Green preparation of holocellulose nanocrystals from burdock and their inhibitory effects against α-amylase and α-glucosidase†
Ying
Li‡
c,
Wei
Liang‡
ac,
Meigui
Huang
 b,
Wuyang
Huang
c and
Jin
Feng
b,
Wuyang
Huang
c and
Jin
Feng
 *c
*c
aSchool of Food and Biological Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang 212013, China
bDepartment of food science and engineering, College of light industry and food engineering, Nanjing forestry university, 159 Longpan Road, Nanjing 210037, China
cInstitute of Agro-product Processing, Jiangsu Academy of Agricultural Sciences, 50 Zhongling Street, Nanjing 210014, China. E-mail: fengjinzju@163.com; Fax: +86-25-84392334; Tel: +86-25-84392334
First published on 8th November 2021
Abstract
In this work, holocellulose nanocrystals (hCNCs) were isolated from burdock insoluble dietary fiber (IDF) by enzymatic hydrolysis and ultrasonic treatment and their inhibitory effects against α-amylase and α-glucosidase were investigated. The hydrodynamic diameter of hCNCs decreased from about 600 to 200 nm with increasing sonication time, accompanied by an improvement in cellulose and glucose contents. Steady-state fluorescence studies suggested that static complexes were formed between hCNCs and α-amylase or α-glucosidase via a spontaneous and endothermic approach, which was driven by both hydrophobic interactions and hydrogen bonding. The median inhibitory concentration (IC50) values of hCNCs against the tested enzymes were positively correlated with their size, and non-competitive and mixed types of inhibition were detected using the Lineweaver–Burk plots. During the simulated digestion, the inclusion of burdock hCNCs obviously retarded the starch hydrolysis in both dose- and size-dependent manners, suggesting their potential in blocking the postprandial serum glucose upsurge.
1. Introduction
According to the definition of the Codex Alimentarius Commission (CAC), dietary fibers (DF) are carbohydrate polymers with ten or more monomeric units that could not be hydrolyzed by endogenous enzymes in the human intestine. DF have been reported to improve satiety, regulate intestinal flora, decrease blood glucose and fat levels, and reduce the risk of cardiovascular diseases of humans.1 The disintegration of insoluble DF (IDF) via chemical (e.g., acid hydrolysis and oxidation), physical (e.g., ultrasonic treatment, high pressure homogenization, and ball milling) or enzymatic (e.g., cellulase hydrolysis) approaches to produce nanocellulose was proven to be an effective way to convert naturally abundant biomass into functional ingredients.2 According to their origin and morphology, nanocellulose can be divided into three categories: cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and bacterial cellulose nanofibrils (BCNFs). The small size, high aspect ratio, and unique crystalline structure endow NCs with special mechanical properties, and they have been widely applied to reinforce and construct nanocomposites, aerogels, active packaging films, and Pickering emulsions.3 Besides, it was revealed that NCs presented superior health-benefiting effects to their parent materials. For example, Yan et al. (2018) suggested that the oil absorption capacities of CNFs and IDF prepared from rice straw were 13.5 and 5.0 g oil per g, respectively;4 the weight of dry feces excreted in a 24 h period by rats fed with CNFs was 20% higher than that of the IDF group. Similarly, a more recent work suggested that 0.75% (w/w) CNCs in heavy fat could reduce over 40% of lipid digestion in vitro, while this value decreased to less than 20% when the CNCs were replaced by DF.5 Besides, about 36% reduction in the postprandial rise in serum triglycerides in healthy rats was observed when the high-fat foods were co-ingested with 1.0% (w/w) of CNCs.The prevalence of type-2 diabetes and the related syndrome has become a public health concern globally.6 Type-2 diabetes is characterized by insulin resistance in the target tissue resulting in reduced glucose absorption. The inhibition of the postprandial serum glucose upsurge is one of the most efficient ways to improve insulin sensitivity. α-Amylase and α-glucosidase are the key enzymes in the small intestine involved in the digestion of starch.7 α-Amylase hydrolyzed the α-(1→4)-glycosidic linkage in starch leading to the formation of maltodextrin products, whereas α-glucosidase catalyzed the cleavage of disaccharides into monosaccharide such as glucose and fructose. Recent studies have proven that NCs, especially the rod-like CNCs with a diameter between 5 and 20 nm and length below 1000 nm, showed potential inhibitory activities against α-amylase and α-glucosidase. Nsor-Atindana and co-authors (2019) revealed that the IC50 values of CNCs of 174.41 nm length and 4.25 nm diameter were 11.36 and 14.60 mg mL−1, respectively, for α-amylase and α-glucosidase inhibition.8 Besides, they could reduce the initial hydrolysis rate of starch by 70.83% during the in vitro digestion of a starch-protein food model, which was favorable for the inhibition of the serum glucose upsurge after the diet. In another work, the authors reported that 0.2% (w/w) of CNCs reduced the activities of α-amylase or α-glucosidase by 10–50%, and they could decrease the fraction of rapidly digestible starch (RDS) and slowly digestible starch (SDS), while improving that of resistant starch (RS) in corn, pea, and potato powders during the simulated digestion test.9
Compared with the more popular natural α-amylase and α-glucosidase inhibitors, such as flavonoids, anthocyanins, carotenoids, polysaccharides, and saponins, CNCs hold advantages owing to their abundant natural resources and high physicochemical stability, which were beneficial for the preservation of their inhibitory effects during product processing, storage, and digestion.7 However, though many green mechanistic methodologies have been applied in the fabrication of CNCs, sulfuric hydrolysis still serves as the predominant approach as it results in high yield and as it is less time-consuming.2,3 Besides, consecutive treatments with alkaline, sodium chlorite, and/or H2O2 were required to remove the hemicellulose and lignin fractions in the IDF, which may cause environmental pollution and restrict their utilization in the food industry.3 There are still demands for green, facile, and easy industrialized approaches. In our preliminary tests, we found that freshly harvested burdock, a traditional Chinese plant that could be utilized in the medical and food industries,10 contains over 20% (w/w) of IDF in dry basis, and only a trace amount of lignin (<5%) was detected in the IDF. We speculated nanomaterials can be fabricated without the delignification process of burdock IDF, and the as-prepared nanomaterials can be defined as holocellulose nanocrystals (hCNCs). Similarly, holocellulose nanomaterials were also prepared in a recent work by ball milling of okara containing a trace amount of lignin as a whole.11
In this work, burdock hCNCs were prepared by combining enzymatic hydrolysis and ultrasonic treatments. The effects of the time of ultrasonic treatment on the physicochemical properties and monosaccharide composition of hCNCs were investigated. The steady-state fluorescence, Fourier-transform infrared (FT-IR), circular dichroism (CD), and UV spectra were utilized to characterize the interactions between burdock hCNCs and α-amylase or α-glucosidase. Besides, the inhibitory effects of burdock hCNCs against the tested enzymes were evaluated and the inhibitory type was revealed using the Lineweaver–Burk plots. Lastly, the starch digestion kinetics in the presence of burdock hCNCs was recorded and analyzed using a fractional conversion model. Information from this work contributed to the development of edible functional ingredients from naturally abundant IDF for the control of postprandial serum glucose levels.
2. Materials and methods
2.1. Materials and reagents
Burdock roots were harvested from Feng County, Xuzhou City, P. R. China, in November 2020. α-Amylase (from pig pancreatin, type VI-B, ≥5 units per mg solid), α-glucosidase (from Saccharomyces cerevisiae, type I, ≥10 units per mg protein), xylanase (from Trichoderma viride, 100–300 units per mg protein), and cellulase (from Trichoderma sp., ≥5000 units per g solid) were purchased from Sigma-Aldrich Corp. (St Louis, USA). Potato starch and p-nitrophenol-α-D-glucopyranoside (p-PN-G) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All other chemicals were of analytical grade and used as purchased.2.2. Preparation of burdock hCNCs
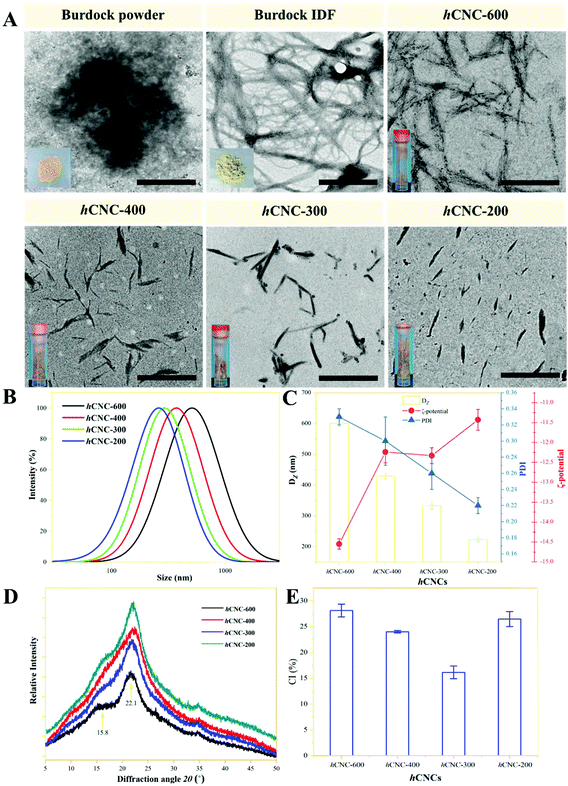
Fresh burdock was sliced and immediately immersed in boiling water to inactivate polyphenol oxidase. Thereafter, 100 g of the sample was mixed with 500 mL of water and pulped thoroughly. The pulp was consecutively treated with 0.15 g of α-amylase (at 60 °C and pH 4.5 for 40 min), 0.20 g of glucoamylase (at 60 °C and pH 6.0 for 40 min), and 1.0 g of papain (at 50 °C and pH 6.0 for 60 min) to remove the starch and protein. Each enzymatic treatment was terminated by boiling the samples in a water bath for 5 min. Centrifugation at 5000g and 10 min affords the burdock IDF as light grey sediment. The IDF was rinsed with water and ethanol several times to remove the trace impurities. Five grams of lyophilized burdock IDF powder was dispersed within 250 mL of citric acid-sodium citrate buffer (50 mM, pH 4.8) containing xylanase (2000 U mL−1) and cellulase (3000 U mL−1), and the mixture was incubated at 50 °C for 24 h under constant stirring of 600 rpm. The sample was then boiled for 10 min to inactivate the enzymes. Afterwards, ultrasonic treatment at 600 W for 0, 1.0, 2.0, or 5.0 h was applied to further liberate CNCs from burdock IDF and reduce their particle size. The mixture was centrifuged at 1000g for 10 min to remove the precipitate. The remaining supernatant was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min three times to collect the sediment, which was rinsed and freeze-dried to afford hCNCs. In general, the hydrodynamic diameter (DZ) of burdock hCNCs decreased with the time of ultrasonic treatment, that is, 600.63, 429.72, 333.85, and 224.19 nm, respectively, at 0, 1.0, 2.0, and 2.0 h (Fig. 1C). Therefore, the hCNCs were nominated as hCNC-600, hCNC-400, hCNC-300, and hCNC-200, respectively.
000g for 30 min three times to collect the sediment, which was rinsed and freeze-dried to afford hCNCs. In general, the hydrodynamic diameter (DZ) of burdock hCNCs decreased with the time of ultrasonic treatment, that is, 600.63, 429.72, 333.85, and 224.19 nm, respectively, at 0, 1.0, 2.0, and 2.0 h (Fig. 1C). Therefore, the hCNCs were nominated as hCNC-600, hCNC-400, hCNC-300, and hCNC-200, respectively.
2.3. Physical properties of burdock hCNCs
For transmission electron microscopy (TEM) observation, an aliquot (20 μL) of the sample was dropped onto the surface of a carbon Formvar-coated copper grid for at least 1 min, after which one drop of aqueous phosphotungstic acid (2.0 wt%) was added to stain the samples for 15–20 s. Then, the excess solution was wicked away using filter paper, and the copper grid was washed with ultrapure water and dried in air for at least 12 h. The morphology of the sample was observed using a JEM-1230 (HR) microscope at a working voltage of 200 kV.The Z-average diameter (DZ), ζ-potential, and polydispersity index (PDI) of the hCNCs (0.01%, w/w) were determined using a commercial Nano ZS90 Zetasizer (Malvern Instrument Ltd, Malvern, UK). Each parameter was calculated as the average of at least triplicate measurements, and each measurement was obtained from the mean of at least 10 readings for a sample.
The XRD pattern of the hCNCs was recorded using an X-ray diffractometer (D8 Advance, Bruker, Germany) using a Cu Kα radiation source at 40 kV and 100 mA. Scattered radiation was detected in the 2θ range of 5–60° at a scan rate of 4° min−1. The crystallinity index (CI) was calculated as follows:12
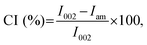 | (1) |
2.4. Composition analysis of the burdock hCNCs
The protein content in the samples was determined using the Dumas combustion method (Rapid N cube, Elementar, Germany) with an N factor of 6.25. The ash content was determined according to the AOAC method. To determine the monosaccharide composition of the IDF or CNCs, the samples were hydrolyzed by trichloroacetic acid at 121 °C for 2 h at first. The resultant hydrolysate was dissolved in ultrapure water, filtered through a 220 nm membrane, and finally analyzed using a Thermo ICS5000+ instrument (Thermo Fisher Scientific, USA) coupled with a Dionex™ CarboPac™ PA10 column (250 × 4.0 mm, 10 μm). The content of each monosaccharide was calculated according to the calibration curve of the corresponding standard.The cellulose content was determined as follows. One gram of the sample (w1) was placed into a sand core crucible, after which 150 mL of preheated H2SO4 (1.25 wt%) and 3–5 droplets of octanol were added. The mixed solution was kept boiling for 30 min. Then, the residue was isolated by vacuum filtration and washed with ultrapure water until the pH of the filtrate was neutral. Then, 150 mL of preheated NaOH solution (1.25 wt%) and several droplets of n-caprylic alcohol were added to the residue, and the mixture was kept boiling for 30 min. Afterward, hydrolysis fluid was discarded and the residue was washed with ultrapure water until the pH of the filtrate was neutral. Fifteen milliliters of acetone were added to rinse the residues and the solvent was separated and discarded via vacuum filtration afterwards, and the process was repeated three times. The residues were heated at 105 °C until the weight (w2) was constant. Thereafter, the residues were transferred to a muffle furnace and heated at 550 °C for 4 h, and the sample was weighed after cooling to ambient temperature (w3). The percentage of cellulose in CNCs was calculated as follows:
| Cellulose (%) = (w2 − w3)/w1 × 100. | (2) |
The hemicellulose content in CNCs was calculated as the difference between the contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF). To determine the NDF content, 1.0 g of samples (w4) was added to a sand core crucible at first. Then, 100 mL of the neutral detergent (18.6 g EDTA, 6.8 g sodium borate decahydrate, 30 g sodium lauryl sulfate, 10 mL triethylene glycerol, and 4.56 g disodium hydrogen phosphate per 1 L) and 2–3 droplets of octanol were added. The mixture was kept boiling for 1 h. The filtrate isolated by vacuum filtration was discarded, and the remaining residue was washed with preheated ultrapure water until no foam was observed in the filtrate. Thereafter, the residue was rinsed with acetone three times and the solvent was removed by vacuum filtration. The sand core crucible containing the sample was heated at 105 °C until the weight reached a constant value (w5). Thereafter, the residues were transferred to a muffle furnace and heated at 550 °C for 4 h, and the sample was weighed (w6) after cooling to ambient temperature. The percentage of NDF in the samples was calculated as follows:
| NDF (%) = (w6 − w5)/w4 × 100. | (3) |
The procedure for ADF determination was same as that for NDF determination with the exception of using an acid detergent (1000 mL of 1.0 M H2SO4 and 20 g of cetyltrimethylammonium bromide). The sample was weighed and coded as w7. After acid detergent hydrolysis, the sand core crucible containing the sample was weighed and coded as w8. After heating at 550 °C for 4 h, the sand core crucible containing the sample was weighed and coded as w9. The percentage of ADF was calculated as follows:
| NDF (%) = (w6 − w5)/w4 × 100. | (4) |
The hemicellulose content was calculated as follows:
| Hemicellulose (%) = NDF (%) − ADF (%). | (5) |
The content of lignin was calculated as follows. One gram of the samples (w7) was added to a sand core crucible, which was then treated with an acid detergent as described above. Afterward, H2SO4 (12 M) was added to the sand core crucible and thoroughly mixed with the residue. The mixture was allowed to react at ambient temperature for 3 h. The liquid was removed by vacuum ultrafiltration and the solid residue was rinsed with hot water until the pH of the filtrate was neutral. The sand core crucible containing the residue was transferred to the oven and heated at 105 °C until the weight was constant (w8). Then, the sample was heated at 550 °C for 4 h. The sand core crucible containing ash was weighed and coded as w9. The percentage of ADL was calculated using the following equation:
| Lignin (%) = (w9 − w8)/w7 × 100. | (6) |
2.5. Steady-state fluorescence analysis
The steady-state fluorescence spectra of α-amylase (4.0 mg mL−1) or α-glucosidase (1.0 mg mL−1) in the presence of different concentrations of hCNCs (0, 0.5, 1.0, 2.0, 3.0 and 4.0 mg mL−1) were recorded using a Cary Eclipse fluorescence spectrometer (Agilent Technologies Co., Santa Clara, USA) at 25 °C. The excitation wavelength was set at 280 nm and the emission spectra were scanned over the range of 300–500 nm. The excitation and emission slit widths were set at 5 nm and 10 nm, respectively.The quenching of the enzyme fluorescence by hCNCs could be categorized into two types: collisional (dynamic) quenching and binding-related (static) quenching. The quenching process can be described using the following Stern–Volmer equation:13
| F0/F = 1 + kqτ0[Q] = 1 + Ksv [Q], | (7) |
For a static quenching process, when the ligand binds independently to a set of equivalent slits on proteins, the binding constant (Kb) and the number of binding sites (n) were calculated based on the following modified Stern–Volmer equation:
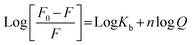 | (8) |
The thermodynamic parameters involved in the hCNC–enzyme interaction herein were calculated according to the van't Hoff equation:14
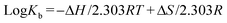 | (9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kbversus 1/T, respectively. The free energy changes (ΔG) were calculated utilizing the following equation:
Kbversus 1/T, respectively. The free energy changes (ΔG) were calculated utilizing the following equation:| ΔG = ΔH − TΔS. | (10) |
2.6. FT-IR, UV, and circular dichroism (CD) measurements
The FT-IR spectra of the pure enzymes and the same in the presence of different hCNCs were recorded using a Tensor 27 instrument (Bruker Co., Karlsruhe, Germany), using a KBr disk with 1% finely ground samples. The spectra were recorded in the range of 400–4000 cm−1 at a resolution of 4 cm−1. The UV spectra of the pure digestive enzymes and the same with different hCNCs were recorded using a UV-6300 spectrophotometer from 220 to 320 nm. The CD spectra of the pure enzymes and the same in the presence of different concentrations of hCNCs were recorded using a J-815 CD spectrometer (JASCO, Tokyo, Japan), using a quartz cuvette of 1.0 mm path length at 50 nm min−1 and 25 °C. All samples were prepared with 10 mM PBS (pH 7.0) to obtain a final protein concentration of 0.2 mg mL−1. The composition of the secondary structure was analyzed using the online program DichroWeb.2.7. Inhibitory activities of burdock hCNCs against α-amylase and α-glucosidase
In brief, 1.0 mL of each hCNC suspension of different concentrations (0.5–8 mg mL−1) was mixed with an equal volume of α-amylase solution (4.0 mg mL−1, pH 7.0 ± 0.2). The mixture was magnetically stirred in an incubator at 600 rpm and 37 °C for 10 min. The hydrolysis was initiated by adding 1.0 mL of potato starch (5.0%), after which the reaction was allowed to proceed under constant stirring for another 20 min. The digestion was terminated by adding 1.0 mL of Na2CO3 (0.2 M). The mixture was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800g for 5 min to separate the supernatant, which was diluted appropriately with ultrapure water and mixed with 2.0 ml of 5-dinitrosalicylic acid (DNS) reagent. The thermal treatment of the mixture at 100 °C for 5.0 min afforded red brown products, and the content of the reduced sugar in the supernatant was proportional to the absorbance of the products at 540 nm. The calibration curve with high linearity was constructed using maltose as the standard.
800g for 5 min to separate the supernatant, which was diluted appropriately with ultrapure water and mixed with 2.0 ml of 5-dinitrosalicylic acid (DNS) reagent. The thermal treatment of the mixture at 100 °C for 5.0 min afforded red brown products, and the content of the reduced sugar in the supernatant was proportional to the absorbance of the products at 540 nm. The calibration curve with high linearity was constructed using maltose as the standard.
The α-glucosidase inhibitory activity of hCNCs was determined according to a previous method with some modifications.15 Solutions of hCNCs of different concentrations (0.25–4.0 mg mL−1), α-glucosidase (1.0 mg mL−1), and p-NP-G (5.0 mM) were prepared using phosphate buffered saline (PBS, 100 mM, pH 6.9) as the solvent. Five hundred milliliters of α-glucosidase were mixed with an equal volume of hCNC solution, and the mixture was incubated at 37 °C for 15 min under magnetic stirring. Then, 500 μL of p-NP-G was added to each reaction vial and further incubated at 37 °C for 20 min. The reaction was terminated by adding 1.0 mL of sodium carbonate (1.0 M), after which the mixture was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. The absorbance of the supernatant at 405 nm was recorded using a microplate reader (Model 550, Bio-Rad, USA).
000g for 5 min. The absorbance of the supernatant at 405 nm was recorded using a microplate reader (Model 550, Bio-Rad, USA).
The inhibition rate of α-amylase (α-glucosidase) was calculated using the following equation:
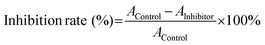 | (11) |
Herein, Acontrol and Ainhibitor represent the absorbance of the supernatant in the absence and presence of hCNCs, respectively.
The IC50 values, representing the concentration of hCNCs when 50% of the inhibition rate was obtained, were calculated by analyzing the plot of the logarithm of the inhibitor concentration against the corresponding inhibition rate.
To characterize the inhibition pattern, the enzymatic reaction was performed with variable concentrations of cooked corn starch (1.0–4.0%) and p-NP-G (1.0–4.0 mM) at two fixed hCNC concentrations (0.5 and 1.0 mg mL−1). The initial velocity values under different substrate concentrations were recorded. A double-reciprocal version of the Michaelis–Menten plot (Lineweaver–Burk plot) was utilized to fit the experimental data:
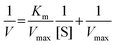 | (12) |
2.8. Simulated digestion of corn starch
The gelatinized corn starch was prepared by boiling a corn starch solution of 5.0% (w/w) for 15 min. After cooling to ambient temperature, the solution of corn starch was mixed with an equal volume of hCNC solution of 0.5, 2.0, or 8.0 mg mL−1. A mixture of 5.0% (w/w) corn starch solution with an equal volume of pure water served as the negative control, whereas a mixture of 5.0% (w/w) corn starch solution with an equal volume of acarbose solution of 2.0 mg mL−1 was utilized as the positive control. Fifteen milliliters of 5.0% NaCl and 5 mL of 0.5 M HCl were added into the mixed solution mentioned above, and the mixture was stirred at 37 °C and 370 rpm for 30 min to mimic the gastric digestion. Then, the digesta was adjusted to pH 7.0 to terminate the gastric digestion, and 5 mL of bile salt solution (50 mg mL−1) and 5 mL of enzyme solution containing 4.0 mg mL−1 α-amylase and 1.0 mg mL−1 α-glucosidase were added. The digesta was allowed to react at 37 °C and 370 rpm for 180 min to mimic the intestinal digestion. An aliquot of 200 μL of the digesta was removed at predetermined time intervals and analyzed for determining the free glucose level using a glucose assay kit.The fractional conversion model was utilized in the present work to fit the data:16,17
| Ct = Cf × (1 − e−kt). | (13) |
Herein, Ct (mM) and Cf (mM) denote the glucose level at digestion time t and the final, maximum glucose level obtained under the simulated digestion conditions, respectively; k (min−1) is the initial reaction rate constant of the studied process.
2.9. Data analysis
All the tests were performed in triplicate, and the data were represented as mean ± standard deviation. PASW Statistics 18 software was used to subject the results to the Tukey–Kramer multiple comparison test in order to analyze the differences. Differences with P < 0.05 were considered to be significant.3. Results and discussion
3.1. Characterization of burdock hCNCs
The most popular way to fabricate CNCs at present is sulphuric acid hydrolysis, and when CNCs were prepared from IDF, the lignin and hemicellulose components are usually required to be removed in advance with the aid of alkali, sodium chlorite, and/or H2O2, causing concerns related to environmental pollution and health risks.11 The IDF in burdock roots, especially those freshly harvested, comprised mainly of cellulose and hemicellulose, with only a trace amount of lignin (below 5%, Table 1). Therefore, in this work, the IDF was disintegrated by enzymatic hydrolysis and ultrasonic power as a whole, without the removal of lignin. Unlike the pure CNCs, the nanoparticles (Fig. 1A) liberated from the IDF contained a considerable amount (40–60%) of the hemicellulose component (Table 1). Therefore, they were denoted as “holocellulose nanocrystals (hCNCs)”. It should be noted that hCNC 600–200 in this work were named by their hydrodynamic diameter (DZ) by assuming that they adopted a spherical structure (Fig. 1B and C). This may reflect the relative size of the sample, but more detailed information about the hCNCs, such as length, width, and aspect ratio should be provided in future work.| Samples | Yield (%) | Chemical composition | ||||
|---|---|---|---|---|---|---|
| Protein (%) | Ash (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | ||
| a The yield was calculated according to the relative weight ratio of IDF to burdock powder. b The yield was calculated according to the relative weight ratio of hCNCs to burdock IDF. Different lowercase letters in the same column represent significant differences in the chemical composition (P < 0.05). | ||||||
| IDF | 21.34 ± 1.23a | 0.32 ± 0.08a | 3.32 ± 0.41b | 58.43 ± 2.12d | 34.32 ± 1.23a | 3.45 ± 0.12c |
| hCNC-600 | 16.17 ± 0.32ab | 1.56 ± 0.14b | 0.34 ± 0.05a | 34.45 ± 1.76a | 57.23 ± 0.32d | 4.32 ± 0.32d |
| hCNC-400 | 21.23 ± 1.18bb | 1.87 ± 0.12c | 0.45 ± 0.21a | 49.43 ± 2.12b | 44.32 ± 2.54c | 3.34 ± 0.23c |
| hCNC-300 | 24.41 ± 0.86cb | 2.32 ± 0.29d | 0.56 ± 0.11a | 54.13 ± 1.79c | 40.45 ± 0.30b | 2.34 ± 0.31b |
| hCNC-200 | 23.48 ± 1.55cb | 2.45 ± 0.22d | 0.38 ± 0.04a | 55.32 ± 2.34c | 39.23 ± 1.76b | 2.11 ± 0.21a |
The super-ground burdock powder displayed an irregular shape of a diameter above 500 nm under TEM observation (Fig. 1A). In contrast, burdock IDF assembled entangled fibril networks owing to its elongated structure. Enzymatic hydrolysis herein effectively disintegrated the clustered fibrils in the IDF and liberated short and individual nanorods (Fig. 1A). Similar non-spherical, needle-like or rod-like structures were reported for CNCs.18 In a previous work, enzyme combination (endoglucanase/hemicellulases) was also utilized for the preparation of CNCs from wood pulp, eucalyptus, and sugarcane bagasse.19 The length of the hCNCs decreased remarkably with the sonication time, which would have resulted from the breakage of the chains by cavitation effects. The modulation effects of the ultrasonic treatment on the length of acid-hydrolyzed CNCs were reported in a recent work, where the average length of the CNCs decreased from 346.28 nm to 276.58, 156.34, and 125.01 nm, respectively, after 5, 10, and 15 min of ultrasonication.20
As summarized in Table 1, about 16% of burdock IDF was converted into hCNC-600 after enzymatic hydrolysis. Compared with IDF, hCNC-600 contained more hemicellulose, indicating that the hemicellulose fibrils were more susceptible to enzymatic hydrolysis than cellulose fibrils in the present work. Both the yield of hCNCs and the cellulose content increased with the sonication time and reached a plateau at 2.0 h, which suggested that ultrasonic treatment promotes the conversion of cellulose microfibrils into nanorods. This is also reflected by the monosaccharide composition of hCNCs (Table S1†). Glucose accounted for 62.66% of the total monosaccharide in hCNC-600, followed by arabinose, galacturonic acid, xylose, galactose, rhamnose, mannose, fructose and fucose, which originated from the hemicellulose portion of burdock IDF. The glucose content in hCNCs increased with the sonication time, suggesting the breakage and liberation of more of the cellulose component from IDF. The weight percentage of protein in burdock hCNCs increased with the proceeding of the ultrasonic treatment as it promoted the release and enhanced the solubility of the protein fraction. All the hCNCs in the present work were negatively charged under neutral conditions (Fig. 1C) owing to anionic monosaccharide galacturonic acid and glucuronic acid (Table S1†), and the magnitude of ζ-potential decreased with the sonication time as a result of the increased proportion of nonionic glucose.
The XRD spectra of the IDF and hCNCs were recorded over the diffraction angle range of 5–50° (Fig. 1D). All the samples showed predominant peaks at 2θ = 22.1° and 15.8°, which were the characteristics of type-I cellulose as reported before.21 The peak at 22.1° was attributed to the plane (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10), whereas the peak at 15.8° was assigned to the plane (110). Ultrasonic treatment did improve the under curve area of the XRD profile, but the shoulder peak at 15.8° became less obvious. The crystalline index of the samples ranged from 15% to 30%, which was remarkably lower than those reported for CNCs prepared by sulphuric acid hydrolysis (about 70%) or ball milling (about 60%) using microcrystalline cellulose as the starting material.21,22 This should be mainly attributed to the fact that the hCNCs in this work contained a large portion of uncrystallized ribbon-like hemicellulose particles (Table 1). In a recent report, the authors suggested that the removal of hemicellulose and lignin with 1% NaOH under ball milling improved the crystallinity of okara from about 30% to 70%.11 The enzymes acted primarily towards the amorphous region and it could improve the overall crystallinity of the nanomaterials.23 Therefore, hCNC-600 liberated by only enzymatic hydrolysis presented the highest crystallinity of about 28%. The cavitation effects of ultrasonic treatment produced free radicals such as OH˙, H˙, and HO2˙, which attacked the glycosidic bonds and improved the yield of hCNCs (Table 1). The crystallinity of the products reduced when the samples were treated for 1.0 or 2.0 h, which may be attributed to the release of more uncrystallized nanorods. A similar phenomenon was observed in another report, where the ball milling treatment of high energy input decreased the crystallinity of CNCs, while increasing their yield.22 Afterwards, the yield of hCNCs reached a plateau and further ultrasonic treatment tended to disrupt the amorphous region in the hCNCs, thereby improving their crystallinity.
10), whereas the peak at 15.8° was assigned to the plane (110). Ultrasonic treatment did improve the under curve area of the XRD profile, but the shoulder peak at 15.8° became less obvious. The crystalline index of the samples ranged from 15% to 30%, which was remarkably lower than those reported for CNCs prepared by sulphuric acid hydrolysis (about 70%) or ball milling (about 60%) using microcrystalline cellulose as the starting material.21,22 This should be mainly attributed to the fact that the hCNCs in this work contained a large portion of uncrystallized ribbon-like hemicellulose particles (Table 1). In a recent report, the authors suggested that the removal of hemicellulose and lignin with 1% NaOH under ball milling improved the crystallinity of okara from about 30% to 70%.11 The enzymes acted primarily towards the amorphous region and it could improve the overall crystallinity of the nanomaterials.23 Therefore, hCNC-600 liberated by only enzymatic hydrolysis presented the highest crystallinity of about 28%. The cavitation effects of ultrasonic treatment produced free radicals such as OH˙, H˙, and HO2˙, which attacked the glycosidic bonds and improved the yield of hCNCs (Table 1). The crystallinity of the products reduced when the samples were treated for 1.0 or 2.0 h, which may be attributed to the release of more uncrystallized nanorods. A similar phenomenon was observed in another report, where the ball milling treatment of high energy input decreased the crystallinity of CNCs, while increasing their yield.22 Afterwards, the yield of hCNCs reached a plateau and further ultrasonic treatment tended to disrupt the amorphous region in the hCNCs, thereby improving their crystallinity.
3.2. Steady-state fluorescence studies
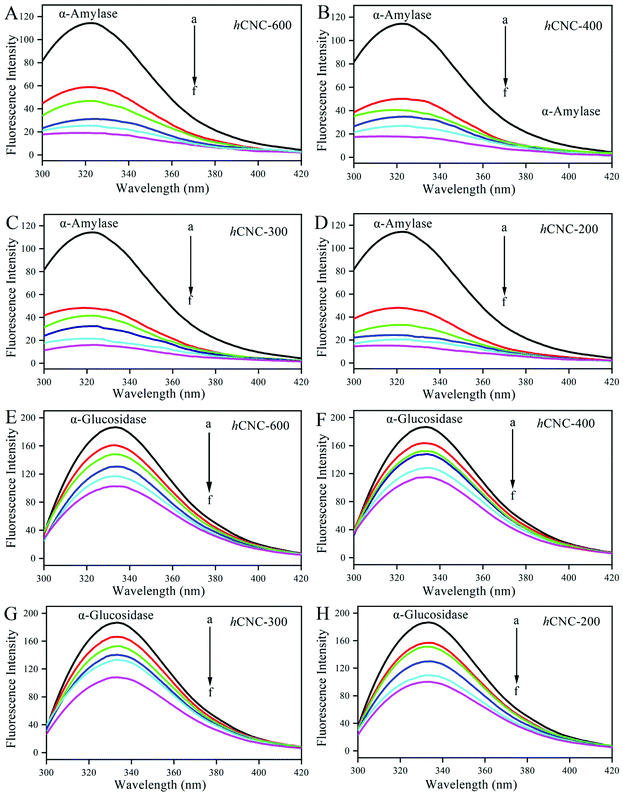
Excitation at 280 nm afforded the fluorescence profile of the Trp residues in α-amylase or α-glucosidase. As illustrated in Fig. 2A–D, pure α-amylase presented a strong fluorescence peak centered around 320 nm, which was consistent with the previous work.20 The emission intensity of α-amylase decreased gradually in the presence of increasing concentrations of hCNCs (0–4.0 mg mL−1). The quenching of the intrinsic fluorescence represents effective interactions between hCNCs and Trp residues in the enzymes, which might result in energy transfer, molecular rearrangements, and ground-state complex formation.24 In addition, a red shift in the fluorescence maxima occurred along with the binding of hCNCs, revealing the change of the tertiary conformation of α-amylase and reorientation of Trp residues to a more polar microenvironment.25 The fluorescence spectrum of α-glucosidase excited at 280 nm showed fluorescence centered at 330 nm with improved intensity compared with that of α-amylase (Fig. 2E–H). The higher the concentration of hCNCs, the lower the fluorescence intensity of α-glucosidase. The fluorescence intensity of α-glucosidase with 4.0 mg mL−1 of hCNCs was only about half of that of α-glucosidase without the hCNCs. Besides, a slight blue shift of the fluorescence peak was observed with increasing hCNC concentration, suggesting that the surrounding microenvironment of Trp residues became more hydrophobic.26The Stern–Volmer plots of the fluorescence quenching of the tested enzymes by hCNCs at 294, 302, and 310 K are presented in Fig. S1a–h.† A linear relationship of a high correlation coefficient (R2 > 0.9) was constructed between F0/F and [Q] at any temperature (Table 2), revealing the presence of a single type of fluorophore that is equally accessible for binding. Similar trends were observed in the interactions between diacylated anthocyanins and α-amylase or α-glucosidase.27 Parameters such as Ksv and kq were extrapolated from the curves. As summarized in Table 2, all kq values were above the maximum diffusion quenching constant for macromolecules (2.0 × 1010 L mol−1 s−1), a threshold suggesting the static binding mode. On the other hand, both Ksv and Kq values of the tested enzymes decreased with increasing temperature. This confirmed that the quenching of fluorescence originated from the formation of a stable complex between the hCNCs and tested enzymes. Otherwise, according to the fluorescence quenching principle, the Ksv values would increase with increasing temperature under dynamic collision conditions.28 The formation of stable complexes was also observed between a water-soluble Rosa roxburghii Tratt fruit polysaccharide and α-glucosidase.15
| α-Amylase | ||||
|---|---|---|---|---|
| Sample | T (K) | K sv (105 mol−1) | K q (1013 mol−1 s−1) | R 2 |
| hCNC-600 | 294 | 4.743 ± 0.011 | 4.743 ± 0.011 | 0.946 |
| 302 | 4.005 ± 0.023 | 4.005 ± 0.023 | 0.975 | |
| 310 | 3.582 ± 0.026 | 3.582 ± 0.026 | 0.994 | |
| hCNC-400 | 294 | 2.022 ± 0.027 | 2.022 ± 0.027 | 0.998 |
| 302 | 1.911 ± 0.021 | 1.911 ± 0.021 | 0.991 | |
| 310 | 1.683 ± 0.015 | 1.683 ± 0.015 | 0.988 | |
| hCNC-300 | 294 | 1.751 ± 0.032 | 1.751 ± 0.032 | 0.988 |
| 302 | 1.388 ± 0.020 | 1.388 ± 0.020 | 0.986 | |
| 310 | 1.227 ± 0.039 | 1.227 ± 0.039 | 0.992 | |
| hCNC-200 | 294 | 0.599 ± 0.051 | 0.599 ± 0.051 | 0.977 |
| 302 | 0.515 ± 0.046 | 0.515 ± 0.046 | 0.959 | |
| 310 | 0.509 ± 0.012 | 0.509 ± 0.012 | 0.975 | |
| α-Glucosidase | ||||
|---|---|---|---|---|
| Sample | T (K) | K sv (105 mol−1) | K q (1013 mol−1 s−1) | R 2 |
| hCNC-600 | 294 | 1.101 ± 0.062 | 1.101 ± 0.062 | 0.999 |
| 302 | 0.900 ± 0.017 | 0.900 ± 0.017 | 0.992 | |
| 310 | 0.873 ± 0.024 | 0.873 ± 0.024 | 0.999 | |
| hCNC-400 | 294 | 0.632 ± 0.043 | 0.632 ± 0.043 | 0.960 |
| 302 | 0.576 ± 0.068 | 0.576 ± 0.068 | 0.983 | |
| 310 | 0.538 ± 0.057 | 0.538 ± 0.057 | 0.936 | |
| hCNC-300 | 294 | 0.422 ± 0.026 | 0.422 ± 0.026 | 0.960 |
| 302 | 0.380 ± 0.087 | 0.380 ± 0.087 | 0.977 | |
| 310 | 0.367 ± 0.068 | 0.367 ± 0.068 | 0.982 | |
| hCNC-200 | 294 | 0.268 ± 0.026 | 0.268 ± 0.026 | 0.997 |
| 302 | 0.241 ± 0.048 | 0.241 ± 0.048 | 0.997 | |
| 310 | 0.214 ± 0.063 | 0.214 ± 0.063 | 0.999 | |
In the present work, at any of the studied temperatures, the binding affinity of hCNCs to the tested enzymes decreased with decreasing particle size. For example, the Kq values for α-glucosidase at 294 K were 4.743, 2.022, 1.751, and 0.599, respectively, for hCNC-600, hCNC-400, hCNC-300, and hCNC-200. There was contrasting evidence showing that the Kq values for α-amylase or α-glucosidase binding were inversely related with the size of CNCs.19 This suggested that the fluorescence quenching of digestive enzymes by hCNCs was a complicated process, which was affected by the size, origin, chemical composition, and microstructure of the hCNCs.
The values of Kb and n at 294 K, 302 K and 310 K were extrapolated from the y-axis intercept and slope of the linear fit regression curves in Fig. S2† and are summarized in Table 3. Kb represented the affinity of the protein to the quencher, with a higher Kb revealing a closer association with stronger binding strength of the quencher. For each case, both the Kb and n values increased with increasing temperature, suggesting the formation of a more stable complex. The high binding constant under physiological conditions suggested the great potential of the hCNCs to interact with the digestive enzymes in the gastrointestinal tract as well as decrease their catalytic activities. Besides, all n values were lower than or approximately 1, which indicated that there was only one hCNC particle bound to the hydrophobic domain where Trp resided. For the interaction between α-glucosidase and hCNCs, the Kb values were inversely correlated with the size of the hCNCs at any of the studied temperatures. Similar results have been reported by a previous report, where the authors suggested that the Kb values were 1.360, 1.768, and 2.281 × 104 L mol−1, respectively, for the interactions between α-glucosidase and MCC (average length: 18.67 μm), nanocrystalline cellulose-1 (average length: 302.5 nm), and nanocrystalline cellulose-2 (average length: 174.41 nm), at 288 K.8 It was reasonable to assume that the decreased size will facilitate the interaction between the CNCs and proteins by reducing the steric hindrance. The CNCs with elongated chains would reduce their accessibility to the Trp residues buried in the hydrophobic interior of the enzyme. In contrast, the binding ability of hCNCs to α-amylase decreased when their particle size decreased from about 600 nm to 400 nm, while increasing with the decrease of particle size afterwards. One possible explanation was that ultrasonic treatment for 1.0 h resulted in a burst release of the cellulose component in hCNCs (from 34.45% to 49.43%), which increased their surface polarity and inhibited the specific hydrophobic interactions between hCNC-400 and α-amylase.
| α-Amylase | ||||
|---|---|---|---|---|
| Sample | T (K) | K b (104 mol−1) | n | R 2 |
| hCNC-600 | 294 | 0.319 ± 0.062 | 0.580 | 0.988 |
| 302 | 0.443 ± 0.031 | 0.623 | 0.999 | |
| 310 | 0.453 ± 0.028 | 0.631 | 0.986 | |
| hCNC-400 | 294 | 0.167 ± 0.058 | 0.556 | 0.963 |
| 302 | 0.196 ± 0.042 | 0.584 | 0.965 | |
| 310 | 0.275 ± 0.038 | 0.644 | 0.925 | |
| hCNC-300 | 294 | 0.315 ± 0.026 | 0.573 | 0.973 |
| 302 | 0.539 ± 0.034 | 0.617 | 0.990 | |
| 310 | 0.579 ± 0.037 | 0.633 | 0.985 | |
| hCNC-200 | 294 | 0.508 ± 0.054 | 0.582 | 0.975 |
| 302 | 0.597 ± 0.017 | 0.619 | 0.995 | |
| 310 | 0.847 ± 0.035 | 0.663 | 0.994 | |
| α-Glucosidase | ||||
| hCNC-600 | 294 | 0.613 ± 0.042 | 0.757 | 0.995 |
| 302 | 0.762 ± 0.076 | 0.788 | 0.987 | |
| 310 | 0.935 ± 0.048 | 0.813 | 0.998 | |
| hCNC-400 | 294 | 0.164 ± 0.034 | 0.707 | 0.922 |
| 302 | 0.251 ± 0.046 | 0.752 | 0.949 | |
| 310 | 0.321 ± 0.075 | 0.788 | 0.940 | |
| hCNC-300 | 294 | 0.143 ± 0.049 | 0.725 | 0.976 |
| 302 | 0.163 ± 0.032 | 0.743 | 0.959 | |
| 310 | 0.206 ± 0.056 | 0.780 | 0.988 | |
| hCNC-200 | 294 | 0.120 ± 0.013 | 0.756 | 0.990 |
| 302 | 0.127 ± 0.026 | 0.764 | 0.988 | |
| 310 | 0.138 ± 0.078 | 0.775 | 0.993 | |
The noncovalent interactions between hCNCs and enzymes included hydrophobic interactions, electrostatic forces, hydrogen bonding, and van der Waals forces. The dominant forces driving the formation of hCNC–enzyme complexes could be identified by analyzing the thermodynamic parameters such as enthalpy change (ΔH) and entropy (ΔS): for ΔH > 0 and ΔS > 0, hydrophobic interaction will be the main force of interaction; for ΔH < 0 and ΔS < 0, van der Waals force and hydrogen bonding will be the main forces of interactions; and for ΔH < 0 and ΔS > 0, the association occurred primarily through an electrostatic force.
As depicted in Table 4, the main driving force for the formation of complexes between hCNCs and the tested enzymes was a hydrophobic interaction, as shown by ΔH > 0 and ΔS > 0, irrespective of the particle size. Besides, all the ΔG values were negative, suggesting that the binding was a spontaneous endothermic process. This is in accordance with the Kb values analyzed by the Stern–Volmer equation, where the values increased with the increase of temperature (Table 3). Similarly, the CNCs could bind and interact with whey protein–bovine serum albumin (BSA) spontaneously through non-covalent forces.29 In another work, the binding of cellulose to α-amylase was considered to be non-specific because the adsorption kinetics decreased rapidly in the presence of excess BSA.30
| α-Amylase | ||||
|---|---|---|---|---|
| Sample | T (K) | ΔH (kJ mol−1) | ΔG (kJ mol−1) | ΔS (J mol−1 K−1) |
| hCNC-600 | 294 | 17.412 | −19.826 | 126.661 |
| 302 | −20.839 | |||
| 310 | −21.853 | |||
| hCNC-400 | 294 | 20.240 | −20.719 | 143.832 |
| 302 | −21.823 | |||
| 310 | −21.850 | |||
| hCNC-300 | 294 | 30.143 | −19.849 | 170.044 |
| 302 | −21.209 | |||
| 310 | −24.872 | |||
| hCNC-200 | 294 | 24.841 | −20.764 | 155.120 |
| 302 | −22.005 | |||
| 310 | −23.246 | |||
| α-Glucosidase | ||||
|---|---|---|---|---|
| Sample | T (K) | ΔH (kJ mol−1) | ΔG (kJ mol−1) | ΔS (J mol−1 K−1) |
| hCNC-600 | 294 | 20.713 | −21.299 | 142.92 |
| 302 | −22.443 | |||
| 310 | −23.586 | |||
| hCNC-400 | 294 | 32.912 | −18.138 | 173.64 |
| 302 | −19.528 | |||
| 310 | −20.917 | |||
| hCNC-300 | 294 | 17.737 | −17.707 | 120.56 |
| 302 | −18.672 | |||
| 310 | −19.636 | |||
| hCNC-200 | 294 | 6.692 | −17.122 | 81.67 |
| 302 | −17.770 | |||
| 310 | −18.418 | |||
3.3. UV, FI-IR, and CD analyses
As depicted in Fig. S3a and b,† tryptophan (Trp) and tyrosine (Tyr) residues in the tested enzymes presented strong absorption peaks centered at 260–280 nm. The addition of hCNCs resulted in a red (a) or blue (b) shift of the predominant band and the formation of new absorption peaks, suggesting that the microenvironment surrounding the chromophores has been changed,31 which was in accordance with the steady-state fluorescence studies (Fig. 2). On the other hand, UV spectroscopy could also further confirm the static fluorescence quenching mechanism of the hCNCs, because the dynamic quenching only affected the excited state of the fluorescent molecules and exerted no influence on their UV absorption spectra.32The amide I band (1700–1600 cm−1) in the FT-IR spectra of the enzymes was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the peptide linkage, while the amide II band (1600–1500 cm−1) was associated with the C–N stretching and N–H bending of the amino groups (Fig. S3c and d†). No new peaks were observed upon binding with the hCNCs, suggesting that covalent interactions were not involved. The presence of hCNCs resulted in a blue shift of the amide I band of α-amylase with improved intensity, and the smaller the size of hCNCs, the stronger the intensity of the amide I band. In contrast, the amide II band of α-amylase became flattened and its position changed slightly in the presence of hCNCs. On the other hand, the hCNCs caused less obvious effects on the FT-IR spectrum of α-glucosidase than on that of α-amylase. Both amide I and II bands of α-glucosidase were shifted to lower wavelengths in the presence of hCNCs. These results suggested that the hCNCs interacted with the C
O stretching vibration of the peptide linkage, while the amide II band (1600–1500 cm−1) was associated with the C–N stretching and N–H bending of the amino groups (Fig. S3c and d†). No new peaks were observed upon binding with the hCNCs, suggesting that covalent interactions were not involved. The presence of hCNCs resulted in a blue shift of the amide I band of α-amylase with improved intensity, and the smaller the size of hCNCs, the stronger the intensity of the amide I band. In contrast, the amide II band of α-amylase became flattened and its position changed slightly in the presence of hCNCs. On the other hand, the hCNCs caused less obvious effects on the FT-IR spectrum of α-glucosidase than on that of α-amylase. Both amide I and II bands of α-glucosidase were shifted to lower wavelengths in the presence of hCNCs. These results suggested that the hCNCs interacted with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups in the protein subunits through hydrogen bonding and hydrophobic forces. The changes in the amide I and amide II bands suggested alterations in the secondary structure of proteins, which were further verified by the subsequent CD analyses.
O and N–H groups in the protein subunits through hydrogen bonding and hydrophobic forces. The changes in the amide I and amide II bands suggested alterations in the secondary structure of proteins, which were further verified by the subsequent CD analyses.
The tested enzymes presented a broad negative band ranging from 210 to 230 nm (Fig. S3e and f†), which could be ascribed to the π → π and n → π* transitions of the peptide linkage, showing a typical α-helical structure.33 In the presence of inhibitors, the negative band became flattened and its magnitude decreased obviously. Native α-amylase contains 32.72% α-helix, 17.55% β-sheet, 21.02% β-turn, and 28.71% unordered. The binding of burdock hCNCs resulted in a remarkable decrease in the α-helical structure and β-turn, accompanied by a parallel increase in β-sheet and unordered. In general, hCNCs of smaller size enabled a more obvious change in the secondary conformation of α-amylase. Similar results were observed regarding the secondary conformation alteration of α-glucosidase in the presence of different hCNCs. As summarized in the inset in Fig. S3f,† the fraction of α-helix in α-glucosidase decreased from 32.72% to 11.44%, 9.02%, 7.94%, and 5.43%, respectively, after binding with hCNC-600, hCNC-400, hCNC-300, and hCNC-200.
3.4. Inhibitory activity of hCNCs against α-amylase and α-glucosidase
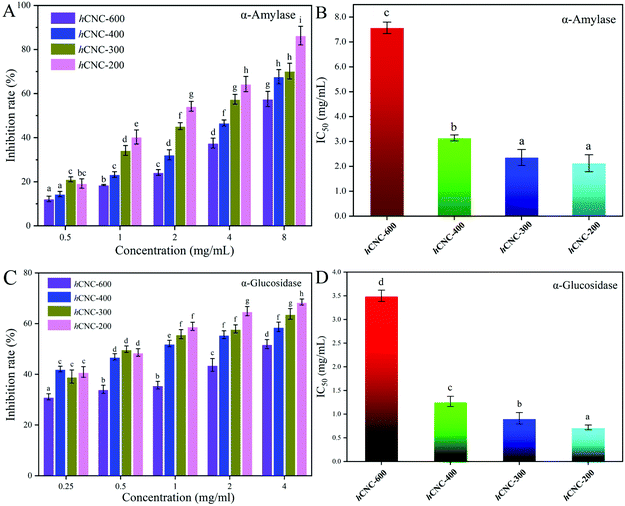
α-Amylase and α-glucosidase are the key enzymes for starch and glycogen hydrolysis in the human digestion tract. The inhibition of the activities of these two enzymes could effectively delay carbohydrate absorption and therefore reduce the postprandial glucose levels. As shown in Fig. 3A, the inhibitory effects of hCNCs against α-amylase were dose-dependent at the studied concentration range. In general, the inhibition rates were below 20% when the concentration of hCNCs was 0.50 mg mL−1, and these values increased to over 60% as the concentration reached 8.0 mg mL−1. At any of the studied concentrations, the inhibition rates for α-amylase decreased with this trend: hCNC-200 > hCNC-300 > hCNC-400 > hCNC-600, suggesting that the inhibitory activity of hCNCs against α-amylase was inversely correlated with their particle size. A similar trend was observed for the inhibitory activities of hCNCs against α-glucosidase with improved inhibition rates at the same concentration (Fig. 3C). The IC50 values herein denoted the concentration of hCNCs under which 50% of enzymatic inhibition was obtained. The IC50 values for α-amylase inhibition were 7.567, 3.143, 2.356, and 2.123 mg mL−1 for hCNC-600, hCNC-400, hCNC-300, and hCNC-200, respectively. Similarly, Nsor-Atindana and co-authors (2020)20 revealed that the IC50 values for α-amylase inhibition decreased from 6.721 to 2.982 mg mL−1 as the length of CNCs decreased from 346.28 to 125.01 nm.16 In another work, these authors suggested that the IC50 values of CNCs with the aspect ratio of 30.72 and 41.04 were 17.00 and 11.36 mg mL−1, respectively, which were much higher than the values in the present work.8 On the other hand, the IC50 values for α-glucosidase inhibition ranged between 0.5 and 3.5 mg mL−1 (Fig. 3D). The exposure of free hydroxyl groups, along with the reduction in the particle size of hCNCs during the ultrasonic treatment, would contribute to their inhibitory effects against the tested enzymes in the present work.The initial velocity values in the presence of different amounts of hCNCs across a wide range of substrate concentrations were utilized to construct the Lineweaver–Burk plots. As depicted in Fig. 4A–D, the Lineweaver–Burk curves for α-amylase inhibition in each subfigure did not run parallel to each other and intersected at the x-axis (a, c), the third quadrant (b), or the second quadrant (d), suggesting different inhibition mechanisms. The binding of hCNC-600 and hCNC-300 to α-amylase resulted in a remarkable decrease in the Vmax values, but the Km values changed slightly (Table S2†). Therefore, the inhibition was characterized to be non-competitive, that is, hCNC-600 and 300 could bind whether or not the substrate has already bound to the digestive enzymes, forming indigestible complexes. This finding was in accordance with a previous report, where the interaction between galactomannan and α-amylase decreased the Vmax values, while did not alter the Km values for gelatinized starch hydrolysis.34 A non-competitive type of inhibition against α-amylase was also observed for the CNCs.30 In contrast, both uncompetitive and noncompetitive modes were involved in the inhibition of hCNC-400 against α-amylase, and were evidenced by the intersection of the curves at the third quadrant (Fig. 4B) and decreased Vmax and Km values (Table S2†). The inhibition of hCNC-200 against α-amylase was characterized to be of a mixed competitive and noncompetitive type, as evidenced by the decreased Vmax values and increased Km values (Table S2†), revealing that hCNC-200 could competitively interact with the binding site of the substrate and other active sites. A mixed competitive and noncompetitive type of inhibition against α-amylase was also observed for tannic acid in a previous study.35
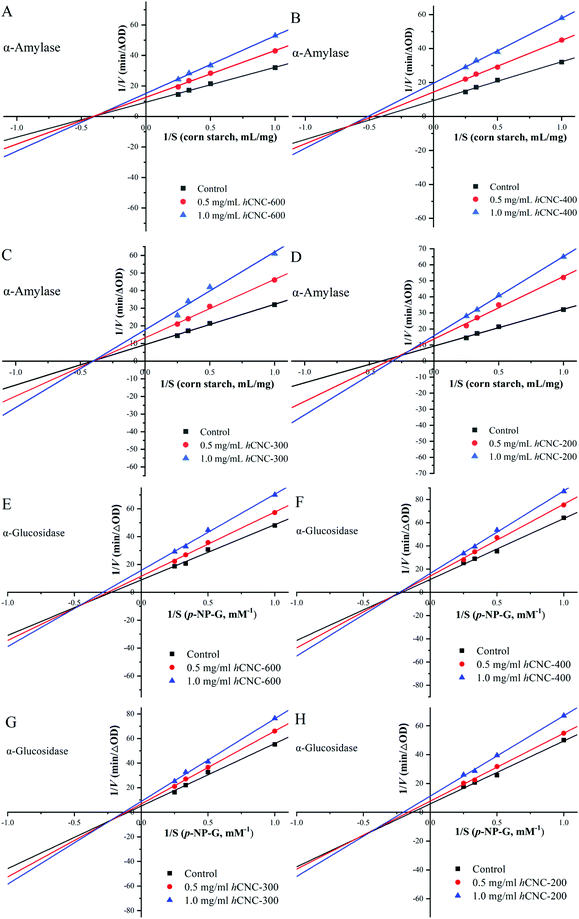 | ||
| Fig. 4 The Lineweaver–Burk plots for α-amylase (A–D) and α-glucosidase (E–H) inhibition in the presence of different concentrations of hCNCs. | ||
In the absence of hCNCs, the Km and Vmax values of α-glucosidase were calculated to be 10.09 mM and 0.198 ΔA405 min−1, respectively (Table S3†). In the presence of any hCNCs, both Vmax and Km values decreased with increasing inhibitor concentration, and the Lineweaver–Burk curves intersected at the third quadrant (Fig. 4E–H). These results indicated that both uncompetitive and noncompetitive types of inhibition were involved. However, a previous work suggested that the inhibition of CNCs against α-glucosidase was mainly noncompetitive with only a minimal level of a mixed mode. We speculated that the inhibitory mode of CNCs was highly dependent on their physicochemical properties, which were influenced by the origin and preparation methods. More work is required in the future to shed light on the relationship between the physicochemical properties of hCNCs and their enzymatic inhibitory effects.
3.5. Simulated digestion
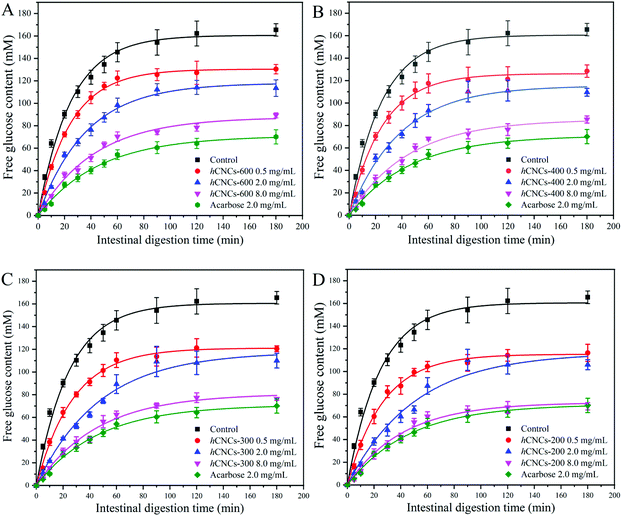
The starch digestion curves can be divided into two regions: rapid digestion at the first 40 min, during which the majority of the postprandial blood glucose rise (as well as the initial insulin demand) occurred after the ingestion of a starchy meal, and prolonged digestion with a much lower hydrolysis rate.36 From Fig. 5, we could learn that even a trace amount of hCNCs could cause inhibitory effects on starch digestion. However, the inhibitory activities were not as effective as acarbose (2.0 mg mL−1).As expected, the evolution patterns of the curves herein were characterized by rapid digestion at first, which slowed down afterwards until a plateau was reached. Therefore, the data were fitted by the fractional conversion model according to eqn (13) and the calculated dynamic parameters are summarized in Table 5. The initial digestion rate constant (k) of the negative control was 0.040 min−1, which changed slightly with 0.5 mg mL−1hCNC-600 or hCNC-400. In contrast, this value decreased to 0.036 and 0.038 min−1, respectively, in the presence of hCNC-300 and hCNC-200 of the same concentration. The inhibitory effect of all hCNCs on the initial rate of starch hydrolysis improved with the increase in their concentrations, and obvious differences in k values could be observed between the control group and those containing 2.0 or 8.0 mg mL−1 of hCNCs (P < 0.05). On the other hand, the k values of the groups containing 8.0 mg mL−1 of hCNCs were similar to that of the acarbose group (2.0 mg mL−1).
| Inhibitor | k (min−1) | C f (mM) | R adj 2 |
|---|---|---|---|
| Different lowercase letters in the same column represent significant differences (P < 0.05). | |||
| Control | 0.040 ± 0.002d | 160.56 ± 3.07h | 0.992 |
| 0.5 mg ml−1 of hCNC-600 | 0.041 ± 0.001d | 130.43 ± 1.25g | 0.998 |
| 2.0 mg ml−1 of hCNC-600 | 0.028 ± 0.002b | 118.01 ± 2.49d | 0.994 |
| 8.0 mg mL−1 of hCNC-600 | 0.023 ± 0.002a | 87.62 ± 2.76c | 0.989 |
| 0.5 mg ml−1 of hCNC-400 | 0.040 ± 0.002d | 126.16 ± 1.58f | 0.997 |
| 2.0 mg ml−1 of hCNC-400 | 0.026 ± 0.002b | 115.45 ± 3.02d | 0.992 |
| 8.0 mg mL−1 of hCNC-400 | 0.022 ± 0.002a | 85.32 ± 2.57c | 0.991 |
| 0.5 mg ml−1 of hCNC-300 | 0.036 ± 0.001c | 121.08 ± 1.51e | 0.996 |
| 2.0 mg ml−1 of hCNC-300 | 0.021 ± 0.002a | 117.64 ± 4.05d | 0.989 |
| 8.0 mg mL−1 of hCNC-300 | 0.022 ± 0.002a | 80.89 ± 2.83b | 0.987 |
| 0.5 mg ml−1 of hCNC-200 | 0.038 ± 0.002c | 115.22 ± 1.56d | 0.996 |
| 2.0 mg ml−1 of hCNC-200 | 0.020 ± 0.002a | 116.55 ± 6.17d | 0.978 |
| 8.0 mg mL−1 of hCNC-200 | 0.024 ± 0.002a | 72.70 ± 0.02a | 0.986 |
| 2.0 mg mL−1 of acarbose | 0.022 ± 0.001a | 71.02 ± 1.76a | 0.994 |
In the present work, all the Cf values of the experimental groups were generally lower than that of the control group, while higher than that of the acarbose group (Table 5). For groups containing the same type of hCNCs, the Cf values were negatively correlated with the concentration of hCNCs. The Cf values decreased with this trend: hCNC-200 > hCNC-300 > hCNC-400 > hCNC-600, when these inhibitors were at the same concentration. These results were consistent with the inhibitory effects of hCNCs against α-amylase and α-glucosidase (Fig. 3), suggesting that the hypoglycemic effects of hCNCs mainly resulted from the blocking of the catalytic activity of the digestive enzymes. The inhibitory effects on starch hydrolysis have also been reported for other dietary fibers such as pectin and pullulan.37,38 However, in the report by Ma et al. (2019), the authors revealed that pectin retarded starch hydrolysis by forming a polysaccharide layer surrounding the surface of the starch granule, which decreased the accessibility of the glycosidic bonds to the digestive enzymes.37 In another work, Chen et al. (2017) demonstrated that the inhibitory effects of pullulan on starch gelatinization and the coating effect of pullulan on the surface of starch granules were responsible for the reduced starch digestibility.38 Therefore, the mechanisms for starch hydrolysis inhibition were different between the long chain polysaccharides and burdock hCNCs in the present work.
4. Conclusion
In this work, hCNCs with rod- or needle-like structures were prepared by combining enzymatic hydrolysis and ultrasonic treatment using burdock IDF as the raw material. The steady-state fluorescence studies suggested that the binding of hCNCs with α-amylase and α-glucosidase was a spontaneous endothermic process, which was driven by hydrogen bonding and hydrophobic interactions and resulted in the formation of a static complex. Burdock hCNCs presented a remarkable inhibitory effect against α-amylase and α-glucosidase, which was negatively correlated with the size of hCNCs. Noncompetitive and mixed types of inhibition were observed for α-amylase, whereas only a mixed type of inhibition was observed for α-glucosidase. The hydrolysis of corn starch during the simulated digestion was retarded in the presence of burdock hCNCs, and the inhibitory effects were both dose- and size-dependent. This work provided a highly efficient, green, and easy scalable methodology to develop hCNCs from naturally abundant IDF, which hold great promise as functional food ingredients in the control of postprandial glucose level and management of diabetes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20180298), the National Natural Science Foundation of China (31801555), the China Postdoctoral Science Foundation Funded Project (2017M621668), the National Postdoctoral Program for Innovative Talents (BX201700101), the Independent Innovation Fund of Agricultural Science and Technology in Jiangsu Province (CX(19)2006), the Key Research and Development Program of Jiangsu Province (BE2020382), and the Open project of Key Laboratory of Agricultural Products Processing, Ministry of Agriculture and Rural Affairs (S2021KFKT-04). It is also a great pleasure to thank Cunfa Xu at Central Laboratory of Jiangsu Academy of Agricultural Sciences for technical supports.References
- A. K. Das, P. K. Nanda, P. Madane, S. Biswas, A. Das, W. Zhang and J. M. Lorenzo, A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods, Trends Food Sci. Technol., 2020, 99, 323–336 CrossRef CAS.
- H. Dai, J. Wu, H. Zhang, Y. Chen, L. Ma, H. Huang, Y. Huang and Y. Zhang, Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge, Trends Food Sci. Technol., 2020, 102, 16–29 CrossRef CAS.
- R. Mu, X. Hong, Y. Ni, Y. Li, J. Pang, Q. Wang, J. Xiao and Y. Zheng, Recent trends and applications of cellulose nanocrystals in food industry, Trends Food Sci. Technol., 2019, 93, 136–144 CrossRef CAS.
- J. Yan, J. Hu, R. Yang, Z. Zhang and W. Zhao, Innovative Nanofibrillated Cellulose from Rice Straw as Dietary Fiber for Enhanced Health Benefits Prepared by a Green and Scale Production Method, ACS Sustainable Chem. Eng., 2018, 6, 3481–3492 CrossRef CAS.
