a given temperature relative to using solely anhydrous ammonia to pretreat dried biomass. However, those moisture levels suitable for AFEX prevent CIII formation during ammonia pretreatment, thus reducing pretreatment effectiveness.3,16,17 COBRA pretreatment is performed on storage-grade, densified biomass with about 10 wt% moisture to prevent microbial decomposition of the biomass. Thus, when the temperature is raised to 100 °C, the pressure increases to about 60 bar, approaching the pressures observed for EA pretreatment. Note that EA pretreatment is also performed using anhydrous ammonia, but on dried loose biomass instead of densified biomass.3 Reducing ammonia loading from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.75
1 to 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM (g/g) during COBRA pretreatment at 75 °C did not significantly impact the glucan and xylan conversion.
BM (g/g) during COBRA pretreatment at 75 °C did not significantly impact the glucan and xylan conversion.
Detailed knowledge on how the biomass bulk density changes during COBRA pretreatment and related thermodynamic property measurements are required to predict the lowest possible ammonia loading for a given operational condition, and to maintain high process sugar yields. Nonetheless, based on the ammonia density at 75 °C, and determining experimentally the water adsorption capacity of SCB pellets as a surrogate for ammonia, while accounting for the respective pellet bulk volume expansion at saturation conditions, we estimate that 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM (g/g) is close to the lower limit required to fully submerge the densified biomass in anhydrous ammonia. Also, preliminary studies confirmed that NH3 loadings below 0.75
BM (g/g) is close to the lower limit required to fully submerge the densified biomass in anhydrous ammonia. Also, preliminary studies confirmed that NH3 loadings below 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g significantly reduced sugar yields for COBRA pretreated CS (data not shown).
BM g/g significantly reduced sugar yields for COBRA pretreated CS (data not shown).
Comparing the three COBRA pretreatment conditions tested in Fig. 2, it is evident that pretreatment performance did not vary significantly for the highest enzyme loading (15 mg g−1 glucan). However, enzyme loading significantly impacts the overall sugar release for all pretreatment conditions, and more so for the lower temperature COBRA pretreatment conditions. Thus, enzyme levels might be reduced for COBRA pretreatment, but might in turn require higher pretreatment temperatures (and pressures). Also, high levels of soluble gluco- and xylo-oligosaccharides were released during high solid loading enzymatic hydrolysis, accounting for 10–15% of the total soluble sugar under some conditions (Fig. 2). The specific properties of these oligosaccharides (including linkage analysis, composition, chemical structure) should be studied so that specific enzymes can be added to the enzyme cocktails to improve the conversion of those soluble carbohydrates to fermentable sugars.
The impact of lignin extraction during COBRA pretreatment was evaluated by removing the liquid ammonia-soluble lignin from the bottom of the reactor, passing it through a sintered filter under pressure (COBRA-LE), as described in the Experimental section. Hereafter the COBRA process with lignin extraction is identified as COBRA-LE. COBRA-LE pretreatment performed at 100 °C extracted about 26% of the original lignin from SCB, resulting in 4% point improvement in glucan conversion (97% overall glucan conversion) over that observed for COBRA pretreatment performed at similar operational conditions without lignin extraction.
Biomass delignification during EA pretreatment has shown a similar effect on enzymatic saccharification to that observed for COBRA-LE. da Costa Sousa et al. reported a glucan conversion improvement of 6% points after removing lignin from CS during EA pretreatment at 120 °C and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g loading, yielding 89% overall glucan conversion.3 However, no significant improvement in total glucan conversion was found for COBRA-LE performed at 75 °C relative to COBRA performed using the same conditions, likely due to low delignification yield (∼19%). Although carbohydrate conversion from COBRA-LE pretreated biomass improved somewhat relative to COBRA, the total sugar yields from pretreated biomass did not improve upon those obtained with COBRA pretreatment. For example, the combined sugar yield at the most severe COBRA-LE pretreatment condition (100 °C, 1
BM g/g loading, yielding 89% overall glucan conversion.3 However, no significant improvement in total glucan conversion was found for COBRA-LE performed at 75 °C relative to COBRA performed using the same conditions, likely due to low delignification yield (∼19%). Although carbohydrate conversion from COBRA-LE pretreated biomass improved somewhat relative to COBRA, the total sugar yields from pretreated biomass did not improve upon those obtained with COBRA pretreatment. For example, the combined sugar yield at the most severe COBRA-LE pretreatment condition (100 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g loading, 3.5 h reaction time) was 65.7 kg sugar per 100 kg untreated SCB, while COBRA achieved 67.4 kg sugar per 100 kg untreated SCB. This is due to the fact that a small fraction of carbohydrates, notably xylan, was extracted with lignin during COBRA-LE pretreatment and was never converted during enzymatic hydrolysis. However, both COBRA and COBRA-LE pretreatments enabled soluble sugar yields, including oligosaccharides, close to the theoretical maximum for the SCB used in this work, i.e., 72.5 kg sugar per 100 kg untreated SCB, using relatively mild operating conditions and low enzyme loadings during high solid loading enzymatic hydrolysis (Fig. 2B).
BM g/g loading, 3.5 h reaction time) was 65.7 kg sugar per 100 kg untreated SCB, while COBRA achieved 67.4 kg sugar per 100 kg untreated SCB. This is due to the fact that a small fraction of carbohydrates, notably xylan, was extracted with lignin during COBRA-LE pretreatment and was never converted during enzymatic hydrolysis. However, both COBRA and COBRA-LE pretreatments enabled soluble sugar yields, including oligosaccharides, close to the theoretical maximum for the SCB used in this work, i.e., 72.5 kg sugar per 100 kg untreated SCB, using relatively mild operating conditions and low enzyme loadings during high solid loading enzymatic hydrolysis (Fig. 2B).
2.2. COBRA pretreatment performance relative to other highly promising alternative pretreatment technologies
Fermentable sugar and ethanol yield for COBRA and COBRA-LE pretreatments carried out at various operational conditions were compared to other leading pretreatment technologies, such as EA, AFEX and StEx (Fig. 3). EA is an alkaline-type pretreatment with similar reaction mechanism to COBRA-LE, i.e., it is an ammonia-based pretreatment that extracts lignin and modifies the cellulose crystalline structure to CIII. AFEX is also an ammonia-based technology, but with established maturity, and unlike COBRA-LE and EA, does not lead to CIII formation nor lignin removal.44,45 Finally, StEx is another industrially-relevant technology that is effective on a wide range of feedstocks. StEx has been very well studied for SCB in previous reports and has been selected to benchmark acidic pretreatments.40,46,47Fig. 3 shows that the latest generation of ammonia pretreatment technologies, such as COBRA, COBRA-LE and EA release significantly higher fermentable sugar levels relative to AFEX and StEx for most operational conditions studied herein. COBRA and COBRA-LE performed at 100 °C resulted in a total sugar yields of approximately 67.4 ± 2.6 and 65.7 ± 1.8 kg per 100 kg of SCB, respectively, a significant improvement over the performance of the other pretreatment technologies in this study and close to the theoretical maximum sugar yield of 72.5 kg per 100 kg of SCB. This result is remarkable if we consider that the enzymatic saccharification for both steam explosion and AFEX-pretreated SCB was performed with 25 mg protein per g glucan enzyme loading, while COBRA, COBRA-LE and EA pretreated SCB used only 15 mg protein per g glucan enzyme loading. Thus, the newer generation of ammonia pretreatment technologies studied herein, which convert CI to CIII and can remove a fraction of the lignin present in SCB, also achieve higher sugar yields with 40% less enzyme relative to AFEX and StEx pretreatments.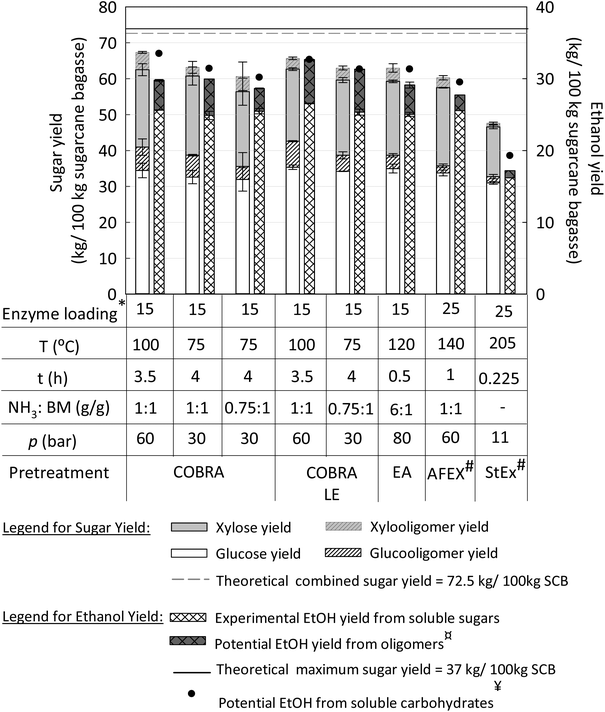 | ||
| Fig. 3 Comparison of COBRA, COBRA-LE, EA, AFEX and steam explosion (StEx) pretreatments in terms of sugar and ethanol (EtOH) yields. Total sugar yields were calculated considering glucose, gluco-oligomers, xylose and xylo-oligomers. Ethanol yields were calculated on the basis of 100 kg of untreated sugarcane bagasse input after 120 min of fermentation time. The theoretical maximum for sugar and ethanol yields was calculated based on the initial glucan and xylan contents in untreated sugarcane bagasse. # The sugar and ethanol yields from “AFEX – bagasse” and “StEx – bagasse – whole slurry” were obtained by Mokomele et al.40¤The potential ethanol yield from oligomers was estimated based on the metabolic yields and sugar consumption obtained in each operational condition (Table S1 in ESI†). ¥The potential ethanol yield from soluble sugars was estimated considering the complete conversion of soluble sugars into ethanol with the highest metabolic yield obtained (97.5%). All the enzymatic hydrolysis liquors were produced at 6% glucan loading (w/w, glucan) for 96 h of hydrolysis time. COBRA, COBRA-LE and EA enzymatic hydrolysis were performed with 15 mg protein per g glucan, while AFEX and StEx were carried out with 25 mg protein per g glucan. | ||
For COBRA-pretreated SCB, the total sugar yield decreases with reducing pretreatment severity, generating lower levels of soluble sugars. However, most of that difference is due to increased oligomeric carbohydrates in the hydrolysate at higher pretreatment severities. There is no substantial difference in monomeric sugar yields between the various COBRA pretreatment conditions: the fermentable sugar yields for the most severe and the least severe COBRA pretreatment conditions were, respectively, 56.0 ± 2.6 and 52.8 ± 4.9 kg per 100 kg SCB. However, if the potential conversion of oligosaccharides to ethanol is also considered, larger differences in ethanol yield can be observed (Fig. 3) between these two conditions. In addition, if we assume that all soluble carbohydrates are converted to ethanol by S. cerevisiae 424A (LNH-ST) with a metabolic yield of 97.5%, the highest metabolic yield observed for all conditions tested herein, 90% of the theoretical ethanol yield from SCB, i.e., 36.9 kg ethanol per 100 kg SCB, could be obtained for the most severe COBRA pretreatment condition.
Significant improvements in ethanol yields were observed when lignin was extracted during COBRA pretreatment (COBRA-LE). Although fermentable sugar yields were slightly lower than those observed for the highest COBRA severity, the fermentation performance on COBRA-LE hydrolysates was much greater than that observed for COBRA hydrolysates, achieving 97.5% metabolic yield (ESI Table S1†). This result is expected, as compounds that inhibited the yeast strain were likely removed during the lignin extraction process.3,48 As previously discussed, a total of 65.7 ± 1.8 kg sugar per 100 kg SCB was solubilized during 6% glucan loading enzymatic hydrolysis for the highest COBRA-LE pretreatment severity. This result includes monomeric and oligomeric sugars, representing about 90% of the theoretical maximum sugar yield from SCB (72.5 kg sugar per 100 kg SCB), of which 76.5% are fermentable monomeric sugars (55.4 kg sugar per 100 kg SCB). The experimental ethanol yield for COBRA-LE pretreatment performed at the highest severity was 26.5 kg ethanol per 100 kg SCB, which is 71.6% of the theoretical. In contrast, the same COBRA pretreatment condition only achieved 69.4% of the theoretical maximum yield, despite generating higher sugar yields. If all soluble sugars, including oligosaccharides, were consumed and converted to ethanol with 97.5% metabolic yield, about 32.7 kg ethanol per 100 kg biomass would be produced, or about 88.4% of the theoretical maximum.
As previously mentioned, these results obtained for COBRA and COBRA-LE pretreatments further highlight the importance of understanding the recalcitrant nature of soluble oligosaccharides present in COBRA-derived hydrolysates. In addition, more robust microorganisms than that used in this study should be developed to maximize ethanol yields from the available sugar present in COBRA-derived hydrolysates. Lower overall xylose consumption (range 84.5 ± 3.7% to 91.1 ± 0.1%) was the main factor responsible for the lower ethanol yields. Improved xylose consumption can be achieved by extracting lignin from SCB or by adding nutrients to the hydrolysate,49 however, both require additional processing costs. For instance, COBRA-LE performed at 100 °C resulted in a delignification yield of 26%, improving the xylose consumption by approximately 8% points relative to COBRA at 100 °C. In addition to enabling a higher ethanol yield, COBRA-LE pretreatment generates a lignin stream that can be further processed and purified, and potentially becomes a revenue source for the biorefinery (see technoeconomic evaluation section below).5,24 Previous studies have demonstrated that the lignin derived from ammonia pretreated biomass maintains the β-aryl–ether linkages from lignin intact, which are critical functionalities to perform controlled lignin depolymerization and avoid C–C condensation reactions.50
Also, EA was performed using 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia to biomass ratio, while the most severe COBRA-LE condition only used a 1
1 ammonia to biomass ratio, while the most severe COBRA-LE condition only used a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to produce a greater yield of fermentable sugars and overall soluble carbohydrates (monomers + oligomers). Although EA pretreatment did not promote higher sugar yields relative to COBRA-LE, it did show better fermentation performance. It may be that the higher lignin extraction efficiency during EA pretreatment relative to COBRA-LE resulted in lower levels of inhibitory compounds for fermentation. However, more in-depth studies are required to determine the inhibitory levels of the extracted lignin to S. cerevisiae 424A (LNH-ST), or even the impact of densification conditions on the potential formation of inhibitory compounds for fermentation.
1 ratio to produce a greater yield of fermentable sugars and overall soluble carbohydrates (monomers + oligomers). Although EA pretreatment did not promote higher sugar yields relative to COBRA-LE, it did show better fermentation performance. It may be that the higher lignin extraction efficiency during EA pretreatment relative to COBRA-LE resulted in lower levels of inhibitory compounds for fermentation. However, more in-depth studies are required to determine the inhibitory levels of the extracted lignin to S. cerevisiae 424A (LNH-ST), or even the impact of densification conditions on the potential formation of inhibitory compounds for fermentation.
As AFEX and StEx pretreatments do not modify cellulose crystallinity, nor remove lignin from biomass, an enzyme loading of 25 mg per glucan was required to maximize sugar yields, as shown by Mokomele et al.40 A total sugar yield of 60.3 ± 1.1 kg per 100 kg SCB was obtained under optimal AFEX conditions (140 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM ratio (g/g) for 1 h), which is comparable to that found for COBRA-LE with the lowest severity tested herein and using only 60% of the enzyme loading added to AFEX-pretreated SCB. Although COBRA-LE is conducted for 4 h, and AFEX for 1 h, COBRA-LE uses densified biomass which occupies significantly less reactor volume per unit of biomass treated. Our findings show that the pretreatment productivity is practically the same for COBRA-LE at 75 °C, with 0.75
BM ratio (g/g) for 1 h), which is comparable to that found for COBRA-LE with the lowest severity tested herein and using only 60% of the enzyme loading added to AFEX-pretreated SCB. Although COBRA-LE is conducted for 4 h, and AFEX for 1 h, COBRA-LE uses densified biomass which occupies significantly less reactor volume per unit of biomass treated. Our findings show that the pretreatment productivity is practically the same for COBRA-LE at 75 °C, with 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM ratio g/g, for 4 h residence time, and for AFEX performed at 140 °C, with 1
BM ratio g/g, for 4 h residence time, and for AFEX performed at 140 °C, with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM ratio (g/g) for 1 h residence time. Thus, in addition to the logistic advantage of using densified biomass for transportation and enabling mega biorefineries, with better economies of scale, COBRA-LE saves both operation and capital costs due to lower pressures, temperatures and ammonia loadings. The cost of biomass densification may not be offset by reduced transportation costs for scenarios with high biomass availability in a relatively small land area but it is still likely to be less expensive to feed densified biomass into the COBRA pretreatment reactor than to feed undensified, loose biomass.
BM ratio (g/g) for 1 h residence time. Thus, in addition to the logistic advantage of using densified biomass for transportation and enabling mega biorefineries, with better economies of scale, COBRA-LE saves both operation and capital costs due to lower pressures, temperatures and ammonia loadings. The cost of biomass densification may not be offset by reduced transportation costs for scenarios with high biomass availability in a relatively small land area but it is still likely to be less expensive to feed densified biomass into the COBRA pretreatment reactor than to feed undensified, loose biomass.
StEX pretreatment led to modest total sugar yields (47.6 ± 0.9 kg per 100 kg SCB), even with 25 mg protein per g glucan enzyme loading. This low sugar yield is mainly due to low carbohydrate recovery after the pretreatment step. StEx pretreatment requires high reaction severities to improve cellulose digestibility, leading to degradation of sugars into e.g., furans, hampering the production of fermentable sugars at high yields. Among the processes studied herein, StEx showed the lowest sugar recovery and lowest product yield, however, techno-economic analysis, as discussed later in this manuscript, clarifies the economic potential of StEx relative to the other pretreatment-based biorefinery models.
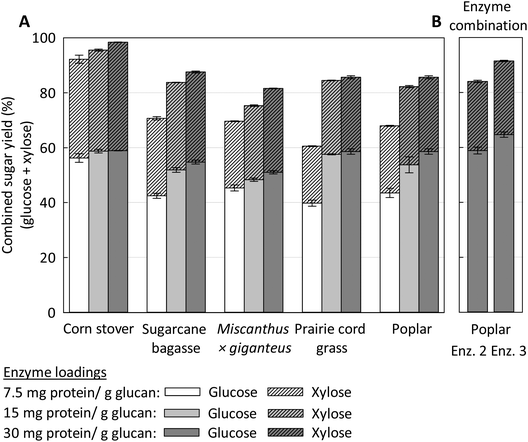
2.3. COBRA effectiveness on a range of feedstock types
The versatility of COBRA to effectively pretreat various biomasses, regardless of their macromolecular composition and morphological structure, has been investigated herein. It is highly desirable to have a practical operational window for effective pretreatment of a wide variety of feedstocks, especially for mega-biorefineries which are supplied with feedstocks available within a very wide radius. Fig. 4 summarizes the influence of COBRA pretreatment performed at 100 °C, 6 h and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM (g/g), on the saccharification performance at 30 mg enzyme protein per g glucan of corn stover, sugarcane bagasse, Miscanthus x giganteus, prairie cordgrass, and poplar after 96 h of enzymatic hydrolysis at various enzyme loadings. Fermentable sugar yields greater than 80% were found for all these materials, despite the inherent differences between woody biomass and grasses in terms of cell wall chemical composition, polysaccharide and lignin linkage types and cell wall ultrastructure. These data suggest that COBRA is robust and can be effective for pretreating a wide range of biomass types, notably herbaceous monocots and dicots, and hardwoods, under relatively mild pretreatment regimes (100 °C, 6 h and 1
BM (g/g), on the saccharification performance at 30 mg enzyme protein per g glucan of corn stover, sugarcane bagasse, Miscanthus x giganteus, prairie cordgrass, and poplar after 96 h of enzymatic hydrolysis at various enzyme loadings. Fermentable sugar yields greater than 80% were found for all these materials, despite the inherent differences between woody biomass and grasses in terms of cell wall chemical composition, polysaccharide and lignin linkage types and cell wall ultrastructure. These data suggest that COBRA is robust and can be effective for pretreating a wide range of biomass types, notably herbaceous monocots and dicots, and hardwoods, under relatively mild pretreatment regimes (100 °C, 6 h and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM g/g), while leading to high fermentable sugar production. The COBRA performance on softwoods was not evaluated herein, as the hemicellulose structure of softwoods, rich in galactoglucomannan, requires a dedicated hemicellulase cocktail that was not available for a fair comparison in this study.
BM g/g), while leading to high fermentable sugar production. The COBRA performance on softwoods was not evaluated herein, as the hemicellulose structure of softwoods, rich in galactoglucomannan, requires a dedicated hemicellulase cocktail that was not available for a fair comparison in this study.
The identification and development of a “feedstock-agnostic” pretreatment that can simultaneously extract lignin from biomass, promote biomass solubilization, and achieve high fermentable sugar yields with minimal use of enzymes and chemicals has been a subject of great interest. For instance, ionic liquid-based pretreatments are claimed to be one of the few “feedstock agnostic” technologies capable of efficiently handling hardwoods, softwoods, agricultural residues, herbaceous dicots and monocots, both as a single and as a blend of various feedstocks.51,52 According to Li et al., [C2mim][OAc] is one of the most effective and versatile ionic liquids reported for biomass pretreatment, as it is able to effectively liberate more than 90% of sugars from eucalyptus and switchgrass during enzymatic saccharification in 24 h.51 However, similar results were not observed for other woody biomasses under the same IL pretreatment (160 °C for 3 h) and enzymatic hydrolysis conditions, as the authors reported only 62% enzymatic digestibility for pine wood, for example. Also, it is important to note that the overall monomeric sugar recovery from pine in that study was only 49.7% after 72 h enzymatic hydrolysis, as a significant portion of the carbohydrates were left in the liquor as oligomers.51 It is worth to mention that a verification experiment on COBRA pretreated pine at 100 °C for 6 h, using with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM loading, followed by 72 h of enzymatic hydrolysis with a non-optimal enzyme cocktail shows nearly 60% monomeric sugar recovery, combined.
BM loading, followed by 72 h of enzymatic hydrolysis with a non-optimal enzyme cocktail shows nearly 60% monomeric sugar recovery, combined.
Thus, as reported for other pretreatments (e.g., DA, AFEX and many others), ionic liquids pretreatment performance also varies with the feedstock due their inherent compositional and structural differences. Sun et al. reported that the type of wood affects the dissolution yields and rates of the feedstocks in [C2mim][OAc].53 For example, red oak dissolves much faster than southern yellow pine. In addition, the performance of a specific pretreatment technology is not only dependent on the type of feedstock, but also on other factors such as enzyme and solids loading, enzyme cocktail, and particle size. As shown in Fig. 4, the total sugar yields for each individual COBRA-pretreated feedstock, except for corn stover, increased significantly with enzyme loading. For instance, an improvement of 29% points in combined sugar yield was found for prairie grass with increasing enzyme loading from 7.5 to 30 mg protein per g glucan. Interestingly, changing enzyme loadings only had a slight effect on total sugar yields achieved from COBRA-pretreated CS.
To better understand the effect of different enzyme combinations on glucan and xylan conversion, we studied the influence of enzyme combinations on poplar, which was pretreated at 100 °C for 6 h with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3
1 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM loading g/g, followed by 96 h enzymatic hydrolysis with an enzyme loading of 30 mg protein per g glucan. As shown in Fig. 4B, there are significant differences in combined fermentable sugar yields for the various enzyme combinations tested for poplar (see data for Poplar, Poplar Enz. 2 and Poplar Enz. 3). An increase of 7% combined sugar yield was obtained using enzyme combination 3 (Enz. 3 in Fig. 4B) relative to the standard enzyme combination previously optimized for SCB. Thus, COBRA pretreatment has significantly improved enzyme access to their substrates, but differences in substrate composition require different ratios of the various enzymes in order to maximize carbohydrate conversion. Unlike acid-based pretreatments which require a washing step, COBRA is basically a dry-to dry pretreatment that preserves polysaccharides with little-to-no degradation of sugars. However, the presence of hemicellulose and pectin requires more robust and complex enzymatic cocktails relative to acidic pretreatments, which typically hydrolyze the non-cellulosic fraction of the biomass. Therefore, ammonia-pretreated mixed feedstocks require non-limiting levels of optimized ratios of cellulases, hemicellulases, pectinases, and other accessory enzymes with synergistic key activities to maximize overall sugar yields.54 This is particularly important not only for COBRA pretreatment, but also for other technologies (e.g., AFEX and EA) that do not hydrolyze hemicellulose linkages.3 Enzymatic deconstruction of hemicellulose is highly dependent on a complex range of enzyme activities that must be understood and fine-tuned to become effective on a wider range of substrates.55
BM loading g/g, followed by 96 h enzymatic hydrolysis with an enzyme loading of 30 mg protein per g glucan. As shown in Fig. 4B, there are significant differences in combined fermentable sugar yields for the various enzyme combinations tested for poplar (see data for Poplar, Poplar Enz. 2 and Poplar Enz. 3). An increase of 7% combined sugar yield was obtained using enzyme combination 3 (Enz. 3 in Fig. 4B) relative to the standard enzyme combination previously optimized for SCB. Thus, COBRA pretreatment has significantly improved enzyme access to their substrates, but differences in substrate composition require different ratios of the various enzymes in order to maximize carbohydrate conversion. Unlike acid-based pretreatments which require a washing step, COBRA is basically a dry-to dry pretreatment that preserves polysaccharides with little-to-no degradation of sugars. However, the presence of hemicellulose and pectin requires more robust and complex enzymatic cocktails relative to acidic pretreatments, which typically hydrolyze the non-cellulosic fraction of the biomass. Therefore, ammonia-pretreated mixed feedstocks require non-limiting levels of optimized ratios of cellulases, hemicellulases, pectinases, and other accessory enzymes with synergistic key activities to maximize overall sugar yields.54 This is particularly important not only for COBRA pretreatment, but also for other technologies (e.g., AFEX and EA) that do not hydrolyze hemicellulose linkages.3 Enzymatic deconstruction of hemicellulose is highly dependent on a complex range of enzyme activities that must be understood and fine-tuned to become effective on a wider range of substrates.55
2.4. Technoeconomic analysis (TEA) and biorefinery systems optimization
A TEA was performed to determine the potential of COBRA and COBRA-LE technologies relative to other benchmarked pretreatments such as AFEX, EA and StEx. In the literature, the optimization and economic evaluation of biorefineries are typically performed around the biorefinery plant itself, where feedstock price at the gate and the size of the biorefinery are fixed variables, and biomass availability is guaranteed. However, feedstock price and availability, and the optimal size of biorefineries will not be the same for every location in the world. In addition, the form in which that feedstock is delivered may depend on the technology used. For example, StEX, AFEX and EA pretreatments are effective in using loose biomass feedstocks, whereas COBRA and COBRA-LE require the use of densified biomass as an integral part of the pretreatment step. Densification should be performed near the farms in biomass processing depots prior to transportation and delivery to the gate of a centralized mega-biorefinery, as depicted in Fig. 1.In fact, AFEX pretreatment can be performed at the depot on loose biomass, and the AFEX-pretreated material can be densified, stored, and transported to a mega-biorefinery for further conversion to biofuels and chemicals.56,57 Since AFEX-treated biomass is an improved animal feed, the AFEX-based depot can provide both animal feed and biorefinery feedstock, thereby helping to “jump-start” a biorefining industry in the same way that the pre-existing use of corn as an animal feed helped jump-start the corn ethanol industry. Furthermore, unpublished work from our group shows that if biomass is AFEX-treated and then pelletized, milder COBRA pretreatment conditions can be used to achieve comparable sugar yields than those obtained using the most severe COBRA conditions reported in this manuscript. However, this scenario (AFEX and pelletization at depots followed by COBRA on AFEX-treated SCB) was not considered in this specific study and will be a topic of a separate manuscript.
Given the many logistics scenarios which will impact feedstock price at the gate of the refinery, this work focuses on comparing centralized lignocellulosic biorefining systems based on various pretreatment technologies applied to SCB processing in the State of São Paulo, Brazil. The geographical region of São Paulo offers high density and availability of SCB where feedstock logistics systems may be easier to implement. Also, considering a region with an unusually high concentration of feedstock does not introduce bias to benefit pretreatment technologies that require densified biomass. On the contrary, it minimizes the economic benefit that densified biomass could have on the overall delivered price of feedstock (ESI Table S2†). As we noted previously, however, transporting, storing, and feeding densified biomass to bioreactors is likely to be considerably more feasible than feeding bulky, loose biomass.
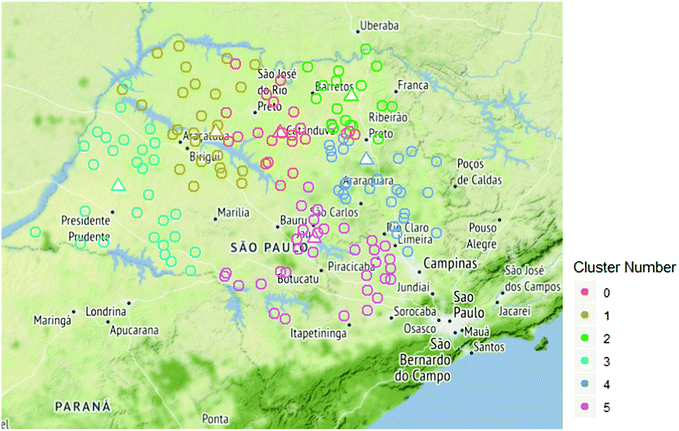
Fig. 5 shows the geolocation of all the active first-generation sugarcane ethanol plants (1G) in the State of São Paulo, Brazil, where a combined excess of 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 Mg per day of dry sugarcane bagasse is potentially available for second generation biofuels.58 Here, each 1G ethanol plant was considered as a Processing Depot (PD), where the sugarcane bagasse produced was dried, pelletized, and stored before being transported to a designated lignocellulosic mega-biorefinery. A conditional K-means algorithm was implemented to determine the number of mega-biorefineries, their optimal location, and the PDs that supply each mega-biorefinery with their SCB, with the objective of minimizing the average biomass transportation distance per Mg of biomass and maximizing the size of the mega-biorefineries within the following constraints: (1) the location of each mega-biorefinery was picked amongst the existing PDs in the State of São Paulo; (2) a maximum mega-biorefinery capacity of 20
679 Mg per day of dry sugarcane bagasse is potentially available for second generation biofuels.58 Here, each 1G ethanol plant was considered as a Processing Depot (PD), where the sugarcane bagasse produced was dried, pelletized, and stored before being transported to a designated lignocellulosic mega-biorefinery. A conditional K-means algorithm was implemented to determine the number of mega-biorefineries, their optimal location, and the PDs that supply each mega-biorefinery with their SCB, with the objective of minimizing the average biomass transportation distance per Mg of biomass and maximizing the size of the mega-biorefineries within the following constraints: (1) the location of each mega-biorefinery was picked amongst the existing PDs in the State of São Paulo; (2) a maximum mega-biorefinery capacity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mg of biomass per day was considered, since the average processing capacity for oil refineries is in that order of magnitude,59 roughly ten times the usually assumed biorefinery size60 and (3) all the available excess sugarcane bagasse could be used by the mega-biorefineries to produce ethanol, electricity and/or lignin.
000 Mg of biomass per day was considered, since the average processing capacity for oil refineries is in that order of magnitude,59 roughly ten times the usually assumed biorefinery size60 and (3) all the available excess sugarcane bagasse could be used by the mega-biorefineries to produce ethanol, electricity and/or lignin.
As depicted in Fig. 5, the clustering algorithm has calculated 6 mega-biorefineries (triangles) and their respective PDs (circles), which are distinguished by the different colors on the map. The capacity and average transportation radius per Mg of biomass for every cluster are described in ESI Table S2.† In summary, the results show that the biomass processing capacity of the 6 mega-biorefineries ranged from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 (cluster 2) to 20
240 (cluster 2) to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (cluster 0) dry Mg per day, and the average transportation radius per Mg of dry biomass ranged from 64.2 (cluster 2) to 121.8 (cluster 3) km Mg−1 SCB. The average delivered price of biomass transported as bales also varied significantly from the biomass transported as pellets. The delivered price of sugarcane bagasse bales varied from $61.5 to $71.3 per dry Mg SCB, whereas the delivered price of pellets varied from $77.6 to $82.1 per dry Mg SCB, corresponding to clusters 2 and 3, respectively.
000 (cluster 0) dry Mg per day, and the average transportation radius per Mg of dry biomass ranged from 64.2 (cluster 2) to 121.8 (cluster 3) km Mg−1 SCB. The average delivered price of biomass transported as bales also varied significantly from the biomass transported as pellets. The delivered price of sugarcane bagasse bales varied from $61.5 to $71.3 per dry Mg SCB, whereas the delivered price of pellets varied from $77.6 to $82.1 per dry Mg SCB, corresponding to clusters 2 and 3, respectively.
The minimum ethanol selling price (MESP) at the mega-biorefinery gate for the clusters shown in Fig. 5 was determined, considering biorefining processes based on different pretreatment technologies. In the present study, the biorefineries based on COBRA and COBRA-LE pretreatment technologies used biomass delivered as pellets, while the remaining biorefineries considered biomass delivered as bales.
Also, three scenarios were examined based on the experimental results and assumptions summarized in Fig. 3. Scenario 1 used the best experimental ethanol yields obtained in this work under the process conditions described in Fig. 3 (highest severity COBRA and COBRA-LE). Scenario 2 assumed that all fermentable sugars were consumed and converted to ethanol with 97.5% metabolic yield (the maximum we have observed). Scenario 3 assumed that all soluble sugars, including oligosaccharides, were consumed and converted to ethanol with 97.5% metabolic yield.
Fig. 6 shows the predicted average MESP for the various scenarios and pretreatment technologies in this study. For Scenario 1, which represents the combined potential of all the technologies used in this work, from pretreatment to fermentation, COBRA-LE and AFEX showed the lowest and very similar MESPs with $1.45 and $1.46 per gallon ethanol, respectively. It is remarkable that COBRA-LE-based biorefineries could still show a slightly lower average MESP than AFEX-based biorefineries, even though the delivered price of densified biomass was significantly higher than baled. This occurs because COBRA-LE achieves higher ethanol yields than any other pretreatment technology tested herein, while requiring 40% less enzyme loading and using the same ammonia loading as AFEX pretreatment.
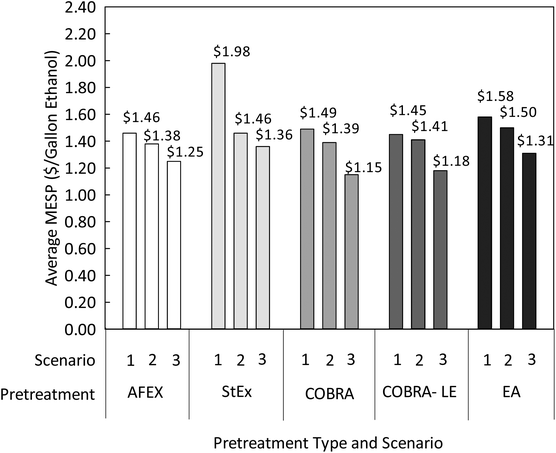 | ||
Fig. 6 Average MESP ($ per gallon ethanol) calculated for mega-biorefinery systems based on various pretreatment technologies, implemented in the State of Sao Paulo, Brazil. COBRA and COBRA-LE pretreatment conditions considered for this study were temperature of 100 °C, residence time of 3.5 h, NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM ratio of 1 BM ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and pressure of 60 bar. The remaining pretreatment conditions were as described in Fig. 3. The enzyme loadings assumed for the techno-economic analysis were 15 mg g−1 glucan for COBRA, COBRA-LE and EA pretreatments, and 25 mg g−1 glucan for AFEX and StEx pretreatments. The base case selling price of the extracted lignin from EA and COBRA-LE was assumed to be $75 per dry Mg of lignin, and all the other non-extracted lignins were converted to electricity in all cases. 1 and pressure of 60 bar. The remaining pretreatment conditions were as described in Fig. 3. The enzyme loadings assumed for the techno-economic analysis were 15 mg g−1 glucan for COBRA, COBRA-LE and EA pretreatments, and 25 mg g−1 glucan for AFEX and StEx pretreatments. The base case selling price of the extracted lignin from EA and COBRA-LE was assumed to be $75 per dry Mg of lignin, and all the other non-extracted lignins were converted to electricity in all cases. | ||
The lignin selling price in this base case for COBRA-LE and EA pretreatment-based biorefineries was similar to the price of densified biomass at $75 per dry Mg lignin, and consequently did not result in any additional value, nor loss, to the biorefinery. The average MESP calculated for StEX-based mega-biorefineries was significantly higher than for any other process studied herein ($1.98 per gallon ethanol), because the experimental ethanol yield obtained was just 16.2 g per 100 kg untreated SCB due to poor xylose yields and conversion by the recombinant S. cerevisiae 424A(LNH-ST) strain used in this work (Fig. 3). If we assume that all fermentable sugars were consumed during fermentation with a metabolic yield of 95.7%, the average MESP for StEx-based mega-biorefineries drops to $1.46 per gallon ethanol. This result shows the importance of improving fermentation strains that can tolerate the presence of inhibitory components in hydrolyzates derived from StEX-pretreated biomass. To a lesser extent, the MESP was also significantly reduced for the other pretreatment technologies in Scenario 2 relative to Scenario 1. In Scenario 2, AFEX pretreatment is predicted to have the lowest MESP at $1.38 per gallon ethanol, followed by COBRA pretreatment at $1.39 per gallon ethanol. Though the ethanol yield is significantly higher for COBRA and COBRA-LE at lower enzyme loadings relative to AFEX pretreatment under Scenario 2, that difference was not sufficient to offset the higher delivered feedstock price of biomass pellets relative to bales. However, MESP differences are not very significant between these three pretreatment technologies, while COBRA technologies still benefit from the better logistics platform offered using densified biomass and the 40% lower enzyme loading relative to AFEX pretreatment. The largest impact of COBRA and COBRA-LE pretreatment technologies on reducing the MESP is showcased in Scenario 3, in which all soluble carbohydrates (monomeric and oligomeric sugars) available after enzymatic hydrolysis are converted to ethanol with a metabolic yield of 97.5%. As shown in Fig. 6, the average MESP of COBRA-LE and COBRA-based biorefineries under Scenario 3 could decrease to $1.18 and $1.15 per gallon ethanol, respectively, whereas the remaining pretreatment technologies would not enable average MESPs below $1.25 per gallon ethanol. This occurs primarily because COBRA pretreatments enable over 90% carbohydrate conversion, including sugar oligomers, which are currently not used by the recombinant S. cerevisiae 424A (LNH-ST) strain.
However, this analysis shows a clear path for improving the economic viability of liquid biofuels, which also applies to other fermentation-based biochemicals. It is critical to understand the fundamental reasons why soluble oligosaccharides accumulate during hydrolysis of COBRA and COBRA-LE pretreated biomass. Enzyme technology could improve, notably better hemicellulase cocktails, to facilitate lignocellulosic biomass deconstruction to fermentable sugars. Alternatively, microorganisms could be developed to effectively address the oligosaccharide conversion problem, in addition to improved sugar consumption and biofuel metabolic yield.
The comparative TEA performed here demonstrates that EA pretreatment, although effectively converting carbohydrates to fermentable sugars, did not show economic advantages relative to COBRA pretreatment technologies, nor relative to AFEX. This was mainly because EA uses a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia-to-biomass ratio, and extracted of a small fraction of the carbohydrates into the lignin stream, thereby giving slightly lower soluble carbohydrate yields relative to COBRA.
1 ammonia-to-biomass ratio, and extracted of a small fraction of the carbohydrates into the lignin stream, thereby giving slightly lower soluble carbohydrate yields relative to COBRA.
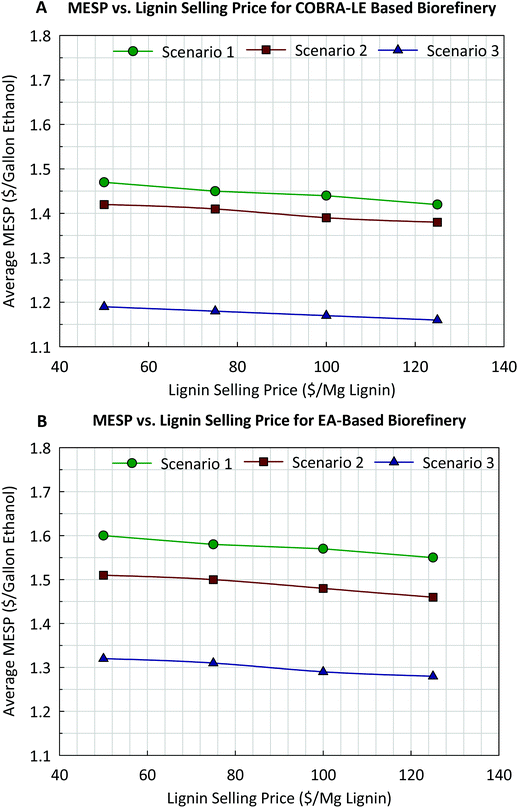
A sensitivity analysis was done to determine how the assumed extracted lignin price affects the average MESP for both types of biorefineries, as shown in Fig. 7. Here, the price of the extracted lignin varied from $50 to $125 per Mg lignin for the 3 scenarios discussed above. Based on Fig. 7, the average MESP is not very sensitive to lignin price, as only 25–30% of the lignin present in SCB was extracted. As such, the average MESP for EA pretreatment-based biorefineries in every scenario calculated in this study was never lower than that shown for AFEX-based biorefineries, even when the price of lignin was assumed to be $125 per Mg lignin.
2.5. Implementation of COBRA-based biorefineries worldwide
This work focused on studying the applicability of COBRA-based biorefineries in the state of Sao Paulo, Brazil. In that example, biomass distribution is highly dense and available, which is not the case in many regions of the world. For areas where biomass productivity is lower or areas where feedstocks are not as available, the biomass collection radius are much wider than for the case of Sao Paulo, Brazil. Therefore, the viability of biorefineries will largely depend on our ability to reduce biomass transportation costs. As such, biomass densification should be required. In Europe or in the United States, for example, corn and other grains are produced, handled, and transported long distances to supply market demands. The lignocellulosic residues derived from that industry, such as corn stover or wheat straw, if densified in local depots, can also be moved the same way grains are moved today, using similar type of infrastructure for handling, storage, and transportation to centralized biorefineries. To use this concept, biomass processing depots need to be implemented, as they do not exist in those regions. They could be located at existing grain elevators, as suggested by Kim et al. 2019.61 That development should go hand in hand with the introduction of lignocellulosic biorefineries that can process biomass pellets, such as COBRA-based biorefineries. A similar concept has been introduced in earlier publications, which proposed that AFEX pretreatment could be performed at the depot level, prior to pelletization and transportation to decentralized biorefineries.61,62 AFEX pretreatment facilitates biomass densification and produces highly digestible pellets that can be used as a feedstock for both biorefineries and animal feed.63 However, performing the pretreatment on densified untreated feedstocks at a centralized facility offers the possibility of energy integration with the rest of the biorefinery, at the pretreatment level, using renewable heat and electricity generated from lignin combustion. In a system where biomass pellets are produced at the depot level, exceptionally large biorefineries can become profitable in various regions of the world. In many cases that would not be the case without leveraging economies of scale, especially in regions of lower biomass availability. Such possibility would allow us to maximize the use of biomass residues to produce biofuels and biobased-chemicals, and improve the sustainability of energy, transportation and chemical industries.3. Materials and methods
3.1. Raw materials and chemicals
Sugarcane bagasse composed of 39.5 ± 0.4% glucan, 25.2 ± 0.1% xylan and 19.4 ± 0.1% lignin was collected from two industrial South African sugarcane sources located in Malelane (TSB Sugar, Mpumalanga) and Mount Edgecombe (SASRI, Kwazulu Natal). The bagasse was milled through a disk mill (Condux LV15 M, Netzch-Condux GmbH, Germany) equipped with a 20 mm screen. The size-reduced bagasse samples were sieved in a stacked-sieve system to remove mineral impurities (e.g., sand), bagasse pith and fines smaller than 600 μm × 600 μm. Prior to pelletization, the sugarcane bagasse was milled through a 40-mesh screen. Corn stover (Pioneer 36H56) composed of 33.7 ± 0.6% glucan, 25.4 ± 0.5% xylan and 14.4 ± 0.8% lignin was harvested from Michigan State University farms (Lansing, MI) in November 2014 and milled through a 40-mesh screen. Miscanthus x giganteus composed of 44.0 ± 0.1% glucan, 17.9 ± 0.4% xylan and 21.8 ± 0.6% lignin, produced at Michigan State University farms (Lansing, MI), was harvested in the Spring of 2014 and milled through a 40-mesh screen prior to further usage. Prairie cord grass composed of 42.1 ± 1.0% glucan, 25.1 ± 0.6% xylan and 18.1% ± 0.2% lignin was harvested in Brookings, SD in 2009 and milled through a 4 mm screen. Hybrid poplar (Populus nigra var. charkoviensis x caudina cv. NE-19) composed of 34.9 ± 0.2% glucan, 12.7 ± 0.1% xylan and 25.3 ± 1.2% lignin was harvested at the University of Wisconsin Arlington Agricultural Research Station in 2010 and milled through a 20-mesh screen prior to further usage. All the feedstocks were stored at 4 °C in Ziplock bags before usage.Anhydrous liquid ammonia cylinders equipped with a dip tube were procured from Airgas (Radnor, PA, USA) for ammonia pretreatment. Solvents, sugar standards, acids and bases were purchased from Sigma Aldrich (St Louis, MO, USA).
Cellic® CTec3 (batch number VDNI0002) and Cellic® HTec3 (batch number VIN00001) enzymes were kindly donated by Novozymes North America, Inc. (Franklinton, NC, USA) and Multifect Pectinase (batch number 4861295753) enzyme was kindly donated by DuPont Industrial Biosciences (Palo Alto, CA, USA). The protein concentration in enzyme solutions was determined using Kjeldahl nitrogen analysis method (AOAC Method 2001.11, Dairy One Cooperative Inc., Ithaca, NY, USA).1,2
3.2. Biomass densification
All untreated biomasses, including sugarcane bagasse, corn stover, poplar, Miscanthus and prairie cord grass, were pelletized using a Buskirk Engineering PM810 (Ossian, IN) flat die pellet mill. Firstly, both roller and dye were heated up to 70 °C by passing AFEX pretreated corn stover through the die. The untreated biomasses were mixed with water until they reached a moisture content of 25% (total weight basis). The moist biomass was stored in a closed container and placed at 4 °C overnight so that the moisture could be fully absorbed by the biomass. The moist biomass was allowed to reach room temperature before being pelletized. No external binder was added as pellet adhesive during densification. The pellets were collected into a plastic container and cooled down at room temperature. Next, they were annealed by subjecting the pellets to oven drying at 50 °C for 48 h and stored at room temperature in sealed plastic bags before usage. The bulk density of biomass pellets was measured filling a tared 500 mL volumetric cylinder with pellets until they reached the 500 mL mark and measuring the weight of the full cylinder to determine the mass of pellets in the volumetric cylinder. The bulk density was obtained by dividing the mass of the pellets by the 500 mL volume in triplicates. The average biomass density of SCB pellets was found to be 560 kg m−3, which is 5.6 times denser than compacted bagasse piles (100 kg m−3).3 Pellets were in average 6.5–7 mm diameter and variable lengths that could go up to approximately 30 mm (Fig. S6†).3.3. COBRA pretreatment of sugarcane bagasse
COBRA pretreatment of sugarcane bagasse was performed in 33 mL in-house designed reactors coupled to a control unit to monitor and to control temperature. The details of the reaction system are given elsewhere.4 The reactors were filled in with the desired amount of pelletized sugarcane bagasse (10% of moisture content (total weight basis)) along with ammonia charged into the reaction with a high-pressure syringe pump (Harvard Apparatus, model PHD 2000, Holliston, MA, USA). Once ammonia was loaded, the reactors were heated up to the desired temperature and maintained according to the reaction time. Both temperature and time were established by the experimental design. After reaching the desired reaction time, a slow (∼2 min) release of ammonia out of the system was performed. Next, the pretreated materials were transferred out of the reactor and left under the fume hood overnight to remove any residual ammonia. After drying, the moisture content of the pretreated sugarcane bagasse was determined using a moisture analyzer (A&D MX-50, A&D Engineering, Inc., San Jose, CA, USA).COBRA pretreatment for high-solid-loading enzymatic hydrolysis was carried out using an in-house built reactor of 700 mL with a similar design as the one of 33 mL. In the case of these reactors, a desired amount of sugarcane bagasse (dry weight basis) was added into the reactor and the ammonia was loaded gravimetrically by weighing the ammonia transferred from a pre-weighed vessel to the reactors. Immediately after filling the system with ammonia, the reactors were heated up and kept at the desired temperature for defined reaction time. All the subsequent steps were identical to those described for the small-scale reactors.
To assess the influence of lignin removal on enzymatic hydrolysis yields, COBRA pretreatment was performed with lignin extraction, hereinafter referred to as COBRA–LE. COBRA–LE was carried out at the same operational conditions as regular COBRA pretreatment. In COBRA–LE pretreatment, the bottom of the reactor was connected to a high-pressure lignin collection vessel, whilst the top of the reactor was connected to a nitrogen line. After reaching the required reaction time, the ammonia was drained along with the dissolved lignin from the reactor to the lignin collection vessel. The exhaust valve from the lignin collector was slowly opened to remove ammonia from the system. Right after, the nitrogen was introduced through the top of the reactor to keep the pressure in the system approximately at 21 bar. This procedure allowed the nitrogen flowing through the system, and helped to flow the liquid ammonia with the dissolved lignin down to the lignin collector, passing through a sintered filter installed in the bottom of the reactor.4 After lignin extraction, the nitrogen flow was cut off to allow the system releasing the pressure slowly. For mass balance purposes, the pretreated sugarcane bagasse was transferred from the reactor to a pre-weighted tray, which was placed under the fume hood for 48 h to remove any potential traces of ammonia. The pretreated sugarcane bagasse was weighted, and its respective moisture content was measured as described above.
3.4. Experimental design for COBRA pretreatment
A statistical design of experiments (DoE) was applied to assess the effect of temperature (X1), reaction time (X2) and ammonia-to-biomass ratio (NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM) (X3) on conversion of glucan and xylan enzymatic into their respective monomers at 72 h. To achieve this, a Box-Behnken DoE was employed using software (Minitab Inc., State College, PA, USA) with 30 experimental points, including replicates and four center point replicates with high and low values of temperature (100 °C and 50 °C), residence time (1 h and 6 h) and NH3
BM) (X3) on conversion of glucan and xylan enzymatic into their respective monomers at 72 h. To achieve this, a Box-Behnken DoE was employed using software (Minitab Inc., State College, PA, USA) with 30 experimental points, including replicates and four center point replicates with high and low values of temperature (100 °C and 50 °C), residence time (1 h and 6 h) and NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM (0.5
BM (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (g/g)), respectively. A quadratic response was carried out on the experimental data as a function of temperature, residence time and NH3
1 (g/g)), respectively. A quadratic response was carried out on the experimental data as a function of temperature, residence time and NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BM ratio (g/g) as independent variables. The interactions between all independent variables were considered in the response surface design. The parameters describing the effect of those variables were considered according to their statistical significance, i.e. p-value (p < 0.05) and model predictive ability (R2predicted). The regression equations describing the response surface design were used to predict the responses of the various effects within the range of experimental domains.
BM ratio (g/g) as independent variables. The interactions between all independent variables were considered in the response surface design. The parameters describing the effect of those variables were considered according to their statistical significance, i.e. p-value (p < 0.05) and model predictive ability (R2predicted). The regression equations describing the response surface design were used to predict the responses of the various effects within the range of experimental domains.
3.5. Low-solid-loading enzymatic hydrolysis
Low-solid-loading enzymatic hydrolysis was performed aiming to evaluate the effect of COBRA operational conditions on both glucan and xylan conversion into their respective monomers. For this purpose, enzymatic hydrolysis was performed in 20 mL screw-cap scintillation vials with 1% glucan loading (w/w, glucan) and 15 mg of protein per g of glucan in 50 mM citrate buffer (pH 4.8), with 15 mL of reaction volume, and incubated at 50 °C in an orbital shaking incubator (New Brunswick, USA) at 250 rpm for 72 h. Sodium azide (0.02% w/v) was added as antibiotic to prevent any microbial contamination during the enzymatic reaction. The enzymes used herein were Cellic® CTec3, HTec3 and Multifect Pectinase. The enzyme ratios (dry weight basis) were 68 wt%, 22 wt% and 10 wt% for CTec3, HTec3 and Multifect Pectinase, respectively. These ratios were previously optimized to maximize total sugar conversion on AFEX-pretreated sugarcane bagasse as described elsewhere.5 After 72 h of enzymatic hydrolysis, the hydrolysates were filtered through a 0.2 μm filter, and soluble sugars, mainly glucose and xylose, were determined using an HPLC equipped with a Bio-Rad Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) as previously reported.63.6. High-solid-loading enzymatic hydrolysis
To evaluating the pretreatment potential under industrially relevant conditions, the enzymatic hydrolysis experiments of COBRA, COBRA–LE and EA pretreated sugarcane bagasse were performed at high solid loading (6% glucan loading, w/w). The experiments were carried out in duplicate in 250 mL Erlenmeyer flasks with 100 mL reaction volume, in 50 mM sodium buffer (pH 4.6), and incubated at 50 °C in an orbital shaking incubator (New Brunswick, USA) at 250 rpm for 96 h. Chloramphenicol (50 μg mL−1) was added to prevent any microbial contamination during the enzymatic and fermentative reaction as well. Previously optimized cocktail of CTec3, HTec3 and Multifect Pectinase was used on a dry protein weight basis for COBRA-pretreated sugarcane bagasse at various enzyme loadings of 15, 10 and 7.5 mg protein per g glucan. In the first 6 h of enzymatic hydrolysis, the pH was monitored and if needed adjusted to 4.8 using 1 M HCl at every 2 h. The blank reactions for both substrate and enzyme complexes were carried out at the same experimental conditions. At the desired enzymatic hydrolysis time (24, 48, 72 and 96 h), 0.5 mL of sample was taken, incubated at 95 °C for 10 min (Eppendorf, Westbury, USA) to denature the enzymes, centrifuged for 4 min at 3500 rpm. The supernatant was sampled, diluted (10-fold), filtered through a 0.2 μm filter and analyzed for monomeric sugars as described elsewhere.6 After 96 h of enzymatic hydrolysis, the slurry was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min to separate the remaining solids from the hydrolysate. The solid streams were washed with 100 mL of water, centrifuged at 10
000g for 30 min to separate the remaining solids from the hydrolysate. The solid streams were washed with 100 mL of water, centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min and the washing water was analyzed in terms of sugar content for mass balance closure purposes. The washed solids were then dried in a freeze-dryer for 72 h before being subjected to compositional analysis. A sample of the hydrolysate was taken, processed, and analyzed for monomeric and oligomeric sugar content. Due to the presence of soluble oligosaccharides in hydrolysates, an acid hydrolysis procedure for estimating the oligomeric sugar content was performed as recommended by NREL/TP-510-42623.7 The oligosaccharide content was determined from the increase in concentration of the monomeric sugars after acid hydrolysis. In preparation for fermentation, the pH of the hydrolysates was adjusted to 5.5 using 10 M KOH, sterilized using a 0.22 μm filter and stored at 4 °C.
000g for 30 min and the washing water was analyzed in terms of sugar content for mass balance closure purposes. The washed solids were then dried in a freeze-dryer for 72 h before being subjected to compositional analysis. A sample of the hydrolysate was taken, processed, and analyzed for monomeric and oligomeric sugar content. Due to the presence of soluble oligosaccharides in hydrolysates, an acid hydrolysis procedure for estimating the oligomeric sugar content was performed as recommended by NREL/TP-510-42623.7 The oligosaccharide content was determined from the increase in concentration of the monomeric sugars after acid hydrolysis. In preparation for fermentation, the pH of the hydrolysates was adjusted to 5.5 using 10 M KOH, sterilized using a 0.22 μm filter and stored at 4 °C.
3.7. Fermentation
The genetically modified xylose-fermenting strain Saccharomyces cerevisiae 424A (LNH-ST) used in the fermentation experiments was kindly provided by Prof. Nancy W.Y. Ho, Purdue University. The seed culture of this strain was prepared in 250 mL Erlenmeyer flasks containing 100 mL YPDX (75 g L−1 glucose, 25 g L−1 xylose, 10 g L−1 yeast extract, 20 g L−1 tryptone) seed culture medium. A frozen glycerol stock stored at −80 °C was used for seed culture inoculation at an initial optical density of 0.1. The seed culture was incubated at 30 °C and 150 rpm under micro-aerobic conditions for 18 h. The seed culture reached at optical density (OD600) of about 12 within 18 h. This seed culture was harvested and used as inoculum for fermentations of the various hydrolysates. The fermentations experiments were initiated with an initial OD600 of 2 (or initial yeast density of 0.96 g L−1). Samples were taken at various time points during the fermentation and cell-free supernatants were submitted for HPLC analysis. The total ethanol yield was determined based on the sugar yield during enzymatic hydrolysis, the sugar consumption and metabolic yield during fermentation.3.8. Chemical analysis
High-solid loading un-hydrolyzed sugarcane bagasse solids were milled in a knife mill to a particle size of 0.5 mm and characterized for their carbohydrate and lignin content according to the NREL/TP-510-42618.1 The composition of carbohydrates was determined using Shimadzu HPLC system equipped with an Aminex HPX-87-H (Bio-Rad, Hercules, CA, USA) column at 50 °C that was eluted with 5 mM H2SO4 at a flow rate of 0.6 mL min−1. The same HPLC analysis conditions were used for the chemical analysis of water-soluble fraction after enzymatic hydrolysis and fermentation. Shimadzu refractive index detector (RID) was used to identify and to quantify glucose, xylose, arabinose, lactic acid and ethanol by means of external calibration. The acid insoluble lignin obtained after acid hydrolysis was quantified gravimetrically and then corrected for the acid insoluble ash that was determined by igniting the content at 550 °C for 5 h. The acid soluble lignin was determined by ultraviolet spectrophotometry of biomass acid hydrolysates at 320 nm and using the absorptivity of 30 L (g cm)−1 as recommended in the literature. Nitrogen analysis of the biomass was performed using the Kjeldahl nitrogen analysis method.3.9. X-ray powder diffraction (XRD)
XRD experiments were carried out on an X-ray powder diffractometer with its beam parallelized by a Gobel mirror (D8 Advance with Lynxeye detector; Bruker, Bruker AXS Inc., Madison, WI, USA). CuKα radiation (wavelength = 1.5418 Å) was generated at 40 kV and 40 mA. The detector slit was set to 2.000 mm. Sample was analyzed using a coupled 2θ/θ scan type with a continuous PSD fast scan mode; 2θ started at 8.000° and ended at 30.0277° with increments of 0.02151°, 136 while θ started at 4.0000° and ended at 15.0138° with increments of 0.01075°. Step time was 1.000 s (i.e., 1025 total steps, effective total time 1157 s per run). Cellulose samples (approximately 0.5 g) were placed in a specimen holder ring made of PMMA with 25 mm diameter and 8.5 mm height, rotating at 5 degrees per minute during analysis.3.10. São Paulo state (Brazil) sugarcane biorefinery clustering optimization
The sugarcane processing information of 159 first-generation (1G) sugarcane bioethanol refineries operating in the state of São Paulo, Brazil, in 2013–2014 season was collected from prior published work.8 About 30 wt% the processed sugarcane in each 1G bioethanol refinery is transformed into wet sugarcane bagasse (SCB), with about 48% moisture.8 Thus, on average, this work considered 15.6 Mg of dry SCB generation per 100 Mg of sugarcane processed. However, not all the SCB can be used for second-generation (2G) biofuels production, as part of that material is used to produce heat and power in current 1G bioethanol plants. It was considered in this study that an average of 74 wt% of the produced SCB is available for 2G biorefining operations,8 which means that 11.5 wt% of the wet sugarcane processed was considered available as dry SCB for 2G biorefining in this study. The geographic coordinates (longitude, latitude) for each sugarcane bioethanol refinery location were determined by Google Maps (https://maps.google.com). Also, the Google Maps application programming interface (API) was used to determine the minimum road distance (in km) between every sugarcane ethanol plant in São Paulo state (https://developers.google.com/maps). In this work, the concept of decentralized biomass pre-processing in the 1G bioethanol plants, also called biomass processing depots (BPDs), prior to being transported to a lignocellulosic mega-biorefinery was evaluated. In BPDs, the available biomass should undergo drying, milling, pelletization or baling (depending on the bioeconomy scenario), and long-term storage. In this study, the biomass was assumed to be transported by truck in the form of untreated pellets for biorefineries based on COBRA and COBRA-LE pretreatment technologies, and in the form of bales for the remaining types of biorefinery scrutinized herein.A conditional K-means clustering algorithm was coded in Python,9 to determine where the lignocellulosic biorefineries (mega-biorefineries) should be located and the group of BPDs that should supply each of those mega-biorefineries with SCB feedstock. The objective of the clustering algorithm was to minimize the average SCB transportation distance per dry ton of biomass from the BPDs to the mega-biorefineries included in the entire system and maximize their individual processing capacity up to a limit of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mg of dry SCB per day. As a set condition in the algorithm, all the available SCB was forced to be included for processing in a mega-biorefinery, and each BPD was set to deliver their entire SCB production to a single mega-biorefinery. The average transportation distance per ton of dry SCB was determined for each of the optimal clusters composed by a mega-biorefinery and respective biomass supplying depots. The mapping of the optimal clusters was performed using the ggmap package in R (https://www.r-project.org) and the georeferenced data labeled with the optimal cluster number assigned by the K-means algorithm.
000 Mg of dry SCB per day. As a set condition in the algorithm, all the available SCB was forced to be included for processing in a mega-biorefinery, and each BPD was set to deliver their entire SCB production to a single mega-biorefinery. The average transportation distance per ton of dry SCB was determined for each of the optimal clusters composed by a mega-biorefinery and respective biomass supplying depots. The mapping of the optimal clusters was performed using the ggmap package in R (https://www.r-project.org) and the georeferenced data labeled with the optimal cluster number assigned by the K-means algorithm.
3.11. Technoeconomic analysis (TEA)
The cost of biomass pre-processing at the BPD level (baling and pelletizing) was assumed to be $12.4 per Mg for square bales and $30.2 per Mg for pellets on a dry biomass basis,10 and was modeled as an added cost to the feedstock. The price of the dried SCB was considered as $42.5 per Mg.11 Also, the truck load was assumed to be paid at $77 per h, assuming an average transportation speed of 50 km h−1. The average amount of SCB transported by truck was assumed as 18 Mg per load for bales and 40 Mg per load for pellets. Based on these assumptions above and the calculated average transportation distance (round trip) per Mg of SCB (Table S2†), the average delivered SCB price was estimated for each of the mega-biorefinery clusters determined by the K-means algorithm, both in the form of bales (for AFEX, EA and StEx) and pellets (for COBRA and COBRA-LE).The minimum ethanol selling price (MESP) at the gate of each of the mega-biorefineries was evaluated considering a 10% internal rate of return (IRR), using a modified version of the Excel-based model developed for prior work for techno-economic modeling of AFEX pretreatment12 and was, in turn, based on the 2012 NREL technoeconomic model.13 AFEX, COBRA, COBRA-LE, StEx and EA pretreatments were simulated.
The biorefinery models assumed the implementation of 6 centralized mega-biorefineries with delivered feedstock prices as described in Table S2.† The installed cost of the equipment used in the pretreatment area was calculated through equipment sizing and equations reported in the literature.14 The installed capital costs used in the model for feedstock handling (area 100) 2000 Mg per day biorefinery were estimated as $24.2 M for AFEX, StEX, and EA and only $4.5 M for COBRA and COBRA-LE based on a biorefinery handling pellets rather than loose biomass.12 The total installed capital costs assumed for pretreatment (area 200) for a 2000 Mg per day biorefinery in the model were estimated as $19.5 M for AFEX, $20.6 M for EA, $6.7 M for StEx, $17.1 M for COBRA, and $17.1 M for COBRA-LE. The remaining process areas in the NREL model (ethanol recovery, wastewater treatment, storage, boiler and utilities) were sized using the six tenths rule and the mass balances obtained from the Excel model. The installed costs of the equipment required for enzyme production were eliminated and it was assumed that the enzymes were purchased from a commercial source. The heat and power required to support the production of ethanol were produced through the combustion of unhydrolyzed solids. The excess electricity produced was assumed to be sold to the grid. The following chemical, biomass, and enzyme costs, and electricity selling price were used in the model calculations (in 2012 dollars): NH3 at $530 per Mg, H2SO4 at $87 per Mg, lime at $107 per Mg, biomass as described in Table S2,† cellulase at $3600 per Mg, hemicellulose at $4500 per Mg, and electricity at $0.0572 per kW h. All monetary values reported in this work are in 2012 US dollars. Ammonia recovery for COBRA, COBRA-LE, EA and AFEX was assumed to be 98% based on nitrogen balances between the untreated and pretreated biomass.
The technoeconomic analysis for the various pretreatment technologies scrutinized in this work were performed using three different scenarios. In Scenario 1, the enzymatic hydrolysis and fermentation performances assumed were those obtained experimentally by the optimal conditions found for each of the pretreatments evaluated. In Scenario 2, the fermentable sugar yields obtained experimentally were considered, however, it was assumed that all sugars were consumed and converted to ethanol with metabolic yield of 97.5%. Finally, Scenario 3 assumed that all soluble sugars generated experimentally during enzymatic hydrolysis, including glucose, xylose and respective oligomers, were converted to ethanol with 97.5% metabolic yield during fermentation.
4. Conclusions
This study considered the new COBRA pretreatment in a more holistic perspective, accounting for feedstock logistics aspects of the technology. COBRA and COBRA-LE pretreatments convert the native CI present in plant cell walls to the more digestible CIII allomorph. COBRA-LE selectively extracted up to 26% of the lignin present in SCB, which might be further processed to aromatic monomer precursors of a range of valuable biobased chemicals.64 COBRA and COBRA-LE pretreatments enabled nearly 80% conversion of the carbohydrates present in SCB to monomeric sugars using 15 mg enzyme per g glucan during 6% glucan loading enzymatic hydrolysis. Also, both pretreatment technologies solubilized >95% of the glucan and xylan-derived carbohydrates present in SCB, both as monomers and oligomers, highlighting the need to develop new enzyme technologies that fully convert the oligomeric carbohydrates, which represent over 15% of the total potential sugar yield. Considering SCB processing in the state of Sao Paulo, Brazil, converting the oligosaccharides into ethanol with metabolic yield of 97.5% reduces the MESP from $1.45 to $1.18 per gallon ethanol for the COBRA-LE-based biorefinery, and from $1.49 to $1.15 per gallon for the COBRA-based biorefinery. However, the base case scenario shows that COBRA-LE and AFEX processes are the most economical for SCB processing with MESPs of $1.45 and $1.46, respectively. Feedstock logistics are much simpler with densified biomass. The technical feasibility of mega-biorefineries using loose feedstocks, like it was assumed for the case of AFEX, StEx and EA, would be very challenging. To address those challenges AFEX pretreatment has been designed for smaller scale operations in a decentralized depot context, which will certainly be the most feasible strategy for its implementation in conjunction with mega-biorefineries for converting AFEX pretreated pellets into biofuel.62COBRA pretreatment has proven to be flexible in terms of the feedstocks that it can handle effectively, from hardwoods to herbaceous monocots and dicots. It is possible that, in the future, it can be effective in softwoods provided that appropriate hemicellulase cocktails are developed to effectively hydrolyze galactoglucomannans. This feature is likely important for implementing mega-biorefineries around the globe. Therefore, COBRA-based pretreatments seem to have most of the traits that one should find in the ideal pretreatment, (1) the ability to treat a variety of densified feedstocks under relatively mild conditions, (2) carbohydrate conversions greater than 95% during high solid loading enzymatic hydrolysis, (3) highly fermentable hydrolysates and (4) a lignin stream with most of the native lignin functionalities for further processing to yield aromatic precursors. Further work should target the hydrolysis of incompletely hydrolyzed oligosaccharides and carbohydrate hydrolase cocktails dedicated to target softwood hemicellulose more effectively.
Conflicts of interest
The authors Leonardo da Costa Sousa and Venkatesh Balan are authors of a patent describing the COBRA pretreatment process studied in this manuscript. All other authors do not have conflicts of interest to declare.Acknowledgements
This project was supported by Michigan Translational Research and Commercialization (MTRAC) Innovation Hub for AgBio, co-funded by the Michigan Economic Development Corporation (MEDC) through the Michigan Strategic Fund. Ana Rita C. Morais is grateful to Fundação para a Ciência e Tecnologia (FCT, Portugal) for her PhD grant (SFRH/BD/94297/2013) and Associated Laboratory for Sustainable Chemistry – Clean Processes and Technologies – LAQV which is financed by national funds from FCT/MEC (UID/QUI/50006/2013) and co-financed by the ERDF under the PT2020 Partnership Agreement (POCI-01-0145-FEDER - 007265). Jian Zhang would like to thank Natural Science Foundation of China (22178105) for supporting this research. The authors would like to thank Professor Antonio Maria Bonomi and his team for providing the data related to sugarcane processing in the refineries of the state of Sao Paulo, Brazil. The authors also thank Professor Johann F Görgens from Stellenbosch University for kindly providing SCB for this study and Novozymes for generously offering Cellic CTec3 and HTec3 enzymes. Professor Bruce E. Dale thanks the DOE Great Lakes Bioenergy Research Center (https://www.greatlakesbioenergy.org), funded by the U. S. Department of Energy through Cooperative Agreement DE-FC02-07ER64494, as well as Michigan State University AgBioResearch and the National Institute for Food and Agriculture of the U. S. Department of Agriculture. Dr Balan would like to thank University Houston and the state of Texas for his startup funds. Dr Lukasik would like to thank to FCT, Portugal for a grant IF/00471/2015.References
- L. da Costa Sousa, S. P. Chundawat, V. Balan and B. E. Dale, Curr. Opin. Biotechnol., 2009, 20, 339–347 CrossRef PubMed.
- T. Renders, G. Van den Bossche, T. Vangeel, K. Van Aelst and B. Sels, Curr. Opin. Biotechnol., 2019, 56, 193–201 CrossRef CAS PubMed.
- L. da Costa Sousa, M. Jin, S. P. Chundawat, V. Bokade, X. Tang, A. Azarpira, F. Lu, U. Avci, J. Humpula and N. Uppugundla, Energy Environ. Sci., 2016, 9, 1215–1223 RSC.
- K. H. Kim, T. Dutta, J. Sun, B. Simmons and S. Singh, Green Chem., 2018, 20, 809–815 RSC.
- L. da Costa Sousa, M. Foston, V. Bokade, A. Azarpira, F. Lu, A. J. Ragauskas, J. Ralph, B. Dale and V. Balan, Green Chem., 2016, 18, 4205–4215 RSC.
- X. Zhu, C. Peng, H. Chen, Q. Chen, Z. K. Zhao, Q. Zheng and H. Xie, ChemistrySelect, 2018, 3, 7945–7962 CrossRef CAS.
- D. S. Argyropoulos and H. I. Bolker, J. Wood Chem. Technol., 1987, 7, 1–23 CrossRef CAS.
- T. Yokoyama, J. Wood Chem. Technol., 2015, 35, 27–42 CrossRef.
- P. J. Deuss, M. Scott, F. Tran, N. J. Westwood, J. G. de Vries and K. Barta, J. Am. Chem. Soc., 2015, 137, 7456–7467 CrossRef CAS PubMed.
- Y. Li, L. Shuai, H. Kim, A. H. Motagamwala, J. K. Mobley, F. Yue, Y. Tobimatsu, D. Havkin-Frenkel, F. Chen and R. A. Dixon, Sci. Adv., 2018, 4, eaau2968 CrossRef PubMed.
- J. V. Vermaas, L. Petridis, X. Qi, R. Schulz, B. Lindner and J. C. Smith, Biotechnol. Biofuels, 2015, 8, 1–16 CrossRef PubMed.
- J. Rahikainen, S. Mikander, K. Marjamaa, T. Tamminen, A. Lappas, L. Viikari and K. Kruus, Biotechnol. Bioeng., 2011, 108, 2823–2834 CrossRef CAS PubMed.
- L. Qin, W.-C. Li, L. Liu, J.-Q. Zhu, X. Li, B.-Z. Li and Y.-J. Yuan, Biotechnol. Biofuels, 2016, 9, 1–10 CrossRef PubMed.
- D. Klein-Marcuschamer, P. Oleskowicz-Popiel, B. A. Simmons and H. W. Blanch, Biotechnol. Bioeng., 2012, 109, 1083–1087 CrossRef CAS PubMed.
- K. Igarashi, M. Wada and M. Samejima, FEBS J., 2007, 274, 1785–1792 CrossRef CAS PubMed.
- A. Mittal, R. Katahira, M. E. Himmel and D. K. Johnson, Biotechnol. Biofuels, 2011, 4, 1–16 CrossRef PubMed.
- L. d. C. Sousa, J. Humpula, V. Balan, B. E. Dale and S. P. Chundawat, ACS Sustainable Chem. Eng., 2019, 7, 14411–14424 CrossRef CAS.
- S. P. Chundawat, G. Bellesia, N. Uppugundla, L. da Costa Sousa, D. Gao, A. M. Cheh, U. P. Agarwal, C. M. Bianchetti, G. N. Phillips Jr. and P. Langan, J. Am. Chem. Soc., 2011, 133, 11163–11174 CrossRef CAS PubMed.
- S. P. Chundawat, L. da Costa Sousa, S. Roy, Z. Yang, S. Gupta, R. Pal, C. Zhao, S.-H. Liu, L. Petridis and H. O'Neill, Green Chem., 2020, 22, 204–218 RSC.
- M. Chen, F. Malaret, A. E. Firth, P. Verdía, A. R. Abouelela, Y. Chen and J. P. Hallett, Green Chem., 2020, 22, 5161–5178 RSC.
- J. R. Bernardo, F. M. Gírio and R. M. Łukasik, Molecules, 2019, 24(4), 808 CrossRef PubMed.
- F. Xu, J. Sun, N. M. Konda, J. Shi, T. Dutta, C. D. Scown, B. A. Simmons and S. Singh, Energy Environ. Sci., 2016, 9, 1042–1049 RSC.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- A. Das, A. Rahimi, A. Ulbrich, M. Alherech, A. H. Motagamwala, A. Bhalla, L. da Costa Sousa, V. Balan, J. A. Dumesic and E. L. Hegg, ACS Sustainable Chem. Eng., 2018, 6, 3367–3374 CrossRef CAS.
- V. Balan, L. D. Sousa, S. P. S. Chundawat, D. Marshall, L. N. Sharma, C. K. Chambliss and B. E. Dale, Biotechnol. Prog., 2009, 25, 365–375 CrossRef CAS PubMed.
- R. J. Garlock, Y. S. Wong, V. Balan and B. E. Dale, BioEnergy Res., 2012, 5, 306–318 CrossRef CAS.
- T. H. Kim and Y. Y. Lee, Bioresour. Technol., 2005, 96, 2007–2013 CrossRef CAS PubMed.
- S. P. Chundawat, R. K. Pal, C. Zhao, T. Campbell, F. Teymouri, J. Videto, C. Nielson, B. Wieferich, L. Sousa, B. E. Dale and V. Balan, JoVE J. Visualized Exp., 2020,(158), e57488 CAS.
- https://www.eia.gov/dnav/pet/pet_pnp_cap1_dcu_nus_a.htm (accessed on 03/19/2021).
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof and M. Worley, Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover, National Renewable Energy Lab.(NREL), Golden, CO (United States), 2011 Search PubMed.
- R. E. Davis, N. J. Grundl, L. Tao, M. J. Biddy, E. C. Tan, G. T. Beckham, D. Humbird, D. N. Thompson and M. S. Roni, Process design and economics for the conversion of Lignocellulosic biomass to hydrocarbon fuels and coproducts: 2018 biochemical design case update; Biochemical deconstruction and conversion of biomass to fuels and products via integrated biorefinery pathways, National Renewable Energy Lab.(NREL), Golden, CO (United States), 2018 Search PubMed.
- P. Lamers, M. S. Roni, J. S. Tumuluru, J. J. Jacobson, K. G. Cafferty, J. K. Hansen, K. Kenney, F. Teymouri and B. Bals, Bioresour. Technol., 2015, 194, 205–213 CrossRef CAS PubMed.
- B. Sharma, R. Clark, M. R. Hilliard and E. G. Webb, Front. Energy Res., 2018, 6, 100 CrossRef.
- S. Kim and B. E. Dale, Biofuels, Bioprod. Biorefin., 2015, 9, 422–434 CrossRef CAS.
- S. P. Chundawat, B. S. Donohoe, L. da Costa Sousa, T. Elder, U. P. Agarwal, F. Lu, J. Ralph, M. E. Himmel, V. Balan and B. E. Dale, Energy Environ. Sci., 2011, 4, 973–984 RSC.
- S. P. Chundawat, R. Vismeh, L. N. Sharma, J. F. Humpula, L. da Costa Sousa, C. K. Chambliss, A. D. Jones, V. Balan and B. E. Dale, Bioresour. Technol., 2010, 101, 8429–8438 CrossRef CAS PubMed.
- D. Tarasov, M. Leitch and P. Fatehi, Biotechnol. Biofuels, 2018, 11, 1–28 CrossRef PubMed.
- X. Li, M. Li, Y. Pu, A. J. Ragauskas, A. S. Klett, M. Thies and Y. Zheng, Renewable Energy, 2018, 123, 664–674 CrossRef CAS.
- C. Krishnan, L. d. C. Sousa, M. Jin, L. Chang, B. E. Dale and V. Balan, Biotechnol. Bioeng., 2010, 107, 441–450 CrossRef CAS PubMed.
- T. Mokomele, L. Costa Sousa, V. Balan, E. van Rensburg, B. E. Dale and J. Görgens, Biotechnol. Biofuels, 2018, 1, 127 CrossRef PubMed.
- N. Uppugundla, L. da Costa Sousa, S. P. Chundawat, X. Yu, B. Simmons, S. Singh, X. Gao, R. Kumar, C. E. Wyman and B. E. Dale, Biotechnol. Biofuels, 2014, 7, 1–14 CrossRef PubMed.
- B. Bals, C. Rogers, M. Jin, V. Balan and B. Dale, Biotechnol. Biofuels, 2010, 3(1), 1–11 CrossRef PubMed.
- V. Sundaram and K. Muthukumarappan, Ind. Crops Prod., 2016, 83, 537–544 CrossRef CAS.
- V. Balan, B. Bals, L. da Costa Sousa, R. Garlock and B. DaleChapter 5: A short review on ammonia-based lignocellulosic biomass pretreatment. Chemical and Biochemical Catalysis for Next Generation Biofuels. ed. B. A. Simmons, Royal Society of Chemistry, 2011, pp. 89–114 Search PubMed.
- X. Tang, L. da Costa Sousa, M. Jin, S. P. Chundawat, C. K. Chambliss, M. W. Lau, Z. Xiao, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2015, 8, 1–17 CrossRef PubMed.
- M. N. Borand, A. Isler Kaya and F. Karaosmanoglu, Energies, 2020, 13, 4552 CrossRef CAS.
- Z.-H. Liu, L. Qin, F. Pang, M.-J. Jin, B.-Z. Li, Y. Kang, B. E. Dale and Y.-J. Yuan, Ind. Crops Prod., 2013, 44, 176–184 CrossRef CAS.
- X. Tang, L. da Costa Sousa, M. Jin, S. P. Chundawat, C. K. Chambliss, M. W. Lau, Z. Xiao, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2015, 8, 1–17 CrossRef PubMed.
- N. Uppugundla, L. D. Sousa, S. P. S. Chundawat, X. R. Yu, B. Simmons, S. Singh, X. D. Gao, R. Kumar, C. E. Wyman, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2014, 7(1), 1–14 CrossRef PubMed.
- R. H. Narron, H. Kim, H.-m. Chang, H. Jameel and S. Park, Curr. Opin. Biotechnol., 2016, 38, 39–46 CrossRef CAS PubMed.
- C. L. Li, L. Sun, B. A. Simmons and S. Singh, BioEnergy Res., 2013, 6, 14–23 CrossRef CAS.
- J. Shi, V. S. Thompson, N. A. Yancey, V. Stavila, B. A. Simmons and S. Singh, Biofuels, 2013, 4, 63–72 CrossRef CAS.
- N. Sun, M. Rahman, Y. Qin, M. L. Maxim, H. Rodriguez and R. D. Rogers, Green Chem., 2009, 11, 646–655 RSC.
- C. Gunawan, S. Xue, S. Pattathil, L. D. Sousa, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2017, 10(1), 1–14 CrossRef PubMed.
- S. S. Xue, N. Uppugundla, M. J. Bowman, D. Cavalier, L. D. C. Sousa, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2015, 8(1), 1–14 CrossRef PubMed.
- P. L. Eranki, B. D. Bals and B. E. Dale, Biofuels, Bioprod. Biorefin., 2011, 5, 621–630 CrossRef CAS.
- B. D. Bals and B. E. Dale, Bioresour. Technol., 2012, 106, 161–169 CrossRef CAS PubMed.
- D. Nunes, Jr., Indicadores de Desempenho da Agroindústria Canavieira - Safras 2012/13 e 2013/14, 2014.
- U. S. E. I. Agency, https://www.eia.gov/energyexplained/oil-and-petroleum-products/refining-crude-oil-refinery-rankings.php (Accessed on 03/17/2021).
- S. Kim and B. E. Dale, Biofuels, Bioprod. Biorefin., 2016, 10, 819–832 CrossRef CAS.
- S. Kim, B. E. Dale, M. Jin, K. D. Thelen, X. Zhang, P. Meier, A. D. Reddy, C. D. Jones, R. Cesar Izaurralde and V. Balan, GCB Bioenergy, 2019, 11, 871–882 CrossRef CAS.
- T. J. Campbell, F. Teymouri, B. Bals, J. Glassbrook, C. D. Nielson and J. Videto, Biofuels, 2013, 4, 23–34 CrossRef CAS.
- P. Mor, B. Bals, A. K. Tyagi, F. Teymouri, N. Tyagi, S. Kumar, V. Bringi and M. VandeHaar, J. Dairy Sci., 2018, 101, 7990–8003 CrossRef CAS PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344(6185), 1246843 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc00488g |
| ‡ ™AFEX is a trademark of MBI International. |
| This journal is © The Royal Society of Chemistry 2022 |