Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enzymatic amide bond formation: synthesis of aminooxo-acids through a Mycobacterium smegmatis acyltransferase†
Michael S.
Christodoulou‡
 *,
Martina Letizia
Contente‡
*,
Martina Letizia
Contente‡
 *,
Sabrina
Dallavalle
and
Andrea
Pinto
*,
Sabrina
Dallavalle
and
Andrea
Pinto

Department of Food, Environmental and Nutritional Sciences (DeFENS), University of Milan, via Celoria 2, 20133, Milan, Italy. E-mail: michail.christodoulou@unimi.it; martina.contente@unimi.it
First published on 25th May 2022
Abstract
A highly-productive strategy based on the use of an acyltransferase from Mycobacterium smegmatis (MsAcT), for the preparation of aminooxo-acids in water was developed. 1 M-scale biotransformations were carried out with excellent yields (68–94%) and rapid reactions (0.5–5 h) starting from anilines and a range of different anhydrides. The high substrate-to-catalyst ratio (Msubstrate/Mcatalyst: 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), enzymatic stability (one month without any activity loss), and excellent protein purification yields (130 mg from 2 g of wet cell paste) made this process a green and cost-efficient approach, which was successfully applied for the preparation of a key intermediate of SAHA synthesis.
000), enzymatic stability (one month without any activity loss), and excellent protein purification yields (130 mg from 2 g of wet cell paste) made this process a green and cost-efficient approach, which was successfully applied for the preparation of a key intermediate of SAHA synthesis.
One of the most important reactions in organic chemistry is the formation of amide bond, which is widely found in many natural compounds, agrochemicals and pharmaceuticals.1–4 Amides are typically synthesized through condensation reactions between amines and carboxylic acids in the presence of an in situ prepared activating agent,5 while other synthetic strategies include the oxidative amidation of alcohols or aldehydes.5 Conventional procedures are characterized by poor atom economy and are usually carried out in organic solvents, requiring a dry environment to avoid reverse hydrolysis reactions. For these reasons, the development of more sustainable and green synthetic strategies for amide bond formation has become a top challenge for scientists in recent years.6
Within the field of biocatalysis, condensation reactions in organic media are usually catalyzed by lipases7 (e.g., CAL B from Candida antarctica), while enzymatic amide syntheses in water mainly rely on penicillin acylases, predominantly applied in the preparation of β-lactam antibiotics,8,9 or acyltransferases with promiscuous activity, such as the one from Pseudomonas protegens, presenting a narrow substrate scope and prolonged reaction times for the obtainment of the desired product (24 h).10 Many esterases belonging to the bacterial hormone-sensitive lipase (bHSL) family have shown promiscuous acyltransferase activity. However, product hydrolysis and low transfer efficiency, requiring critical and careful reaction optimization, have been a major drawback for their application on a preparative scale.11 A better biocatalyst with similar behaviour is represented by EstCE1, a family VIII carboxylesterase with a strong preference for the water amidation and carbamoylation of aromatic compounds.12 Its crystal structure was recently solved and the amino acid motif important for promiscuous acyltransferase activity finally elucidated.13 With the aim of developing green chemistry approaches for amide synthesis, an acyltransferase from Mycobacterium smegmatis (MsAcT), has been exploited both in buffer and solvent media, in batch or in continuous mode, starting from primary amines and reactive short-chain esters.14–18 A further process implementation was obtained by its evolution through rational design (MsAcT S11C), unlocking the possibility of synthesizing tertiary amides.19 MsAcT, which was initially characterized for its capability to perform trans-esterification reactions in water,20–22 demonstrated a remarkable activity even at high substrate concentrations (up to 0.5 M).16,23,24 Although vinyl esters have shown superior reactivity as acyl donors with respect to ethyl ones,14,16,21 their procurement remains a concern with just a few commercially available and at high costs. Furthermore their synthesis is based on Pd-catalysis and harsh reaction conditions (i.e., anhydrous THF, high temperature), which are not considered environmentally-friendly.15,17,18 To the best of our knowledge, no biocatalytic reactions or green processes for the preparation of vinyl esters have been reported to date. Within this framework, and considering the broad substrate scope of MsAcT, a rapid and robust enzymatic approach for the preparation of amides starting from amines and various anhydrides has been developed. Anhydrides not only are commercially available on a large variety, but they are also low-cost reagents. In addition, the new MsAcT-mediated synthetic strategy gave access to a range of different aminooxo-acids in water.
To increase the appeal of this procedure suberanilic acid, a key intermediate for the preparation of SAHA (i.e., suberoylanilide hydroxamic acid), a potent inhibitor of histone deacetylases (HDACs),25 was synthesized in one single step, at high substrate loading and short reaction time, further increasing the potential of this enzyme in condensation reactions in water environment.
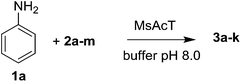
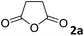
To monitor the MsAcT stability overtime, its activity was assayed (see ESI†) each week after its purification. No loss in the enzymatic performance was observed after 4 weeks of evaluation, storing the pure MsAcT at 4 °C in phosphate buffer (0.1 M pH 8.0). Optimization of the reaction parameters was firstly carried out using aniline (1a) and succinic anhydride (2a) (Table 1).
| Entry | Anhydride | Product | Yielda (%) |
|---|---|---|---|
| Timeb (h) | |||
Reactions conditions: 1 M substrates, 1 mg mL−1 enzyme (120 U mL−1; 0.00004 M), 25 °C in phosphate buffer (0.1 M, pH 8.0); substrate-to-catalyst ratio 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. To increase the substrate solubility 10% DMSO was employed as cosolvent.a Isolated yield.b Time corresponding to the maximum yield. No reaction was observed by adding the substrates in the same reaction conditions without the catalyst. 000. To increase the substrate solubility 10% DMSO was employed as cosolvent.a Isolated yield.b Time corresponding to the maximum yield. No reaction was observed by adding the substrates in the same reaction conditions without the catalyst. |
|||
| 1 |
|
|
90 |
| 1 | |||
| 2 |
|
|
88 |
| 0.5 | |||
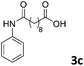
| 3 |
|
|
80 |
| 1 | |||
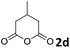
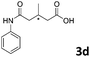
| 4 |
|
|
76 |
| 2 | |||
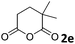
| 5 |
|
|
94 |
| 2 | |||
| 6 |
|
|
74 |
| 3 | |||
| 7 |
|
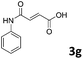
|
77 |
| 2 | |||
| 8 |
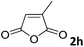
|
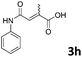
|
69 |
| 3 | |||
| 9 |
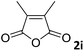
|
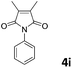
|
93 |
| 3 | |||
| 10 |
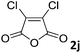
|
|
68 |
| 3 | |||
| 11 |
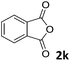
|
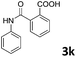
|
83 |
| 2 | |||
| 12 |
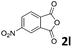
|
— | |
| 48 | |||
| 13 |
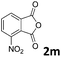
|
— | |
| 48 | |||
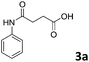
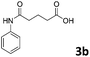
Different substrate concentrations (0.1–1 M) were tested. The formation of 3a (i.e., 4-anilino-4-oxobutanoic acid) was obtained with excellent conversion (90%) and short reaction time (1 h) using 1 M aniline, 1 eq. anhydride and 1 mg mL−1 MsAcT (see ESI†).
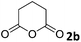
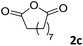
The substrate scope was further evaluated employing aniline (1a) in the presence of various anhydrides (2b–2m), the results are summarized in Table 1. The optimized reaction conditions have been applied.
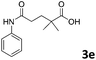
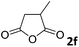
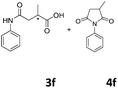
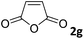
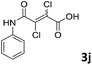
The anhydrides corresponding to the entries 1–11, Table 1 provided the corresponding aminooxo-acids 3 in high yields (68–94%) and short reaction times (0.5–3 h). No reaction was observed only when NO2-phthalic anhydrides (entries 12 and 13, Table 1) were employed as donors, probably due to the steric hindrance, and the presence of a strong EWG (i.e., electron-withdrawing group). Among the non-symmetrical anhydrides (entries 5, 6 and 8, Table 1) 2e and 2h furnished the desired products with anhydride opening from the less hindered portion. Interestingly, a mixture of closed and open compounds was observed for 2f (entry 6, Table 1). 2i was the only anhydride giving cyclic 4i as the single product (entry 9, Table 1) as previously reported in the literature.26 Notably, previous attempts to produce these aminooxo-acids starting from aniline and anhydrides were performed in organic solvents (e.g., diethyl ether, toluene, acetonitrile, dichloromethane), using high or very low temperatures (e.g., 90 °C or 5 °C) in the presence of strong bases or expensive catalysts.26–29
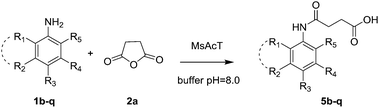
Subsequently, various anilines (1b–q) keeping constant succinic anhydride 2a were assayed (Table 2).
| Entry | Aniline | R1 | R2 | R3 | R4 | R5 | Pa | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| Timec (h) | ||||||||
Reactions conditions: 1 M substrates, 1 mg mL−1 enzyme (120 U mL−1; 0.00004 M), 25 °C in phosphate buffer (0.1 M, pH 8.0); substrate-to-catalyst ratio 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. To increase the substrate solubility 10% DMSO was employed as cosolvent.a P: Product.b Isolated yield.c Time corresponding to the maximum yield. No reaction was observed by adding the substrates in the same reaction conditions without the catalyst. 000. To increase the substrate solubility 10% DMSO was employed as cosolvent.a P: Product.b Isolated yield.c Time corresponding to the maximum yield. No reaction was observed by adding the substrates in the same reaction conditions without the catalyst. |
||||||||
| 1 | 1b | Me | H | H | H | H | 5b | 94 |
| 1 | ||||||||
| 2 | 1c | H | Me | H | H | H | 5c | 93 |
| 1 | ||||||||
| 3 | 1d | H | H | Me | H | H | 5d | 94 |
| 1 | ||||||||
| 4 | 1e | Me | H | Me | H | Me | 5e | 90 |
| 1.5 | ||||||||
| 5 | 1f | OMe | H | H | H | H | 5f | 84 |
| 2 | ||||||||
| 6 | 1g | H | H | OMe | H | H | 5g | 85 |
| 2 | ||||||||
| 7 | 1h | H | OMe | H | OMe | H | 5h | 87 |
| 3 | ||||||||
| 8 | 1i | H | OMe | OMe | H | H | 5i | 85 |
| 3 | ||||||||
| 9 | 1j | Cl | H | H | H | H | 5j | 82 |
| 2 | ||||||||
| 10 | 1k | H | Cl | H | H | H | 5k | 83 |
| 2 | ||||||||
| 11 | 1l | H | H | Cl | H | H | 5l | 85 |
| 2 | ||||||||
| 12 | 1m | NO2 | H | H | H | H | — | — |
| 48 | ||||||||
| 13 | 1n | H | NO2 | H | H | H | — | — |
| 48 | ||||||||
| 14 | 1o | H | H | NO2 | H | H | — | — |
| 48 | ||||||||
| 15 | 1p | Phenyl | H | H | H | 5p | 88 | |
| 5 | ||||||||
| 16 | 1q | H | H |
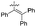
|
H | H | 5q | 93 |
| 5 | ||||||||
Aniline substitution with EDG (i.e., electron-donor groups) such as methyl or methoxy moieties did not affect the enzymatic performance, providing the corresponding compounds 5b–i in high yields (84–95%) and short reaction times (1–3 h) (entries 1–8, Table 2). Similar results were obtained with chloro-anilines (entries 9–11, Table 2). As previously described for NO2-substituted phthalic anhydrides, when a strong EWG was introduced to the substrates (entries 12–14, Table 2), no biotransformation was observed even at prolonged reaction times (48 h). The replacement of the phenyl ring with the naphthyl one (1p) (entry 15, Table 2) was well tolerated by MsAcT. Finally, the bulkier amine 4-(1,2,2-triphenylvinyl)aniline (1q) furnished compound 5q in excellent yield (93%) in 5 h (entry 16, Table 2).
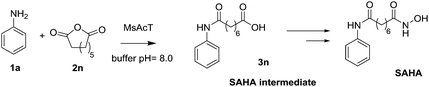
Although the developed strategy clearly shows the versatility of MsAcT, giving an easy and sustainable access to a wide set of aminooxo-acids, the real leap in its application is the preparation of SAHA intermediate in a green and highly-productive way (3n, Fig. 1).
Vorinostat, the commercial name of SAHA, belongs to the histone deacetylase inhibitors (HDI). Presenting a broad spectrum of epigenetic activities, it is considered a potent anticancer agent, especially for tumour relapses. Compound 3n is a key intermediate for the preparation of SAHA and its derivatives, which are under investigation to ameliorate the pharmacokinetic profile, tolerability or for the treatment of different diseases such as human leishmaniasis, among others.30–323n here prepared in water environment, was obtained in excellent yield and reaction time (90%, 1 h) and easily purified by an acid work-up and recrystallization from MeOH.
In conclusion, the high substrate loading (1 M), and yields (68–94%), together with the rapid reactions (0.5–5 h) described in this work for the preparation of a range of aminooxo-acids starting from anilines and anhydrides, has completely overcome the perception about biocatalysis ineffectiveness when compared with traditional synthesis. The efficiency and versatility of MsAcT catalyst, here employed as pure enzyme, are noteworthy. Although protein purification is usually considered a time-, energy- and cost-consuming technique, the catalyst stability (more than one month without activity losses) together with the high purification yields, allowed for just one purification cycle, dramatically decreasing the overall process-related costs and making this strategy further appealing from an economic perspective.
The remarkably highly-productive system here described can compete with conventional methods for the amide production at preparative scale. Finally, the intermediate for the synthesis of SAHA and its derivatives was efficiently prepared in water, demonstrating the high potential of MsAcT of catalyzing condensation reactions in a sustainable manner.
Author contributions
Conceptualization, M. C. and M. L. C.; methodology, M. C. and M. L. C.; investigation M. C. and M. L. C.; resources, A. P. and S. D.; data curation, M. C., M. L. C.; writing – review and editing, all authors. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Notes and references
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- J. Boström, D. G. Brown, R. J. Young and G. M. Keserü, Nat. Rev. Drug Discovery, 2018, 17, 709–727 CrossRef PubMed.
- S. K. Sharma, A. S. R. Paniraj and Y. B. Tambe, J. Agric. Food Chem., 2021, 69, 14761–14780 CrossRef CAS PubMed.
- L. Bering, E. J. Craven, S. A. Sowerby Thomas, S. A. Shepherd and J. Micklefield, Nat. Commun., 2022, 13, 1–10 Search PubMed.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–442 RSC.
- V. Gotor, Bioorg. Med. Chem., 1999, 7, 2189–2197 CrossRef CAS.
- M. R. Petchey and G. Grogan, Adv. Synth. Catal., 2019, 361, 3895–3914 CrossRef CAS.
- A. Brugging, E. C. Roos and E. De Vroom, Org. Process Res. Dev., 1998, 2, 128–133 CrossRef.
- A. Zadło-Dobrowolska, N. G. Schmidt and W. Kroutil, Chem. Commun., 2018, 54, 3387–3390 RSC.
- H. Müller, A. K. Becker, G. J. Palm, L. Berndt, C. P. S. Badenhorst, S. P. Godehard, L. Reisky, M. Lammers and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2020, 59, 11607–11612 CrossRef.
- C. Elend, C. Schmeisser, C. Leggewie, P. Babiak, J. Carballeira, H. L. Steele, J.-L. Reymond, K.-E. Jaeger and W. R. Streit, Appl. Biochem. Biotechnol., 2013, 169, 15–28 CrossRef PubMed.
- H. Müller, S. P. Godehard, G. J. Palm, L. Berndt, C. P. S. Badenhorst, A. K. Becker, M. Lammers and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 2013–2017 CrossRef PubMed.
- M. L. Contente, A. Pinto, F. Molinari and F. Paradisi, Adv. Synth. Catal., 2018, 360, 4814–4819 CrossRef CAS.
- F. Annunziata, M. L. Contente, D. Betti, C. Pinna, F. Molinari, L. Tamborini and A. Pinto, Catalysts, 2020, 10, 1–8 CrossRef.
- M. L. Contente, S. Farris, L. Tamborini, F. Molinari and F. Paradisi, Green Chem., 2019, 21, 3263–3266 RSC.
- D. R. Padrosa and M. L. Contente, Tetrahedron Lett., 2021, 86, 153453 CrossRef CAS.
- C. Pinna, P. A. Martino, G. Meroni, V. M. Sora, L. Tamborini, S. Dallavalle, M. L. Contente and A. Pinto, J. Agric. Food Chem., 2022, 70, 223–228 CrossRef CAS PubMed.
- M. L. Contente, D. Roura Padrosa, F. Molinari and F. Paradisi, Nat. Catal., 2020, 3, 1020–1026 CrossRef CAS.
- I. Mathews, M. Soltis, M. Saldajeno, G. Ganshaw, R. Sala, W. Weyler, M. A. Cervin, G. Whited and R. Bott, Biochemistry, 2007, 46, 8969–8979 CrossRef CAS PubMed.
- N. de Leeuw, G. Torrelo, C. Bisterfeld, V. Resch, L. Mestrom, E. Straulino, L. van der Weel and U. Hanefeld, Adv. Synth. Catal., 2018, 360, 242–249 CrossRef CAS.
- L. Mestrom, J. G. R. Claessen and U. Hanefeld, ChemCatChem, 2019, 11, 2004–2010 CrossRef CAS.
- I. Chiarelli Perdomo, S. Gianolio, A. Pinto, D. Romano, M. L. Contente, F. Paradisi and F. Molinari, J. Agric. Food Chem., 2019, 67, 6517–6522 CrossRef PubMed.
- M. L. Contente, L. Tamborini, F. Molinari and F. Paradisi, J. Flow Chem., 2020, 10, 235–240 CrossRef CAS.
- P. A. Marks and R. Breslow, Nat. Biotechnol., 2007, 25, 84–90 CrossRef CAS PubMed.
- M. B. Richardson, K. N. Gabriel, J. Garcia, S. Ashby, J. Kim, C. Lau, J. Hong, R. J. Le Tourneau, S. Sen, D. Narel, B. B. Katz, J. W. Ziller, S. Majumdar, P. G. Collins and G. A. Weiss, Bioconjugate Chem., 2020, 31, 1449–1462 CrossRef CAS PubMed.
- Y. Ma, X. Yang, H. Han, Z. Wen, M. Yang, Y. Zhang, J. Fu, X. Wang, T. Yin, G. Lu, J. Qi, H. Lin, X. Wang and Y. Yang, Bioorg. Chem., 2021, 111, 104872 CrossRef CAS PubMed.
- K. Voos, E. Schönauer, A. Alhayek, J. Haupenthal, A. Andreas and C. Ducho, ChemMedChem, 2021, 16, 1257–1267 CrossRef CAS PubMed.
- P. Hermant, D. Bosc, C. Piveteau, R. Gealageas, B. Lam, C. Ronco, M. Roignant, H. Tolojanahary, L. Jean, P. Renard, M. Lemdani, M. Bourotte, A. Herledan, C. Bedart, A. Biela, F. Leroux, B. Deprez and R. Deprez-poulain, J. Med. Chem., 2017, 60, 9067–9089 CrossRef CAS PubMed.
- E. D. D. Calder, A. Skwarska, D. Sneddon, L. K. Folkes, I. N. Mistry, S. J. Conway and E. M. Hammond, Tetrahedron, 2020, 76, 131170 CrossRef CAS.
- V. Corpas-López, M. Tabraue-Chávez, Y. Sixto-López, S. Panadero-Fajardo, F. Alves De Lima Franco, J. F. Domínguez-Seglar, F. Morillas-Márquez, F. Franco-Montalbán, M. Díaz-Gavilán, J. Correa-Basurto, J. López-Viota, M. López-Viota, J. Pérez Del Palacio, M. De La Cruz, N. De Pedro, J. Martín-Sánchez and J. A. Gómez-Vidal, J. Med. Chem., 2020, 63, 5734–5751 CrossRef PubMed.
- C. B. Botta, W. Cabri, E. Cini, L. De Cesare, C. Fattorusso, G. Giannini, M. Persico, A. Petrella, F. Rondinelli, M. Rodriquez, A. Russo and M. Taddei, J. Med. Chem., 2011, 54, 2165–2182 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc00655c |
| ‡ These authors contributed equally to the paper. |
| This journal is © The Royal Society of Chemistry 2022 |