- G. M. DeLoid, I. S. Sohal, L. R. Lorente, R. M. Molina, G. Pyrgiotakis, A. Stevanovic, R. Zhang, D. J. McClements, N. K. Geitner, D. W. Bousfield, K. W. Ng, S. C. J. Loo, D. C. Bell, J. Brain and P. Demokritou, Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose, ACS Nano, 2018, 12, 6469–6479 CrossRef CAS PubMed.
- N. Becerra-Tomas, I. Paz-Graniel, P. Hernandez-Alonso, D. J. A. Jenkins, C. W. C. Kendall, J. L. Sievenpiper and J. Salas-Salvado, Nut consumption and type 2 diabetes risk: a systematic review and meta-analysis of observational studies, Am. J. Clin. Nutr., 2021, 113, 960–971 CrossRef PubMed.
- K. Papoutsis, J. Zhang, M. C. Bowyer, N. Brunton, E. R. Gibney and J. Lyng, Fruit, vegetables, and mushrooms for the preparation of extracts with alpha-amylase and alpha-glucosidase inhibition properties: A review, Food Chem., 2021, 338, 128119 CrossRef CAS PubMed.
- J. Nsor-Atindana, H. D. Goff, M. N. Saqib, M. Chen, W. Liu, J. Ma and F. Zhong, Inhibition of alpha-amylase and amyloglucosidase by nanocrystalline cellulose and spectroscopic analysis of their binding interaction mechanism, Food Hydrocolloids, 2019, 90, 341–352 CrossRef CAS.
- N. Ji, C. Liu, M. Li, Q. Sun and L. Xiong, Interaction of cellulose nanocrystals and amylase: Its influence on enzyme activity and resistant starch content, Food Chem., 2018, 245, 481–487 CrossRef CAS PubMed.
- S. Wu, M. Huang, C. Li, Z. Chai, L. Cui, W. Huang, Y. Li and J. Feng, Fabrication of ovalbumin-burdock polysaccharide complexes as interfacial stabilizers for nanostructured lipid carriers: Effects of high-intensity ultrasound treatment, Food Hydrocolloids, 2021, 111, 106407 CrossRef CAS.
- T. Yang and C. H. Tang, Holocellulose nanofibers from insoluble polysaccharides of okara by mild alkali planetary ball milling: Structural characteristics and emulsifying properties, Food Hydrocolloids, 2021, 115, 106625 CrossRef CAS.
- Y. Xiao, Y. Liu, X. Wang, M. Li, H. Lei and H. Xu, Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment, Int. J. Biol. Macromol., 2019, 140, 225–233 CrossRef CAS PubMed.
- A. Bhogale, N. Patel, P. Sarpotdar, J. Mariam, P. M. Dongre, A. Miotello and D. C. Kothari, Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy, Colloids Surf., B, 2013, 102, 257–264 CrossRef CAS PubMed.
- V. D. Suryawanshi, L. S. Walekar, A. H. Gore, P. V. Anbhule and G. B. Kolekar, Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin, J. Pharm. Anal., 2016, 6, 56–63 CrossRef PubMed.
- L. Wang, C. Chen, B. Zhang, Q. Huang, X. Fu and C. Li, Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its alpha-glucosidase inhibitory activity, Food Funct., 2018, 9, 3974–3985 RSC.
- J. Feng, M. Huang, Z. Chai, C. Li, W. Huang, L. Cui and Y. Li, The influence of oil composition on the transformation, bioaccessibility, and intestinal absorption of curcumin in nanostructured lipid carriers, Food Funct., 2020, 11, 5223–5239 RSC.
- N. Wang, L. Wu, S. Huang, Y. Zhang, F. Zhang and J. Zheng, Combination treatment of bamboo shoot dietary fiber and dynamic high-pressure microfluidization on rice starch: Influence on physicochemical, structural, and in vitro digestion properties, Food Chem., 2021, 350, 128724 CrossRef CAS PubMed.
- W. Wang, G. Du, C. Li, H. Zhang, Y. Long and Y. Ni, Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis L.) and their applications to palm oil/water Pickering emulsion, Carbohydr. Polym., 2016, 151, 1–8 CrossRef CAS PubMed.
- R. S. Sobral Teixeira, A. S. A. da Silva, J. H. Jang, H. W. Kim, K. Ishikawa, T. Endo, S. H. Lee and E. P. S. Bon, Combining biomass wet disk milling and endoglucanase/beta-glucosidase hydrolysis for the production of cellulose nanocrystals, Carbohydr. Polym., 2015, 128, 75–81 CrossRef PubMed.
- J. Nsor-atindana, M. Yu, H. D. Goff, M. Chen and F. Zhong, Analysis of kinetic parameters and mechanisms of nanocrystalline cellulose inhibition of alpha-amylase and alpha-glucosidase in simulated digestion of starch, Food Funct., 2020, 11, 4719–4731 RSC.
- X. Li, J. Li, J. Gong, Y. Kuang, L. Mo and T. Song, Cellulose nanocrystals (CNCs) with different crystalline allomorph for oil in water Pickering emulsions, Carbohydr. Polym., 2018, 183, 303–310 CrossRef CAS PubMed.
- X. Kang, S. Kuga, C. Wang, Y. Zhao, M. Wu and Y. Huang, Green Preparation of Cellulose Nanocrystal and Its Application, ACS Sustainable Chem. Eng., 2018, 6, 2954–2960 CrossRef CAS.
- X. Q. Chen, G. X. Pang, W. H. Shen, X. Tong and M. Y. Jia, Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers, Carbohydr. Polym., 2019, 207, 713–719 CrossRef CAS PubMed.
- F. Hua, P. Zhou, H. Y. Wu, G. X. Chu, Z. W. Xie and G. H. Bao, Inhibition of alpha-glucosidase and alpha-amylase by flavonoid glycosides from Lu'an GuaPian tea: molecular docking and interaction mechanism, Food Funct., 2018, 9, 4173–4183 RSC.
- J. Feng, H. Xu, L. Zhang, H. Wang, S. Liu, Y. Liu, W. Hou and C. Li, Development of Nanocomplexes for Curcumin Vehiculization Using Ovalbumin and Sodium Alginate as Building Blocks: Improved Stability, Bioaccessibility, and Antioxidant Activity, J. Agric. Food Chem., 2019, 67, 379–390 CrossRef CAS PubMed.
- J. Feng, S. Wu, H. Wang and S. Liu, Gliadin nanoparticles stabilized by a combination of thermally denatured ovalbumin with gemini dodecyl O-glucoside: The modulating effect of cosurfactant, Colloids Surf., A, 2017, 516, 94–105 CrossRef CAS.
- Y. Yang, J.-L. Zhang, L.-H. Shen, L.-J. Feng and Q. Zhou, Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) against alpha-amylase and alpha-glucosidase, Food Chem., 2021, 359, 129934–129934 CrossRef CAS PubMed.
- Y. Li, F. Gao, F. Gao, F. Shan, J. Bian and C. Zhao, Study on the Interaction between 3 Flavonoid Compounds and alpha-Amylase by Fluorescence Spectroscopy and Enzymatic Kinetics, J. Food Sci., 2009, 74, C199–C203 CrossRef CAS PubMed.
- F. Liu, J. Zheng, C.-H. Huang, C.-H. Tang and S. Y. Ou, Pickering high internal phase emulsions stabilized by protein-covered cellulose nanocrystals, Food Hydrocolloids, 2018, 82, 96–105 CrossRef CAS.
- S. Dhital, M. J. Gidley and F. J. Warren, Inhibition of alpha-amylase activity by cellulose: Kinetic analysis and nutritional implications, Carbohydr. Polym., 2015, 123, 305–312 CrossRef CAS PubMed.
- S. Jiang, M. Li, R. Chang, L. Xiong and Q. Sun, In vitro inhibition of pancreatic alpha-amylase by spherical and polygonal starch nanoparticles, Food Funct., 2018, 9, 355–363 RSC.
- X. Cai, J. Yu, L. Xu, R. Liu and J. Yang, The mechanism study in the interactions of sorghum procyanidins trimer with porcine pancreatic alpha-amylase, Food Chem., 2015, 174, 291–298 CrossRef CAS PubMed.
- G. Zhang, L. Wang, P. Fu and M. Hu, Mechanism and conformational studies of farrerol binding to bovine serum albumin by spectroscopic methods, Spectrochim. Acta, Part A, 2011, 82, 424–431 CrossRef CAS PubMed.
- S. L. Slaughter, P. R. Ellis, E. C. Jackson and P. J. Butterworth, The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic alpha-amylase, Biochim. Biophys. Acta, Gen. Subj., 2002, 1571, 55–63 CrossRef CAS.
- H. Xiao, B. Liu, H. Mo and G. Liang, Comparative evaluation of tannic acid inhibiting alpha-glucosidase and trypsin, Food Res. Int., 2015, 76, 605–610 CrossRef CAS PubMed.
- P. J. Butterworth, F. J. Warren, T. Grassby, H. Patel and P. R. Ellis, Analysis of starch amylolysis using plots for first-order kinetics, Carbohydr. Polym., 2012, 87, 2189–2197 CrossRef CAS.
- Y. S. Ma, Y. Pan, Q. T. Xie, X. M. Li, B. Zhang and H. Q. Chen, Evaluation studies on effects of pectin with different concentrations on the pasting, rheological and digestibility properties of corn starch, Food Chem., 2019, 274, 319–323 CrossRef CAS PubMed.
- L. Chen, Y. Tian, Z. Zhang, Q. Tong, B. Sun, M. M. A. Rashed and Z. Jin, Effect of pullulan on the digestible, crystalline and morphological characteristics of rice starch, Food Hydrocolloids, 2017, 63, 383–390 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo02012a |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |