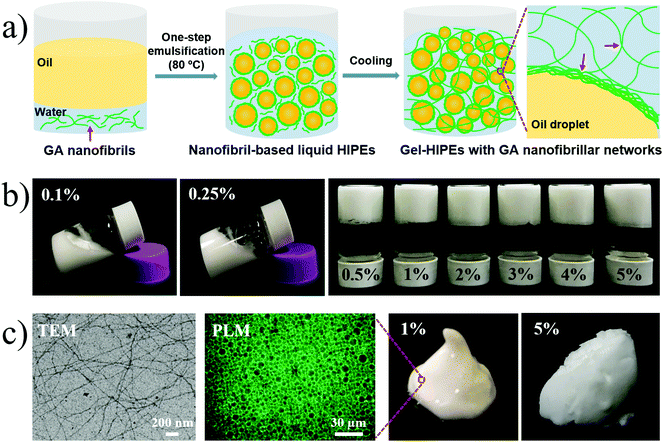
Robust and highly adaptable high internal phase gel emulsions stabilized solely by a natural saponin hydrogelator glycyrrhizic acid†
Mengyue
Xu‡
a,
Lulu
Ma‡
a,
Qing
Li
a,
Jiahao
Wu
b,
Zhili
Wan
 *abc,
To
Ngai
*abc,
To
Ngai
 *b and
Xiaoquan
Yang
*b and
Xiaoquan
Yang
 a
a
aLaboratory of Food Proteins and Colloids, School of Food Science and Engineering, Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, South China University of Technology, Guangzhou 510640, China
bDepartment of Chemistry, The Chinese University of Hong Kong, Shatin, N. T., Hong Kong, China. E-mail: zhiliwan@scut.edu.cn; tongai@cuhk.edu.hk; Fax: +(086) 20-87114263; Fax: +(852) 26035057; Tel: +(852)39431223
cOverseas Expertise Introduction Center for Discipline Innovation of Food Nutrition and Human Health (111 Center), Guangzhou 510640, China
First published on 22nd November 2021
Abstract
Herein, we report a new class of high internal phase gel emulsions (gel-HIPEs) that are mechanically robust, adaptable, and processable. They can be synthesized facilely by using the natural food-grade saponin glycyrrhizic acid (GA) as the sole stabilizer, which is shown to be versatile for various oils. The structural properties of these HIPEs including appearance, viscoelasticity and processability are well controlled by simply changing the concentration of GA nanofibrils. When the GA nanofibril concentration exceeds 0.3 wt%, the unique gel-HIPEs can be produced through the formation of fibrillar hydrogel networks in the continuous phase. When the nanofibril concentration only increases to 5 wt%, it is surprising to see that these gel-HIPEs display an extremely high mechanical strength, and the storage moduli as well as the yield stress values can reach 408.5 kPa and 3340 Pa (or even more), respectively. We conjecture that such remarkable mechanical performance is mainly attributed to the highly viscoelastic GA nanofibrillar networks in the continuous phase of gel-HIPEs, which can actively trap the nanofibril-coated emulsion droplets and thus strengthen the gel matrix. Consequently, the robust gel-HIPEs can be used as a solid template to fabricate stable porous materials without the need for crosslinking of the continuous phase, and the open- and closed-cell foam microstructures are controlled by the nanofibril concentration. Furthermore, the nanofibril-based HIPEs are promising long-term delivery vehicles with controlled-release properties for lipophilic active cargoes, since the strong fibrillar networks at the droplet surfaces and in the continuous phase can effectively retard the active release.
1. Introduction
Gel emulsions are complex structured soft materials, which display both the properties of traditional multiphase emulsions and the behaviors of physical gels.1 Due to their unique rheological properties and mesoscale structures, gel emulsions are of outstanding importance in various applications, such as in foods with structured oils, cosmetics, pharmaceuticals, detergency, and tissue engineering, or serve as liquid templates for the synthesis of porous materials.2,3 As one of the most common and unique types of gel emulsions, high internal phase emulsions (HIPEs) that possess an internal phase volume fraction exceeding 0.74 have shown great potential in various applications owing to their interesting structural features and a solid-like viscoelastic behavior arising from the tight packing of the dispersed droplets.1,4In view of their wide applications, the efficient and facile fabrication of stable HIPEs is highly required and remains an important issue in emulsion science due to their ultrahigh internal phase fraction and thermodynamic instability. Typically, the formation of HIPEs generally requires the addition of interfacial stabilizers, such as small surfactants (e.g., sorbitan monooleate and CTAB),5,6 amphiphilic polymers,7 or colloidal particles (e.g., silica and PNIPAM-based microgel particles).8,9 In comparison with these surfactant-stabilized HIPEs, the HIPEs stabilized by colloidal particles, known as Pickering HIPEs, are less susceptible to coalescence, Ostwald ripening, and creaming due to the irreversible adsorption of the particles at the interface, which makes them highly stable for months or even years.10 However, the preparation of Pickering HIPEs requires specially surface-modified particles with appropriate amphiphilicity,9,10 and the formed emulsions are sometimes unstable and may undergo phase inversion at a high volume fraction (around 0.7) of the dispersed droplets.11,12 In recent years, considering the environmental drawbacks and even the potential safety concerns of synthetic surfactants or inorganic particles, there is growing interest in the development of food-grade Pickering HIPEs by using particles of natural origin as stabilizers, such as particles made of animal and plant proteins,13–15 modified starch,16 cellulose,17 and chitin.18 Unfortunately, these natural building blocks are often restricted by relatively poor solubility and uncontrollable particle wettability, and thus require complicated and time-consuming fabrication and surface modification processes, which limit their use as efficient and robust stabilizers to make food-grade HIPEs. Therefore, it remains highly desirable to explore new building blocks from natural edible substances, for the facile synthesis of stable and sustainable HIPEs for their safe applications in food, pharmaceutical, and personal care products.
Recently, we reported a new kind of food-grade oil-in-water (O/W) gel emulsion which was solely stabilized by a naturally occurring triterpenoid saponin glycyrrhizic acid (GA).19,20 GA is the main active compound ingredient of licorice root extract and is widely used as a sweetening agent in candies and sweets or as drugs for treating liver and skin diseases due to its wide biological activities. As a chiral molecule, GA has a hierarchical self-assembly in water forming long semiflexible nanofibrils (few nm thickness), which then form a nematic supramolecular hydrogel with a hydrogen-bond 3D fibrillar network when the nanofibril concentration is above 0.3 wt%.19,21 These amphiphilic nanofibrils display spatially controllable self-assembly at liquid–liquid interfaces and in the aqueous phase,22–25 forming fibrillar gel networks in the continuous phase as well as on the surfaces of emulsion droplets after emulsification, which thus leads to the formation of oil-in-water gel emulsions. The most prominent feature of our GA nanofibril-based gel emulsions is that their gelation behaviors mainly rely on the assembled fibrillar hydrogel network in bulk, rather than the tight stacking of the dispersed droplets covered by GA nanofibrils; therefore, the volume fraction of the dispersed phase of our GA nanofibril gel emulsions can be varied continuously (below and above 0.74), which does not significantly affect the stability and structural properties of the whole emulsions. Recent studies have demonstrated that the gel emulsion strategy based on the use of suitable low-molecular-weight gelator molecules is a potential solution to overcome the common problems of traditional HIPEs.26,27 For example, the mechanical and long-term storage stabilities of HIPEs can be obviously increased by the gelation of the continuous phase as well as the compact stacking of the dispersed droplets, and their structural properties also can be highly controlled by tuning the amounts of gelators.26,27 Therefore, there is no doubt that this structuring strategy can broaden the practical applications of HIPEs.
Herein, we exploit the use of natural GA nanofibrils as a sole stabilizer for the facile and efficient synthesis of a new class of structured O/W HIPEs. Stable and processable gel-HIPEs can be rapidly fabricated by adopting a simple one-step emulsification at high temperature (80–90 °C) with subsequent cooling (Fig. 1a), which has the advantage of not requiring specialized equipment, the addition of any synthetic surfactants, particles, or polymers, or any surface modification processes. During the hot homogenization (80–90 °C), GA nanofibrils with favorable interfacial activity form multilayer interfacial networks to stabilize the generated emulsion droplets (Fig. 1a and c).19,20,22 The subsequent cooling can strengthen the interfibrillar hydrogen-bond interactions leading to the formation of viscoelastic hydrogel networks in the continuous phase and at the droplet surfaces, and then the gel-HIPEs can be created (Fig. 1). We then characterized the mechanical properties and microstructure of gel-HIPEs by performing a small-deformation rheological test and a range of microscopy techniques, respectively. Finally, the potential applications of these gel-HIPEs as templates for the synthesis of biosafe porous materials or as natural delivery vehicles for hydrophobic functional ingredients (e.g., β-carotene) were evaluated.
2. Experimental section
2.1 Materials
Glycyrrhizic acid mono ammonium salt (GA, purity >98%) was purchased from Acros Organics, USA. Sunflower oil (Standard Foods Co., Shanghai, China), algal oil (Type II, Runke Bioengineering Co., Ltd, Shantou, China), medium chain triglycerides (MCTs) (Yuanye Biotechnology Co., Ltd, Shanghai, China), mineral oil (Alfa Aesar, Tianjin, China), castor oil (Aladdin, Shanghai, China), dodecane (Tokyo Chemical Industry (TCI), Tokyo, Japan), and hexane (TCI, Tokyo, Japan) were used without further purification. Nile Red was purchased from Sigma-Aldrich (St Louis, MO, USA). β-Carotene (purity >96%) was obtained from Aladdin (Shanghai, China). Milli-Q water (18.2 MΩ cm) was used throughout this work. All other chemicals used were of analytical grade.2.2 Preparation of GA nanofibril-stabilized HIPEs
A stock solution of GA nanofibrils (25 wt%) was prepared by dissolving GA powder in water in sealed glass vials (2.5 cm internal diameter, 9 cm height), and heating it at 90 °C under mild agitation to obtain a clear solution. The HIPEs were prepared by first dispersing oils in hot GA nanofibril solutions (80–90 °C) under mild agitation for 3 min, and then the resulting dispersions were immediately homogenized at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 s using an Ultra-Turrax T10 (IKA-Werke GmbH & Co., Germany). The resultant HIPEs were cooled and then stored overnight (12 h) at room temperature (25 °C) before further use. To obtain the dried gel-HIPEs, the volatile hexane was chosen as the oil phase to prepare the nanofibril-based HIPEs. The prepared HIPE samples were then subjected to air-drying in an oven at 25 °C for 24 h, or to lyophilization, where the samples were first frozen at −40 °C, followed by drying for 24 h in a Christ DELTA 1-24 LSC freeze-dryer (Christ, Germany).
000 rpm for 30 s using an Ultra-Turrax T10 (IKA-Werke GmbH & Co., Germany). The resultant HIPEs were cooled and then stored overnight (12 h) at room temperature (25 °C) before further use. To obtain the dried gel-HIPEs, the volatile hexane was chosen as the oil phase to prepare the nanofibril-based HIPEs. The prepared HIPE samples were then subjected to air-drying in an oven at 25 °C for 24 h, or to lyophilization, where the samples were first frozen at −40 °C, followed by drying for 24 h in a Christ DELTA 1-24 LSC freeze-dryer (Christ, Germany).
2.3 Transmission electron microscopy (TEM)
A droplet of hot GA nanofibril solution (0.1 and 0.5 wt%) was deposited onto a carbon-coated copper grid, and then a droplet of 2% uranyl acetate was added onto the grids to negatively stain the samples for 30 s. Observations were carried out with a JEM-2100F transmission electron microscope operating at 200 kV (JEOL, Japan).2.4 Polarized light microscopy (PLM)
PLM observations were performed on a Zeiss Axioscope 40 Pol/40A Pol polarized light microscope (Zeiss, Germany) equipped with a Power Shot G5 camera (Canon, Japan). The HIPE samples were carefully transferred onto a flat slide and covered by a coverslip. Each image was acquired under normal and polarized light.2.5 Confocal laser scanning microscopy (CLSM)
CLSM observations for HIPEs were performed with a Leica TCS SP5 confocal microscope (Leica Microsystems, Germany). Nile Red (0.1 wt%), a fluorescent dye for oil, was first dissolved in sunflower oil, which was then used to prepare HIPEs. The prepared HIPEs were carefully placed on concave confocal microscope slides and covered with coverslips. They were observed under an argon krypton laser (ArKr, 488 nm) with a 100× oil immersion objective lens at room temperature (25 °C). The oil phase dyed with Nile Red is green.2.6 Field emission scanning electron microscopy (FE-SEM)
A Zeiss Merlin field emission scanning electron microscope (Zeiss, Germany) was used to observe the macroporous structure of dried HIPEs. The samples were carefully transferred and adhered onto a holder, and then sputter-coated with gold (JEOL JFC-1200 fine coater, Japan) before imaging.2.7 Rheology measurements
The rheological properties of HIPEs were investigated using a Haake MARS60 rheometer (Thermo Scientific, Germany), and a plate-plate geometry (35 mm diameter, 1 mm gap) was used. All HIPE samples were carefully scooped onto the rheometer plate and then allowed to relax for 3 min before testing. Dynamic oscillatory shear tests including amplitude sweeps (stress = 0.1–5000 Pa, frequency = 1 Hz) and frequency sweeps (0.01–100 Hz, stress = 100 Pa, within the linear viscoelastic region) were performed to determine the variation of the elastic modulus (G′) and viscous modulus (G′′). All the measurements were performed at 25 °C.2.8 In vitro cargo release
To evaluate the potential of the GA nanofibril-stabilized HIPEs as nutraceutical/drug delivery vehicles, we selected β-carotene as a model hydrophobic active cargo to prepare the functional HIPEs. The β-carotene (0.1 wt% of oil) was incorporated into the sunflower oil prior to the emulsification step, and then the in vitro release of β-carotene from the HIPEs was determined based on the membrane-free model.14,28 Approximately 0.5 g of the β-carotene-loaded HIPEs was weighed into glass vials, followed by the careful addition of 4 mL of Tween 80 solution (3 wt%) and 10 mL of hexane. The glass vials were incubated in a shaking chamber at 100 rpm and 25 °C. An aliquot (100 μL) of the supernatants was periodically removed, and the concentration of released β-carotene was measured at 450 nm using a UV-vis spectrophotometer (C40 Touch, Implen, Germany). The cumulative percentage released was integrated from each measurement, according to a previously performed calibration curve.2.9 Statistical analysis
Unless specified in our experiments, at least three freshly prepared samples were characterized. Analysis of variance (ANOVA) of the data was performed using the SPSS 19.0 statistical analysis system. Duncan's test was used for the comparison of mean values among three treatments using a level of significance of 5%.3. Results and discussion
In the present study, we investigated the use of natural GA nanofibrils as a sole building block for the efficient synthesis of robust and processable gel-HIPEs. These GA nanofibril-stabilized HIPEs were rapidly fabricated by adopting a simple one-step emulsification at high temperature (80–90 °C, above the melting temperature of the GA hydrogel at 55–60 °C) followed by subsequent cooling (Fig. 1a). The formation of GA fibrillar hydrogel networks in the continuous phase and the multilayer attachment of GA nanofibrils at the droplet surfaces lead to the nanofibril-stabilized gel-HIPEs (Fig. 1a).19,20,29 The impact of GA nanofibril concentration (0.1–5 wt%) on the formation and stability of HIPEs was studied at a constant sunflower oil volume fraction of 80% (Fig. 1b). As can be seen, a series of fine and stable HIPEs with homogeneous appearance were obtained, and even at a relatively low nanofibril concentration of 0.1 wt%, the HIPEs can remain unchanged without obvious phase separation for at least one month at room temperature (25 °C) (Fig. S2†). The superior long-term stability of the HIPEs, especially at 0.5–5 wt% GA nanofibrils, is mainly attributed to the fibrillar hydrogel network in the continuous phase, which can trap the dispersed oil droplets and restrict their movement, thus preventing the flocculation and coalescence of emulsions.19,20 Besides, previous studies have shown that the multilayer fibril shell around the droplets with high electrostatic repulsive forces also can provide high electrostatic stability for the GA nanofibril-stabilized HIPEs.19,20The structural rigidness and processability of HIPEs are found to be highly dependent on the concentration of GA nanofibrils (0.1–5 wt%). At low nanofibril concentrations (0.1 and 0.25 wt%), which are those below the critical gelation concentration of GA nanofibrils in water (0.3 wt%), the prepared HIPEs are fluid because the amount of free GA nanofibrils is not sufficient to gel the continuous phase (Fig. 1b). When the nanofibril concentration is above 0.3 wt% (0.5–5 wt%), as expected, the self-standing gel-HIPEs were obtained due to the formation of a viscoelastic GA hydrogel in the continuous phase (Fig. S1†). The increasing GA nanofibril concentrations significantly promote the structural rigidness and solid-like appearance of these gel-HIPEs (Fig. 1c). This can be explained by the formation of stronger GA fibrillar hydrogel networks in the continuous phase which thus entrap the emulsion droplets more compactly. A further increase in the volume fraction of internal phase (up to 90%) can also lead to stable and homogeneous gel-HIPEs (Fig. S3†). These results reveal that GA nanofibrils are natural and efficient building blocks to produce stable and processable gel-HIPEs, and their structural properties (e.g., appearance, viscoelasticity, and stability) can be highly controlled by simply changing the concentration of the stabilizer (GA nanofibrils), which is difficult to realize in traditional HIPEs, where the ratio of the two immiscible phases can only be adjusted within a narrow range due to the geometrical requirement, resulting in limited formulations for practical applications.4,5,8,27 This is beneficial for the applications of our GA nanofibril HIPEs in food, cosmetic, and pharmaceutical products.
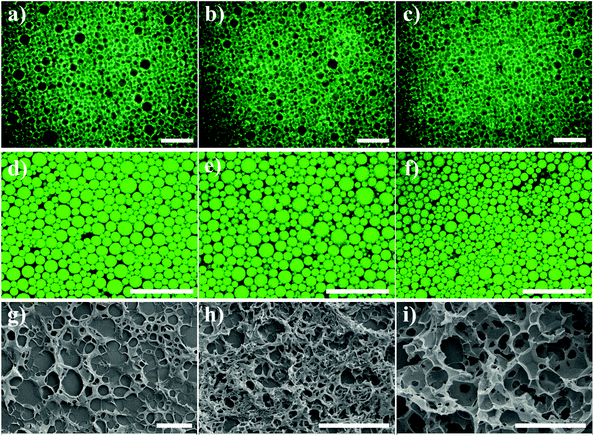
To visualize the GA fibrillar networks and the arrangement of emulsion droplets within the HIPEs, we adopted polarized light microscopy (PLM) and confocal laser scanning microscopy (CLSM) to observe the microstructure of gel-HIPEs with 80 vol% sunflower oil. As shown in Fig. 2a–c, all gel emulsions showed strong birefringence under PLM, which is attributed to the presence of anisotropic GA assemblies including a multilamellar nanofibril shell at the surfaces of the droplets and a fibrillar hydrogel network in the continuous phase. Our previous studies have demonstrated that the multilayer adsorption of GA nanofibrils at the liquid interfaces and the formation of the viscoelastic GA hydrogel networks in the continuous phase can provide superior stability to the nanofibril-stabilized emulsions and foams.19,20,23,24 A porous honeycomb-like network formed by the thickened planar adhesion junctions between emulsion droplets can be clearly seen from these PLM images, and these nanofibril-coated droplets appear to be closely packed together in the continuous matrix. The polyhedral shape and size of these tightly packed oil droplets were observed more directly in the CLSM images of the gel-HIPEs dyed with Nile Red (Fig. 2d–f, and S4†), which is in good agreement with the PLM results. We further used volatile hexane as the oil phase (80 vol%) to produce the HIPEs, and then prepared the samples for SEM observations by evaporation under air-drying conditions. The use of hexane as the oil phase did not affect the formation of nanofibril HIPEs. As seen from Fig. 2g–i, a fibrous network in the continuous phase is clearly observed, and the networks become more evident and integrated with increasing GA nanofibril concentration (0.25–1 wt%). These results confirm the presence of the GA fibrillar network within the continuous phase of HIPEs, which is formed through the interfibrillar hydrogen-bond junctions and entanglements.19,20,23,24
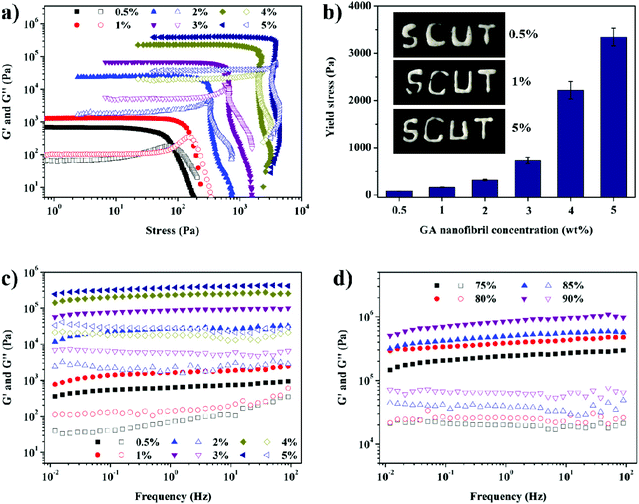
We then studied the mechanical properties of the GA nanofibril-stabilized gel-HIPEs by performing oscillatory amplitude and frequency sweep measurements. Fig. 3a and c show the storage (G′) and loss (G′′) moduli as a function of stress and frequency for the gel-HIPEs (80 vol% sunflower oil) with different GA nanofibril concentrations (0.5–5 wt%), respectively. As seen in Fig. 3a, for all samples, the storage modulus, G′, is always much higher than the loss modulus G′′ in the linear viscoelastic regime (LVR), which indicates the elastic solid-like behaviors of these gel-HIPEs. With increasing nanofibril concentration from 0.5 to 5 wt%, the values of G′ increase from 688.5 Pa to 408.5 kPa (Fig. 3a, within the LVR), and the corresponding critical yield stress increases from 83.5 Pa to 3340 Pa (Fig. 3b, defined as the crossover point of G′ and G′′), which means that the mechanical strength and the elasticity of the gel-HIPEs strongly depend on the GA nanofibril concentration. This can be further confirmed by the frequency sweep measurements (Fig. 3c), which show that the values of G′ are higher than those of G′′ within the applied frequency range, and both G′ and G′′ display a weak frequency dependence, indicating that these gel-HIPEs have an excellent tolerance to external forces. The increase of the oil phase volume fraction leads to a further increase in the gel strength (Fig. 3d), and the values of G′ and yield stress for the 5 wt% GA nanofibril-stabilized gel-HIPE with 90 vol% oil can reach 860.1 kPa and 7069.5 Pa, respectively. Such surprising high storage moduli (within the LVR) are unprecedented, which are nearly 2–3 orders of magnitude larger than those for previously reported HIPEs with relatively obvious viscoelastic characteristics, such as the microgel-stabilized Pickering HIPEs (around 200 Pa),30 the HIPEs stabilized by linear homopolymers (7100 Pa),31 depletion attraction-induced Pickering HIPEs (<400 Pa),32 and Pickering HIPEs stabilized by proteins and protein-based particles (<3000 Pa).13,14,33,34 This can be explained by the fact that for those previously reported HIPEs, their solid-like viscoelastic properties are mainly dominated by the tight packing of the dispersed oil droplets,1,4,8,27 and the interdroplet interactions are also relatively weak for reinforcing the continuous matrix of HIPEs. In contrast, for our GA nanofibril-stabilized gel-HIPEs, the highly viscoelastic behaviors mainly rely on the nanofibrillar networks in the continuous phase and at the droplet surfaces (Fig. 1a), which are formed through the strong interfibrillar hydrogen-bond interactions.19,20,24 Additionally, the GA nanofibril-coated emulsion droplets are packed and connected very closely within the continuous network due to the interfibrillar interactions (Fig. 2), which can provide stronger droplet–droplet and droplet–matrix interactions, and thereby further contribute to strengthening the gel matrix. These factors therefore endow the nanofibril-stabilized gel-HIPEs with such spectacular mechanical elasticity. Due to their high structural viscoelasticity, the nanofibril-stabilized HIPEs are rigid and processable, and the processability appears to be largely dependent on the nanofibril concentration (Fig. 3b, inset image). These results taken together reveal that the GA nanofibril-stabilized gel-HIPEs are highly elastic, solid-like yield stress materials.
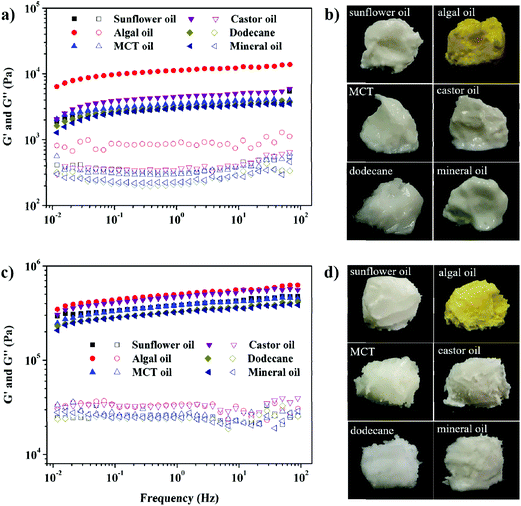
We demonstrated the universality of the GA nanofibrils as natural emulsifiers for producing stable and processable gel-HIPEs containing a variety of oil phases, such as sunflower oil, algal oil, MCT oil, castor oil, dodecane, and mineral oil. These liquid oils are commonly used in applications but have significantly different compositions and polarities. The same emulsification procedure as that used with sunflower oil gel-HIPEs was performed (Fig. 1a). As seen in Fig. 4, for all the cases, rigid and processable gel-HIPEs are formed, and their structural properties including appearance and mechanical viscoelasticity can be well tuned by the GA nanofibril concentrations (1 and 5 wt%). This indicates the versatility and robustness of the GA nanofibrils as a sole and highly efficient stabilizer for the facile synthesis of gel-HIPEs, and these soft solid oil products are expected to find a broad range of applications in various fields.
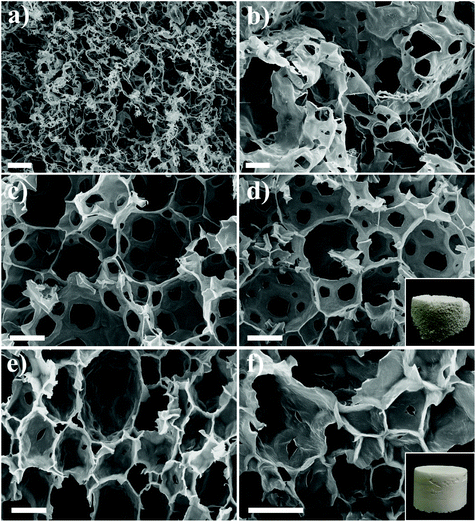
The applicability of our nanofibril-stabilized gel-HIPEs was investigated. We showed that the GA nanofibril-based porous foams can be obtained by using these robust gel-HIPEs as a precursor template, which were prepared by using volatile hexane (80 vol%) as the oil phase and then evaporated by freeze-drying. From the FE-SEM images (Fig. 5), we can see that the microstructure of these porous materials can be controlled by tuning the GA nanofibril concentrations. Note that at a low GA nanofibril concentration (0.1 wt%), no porous network structure can be observed, and the walls of the droplets appear to completely collapse after drying (Fig. 5a), due to the absence of the viscoelastic fibrillar networks in the precursor HIPEs (Fig. 1b). The gel-HIPEs prepared at 1 and 3 wt% GA nanofibrils show a typical open porous network, and the pore size is generally consistent with the droplet size of precursor HIPEs (Fig. 2), suggesting the structural integrity of the GA nanofibril-coated droplets during drying. Interestingly, with the increase of the nanofibril concentration to 5 wt%, the changes of the obtained porous foam from open- to closed-cell structures are clearly observed (Fig. 5 and S5†), and the solid foam shows a more homogeneous appearance (Fig. 5d and f, inset images). It is noted that there is no obvious pore throat within the pore walls, which are formed from the drying of the GA nanofibrillar networks around the droplets and in the continuous phase. This can be explained by the fact that the viscoelastic fibrillar networks inside the gel-HIPEs become stronger at higher GA nanofibril concentration (Fig. 3) and therefore do not rupture to form pore throats under freeze-drying. We thus show that our robust gel-HIPEs stabilized solely by natural GA nanofibrils can be used as a simple and solid template for the synthesis of a variety of porous materials without the crosslinking of the continuous phase. These results indicate that the highly viscoelastic GA nanofibrillar networks can convey the superior stability to these nanofibril-based gel-HIPE templates during drying, and thus are the key factors to produce stable and controllable porous structures.
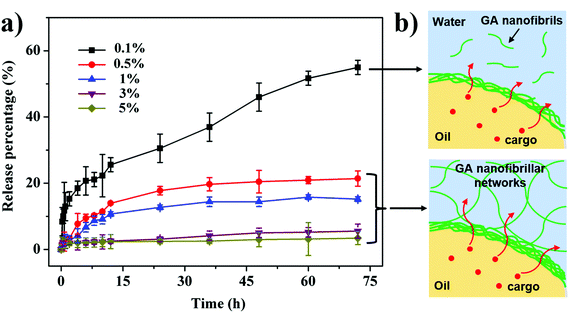
Another important application for O/W HIPEs is their use as delivery systems for the encapsulation and release of oil-soluble bioactive ingredients in foods, pharmaceuticals, and cosmetics. Herein, we further fabricated functional HIPEs by encapsulating a model hydrophobic active cargo (i.e., β-carotene) into the sunflower oil phase and then investigated the in vitro release behavior of β-carotene from these nanofibril-stabilized HIPEs. As seen from Fig. 6a, the release kinetic profiles of β-carotene from the HIPEs strongly depend on the concentration of GA nanofibrils (0.1–5 wt%). At a low nanofibril concentration (0.1 wt%), the release kinetics of β-carotene from the obtained fluid HIPEs (Fig. 1b) can be described as a two-step biphasic process, that is, an initial fast release stage (within 1 h) followed by a subsequent slow and sustained release. Compared with the liquid-HIPE at 0.1 wt% GA nanofibrils, the gel-HIPEs prepared using 0.5–5 wt% nanofibrils (Fig. 1b) display significantly different release profiles, which show that the release rates and the accumulative amounts of β-carotene released largely decrease with increasing nanofibril concentration. Particularly, for these gel-HIPEs at higher GA nanofibril concentrations (3 and 5 wt%), the detectable amounts of β-carotene are very low (3.4–5.6%) during the entire release period (72 h). This can be explained by the fact that a higher concentration of GA nanofibrils results in a higher packing density of the multilayer fibril shell around emulsion droplets (Fig. 2), which thus can effectively inhibit the transport of β-carotene incorporated into the oil phase through the droplet surfaces (Fig. 6b). More importantly, at high GA nanofibril concentrations (0.5–5 wt%), the viscoelastic GA nanofibrillar networks in the continuous phase of these gel-HIPEs (Fig. 2) play a more dominant role in retarding the release. The stronger fibrillar networks at the surfaces of the droplets and in the continuous phase formed at higher nanofibril concentrations can generate higher diffusion resistance (Fig. 6b), and consequently the release of β-carotene is markedly decreased, especially in these gel-HIPEs with 3 and 5 wt% GA nanofibrils. These results indicate that the O/W HIPEs stabilized by GA nanofibrils are promising long-term delivery vehicles for hydrophobic active cargoes, and the cargo release properties can be controlled by simply tuning the concentration of GA nanofibrils in the HIPE formulations.
4. Conclusions
In summary, we have shown that a new class of high internal phase gel emulsions (gel-HIPEs) that are mechanically robust and processable can be produced simply and efficiently by using the natural food-grade saponin GA nanofibrils as the sole stabilizer. The structural properties including appearance, viscoelasticity and processability of gel-HIPEs are controllable by simply tuning the concentration of GA nanofibrils. More importantly, these gel-HIPEs display an extremely high mechanical strength, and their storage moduli and yield stress values can reach 408.5 kPa and 3340 Pa, respectively, when the concentration of GA nanofibrils only increases to 5 wt%. This unprecedented result has not been reported before for those HIPEs stabilized by surfactants, polymers, or colloidal particles. Such spectacular mechanical strength is mainly ascribed to the formation of the highly viscoelastic GA nanofibrillar networks in the continuous phase, which can actively trap the nanofibril-coated emulsion droplets and thus strengthen the gel structure. Accordingly, the nanofibril-stabilized robust gel-HIPEs can be used as a solid template to make natural, biosafe porous materials without the necessity for crosslinking of the continuous phase, which have controlled open- and closed-cell microstructures by adjusting the nanofibril concentration. Moreover, these nanofibril HIPEs are also shown to be promising long-term delivery systems with controlled-release properties for lipophilic active cargoes. This natural GA nanofibril system is found to be versatile for different oil phases, and the fabrication procedure of HIPEs is also facile, fast, and environmentally friendly. We therefore expect that these novel gel-HIPE materials may find a broad range of sustainable applications in healthy foods, cosmetics, and biological and medical fields and also may be of interest to be applied as templates for advanced materials design.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (32172347 and 31801476), the Natural Science Foundation of Guangdong Province (2021A1515011000), the Fundamental Research Funds for the Central Universities (2020ZYGXZR092), and the 111 Project (B17018).References
- B. P. Binks, Modern Aspects of Emulsion Science, Royal Society of Chemistry, Cambridge, 1998 Search PubMed.
- E. Dickinson, Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets, Food Hydrocolloids, 2012, 28, 224–241 CrossRef CAS.
- N. R. Cameron, High internal phase emulsion templating as a route to well-defined porous polymers, Polymer, 2005, 46, 1439–1449 CrossRef CAS.
- K. J. Lissant, The geometry of high-internal-phase-ratio emulsions, J. Colloid Interface Sci., 1966, 22, 462–468 CrossRef CAS.
- J. M. Williams, High internal phase water-in-oil emulsions: Influence of surfactants and cosurfactants on emulsion stability and foam quality, Langmuir, 1991, 7, 1370–1377 CrossRef CAS.
- S. Zhang, J. Chen and V. T. Perchyonok, Stability of high internal phase emulsions with sole cationic surfactant and its tailoring morphology of porous polymers based on the emulsions, Polymer, 2009, 50, 1723–1731 CrossRef CAS.
- Q. Chen, X. Cao, H. Liu, W. Zhou, L. Qin and Z. An, pH-responsive high internal phase emulsions stabilized by core cross-linked star (CCS) polymers, Polym. Chem., 2013, 4, 4092–4102 RSC.
- Z. Li, T. Ming, J. Wang and T. Ngai, High internal phase emulsions stabilized solely by microgel particles, Angew. Chem., Int. Ed., 2009, 48, 8490–8493 CrossRef CAS PubMed.
- V. O. Ikem, A. Menner and A. Bismarck, High internal phase emulsions stabilized solely by functionalized silica particles, Angew. Chem., Int. Ed., 2008, 47, 8277–8279 CrossRef CAS.
- J. J. Blaker, K. Y. Lee, X. Li, A. Menner and A. Bismarck, Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates, Green Chem., 2009, 11, 1321–1326 RSC.
- B. P. Binks and S. O. Lumsdon, Catastrophic phase inversion of water-in-oil emulsions stabilized by hydrophobic silica, Langmuir, 2000, 16, 2539–2547 CrossRef CAS.
- B. P. Binks and S. O. Lumsdon, Influence of particle wettability on the type and stability of surfactant-free emulsions, Langmuir, 2000, 16, 8622–8631 CrossRef CAS.
- Y. Q. Hu, S. W. Yin, J. H. Zhu, J. R. Qi, J. Guo, L. Y. Wu, C. H. Tang and X. Q. Yang, Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles, Food Hydrocolloids, 2016, 61, 300–310 CrossRef CAS.
- H. Tan, G. Sun, W. Lin, C. Mu and T. Ngai, Gelatin particle-stabilized high internal phase emulsions as nutraceutical containers, ACS Appl. Mater. Interfaces, 2014, 6, 13977–13984 CrossRef.
- S. Zamani, N. Malchione, M. J. Selig and A. Abbaspourrad, Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels, Food Funct., 2018, 9, 982–990 RSC.
- A. Marefati, M. Sjöö, A. Timgren, P. Dejmek and M. Rayner, Fabrication of encapsulated oil powders from starch granule stabilized W/O/W Pickering emulsions by freeze-drying, Food Hydrocolloids, 2015, 51, 261–271 CrossRef.
- I. Capron and B. Cathala, Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals, Biomacromolecules, 2013, 14, 291–296 CrossRef PubMed.
- E. Perrin, H. Bizot, B. Cathala and I. Capron, Chitin nanocrystals for Pickering high internal phase emulsions, Biomacromolecules, 2014, 15, 3766–3771 CrossRef PubMed.
- Z. Wan, Y. Sun, L. Ma, J. Guo, J. Wang, S. Yin and X. Yang, Thermoresponsive structured emulsions based on the fibrillar self-assembly of natural saponin glycyrrhizic acid, Food Funct., 2017, 8, 75–85 RSC.
- Z. Wan, Y. Sun, L. Ma, X. Yang, J. Guo and S. Yin, Responsive emulsion gels with tunable properties formed by self-assembled nanofibrils of natural saponin glycyrrhizic acid for oil structuring, J. Agric. Food Chem., 2017, 65, 2394–2405 CrossRef.
- A. Saha, J. Adamcik, S. Bolisetty, S. Handschin and R. Mezzenga, Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features, Angew. Chem., 2015, 127, 5498–5502 CrossRef.
- Q. Li, M. Xu, J. Xie, E. Su, Z. Wan, L. M. C. Sagis and X. Yang, Large amplitude oscillatory shear (LAOS) for nonlinear rheological behavior of heterogeneous emulsion gels made from natural supramolecular gelators, Food Res. Int., 2021, 140, 110076 CrossRef CAS.
- Z. Wan, Y. Sun, L. Ma, F. Zhou, J. Guo, S. Hu and X. Yang, Long-lived and thermoresponsive emulsion foams stabilized by self-assembled saponin nanofibrils and fibrillar network, Langmuir, 2018, 34, 3971–3980 CrossRef CAS PubMed.
- L. Ma, Q. Li, Z. Du, E. Su, X. Liu, Z. Wan and X. Yang, A natural supramolecular saponin hydrogelator for creation of ultrastable and thermostimulable food-grade foams, Adv. Mater. Interfaces, 2019, 6, 1900417 CrossRef.
- E. Su, Q. Li, M. Xu, Y. Yuan, Z. Wan, X. Yang and B. P. Binks, Highly stable and thermo-responsive gel foams by synergistically combining glycyrrhizic acid nanofibrils and cellulose nanocrystals, J. Colloid Interface Sci., 2021, 587, 797–809 CrossRef CAS.
- X. Chen, K. Liu, P. He, H. Zhang and Y. Fang, Preparation of novel W/O gel-emulsions and their application in the preparation of low-density materials, Langmuir, 2012, 25, 9275–9281 CrossRef.
- T. Yan, B. Song, X. Pei, Z. Cui, B. P. Binks and H. Yang, Widely adaptable oil-in-water gel emulsions stabilized by an amphiphilic hydrogelator derived from dehydroabietic acid, Angew. Chem., 2020, 132, 647–651 CrossRef.
- S. A. Wissing and R. H. Müller, Solid lipid nanoparticles as carrier for sunscreens: In vitro release and in vivo skin penetration, J. Controlled Release, 2002, 81, 225–233 CrossRef CAS PubMed.
- Q. Li, Q. He, M. Xu, J. Li, X. Liu, Z. Wan and X. Yang, Food-grade emulsions and emulsion gels prepared by soy protein-pectin complex nanoparticles and glycyrrhizic acid nanofibrils, J. Agric. Food Chem., 2020, 68, 1051–1063 CrossRef CAS PubMed.
- Z. Li and T. Ngai, Stimuli-responsive gel emulsions stabilized by microgel particles, Colloid Polym. Sci., 2011, 289, 489–496 CrossRef CAS.
- Q. Chen, M. R. Hill, W. L. Brooks, A. Zhu, B. S. Sumerlin and Z. An, Boronic acid linear homopolymers as effective emulsifiers and gelators, ACS Appl. Mater. Interfaces, 2015, 7, 21668–21672 CrossRef CAS PubMed.
- K. Kim, S. Kim, J. Ryu, J. Jeon, S. G. Jang, H. Kim, D.-G. Gweon, W. B. Im, Y. Han, H. Kim and S. Q. Choi, Processable high internal phase Pickering emulsions using depletion attraction, Nat. Commun., 2017, 8, 14305 CrossRef CAS PubMed.
- Y. T. Xu, C. H. Tang and B. P. Binks, Ultraefficient stabilization of high internal phase emulsions by globular proteins in the presence of polyols: Importance of a core-shell nanostructure, Food Hydrocolloids, 2020, 107, 105968 CrossRef.
- S. Zamani, N. Malchione, M. J. Selig and A. Abbaspourrad, Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels, Food Funct., 2018, 9, 982–990 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo01656c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |