Non-fullerene acceptors with hetero-dihalogenated terminals induce significant difference in single crystallography and enable binary organic solar cells with 17.5% efficiency†
Lai
Wang
,
Qiaoshi
An
 *,
Lu
Yan
,
Hai-Rui
Bai
,
Mengyun
Jiang
,
Asif
Mahmood
*,
Lu
Yan
,
Hai-Rui
Bai
,
Mengyun
Jiang
,
Asif
Mahmood
 ,
Can
Yang
,
Hongfu
Zhi
and
Jin-Liang
Wang
,
Can
Yang
,
Hongfu
Zhi
and
Jin-Liang
Wang
 *
*
Key Laboratory of Cluster Science of Ministry of Education Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China. E-mail: jinlwang@bit.edu.cn; qsan@bit.edu.cn
First published on 6th October 2021
Abstract
Despite the dihalogenation of terminals being an effective strategy to produce efficient nonfullerene acceptor (NFA)-based organic solar cells (OSCs), hetero-dihalogenated terminals are quite difficult to prepare. Here, we synthesized, for the first time, two new hetero-dihalogenated terminals (FCl-IC and FBr-IC) with a fluorine/chlorine or fluorine/bromine pair at one terminal and three NFAs (Y-BO-FCl, Y-BO-FBr, and Y-BO-ClBr) with three hetero-dihalogenated terminals (FCl-IC, FBr-IC, and ClBr-IC) using a general process for OSCs, respectively. A neat film of Y-BO-FCl presents a slightly lower energy level in comparison to those of Y-BO-FBr and Y-BO-ClBr. We, for the first time, obtained single crystals of hetero-dihalogenated NFAs. On going from Y-BO-ClBr single crystals to fluorinated acceptor single crystals, the crystal systems and intermolecular packing motifs were significantly improved. The crystallographic and theoretical analysis indicate that Y-BO-FCl exhibits the most planar molecular geometry, the shortest intermolecular packing distance and largest π–π electronic coupling among these acceptors. Moreover, PM6:Y-BO-FCl blend films present more ordered face-on orientation crystallinity, more suitable fiber-like phase separation, higher and more balanced charge mobility, and weaker charge recombination in comparison with those of PM6:Y-BO-FBr and PM6:Y-BO-ClBr. As a result, a performance of up to a remarkable power conversion efficiency of 17.52% with an enhanced FF value of ca. 78% was achieved in binary Y-BO-FCl:PM6 devices compared to those of PM6:Y-BO-FBr (16.47%) and PM6:Y-BO-ClBr (13.61%), which to date is the highest efficiency for a hetero-halogenated NFAs-based OSC. Our investigations demonstrate that a fluorine/chlorine hetero-dihalogenated terminal represents a new and effective synergistic strategy to induce significant differences in single crystals and produce high-performance hetero-halogenated NFA-based OSCs.
Broader contextOrganic solar cells (OSCs) have attracted more and more attention with flexible and large area fabrication properties. Modifying small molecular acceptors (SMAs) with halogenation of end groups has been considered as a relatively simple but an effective strategy to improve device performance. However, dihalogenated terminals were limited to merely homogenous dihalogenated ones and hetero-dihalogenated terminals are quite difficult to obtain, which is a key challenge for highly efficient SMAs. Herein, we facile developed a novel method to synthesize three hetero-dihalogenated terminals. Three SMAs with hetero-dihalogenated terminals were synthesized to systematically probe the effects of different pairs of two halogens on the molecular optoelectronic property and crystal packing. We found that from Y-BO-ClBr to Y-BO-FCl/Y-BO-FBr with fluorine substituent significantly alters the crystal system and intermolecular packing motif. Finally, the device based on PM6:Y-BO-FCl achieved the highest PCE of 17.52% owing to the largest and well-balanced charge mobility and the most suitable blend morphology, which is the highest value for the hetero-halogen-modified acceptors in binary OSCs. Our systematic studies open a new avenue for dihalogenated terminal engineering of SMAs by incorporation of fluorine/chlorine hetero-dihalogenated terminal to enhance the crystal intermolecular packing and achieve optimal film morphology and excellent photovoltaic performance. |
Introduction
Organic solar cells (OSCs) are considered to be a prospective energy source, due to their unique advantages of flexibility, their large area fabrication, and solution processability.1–16 Over the past few decades, significant progress has been made in the power conversion efficiencies (PCEs) of OSCs with non-fullerene acceptors (NFAs), benefiting from the tremendous efforts in new materials design and processing optimization.17–36 Recently, NFAs with A–D–A and A–DA′D–A structures have dominated the improvement in OSC performance owing to their strong and broader absorption and easy chemical modification.37–45 To date, binary OSCs with A–D–A or A–DA′D–A type small molecular acceptors have exhibited over 17% efficiency.29,46–48 Generally, molecular structure engineering plays a crucial role in the device performance of NFAs.49–56 However, how to precisely use molecular engineering to achieve a perfect trade-off among device parameters and further boost efficiency is still a challenge.Many molecular modulation approaches have been employed to tune the intermolecular packing, absorption spectra, and energy levels of NFAs.57–73 Among these, the halogenation of end groups with different number of halogen elements, at different substituted positions or on different aromatic units, has been considered as a relatively simple but significantly effective strategy to change the absorption range, optimize the energy levels, and enhance the intermolecular interactions of the resultant acceptors, leading to better blend morphology and device performance.74–81 For example, compared to nonfluorinated acceptors, acceptors with difluorinated 1,1-dicyanomethylene-3-indanone (IC) show obviously better crystallinity and device performance owing to their high electronegativity and the small size of the fluorine atoms.78,82 What is more, different types of halogen atoms on the end groups of NFAs have different influences on the optoelectronic, charge transport, and photovoltaic properties.83,84 However, the common multiply halogenated acceptors usually have only homogenous halogen atoms attached to the two terminals of the acceptors, which may impede the development of high-performance multiply halogenated acceptors. Recently, the strategy of preparing asymmetric acceptors with two different halogenated terminals (A1 and A2) has gradually attracted much attention, as these acceptors combine the advantages of two halogenated terminals and achieve better trade-off among device parameters and further improve the device performance in comparison to acceptors with two symmetric homogeneous halogenated terminals.46,47,82–84 However, due to the reversibility of Knoevenagel condensation, these asymmetric acceptors normally require a comparatively complex synthesis and purification process to remove the symmetric acceptor byproduct. Therefore, it is necessary to find more effective and alternative strategies to obtain novel multiply halogenated acceptors with different halogenated atoms, and to further improve the device performance.
On the other hand, heterogenous dihalogenated IC, containing two different halogen atoms on the same IC unit, has just emerged recently and has thus received less attention, but represents an effective and alternative approach to obtain heterogenous halogenated NFAs.36,85–87 For example, we have recently synthesized a new hetero-dihalogenated IC-based NFA containing bromine and chlorine atoms, which presents more balanced carrier mobility in comparison with blend films composed of homo-halogenated acceptors, resulting in obviously better device efficiency.87 However, compared to the widely used homogenous difluorinated and dichlorinated IC, hetero-dihalogenated IC-based NFA with both a F atom and Cl atom at one IC terminal seems to have been ignored and has never been reported to the best of our knowledge, which might due to the lack of an appropriate method by which to afford hetero-dihalogenated IC with both F and Cl atoms in a high isolated yield. Recently, single-crystal structures of some homogenous halogenated NFAs have been reported,88,89 which are beneficial towards gaining an in-depth understanding of the effect that halogenated terminals have on molecular packing, morphology and charge transport properties. Introducing different halogenated terminals might lead to a sophisticated divergence in intermolecular packing and crystallinity.83,86,87 However, to date, systematic studies incorporating detailed single-crystal and theoretical analysis to investigate the effect that different types of halogen atoms on the end groups of acceptors have on the intermolecular packing, electronic coupling, morphology of blend films and device performance of a hetero-halogenated NFA series have not been reported, which might be due to asymmetric terminals with multiple conformations making it difficult to achieve high quality single crystals. Therefore, to extend the scope of hetero-dihalogenated IC-based NFAs and enhance their device performance, it is particularly important and necessary to synthesize a series of hetero-dihalogenated IC-based NFAs containing fluorine, chlorine, and bromine atoms, and systematically explore the influence that a different pair of halogen atoms on the end group of acceptors have on their intermolecular crystal packing, crystallinity, film morphology and photovoltaic performance.
In this contribution, we facilely synthesized two new hetero-dihalogenated terminals (FCl-IC and FBr-IC), which contain a fluorine/chlorine or fluorine/bromine pair on the same IC skeleton, respectively. Then, three NFAs based on a fused benzothiadiazole core, named Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr (see Fig. 1), with three distinct hetero-dihalogenated IC terminals (FCl-IC, FBr-IC, and ClBr-IC), were successfully synthesized, respectively, which allowed us to systematically probe the effects that different pairs of halogen atoms on the end groups of acceptors have on their single-crystal packing, film morphology and photovoltaic performance. Compared to Y-BO-ClBr, the fluorinated acceptors (Y-BO-FCl and Y-BO-FBr) display slightly downshifted highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels. Single-crystal structures suggest that introducing a fluorine substituent from Y-BO-ClBr to Y-BO-FCl/Y-BO-FBr significantly alters the crystal system and intermolecular packing motif. Moreover, density functional theory (DFT) calculations and single-crystal structures suggest that Y-BO-FCl presents the most planar molecular geometry, shortest intermolecular packing distance, and largest π–π electronic coupling among these three acceptors. Morphology analysis revealed that the PM6:Y-BO-FCl blend film presents the most preferential face-on orientation with the longest crystal coherence lengths and best nanofiber-like interpenetrated network structure with proper phase-separation among these three blend films. Finally, the device based on PM6:Y-BO-FCl achieved the highest PCE of 17.52%, which is obviously higher than that of PM6:Y-BO-FBr (16.47%) and dramatically higher than that of PM6:Y-BO-ClBr (13.61%). The highest PCE based on a PM6:Y-BO-FCl blend film was attributed to it having the weakest charge recombination, the highest and most well-balanced charge mobility, and the most suitable blend morphology. As far as we know, the remarkable PCE of 17.52% is the highest value reported for a hetero-halogen-modified acceptor in a binary OSCs. This systematic study suggested that the incorporation of fluorine/chlorine hetero-dihalogenated IC is an effective method for enhancing the crystal intermolecular packing and film morphology of NFAs and achieving excellent photovoltaic performance.
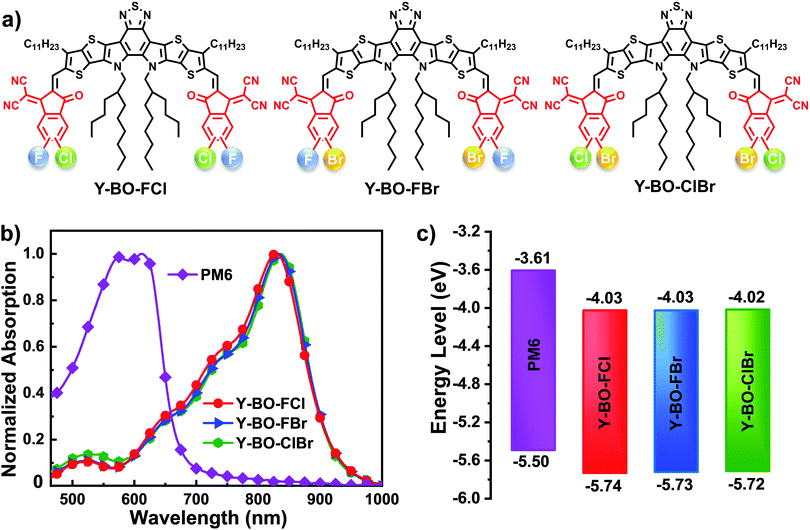 | ||
| Fig. 1 (a) Molecular structure of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr. (b) Absorption spectra of acceptors and PM6 in neat films. (c) The energy levels of acceptors and PM6 (the chemical structure of PM6 is shown in Fig. S1, ESI†) in neat films. | ||
Results and discussion
The challenge is how to synthesize these new hetero-halogenated IC moieties with both fluorine and chlorine or bromine on the same end group. As presented in Scheme 1, compound 1 was obtained by nitration of commercially available 4-fluoro-2-methylbenzonitrile and then underwent a reduction in 67% yield. 1 was converted to the corresponding chlorination product 2a or bromination product 2bvia a Sandmeyer reaction involving cuprous chloride in hydrochloric acid or cuprous bromide in hydrobromic acid in 77% and 68% yields, respectively. The monocarboxylic acid 3a and 3b were separately prepared from 2a and 2bvia a hydrolysis reaction in 90% and 87% yield, respectively. Subsequently, 3a and 3b were separately subjected to an oxidization reaction with KMnO4 to obtain the dicarboxylic acids 4a and 4b in 64% and 69% yield, which were further treated with acetic anhydride to afford anhydrides 5a and 5b in high yield, respectively. Then, 5a and 5b were separately treated with acetic anhydride, triethylamine, and ethyl acetoacetate to obtain 6a and 6b, followed by treatment with malononitrile to produce the hetero-halogenated end groups FCl-IC and FBr-IC with two isomers in 56% and 60% yield, respectively. The determined ratios of the two isomers for FCl-IC and FBr-IC based on the 1H NMR spectra are ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.19 for FCl-IC1
0.19 for FCl-IC1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FCl-IC2 and ca. 1
FCl-IC2 and ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67 for FBr-IC1
0.67 for FBr-IC1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FBr-IC2, respectively. Notably, our synthesis of FCl-IC and FBr-IC started from a cheap fluorinated precursor, which was beneficial for avoiding the toxicity of fluorination and utilizing a facile and economic synthetic process. Moreover, the hybrid synthetic method is a general process and is fit for synthesizing other hetero-fluorinated ICs. ClBr-IC with two isomers was synthesized using our previously reported procedure.87 Finally, these products Y-BO-FCl, Y-BO-FBr, and Y-BO-ClBr86 were obtained via Knoevenagel condensation reactions between Y-BO-CHO90 and these hybrid dihalogenated end groups FCl-IC, FBr-IC, ClBr-IC in 86%, 88% and 85% yields, respectively. The key new intermediate and desired acceptors were clearly characterized by 1H NMR, 13C NMR, and mass spectra (see the ESI†). Based on the curves of thermogravimetric analysis (see Fig. S2, ESI†), the thermal decomposition temperatures (at 5% weight loss) of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr are 310 °C, 309 °C and 308 °C, respectively, which indicates that these hetero-dihalogenated end groups-based acceptors have comparable thermal stability for OSC fabrication.
FBr-IC2, respectively. Notably, our synthesis of FCl-IC and FBr-IC started from a cheap fluorinated precursor, which was beneficial for avoiding the toxicity of fluorination and utilizing a facile and economic synthetic process. Moreover, the hybrid synthetic method is a general process and is fit for synthesizing other hetero-fluorinated ICs. ClBr-IC with two isomers was synthesized using our previously reported procedure.87 Finally, these products Y-BO-FCl, Y-BO-FBr, and Y-BO-ClBr86 were obtained via Knoevenagel condensation reactions between Y-BO-CHO90 and these hybrid dihalogenated end groups FCl-IC, FBr-IC, ClBr-IC in 86%, 88% and 85% yields, respectively. The key new intermediate and desired acceptors were clearly characterized by 1H NMR, 13C NMR, and mass spectra (see the ESI†). Based on the curves of thermogravimetric analysis (see Fig. S2, ESI†), the thermal decomposition temperatures (at 5% weight loss) of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr are 310 °C, 309 °C and 308 °C, respectively, which indicates that these hetero-dihalogenated end groups-based acceptors have comparable thermal stability for OSC fabrication.
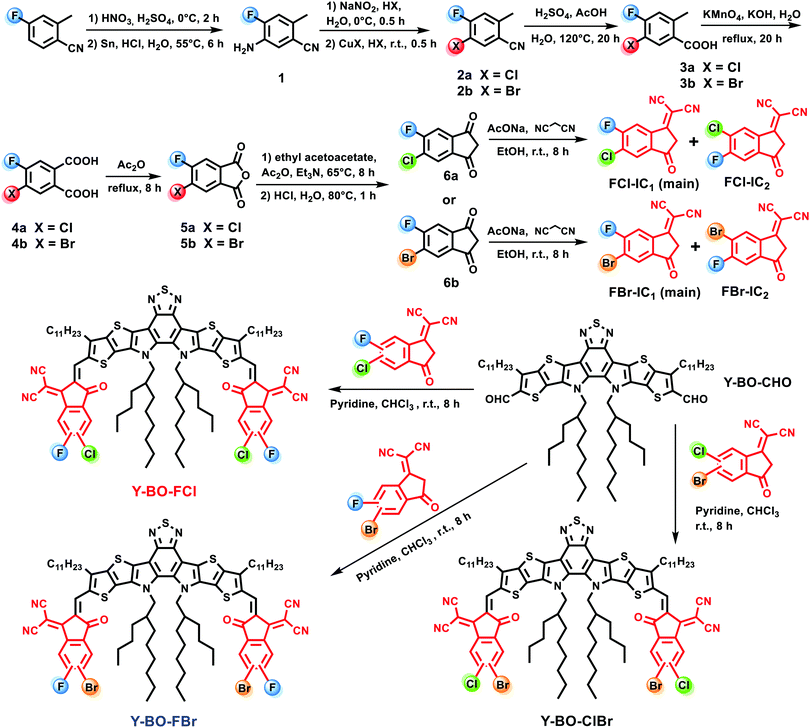 | ||
| Scheme 1 Synthetic routes of terminals FCl-IC and FBr-IC and acceptors Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr. | ||
Density functional theory (DFT) calculations were carried out using Gaussian 09 at the B3LYP/6-31g** level to determine the influence that different end groups have on molecular skeletons, frontier molecular orbitals and dipole moments of the whole molecules. These three regioisomers in the same hybrid-dihalogenated end groups displayed similar calculated values (Table S1, ESI†), suggesting that influence of the regioselective hetero-dihalogenated end groups could be ignored. As shown in Fig. S3 (ESI†), the optimized molecular skeletons of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr exhibited similar nearly planar banana-shaped geometry owing to intramolecular S⋯O non-covalent interactions. Moreover, the dipole moment of Y-BO-FBr is ca. 0.80 Debye, which is slightly higher than that of Y-BO-FCl (0.23 Debye) and Y-BO-ClBr (0.49 Debye) due to the relatively larger difference between the F and Br atoms in terms of atom radius and electronegativity in comparison with the other pair of hetero-dihalogen atoms. A higher dipole moment may enhance the strength of intramolecular charge transfer, which is beneficial toward achieving intense absorption of Y-BO-FBr. The HOMO orbitals of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr are mainly located on the DA′D central cores and the calculated values are −3.60, −3.59, and −3.58 eV, respectively. The LUMO orbitals are widely delocalized over the entire backbone and relatively more distributed at the hetero-dihalogenated end groups, with calculated values of −5.62, −5.61, and −5.60 eV, respectively. The lowest HOMO and LUMO energy levels of Y-BO-FCl were attributed to FCl-IC having the strongest electron-withdrawing ability in comparison to Y-BO-FBr and Y-BO-ClBr.
