Protective and restorative effects of sophorolipid on intestinal dystrophy in dextran sulfate sodium-induced colitis mouse model
Min-Jin
Kwak
 ac,
Dong-Jin
Ha
a,
Yong-Soon
Choi
a,
Hanbae
Lee
b and
Kwang-Youn
Whang
*a
ac,
Dong-Jin
Ha
a,
Yong-Soon
Choi
a,
Hanbae
Lee
b and
Kwang-Youn
Whang
*a
aDepartment of Biotechnology, Korea University, Seoul 02841, Republic of Korea. E-mail: kwhang@korea.ac.kr; ics1051@korea.ac.kr; djha@ctcbio.com; dj1235@korea.ac.kr; Fax: +82-2-3290-3499; Tel: +82-2-3290-3492
bPathway Intermediates, Seoul 02841, Republic of Korea. E-mail: hanbae.lee@pathway-intermediates.com
cDivision of Interdisciplinary Program in Precision Public Health (BK21 FOUR Program), Department of Biomedical Engineering, Korea University, Seoul 02841, Republic of Korea
First published on 26th November 2021
Abstract
The public has gradually begun to regard inflammatory bowel disease (IBD) as a crucial health issue; however, its mode of action has not been fully elucidated. Sophorolipid (SPL), a glycolipid-type biosurfactant, could be used as a potential treatment in physical intestinal dystrophy. We conducted a 2 × 2 factorial experiment to investigate the protective effect of SPL in a dextran sulfate sodium (DSS)-induced colitis mouse model (first factor, presence of SPL in feed; second factor, presence of DSS in water). Forty C57BL/6 mice (8-week-old) were used, and they were allocated to treatments according to their initial body weight. After a 7 d adjustment period, the DSS treatment was initiated in specific groups. At day 14, DSS was withdrawn from mice, and half of the mice were randomly selected and euthanized to collect colon and colon content samples. Three days after the end of DSS treatment, the rest of the mice were euthanized to investigate the therapeutic effect of SPL. Dietary SPL improved the growth performance in 3 d after DSS treatment, and the histopathological score was lower in the DSS-treated SPL group than in the DSS-treated control group. Mucosal thickness and goblet cell numbers significantly increased in the SPL-supplemented groups compared to in the control group. Similarly, SPL supplementation upregulated the gene expression levels of mucin-2, interleukin-10, and transforming growth factor-β, and increased the concentration of short chain fatty acid compared to the control groups. In conclusion, dietary supplementation with SPL attenuated the pathological response against acute and chronic inflammation by the maintenance of the mucosal barrier and wound healing capacity.
1. Introduction
Inflammatory bowel disease (IBD) is an intestinal disorder, classified as ulcerative colitis or Crohn's disease. However, its exact mechanism of etiology or pathogenesis is not completely understood.1 IBD generally manifests pathologically as diarrhea, rectal bleeding, weight loss, and mucosal destruction.2 Moreover, the local inflammation and oxidative stress in IBD can extend to other organs through the systemic circulation.3 Therefore, further studies on IBD are needed because it has become a globally prevalent disease and can also induce fetal damage in humans.4Recently, dextran sulfate sodium (DSS)-induced ulcerative colitis has been accepted as a well-characterized IBD model morphologically and biochemically, and it is a useful and popular model to study the inflammatory mechanisms of colitis due to its simplicity and reproducibility.5 It is generally assumed that DSS can cause colitis by the bacterial production of inflammatory cytokines through direct toxicity to the gut epithelial membrane and mucosal barrier integrity.6 Thus, the DSS-induced colitis mouse model is suitable for an experiment to evaluate the protective effect of the gut against external stress factors.
Our previous studies revealed that dietary supplementation of sophorolipid (SPL), a glycolipid bio-surfactant, could improve the gut defense environment, including tight junctions, mucosal production, and the microbiota population.7 SPLs are produced by non-pathogenic yeasts, such as Candida bombicola, and are composed of a dimeric sugar linked by a glycosidic bond to a hydroxyl fatty acid.8 SPLs are more biodegradable emulsifiers, and dietary SPL supplementation can accelerate the wound healing process by stimulating collagen production.9–11 In addition, Kim et al. demonstrated that SPL is a safe ingredient to inhibit the growth of Propionibacterium acnes by orally administration up to 5 g of SPL per kg body weight.12 Therefore, we conducted an experiment using a DSS-induced colitis mouse model to evaluate the protective or restorative effects of SPL on intestinal dystrophy.
2. Materials and methods
2.1. Animals and diets
All studies were conducted in accordance with the guidelines and regulations of the Animal Ethics Committee approved by Korea University (Seoul, Republic of Korea; Approval number: KUIACUC-2020-0097). A total of 40, 8-week-old C57BL/6 mice (initial body weight: 21.14 g) were obtained from Samtako (Kyung-gi, Republic of Korea). The basal diet was a NIH-41 diet and its feed composition and calculated nutritional value are shown in Table 1. The feed and SPL were provided by EASY BIO Inc. (Seoul, Republic of Korea).
a Provided per kilogram of complete diet: vitamin A, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 IU; vitamin D3, 2500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; pantothenic acid, 15 mg; nicotinic acid, 40 mg; choline, 400 mg; vitamin B12, 12 μg; iron, 90 mg; copper, 8.8 mg; zinc, 100 mg; manganese, 54 mg; iodine, 0.35 mg; selenium, 0.30 mg. 000 IU; vitamin D3, 2500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; pantothenic acid, 15 mg; nicotinic acid, 40 mg; choline, 400 mg; vitamin B12, 12 μg; iron, 90 mg; copper, 8.8 mg; zinc, 100 mg; manganese, 54 mg; iodine, 0.35 mg; selenium, 0.30 mg.
|
|
|---|---|
| Ingredients, g kg −1 | |
| Ground whole hard wheat | 34.90 |
| Ground #2 yellow corn | 21.00 |
| Ground whole oats | 10.00 |
| Wheat middlings | 10.00 |
| Fish meal | 9.00 |
| Soy oil | 2.00 |
| Soybean meal | 5.00 |
| Alfalfa meal | 2.00 |
| Corn gluten meal | 2.00 |
| Dicalcium phosphate | 1.50 |
| Yeast-Brewers | 1.00 |
| Premixesa | 0.60 |
| Grounded limestone | 0.50 |
| Salt | 0.50 |
| Calculated composition | |
| Crude protein, g kg−1 | 180.0 |
| Crude fat, g kg−1 | 50.0 |
| Crude fiber, g kg−1 | 50.0 |
| Ash, g kg−1 | 80.0 |
| Calcium, g kg−1 | 10.0 |
| Total phosphorous, g kg−1 | 8.5 |
| Lysine, g kg−1 | 8.5 |
| Methionine, g kg−1 | 3.5 |
2.2. Experimental procedures and sample collection
Ten mice were randomly allocated to four treatments according to their initial body weight in a 2 × 2 factorial design. One factor was dietary supplementation (CON, NIH-41; SPL, 10 mg kg−1 of sophorolipid-supplemented diet) and the other factor was their drinking water (TW, tap water; DSS, 3% DSS treated water). DSS (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
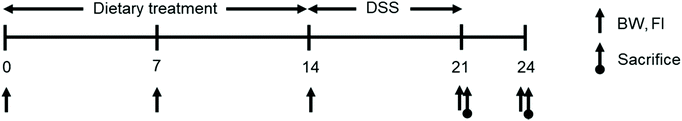
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW) was supplied by MP Biomedicals (Shanghi, China). Mice had free access to feed and water during the experiment, and their BW and feed intake were measured on days 0, 7, 14, and 17 to calculate average daily gain, average daily feed intake, and feed efficiency. The overall experimental scheme is represented in Fig. 1. On day 7, the CON-DSS and SPL-DSS groups received 3% DSS in their drinking water ad libitum for a further seven days. At the end of the DSS treatment, five mice from each treatment group were sacrificed to collect colon and cecal content samples. After three days of restoration, the other five mice in each treatment were also sacrificed. Samples were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis. Parts of the colon samples were fixed in 4% formalin solution for histopathological analysis.
000 MW) was supplied by MP Biomedicals (Shanghi, China). Mice had free access to feed and water during the experiment, and their BW and feed intake were measured on days 0, 7, 14, and 17 to calculate average daily gain, average daily feed intake, and feed efficiency. The overall experimental scheme is represented in Fig. 1. On day 7, the CON-DSS and SPL-DSS groups received 3% DSS in their drinking water ad libitum for a further seven days. At the end of the DSS treatment, five mice from each treatment group were sacrificed to collect colon and cecal content samples. After three days of restoration, the other five mice in each treatment were also sacrificed. Samples were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis. Parts of the colon samples were fixed in 4% formalin solution for histopathological analysis.
