Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deep eutectic solvents for the preservation of concentrated proteins: the case of lysozyme in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) glycerol†
glycerol†
Adrian
Sanchez-Fernandez
 *ab,
Sylvain
Prevost
*ab,
Sylvain
Prevost
 c and
Marie
Wahlgren
c and
Marie
Wahlgren
 b
b
aCentro Singular de Investigación en Química Biolóxica e Materiais Moleculares, Universidade de Santiago de Compostela, Rúa de Jenaro de la Fuente, s/n, 15705 Santiago de Compostela, Spain. E-mail: adriansanchez.fernandez@usc.es
bFood technology, Engineering and Nutrition, Lund University, Box 117, SE-221 00 Lund, Sweden. E-mail: adrian.sanchez-fernandez@food.lth.se
cInstitut Laue-Langevin, DS/LSS, 71 avenue des Martyrs, 38000, Grenoble, France
First published on 6th May 2022
Abstract
The stability and activity of a model protein, lysozyme, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol was characterised across a wide range of protein concentrations, i.e., between 4 and 143 mg ml−1, where the protein is soluble and physically stable. Lysozyme folds into a globular conformation in this deep eutectic solvent like that in the native state, but with subtle variations in its internal structure. The protein remained stable even after 40 days of storage at room temperature and retrieved its native conformation and activity upon rehydration. These results show the potential of deep eutectic solvents as sustainable, non-toxic, and synthetically accessible media for the storage and preservation of proteins in a concentrated regime without the requirement of other excipients.
glycerol was characterised across a wide range of protein concentrations, i.e., between 4 and 143 mg ml−1, where the protein is soluble and physically stable. Lysozyme folds into a globular conformation in this deep eutectic solvent like that in the native state, but with subtle variations in its internal structure. The protein remained stable even after 40 days of storage at room temperature and retrieved its native conformation and activity upon rehydration. These results show the potential of deep eutectic solvents as sustainable, non-toxic, and synthetically accessible media for the storage and preservation of proteins in a concentrated regime without the requirement of other excipients.
The protection of vulnerable biomacromolecules (e.g., proteins) poses a major challenge for the current logistic and storage technologies.1 This becomes especially challenging when requiring high protein concentrations as these are prone to degrade faster than dilute solutions.2 The goal is to protect those against the different chemical and physical degradation routes, and various methods have been developed to increase the stability of labile biomolecules. Aqueous environments often present a harsh environment where e.g., deamidation and aggregation can have a critical impact on the integrity of proteins.3 The addition of neutral osmolytes, such as glycerol, can improve the stability of proteins due to the changes in the protein dynamics and structure, thus hindering chemical reactions and the unfolding propensity.4–6 The use of lyophilisation to store proteins as dry powders is also a common method. However, many proteins are prone to degradation through physical/interfacial stress and they cannot be stabilised through lyophilisation or precipitation.7 Moreover, further development of new methods for protein preservation is required to make formulated biologics (e.g., therapeutic proteins) widely available without requiring costly refrigeration or cryo-preservation technologies. In this sense, deep eutectic solvents (DESs) and ionic liquids have emerged as promising sustainable alternatives for the stabilisation of proteins.8–11
DESs are sustainable liquids obtained through the complexation of simple organic compounds (e.g., choline chloride and glycerol) at the mixture's eutectic composition.12–15 They are synthetically accessible, tailorable through the chemical selection of the constituents, chemically stable, and often show negligible toxicity.16–18 Previous reports have shown that proteins follow non-native folding pathways in anhydrous DESs, often decreasing the backbone mobility and increasing their stability.19–22 Also, recent reports have shown that the functional integrity of proteins can be retained in DESs. The stability of human interferon increases in sugar-based DESs compared to that in aqueous buffers during long-term storage (90 days), even at elevated temperatures.23 A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline
2 choline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) geranate solution was shown to preserve the physical integrity of insulin while retaining its activity.24 Similarly, the stabilities of α-chymotrypsin and Trp-cage protein at high temperatures increase in choline-based DESs compared to aqueous solutions.25,26 DESs have also been proposed to act as cryo-protectants of biomolecules in biological systems.27 The restricted solute mobility in DESs has been also hypothesised to hinder the chemical degradation of beta-lactams, resulting in an increase in their stability by several orders of magnitude.28 Therefore, DESs pose a novel approach for the protection of sensitive biomolecules against chemical, physical and thermal denaturation.
geranate solution was shown to preserve the physical integrity of insulin while retaining its activity.24 Similarly, the stabilities of α-chymotrypsin and Trp-cage protein at high temperatures increase in choline-based DESs compared to aqueous solutions.25,26 DESs have also been proposed to act as cryo-protectants of biomolecules in biological systems.27 The restricted solute mobility in DESs has been also hypothesised to hinder the chemical degradation of beta-lactams, resulting in an increase in their stability by several orders of magnitude.28 Therefore, DESs pose a novel approach for the protection of sensitive biomolecules against chemical, physical and thermal denaturation.
However, these investigations are limited to very dilute conditions (e.g., nM to μM protein concentrations), and the behaviour of proteins in DESs at technologically relevant concentrations has remained unexplored until now. This knowledge gap limits the prospective applications of DESs in protein stabilisation, as very large amounts of solvents would be required to store or formulate proteins in dilute conditions, which would be inefficient. Here, we report the ability of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (ChCl
glycerol (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc) to preserve the physical integrity of lysozyme (Lyz) at high protein concentrations. Lyz has extensively been used as a robust model to study the stability of proteins under different conditions. For instance, Lyz is often used to test the behaviour of high concentrations of proteins in aqueous solutions.29 In addition, the stability of Lyz has been previously studied in organic solvents, ionic liquids and deep eutectic solvents.4,19,30 Therefore, Lyz constitutes a valuable model protein to investigate its conformation and function over a wide concentration range when dissolved in a DES.
Glyc) to preserve the physical integrity of lysozyme (Lyz) at high protein concentrations. Lyz has extensively been used as a robust model to study the stability of proteins under different conditions. For instance, Lyz is often used to test the behaviour of high concentrations of proteins in aqueous solutions.29 In addition, the stability of Lyz has been previously studied in organic solvents, ionic liquids and deep eutectic solvents.4,19,30 Therefore, Lyz constitutes a valuable model protein to investigate its conformation and function over a wide concentration range when dissolved in a DES.
Initially, we investigated the behaviour of Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc in dilute conditions using second-derivative UV-vis absorption spectroscopy and small-angle neutron scattering (SANS). Importantly, no signs of aggregation were found during sample preparation or measurement. It should be noted that SANS experiments were performed using isotopically labelled DES, i.e., 1
Glyc in dilute conditions using second-derivative UV-vis absorption spectroscopy and small-angle neutron scattering (SANS). Importantly, no signs of aggregation were found during sample preparation or measurement. It should be noted that SANS experiments were performed using isotopically labelled DES, i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 d9-choline chloride
2 d9-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
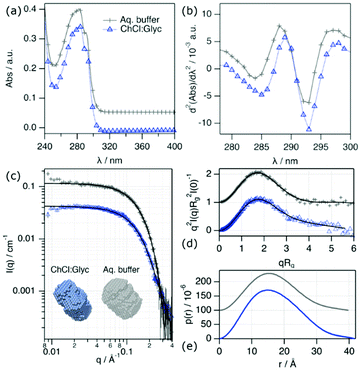
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d8-glycerol, and the deuterium labelling has previously been shown to not affect the behaviour of Lyz in DESs.20 The data are displayed in Fig. 1 and the main results are presented in Table 1. The results for Lyz in aqueous buffer (10 mM sodium phosphate, pH 7) are presented for comparison. Details on the analysis of the data are provided in the ESI.†
d8-glycerol, and the deuterium labelling has previously been shown to not affect the behaviour of Lyz in DESs.20 The data are displayed in Fig. 1 and the main results are presented in Table 1. The results for Lyz in aqueous buffer (10 mM sodium phosphate, pH 7) are presented for comparison. Details on the analysis of the data are provided in the ESI.†
| D max/Å | R g/Å | N agg | λ d 2Abs,Tyr/nm | d 2Abs287/d2Abs295 |
|---|---|---|---|---|
Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Glyc Glyc
|
||||
| 41.7 ± 1.0 | 13.3 ± 0.2 | 1.08 ± 0.06 | 288.9 ± 0.3 | 0.662 ± 0.003 |
| Lyz in aqueous buffer | ||||
| 39.8 ± 1.0 | 12.9 ± 0.1 | 1.08 ± 0.04 | 288.2 ± 0.2 | 0.725 ± 0.002 |
Our results show that Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc folds into a globular structure with similar dimensions (Dmax, Rg) to those in an aqueous buffer and retains its monomeric form (Nagg).31 However, the spectroscopy data shows a subtle bathochromic shift in λd2Abs,Tyr for the protein in DES. This variation must be attributed to a change in the solvation environment of the protein chromophores, i.e., tyrosine (Tyr) and tryptophan (Trp), where the increase in the wavelength potentially results from the exposure of those to a more apolar environment.32,33 Similarly, a decrease in d2Abs287/d2Abs295 results from a decrease in the polarity of the Tyr and Trp environments. In addition, the high q expansion of the scattering data, highlighted in the Kratky representation (qRg > 3), is different between the signals in the DES and the aqueous buffer. This change in the slope of the scattering data must relate to a subtle disruption of the internal native structure of Lyz. A 3D reconstruction of the conformation of the solvated Lyz has been performed using ab initio bead modelling (see Fig. 1c).34 These models confirm that Lyz remains folded in both the DES and aqueous buffer, with minimal structural differences in its overall conformation. Thus, the results from the characterisation studies confirm that Lyz remains folded in the DES with an internal structure somehow different to that in the native state. This behaviour has been previously reported for Lyz in 1
Glyc folds into a globular structure with similar dimensions (Dmax, Rg) to those in an aqueous buffer and retains its monomeric form (Nagg).31 However, the spectroscopy data shows a subtle bathochromic shift in λd2Abs,Tyr for the protein in DES. This variation must be attributed to a change in the solvation environment of the protein chromophores, i.e., tyrosine (Tyr) and tryptophan (Trp), where the increase in the wavelength potentially results from the exposure of those to a more apolar environment.32,33 Similarly, a decrease in d2Abs287/d2Abs295 results from a decrease in the polarity of the Tyr and Trp environments. In addition, the high q expansion of the scattering data, highlighted in the Kratky representation (qRg > 3), is different between the signals in the DES and the aqueous buffer. This change in the slope of the scattering data must relate to a subtle disruption of the internal native structure of Lyz. A 3D reconstruction of the conformation of the solvated Lyz has been performed using ab initio bead modelling (see Fig. 1c).34 These models confirm that Lyz remains folded in both the DES and aqueous buffer, with minimal structural differences in its overall conformation. Thus, the results from the characterisation studies confirm that Lyz remains folded in the DES with an internal structure somehow different to that in the native state. This behaviour has been previously reported for Lyz in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea using MD simulations, where the internal (secondary) structure of the protein was altered but the overall globularity of Lyz was retained compared to its native state.20,22 This variation in the internal structure was attributed to specific interactions between choline ions and the aromatic and charged residues of the protein.35
urea using MD simulations, where the internal (secondary) structure of the protein was altered but the overall globularity of Lyz was retained compared to its native state.20,22 This variation in the internal structure was attributed to specific interactions between choline ions and the aromatic and charged residues of the protein.35
Subsequently, the behaviour of Lyz at concentrations between 14 mg ml−1 (0.98 mM) and 224 mg ml−1 (15.7 mM) was investigated. The sample originally containing 224 mg ml−1 of Lyz showed the presence of a solid residue after freeze-drying. The sample was centrifuged and the amount of undissolved Lyz was determined. The solubility of Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc was found to be ca. 143 mg ml−1 (10.0 mM). Thus, Lyz solubility at room temperature in this DES is of the same order of magnitude as that in water.29 However, the presence of salts significantly decreases its solubility.36 For instance, the solubility of Lyz is 64 mg ml−1 and 9.4 mg ml−1 at 250 mM and 350 mM salt concentrations, respectively. Considering that the effective ionic strength of ChCl
Glyc was found to be ca. 143 mg ml−1 (10.0 mM). Thus, Lyz solubility at room temperature in this DES is of the same order of magnitude as that in water.29 However, the presence of salts significantly decreases its solubility.36 For instance, the solubility of Lyz is 64 mg ml−1 and 9.4 mg ml−1 at 250 mM and 350 mM salt concentrations, respectively. Considering that the effective ionic strength of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc is potentially higher than those aqueous electrolytes,37 the solubility of Lyz in the DES is significantly higher than that in saline aqueous buffers. Also, the solubility of Lyz in ChCl
Glyc is potentially higher than those aqueous electrolytes,37 the solubility of Lyz in the DES is significantly higher than that in saline aqueous buffers. Also, the solubility of Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc is higher than that in glycerol.38
Glyc is higher than that in glycerol.38
The conformational landscape of Lyz at higher concentrations, i.e., between 14 mg ml−1 and 112 mg ml−1 (7.83 mM), in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
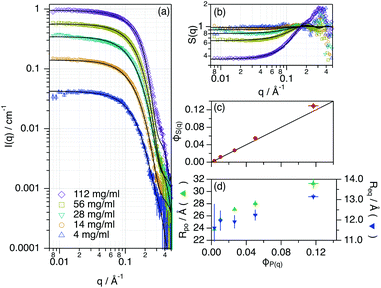
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc was probed using SANS. This technique enables the morphological characterisation of proteins in solution (e.g., folding) as well as the interactions in the colloidal domain (e.g., self-association), and it is very sensitive to protein aggregation.39,40 In addition, the use of isotope-labelled compounds in SANS is a suitable method to resolve the structure of macromolecular systems in DES due to the high contrast between the macromolecule and the solvent.20,41 Data and best models are presented in Fig. 2. For detailed information on the data analysis, see the ESI.†
Glyc was probed using SANS. This technique enables the morphological characterisation of proteins in solution (e.g., folding) as well as the interactions in the colloidal domain (e.g., self-association), and it is very sensitive to protein aggregation.39,40 In addition, the use of isotope-labelled compounds in SANS is a suitable method to resolve the structure of macromolecular systems in DES due to the high contrast between the macromolecule and the solvent.20,41 Data and best models are presented in Fig. 2. For detailed information on the data analysis, see the ESI.†
While retaining its globular conformation with no signs of aggregation, Lyz only experiences a subtle change in the conformation (rpo, req) when increasing the volume fraction of the protein. Considering the small magnitude of these changes, they probably relate to subtle variations in the tertiary structure of the protein. Unlike the observations of the aqueous behaviour of Lyz at high concentrations, no changes in self-association were observed and Lyz remains as a monomer in the DES.29 The effective structure factor (S(q), obtained as the ratio between the experimental scattering data and the form factor of the protein, see the ESI†), attributed to the protein–protein interactions in the concentrated regime, was analysed using an apparent excluded volume effect. The results showed that the volume fraction of the apparent interaction, parameterised as ϕS(q), should not be a constraint to ϕP(q) to obtain a good fit of the data. Thus, there are weak interactions that act upon the system beyond the protein–protein excluded volume (hard sphere) effects. This excess contribution could be attributed to the weak protein–protein electrostatic repulsion, which in turn could sustain the colloidal stability of the system.37 Another possible explanation for this effect could be the presence of a solvation layer with the DES constituents tightly bound to the protein, which would affect the protein–protein correlations.22
One of the key aspects of the potential use of DESs as storage media is the capacity of the protein to remain stable in DESs and retrieve the native conditions upon reincorporation into an aqueous buffer. To test this, we have stored 28 mg ml−1 Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
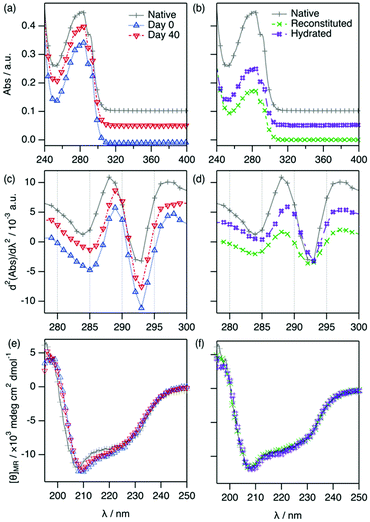
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc for a period of 40 days at room temperature (ca. 22 °C) under dry conditions. For subsequent analysis, the samples were diluted to a working concentration of 1.43 mg ml−1 (100 μM) after storage. The results from the UV-vis and far-UV circular dichroism (CD) characterisation of Lyz stored in ChCl
Glyc for a period of 40 days at room temperature (ca. 22 °C) under dry conditions. For subsequent analysis, the samples were diluted to a working concentration of 1.43 mg ml−1 (100 μM) after storage. The results from the UV-vis and far-UV circular dichroism (CD) characterisation of Lyz stored in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc are presented in Fig. 3.
Glyc are presented in Fig. 3.
For the samples with protein in DES, the absorbance data and the second-derivative spectra exhibit only very minor differences between 0 and 40 days (λd2Abs,Tyr = 288.9 ± 0.4 nm, d2Abs287/d2Abs295 = 0.674 ± 0.008 for the 40 days sample). Similarly, the CD spectra of the protein in DES at 0 and 40 days are practically identical, showing that no changes in the secondary structure of the protein occur during storage.
Subsequently, two approaches were followed to study the activity of Lyz after storage in the DES: (i) reconstitution and (ii) hydration. In the reconstitution approach (i), the DES components were removed through extensive dialysis against aqueous buffer (sample labelled as reconstituted). The second-derivative spectra show that the position of the peaks from the reconstituted Lyz are the same as those of the native protein in aqueous buffer. Thus, the chromophores retrieve their native environment upon the removal of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc, showing that Lyz solvation in DES does not cause any irreversible changes in the protein even after 40 days of storage. This recovery has previously been shown for insulin in 1
Glyc, showing that Lyz solvation in DES does not cause any irreversible changes in the protein even after 40 days of storage. This recovery has previously been shown for insulin in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline geranate and for lysozyme in ChCl
2 choline geranate and for lysozyme in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc, both in dilute conditions; upon dialysis of the DES components, the proteins retrieved their native characteristics.24 Here, it is demonstrated that high concentrations of protein can also retrieve their native conditions after being stored in DES without showing any aggregation or degradation. Upon reconstitution in an aqueous buffer, a decrease in the absorbed intensity is observed, as observed in Fig. 3c. This is attributed to a decrease in protein concentration because of the dialysis process. To increase the dialysis rate, a 3-fold dilution of the protein solubilised in DES was performed using water before dialysing the sample. Still, a limitation associated with the dialysis process is that it is slow (24 h).
Glyc, both in dilute conditions; upon dialysis of the DES components, the proteins retrieved their native characteristics.24 Here, it is demonstrated that high concentrations of protein can also retrieve their native conditions after being stored in DES without showing any aggregation or degradation. Upon reconstitution in an aqueous buffer, a decrease in the absorbed intensity is observed, as observed in Fig. 3c. This is attributed to a decrease in protein concentration because of the dialysis process. To increase the dialysis rate, a 3-fold dilution of the protein solubilised in DES was performed using water before dialysing the sample. Still, a limitation associated with the dialysis process is that it is slow (24 h).
To probe an alternative method (ii), we tested the possibility of hydrating DES-containing Lyz (sample labelled as hydrated) as it has been previously shown that high levels of DES hydration prompt the recovery of the native behaviour of proteins.19,20 This approach involves mixing the DES containing the protein (previously diluted to 1.43 mg ml−1 using DES) with water to a final water content of 67 wt%. The results from the characterisation are presented in Fig. 3. The characteristic parameters from the absorbance spectra for the hydrated sample are very similar to those of the native protein (λd2Abs,Tyr = 288.4 ± 0.2 nm, d2Abs287/d2Abs295 = 0.713 ± 0.005). An obvious reduction in protein concentration is observed, as expected after the hydration process. Importantly, no evidence of protein degradation is again observed. In addition, the far-UV CD spectrum of the hydrated sample is identical to that of Lyz and, hence, the protein secondary structure is unchanged.
Importantly, no signs of protein degradation or aggregation were observed during any of these processes. The wide solubility range of Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc allows relevant protein concentrations to be achieved a variety of technical applications, i.e., up to 50 mg ml−1.2 The main difference resides in the resulting solution: (i) the dialysis product provides a practically DES-free solution, although it is time consuming, and (ii) the hydration product is comparatively much faster but a residual amount of the DES components remains in the protein solution.
Glyc allows relevant protein concentrations to be achieved a variety of technical applications, i.e., up to 50 mg ml−1.2 The main difference resides in the resulting solution: (i) the dialysis product provides a practically DES-free solution, although it is time consuming, and (ii) the hydration product is comparatively much faster but a residual amount of the DES components remains in the protein solution.
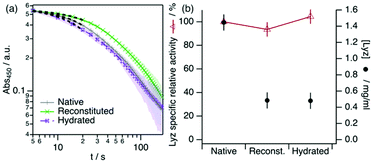
To demonstrate that the functionality of Lyz is retrieved after storage in DES, we have measured the activity of reconstituted and hydrated Lyz after 40 days of storage in the DES at room temperature. The results from the activity assays are presented in Fig. 4.
Our results show that Lyz in the reconstituted and hydrated samples effectively retains its normalised activity after storage in neat DES within the error. This agrees with previous reports on enzymatic activity in DES, as it has been shown that Lyz retains its activity when dissolved or surface-immobilised in DES.19,42 Similarly, other enzymes were shown to remain functional in other choline-based neoteric solvents, such as L-asparaginase,43 Lyz,44 and lactate oxidase,45 among others. Although only a subtle decrease in activity is observed for Lyz in aqueous buffers after long-term storage, this is not a general case. For instance, Lyz loses ca. 40% of its activity in choline dihydrogen phosphate ionic liquid after 4 weeks of storage.44 In addition, aqueous ionic liquids can have a negative impact on the activity of Lyz after long-term storage.46 The stability and functional resilience are potentially attributed to the physicochemical properties of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc compared to other neoteric solvents, as more cohesive solvents are suggested to promote protein folding better than those less cohesive and, thus, better preserve the integrity of the protein.47 This parallels the behaviour of Lyz in glycerol, which conforms into a stable and functional molten globule.4 Thus, DESs offer a new perspective on the design of suitable environments for a variety of labile biomacromolecules.
Glyc compared to other neoteric solvents, as more cohesive solvents are suggested to promote protein folding better than those less cohesive and, thus, better preserve the integrity of the protein.47 This parallels the behaviour of Lyz in glycerol, which conforms into a stable and functional molten globule.4 Thus, DESs offer a new perspective on the design of suitable environments for a variety of labile biomacromolecules.
Conclusions
In summary, we have studied the behaviour of Lyz in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc over a wide range of concentrations, ranging from dilute (1.4 mg ml−1, 0.100 mM) to concentrated (142 mg ml−1, 9.9 mM) conditions. The internal structure of the protein in the DES changes from that in aqueous buffer. This is probably attributed to the change in the environment of the amino acid residues, resulting in a rearrangement of the secondary structure of Lyz. Despite this, Lyz retains its globularity in the DES with an overall structure similar to that in aqueous buffer, which potentially enables the protein to remain physically stable even after long periods of storage in the DES. After 40 days of storage in ChCl
Glyc over a wide range of concentrations, ranging from dilute (1.4 mg ml−1, 0.100 mM) to concentrated (142 mg ml−1, 9.9 mM) conditions. The internal structure of the protein in the DES changes from that in aqueous buffer. This is probably attributed to the change in the environment of the amino acid residues, resulting in a rearrangement of the secondary structure of Lyz. Despite this, Lyz retains its globularity in the DES with an overall structure similar to that in aqueous buffer, which potentially enables the protein to remain physically stable even after long periods of storage in the DES. After 40 days of storage in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glyc at room temperature, the protein showed the same characteristics as the native state, proving the capacity of DES to preserve the integrity of Lyz. Importantly, the enzymatic activity of the reconstituted and hydrated Lyz solutions is totally retained after storage in DES.
Glyc at room temperature, the protein showed the same characteristics as the native state, proving the capacity of DES to preserve the integrity of Lyz. Importantly, the enzymatic activity of the reconstituted and hydrated Lyz solutions is totally retained after storage in DES.
These results show the potential of DES as suitable media for proteins at high concentrations, overcoming the limitations of molecular solvents where physical and chemical degradation often affects the integrity of biomolecules. Thus, the use of DESs for protein stabilisation presents several benefits: (1) the wide variety of DESs and their concomitant wide range of properties will allow to develop environments with design properties to protect proteins with specific requirements; (2) DES can solubilise a wider range of amphiphilic molecules of importance in formulation technologies, e.g., lipids and surfactants,48,49 than other organic solvents, such as glycerol;50 and (3) they can be produced from sustainably sourced materials.18
Experimental section
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol and the deuterated analogue were prepared by mixing the components and heating at 60 °C until a transparent, homogeneous liquid had formed.12 Samples were prepared using a freeze drying protocol using an Epsilon 2-6D LSCplus from Martin Christ.20 Control samples of the DES without protein were tested for water content using Karl-Fischer titration. The “neat” DES contained 0.47 wt% water and 0.58 wt% water before and after incorporating the protein, respectively. The UV-vis absorption measurements were performed using a Varian Cary 50 UV-vis spectrometer (190–500 nm, 600 nm min−1) at 25 °C. CD measurements were performed using a Jasco J-715. The activity of Lyz was determined by measuring the turbidimetric rate of lysis of a Micrococcus lysodeikticus suspension as catalysed by Lyz in aqueous buffer using a Biochrom Libra S60 spectrometer. SANS data were acquired on D22 (Institut Laue-Langevin, France) at 25 °C in a q-range between 0.006–0.65 Å−1. Data are available at https://doi.org/10.5291/ILL-DATA.8-03-1049. The analysis of the SANS data was performed using ATSAS 2.8.3 and SasView 5.0.3.34,51
glycerol and the deuterated analogue were prepared by mixing the components and heating at 60 °C until a transparent, homogeneous liquid had formed.12 Samples were prepared using a freeze drying protocol using an Epsilon 2-6D LSCplus from Martin Christ.20 Control samples of the DES without protein were tested for water content using Karl-Fischer titration. The “neat” DES contained 0.47 wt% water and 0.58 wt% water before and after incorporating the protein, respectively. The UV-vis absorption measurements were performed using a Varian Cary 50 UV-vis spectrometer (190–500 nm, 600 nm min−1) at 25 °C. CD measurements were performed using a Jasco J-715. The activity of Lyz was determined by measuring the turbidimetric rate of lysis of a Micrococcus lysodeikticus suspension as catalysed by Lyz in aqueous buffer using a Biochrom Libra S60 spectrometer. SANS data were acquired on D22 (Institut Laue-Langevin, France) at 25 °C in a q-range between 0.006–0.65 Å−1. Data are available at https://doi.org/10.5291/ILL-DATA.8-03-1049. The analysis of the SANS data was performed using ATSAS 2.8.3 and SasView 5.0.3.34,51
Author contributions
A. S.-F.: Conceptualisation, methodology, investigation, formal analysis, writing – original draft, writing – review & editing, and funding acquisition; S. P.: Data curation, resources, methodology, investigation, and writing – review & editing; M. W.: Writing – review & editing and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. S.-F. acknowledges the Spanish Ministerio de Universidades for the awarded Maria Zambrano fellowship. The research in this study was also performed with financial support from Vinnova – a Swedish Governmental Agency for Innovation Systems within the NextBioForm Competence Centre and the Crafoord Foundation (Grant 20190750). The authors thank the Institut Laue-Langevin for the awarded beamtime (8-03-1049). This work benefited from the use of the SasView application, originally developed under the NSF award DMR-0520547. SasView contains the code developed with funding from the European Union's Horizon 2020 Research and Innovation Programme under the SINE2020 project, Grant Agreement No. 654000.Notes and references
- S. J. Shire, Z. Shahrokh and J. Liu, J. Pharm. Sci., 2004, 93, 1390–1402 CrossRef CAS PubMed.
- M. C. Manning, J. Liu, T. Li and R. E. Holcomb, in Advances in Protein Chemistry and Structural Biology, ed. R. Donev, Academic Press, 2018, vol. 112, pp. 1–59 Search PubMed.
- W. Wang, S. Nema and D. Teagarden, Int. J. Pharm., 2010, 390, 89–99 CrossRef CAS PubMed.
- R. V. Rariy and A. M. Klibanov, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 13520–13523 CrossRef CAS PubMed.
- M. Hirai, S. Ajito, M. Sugiyama, H. Iwase, S.-I. Takata, N. Shimizu, N. Igarashi, A. Martel and L. Porcar, Biophys. J., 2018, 115, 313–327 CrossRef CAS PubMed.
- I. Ramm, A. Sanchez-Fernandez, J. Choi, C. Lang, J. Fransson, H. Schagerlöf, M. Wahlgren and L. Nilsson, Pharmaceutics, 2021, 13, 1853 CrossRef CAS PubMed.
- I. Roy and M. N. Gupta, Biotechnol. Appl. Biochem., 2004, 39, 165–177 CrossRef CAS PubMed.
- M. S. Álvarez and Y. Zhang, J. Controlled Release, 2019, 311–312, 225–232 CrossRef PubMed.
- A. Sanchez-Fernandez and A. J. Jackson, in Eutectic Solvents and Stress in Plants, Academic Press, 2021, pp. 69–94, DOI:10.1016/bs.abr.2020.09.003.
- K. D. Weaver, H. J. Kim, J. Sun, D. R. MacFarlane and G. D. Elliott, Green Chem., 2010, 12, 507–513 RSC.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155–1157 RSC.
- A. P. Abbott, R. C. Harris, K. S. Ryder, C. D'Agostino, L. F. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82–90 RSC.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Green Chem., 2016, 18, 2736–2744 RSC.
- O. S. Hammond, D. T. Bowron, A. J. Jackson, T. Arnold, A. Sanchez-Fernandez, N. Tsapatsaris, V. Garcia Sakai and K. J. Edler, J. Phys. Chem. B, 2017, 121, 7473–7483 CrossRef CAS PubMed.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- I. P. E. Macario, H. Oliveira, A. C. Menezes, S. P. M. Ventura, J. L. Pereira, A. M. M. Goncalves, J. A. P. Coutinho and F. J. M. Goncalves, Sci. Rep., 2019, 9, 3932 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- R. Esquembre, J. M. Sanz, J. G. Wall, F. del Monte, C. R. Mateo and M. L. Ferrer, Phys. Chem. Chem. Phys., 2013, 15, 11248–11256 RSC.
- A. Sanchez-Fernandez, K. J. Edler, T. Arnold, D. Alba Venero and A. J. Jackson, Phys. Chem. Chem. Phys., 2017, 19, 8667–8670 RSC.
- H. Monhemi, M. R. Housaindokht, A. A. Moosavi-Movahedi and M. R. Bozorgmehr, Phys. Chem. Chem. Phys., 2014, 16, 14882–14893 RSC.
- P. Kumari, M. Kumari and H. K. Kashyap, J. Phys. Chem. B, 2020, 124, 11919–11927 CrossRef CAS PubMed.
- M. S. Lee, K. Lee, M. W. Nam, K. M. Jeong, J. E. Lee, N. W. Kim, Y. Yin, S. Y. Lim, D. E. Yoo, J. Lee and J. H. Jeong, J. Ind. Eng. Chem., 2018, 65, 343–348 CrossRef CAS.
- A. Banerjee, K. Ibsen, Y. Iwao, M. Zakrewsky and S. Mitragotri, Adv. Healthcare Mater., 2017, 6, 1601411 CrossRef PubMed.
- N. Yadav, K. Bhakuni, M. Bisht, I. Bahadur and P. Venkatesu, ACS Sustainable Chem. Eng., 2020, 8, 10151–10160 CrossRef CAS.
- S. Pal, R. Roy and S. Paul, J. Phys. Chem. B, 2020, 124, 7598–7610 CrossRef CAS PubMed.
- A. Gertrudes, R. Craveiro, Z. Eltayari, R. L. Reis, A. Paiva and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2017, 5, 9542–9553 CrossRef CAS.
- B. Olivares, F. Martínez, L. Rivas, C. Calderón, J. M. Munita and P. R. Campodonico, Sci. Rep., 2018, 8, 14900 CrossRef PubMed.
- L. Porcar, P. Falus, W.-R. Chen, A. Faraone, E. Fratini, K. Hong, P. Baglioni and Y. Liu, J. Phys. Chem. Lett., 2010, 1, 126–129 CrossRef CAS.
- J. P. Mann, A. McCluskey and R. Atkin, Green Chem., 2009, 11, 785–792 RSC.
- H. B. Stuhrmann and H. Fuess, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 67–74 CrossRef.
- R. Lange and C. Balny, Biochim. Biophys. Acta, 2002, 1595, 80–93 CrossRef CAS.
- M. S. Weiss, G. J. Palm and R. Hilgenfeld, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2000, 56, 952–958 CrossRef CAS PubMed.
- M. V. Petoukhov, D. Franke, A. V. Shkumatov, G. Tria, A. G. Kikhney, M. Gajda, C. Gorba, H. D. T. Mertens, P. V. Konarev and D. I. Svergun, J. Appl. Crystallogr., 2012, 45, 342–350 CrossRef CAS PubMed.
- B. D. Belviso, F. M. Perna, B. Carrozzini, M. Trotta, V. Capriati and R. Caliandro, ACS Sustainable Chem. Eng., 2021, 9, 8435–8449 CrossRef CAS.
- C. Gripon, L. Legrand, I. Rosenman, O. Vidal, M. C. Robert and F. Boué, J. Cryst. Growth, 1997, 177, 238–247 CrossRef CAS.
- A. Sanchez-Fernandez, A. J. Jackson, S. F. Prevost, J. J. Doutch and K. J. Edler, J. Am. Chem. Soc., 2021, 143, 14158–14168 CrossRef CAS PubMed.
- G. Houen, K. Bechgaard, J. Songstad, M. Leskelä, M. Polamo, M. Homsi, F. Kuske, M. Haugg, N. Trabesinger-Rüf and E. Weinhold, Acta Chem. Scand., 1996, 50, 68–70 CrossRef CAS.
- E. J. Yearley, I. E. Zarraga, S. J. Shire, T. M. Scherer, Y. Gokarn, N. J. Wagner and Y. Liu, Biophys. J., 2013, 105, 720–731 CrossRef CAS PubMed.
- W. Heller, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 1213–1217 CrossRef CAS PubMed.
- A. Sanchez-Fernandez, A. E. Leung, E. G. Kelley and A. J. Jackson, J. Colloid Interface Sci., 2021, 581, 292–298 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker and S. Holmes, J. Mol. Catal. B: Enzym., 2011, 72, 163–167 CrossRef CAS PubMed.
- A. Magri, T. Pecorari, M. M. Pereira, E. M. Cilli, T. L. Greaves and J. F. B. Pereira, ACS Sustainable Chem. Eng., 2019, 7, 19720–19731 CrossRef CAS.
- K. D. Weaver, R. M. Vrikkis, M. P. Van Vorst, J. Trullinger, R. Vijayaraghavan, D. M. Foureau, I. H. McKillop, D. R. MacFarlane, J. K. Krueger and G. D. Elliott, Phys. Chem. Chem. Phys., 2012, 14, 790–801 RSC.
- V. F. Curto, S. Scheuermann, R. M. Owens, V. Ranganathan, D. R. MacFarlane, F. Benito-Lopez and D. Diamond, Phys. Chem. Chem. Phys., 2014, 16, 1841–1849 RSC.
- L. Satish, S. Millan and H. Sahoo, J. Mol. Liq., 2019, 278, 329–334 CrossRef CAS.
- E. C. Wijaya, F. Separovic, C. J. Drummond and T. L. Greaves, Phys. Chem. Chem. Phys., 2016, 18, 25926–25936 RSC.
- A. Sanchez-Fernandez, T. Arnold, A. J. Jackson, S. L. Fussell, R. K. Heenan, R. A. Campbell and K. J. Edler, Phys. Chem. Chem. Phys., 2016, 18, 33240–33249 RSC.
- S. J. Bryant, R. Atkin and G. G. Warr, Langmuir, 2017, 33, 6878–6884 CrossRef CAS PubMed.
- N. R. Agrawal, X. Yue, Y. Feng and S. R. Raghavan, Langmuir, 2019, 35, 12782–12791 CrossRef CAS PubMed.
- O. Glatter, J. Appl. Crystallogr., 1977, 10, 415–421 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Further information on the preparation of the samples, experimental details and data analysis. See DOI: https://doi.org/10.1039/d1gc04378a |
| This journal is © The Royal Society of Chemistry 2022 |