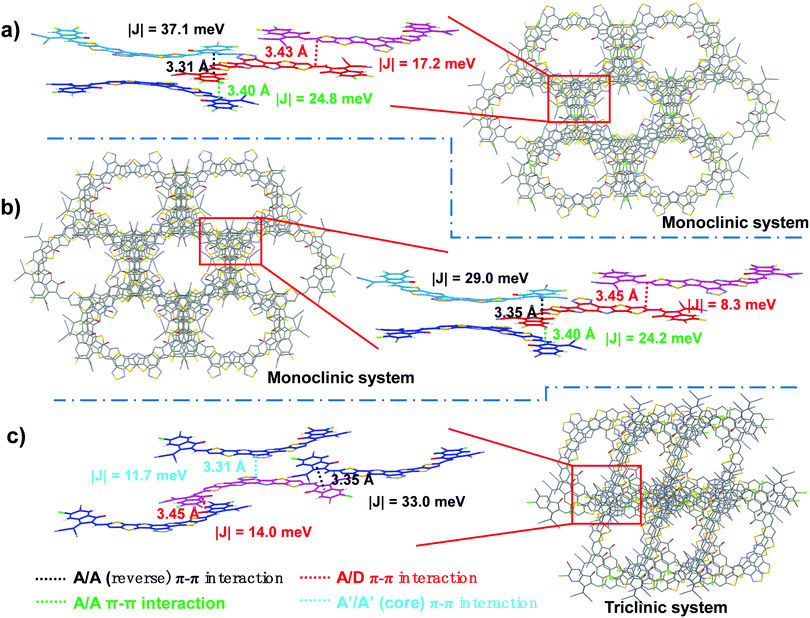
To investigate the effect that the three different hetero-dihalogenated end groups have on the monomolecular geometry and intermolecular packing, single-crystal X-ray diffraction measurements of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr were performed. The detailed crystal parameters and ORTEP drawings of the “part-occupancy” of the halogens are listed in Table S2 and Fig. S4 (ESI†). The single crystals of Y-BO-FCl and Y-BO-FBr belong to a monoclinic system, which is different from that of Y-BO-ClBr, which is in a triclinic system. As shown in Fig. S4 (ESI†), owing to the existence of three regioisomers with alternating positions of the halogens at the terminals, displacement ellipsoids are shown for the terminal halogen atoms with ca. 50%/50% or ca. 75%/25%-occupancy, dependent on the different chemical regioselectivity of the terminal halogen atoms and most reasonable atomic ellipsoid. As shown in Fig. 2, Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr present a similar banana-curved and helical molecular geometry due to an intramolecular S⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C conformational lock. The molecular torsion angle within the two planes respectively built from the two end groups in one molecular Y-BO-FCl is 3.05°, indicating the relative planar conformation. While, the molecular torsion angles are 3.79° for Y-BO-FBr and 10.91° for Y-BO-ClBr. This indicates that the incorporation of a relatively larger sized halogen atom substitution on the end group leads to a slight increase in the molecular torsion angles, especially for Y-BO-ClBr. As is known, A–DA′D–A type SMAs tend to stack together between nearby terminals via A/A (reverse or non-reverse) π–π interactions, which is the major driving force to determine the crystal structure of these molecules. Therefore, as shown in Fig. 3, different hetero-dihalogenated end groups with different molecular torsion angles also led to dramatically different intermolecular packing modes. Fluorine-based Y-BO-FCl and Y-BO-FBr crystals have four independent molecular conformations with three types of π⋯π interactions between neighbor molecules, including the π⋯π interactions between two terminal groups (A/A) and the π–π stacking between one terminal group and half central core of an adjacent molecule (A/D). Specifically, the π–π stacking distance between one terminal group and central core in Y-BO-FCl crystal is ca. 3.43 Å, which is slightly shorter than that in the Y-BO-FBr crystals (ca. 3.45 Å). Moreover, the π–π stacking distance between two antiparallel terminal groups in the Y-BO-FCl crystal is ca. 3.31 Å, which is obviously shorter than that in the Y-BO-FBr crystals (ca. 3.35 Å). The Y-BO-FCl and Y-BO-FBr crystals exhibit another similar π–π stacking distance between two non-reverse parallel terminals (3.40 Å). In contrast, the non-fluorine-based Y-BO-ClBr crystal exists in two independent molecular conformations, which results in only one type of π–π stacking between two terminals (A/A), with a distance of 3.35 Å and π–π stacking between one terminal group and one central core of the adjacent molecule (A/D) with a π–π distance of ca. 3.45 Å. Both distances are slightly longer than those of Y-BO-FCl and are comparable to those of the Y-BO-FBr crystal due to the large size and steric hindrance of terminal Cl and Br atoms, suggesting that Y-BO-ClBr adopts an unfavorable intermolecular stacking mode with less and weaker π–π stacking from the terminals. Moreover, as shown in Fig. S5 (ESI†), A/A (reverse) π–π interactions for the Y-BO-ClBr crystal also present a torsion angle from the vertical direction. Besides this, special π–π stacking between the central benzothiadiazole unit with a distance of 3.31 Å was observed in the Y-BO-ClBr crystal. These led to a change in the crystal system from a monoclinic system for Y-BO-FCl to a triclinic system for Y-BO-ClBr. Moreover, with the successive increase in the size of the halogen atom substitution on the end group, the π–π distance gradually increased and the intermolecular aggregation gradually became weaker to a certain extent on going from Y-BO-FCl to Y-BO-ClBr. Apart from the above S⋯O
C conformational lock. The molecular torsion angle within the two planes respectively built from the two end groups in one molecular Y-BO-FCl is 3.05°, indicating the relative planar conformation. While, the molecular torsion angles are 3.79° for Y-BO-FBr and 10.91° for Y-BO-ClBr. This indicates that the incorporation of a relatively larger sized halogen atom substitution on the end group leads to a slight increase in the molecular torsion angles, especially for Y-BO-ClBr. As is known, A–DA′D–A type SMAs tend to stack together between nearby terminals via A/A (reverse or non-reverse) π–π interactions, which is the major driving force to determine the crystal structure of these molecules. Therefore, as shown in Fig. 3, different hetero-dihalogenated end groups with different molecular torsion angles also led to dramatically different intermolecular packing modes. Fluorine-based Y-BO-FCl and Y-BO-FBr crystals have four independent molecular conformations with three types of π⋯π interactions between neighbor molecules, including the π⋯π interactions between two terminal groups (A/A) and the π–π stacking between one terminal group and half central core of an adjacent molecule (A/D). Specifically, the π–π stacking distance between one terminal group and central core in Y-BO-FCl crystal is ca. 3.43 Å, which is slightly shorter than that in the Y-BO-FBr crystals (ca. 3.45 Å). Moreover, the π–π stacking distance between two antiparallel terminal groups in the Y-BO-FCl crystal is ca. 3.31 Å, which is obviously shorter than that in the Y-BO-FBr crystals (ca. 3.35 Å). The Y-BO-FCl and Y-BO-FBr crystals exhibit another similar π–π stacking distance between two non-reverse parallel terminals (3.40 Å). In contrast, the non-fluorine-based Y-BO-ClBr crystal exists in two independent molecular conformations, which results in only one type of π–π stacking between two terminals (A/A), with a distance of 3.35 Å and π–π stacking between one terminal group and one central core of the adjacent molecule (A/D) with a π–π distance of ca. 3.45 Å. Both distances are slightly longer than those of Y-BO-FCl and are comparable to those of the Y-BO-FBr crystal due to the large size and steric hindrance of terminal Cl and Br atoms, suggesting that Y-BO-ClBr adopts an unfavorable intermolecular stacking mode with less and weaker π–π stacking from the terminals. Moreover, as shown in Fig. S5 (ESI†), A/A (reverse) π–π interactions for the Y-BO-ClBr crystal also present a torsion angle from the vertical direction. Besides this, special π–π stacking between the central benzothiadiazole unit with a distance of 3.31 Å was observed in the Y-BO-ClBr crystal. These led to a change in the crystal system from a monoclinic system for Y-BO-FCl to a triclinic system for Y-BO-ClBr. Moreover, with the successive increase in the size of the halogen atom substitution on the end group, the π–π distance gradually increased and the intermolecular aggregation gradually became weaker to a certain extent on going from Y-BO-FCl to Y-BO-ClBr. Apart from the above S⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C intramolecular interactions and π⋯π intermolecular interactions, multiple other weak non-covalent intermolecular interactions exist, such as F⋯N, Cl⋯N, Cl⋯O, Cl⋯π, and F⋯π (Fig. S6, ESI†). For example, fluorine-based Y-BO-FCl and Y-BO-FBr crystals exhibit weak F⋯N non-covalent intermolecular interactions with a distance of 3.41 and 3.45 Å, respectively, which are induced from the fluorine on the terminals to the central benzothiadiazole unit. While, an obvious Cl⋯N interaction for the Y-BO-ClBr crystal with a distance of 3.00 Å was also observed. In addition, the further 3D interpenetrated packing networks of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr from the top view are presented in Fig. 3. Each of the elliptical frame structures of the Y-BO-FCl and Y-BO-FBr crystals were formed by four molecules owing to the multiple intermolecular interactions, which is obviously different from that of the elliptical frame structure of the Y-BO-ClBr crystal with only two molecules. These results demonstrate that Y-BO-FCl presents the most planar monomolecular structure, the tightest molecular packing, and the best ordered 3D molecular packing network owing to the relatively small radius of F and Cl atoms, which facilitate an improvement in its molecular crystallinity and charge transportation in multiple directions in thin films.
C intramolecular interactions and π⋯π intermolecular interactions, multiple other weak non-covalent intermolecular interactions exist, such as F⋯N, Cl⋯N, Cl⋯O, Cl⋯π, and F⋯π (Fig. S6, ESI†). For example, fluorine-based Y-BO-FCl and Y-BO-FBr crystals exhibit weak F⋯N non-covalent intermolecular interactions with a distance of 3.41 and 3.45 Å, respectively, which are induced from the fluorine on the terminals to the central benzothiadiazole unit. While, an obvious Cl⋯N interaction for the Y-BO-ClBr crystal with a distance of 3.00 Å was also observed. In addition, the further 3D interpenetrated packing networks of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr from the top view are presented in Fig. 3. Each of the elliptical frame structures of the Y-BO-FCl and Y-BO-FBr crystals were formed by four molecules owing to the multiple intermolecular interactions, which is obviously different from that of the elliptical frame structure of the Y-BO-ClBr crystal with only two molecules. These results demonstrate that Y-BO-FCl presents the most planar monomolecular structure, the tightest molecular packing, and the best ordered 3D molecular packing network owing to the relatively small radius of F and Cl atoms, which facilitate an improvement in its molecular crystallinity and charge transportation in multiple directions in thin films.
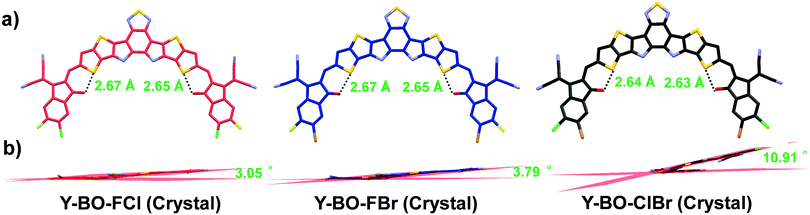 | ||
| Fig. 2 Monomolecular single crystallographic structures of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr in (a) top-view and (b) side-view (the alkyl chains and hydrogen atoms are omitted for clarity). Detailed ORTEP drawings of the “part-occupancy” of two terminal halogens in the crystallographic structures of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr are shown in Fig. S4 (ESI†). | ||
We next assessed the crystal transport properties by estimating the intermolecular electronic coupling along different types of π⋯π interactions using DFT at the ωB97XD/6-31G(d,p) level.80,91 The detailed electronic coupling values for different types of π⋯π interactions are given in Fig. 3. Note that the value of electronic couplings arising from cofacial π–π interactions from two antiparallel terminal groups and two non-reverse parallel terminal groups in Y-BO-FCl are ca. 37.1 and 24.8 meV, respectively, which are much larger than those of the corresponding electronic couplings in Y-BO-FBr (ca. 29.0 and 24.2 meV) and fluorine-free Y-BO-ClBr (ca. 33.0 meV). These results reflect the shortest π–π distances and most favorable orbital overlap between two end groups in the Y-BO-FCl crystal. Moreover, the value of electronic couplings arising from the π–π stacking between one terminal group and central core in Y-BO-FCl is ca. 17.2 meV, which is much larger than those corresponding electronic couplings in Y-BO-FBr (ca. 8.3 meV) and fluorine-free Y-BO-ClBr (ca. 14.0 meV). These results also reflect the shortest π–π distances and most favorable orbital overlap between one terminal group and central core in the Y-BO-FCl crystal. Meanwhile, the electronic coupling of π–π stacking between the central benzothiadiazole unit and pyrrole ring (11.7 meV) only exists between the Y-BO-ClBr molecules, which is obviously smaller than the other types of π–π interactions (A/D or A/A π–π interactions) between the Y-BO-FCl molecules. This reflects the less efficient orbital overlap of the Y-BO-ClBr central benzothiadiazole unit due to a more staggered packing arrangement. In contrast, the Y-BO-FCl crystal exhibits large electronic coupling along all types of π⋯π interactions, indicating its highly efficient three-dimensional electron transport features. Note that the trend in the computed electronic couplings is consistent with the following results of their experimental electron mobilities in neat films, showing that the electron mobility of Y-BO-FCl (7.78 × 10−4 cm2 V−1 s−1) is higher than those of Y-BO-FBr (5.32 × 10−4 cm2 V−1 s−1) and Y-BO-ClBr (2.33 × 10−4 cm2 V−1 s−1) (Fig. S7, ESI†). This indicates that the stacking distance and packing mode of the acceptor crystals play a significant role in electron transportation in neat acceptor films. Such results suggest that Y-BO-FCl indeed exhibits the most efficient electron transport among the three hetero-dihalogenated acceptors.
The absorption spectra of Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr in dilute solution and thin films are shown in Fig. 1a and Fig. S8a (ESI†). All of the acceptors present a broad absorption in the range of 450–800 nm in solution, and their maximum absorptions are located at 740 nm for Y-BO-FCl, 743 nm for Y-BO-FBr, and 747 nm for Y-BO-ClBr, respectively (Table 1). Compared to Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr in solution show slightly red-shifted maximum absorption peaks with a slightly larger molar extinction coefficient, which might be attributed to the relative larger atomic orbitals and enhanced conjugated effect of the chlorine and bromine atoms in comparison with that of the fluorine atom. On going from dilute chloroform solutions to neat films, the absorption spectra of these three acceptors are obviously redshifted by ca. 90 nm, with a wider absorption in the range of 500–950 nm (see Fig. 1b) owing to the obvious enhanced π–π stacking in thin films. The optical bandgaps of Y-BO-FCl, Y-BO-FBr, Y-BO-ClBr in thin films calculated from the absorption edge are 1.33, 1.32, and 1.33 eV, respectively. The HOMO and LUMO energy levels of Y-BO-FCl, Y-BO-FBr, Y-BO-ClBr in thin films can be estimated from their reduction/oxidation curves, with corresponding values of −5.74/−4.03 eV, −5.73/−4.03 eV, and −5.72/−4.02 eV, respectively (Fig. S8b, ESI† and Table 1). Notably, from Y-BO-FCl to Y-BO-ClBr, the successive decrease in the electron-withdrawing ability of halogen atoms results in gradually upshifted HOMO and LUMO energy levels (Fig. 1c), which shows the same trend as the DFT calculation results above. The highest LUMO energy of Y-BO-ClBr might contribute to it having the largest VOC value Clearly, the different hetero-halogenation of the end groups on the acceptors to some extent has an effect on their absorption and molecular energy levels.
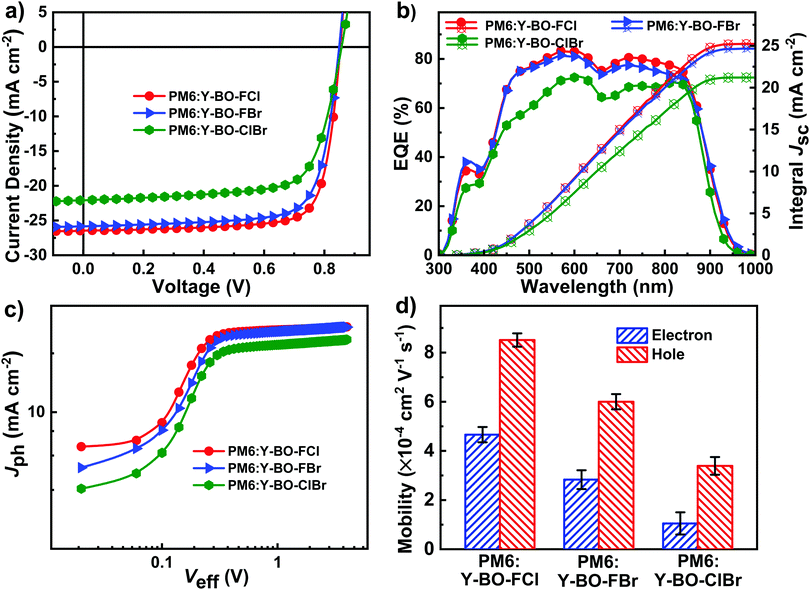
We fabricated three groups of OSCs with a conventional binary device structure of ITO/PEDOT:PSS/PM6:Y-BO-FCl or Y-BO-FBr or Y-BO-ClBr/PDIN/Ag. The blend film was spin-cast from these mixtures in chlorobenzene and then thermal annealing was carried out at 80 °C. The optimized J–V curves of OSCs are plotted in Fig. 4a and the best device parameters are summarized in Table 2. Histograms of the efficiency and distribution of the photovoltaic parameters in different batches of Y-BO-FCl:PM6, Y-BO-FBr:PM6, and Y-BO-ClBr:PM6-based devices are shown in Fig. S9 and S10 (ESI†). Notably, the PM6:Y-BO-FCl-based device yields an impressive PCE of up to 17.52%, with a JSC value of 26.45 mA cm−2, a VOC value of 0.85 V, and an impressive FF value of 77.92%, indicating the advantage of the hetero-halogenation of end groups with fluorine and chlorine atoms.77 Compared to Y-BO-FCl:PM6, although the Y-BO-FBr:PM6-based device exhibits a comparable VOC value of 0.85 V and JSC of 25.83 mA cm−2, it shows a worse FF value of 75.02%, resulting in a relatively lower PCE of 16.47%. Although the Y-BO-ClBr:PM6-based device presents a larger VOC value of 0.86 V, it still has the worst PCE of 13.61%, which is due to its greatly decreased FF (71.62%) and JSC (22.09 mA cm−2) values. Overall, from Y-BO-FCl and Y-BO-FBr to the Y-BO-ClBr-based devices, the increased VOC values match well with the gradually increased LUMO values of the acceptors. The lower JSC and FF values might result from the poor electron transport ability and slightly poor blend morphology of Y6-BO-ClBr in comparison with those of Y-BO-FCl and Y-BO-FBr, which might be due to the worst planar monomolecular structure and loose molecule packing of Y6-BO-ClBr. To the best of our knowledge, the remarkably PCE of 17.52% for Y-BO-FCl is the highest value for reported heterohalogen-modified acceptor-based binary PSCs (see Table S3 and Chart S1, ESI†).46,47,82–85 These results demonstrate that the hetero-halogenation of end groups with fluorine and chlorine atoms is more beneficial in boosting the FF, JSC, and PCE values.
| Active layers | V OC (V) | J SC (mA cm−2) | FFa (%) | PCEa (%) |
|---|---|---|---|---|
| a The values in parentheses are the average values calculated from 20 devices with standard deviation. | ||||
| PM6:Y-BO-FCl | 0.85 (0.85 ± 0.003) | 26.45 (26.47 ± 0.13) | 77.92 (76.72 ± 0.71) | 17.52 (17.33 ± 0.12) |
| PM6:Y-BO-FBr | 0.85 (085 ± 0.002) | 25.83 (25.79 ± 0.22) | 75.02 (74.15 ± 0.77) | 16.47 (16.27 ± 0.12) |
| PM6:Y-BO-ClBr | 0.86 (0.86 ± 0.002) | 22.09 (21.98 ± 0.26) | 71.62 (70.70 ± 0.97) | 13.61 (13.23 ± 0.34) |
The external quantum efficiency (EQE) spectra of the devices based on PM6:Y-BO-FCl, PM6:Y-BO-FBr, and PM6:Y-BO-ClBr were measured and present different JSC values from the J–V curves. As shown in Fig. 4b, PM6:Y-BO-FCl and PM6:Y-BO-FBr show a higher and broader photocurrent response than the PM6:Y-BO-ClBr-based device, which might be due to their different π–π intermolecular packing types and favorable blend morphologies. The highest EQE value of the PM6:Y-BO-FCl-based device is 82.3% at 570 nm, which is obviously higher than those of PM6:Y-BO-FBr (81.5% at 570 nm) and PM6:Y-BO-ClBr (72.8% at 610 nm). Moreover, although the EQE spectrum of the PM6:Y-BO-FCl exhibits a lower photocurrent response in the range of 300–400 nm and a slightly less extended photon response in the band tail, it is compensated for by the excellent photon response in the range of 500–900 nm compared with that of PM6:Y-BO-FBr, thus leading to a slightly higher JSC value. The calculated current densities obtained from the corresponding EQE curves are 25.34, 24.97, and 21.23 mA cm−2, respectively, which represents less than 5% mismatch with the JSC values from the J–V curves.
To understand the difference in the exciton dissociation and charge collection of these hetero-halogenated acceptors, the relationship between the photocurrent density (Jph) versus the effective voltage (Veff) was studied. As shown in Fig. 4c, the exciton dissociation efficiency (ηdiss) was obtained using the the equation ηdiss = JSC/Jsat under short-circuit conditions. Therefore, the ηdiss values for PM6:Y-BO-FCl, PM6:Y-BO-FBr and PM6:Y-BO-ClBr are 0.96, 0.95, and 0.94, respectively. To further evaluate the efficiency of charge collection, ηcoll = Jmax/Jsat (where Jmax is the current density at maximum power) was calculated. The ηcoll values of the PM6:Y-BO-FCl, PM6:Y-BO-FBr and PM6:Y-BO-ClBr-based devices are 0.89, 0.87, and 0.83, respectively, which are consistent with the trend in the ηdiss values. The highest ηdiss and ηcoll values for PM6:Y-BO-FCl suggest that the Y-BO-FCl-based device exhibits the most effective exciton dissociation and charge collection, which might explain the highest JSC value for the corresponding device. We further explored the relationship between JSC/VOC and light intensity (Plight). Under different Plight, the corresponding JSC follows the equation of JSC ∝ Pαlight. When α is close to 1, weak recombination was achieved. As shown in Fig. S11a (ESI†), the recombination parameters of α are 0.96, 0.95 and 0.93 for the Y-BO-FCl-, Y-BO-FBr- and Y-BO-ClBr-based devices, respectively. The highest α of the Y-BO-FCl-based device suggests that it exhibits the lowest degree of bimolecular recombination loss of the three devices. In addition, the relationships between VOC and ln(Plight) of the devices suggest the possibility of trap-assisted recombination. As illustrated in Fig. S11b (ESI†), the slopes of the Y-BO-FCl- and Y-BO-FBr-based OSCs are 1.02kT/q and 1.06kT/q, respectively, which are obviously lower than that of the Y-BO-ClBr-based device (1.47kT/q). This indicates that the monomolecular recombination loss or trap-assisted recombination was obviously suppressed in the Y-BO-FCl and Y-BO-FBr-based OSCs, which contribute towards the higher FF and JSC values of the corresponding devices. As a result, the extent of charge recombination is in good agreement with the corresponding device parameters.
To explore the effect of hetero-halogenated end groups on the charge transport properties, we used a space-charge-limited current (SCLC) model to measure the electron mobility (μe) and hole mobility (μh) of these blend films and the results are shown in Fig. 4d and Fig. S12 (ESI†). The electron mobilities (μe) and hole mobilities (μh) of the PM6:Y-BO-FCl, PM6:Y-BO-FBr, and PM6:Y-BO-ClBr blend films are calculated to be 4.46 × 10−4/8.51 × 10−4 cm2 V−1 s−1, 2.83 × 10−4/6.00 × 10−4 cm2 V−1 s−1, and 1.05 × 10−5/3.39 × 10−4 cm2 V−1 s−1, respectively. Therefore, the ratios of electron mobility to hole mobility (μe/μh) of the PM6:Y-BO-FCl, PM6:Y-BO-FBr, and PM6:Y-BO-ClBr blend films were calculated to be 0.52, 0.47 and 0.31, respectively. Obviously, the choice of F and Cl atoms in the modified NFAs could effects the enhancement of the charge mobilities and a more balanced μh/μe ratio is achieved, which leads to the PM6:Y-BO-FCl-based device exhibiting the highest JSC and FF values in in comparison to the other two heterohalogenated NFAs-based devices.
To understand the influence that different hetero-halogenated terminals has on the phase separation, we applied atomic force microscopy (AFM) and transmission electron microscopy (TEM) techniques to these blend films. As shown in the AFM height images (Fig. 5a–c), the three blends exhibit a uniform and relative smooth surface morphology, and the root-mean-square roughnesses for PM6:Y-BO-FCl, PM6:Y-BO-FBr and PM6:Y-BO-ClBr are 2.7, 1.9, and 0.9 nm, respectively. In addition, from the phase images (Fig. 5d–f), the PM6:Y-BO-FCl and PM6:Y-BO-FBr blends present obvious fiber-like surface morphology, which is not observed for the PM6:Y-BO-ClBr blend. This can be attributed to the more ordered crystallization tendency induced by these specific heterohalogenated end groups. From the TEM image (Fig. 5g–i), PM6:Y-BO-ClBr still shows weak phase-separated features. In contrast, more obvious nanofiber-like interpenetrated structures with proper phase-separated morphologies were observed in the other materials, especially for the PM6:Y-BO-FCl blend film, which is consistent with the electron mobility trends of these blend films. These results indicate that the fluorine/chlorine hetero-halogenated acceptor is beneficial to achieving suitable donor/acceptor distribution and phase separation scale in the blend films, leading to efficient charge transport and an enhanced FF value.
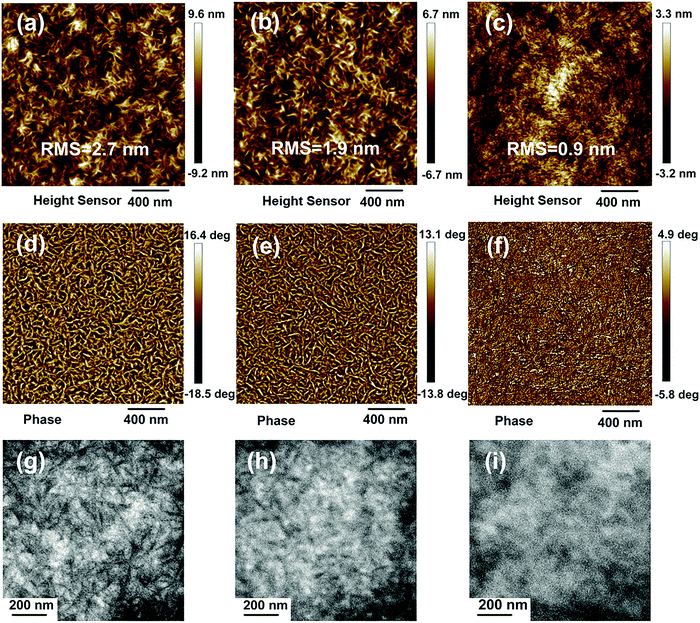 | ||
| Fig. 5 (a–c) AFM height images, (d–f) AFM phase images, and (g–i) high-resolution TEM images of the PM6:Y-BO-FCl, PM6:Y-BO-FBr and PM6:Y-BO-ClBr blend films. | ||
To better understand the effects that different hetero-halogenated end groups have on the intermolecular packing mode and orientation of the crystallinity in the single component and blend films, two-dimensional grazing incidence X-ray diffraction (2D-GIXD) was carried out.90 As shown in Fig. S13 (ESI†), the neat Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr films exhibit a distinct π–π stacking (010) peak at ca. 1.79, 1.78, and 1.78 Å−1 in the out-of-plane (OOP) direction with π–π stacking distances of ca. 3.51, 3.52, and 3.52 Å, respectively, suggesting predominant face-on molecular packing patterns. Moreover, the neat Y-BO-FCl film shows a slightly higher diffraction intensity than those of the Y-BO-FBr and Y-BO-ClBr neat films. These results imply that the acceptor Y-BO-FCl with fluorine and chlorine atoms exhibit higher crystallinity and better intermolecular packing in comparison with those of Y-BO-FBr and Y-BO-ClBr, in agreement with the trend of the single crystals. The 2D images of the three blend films and corresponding 1D line-cuts are presented in Fig. 6. Although the blend films present face-on orientation with the (100) diffraction peaks at ca. 0.30 Å−1 in the IP direction and the (010) diffraction peaks at ca. 1.76 Å−1 in the OOP direction, the PM6:Y-BO-FCl blend films show obviously higher diffraction intensity than that of the PM6:Y-BO-FBr and PM6:Y-BO-ClBr blend films. Moreover, the corresponding crystal coherence lengths (CCLs) of (010) π–π stacking peaks in the OOP direction and the (100) lamellar diffraction peaks in the IP direction are estimated to be 24.9 Å and 165.8 Å for PM6:Y-BO-FCl, 24.3 and 145.7 Å for PM6:Y-BO-FBr, and 23.8 and 130.9 Å for PM6:Y-BO-ClBr. This gradually decreased crystallinity was consistent with the trend of AFM and TEM images. Therefore, the PM6:Y-BO-FCl blend films exhibit the most favorable face-on orientation and the largest CCL among the three blend films, which explains their highest charge mobilities. Our results indicate that precisely chosen hetero-halogenated end groups with a relatively small radius and more electronegative halogens can effectively enhance the crystallization of the acceptors and face-on orientation of the corresponding blend films, leading to higher JSC and FF values in the corresponding OSCs.
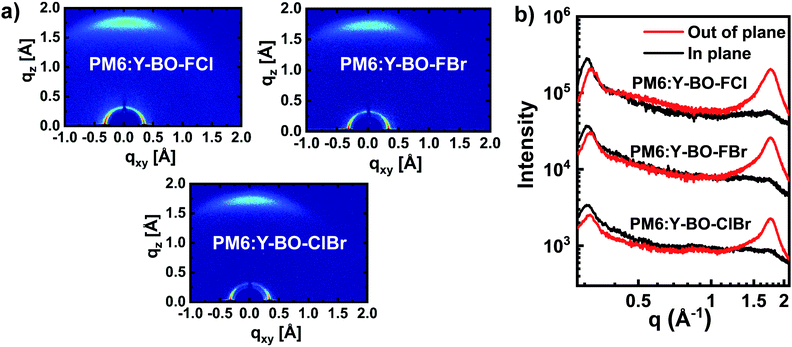 | ||
| Fig. 6 (a) 2D-GIXD patterns and (b) 1D GIXD line-cuts of blend films of PM6:Y-BO-FCl, PM6:Y-BO-FBr, and PM6:Y-BO-ClBr. | ||
Conclusions
In conclusion, we successfully synthesized two new hetero-dihalogenated end groups (FCl-IC and FBr-IC) with fluorine/chlorine or fluorine/bromine atoms in one IC unit, respectively, in a facile and economic synthetic process. After that, we also synthesized a series of NFAs (Y-BO-FCl, Y-BO-FBr and Y-BO-ClBr) by changing the hetero-dihalogenated terminals to FCl-IC, FBr-IC, and ClBr-IC, respectively. The impacts that different hetero-dihalogenated terminals have on their optoelectronic and intermolecular aggregation properties, and eventually device performance of these NFAs were exhaustively investigated for the first time. The Y-BO-FCl neat film exhibits deeper energy levels in comparison with films of Y-BO-FBr and Y-BO-ClBr. Dramatic changes in intermolecular packing features are revealed from a non-fluorinated acceptor single crystal to a fluorinated acceptor single crystal, indicating that fluorine atoms play an essential role in intermolecular interactions. Crystallographic and theoretical analysis indicate that Y-BO-FCl displays a relatively planar molecular skeleton, obviously tighter intermolecular packing and enhanced π–π electronic coupling in comparison to Y-BO-FBr and Y-BO-ClBr, resulting in the Y-BO-FCl film exhibiting the strongest crystallinity. Moreover, PM6:Y-BO-FCl blend films present more apparent face-on orientation crystallinity and more suitable fiber-like phase separation in comparison with those of PM6:Y-BO-FBr and PM6:Y-BO-ClBr, which effectively suppresses charge recombination and promotes the high and balanced charge mobility. Consequently, the PCE of the Y-BO-FCl:PM6-based devices reached 17.52%, which is superior to those of the PM6:Y-BO-FBr (16.47%) and PM6:Y-BO-ClBr-based devices (13.61%). Up to now, this impressive PCE of 17.52% is the highest efficiency reported for hetero-halogen-functionalized acceptors in binary OSCs. Our investigation demonstrates that the types of hetero-dihalogenated end groups play an important role in modulating the intermolecular aggregation and packing properties, blend morphology, and device performance of the acceptors. Our work highlights the synergistic effects of fluorine and chlorine substitutions at one end group (FCl-IC) in affording efficient OSCs. In addition, upon consideration of the diversification of the substitution at the terminals, it can be speculated that the careful screening of other pairs of hetero-substitutions will further promote the device performance of hetero-substitution-based acceptors.Author contributions
Jin-Liang Wang conceived the idea. Lai Wang synthesized the new hetero-dihalogenated end groups and NFAs and performed the data collection and photoelectrical characterization. Qiaoshi An and Lu Yan designed and performed device fabrication and optimization. Lu Yan, Hai-Rui Bai, Mengyun Jiang, and Hong-Fu Zhi completed the device characterization and morphology characterization. Hai-Rui Bai and Can Yang carried out the 2D GIXD measurements. Asif Mahmood conducted the DFT calculations. Lu Yan supplied FBr-IC. Jin-Liang Wang completed the single-crystal preparation of the three NFAs and crystallographic analysis. Jin-Liang Wang, Lai Wang, and Qiaoshi An wrote and revised the manuscript. All authors discussed the results. Jin-Liang Wang and Qiaoshi An supervised the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by grants from the Natural Science Foundation of China (No. 21971014, 21672023) and the National Key Research and Development Program of China (2018YFA0901800). Jin-Liang Wang was supported by the Thousand Youth Talents Plan of China and BIT Teli Young Fellow Recruitment Program. The authors thank the Analysis & Testing Center, Beijing Institute of Technology for NMR, TGA, AFM, device preparation, and characterization. Lai Wang thanks Prof. Qiaoshi An (Beijing Institute of Technology) for device optimization and helping drawing the figures of device characterization. Lai Wang thanks Prof. Jin-Liang Wang (Beijing Institute of Technology) for single-crystal preparation. Lai Wang thanks Zhao Jiang (Beijing Institute of Technology) for help with the synthesis of ClBr-IC. Lai Wang thanks Lu Yan (Beijing Institute of Technology) for supplying FBr-IC. Lai Wang thanks Xin Zhao (Beijing Institute of Technology) for supplying FCl-IC to obtain MS result. Lai Wang thanks Lu Yan, Hai-Rui Bai, Hongfu Zhi, and Mengyun Jiang (Beijing Institute of Technology) for the device characterization. The authors thank Prof. Hai-Jun Fan, Prof. Xiaozhang Zhu, and Dr Yongjie Chen (Chinese Academy of Sciences) for help with the single-crystal measurements. The authors thank Prof. Xiaoyu Cao and Dr Hang Qu (Xiamen University) for help with the crystallographic refinement. The authors are grateful for having been able to use beamline BL14B1 (Shanghai Synchrotron Radiation Facility) for the 2D GIXD experiments. The authors thank Prof. Jian Pei and Dr Shi-Sheng Wan (Peking University) for the MS experiments. The authors thank Prof. Xiao Feng and Dr Qingnuan Zhang (Beijing Institute of Technology) for the IR experiments.Notes and references
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006–7043 CrossRef PubMed.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef PubMed.
- H.-H. Gao, Y. Sun, Y. Cai, X. Wan, L. Meng, X. Ke, S. Li, Y. Zhang, R. Xia, N. Zheng, Z. Xie, C. Li, M. Zhang, H.-L. Yip, Y. Cao and Y. Chen, Adv. Energy Mater., 2019, 9, 1901024 CrossRef.
- X. Guo, Q. Fan, J. Wu, G. Li, Z. Peng, W. Su, J. Lin, L. Hou, Y. Qin, H. Ade, L. Ye, M. Zhang and Y. Li, Angew. Chem., Int. Ed., 2021, 60, 2322–2329 CrossRef PubMed.
- D. Hu, Q. Yang, H. Chen, F. Wobben, V. M. Le Corre, R. Singh, T. Liu, R. Ma, H. Tang, L. J. A. Koster, T. Duan, H. Yan, Z. Kan, Z. Xiao and S. Lu, Energy Environ. Sci., 2020, 13, 2134–2141 RSC.
- L. Ye, Y. Xiong, Z. Chen, Q. Zhang, Z. Fei, R. Henry, M. Heeney, B. T. O'Connor, W. You and H. Ade, Adv. Mater., 2019, 31, 1808153 CrossRef PubMed.
- Q. Fan, Q. An, Y. Lin, Y. Xia, Q. Li, M. Zhang, W. Su, W. Peng, C. Zhang, F. Liu, L. Hou, W. Zhu, D. Yu, M. Xiao, E. Moons, F. Zhang, T. D. Anthopoulos, O. Inganäs and E. Wang, Energy Environ. Sci., 2020, 13, 5017–5027 RSC.
- S. Pang, Z. Wang, X. Yuan, L. Pan, W. Deng, H. Tang, H. Wu, S. Chen, C. Duan, F. Huang and Y. Cao, Angew. Chem., Int. Ed., 2021, 60, 8813–8817 CrossRef CAS PubMed.
- Z. Liang, M. Li, Q. Wang, Y. Qin, S. J. Stuard, Z. Peng, Y. Deng, H. Ade, L. Ye and Y. Geng, Joule, 2020, 4, 1278–1295 CrossRef CAS.
- K. Gao, Y. Kan, X. Chen, F. Liu, B. Kan, L. Nian, X. Wan, Y. Chen, X. Peng, T. P. Russell, Y. Cao and A. K. Jen, Adv. Mater., 2020, 32, 1906129 CrossRef PubMed.
- X. Li, I. Angunawela, Y. Chang, J. Zhou, H. Huang, L. Zhong, A. Liebman-Pelaez, C. Zhu, L. Meng, Z. Xie, H. Ade, H. Yan and Y. Li, Energy Environ. Sci., 2020, 13, 5028–5038 RSC.
- A. Wadsworth, M. Moser, A. Marks, M. S. Little, N. Gasparini, C. J. Brabec, D. Baran and I. McCulloch, Chem. Soc. Rev., 2019, 48, 1596–1625 RSC.
- T. Liu, R. Ma, Z. Luo, Y. Guo, G. Zhang, Y. Xiao, T. Yang, Y. Chen, G. Li, Y. Yi, X. Lu, H. Yan and B. Tang, Energy Environ. Sci., 2020, 13, 2115–2123 RSC.
- G. Wang, F. S. Melkonyan, A. Facchetti and T. J. Marks, Angew. Chem., Int. Ed., 2019, 58, 4129–4142 CrossRef.
- X. Du, T. Heumueller, W. Gruber, A. Classen, T. Unruh, N. Li and C. Brabec, Joule, 2018, 3, 215–226 CrossRef.
- G. P. Kini, S. J. Jeon and D. K. Moon, Adv. Mater., 2020, 32, 1906175 CrossRef.
- L. Zhu, J. Zhang, Y. Guo, C. Yang, Y. Yi and Z. Wei, Angew. Chem., Int. Ed., 2021, 60, 15348–15353 CrossRef CAS.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef CAS.
- C. Yang, Q. An, H.-R. Bai, H.-F. Zhi, H. S. Ryu, A. Mahmood, X. Zhao, S. Zhang, H. Y. Woo and J.-L. Wang, Angew. Chem., Int. Ed., 2021, 60, 19241–19252 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Nat. Commun., 2019, 10, 2515 CrossRef PubMed.
- S. Park, H. Ahn, J.-Y. Kim, J. B. Park, J. Kim, S. H. Im and H. J. Son, ACS Energy Lett., 2019, 5, 170–179 CrossRef.
- T. Yan, W. Song, J. Huang, R. Peng, L. Huang and Z. Ge, Adv. Mater., 2019, 31, 1902210 CrossRef PubMed.
- Y. Xu, H. Yao, L. Ma, L. Hong, J. Li, Q. Liao, Y. Zu, J. Wang, M. Gao, L. Ye and J. Hou, Angew. Chem., Int. Ed., 2020, 59, 9004–9010 CrossRef CAS.
- C. Zhao, Z. Zhang, F. Han, D. Xia, C. Xiao, J. Fang, Y. Zhang, B. Wu, S. You, Y. Wu and W. Li, Angew. Chem., Int. Ed., 2021, 60, 8526–8531 CrossRef CAS.
- J. Wu, J. Lee, Y.-C. Chin, H. Yao, H. Cha, J. Luke, J. Hou, J.-S. Kim and J. R. Durrant, Energy Environ. Sci., 2020, 13, 2422–2430 RSC.
- G. Li, X. Zhang, L. O. Jones, J. M. Alzola, S. Mukherjee, L. W. Feng, W. Zhu, C. L. Stern, W. Huang, J. Yu, V. K. Sangwan, D. M. DeLongchamp, K. L. Kohlstedt, M. R. Wasielewski, M. C. Hersam, G. C. Schatz, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2021, 143(16), 6123–6139 CrossRef CAS PubMed.
- L.-W. Feng, J. Chen, S. Mukherjee, V. K. Sangwan, W. Huang, Y. Chen, D. Zheng, J. W. Strzalka, G. Wang, M. C. Hersam, D. DeLongchamp, A. Facchetti and T. J. Marks, ACS Energy Lett., 2020, 5, 1780–1787 CrossRef CAS.
- W. Gao, Q. An, M. Hao, R. Sun, J. Yuan, F. Zhang, W. Ma, J. Min and C. Yang, Adv. Funct. Mater., 2020, 30, 1908336 CrossRef CAS.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272–275 CrossRef CAS.
- M. Mainville and M. Leclerc, ACS Energy Lett., 2020, 5, 1186–1197 CrossRef CAS.
- H. Tang, H. Chen, C. Yan, J. Huang, P. W. K. Fong, J. Lv, D. Hu, R. Singh, M. Kumar, Z. Xiao, Z. Kan, S. Lu and G. Li, Adv. Energy Mater., 2020, 10, 2001076 CrossRef CAS.
- J. Wan, L. Zhang, Q. He, S. Liu, B. Huang, L. Hu, W. Zhou and Y. Chen, Adv. Funct. Mater., 2020, 30, 1909760 CrossRef CAS.
- J.-L. Wang, K.-K. Liu, J. Yan, Z. Wu, F. Liu, F. Xiao, Z.-F. Chang, H.-B. Wu, Y. Cao and T. P. Russell, J. Am. Chem. Soc., 2016, 138, 7687–7697 CrossRef CAS PubMed.
- H. Yu, L. Arunagiri, L. Zhang, J. Huang, W. Ma, J. Zhang and H. Yan, J. Mater. Chem. A, 2020, 8, 6501–6509 RSC.
- Q. Yue, W. Liu and X. Zhu, J. Am. Chem. Soc., 2020, 142, 11613–11628 CrossRef CAS.
- F. Peng, K. An, W. Zhong, Z. Li, L. Ying, N. Li, Z. Huang, C. Zhu, B. Fan, F. Huang and Y. Cao, ACS Energy Lett., 2020, 5, 3702–3707 CrossRef CAS.
- B. Liu, H. Sun, J.-W. Lee, J. Yang, J. Wang, Y. Li, M. Xu, Q. Liao, B. Li, W. Zhang, D. Han, L. Niu, H. Meng, B. Kim and X. Guo, Energy Environ. Sci., 2021, 14, 4499–4507 RSC.
- Q. Fan, H. Fu, Q. Wu, Z. Wu, F. Lin, Z. Zhu, J. Min, H. Y. Wu and A. K. Y. Jen, Angew. Chem., Int. Ed., 2021, 60, 15935–15943 CrossRef CAS PubMed.
- Z.-Q. Jiang, T.-T. Wang, F.-P. Wu, J.-D. Lin and L.-S. Liao, J. Mater. Chem. A, 2018, 6, 17256–17287 RSC.
- C. Zhu, J. Yuan, F. Cai, L. Meng, H. Zhang, H. Chen, J. Li, B. Qiu, H. Peng, S. Chen, Y. Hu, C. Yang, F. Gao, Y. Zou and Y. Li, Energy Environ. Sci., 2020, 13, 2459–2466 RSC.
- Z. Fei, F. D. Eisner, X. Jiao, M. Azzouzi, J. A. Röhr, Y. Han, M. Shahid, A. S. R. Chesman, C. D. Easton, C. R. McNeill, T. D. Anthopoulos, J. Nelson and M. Heeney, Adv. Mater., 2018, 30, 1705209 CrossRef PubMed.
- Y.-N. Chen, M. Li, Y. Wang, J. Wang, M. Zhang, Y. Zhou, J. Yang, Y. Liu, F. Liu, Z. Tang, Q. Bao and Z. Bo, Angew. Chem., Int. Ed., 2020, 59, 22714–22720 CrossRef CAS.
- L. Zhan, S. Li, T.-K. Lau, Y. Cui, X. Lu, M. Shi, C.-Z. Li, H. Li, J. Hou and H. Chen, Energy Environ. Sci., 2020, 13, 635–645 RSC.
- H. Yu, M. Pan, R. Sun, I. Agunawela, J. Zhang, Y. Li, Z. Qi, H. Han, X. Zou, W. Zhou, S. Chen, J. Y. L. Lai, S. Luo, Z. Luo, D. Zhao, X. Lu, H. Ade, F. Huang, J. Min and H. Yan, Angew. Chem., Int. Ed., 2021, 60, 10137–10146 CrossRef CAS.
- Y. Wei, J. Yu, L. Qin, H. Chen, X. Wu, Z. Wei, X. Zhang, Z. Xiao, L. Ding, F. Gao and H. Huang, Energy Environ. Sci., 2021, 14, 2314–2321 RSC.
- Z. Luo, R. Ma, T. Liu, J. Yu, Y. Xiao, R. Sun, G. Xie, J. Yuan, Y. Chen, K. Chen, G. Chai, H. Sun, J. Min, J. Zhang, Y. Zou, C. Yang, X. Lu, F. Gao and H. Yan, Joule, 2020, 4, 1236–1247 CrossRef CAS.
- H. Chen, H. Lai, Z. Chen, Y. Zhu, H. Wang, L. Han, Y. Zhang and F. He, Angew. Chem., Int. Ed., 2021, 60, 3238–3246 CrossRef CAS PubMed.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed.
- J.-W. Lee, D. Jeong, D. J. Kim, T. N.-L. Phan, J. S. Park, T.-S. Kim and B. J. Kim, Energy Environ. Sci., 2021, 14, 4067–4076 RSC.
- J. Qu, H. Chen, J. Zhou, H. Lai, T. Liu, P. Chao, D. Li, Z. Xie, F. He and Y. Ma, ACS Appl. Mater. Interfaces, 2018, 10, 39992–40000 CrossRef CAS.
- C. Yang, S. Zhang, J. Ren, M. Gao, P. Bi, L. Ye and J. Hou, Energy Environ. Sci., 2020, 13, 2864–2869 RSC.
- H. Lai, H. Chen, J. Zhou, J. Qu, P. Chao, T. Liu, X. Chang, N. Zheng, Z. Xie and F. He, iScience, 2019, 17, 302–314 CrossRef CAS PubMed.
- A. Karki, J. Vollbrecht, A. J. Gillett, S. S. Xiao, Y. Yang, Z. Peng, N. Schopp, A. L. Dixon, S. Yoon, M. Schrock, H. Ade, G. N. M. Reddy, R. H. Friend and T.-Q. Nguyen, Energy Environ. Sci., 2020, 13, 3679–3692 RSC.
- L. Yang, X. Song, J. Yu, H. Wang, Z. Zhang, R. Geng, J. Cao, D. Baran and W. Tang, J. Mater. Chem. A, 2019, 7, 22279–22286 RSC.
- A. Mahmood and J.-L. Wang, Energy Environ. Sci., 2021, 14, 90–105 RSC.
- J. Ge, L. Xie, R. Peng, B. Fanady, J. Huang, W. Song, T. Yan, W. Zhang and Z. Ge, Angew. Chem., Int. Ed., 2020, 59, 2808–2815 CrossRef CAS PubMed.
- J. Zhang, C. Yan, W. Wang, Y. Xiao, X. Lu, S. Barlow, T. C. Parker, X. Zhan and S. R. Marder, Chem. Mater., 2018, 30, 309–313 CrossRef CAS.
- M. Jiang, H. Bai, H. Zhi, H. Y. Woo, L. Tong, J.-L. Wang, F. Zhang and Q. An, Energy Environ. Sci., 2021, 14, 3945–3953 RSC.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Joule, 2019, 3, 3020–3033 CrossRef CAS.
- R. Wang, J. Yuan, R. Wang, G. Han, T. Huang, W. Huang, J. Xue, H. C. Wang, C. Zhang, C. Zhu, P. Cheng, D. Meng, Y. Yi, K. H. Wei, Y. Zou and Y. Yang, Adv. Mater., 2019, 31, 1904215 CrossRef CAS PubMed.
- R. Sun, Y. Wu, J. Guo, Y. Wang, F. Qin, B. Shen, D. Li, T. Wang, Y. Li, Y. Zhou, G. Lu, Y. Li and J. Min, Energy Environ. Sci., 2021, 14, 3174–3183 RSC.
- M. Chang, L. Meng, Y. Wang, X. Ke, Y.-Q.-Q. Yi, N. Zheng, W. Zheng, Z. Xie, M. Zhang, Y. Yi, H. Zhang, X. Wan, C. Li and Y. Chen, Chem. Mater., 2020, 32, 2593–2604 CrossRef CAS.
- X. Chen, B. Kan, Y. Kan, M. Zhang, S. B. Jo, K. Gao, F. Lin, F. Liu, X. Peng, Y. Cao and A. K. Y. Jen, Adv. Funct. Mater., 2020, 30, 1909535 CrossRef CAS.
- W. Gao, T. Liu, R. Sun, G. Zhang, Y. Xiao, R. Ma, C. Zhong, X. Lu, J. Min, H. Yan and C. Yang, Adv. Sci., 2020, 7, 1902657 CrossRef CAS PubMed.
- Y. Wang, Y. Wang, L. Zhu, H. Liu, J. Fang, X. Guo, F. Liu, Z. Tang, M. Zhang and Y. Li, Energy Environ. Sci., 2020, 13, 1309–1317 RSC.
- G.-Y. Ge, J.-T. Li, J.-R. Wang, M. Xiong, X. Dong, Z.-J. Li, J.-L. Li, X.-Y. Cao, T. Lei and J.-L. Wang, Adv. Funct. Mater., 2021 DOI:10.1002/adfm.202108289.
- Z. Luo, R. Sun, C. Zhong, T. Liu, G. Zhang, Y. Zou, X. Jiao, J. Min and C. Yang, Sci. China: Chem., 2020, 63, 361–369 CrossRef CAS.
- G. Chai, Y. Chang, J. Zhang, X. Xu, L. Yu, X. Zou, X. Li, Y. Chen, S. Luo, B. Liu, F. Bai, Z. Luo, H. Yu, J. Liang, T. Liu, K. S. Wong, H. Zhou, Q. Peng and H. Yan, Energy Environ. Sci., 2021, 14, 3469–3479 RSC.
- Z. Zhang, J. Yu, X. Yin, Z. Hu, Y. Jiang, J. Sun, J. Zhou, F. Zhang, T. P. Russell, F. Liu and W. Tang, Adv. Funct. Mater., 2018, 28, 1705095 CrossRef.
- K.-K. Liu, X. Xu, J.-L. Wang, C. Zhang, G.-Y. Ge, F.-D. Zhuang, H.-J. Zhang, C. Yang, Q. Peng and J. Pei, J. Mater. Chem. A, 2019, 7, 24389–24399 RSC.
- G. Zhang, X.-K. Chen, J. Xiao, P. C. Y. Chow, M. Ren, G. Kupgan, X. Jiao, C. C. S. Chan, X. Du, R. Xia, Z. Chen, J. Yuan, Y. Zhang, S. Zhang, Y. Liu, Y. Zou, H. Yan, K. S. Wong, V. Coropceanu, N. Li, C. J. Brabec, J.-L. Bredas, H.-L. Yip and Y. Cao, Nat. Commun., 2020, 11, 3943 CrossRef CAS PubMed.
- Y. Ma, D. Cai, S. Wan, P. Wang, J. Wang and Q. Zheng, Angew. Chem., Int. Ed., 2020, 59, 21627–21633 CrossRef CAS PubMed.
- X. Xu, L. Yu, H. Yan, R. Li and Q. Peng, Energy Environ. Sci., 2020, 13, 4381–4388 RSC.
- T. Li, Y. Wu, J. Zhou, M. Li, J. Wu, Q. Hu, B. Jia, X. Pan, M. Zhang, Z. Tang, Z. Xie, T. P. Russell and X. Zhan, J. Am. Chem. Soc., 2020, 142, 20124–20133 CrossRef CAS.
- S. Dai and X. Zhan, Adv. Energy Mater., 2018, 8, 1800002 CrossRef.
- Y. Li, J.-D. Lin, X. Che, Y. Qu, F. Liu, L.-S. Liao and S. R. Forrest, J. Am. Chem. Soc., 2017, 139, 17114–17119 CrossRef CAS PubMed.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Tang, B. Lin, K. Xian, B. Gao, C. An, P. Bi, W. Ma and J. Hou, Natl. Sci. Rev., 2020, 7, 1239–1246 CrossRef CAS PubMed.
- J.-L. Wang, K.-K. Liu, L. Hong, G.-Y. Ge, C. Zhang and J. Hou, ACS Energy Lett., 2018, 3, 2967–2976 CrossRef CAS.
- S. M. Swick, J. M. Alzola, V. K. Sangwan, S. H. Amsterdam, W. Zhu, L. O. Jones, N. Powers-Riggs, A. Facchetti, K. L. Kohlstedt, G. C. Schatz, M. C. Hersam, M. R. Wasielewski and T. J. Marks, Adv. Energy Mater., 2020, 10, 2000F Search PubMed.
- T.-J. Wen, Z.-X. Liu, Z. Chen, J. Zhou, Z. Shen, Y. Xiao, X. Lu, Z. Xie, H. Zhu, C.-Z. Li and H. Chen, Angew. Chem., Int. Ed., 2021, 60, 12964–12970 CrossRef CAS.
- H. Wang, T. Liu, J. Zhou, D. Mo, L. Han, H. Lai, H. Chen, N. Zheng, Y. Zhu, Z. Xie and F. He, Adv. Sci., 2020, 7, 1903784 CrossRef CAS.
- T. Liu, Y. Zhang, Y. Shao, R. Ma, Z. Luo, Y. Xiao, T. Yang, X. Lu, Z. Yuan, H. Yan, Y. Chen and Y. Li, Adv. Funct. Mater., 2020, 30, 2000456 CrossRef CAS.
- H. Lai, H. Chen, Y. Zhu, L. Chen, H.-H. Huang and F. He, J. Mater. Chem. A, 2020, 8, 9670–9676 RSC.
- S. Li, L. Zhan, Y. Jin, G. Zhou, T. K. Lau, R. Qin, M. Shi, C. Z. Li, H. Zhu, X. Lu, F. Zhang and H. Chen, Adv. Mater., 2020, 32, 2001160 CrossRef CAS.
- H. Yu, M. Pan, R. Sun, I. Agunawela, J. Zhang, Y. Li, Z. Qi, H. Han, X. Zou, W. Zhou, S. Chen, J. Y. L. Lai, S. Luo, Z. Luo, D. Zhao, X. Lu, H. Ade, F. Huang, J. Min and H. Yan, Angew. Chem., Int. Ed., 2021, 60, 10137–10146 CrossRef CAS.
- Z. Luo, R. Ma, Z. Chen, Y. Xiao, G. Zhang, T. Liu, R. Sun, Q. Zhan, Y. Zou, C. Zhong, Y. Chen, H. Sun, G. Chai, K. Chen, X. Guo, J. Min, X. Lu, C. Yang and H. Yan, Adv. Energy Mater., 2020, 10, 2002649 CrossRef CAS.
- S.-S. Wan, X. Xu, Z. Jiang, J. Yuan, A. Mahmood, G.-Z. Yuan, K.-K. Liu, W. Ma, Q. Peng and J.-L. Wang, J. Mater. Chem. A, 2020, 8, 4856–4867 RSC.
- L. Zhu, M. Zhang, G. Zhou, T. Hao, J. Xu, J. Wang, C. Qiu, N. Prine, J. Ali, W. Feng, X. Gu, Z. Ma, Z. Tang, H. Zhu, L. Ying, Y. Zhang and F. Liu, Adv. Energy Mater., 2020, 10, 1904234 CrossRef CAS.
- G.-Y. Ge, W. Xiong, K.-K. Liu, H. S. Ryu, S.-S. Wan, B. Liu, A. Mahmood, H.-R. Bai, J.-F. Wang, Z. Wang, H. Y. Woo, Y. Sun and J.-L. Wang, J. Mater. Chem. C, 2021, 9, 1923 RSC.
- L. Hong, H. Yao, Z. Wu, Y. Cui, T. Zhang, Y. Xu, R. Yu, Q. Liao, B. Gao, K. Xian, H. Y. Woo, Z. Ge and J. Hou, Adv. Mater., 2019, 31, 1903441 CrossRef PubMed.
- Y. Hu, K. Chaitanya, J. Yin and X.-H. Ju, J. Mater. Sci., 2016, 51, 6235–6248 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of NMR and MS spectra, additional experimental results, and crystallographic data for CCDC 2062921, 2062922 and 2070537. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ee01832a |
| This journal is © The Royal Society of Chemistry 2022 |