2.3. Histological analysis in colon
Fixed colon samples were embedded into paraffin blocks, and 5 μm cross-sections were prepared using a rotary microtome CUT 5062 (SLEE MAINZ, Mainz, Germany). These sections were stained using the Alcian blue staining method. A single observer measured the thickness of the mucus and counted the goblet cell numbers. Colon disruptions induced by DSS treatment were scored according to the scoring system described by Villanacci et al., and the scoring criteria are presented in Table 2.13| Indices | Score | Intensity | Histological evidence |
|---|---|---|---|
| Chronic inflammation | 0 | No increase | Normal number of inflammatory cells |
| 1 | Moderate | Moderate number of inflammatory cells Aggregated between crypts | |
| 2 | Severe | Marked increase in chronic inflammation | |
| Active inflammation | 0 | Normal | Neutrophils not present |
| 1 | Low grade | Neutrophils present transmigrating through the crypt epithelium or within crypt lumina in <20% of crypts | |
| 2 | Moderate | Neutrophilic infiltration in >20% of crypts or presence of erosions | |
| 3 | High grade | Presence of ulcers | |
| Crypt distortion | 0 | None | Crypts have normal outlines |
| 1 | Mild | Scattered crypts showing irregular outline | |
| 2 | Moderate | 25%–50% of crypts with an irregular outline | |
| 3 | Severe | >50% of crypts with an irregular outline | |
2.4. Cecal short chain fatty acid measurement at the end of DSS-treatment
The short chain fatty acid (SCFA) concentration in the colon of the colitis-induced mice was measured using a gas chromatography-mass spectrophotometer according to the method of Furusawa et al.14 Cecal contents (10 mg) were homogenized with an extraction solution consisting of 100 μL of internal standard (100 μmol L−1 crotonic acid), 100 μL hydrochloric acid, and 200 μL of ether. After vigorous vortexing for 10 min, the homogenates were centrifuged at 1000g for 10 min, and 80 μL of the supernatant was transferred into new glass vials. Aliquots were mixed with 16 μL of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide and sealed tightly. The glass vials were heated at 80 °C for 20 min in a water bath and then left at room temperature for 48 h for derivatization. The derivatized samples were run through a 6890N Network GC System with an HP-5MS column and 5973N network mass selective detector. Pure helium was used as the carrier gas and delivered at a 1.2 mL min−1 flow rate. The head pressure was set to 97 kPa with a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split. The inlet temperature was 250 °C, and the transfer line temperature was 260 °C. The temperature program was as follows: 60 °C for 3 min, 60–120 °C (5 °C min−1), and 120–300 °C (20 °C min−1). The run time was 30 min, and the SCFA concentrations were quantified by comparing their peak areas with standard controls.
1 split. The inlet temperature was 250 °C, and the transfer line temperature was 260 °C. The temperature program was as follows: 60 °C for 3 min, 60–120 °C (5 °C min−1), and 120–300 °C (20 °C min−1). The run time was 30 min, and the SCFA concentrations were quantified by comparing their peak areas with standard controls.
2.5. Analysis of colon contents after DSS-treatment using qRT-PCR
Total RNA from colon samples was extracted using TRIzol® (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions, and the concentration and purity of RNA were determined using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Subsequently, cDNA samples were synthesized using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions. Gene expression levels of inflammatory cytokines, including mucin-2 (MUC2), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) in the colon, were determined using a RealHelixTM Premier qPCR kit (NanoHelix, Daejun, Korea) with the StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA); the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. The 2−ΔΔCT method was used to quantify the relative mRNA expression levels. The primers for the target genes are listed in Table 3.| Gene name | Sequence (forward, reverse) | Ref. |
|---|---|---|
| GAPDH | 5′-ATGGGAAGCTGGTCATCAAC-3′ | 37 |
| 5′-TGTGAGGGAGATGCTCAGTG-3′ | ||
| MUC2 | 5′-CGACACCAGGGATTTCGCTTAAT-3′ | 38 |
| 5′-CACTTCCACCCTCCCGGCAAAC-3′ | ||
| TNF-α | 5′-AAGCCTGTAGCCCACGTCGTA-3′ | 39 |
| 5′-GGCACCACTAGTTGGTTGTCTTTG-3′ | ||
| IL-10 | 5′-AAGTGATGCCCCAGGCA-3′ | 40 |
| 5′-TCTCACCCAGGGAATTCAAA-3′ | ||
| TGF-β | 5′-GCTAATGGTGGACCGCAACAAC-3′ | 41 |
| 5′-CACTGCTTCCCGAATGTCTGAC-3′ |
2.6. Statistical analysis
All data were analyzed by analysis of variance (ANOVA) using Statistical System 9.4 (SAS Institute, Cary, NC, USA). Significant differences between treatments were determined using Duncan's multiple-range tests and were defined at a significance level of P < 0.05.3. Results
3.1. Growth performance
The growth performance of colitis-induced mice fed the experimental diets during the experimental period is summarized in Table 4. Mice drinking DSS-containing water had significantly lower growth performance indicators (body weight, average daily gain, average daily feed intake, and feed efficiency) compared to tap water drinking mice. Three days after DSS treatment, the average daily gain and feed efficiency in mice fed SPL-supplemented feed numerically recovered by 8.5% and 7.1%, respectively, compared to in the mice in the CON group.| CON | SPL | SEM | p-value | |||
|---|---|---|---|---|---|---|
| TW | DSS | TW | DSS | |||
| a Treatments: CON, control group fed with basal diet; SPL, group fed with 10 mg kg−1 of sophorolipid supplemented diet; TW, tap water drinking group; DSS, DSS-containing water drinking group; SEM, standard error of mean. b BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FE, feed efficiency. c A mouse is an experimental unit; 5 replications (pens) per treatment. a,b Mean values within a row have different superscript letters were significantly different (P < 0.05). | ||||||
| BW, g | ||||||
| Day 0 | 21.06 | 21.37 | 21.06 | 21.17 | 0.149 | 0.913 |
| Day 7 | 23.44 | 23.90 | 23.64 | 24.00 | 0.310 | 0.938 |
| Day 14 | 24.26a | 21.00b | 24.42a | 20.90b | 0.519 | 0.003 |
| Day 17 | 24.36a | 17.93b | 24.52a | 18.07b | 0.885 | 0.000 |
| ADG, g day−1 | ||||||
| Day 0–7 | 0.34 | 0.36 | 0.37 | 0.40 | 0.028 | 0.908 |
| Day 7–14 | 0.12a | −0.41b | 0.11a | −0.44b | 0.070 | 0.000 |
| Day 14–17 | 0.03a | −1.02b | 0.03a | −0.94b | 0.136 | 0.000 |
| ADFI, g day−1 | ||||||
| Day 0–7 | 4.14 | 3.88 | 3.80 | 4.16 | 0.066 | 0.103 |
| Day 7–14 | 3.79a | 3.48b | 3.96a | 3.35b | 0.078 | 0.007 |
| Day 14–17 | 4.29a | 1.83b | 3.99a | 1.81b | 0.296 | 0.000 |
| FE | ||||||
| Day 0–7 | 0.082 | 0.093 | 0.097 | 0.097 | 0.006 | 0.767 |
| Day 7–14 | 0.031a | −0.119b | 0.028a | −0.132b | 0.020 | 0.000 |
| Day 14–17 | 0.008a | −0.559b | 0.008a | −0.522b | 0.078 | 0.000 |
3.2. Colon histology
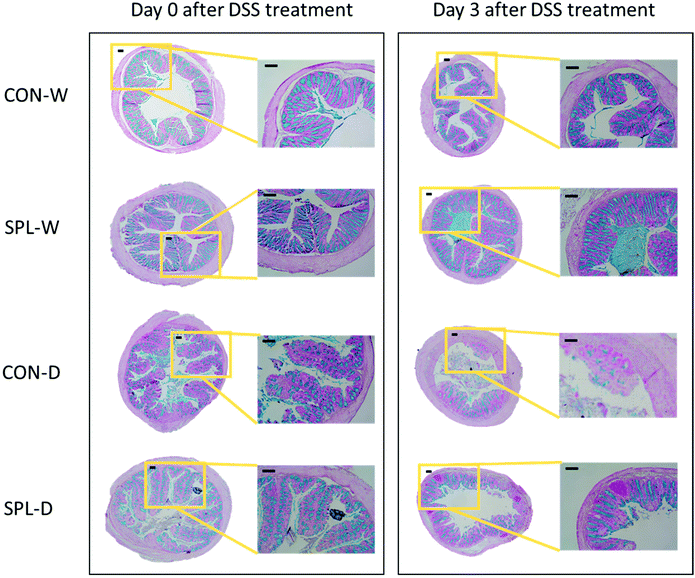
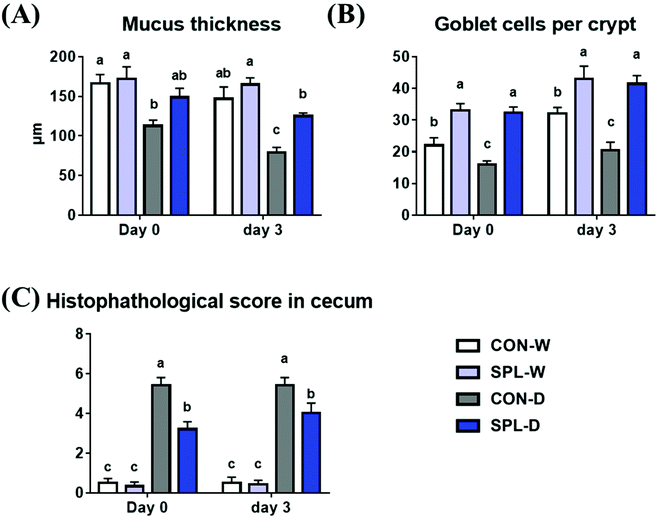
Representative images of the colon in colitis-induced mice at days 0 and 3 after DSS treatment are shown in Fig. 2, and the results of the histological analysis are presented in Fig. 3. The crypt outline in the colon of mice fed the SPL-supplemented diet collapsed less than in in the colon of mice in the CON group. Similarly, the mucosal thickness of the colitis-induced SPL group was higher than of the colitis-induced CON group (P < 0.05) and dietary SPL supplementation in both the TAP and DSS treatments increased the goblet cell count in the colon compared to the CON group (P < 0.05). Moreover, dietary SPL supplementation lowered the histopathological score compared to the CON group after the 7-day DSS treatment (P < 0.05).3.3. Cecal SCFA analysis after DSS-treatment
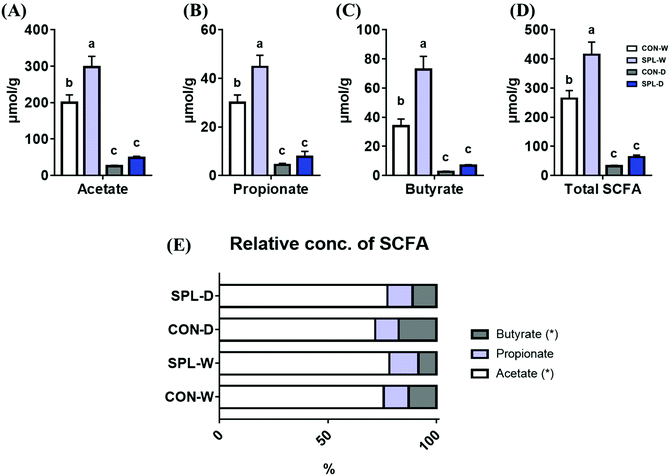
The absolute concentrations of SCFA (acetate, propionate, butyrate, and total) and the relative concentrations of SCFAs are shown in Fig. 4. The concentrations of all SCFAs (acetate, propionate, butyrate, and total) were increased by dietary SPL supplementation in the TAP groups compared to the CON group (P < 0.05). In addition, dietary SPL supplementation in colitis-induced mice also numerically increased the SCFA concentration compared to the colitis-induced CON group.3.4. The qRT-PCR analysis 3 d after DSS-treatment
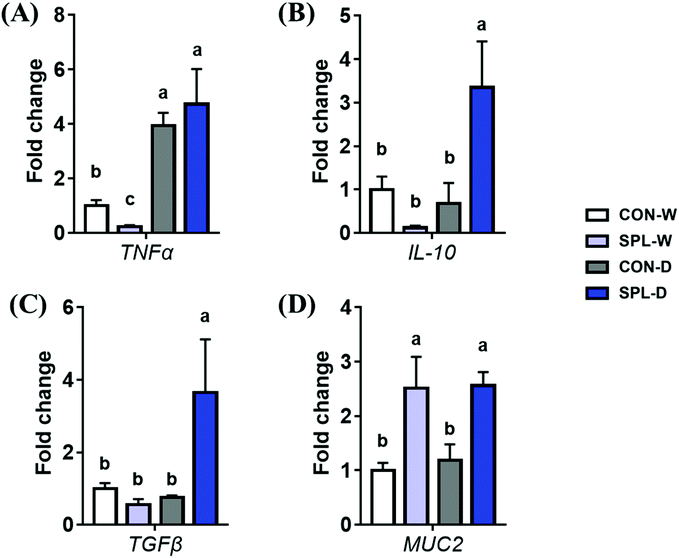
The gene expression levels of TNF-α, IL-10, TGF-β, and MUC2 in the colon of mice are shown in Fig. 5. The expression level of TNF-α was significantly increased by DSS-treatment in both CON and SPL groups (P < 0.05). However, the expression level of MUC2 were increased by dietary SPL supplementation in both the TAP and DSS groups (P < 0.05). Additionally, dietary SPL supplementation increased the gene expression levels of IL-10 and TGF-β after colitis induction (P < 0.05).4. Discussion
A chronic gut inflammatory disease, IBD, has recently been identified as a public health issue because of its rapidly increasing incidence worldwide.15 The pathogenesis of IBD is still unknown, and various studies have proposed that genetic and environmental factors as the main reasons.16 One of the critical pathologies of IBD is intestinal barrier dysfunction by destruction of epithelial mucus and an increase in proinflammatory cytokine levels.17 Several clinical methods have been employed to treat IBD, including drugs, immunomodulating factors, antibiotics, and surgical methods; however, they induce severe side effects or incur enormous costs.18 Consequently, various researchers have searched for novel, highly efficient, and low-risk therapeutic approaches to overcome the public fear of IBD.In this study, we proposed that dietary SPL supplementation during the experimental period could improve growth performance after removal of DSS-containing water. SPL is a type of glycolipid type biosurfactant, and it has already been applied in the hygiene and medicine industry to prevent secondary infection.19 In accordance with this study, Gu et al. demonstrated that tilapia head glycolipids, a candidate prebiotic, could ameliorate damage from DSS-treatment by the upregulation of the beneficial bacteria in the gut microbiota.20 Various studies have postulated a shift in gut bacteria from pathogenic to beneficial populations as a novel therapeutic approach to IBD.21,22 In our previous study, dietary SPL supplementation replaced the use of antibiotics in broiler chickens through a rapid modulation of the gut microbial population.23 However, further study is needed to elucidate the therapeutic effect of SPL on gut microbiota in a DSS-induced colitis mouse model.
The continuous mucus layer that covers the colon's epithelium can produce a symbiotic homeostasis between the gut microbiota and the host.24 During inflammation in the colon, the homeostatic balance shifts negatively by disruption of the mucus layer, and the immune system and inflammation are activated.25 Petersson et al. demonstrated that mucosal thickness was correlated with colitis progression using DSS, and the regeneration of mucus could be one of the treatments for chronic colitis.26 Our previous studies demonstrated that dietary SPL could ameliorate intestinal dystrophy by the strengthened mucus barrier integrity and structure through increment of mucus-presenting cells, resulting in acceleration of growth in the early-weaned rat.7 Therefore, we also performed histological evaluation in cecum or DSS-treated mouse, and our results suggest that dietary SPL supplementation can also protect mucosal destruction after DSS-treatment through upregulation of the mucus-presenting cell numbers. Nishida et al. suggested that MUC2 plays an important role in the gut defense system by lowering inflammation and improving gut integrity because it is the core protein of membrane-linked mucin.27 Similarly, the results of the current study also demonstrate that the dietary addition of an adequate dosage of SPL improves colonic mucosal layer integrity regardless of DSS treatment. Consequently, our study proposed that adequate dietary SPL supplementation had the potential to increase mucosal generation in the gastrointestinal tract.
Furthermore, cecal SCFAs are known to be signaling molecules that are regulated by the gut microbiota population and they could maintain the metabolic status of colonic epithelial cell layers.28 SCFAs (acetate, propionate, and butyrate) protect the intestinal defense layer by fortifying the tight junctions against external injury and increasing the fuel energy available to enterocytes.29,30 In our study, dietary SPL supplementation upregulated the production of all SCFAs regardless of DSS treatment. Xu et al. demonstrated that SPL could be applied in the hygiene industry to remove waste-activated sludge by producing SCFAs in domestic wastewater.31 They demonstrated that SPL in waste-activated sludge solubilization could enhance SCFA production by increasing the population of anaerobic hydrolytic microbes and decreasing the population of methanogens. In our previous study, dietary SPL supplementation increased the abundance of the SCFA-producing bacteria, Lactobacillus helveticus and Akkermansia muciniphila in the cecum of broiler chickens.23 Also, Willem suggested that Akkermansia muciniphila conserve intestinal symbiont by induction of signaling pathways related to gut immune and metabolic mechanisms through producing SCFA with degradation of gut mucin.32 However, further studies will be needed to investigate the shift of intestinal bacterial communities by DSS treatment and SPL addition in the feed.
Various studies have reported that SPL exerts wound healing activity through antibiotic and immunomodulating effects,33,34 and this is the first study to indicate the ameliorating effects of SPL in a DSS animal model. In 2017, Ihara suggested that TGF-β signaling pathway-related adhesion molecules could be the next candidates for IBD treatment, because the TGF-β pathway is implicated in the pathogenesis of IBD.35 In accordance with this study, we also found that SPL-fed mice had higher gene expression of TGF-β in colonic tissues. Moreover, several reports have indicated that IL-10 plays an important role in the maintenance of intestinal homeostasis.36 Keubler et al. proposed that IL-10, produced by Treg cells, sufficiently suppressed innate and adaptive immune responses to establish a counterbalance after acute immune responses. The increasing effect of SPL on the expression level of IL-10 might be related to the preservation of intestinal barrier integrity after DSS treatment. Dietary addition of SPL could directly upregulate tissue remodeling and mucin-presenting capacities and preserve gut homeostasis after acute inflammatory induction.
Collectively, dietary sophorolipid treatment in colitis-induced mice ameliorated epithelial damage in the colon by increasing the mucosal layer and mucus-presenting cells via colonal SCFA. The gene expression of anti-inflammatory cytokines and tissue remodeling factors was also increased by dietary SPL, resulting in improved growth and feed efficiency after colitis induction. Further studies will be needed to elucidate whether the therapeutic effect of SPL occurs as a result of the direct action of SPL, or indirectly via modulation of the gut microbiota.
Author contributions
Min-Jin Kwak and Dong-Jin Ha contributed to this work equally and they are regarded as co-first authors. They had contributed conceptualization, analysis, data curation, writing, and revision. Min-Jin Kwak and Yong-Soon Choi had contributed investigation and data curation. Hanbae Lee corresponded for the resources and funding. Kwang-Youn Whang corresponded for conceptualization, supervision, and project administration.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by Intermediate Pathway, Inc., and Korea University [Q1813691].References
- X. Ma, Y. Hu, X. Li, X. Zheng, Y. Wang, J. Zhang, C. Fu and F. Geng, Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation, Front. Pharmacol., 2018, 9, 944–959 CrossRef PubMed.
- B. O. Ajayi, J. A. Adedara and E. O. Farombi, Pharmacological activity of 6-gingerol in dextran sulphate sodium–induced ulcerative colitis in BALB/c mice, Phytother. Res., 2015, 29, 566–572 CrossRef CAS PubMed.
- A. R. Parmar, P. P. Trivedi and G. B. Jena, Dextran sulfate sodium-induced ulcerative colitis leads to testicular toxicity in mice: Role of inflammation, oxidative stress and DNA damage, Reprod. Toxicol., 2014, 49, 171–184 CrossRef CAS PubMed.
- M. Mohamadzadeh, E. A. Pfeiler, J. B. Brown, M. Zadeh, M. Gramarossa, E. Managlia, P. Bere, B. Sarraj, M. W. Khan, K. C. Pakanati, M. J. Ansari, S. O'Flaherty, K. T. Barrett and T. R. Klaenhammer, Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4623–4630 CrossRef CAS PubMed.
- B. Chassaing, J. D. Aitken, M. Malleshappa and M. Vijay-Kumar, Dextran sulfate sodium (DSS)–induced colitis in mice, Curr. Protoc. Immunol., 2014, 104, 15–25 Search PubMed.
- T. C. Huang, S. S. Tsai, L. F. Liu, Y. L. Liu, H. J. Liu and K. P. Chuang, Effect of Arctium lappa L. in the dextran sulfate sodium colitis mouse model, World J. Gastroenterol., 2010, 16, 4193–4199 CrossRef PubMed.
- M. J. Kwak, M. Y. Park, J. Kim, H. B. Lee and K. Y. Whang, Curative effects of sophorolipid on physical wounds: in vitro and in vivo studies, Vet. Med. Sci., 2021, 2021, 1–9 Search PubMed.
- D. W. Develter and L. M. Lauryssen, Properties and industrial applications of sophorolipids, Eur. J. Lipid Sci. Technol., 2010, 112, 628–638 CrossRef CAS.
- W. R. Finnerty, Biosurfactants in environmental biotechnology, Curr. Opin. Biotechnol., 1994, 5, 291–295 CrossRef CAS.
- J. D. Desai and I. M. Banat, Microbial production of surfactants and their commercial potential, Microbiol. Mol. Biol. Rev., 1997, 61, 47–64 CAS.
- K. J. Cho, Y. B. Kim and E. K. Kim, Production and application of sophorolipid, a microbial surfactant, KSBB J., 1999, 14, 747–753 Search PubMed.
- K. Kim, D. You, Y. Kim, B. Lee, D. Shin and E. K. Kim, Characteristics of sophorolipid as an antimicrobial agent, J. Microbiol. Biotechnol., 2002, 12, 235–241 CAS.
- V. Villanacci, E. Antonelli, K. Geboes, G. Casella and G. Bassotti, Histological healing inflammatory bowel disease: A still unfulfilled promise, World J. Gastroenterol., 2013, 19, 968–978 CrossRef PubMed.
- Y. Furusawa, Y. Obata, S. Fukuda, T. A. Endo, G. Nakato, D. Takahashi, Y. Nakanishi, C. Uetake, K. Kato, T. Kato, M. Takahashi, N. N. Fukuda, S. Murakami, E. Miyauchi, S. Hino, K. Atarashi, S. Onawa, Y. Fujimura, T. Lockett, J. M. Clarke, D. L. Topping, M. Tomita, S. Hori, O. Ohara, T. Morita, H. Koseki, J. Kikuchi, K. Honda, K. Hase and H. Ohno, Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells, Nature, 2013, 504, 446–450 CrossRef CAS PubMed.
- G. G. Kaplan and S. C. Ng, Understanding and preventing the global increase of inflammatory bowel disease, Gastroenterology, 2017, 152, 313–321 CrossRef PubMed.
- Y. Wang, N. Zhang, J. Kan, X. Zhang, X. Wu, R. Sun, S. Tang, J. Liu, C. Qian and C. Jin, Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice, Carbohydr. Polym., 2019, 213, 89–99 CrossRef CAS PubMed.
- S. Yin, H. Yang, Y. Tao, S. Wei, L. Li, M. Liu and J. Li, Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response, Inflammation, 2020, 43, 765–776 CrossRef CAS PubMed.
- Y. S. Chiou, J. L. Ma, S. Sang, C. T. Ho, Y. J. Wang and M. H. Pan, Peracetylated (-)-epigallocatechin 3-gallate (AcEGCG) potently suppresses detran sulfate sodium-induced colitis and colon tumorigenesis in mice, J. Agric. Food Chem., 2012, 60, 3441–3451 CrossRef CAS PubMed.
- C. Valotteau, N. Baccile, V. Humblot, S. Roelants, W. Soetaert, C. V. Stevens and Y. F. Dufrêne, Nanoscale antiadhesion properties of sophorolipid-coated surfaces against pathogenic bacteria, Nanoscale Horiz., 2019, 4, 975–982 RSC.
- Z. Gu, Y. Zhu, S. Jiang, G. Xia, C. Li, X. Zhang, J. Zhang and X. Shen, Tilapia head glycolipids reduce inflammation by regulating the gut microbiota in dextran sulphate sodium-induced colitis mice, Food Funct., 2020, 11, 3245–3255 RSC.
- K. Praengam, Y. Sahasakul, P. Kupradinun, S. Sakarin, W. Sanitchua, A. Rungsipipat, K. Rattanapinyopituk, P. Angkasekwinai, K. Changsri, W. Mhuantong, S. Tangphatsornruang and S. Tuntipopipat, Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice, Food Funct., 2017, 8, 4630–4643 RSC.
- K. Sheng, G. Zhang, M. Sun, S. He, X. Kong, J. Wang, F. Zhu, X. Zha and Y. Wang, Grape seed proanthocyanidin extract ameliorate dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation, Food Funct., 2020, 11, 7817–7829 RSC.
- M. J. Kwak, M. Y. Park, Y. S. Choi, J. Cho, D. Pathiraja, J. Kim, H. Lee, I. G. Choi and K. Y. Whang, Dietary sophorolipid accelerates growth by modulation of gut microbiota population ant intestinal environments in broiler chickens, J. Anim. Sci. Biotechnol., 2021, 12, 1–9 Search PubMed.
- F. Backhed, R. E. Ley, J. L. Sonnenburg, D. A. Peterson and J. I. Gordon, Host-bacterial mutualism in the human intestine, Science, 2005, 307, 1915–1920 CrossRef PubMed.
- M. T. Abreu, Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function, Nat. Rev. Immunol., 2010, 10, 131–144 CrossRef CAS PubMed.
- J. Peterson, O. Schreiber, G. C. Hansson, S. J. Gendler, A. Velcich, J. O. Lundberg, S. Roos, L. Holm and M. Phillipson, Importance and regulation of the colonic mucus barrier in a mouse model of colitis, Am. J. Physiol.: Gastrointest. Liver Physiol., 2011, 300, G327–G333 CrossRef PubMed.
- K. Nishida, M. Kamizato, T. Kawai, K. Masuda, K. Takeo, S. Teshima-Kondo, T. Tanahashi and K. Rokutan, Interleukin–18 is a crucial determinant of vulnerability of the mouse rectum to psychosocial stress, FASEB J., 2009, 23, 1797–1805 CrossRef CAS PubMed.
- S. Murugesan, K. Nirmalkar, C. Hoyo-Vadillo, M. Espitia, D. Ramírez-Sánchez and J. Garcia-Mena, Gut microbiome production of short-chain fatty acids and obesity in children, Eur. J. Clin. Microbiol. Infect. Dis., 2018, 37, 621–625 CrossRef CAS PubMed.
- Z. Zha, Y. Lv, H. Tang, T. Li, Y. Miao, J. Cheng, G. Wang, Y. Tan, Y. Zhu, X. Xiao, D. Kang, W. Ying and Y. Hongping, An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis, Int. J. Biol. Macromol., 2020, 156, 1217–1233 CrossRef CAS PubMed.
- P. Thomson, D. Medina, M. Ortúzar, M. Gotteland and D. Garrido, Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides, Food Res. Int., 2018, 109, 14–23 CrossRef CAS PubMed.
- Q. Xu, X. Liu, D. Wang, Y. Liu, Q. Wang, B. J. Ni, X. Li, Q. Yang and H. Li, Enhanced short-chain fatty acids production from waste activated sludge by sophorolipid: Performance, mechanism, and implication, Bioresour. Technol., 2019, 284, 456–465 CrossRef CAS PubMed.
- M. Willem, Microbe Profile: Akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa, Microbiology, 2017, 163, 646–648 CrossRef PubMed.
- H. L. Lydon, N. Baccile, B. Callaghan, R. Marchant, C. A. Mitchell and I. M. Banat, Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing, Antimicrob. Agents Chemother., 2017, 61, e02547–16 CrossRef CAS PubMed.
- S. V. More, S. S. Koratkar, N. Kadam, S. Agawnae and A. Prabhune, Formulation and evaluation of wound healing activity of sophorolipid-sericin gel in wistar rats, Pharmacogn. Mag., 2019, 15, 123–127 CAS.
- S. Ihara, Y. Hirata and K. Koike, TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota, J. Gastroenterol., 2017, 52, 777–787 CrossRef CAS PubMed.
- L. M. Keubler, M. Buettner, C. Hager and A. Bleich, A multihit model: colitis lessons from the inerleukin-10-deficient mouse, Inflamm. Bowel Dis., 2015, 21, 1967–1975 CrossRef PubMed.
- R. C. Brown, A. P. Morris and R. G. O'Neil, Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells, Brain Res., 2007, 1130, 17–30 CrossRef CAS PubMed.
- P. Garg, A. Ravi, N. R. Patel, J. Roman, A. T. Gewirtz, D. Merlin and S. V. Sitaraman, Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation, Gastroenterology, 2007, 132, 1877–1889 CrossRef CAS PubMed.
- P. Khare, S. Jagtap, Y. Jain, R. K. Baboota, P. Mangal, R. K. Boparai, K. K. Bhutani, S. S. Sharma, L. S. Premkumar, K. K. Kondepudi and K. Chopra, Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice, BioFactors, 2016, 42, 201–211 CrossRef CAS PubMed.
- Y. Zhang, Z. Wang, H. Xiao, X. Liu, G. Zhu, D. Yu, G. Han, G. Chen, C. Hou, N. Ma, B. Shen, Y. Li, T. Wang and R. Wang, Foxd3 suppresses interleukin-10 expression in B cells, Immunology, 2016, 150, 478–488 CrossRef PubMed.
- A. Rodríguez-Nogales, F. Algieri, J. Garrido-Mesa, T. Vezza, M. P. Utrilla, N. Chueca, F. Garcia, M. Olivares, M. E. Rodríguez-Cabezas and J. Gálvez, Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition, Mol. Nutr. Food Res., 2017, 61, 1700144 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |