Life cycle assessment of multistep benzoxazole synthesis: from batch to waste-minimised continuous flow systems†
Jose
Osorio-Tejada
 a,
Francesco
Ferlin
a,
Francesco
Ferlin
 b,
Luigi
Vaccaro
b,
Luigi
Vaccaro
 b and
Volker
Hessel
b and
Volker
Hessel
 ac
ac
aDepartment of Chemical Engineering, School of Engineering, University of Warwick, Coventry, UK. E-mail: volker.hessel@adelaide.edu.au
bLaboratory of Green S.O.C. – Dipartimento di Chimica, biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, 06123 – Perugia, Italy. E-mail: luigi.vaccaro@unipg.it; Web: http://greensoc.chm.unipg.it/
cSchool of Chemical Engineering and Advanced Materials, University of Adelaide, Adelaide, Australia
First published on 29th November 2021
Abstract
In this contribution we have focused on the progress of synthesis methods for the preparation of 2-aryl benzoxazoles as highly interesting materials with increasing relevance in the pharmaceutical industry as well as in optical applications. The traditional production methods of 2-aryl benzoxazoles clearly have some drawbacks related to the use of strong acids and/or toxic reagents leading to a large production of waste. Importantly, comprehensive analysis of the associated risk in terms of safety, environmental impact and disposal cost is lacking. In this regard, the life cycle assessment (LCA) methodology is herein applied to ultimately evaluate the environmental profile of the available routes to access 2-aryl benzoxazoles. Seven batch synthesis approaches and two continuous-flow (CF) approaches (small and large scale) are closely compared. The superiority of the CF technology is ultimately proven among the analysed environmental impact categories. The main finding is that the oxygen-flow chemistry intensification fortified the sustainability of the green chemistry principles (towards the catalyst/solvent) themselves by ensuring the regeneration of OMS catalysts and reduction of manganese leaching to the minimum by the CPME solvent, which also provided high solvent recyclability. In this way, it adds circularity in the sense of its 10R framework (e.g. R standing for recycle, repair, rethink, and refuse). As a result, for example, our flow approach reduces carbon emissions by 85% in comparison with our batch approach, the latter exhibiting lower environmental impact than the six batch approaches from the literature. In addition, our flow chemistry process has lower energy consumption and solvent load, whose share is up to 88% of the environmental impact.
1. Introduction
Most of the common green chemistry metrics such as E-factor, reaction mass efficiency (RME), and atom economy (AE)1–4 are useful tools to focus on and measure a specific parameter of a process in order to optimize its efficiency and minimize the associated environmental impact. Slightly wider metrics such as EcoScale5 or environmental quotient (EQ)6 can evaluate the environmental profiles of processes or materials but provide limited information on their potential environmental burden and toxicity during and after usage. In the real world, for obtaining a precise picture of the actual environmental impact, there is always a more stringent need for a comprehensive tool able to analyse and quantify the environmental impacts of materials and energy usage, from the initial access to the feedstock to the final disposal of waste. This is true relevant in the production of bulk or fine chemicals.7–10Life cycle assessment (LCA) methodology11 fulfils these requirements and evaluates the environmental impacts of water, air, and soil emissions in different areas of protection, such as ecosystems, human health, and resources. In the search for solutions to optimise the sustainability of a chemical production process, a careful comprehensive assessment is needed to individuate the most promising routes.
The most promising route is the use of innovative enabling technologies that have proven to be effective in opening new production routes and offering effective energetic and practical solutions as is the case of flow chemistry.12–14 This technology has shown to be able to open new scenarios in terms of control of reactivity or downstream process manipulation. Indeed, by performing the reactions in flow, advantages such as the increase in energy and mass transfer or the reduction or elimination of wasteful purification steps make flow procedures generally appealing. Moreover, flow chemistry can be a bridge between academic experiments and industrial processes because it generally provides safe, efficient, reproducible, and scalable chemical reaction methods.15
Anyway, it is still very rare to find reports on the actual assessment of sustainability advantages achieved by using flow technology compared to the classic batch approach. In fact, the cases where chemists quantify the advances achieved in flow by calculating appropriate mass green metrics are very rare16–21 and it is very difficult to find more complete examples where chemists and/or engineers quantify the efficiency of a production strategy in flow using LCA22–25 and circular economy assessments.26–29
To evaluate the actual advances achieved in flow and therefore its possible implementation in real cases, this step forward is needed and LCA and circular economy assessments are needed. While green chemistry means to look at the efficiency of the present, the newly defined flow process, LCA means to look at the present and at the past of the process, evaluating not only the actual existing flow process but also its “ecological backpack”. Finally, circularity also means to additionally look at the future of the flow process including the possible market use and recovery and recycling of the materials.
Therefore, it is important to carry out appropriate LCA assessments on processes realized in flow and possibly highlight the limits or the opportunities available when moving from batch to flow in order to make the final step including circularity in the process.
For these reasons we report the present study on the detailed LCA of the available procedures for the synthesis of 2-aryl benzoxazoles comparing the batch and flow approaches aiming at highlighting the importance of every single parameter and component of the processes focusing on the pros and cons of different solutions (Scheme 1).
Benzoxazoles are molecular entities of general interest, given the increasing use of this class of scaffolds in the pharmaceutical industry.30,31 In particular, 2-aryl benzoxazole derivatives have shown potential antitumor, antimicrobial, and inhibitory activities,32–38 as well as noteworthy properties for optical applications.39–44 Traditional methods for obtaining 2-substituted benzoxazoles, such as the condensation of 2-aminophenol and carboxylic acid45 or the oxidative cyclization of phenolic Schiff bases,46 have drawbacks related to the use of strong acids, toxic reagents and harsh reaction conditions, and the generation of dangerous peroxides and salt waste.47–51
In the last decade, many methods have been proposed for the optimization of 2-aryl benzoxazole synthesis featuring improvements in terms of reaction time, waste generation, milder temperature or pressure, and avoidance of dangerous compounds,52–57 but none of them have thus far demonstrated the reduction of the environmental impacts in a comprehensive and analytical manner.
Recent works have used classic green mass-balance indicators to demonstrate the efficiency of the process in the usage of resources such as atom and carbon economy,58 yield,59 reaction and effective mass efficiency,60 the BioLogicTool plots61 or the environmental factor (E-factor),62 where e.g. a low E-factor indicates low waste generation.
However, when the reduction of wastes implies a change in the production method (temperature, pressure, time, etc.) or materials (catalysts, bases, additives, and solvents), environmental impacts could increase or emerge due to the increase in the energy usage in the process or during the extraction, production, and distribution of new materials. For instance, the traditional route by cyclization of phenolic Schiff bases uses manganese triacetate, a moderately toxic reagent, in a batch process with toluene at 110 °C for 1 hour.46 This process was optimized by Khalafi-Nezhad and Panahi in 201454 by replacing the toxic reagent with the base triethylenediamine, a relatively benign compound, but increasing the residence time to 24 hours, which requires extra energy and, hence, potentially higher negative environmental impacts.
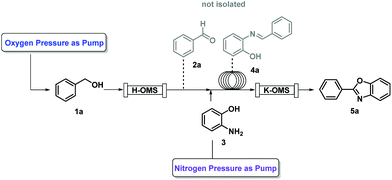
Recently, we have proposed a novel, safe and fast waste-minimized reaction protocol for 2-aryl benzoxazole synthesis promoted by heterogeneous mixed valence manganese octahedral molecular sieves (OMS) and cyclopentyl methyl ether (CPME)63 (Scheme 2). The OMS catalyst and its molecular structure improve the reactivity and selectivity of the system, while the use of CPME as the reaction medium allowed a minimal loss of 0.002% of manganese. This protocol, applied in a continuous flow (CF) system using oxygen flow, ensured complete regeneration of the catalyst resulting in high durability and reusability with excellent product yields and good hourly productivity. In terms of environmental impacts, besides the benefits of the use of CPME64 such as low safety hazard, stability under either basic or acidic conditions, and low tendency to produce peroxides,65 its easy and almost complete recovery by distillation implies a very low additional solvent input in each synthesis process, and also its relatively low enthalpy of vaporization might imply low energy requirements for its recovery. Moreover, the improved reaction yield promoted by the manganese OMS catalyst would reduce the emissions related to materials waste. Also, the use of the environmentally friendly and abundant O2 and N2 in the CF protocol could reduce the energy requirements for distillation and regeneration of the catalysts after the synthesis process.
However, the above benefits of the strategies proposed in the novel protocol must be demonstrated by quantitative comprehensive and trustworthy indicators as the LCA methodology.
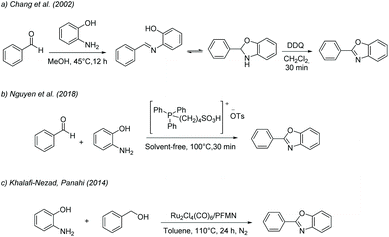
To date, none of the different routes for benzoxazole synthesis have been evaluated through an LCA study. Therefore, the main aim of this study is to evaluate the extent to which the strategies proposed for this waste-minimized reaction protocol for 2-aryl benzoxazole synthesis, promoted by the manganese OMS systems and CPME (Scheme 2), reduced the global warming potential and the overall environmental impact of this product through the life cycle perspective. Additionally, the LCA results for this protocol are benchmarked against other optimised and conventional batch synthesis processes, as summarized in Table 1. The approaches of Chang et al. (2002),52 Nguyen et al. (2018),53 and Khalafi-Nezhad and Panahi (2014)54 were selected due to the similarity to our process because they also use aminophenol (3) as the starting reagent with benzaldehyde (2a).
| Approach | Reference | Approach characteristics | Remarks | Green Chemistry principles (ref. 66) | Circularity Principles (10R framework) (ref. 67) | |
|---|---|---|---|---|---|---|
| Pathway | Process Time/Temp. | |||||
| Ours | Ferlin et al. (2019)63 | Flow via manganese OMS systems and CPME medium and O2 and N2 gas | 78 min/106 °C | Safe, fast, and waste-minimized reaction protocol with direct catalyst regeneration | 1, 4, 5, 6, 9, 12 | Recycling R8, repair R4, rethink R1, reduce R2, redesign |
| Ferlin et al. (2019)63 | Flow via manganese OMS systems and CPME medium and O2 and N2 gas | 24 h/106 °C | Safe, fast, and waste-minimized reaction protocol with direct catalyst regeneration, large scale production | 1, 4, 5, 6, 9, 12 | Recycling R8, repair R4, rethink R1, reduce R2, redesign | |
| Ferlin et al. (2019)63 | Batch via manganese OMS systems in CPME medium | 70 min/106 °C | Safe, fast, and waste-minimized reaction protocol | 1, 4, 5, 6, 9 | Recycling R8, rethink R1 | |
| Optimised | Chang et al. (2002)52 | Batch via DDQ in methanol and DCM medium | 12 h/45 °C | Mild and efficient protocol | 3 | Reduce R2 |
| Nguyen et al. (2018)53 | Batch via phosphonium acidic ionic liquid in a solvent-free medium | 30 min/100 °C | Fast solvent-free reaction | 5 | Refuse R0 | |
| Khalafi and Panahi (2014)54 | Batch via Ru-based catalyst with DABCO as the base in toluene medium | 24 h/110 °C | Chemical diversity: applicability to a wide substrates scope protocol | — | Rethink R1 | |
| Conventional | Saha et al. (2009)55 | Batch via CuO nanoparticles in DMSO and potassium carbonate medium | 16 h/110 °C | Chemical diversity: applicability to a wide substrates scope protocol. Efficient method | — | Rethink R1 |
| Praveen et al. (2008)56 | Batch via silica supported pyridinium chlorochromate (PCC) in dichloromethane | 30 min/room temperature | Mild process | 3 | Reduce R2 | |
| Wang et al. (2013)57 | Batch via acyl chloride with chlorobenzene and water in potassium carbonate medium | 12 h/140 °C | New reaction path: alternative arylation method | 8 | Rethink R1 | |
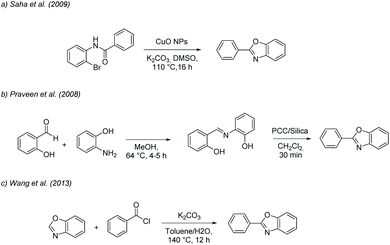
These processes can be referred to as “optimised” as they feature several advances in terms of sustainability such as the use of a recoverable catalyst or ligand, or the adoption of solvent-free conditions (Scheme 3). On the other hand, the approaches of Saha et al. (2009),55 Praveen et al. (2008)56 and Wang et al. (2013)57 were selected as they can be considered representative of many common strategies as the material used are very common in these transformations.
The analysis of each proposed approach in the literature relies on the development of new chemistry ideas by providing an attributive LCA pointing at hotspots, which can be useful for creating a combination of concrete green process chemistry proposals (e.g., unusual process temperature, new catalysts, recycling concepts, and green reactants) to be introduced in future scalable experiments for the synthesis of any compound.
In the remainder of this article, we describe the LCA study methods and their application to our approach for 2-aryl benzoxazole synthesis for the CF and batch experiments.63 We also describe in detail the selected three similarly optimized protocols,52–54 and the other three different synthesis routes.55–57 The results of the critical comparative assessment and the scenario analyses are presented and also discussed.
2. Methods
This study followed the four phases described in the LCA methodology.12 Firstly, the goal, scope and typology of the study are defined. Then, the procedures for the development of materials and energy flow inventories are detailed for each of the synthesis processes, as well as the followed methods and assumptions. Subsequently, the impact assessment method and the interpretation of results are described.2.1 Goal and scope definition
The goal of this LCA study was to assess the environmental impacts of different routes for the synthesis of 2-aryl benzoxazoles proposed in the literature from conventional batch processes to waste-minimized CF systems. In this study a total of nine different synthesis approaches were analysed. The assessed synthesis proposals were set only to produce 2-aryl benzoxazoles, without by-products. Hence, a cut-off approach system model based on mass allocation was defined. The functional unit was defined as 1 g of the final product. The system boundaries were defined based on a cradle-to-gate approach, considering the emissions and resource usage for the extraction, production and transportation of all materials and energy, and the emissions to water, air, and soil from the process itself.The LCA studies did not include the manufacturing of manual or computer support tools, machinery, or equipment such as reactors, heat exchangers, pumps, cylinders, etc. Regarding the data quality, in order to maintain consistency and low uncertainty, European datasets for general industry practices were referred for all process flows. The system functions definitions for each evaluated process are explained below.
The first analysed pathways were the protocols based on the use of heterogeneous manganese systems with CPME and oxygen (depicted in Scheme 2).63 These processes from benzyl alcohol and aminophenol were firstly tested in batch using CPME as the reaction medium and manganese OMS as catalysts with a residence time of 70 min at 106 °C, affording 94% yield. From this optimized approach a multistep CF process was proposed using compressed nitrogen and oxygen as the terminal oxidants, which besides ensuring a complete regeneration of the catalyst, allows the maintenance of a continuous production flow rate up to 2.3 g per hour with 98% yield. For these batch, CF small scale and CF multigram scale experiments, the obtained product quantities (and residence times) were 0.037 g (70 min), 0.708 g (78 min), and 53.5 g (24 h), with E-factors of 42, 6.4, and 1.7, respectively. These experiments reached high yields, even without using the wasteful silica-gel column purification step required for the other considered protocols.
Subsequently, the following approaches were selected for the LCA comparison as they can be considered representative of many common strategies as the materials used (catalysts, bases, additives, and solvents) are very common in these transformations.
The first approach, detailed in Saha et al. (2009),55 is a general and efficient method from haloanilide based on frequently used solvent dimethyl sulfoxide (DMSO), using a common Cu-based catalyst (CuO nanoparticles) in potassium carbonate for 16 h at 110 °C, affording 95% yield, (Scheme 4a). The catalyst can be recovered by centrifugation, washing, and drying under vacuum, without a significant loss in its properties.
The second approach by Praveen et al. (2008),56 is a mild process based on heterogeneously catalysed oxidative cyclization of a preformed imine (Schiff's base from o-aminophenol and salicylaldehyde refluxed in methanol for 4–5 h) and the use of a suspension of silica supported pyridinium chlorochromate (PCC) in dichloromethane for 30 min at room temperature, affording 91% yield, (Scheme 4b).
The third strategy by Wang et al. (2013),57 is an alternative arylation method for preformed benzoxazoles using acyl chloride as the coupling partner and chlorobenzene and water in potassium carbonate for 12 h at 140 °C, affording 82% yield (Scheme 4c).
2.2 Inventory analysis
The elaboration of inventories for each of the nine synthesis processes is a time-consuming task due to the lack of data in most experimental procedures. Indeed, generally, the energy utilized for heating, cooling, or stirring procedures is not measured or documented in the publications. In addition, some experiments neither quantify the water or solvents used for washing the products, nor the quantity of the outputs of these unreacted compounds. For these reasons, in order to avoid assumptions, we estimated the quantity of energy and materials that were not documented for each assessed process.Another difficulty in the development of inventories for each process was the availability of some specific compounds in life cycle inventory (LCI) databases. In the present study we used the most recent and complete database Ecoinvent v3.7.1, in which, a total of 12 compounds were not available in the database, being necessary to create new inventories to be included in the processes modelling. A simple solution can be data extrapolation from similar available compounds or the use of generic inventories, such as “market for chemical, organic”, which are not generally recommended68 since average impacts might be distant from its real environmental profile. Another solution for this lack of information can be the use of retrosynthetic analysis to find simpler precursor compounds that might be available in the database and assuming a yield of 95% for quantity estimation via stoichiometric equations.69 However, through retrosynthetic analysis, the quantities of catalysts, reagents, solvents, and energy used cannot be estimated, but are necessary to make assumptions, and this increases uncertainty. Therefore, for the creation of new inventories, we used a process-specific method by performing a deep literature review to find detailed descriptions to produce the target compounds, selecting the most basic or conventional synthesis process when different approaches were found for the same compound.
For all unavailable compounds which had to be specifically modelled, general assumptions were made regarding facilities and transportation, considering an organic chemical plant of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t production per year with a 50 years lifespan and transport distances of 100 km by lorry > 16 t and 600 km by train.69,70 The process energy of 2 MJ in the form of steam and 0.333 kW h of electricity per kg of the compound was assumed.68,71 The energy for lighting and heating offices was excluded in this study. For the experiments and precursor inventories, a solvent recovery of 71% was considered,72 except for CPME in the manganese OMS system route in which a 98% recovery rate was obtained. Solvent usage in the purification step by column chromatography of 1 mL per gram of product was assumed. When the solvent names were not specified, a mixture of 0.5 mL of petroleum ether and 0.5 mL of ethyl acetate was used.
000 t production per year with a 50 years lifespan and transport distances of 100 km by lorry > 16 t and 600 km by train.69,70 The process energy of 2 MJ in the form of steam and 0.333 kW h of electricity per kg of the compound was assumed.68,71 The energy for lighting and heating offices was excluded in this study. For the experiments and precursor inventories, a solvent recovery of 71% was considered,72 except for CPME in the manganese OMS system route in which a 98% recovery rate was obtained. Solvent usage in the purification step by column chromatography of 1 mL per gram of product was assumed. When the solvent names were not specified, a mixture of 0.5 mL of petroleum ether and 0.5 mL of ethyl acetate was used.
The energy needed for heating and stirring the mixtures was directly measured with an electric monitor in our experiments for 2-aryl benzoxazole synthesis via heterogeneous manganese systems. In addition, the necessary energy was estimated thermodynamically, considering also heat losses by radiation, from which we determined an electrical efficiency of 59.4% for the heating plate. Therefore, the energy for other synthesis processes was thermodynamically estimated and adjusted with this efficiency rate, assuming that in all the experiments a similar heating device was used. These energy expenses were estimated for the complete reported procedures for each experiment, including all required workup and materials recovery. Regarding the energy for stirring, our measures found that the power consumption of the stirring motor only depends on the defined speed, that is, the consumption is the same regardless of the quantity or the density of the mixture. In this sense because each experiment uses different quantities, to establish fair energy allocation, a default additional power of 3.2 W (measured at 700 rpm) was assumed for 10 g of the mixture and consequently extrapolated to each experiment.
In the inventories, were also quantified, the emissions to air during the synthesis processes (0.2% volatile input materials)69 and air and water (river) emissions after wastewater treatment; no emissions to the soil were determined since no agricultural destination of the digested sludge was considered. In this wastewater treatment, 65.8% of the organic compounds were retained in the sludge, 24.5% were oxidized and emitted to air basically in the form of CO2, and the remaining 9.7% were released into the river.73 Nitrogen compounds during the sludge digestion are mostly released into air as N2, while NO2, NH3, and N2O emissions are negligible, thus not inventoried in this study.74 Transfer coefficients to sludge and effluent of inorganic compounds were based on the elimination rates for each specific element.73,75,76
The elaborated inventories for the nine compared synthesis processes for 2-aryl benzoxazoles and the twelve unavailable precursor compounds (CPME, K-OMS, H-OMS, dichlorodicyanobenzoquinone (DDQ), phosphonium acidic ionic liquid, triethylenediamine (DABCO), ruthenium(I) carbonyl complex (Ru2Cl4(CO)6, N-(2-bromophenyl)benzamide, copper oxide (CuO) nanoparticles, imine Schiff's base, silica supported pyridine-chlorochromate (PCC), and benzo[d]oxazole) are detailed in the ESI1.†
2.3 Impact assessment and interpretation
The impact assessment was performed using software SimaPro 9.2 and the method ReCiPe 2016.77 Among the included impact categories in ReCiPe, ten relevant impact categories in chemical production were considered:78,79 global warming, ozone formation (on human health), ozone formation (on terrestrial ecosystems), terrestrial acidification, freshwater eutrophication, terrestrial ecotoxicity, freshwater ecotoxicity, human carcinogenic toxicity, human non-carcinogenic toxicity, and fossil resource scarcity. Midpoint impact categories and endpoint damage areas (human health, ecosystems, and resources) under the hierarchical perspective (100 years) were analysed. Long-term emissions, which affect scenarios beyond 100 years, were excluded due to their high uncertainties80–83 and their relationship to heavy metal toxicity, therefore not very relevant in organic chemical processing.84 Among the impact categories, special attention was paid to global warming, ozone formation and freshwater ecotoxicity because they are greatly affected by emissions to air and water caused by material waste during the synthesis processes.The results for the CF small scale protocol are presented and analysed in midpoints and compared to results from the batch experiment, which were also performed on small scales. Subsequently, the results are weighted and normalized in endpoint damage areas to compare our approach against others by a single indicator as a benchmark of the global environmental impact. The endpoint or damage assessment refers to the potential impacts of each impact category in protection areas such as human health, ecosystem quality and fossil resource scarcity. In this process, midpoint characterization results are converted to intermediate units to be weighted and normalized to represent, in micropoints (μPts), the relative impact of the results according to their severity in a global context. In this endpoint comparison, the CF multigram experiment was also included to see how higher materials efficiency impacts the LCA results.
Given that the assumed rate for solvents recovery and power of stirring mixtures could generate high uncertainty, the extent to which these assumptions affect the results is assessed through sensitivity analyses.
3. Results and discussion
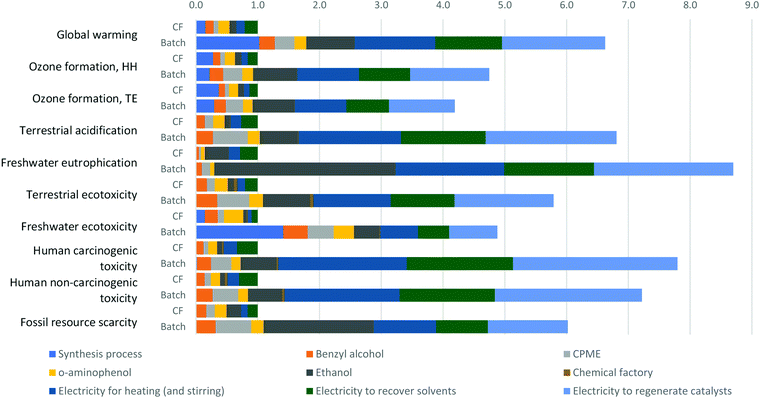
The contributions of each material and energy input for the environmental impact categories of our CF small scale approach for producing one gram of 2-aryl benzoxazoles are depicted in Fig. 1. The contributions of K- and H-OMS catalysts are not shown in the charts because their contributions in all impact categories were insignificant. The shares of different inputs in all impact categories were very diverse. The impacts of the process itself (in light blue) are predominant in global warming due to the CO2 emitted by the oxidation of solvent wastes in the wastewater treatment process; in the ozone formation category mainly due to the volatile emissions of CPME; and freshwater ecotoxicity due to small fraction of waste released to rivers which are not retained in the wastewater process. The energy spent for solvent recovery (in green) and heating (in dark blue) have relevant contributions, especially in the categories of terrestrial acidification, freshwater eutrophication, and human toxicity, where both inputs account for around half of the impact.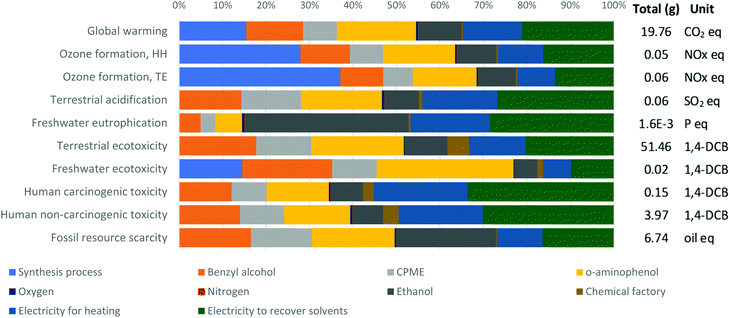 | ||
| Fig. 1 ReCiPe midpoint characterization shares – 1 g of 2-aryl benzoxazoles via the CF small scale OMS system. | ||
The production of most of the materials utilized in the process had relevant contributions, where o-aminophenol (in yellow) had the highest impacts in most of the impact categories, except for the categories of freshwater eutrophication and fossil resource scarcity, in which ethanol production has higher impacts. The O2 and N2 released into air do not cause negative impacts, but their production and transportation do. However, the contributions of O2 and N2 were very low, i.e., 0.341% and 0.093%, respectively, of the total 19.76 g CO2 eq. emitted per functional unit. Besides their low impacts, the use of these environmentally friendly gases in the CF protocol allowed the automatic regeneration of the catalyst, unlike the batch process, where the catalysts must be separated from the product and solvents and be placed in a Schlenk pressure tube and heated at 100 °C during 1 h to regenerate them.
For the above reasons, in order to analyse the extent to which the CF protocol allows the reduction of the environmental impact, the totals shown in Fig. 2 were normalized to 1.0 and compared to the batch experiment following the same OMS based route shown in Fig. 2.
From Fig. 2 we can affirm that the strategy of introducing a CF process using O2 and N2 can reduce carbon emission by 85%, and, in general, obtain environmental impact reductions between 76% (e.g., ozone formation, TE) and 89% (e.g., freshwater eutrophication). As expected, the energy required to recover the catalysts (in light purple) had very relevant contributions in most of the impact categories for the batch approach. This energy consumption, together with the energy spent for solvent recovery and heating (and stirring), represents most of the impacts, except for the category of freshwater ecotoxicity where energy shares 38.8% of the impact. In this category, as well as in global warming, the impact of the batch process itself has relevant contributions mainly due to the fraction of unreacted benzyl alcohol that is released to rivers and the CO2 emitted for the oxidation of ethanol wastes.
In general, the high impacts of the batch process due to the energy consumption in the categories related to toxicity issues are associated with copper used in the electricity distribution network. Although both processes are performed at the same temperature, the energy inputs for heating and recovering solvents are higher in the batch approach because in this process more quantities of solvents are used per functional unit than in the CF process.
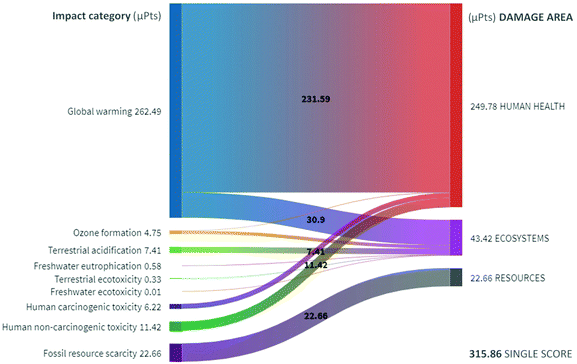
In both approaches the high recovery rate of 98% for the CPME made the contribution of its production and wastes very low in the LCA of the manganese OMS system. Though this route requires a higher process temperature (106 °C) than other mild reaction approaches, therefore it is possible that other protocols will be more environmentally friendly than the proposed method. In this sense, to perform a simple comparison of the overall environmental impacts, the nine approaches are assessed by the ReCiPe endpoint method throughout a weighted aggregation in a single score.
Firstly, the impact category contribution to damage areas for the CF small scale process is presented in Fig. 3, where 83% of the total 315.86 μPts are final impacts on human health. The global warming impact category contributes to 93% and 71% of the total damage to human health and ecosystems, respectively. This high contribution is because global warming greatly affects the availability of water for drinking and food production, causing damage to human health and living species.
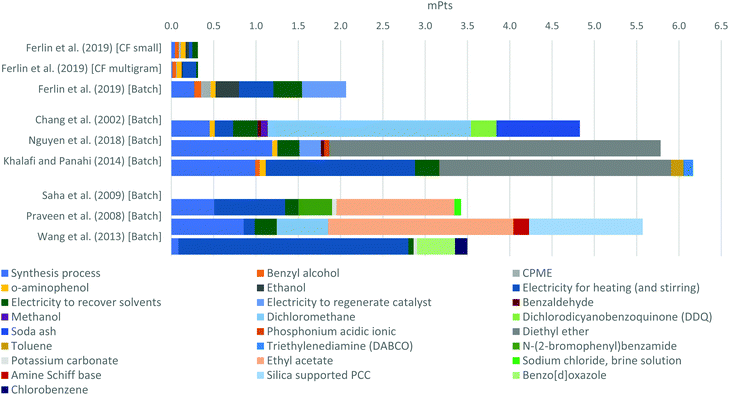
The global warming impact category was also the main contributor to the single scores in other evaluated approaches in this study. Therefore, the endpoint analysis basically represents the benchmark of the approaches on the global warming impacts. In Fig. 4, the previous two processes, including the results for a CF multigram scale, are compared against the other six batch approaches proposed in the literature. To reduce the complexity of the chart, inputs with contributions lower than 0.5% of the total single score were not included, such as the chemical factory, solvents used in column chromatography, and catalysts in most of the approaches. Solvents used in the reaction or for washing the product were not grouped since in some approaches they had high contributions, being relevant for the identification of the specific solvent name.
Despite the better E-factor of the CF multigram scale experiment, this experiment obtained the same environmental score as the small scale one. This was because the multigram scale experiment was performed in a long 24 h run, therefore, the lower impacts generated by the higher material usage efficiency were compensated by the higher energy usage for heating the system for more time. In this sense, the contribution of energy for heating increased from 13.4% to 51.7%. However, this high share of the heating input in this approach gives broad opportunity for reducing its environmental impact through the use of clean energy sources to heat the system, since in this case the energy used came from a European electricity mix which generates significant negative impacts due to its distribution networks and the use of 38% from fossil energy sources.85
Among the batch approaches, the one using the manganese OMS system achieved the best environmental profile. Similar to the CF multigram approach, the majority of the impacts in this approach are energy related, making it possible to reduce its impacts even more. Yet, in the scenario where zero impact of energy inputs was assumed, the protocol proposed by Wang et al.57via acyl chloride would be the best route among the batch approaches since in this case the energy input shares 80% of the impacts due to the required 12 h of heating and stirring for the reaction at 140 °C.
The approach using phosphonium liquid53 requires high temperatures, but the energy consumption share was very low because it is a fast reaction process. This shows the relevance of time reaction in energy consumption where stirring also had relevant impacts such as 22% share of the total energy related impacts in the Wang et al. (2013)57 approach.
The fast and mild approaches where energy contribution was very low also show opportunity for further optimizations due to the high impacts of solvents used for the reactions and for washing the final products. This is evident in the Nguyen53et al. approach using phosphonium liquid where 88% of the impacts are related to the high quantity of diethyl ether used to wash the product and its related emissions due to its corresponding wastes. Similarly, the mild approaches via DDQ52 and PCC56 have very low impacts related to energy consumption, but they have high impacts because of the use of solvents, such as dichloromethane (49.5% share in the DDQ approach) and ethyl acetate (39% share in the PCC approach).
According to the previous analyses, among the nine benchmarked approaches, the experiment with the worst environmental profile, performed by Khalafi & Panahi54 (2014) using the Ru-catalyst, would exhibit significant impact reduction if clean energy and solvent optimization are applied. However, in this experiment, as well as in the other approaches affected by the solvent usage and their process wastes,52,55–57 assumptions of lower solvent quantities usage cannot be guessed because it depends on each experiment. Yet, these high solvent usages impact could be reduced by up to 66% if higher recovery rates were assumed, such as 90%. That is, the solvent wastes would be reduced from 0.29 to 0.10 kg kg−1. Also, this reduction in solvent waste would automatically decrease the emissions of the synthesis processes since a lower quantity of organic compounds would end in wastewater treatment processes.
Additionally, it is important to note that further optimization in the energy quantity can be obtained by a modification of the assumption made for the stirring input. The stirring motor consumption was assumed 3.2 W for a default quantity of 10 g of the mixture and extrapolated to each quantity. Otherwise, if considering the same stirring power for 14.1 g (in Praveen et al., (2008)56), and for 0.5 g (in Wang et al., (2013)57), the energy required per gram of product in the last experiment would be unfairly overestimated. However, although this assumption allowed a fair allocation of this input to the functional unit, the contribution of this input in each approach could change if the default stirred quantity or the assumed speed change.
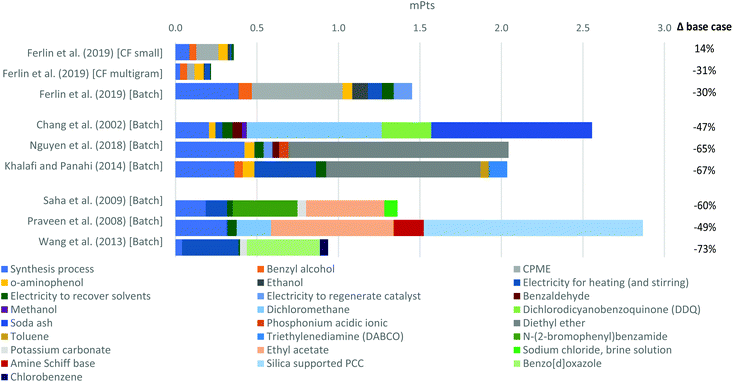
In accordance with the above, a scenario is proposed with a combination of input optimizations that would benefit all the approaches to different degrees through a sensitivity analysis. In this sense, the recovery rate of the solvent was improved from 71% to 90%. In this regard, to balance the experimental conditions, the 98% recovery rate for CPME in the manganese OMS systems was reduced to 90%, but at the same time the assumed recovery rate for the ethanol used in this route was improved. Regarding the energy for stirring, this input was optimized by assuming that 3.2 W was consumed for stirring 40 g instead of 10 g of mixture. In addition, the energy from grid electricity was replaced by solar photovoltaic electricity. The results for this scenario are presented in Fig. 5.
All the approaches obtained significant improvements in their environmental profiles, except for the CF small scale approach because it was more affected by the reduction of the CPME recovery rate than by the improvement of the ethanol recovery rate, as well as using solar electricity since the energy contribution was not very high. In contrast, given that in the CF multigram scale approach the energy had relevant contribution, this approach showed the best environmental impact in this scenario.
Among the batch approaches, the route via acyl chloride obtained the highest reduction rate because this approach had very high contribution of the energy used for heating and stirring. The other route via the Ru-catalyst also had a very high reduction rate, but to a lower degree because this approach does not need stirring and the electricity for heating did not have as much contribution as the solvent. Although the considered electricity from multi-Si solar photovoltaic 570 kWp open ground installation does not have zero environmental impacts, it has about 78% lower environmental impacts than the European grid electricity (compared on single scores from the endpoint results for the impact categories considered in this study), which make this input the main contributor to impact reductions.
Regardless of the important impact reductions caused by the 90% recovery rate, the fraction of new dichloromethane, diethyl ether or ethyl acetate that must be added in each run still has large contributions to the environmental impacts.
4. Conclusions
This study allowed us to perform a hotspot analysis of the comprehensive environmental impacts of different routes for 2-aryl benzoxazole synthesis through the LCA methodology. It compares our flow chemistry approach, its batch equivalent, and six different batch approaches reported in the literature; three being similarly optimised and the other three different synthesis routes. Our main purpose was to show how innovations in green chemistry and circularity motivation finally result in real sustainability benefits. It helps in the economic growth of flow chemistry, as an industry that increasingly needs to support its developments and products with a good environmental profile.The analysed batch approaches, including ours, root in sustainability thinking steered by the 12 Principles of Green Chemistry and related, later formulated, more complex green chemistry concepts. Yet, with our batch process we go a step further. First, we prepare to benefit from circularity approaches, by allowing recycling of the solvent and reuse of the catalyst we employ (as measures of circularity's 10R framework). This is fully exploited in our flow approach and, in addition, we profit from process intensification by microfluidic processing by virtue of maximising transport processes. The outcomes achieved underline that a holistic and up-to-date approach, both on the chemistry and chemical engineering/process tool side, can massively optimise the environmental friendliness, in our case by a trifold sustainability approach. The 12 Principles of Green Chemistry are meanwhile three decades old and were majorly postulated to enhance the safety of chemical plant operation on the background of chemical pollution and even plant operation disasters at that time. Even microreactors and flow chemistry turn to be almost two decades old and achieve a better process outcome when guided by sustainability principles and assessment.
One key green principle employed is the use of an advanced catalyst, based on manganese OMS systems. OMS catalysts have been claimed to improve reactivity and selectivity, thus showing high productivity. The use of oxygen flow and its favourable mass transfer by means of multiphase microfluidics ensure the regeneration of catalysts. Another key green principle employed is the use of an advanced solvent, CPME, proposed as an environmentally friendly solvent reaction medium, which exhibits only minimal metal leaching from catalysts and has high solvent reusability. The results of this optimised synthesis approach were benchmarked against the other three recently optimised approaches and three conventional routes presented in the literature. These different approaches have been proposed basically in order to replace the use of strong acids and toxic reagents and to reduce waste with the use of recyclable materials. However, our life-cycle results show that the benefits of each optimisation are undermined by high environmental impacts due to the production of new materials or the higher energy usage for additional heating and stirring requirements. The life-cycle impact assessment also shows that inefficient material usage (by low yield) causes a secondary negative contribution to the environment, besides producing waste. The waste additionally increases global warming, ozone formation and freshwater ecotoxicity due to the emissions to air and water after the synthesis processes. Besides the recovery rate, the quantity and type of solvents used are relevant: altogether this sums up to 88% of the environmental impact in the process based on a phosphonium acidic ionic liquid and using diethyl ether to separate the product.
The flow chemistry approach generated an average of 20 g CO2 eq. per gram of 2-aryl benzoxazole, which represents an 85% emission reduction in comparison with the batch equivalent approach. This is caused by the common advantage of continuous flow approaches to provide higher yields than the batch ones. A specific flow advantage is the use of environmentally friendly O2 and N2 flows, which reduce the energy consumption to heat the mixtures and to regenerate the catalyst. These features are even evident for the equivalent batch approach, using the same manganese OMS systems and CPME solvent. In a scenario analysis it is observed that even if the solvent disadvantage is taken away from the other six approaches (by assuming a higher solvent recovery rate), the high energy related impacts still cause an overall inferior environmental profile. Only when assuming using renewable energy and 90% solvent recovery rate, one batch approach was finally much better than the others. Yet, in this scenario, the scaled-up flow approach obtained the best results given the ease of solvent load optimisation per unit of reagents in this multigram experiment.
As an outlook, the main future promise lies in the reduction of the solvent quantity and replacement of the current solvents dichloromethane, diethyl ether, and ethyl acetate with greener solvent types.
Author contributions
J. Osorio-Tejada: Writing – original draft, investigation, conceptualization, methodology. F. Ferlin: Visualization, investigation. L. Vaccaro: Writing – review & editing. V. Hessel: Writing – reviewing and editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Università degli Studi di Perugia and MIUR are acknowledged for financial support to the project AMIS, through the program “Dipartimenti di Eccellenza – 2018-2022”.Notes and references
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov and C. H. Lam, et al. , Green Chem., 2018, 20, 1929–1961 RSC.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2(3) DOI:10.1186/1860-5397-2-3.
- E. Heinzle, D. Weirich, F. Brogli, V. H. Hoffmann, G. Koller, M. A. Verduyn and K. Hungerbühler, Ind. Eng. Chem. Res., 1998, 37, 3395–3407 CrossRef CAS.
- G. Wernet, S. Conradt, H. P. Isenring, C. Jiménez-González and K. Hungerbühler, Int. J. Life Cycle Assess., 2010, 15, 294–303 CrossRef CAS.
- W. De Soete, J. Dewulf, P. Cappuyns, G. Van Der Vorst, B. Heirman, W. Aelterman, K. Schoeters and H. Van Langenhove, Green Chem., 2013, 15, 3039–3048 RSC.
- J. Provo, J. Fava and S. Baer, Curr. Opin. Chem. Eng., 2013, 2, 278–281 CrossRef.
- M. Margallo, A. Dominguez-Ramos and R. Aldaco, Educ. Chem. Eng., 2019, 26, 89–96 CrossRef.
- ISO, ISO 14040:2006. Environmental management—Life cycle assessment—Principles and framework, vol. 2006. 2006.
- V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, ChemSusChem, 2013, 6, 746–789 CrossRef CAS.
- L. Vaccaro, D. Lanari, A. Marrocchi and G. Strappaveccia, Green Chem., 2014, 16, 3680–3704 RSC.
- F. Ferlin, D. Lanari and L. Vaccaro, Green Chem., 2020, 22, 5937–5955 RSC.
- S. Newman and K. Jensen, Green Chem., 2013, 15, 1456–1472 RSC.
- A.-C. Bedard, A. R. Longstreet, J. Britton, Y. Wang, H. Moriguchi, R. W. Hicklin, W. H. Green and T. F. Jamison, Bioorg. Med. Chem., 2017, 25, 6233–6241 CrossRef CAS.
- C. Dai, D. R. Snead, P. Zhang and T. F. Jamison, J. Flow Chem., 2015, 5, 133–138 CrossRef.
- M. G. Russel and T. F. Jamison, Angew. Chem., Int. Ed., 2019, 58, 7678–7681 CrossRef.
- F. Ferlin, D. Sciosci, F. Valentini, G. Cravotto, J. Menzio and K. Martina, Green Chem., 2021, 23, 7210–7218 RSC.
- F. Ferlin, F. Valentin, G. Brufani, D. Lanari and L. Vaccaro, ACS Sustainable Chem. Eng., 2021, 9, 5740–5749 CrossRef CAS.
- F. Ferlin, I. Anastasiou, L. Carpisassi and L. Vaccaro, Green Chem., 2021, 23, 6576–6582 RSC.
- V. Hessel, M. Escribà-Gelonch, J. Bricout, N. N. Tran, A. Anastasopoulou, F. Ferlin, F. Valentini, D. Lanari and L. Vaccaro, ACS Sustainable Chem. Eng., 2021, 9, 9508–9540 CrossRef CAS.
- V. Klug, J.-P. Schöggl, D. Dallinger, K. Hiebler, C. Stueckler, A. Steiner, A. Arzt, C. O. Kappe and R. J. Baumgartner, ACS Sustainable Chem. Eng., 2021, 9, 8980–8989 CrossRef CAS.
- A. A. Lapkin and P. Yaseneva, Life cycle assessment of flow chemistry processes, in Sustainable flow chemistry, ed. L. Vaccaro, Wiley-VCH, Weinheim, 2017, pp. 249–276 Search PubMed.
- P. Yaseneva, C. F. Marti, E. Palomares, X. Fan, T. Morgan, P. S. Perez, M. Ronning, F. Huang, T. Yuranova, L. KiwiMinsker, S. Derrouiche and A. A. Lapkin, Chem. Eng. J., 2014, 248, 230–241 CrossRef CAS.
- D. Ott, S. Borukhova and V. Hessel, Green Chem., 2016, 18, 1096–1116 RSC.
- V. Hessel and L. Vaccaro, Chim. Oggi – Chem. Today, 2021, 39, 16 Search PubMed.
- M. Escriba-Gelonch, J. Bricout and V. Hessel, ACS Sustainable Chem. Eng., 2021, 9, 1867–1879 CrossRef CAS.
- Q. H. Pho, M. Escriba-Gelonch, D. Losic, E. V. Rebrov, N. N. Tran and V. Hessel, ACS Sustainable Chem. Eng., 2021, 9, 4755–4770 CrossRef CAS.
- C. S. Demmer and L. Bunch, Eur. J. Med. Chem., 2015, 97, 778–785 CrossRef CAS.
- H. Z. Zhang, Z. L. Zhao and C. H. Zhou, Eur. J. Med. Chem., 2018, 144, 444–492 CrossRef CAS.
- Ö. Temiz, I. Ören, E. Şener, I. Yalçin and N. Uçartürk, Farmaco, 1998, 53, 337–341 CrossRef PubMed.
- T. Aotsuka, N. Wagatsuma, H. Kato and N. Ashizawa, Pyrazole derivatives and cox inhibitors containing them, WO/1998/046594, 1998.
- M. R. DeLuca and S. M. Kerwin, Tetrahedron Lett., 1997, 38, 199–202 CrossRef CAS.
- D. W. Dunwell, D. Evans and T. A. Hicks, J. Med. Chem., 1975, 18, 1158–1159 CrossRef CAS PubMed.
- E. Oksuzoglu, B. Tekiner-Gulbas, S. Alper, O. Temiz-Arpaci, T. Ertan, I. Yildiz, N. Diril, E. Sener-Aki and I. Yalcin, J. Enzyme Inhib. Med. Chem., 2008, 23, 37–42 CrossRef CAS.
- E. Oksuzoglu, O. Temiz-Arpaci, B. Tekiner-Gulbas, H. Eroglu, G. Sen, S. Alper, I. Yildiz, N. Diril, E. Aki-Sener and I. Yalcin, Med. Chem. Res., 2008, 16, 1–14 CrossRef.
- D. Kumar, M. R. Jacob, M. B. Reynolds and S. M. Kerwin, Bioorg. Med. Chem., 2002, 10, 3997–4004 CrossRef CAS.
- M. A. Fourati, T. Maris, W. G. Skene, C. G. Bazuin and R. E. Prudhomme, J. Phys. Chem. B, 2011, 115, 12362–12369 CrossRef CAS.
- A. Ghodbane, S. D'Altério, N. Saffon, N. D. McClenaghan, L. Scarpantonio, P. Jolinat and S. Fery-Forgues, Langmuir, 2012, 28, 855–863 CrossRef CAS.
- T. Ogura, K. T. Yamaguchi, Y. Shibasaki and M. Ueda, Polym. J., 2007, 39, 245–251 CrossRef CAS.
- T. Ogoshi, J. Miyake and Y. Chujo, Macromolecules, 2005, 38, 4425–4431 CrossRef CAS.
- S. Park, S. Kim, J. Seo and S. Y. Park, Macromolecules, 2005, 38, 4557–4559 CrossRef CAS.
- P. Chen, Y. Xu, W. Du, G. Zhang, X. Chen and Z. An, Mol. Cryst. Liq. Cryst., 2014, 592, 44–62 CrossRef CAS.
- Y. H. So and J. P. Heeschen, J. Org. Chem., 1997, 62, 3552–3561 CrossRef CAS.
- R. S. Varma and D. Kumar, J. Heterocycl. Chem., 1998, 35, 1539–1540 CrossRef CAS.
- J. Peng, C. Zong, M. Ye, T. Chen, D. Gao, Y. Wang and C. Chen, Org. Biomol. Chem., 2011, 9, 1225–1230 RSC.
- O. Koleda, T. Broese, J. Noetzel, M. Roemelt, E. Suna and R. Francke, J. Org. Chem., 2017, 82, 11669–11681 CrossRef CAS.
- A. Patra, A. James, T. K. Das and A. T. Biju, J. Org. Chem., 2018, 83, 14820–14826 CrossRef CAS PubMed.
- C. Yu, X. Guo, B. Shen, Z. Xi, Q. Li, Z. Yin, H. Liu, M. Muzzio, M. Shen, J. Li, C. T. Seto and S. Sun, J. Mater. Chem. A, 2018, 6, 23766–23772 RSC.
- J. He, F. Lin, X. Yang, D. Wang, X. Tan and S. Zhang, Org. Process Res. Dev., 2016, 20, 1093–1096 CrossRef CAS.
- J. Chang, K. Zhao and S. Pan, Tetrahedron Lett., 2002, 43, 951–954 CrossRef CAS.
- Q. T. Nguyen, A. H. Thi Hang, T. L. Ho Nguyen, D. K. Nguyen Chau and P. H. Tran, RSC Adv., 2018, 8, 11834–11842 RSC.
- A. Khalafi-Nezhad and F. Panahi, ACS Catal., 2014, 4, 1686–1692 CrossRef CAS.
- P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719–8725 CrossRef CAS PubMed.
- C. Praveen, K. H. Kumar, D. Muralidharan and P. T. Perumal, Tetrahedron, 2008, 64, 2369–2374 CrossRef CAS.
- L. Wang, X. Ren, J. Yu, Y. Jiang and J. Cheng, J. Org. Chem., 2013, 78, 12076–12081 CrossRef CAS PubMed.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259–281 CrossRef CAS.
- R. H. Petrucci, F. G. Herring, J. D. Madura and C. Bissonnette, in General Chemistry: principles and modern applications, 10th, Pearson Canada, Toronto, Ontario, 2010 Search PubMed.
- T. Hudlicky, Chem. Rev., 1996, 96, 3–30 CrossRef CAS PubMed.
- Y. Lie, P. Ortiz, R. Vendamme, K. Vanbroekhoven and T. J. Farmer, Ind. Eng. Chem. Res., 2019, 58, 15945–15957 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- F. Ferlin, M. K. Van Der Hulst, S. Santoro, D. Lanari and L. Vaccaro, Green Chem., 2019, 21, 5298–5305 RSC.
- U. Azzena, M. Carraro, L. Pisano, S. Monticelli, R. Bartolotta and V. Pace, ChemSusChem, 2019, 12, 40–70 CrossRef CAS PubMed.
- M. Shang, T. Noel, Y. Su and V. Hessel, Ind. Eng. Chem. Res., 2016, 55, 2669–2676 CrossRef CAS.
- P. T. Anastas and J. Warner, 12 Principles of Green Chemistry, in Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 30 Search PubMed.
- P. Morseletto, Targets for a circular economy, in Resour. Conserv. Recycl, vol. 153, p. 104553, 2020, DOI:10.1016/J.RESCONREC.2019.104553.
- G. Wernet, S. Hellweg and K. Hungerbühler, Int. J. Life Cycle Assess., 2012, 17, 720–728 CrossRef CAS.
- R. Hischier, S. Hellweg, C. Capello and A. Primas, Int. J. Life Cycle Assess., 2005, 10, 59–67 CrossRef CAS.
- R. Frischknecht, N. Jungbluth, H. J. Althaus, G. Doka, R. Dones, T. Heck, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer and M. Spielmann, Int. J. Life Cycle Assess., 2005, 10, 3–9 CrossRef CAS.
- Gendorf, Environmental declaration of the chemical plants at the Gendorf site in Burgkirchen, Germany., 2000.
- C. Capello, S. Hellweg, B. Badertscher and K. Hungerbühler, Environ. Sci. Technol., 2005, 39, 5885–5892 CrossRef CAS PubMed.
- G. Doka, Life Cycle Inventories of Waste Treatment Services. ecoinvent report No. 13. Swiss Centre for Life Cycle Inventories, Dübendorf, 2009, 2009.
- A. Köhler, S. Hellweg, E. Recan and K. Hungerbühler, Environ. Sci. Technol., 2007, 41, 5515–5522 CrossRef PubMed.
- P. Koppe and A. Stotzek, Kommunales Abwasser. Vulkan Verlag, Essen, Germany, 1993 Search PubMed.
- M. Boller and M. Hafliger, Verbleib von Schwermetallen bei unterschiedlicher Meteorwasserentsorgung, Gas Wasser Abwasser, No.1/1996/Zurich, Switzerland, 1996 Search PubMed.
- M. A. J. Huijbregts et al. , ReCiPe 2016 v1.1. A harmonized life cycle impact assessment method at midpoint and endpoint level. Report I: Characterization, Bilthoven, 2017. [Online]. Available: http://www.rivm.nl/en.
- S. Maranghi and C. Brondi, Life Cycle Assessment in the Chemical Product Chain, Springer, Siena, 2020 Search PubMed.
- O. M. Morales-Gonzalez, M. Escribà-Gelonch and V. Hessel, Int. J. Life Cycle Assess., 2019, 24, 2111–2127 CrossRef CAS.
- E. Igos, R. Mailler, R. Guillossou, V. Rocher and J. Gasperi, J. Cleaner Prod., 2021, 287, 125067 CrossRef CAS.
- L. Zampori, E. Saouter, E. Schau, J. Cristobal, V. Castellani and S. Sala, Guide for interpreting life cycle assessment result, Luxembourg, 2016, DOI:10.2788/171315.
- S. A. Morais and C. Delerue-Matos, J. Hazard. Mater., 2010, 175, 12–22 CrossRef CAS.
- L. Rigamonti, A. Falbo, L. Zampori and S. Sala, Int. J. Life Cycle Assess., 2017, 22, 1278–1287 CrossRef.
- I. Bakas, M. Z. Hauschild, T. F. Astrup and R. K. Rosenbaum, Int. J. Life Cycle Assess., 2015, 20, 1444–1455 CrossRef CAS.
- BP, Electricity production from fossil fuels, nuclear and renewables, Europe, in BP Statistical Review of World Energy, 2020. https://ourworldindata.org/grapher/elec-fossil-nuclear-renewables?country=~Europe (accessed Jul. 25, 2021) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03202j |
| This journal is © The Royal Society of Chemistry 2022 |