Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A trans-Pt(II) hedgehog pathway inhibitor complex with cytotoxicity towards breast cancer stem cells and triple negative breast cancer cells†
Aisling L.
Ryan
 ab,
Joshua
Northcote-Smith
c,
Aoife
McKeon
a,
Andrew
Roe
d,
Paul
O'Dowd
ab,
Joshua
Northcote-Smith
c,
Aoife
McKeon
a,
Andrew
Roe
d,
Paul
O'Dowd
 ab,
Brendan
Twamley
e,
Triona
Ní Chonghaile
d,
Kogularamanan
Suntharalingam
ab,
Brendan
Twamley
e,
Triona
Ní Chonghaile
d,
Kogularamanan
Suntharalingam
 *c and
Darren M.
Griffith
*c and
Darren M.
Griffith
 *ab
*ab
aDepartment of Chemistry, RCSI, 123 St. Stephens Green, Dublin 2, Ireland. E-mail: dgriffith@rcsi.com
bSSPC, Synthesis and Solid State Pharmaceutical Centre, Ireland
cSchool of Chemistry, University of Leicester, Leicester LE1 7RH, UK
dDepartment of Physiology and Medical Physics, Royal College of Surgeons in Ireland, Dublin, Ireland
eSchool of Chemistry, Trinity College Dublin, University of Dublin, Dublin 2, Ireland
First published on 10th November 2022
Abstract
The first example of a Pt complex of GANT61, a hedgehog (Hh) pathway inhibitor is reported. Reaction of cis-[Pt(II)Cl2(dmso)2] with one equivalent of 4-pyridine carboxaldehyde (4-PCA, control ligand) or one equivalent of GANT61 (Hh pathway inhibitor) in acetone at rt for 30 minutes afforded trans-[Pt(II)Cl2(dmso)(4-PCA)] (1) and trans-[Pt(II)Cl2(dmso)(GANT61)] (2) respectively, where 4-PCA and GANT61 are N-donor ligands. The structures of 1 and 2 were fully characterised by elemental analysis, 1H NMR, 13C NMR and IR spectroscopy and X-ray crystallography. 1 and 2 undergo isomerisation from trans- to cis-in solution and therefore the biological activity of 2 is also associated with the cis-configuration. The in vitro cytotoxicity data show that 2 is a potent inhibitor of the growth of breast CSC-depleted HMLER and breast CSC-enriched HMLER-shEcad cells. Furthermore 2 markedly reduced the size and viability and significantly reduced the number of CSC-enriched HMLER-shEcad mammospheres formed. 2 also induced apoptosis with low micromolar IC50 values against two triple negative breast cancer lines, MDA-MB-231 (MDA231) and BT549. 2, which possesses the Hh pathway inhibitor GANT61 as an N donor ligand exhibits far superior anti-CSC activity including in the CSC-enriched mammosphere model and activity against TNBC cells as compared to its control analogue, the trans-Pt(II) 4-PCA complex 1. The trans-Pt GANT61 complex 2 has also been shown to cause DNA damage and inhibit the Hh pathway at the level of GLI.
Introduction
Platinum (Pt)-based compounds exhibit efficacy against many solid tumours and are in turn an important and established class of anticancer chemotherapeutics.1The three Pt(II) anticancer drugs with worldwide approval, cisplatin, carboplatin and oxaliplatin, Fig. 1, are administered singularly or in anticancer regimens together with other anticancer agents such as doxorubicin, etoposide, gemcitabine, paclitaxel and 5-fluorouracil.2
The cytotoxicity of Pt anticancer drugs is primarily attributed to electrophilic Pt(II) centres covalently binding nuclear DNA, which induces DNA perturbation damage responses and ultimately programmed cell death, apoptosis. Pt(II) centres are also increasingly accepted to react with many cytoplasmic nucleophiles, including mitochondrial DNA, RNA as well as multiple mitochondrial and extramitochondrial proteins.1
Despite the unquestionable success of Pt(II) anticancer drugs, their clinical efficacy is limited by toxic side effects3 and resistance (intrinsic or acquired).4 The underlying resistance mechanisms are complex and include decreased accumulation, detoxification, increased DNA damage repair, and abnormal signaling pathways.4,5
Drug treatment failure and cancer relapse are also associated with the self-renewal properties associated with a small population of tumour cells called cancer stem cells (CSCs). CSCs have the ability to differentiate, self-renew and seed the formation of new metastatic tumors at secondary sites.5,6 Significantly CSCs are typically more resistant to chemotherapeutic agents, including Pt-based anticancer drugs, as compared to more differentiated cellular subtypes from the same tissue.7,8 Therefore clinical Pt(II) anticancer drugs cannot effectively eradicate CSCs.
The Hedgehog (Hh) signalling pathway regulates cell differentiation, cell proliferation and stem cell maintenance during embryonic development. Signaling in the Hh pathway ultimately results in downstream transcription of three glioma-associated oncogene homologue (GLI) transcription factor proteins, GLI1, GLI2 and GLI3. GLI1 and GLI2 behave as activating proteins.9 The Hh pathway is usually silent in adult tissues but abnormal Hh signalling does occur and is strongly associated with tumour growth, tumour resistance to drug treatment, and metastasis.9,10 In addition, the Hh pathway plays a role in the maintenance and differentiation of CSCs including breast cancer stem cells (BCSCs).11,12 Inhibition of the hedgehog pathway therefore represents an important therapeutic strategy to tackle resistance and target CSCs.
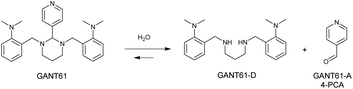
GANT61 is a small molecule inhibitor of the Hh pathway that acts downstream at the level of GLI. It has been shown to block GLI function in the nucleus, suppress both GLI1- and GLI2-mediated transcription, and inhibit the binding of GLI1 with DNA.13,14 GANT61 exhibits antiproliferative/antitumour activity in vitro and in vivo15,16 and was also demonstrated to attenuate stem cell phenotypes such as CD44+/CD24−ve cells and sphere forming capacity in triple negative breast cancer (TNBC) cell lines.17 Significantly GANT61 hydrolyses in vivo to give 4-pyridine carboxaldehyde (4-PCA), GANT61-A, and the bioactive diamine derivative, GANT61-D (Scheme 1). GANT61-D is responsible for the inhibition of GLI-mediated transcription and is reported to bind to an active zinc finger site in GLI1.18,19
Previously we developed Ni(II), Pd(II), and Pt(II) complexes of the Hh pathway inhibitor GANT61-D, where the Ni(II) and Pd(II) complexes exhibited noteworthy in vitro cytotoxicity against medulloblastoma cancer cells.20
In this study we hypothesised that a Pt(II) Hh pathway inhibitor complex may target bulk tumour cells via Pt(II) DNA binding and subpopulations of cancer stem cells via Hh inhibition and in turn combat resistance in breast cancer.
Numerous preclinical and non-traditional classes of Pt anticancer drug candidates have been developed to date.21trans-[Pt(II)Cl2(dmso)L] type complexes, where L is a planar N-donor aromatic ligand or heterocyclic ligand, exhibit appreciable anticancer activity though in most cases inferior activity as compared to cisplatin.22–24
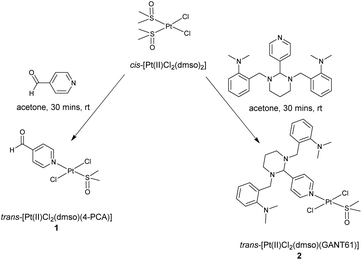
Given GANT61 would likely behave as an effective N-donor ligand via the pyridyl nitrogen, a trans Pt(II) dichlorido, dimethylsulfoxido complex of the Hh pathway inhibitor GANT61, trans-[Pt(II)Cl2(dmso)(GANT61)], was developed. It is expected that the trans-Pt(II) GANT61 complex will release the bioactive Hh pathway inhibitor GANT61-D, in a similar manner to GANT61, whilst 4-PCA would remain bound to the Pt(II) centre as the stable amine carrier ligand (Scheme 2).
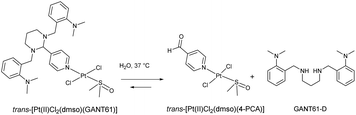 | ||
| Scheme 2 The proposed hydrolysis of the GANT61 ligand in trans-[Pt(II)Cl2(dmso)(GANT61)] to release hydrolysis products GANT61-D. | ||
Herein we report the in vitro anti-CSC properties including against CSC-enriched mammospheres and cytotoxicity of the trans-Pt GANT61 complex against triple negative breast cancer (TNBC) cells. Furthermore we investigated the potential of this complex to cause DNA damage and inhibit the Hh pathway at the level of GLI.
Results and discussion
Synthesis of trans-[Pt(II)Cl2(dmso)L] type complexes
It is well established that the reaction of one equivalent of an amine N-donor ligand with cis-[Pt(II)Cl2(dmso)2], initially affords the trans-isomer of a [Pt(II)Cl2(dmso)L] type complex, which can be isolated and characterised in full.22,25,26 Reaction of cis-[Pt(II)Cl2(dmso)2] with either one equivalent of 4-PCA or one equivalent of GANT61 in acetone at rt for 30 minutes afforded either trans-[Pt(II)Cl2(dmso)(4-PCA)] (1) and trans-[Pt(II)Cl2(dmso)(GANT61)] (2) respectively, Scheme 3.It is noteworthy that (i) reaction of one equivalent of GANT61 with cis-[Pt(II)Cl2(dmso)2] at an extended reaction time and at reflux will result in the synthesis of the cis-isomer, cis-[Pt(II)Cl2(dmso)(GANT61)] and (ii) subsequent reaction of cis-[Pt(II)Cl2(dmso)(GANT61)] with two equivalents of GANT61 affords cis-[Pt(II)Cl2(GANT61)2], which is completely insoluble in aqueous mixtures.27
Both 1 and 2 were fully characterised by elemental analysis, 1H NMR, 13C NMR and IR spectroscopy and X-ray crystallography (Fig. S1–S6†).
Briefly with regards to the characterisation of the trans-Pt(II) GANT61 complex 2 as a representative example, the elemental analysis was fully consistent with two chlorido ligands, one dmso ligand, one dmso solvent of crystallisation and one GANT61 ligand per Pt(II) centre. In the 1H NMR spectrum of 2 (CDCl3, Fig. S4†) the twelve aromatic protons associated with the three aromatic rings of GANT61 for example are observed across five signals ranging from 7.01 to 8.63 ppm. Coordination of GANT61 to Pt(II) via the pyridyl N-donor atom resulted in a distinctive chemical shift of the two pyridyl aromatic signals from to 7.64 and 8.54 ppm in the free ligand to 7.76 and 8.63 ppm in the complex. The dimethylaniline protons are for instance observed as a singlet at 2.58 ppm and integrate for twelve as expected. The singlet at 3.45 ppm is attributed to the six protons of the coordinated dmso ligand and a second singlet at 2.61 ppm is attributed to the DMSO solvent of crystallisation, which is evidenced in the single X-ray crystal structure. In the 13C NMR spectrum of 2 (Fig. S5†) the aromatic carbons are found between 156 and 119 ppm, the four dimethylaniline (DMA) methyl carbons signal is present at 45 ppm, and the signal for the coordinated dmso methyl carbons at 41 ppm. In the IR spectrum of 2 (Fig. S6†) the distinctive ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the coordinated dmso is observed at 1143 cm−1.
O) of the coordinated dmso is observed at 1143 cm−1.
X-ray crystal structures
The X-ray crystal structure of 1 and 2 highlight the trans configuration of both Pt complexes, Fig. 2 and 3 with Cl–Pt–Cl angles of 175.18(3) Å and 177.37(3) Å respectively. Metal bond lengths are as expected with trans substitution. The angle between the pyridine ring plane and the Pt–Cl–S plane is 55.39(9)° in 1 and 51.85(10)° in 2. A similar acute angle (ca. 59°) is seen in the unsubstituted trans-[Pt(II)Cl2(dmso)(py)] where py is pyridine.28 In 1, this twist enables the pyridine ring to form weak hydrogen bonds to the DMSO oxygen. The steric bulk of the GANT61 ligand in 2 forces a more crowded environment, with one dimethylaniline group turned upwards, the other downwards towards the pyridine ring with enough space in the lattice to co-crystallize solvent.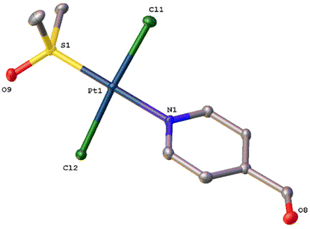 | ||
| Fig. 2 Molecular structure of 1 with atomic displacement shown at 50% probability. Hydrogen atoms omitted. | ||
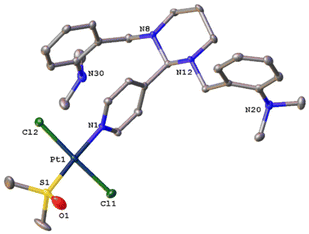 | ||
| Fig. 3 Molecular structure of 2 with atomic displacement at 50% probability. Hydrogen atoms and mixed partially occupied DMSO/acetone solvent omitted (see ESI† for complete structure). | ||
Isomerisation of 1 and 2
Both 1 and 2 were observed to isomerise from the trans isomers to the corresponding cis-isomers on redissolving the complexes in CDCl3 with stirring at rt. Farrell and coworkers had previously demonstrated the isomerisation in DMSO of trans-[Pt(II)Cl2(dmso)(quinoline)] to cis-[Pt(II)Cl2(dmso)(quinoline)].22The isomerisation can be monitored by 1H NMR. A downfield shift for example in key aliphatic signals are observed in the 1H NMR spectra (CDCl3) on the isomerisation of trans-[Pt(II)Cl2(dmso)(GANT61)] to cis-[Pt(II)Cl2(dmso)(GANT61)]. For instance the two sets of CH2 protons adjacent to the aniline groups shift from 3.68 to 3.83 ppm and the methine proton signal from 4.18 to 4.28 ppm (Fig. S9†).
2 was also observed to isomerise in D6-DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O (90
D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at RT with the same shift in the CH2 protons adjacent to the aniline groups shift from 3.74 to 4.04 ppm and the methine proton from 4.53 to 4.57 ppm (Fig. S10†).
10) at RT with the same shift in the CH2 protons adjacent to the aniline groups shift from 3.74 to 4.04 ppm and the methine proton from 4.53 to 4.57 ppm (Fig. S10†).
Hydrolysis of 2 to release GANT61-D
A UV-Vis, HR MS and 1H NMR study was undertaken to gain an insight into the behaviour of complex 2 in solution (Fig. S10–S12†). With regards to the UV study, 2 is stable in pure DMSO, over a 24 h period. In PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (200
DMSO (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S11†) and DMEM
1) (Fig. S11†) and DMEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (200
DMSO (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S12†) mixtures, 2 is less stable with a decrease in absorption observed, starting after 1 hour and particularly after 8 hours.
1) (Fig. S12†) mixtures, 2 is less stable with a decrease in absorption observed, starting after 1 hour and particularly after 8 hours.
After 72 h incubation of 2 in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (10
DMSO (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 37 °C, ion signals at 534.2197, 612.2338 and 695.2444 amu associated with [Pt(GANT61–C6H5–N(CH3)2)(OCH3)]+ (C21H29N3OPt), [Pt(GANT61–N(CH3)2)(OCH3)]+ (C26H33N4OPt) and [Pt(GANT61)(OCH3)]+ + K + H (C28H39N5OKPt) were observed in the positive mode of the high resolution mass spectrum (Fig. S13†). In each case, the molecular ions match the expected isotopic pattern and the elemental composition analysis. Furthermore, the peaks match within 10 ppm resolution of the expected values. This shows that some GANT61 remains bound to the Pt(II) centre in aqueous solution. Significantly an ion signal associated with GANT61 + H+ is clearly observed as the major signal at 341.2703 amu (Fig. S13†) indicating the presence of free bioactive molecule GANT61-D.
1) at 37 °C, ion signals at 534.2197, 612.2338 and 695.2444 amu associated with [Pt(GANT61–C6H5–N(CH3)2)(OCH3)]+ (C21H29N3OPt), [Pt(GANT61–N(CH3)2)(OCH3)]+ (C26H33N4OPt) and [Pt(GANT61)(OCH3)]+ + K + H (C28H39N5OKPt) were observed in the positive mode of the high resolution mass spectrum (Fig. S13†). In each case, the molecular ions match the expected isotopic pattern and the elemental composition analysis. Furthermore, the peaks match within 10 ppm resolution of the expected values. This shows that some GANT61 remains bound to the Pt(II) centre in aqueous solution. Significantly an ion signal associated with GANT61 + H+ is clearly observed as the major signal at 341.2703 amu (Fig. S13†) indicating the presence of free bioactive molecule GANT61-D.
An 1H NMR study was undertaken at 37 °C in D6-DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O (90
D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) over a 72 hours period. This 1H NMR study is complex and multiple processes are at play over the 72 hours. At time 0 minor additional signals are observed which are not associated with the signals of the parent Pt complex 2 (Fig. S14†). From 1 hour the signals associated with the GANT61 ligand bound to Pt(II), for example the 5 aromatic signals are primarily gone. From 1 hour the evolution of signals associated with a 4-PCA system, i.e. two aromatic doublets at 8.22 and 9.22 ppm and an aldehydic proton at 10.44 ppm, with the correct integration are observed. These signals increase up to 72 hours and support the release of GANT61-D, where the 4-PCA is still bound to Pt(II). Signals associated with GANT61-D are also present. For example the aromatic signals for GANT61-D are found from ca. 7.46–8.02 ppm though they are poorly resolved.
10) over a 72 hours period. This 1H NMR study is complex and multiple processes are at play over the 72 hours. At time 0 minor additional signals are observed which are not associated with the signals of the parent Pt complex 2 (Fig. S14†). From 1 hour the signals associated with the GANT61 ligand bound to Pt(II), for example the 5 aromatic signals are primarily gone. From 1 hour the evolution of signals associated with a 4-PCA system, i.e. two aromatic doublets at 8.22 and 9.22 ppm and an aldehydic proton at 10.44 ppm, with the correct integration are observed. These signals increase up to 72 hours and support the release of GANT61-D, where the 4-PCA is still bound to Pt(II). Signals associated with GANT61-D are also present. For example the aromatic signals for GANT61-D are found from ca. 7.46–8.02 ppm though they are poorly resolved.
Collectively, the UV-Vis, HR mass spectrometry and 1H NMR study supports the isomerisation of 2 from trans to cis and hydrolysis of the GANT61 ligand to release GANT61-D over the 72 experimental time point.
In vitro cytotoxicity
To determine the bulk breast cancer cell and breast CSC potency and investigate potential specificity of the trans-Pt(II) GANT61 complex 2, two human mammary epithelial cell lines were initially used; HMLER and HMLER-shEcad cells. HMLER cells exhibit a stable CSC-like population of 5–8%, whereas HMLER-shEcad cells display a significantly larger CSC-like population (ca. 90%).29The in vitro cytotoxic properties of Hh pathway inhibitors (GANT61, GANT61-D), 4-PCA, Pt complexes (trans-[Pt(II)Cl2(dmso)(4-PCA)] 1, trans-[Pt(II)Cl2(dmso)(GANT61)] 2, cisplatin and carboplatin), and salinomycin against HMLER cells and HMLER-shEcad cells were assessed using the MTT assay. Salinomycin, a breast CSC-specific compound was used as a positive control.29 Cisplatin and carboplatin were control Pt compounds and 4-PCA a control for the N-donor ligands. The IC50 values, the concentration required to reduce viability by 50%, were determined from dose–response curves (Fig. S15–S20†) and are summarized in Table 1.
| Compound | HMLER IC50 (μM) | HMLER-shEcad IC50 (μM) | Mammosphere IC50 (μM) |
|---|---|---|---|
| GANT61 | 16.0 ± 0.6 | 14.9 ± 0.1 | 16.0 ± 0.4 |
| GANT61-D | 25.8 ± 1.8 | 23.3 ± 1.2 | 16.4 ± 1.0 |
| 4-PCA | >100 | >100 | >133 |
| 1 | 36.3 ± 1.9 | 36.9 ± 0.1 | >133 |
| 2 | 1.0 ± 0.02 | 2.6 ± 0.2 | 4.4 ± 0.01 |
| Cisplatin29,30 | 2.6 ± 0.02 | 5.7 ± 0.3 | 13.5 ± 2.3 |
| Carboplatin29,30 | 67.3 ± 2.8 | 72.4 ± 8.0 | 18.1 ± 0.4 |
| Salinomycin29,30 | 11.4 ± 0.4 | 4.2 ± 0.4 | 18.5 ± 1.5 |
Both the Hh pathway inhibitor GANT61 and its bioactive hydrolysis product GANT61-D exhibited similar activity against both HMLER and HMLER-shEcad cells with IC50 values ranging from 15 to 26 μM. No selectivity for the CSC-enriched HMLER-shEcad cell line over the HMLER cell line was observed for either. 4-PCA was observed to be non-toxic within the concentration range tested (IC50 value > 100 μM).
It is notable that the trans-Pt(II) GANT61 complex 2, which possesses GANT61, is the most potent of all the test compounds with IC50 values of 1.0 and 2.6 μM against HMLER and HMLER-shEcad cells, respectively. No selectivity for the CSC-enriched cells was observed, which is in line with the observation for the Hh pathway inhibitors GANT61 and GANT61-D.
Significantly though trans-Pt GANT61 complex 2, is ca. thirty six times more cytotoxic against HMLER and ca. fourteen times more cytotoxic against HMLER-shEcad cells as compared to the trans-Pt(II) 4-PCA analogue and control compound 1.
Furthermore 2 has slightly lower IC50 values as compared to cisplatin (2.6 & 5.7 μM) and salinomycin (11.4 & 4.2 μM) and considerably lower IC50 values as compared to carboplatin (67.3 & 72.4 μM) against HMLER and HMLER-shEcad cells.
Overall, the in vitro cytotoxicity data shows that 2 is a potent inhibitor of the growth of breast CSC-depleted HMLER and breast CSC-enriched HMLER-shEcad cells.
Complex 2 did not exhibit notable selectivity for the cancer cells investigated (IC50's = 1.0 & 2.6 μM) over normal cells, given an IC50 of 2.3 ± 0.1 μM (Fig. S11†) was determined against the MCF10A cell line, a human mammary epithelial cell line.
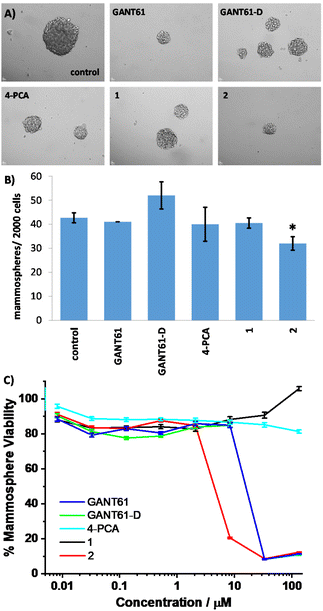
Breast CSCs, due to their stem cell-like character, have the ability to form multicellular three-dimensional structures called mammospheres. The ability of test compounds to reduce CSC-enriched HMLER-shEcad mammosphere formation and viability was assessed using an inverted microscope and the colorimetric resazurin-based reagent TOX8, respectively (Fig. 4 and Table 1).
The addition of GANT61, GANT61-D, 4-PCA, 1, or 2 (at their IC20 values) to single cell suspensions of HMLER-shEcad cells markedly reduced the size of mammospheres formed after 5 days incubation (Fig. 4A). Only the trans-Pt(II) GANT61 complex 2 significantly reduced (p < 0.05) the number of mammospheres formed (Fig. 4B).
In terms of mammosphere viability, the trans-Pt(II) GANT61 complex 2 was the most potent of all the test compounds with an IC50 (concentration required to reduce mammosphere viability by 50%) value of 4.4 ± 0.01 μM and was three times more potent than cisplatin (Fig. 4C). The trans-Pt(II) GANT61 complex 2 was ca. four times more potent towards mammospheres than hedgehog pathway inhibitors GANT61 and GANT61-D (Fig. 4C). Significantly the trans-Pt(II) 4-PCA analogue and control compound 1 was not active at the highest concentration investigated, 133 μM (Fig. 4C). Collectively, the mammosphere studies show that 2 is able to markedly reduce breast CSC mammosphere formation, size, and viability.
Cell death analysis
Apoptosis was assessed by flow cytometry using annexin V/PI staining in two TNBC cell lines, MDA-MB-231 (MDA231) and BT549, following treatment with test compounds. TNBC is a highly aggressive subtype of breast cancer that lacks expression of progesterone receptor (PR), estrogen receptor (ER) and human epidermal growth factor 2 (HER2).31The IC50 values, the concentration required to induce apoptosis in 50% of cell population, were determined from dose–response curves (Fig. S21†) and are summarized in Table 2.
| Compound | MDA231 IC50 (μM) | BT549 IC50 (μM) |
|---|---|---|
| GANT61 | >100 | 15.62 |
| GANT61-D | 89.96 | 14.28 |
| 1 | >100 | 12.78 |
| 2 | 3.60 | 4.00 |
| Cisplatin | 2.97 | 0.41 |
The BT549 cell line was significantly more sensitive to treatment with all test compounds as compared to the MDA231 cells. Cisplatin was found to be the most potent of the test compounds with IC50 values of 2.97 and 0.41 μM against MDA23 and BT549 TNBC cells respectively. Hh pathway inhibitors GANT61 and GANT61-D were relatively non-toxic towards the MDA231 cells in contrast to the cytotoxicity they exhibited against the BT59 cell line with IC50's of 15.6 and 14.3 μM respectively.
Significantly the trans-Pt(II) GANT61 complex 2 exhibited good activity against both cell lines with IC50 values of 3.6 and 4.0 μM against MDA231 cells and BT549 cells respectively and was more than 30 times more active than the trans-Pt(II) 4-PCA complex 1 against the MDA231 cells and 3 times more active against the BT549 cells.
We hypothesised that a Pt(II) complex possessing GANT61 as an N-donor ligand would release the bioactive Hh pathway inhibitor GANT61-D, in a similar manner to GANT61, whilst 4-PCA would remain bound to the Pt(II) centre as the stable amine carrier ligand. In summary the trans-Pt(II) complex 2, which possesses the Hh pathway inhibitor GANT61 as an N donor ligand exhibits far superior anti-CSC activity including in a CSC-enriched mammosphere model and activity against TNBC cells as compared to its control analogue, the trans-Pt(II) 4-PCA complex 1.
DNA damage and hedgehog pathway inhibition at the level of GLI
We hypothesised that a Pt(II) complex possessing GANT61 as an N-donor ligand should possess DNA binding ability and Hh inhibitory activity. The potential of 2 to damage genomic DNA was probed by monitoring the expression of a biomarker related to the DNA damage pathway using immunoblotting methods. HMLER-shEcad cells incubated with 2 (IC50/2 & IC50 for 72 h) displayed a marked increase in the expression of the phosphorylated form of H2AX (γH2AX), indicative of DNA damage (Fig. 5).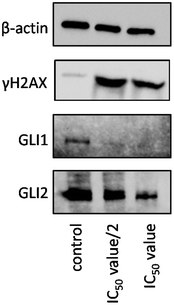 | ||
| Fig. 5 Immunoblotting analysis of proteins related to the DNA damage and GLI pathways. Protein expression in HMLER-shEcad cells following treatment with 2 (IC50/2 value and IC50 value for 72 h). | ||
HMLER-shEcad cells treated with 2 (IC50/2 & IC50 for 72 h) also exhibited decreased expression of GLI-1 and GLI-2 (Fig. 5). As previously highlighted, GANT61 inhibits the Hh pathway downstream at the level of GLI. It has been shown to block GLI function in the nucleus, suppress both GLI1- and GLI2-mediated transcription, and inhibit the binding of GLI1 with DNA.13,14
In summary the trans-Pt GANT61 complex 2 has been shown to cause DNA damage and inhibit the Hh pathway at the level of GLI.
Experimental
Materials and instrumentation
Potassium tetrachloroplatinate(II) was purchased from Alfa Aesar (Heysham LA3 2XY, United Kingdom) and used without further purification. All other commercially available reagents and solvents, including deuterated solvents, were purchased from Merck/Sigma-Aldrich (Arklow, Co. Wicklow, Ireland), and used without further purification unless otherwise stated. GANT61,32 GANT61-D32 and cis-[Pt(II)Cl2(dmso)2]33 were synthesised as previously reported. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 NMR spectrometer. The spectra were analysed using MestReNova software. The residual undeuterated solvent signals were used as internal references.34 Elemental analysis (C, H, N and Cl) was performed at the Microanalytical Laboratory, School of Chemistry and Chemical Biology, University College Dublin, Ireland. Fourier Transform Infrared (FT-IR) spectra were recorded in-house using a Nicolet iS10 FT-IR spectrometer with spectra recorded from 4000–400 wavenumbers (cm−1) and analysed using OMNICTM software.Synthetic procedures
1H NMR (400 MHz, CDCl3) δ 10.14 (s, 1H), 9.07 (d, J = 4.0 Hz, 2H), 7.86 (d, J = 4.0 Hz, 2H), 3.49 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 188.99 (s), 153.80 (s), 142.93 (s), 123.97 (s), 44.34 (s). C8H11Cl2NO2PtS requires C, 21.29; H, 2.46; N, 3.10; Cl, 15.71%, found C, 21.30; H, 2.33; N, 2.86; Cl, 15.74%. FT-IR νmax (cm−1): 3105.1, 1714.4 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1424.3, 1319.2, 1139.0 ν(S
O), 1424.3, 1319.2, 1139.0 ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1026.1, 820.4.
O), 1026.1, 820.4.
1H NMR (400 MHz, CDCl3) δ 8.63 (d, J = 4.0 Hz, 2H), 7.76 (d, J = 4.0 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H), 7.20–7.17 (m, 2H), 7.05–7.01 (m, 4H), 4.18 (s, 1H), 3.72 (dd, J = 20 Hz, 12 Hz, 4H), 3.45 (s, 6H), 2.93–2.89 (m, 2H), 2.61 (s, 6H), 2.58 (s, 12H), 2.43–2.38 (m, 2H), 1.83–1.82 (m, 1H), 1.48–1.46 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 156.75, 153.04, 151.30, 133.26, 129.57, 127.66, 126.27, 123.29, 119.21, 83.10, 52.96, 48.97, 45.30, 44.30, 41.14, 20.94. C29H41Cl2N5OPtS·SO(CH3)2 requires C, 43.71; H, 5.56; N, 8.22; Cl, 8.32%, found C, 44.16; H, 5.48; N, 8.05; Cl, 8.45%. FT-IR νmax (cm−1): 2942.9, 2792.4, 1491.6, 1301.3, 1142.8 ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1035.1, 936.9.
O), 1035.1, 936.9.
X-ray crystallography
Data for 1 were collected on a Bruker APEX DUO and for 2 on a Bruker D8 Quest ECO using Mo Kα radiation (λ = 0.71073 Å). Each sample was mounted on a MiTeGen cryoloop and data collected at 100(2) K. Bruker APEX software35 was used to collect and reduce data. Absorption corrections were applied using SADABS.36 Structures were solved with the SHELXT structure solution program37 using Intrinsic Phasing. Data were refined using least squares method on F2 with SHELXL.38 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were assigned to calculated positions using a riding model with appropriately fixed isotropic thermal parameters. Molecular graphics were generated using OLEX2.39 Crystal data, details of data collection and refinement are given in Table S1.†Disordered solvents are present in the void in 2 with DMSO, 60% occupied and acetone, 15% occupied. Both modelled with a rigid model. Displacement constraints (EADP) were used to model the oxygen and carbon atoms. The large residual density is located near the heavy Pt atom in both 1 and 2, and the intensity (1.2 and 1.64 e Å−3, 0.61 Å and 0.93 Å from Pt1 in both 1 and 2) is smaller than the 0.1 × Z e Å−3 (Z = 78 for Pt). This originates from absorption. The deepest hole is −1.43 and −1.15 e Å−3, 0.73 Å and 1.13 Å from Pt1 and C19 respectively in 1 and 2.
Crystallographic data for the structure in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. 2196198 and 2196199.†
Cell lines and cell culture conditions
The human mammary epithelial cell lines, HMLER and HMLER-shEcad were kindly donated by Prof. R. A. Weinberg (Whitehead Institute, MIT). The human epithelial breast MCF710A cell line was acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA). HMLER, HMLER-shEcad, and MCF10A cells were maintained in Mammary Epithelial Cell Growth Medium (MEGM) with supplements and growth factors (BPE, hydrocortisone, hEGF, insulin, and gentamicin/amphotericin-B). The cells were grown at 310 K in a humidified atmosphere containing 5% CO2.Cytotoxicity MTT assay
The colorimetric MTT assay was used to determine the toxicity of GANT61, GANT61-D, 4-PCA, 1, and 2. HMLER, HMLER-shEcad, and MCF10A cells (5 × 103) were seeded in each well of a 96-well plate. After incubating the cells overnight, various concentrations of the compounds (0.0004–100 μM), were added and incubated for 72 h (total volume 200 μL). Stock solutions of the compounds were prepared as 10 mM solutions in DMSO and diluted using media. The final concentration of DMSO in each well was 0.5% and this amount was present in the untreated control as well. After 72 h, 20 μL of a 4 mg mL−1 solution of MTT in PBS was added to each well, and the plate was incubated for an additional 4 h. The MEGM/MTT mixture was aspirated and 200 μL of DMSO was added to dissolve the resulting purple formazan crystals. The absorbance of the solutions in each well was read at 550 nm. Absorbance values were normalised to (DMSO-containing) control wells and plotted as concentration of test compound versus % cell viability. IC50 values were interpolated from the resulting dose dependent curves. The reported IC50 values are the average of three independent experiments (n = 18).Tumorsphere formation and viability assay
HMLER-shEcad cells (5 × 103) were plated in ultralow-attachment 96-well plates (Corning) and incubated in MEGM supplemented with B27 (Invitrogen), 20 ng mL−1 EGF, and 4 μg mL−1 heparin (Sigma) for 5 days. Studies were also conducted in the presence of GANT61, GANT61-D, 4-PCA, 1, and 2 (0–133 μM). Mammospheres treated with GANT61, GANT61-D, 4-PCA, 1, and 2 (at their respective IC20 values, 5 days) were counted and imaged using an inverted microscope. The viability of the mammospheres was determined by addition of a resazurin-based reagent, TOX8 (Sigma). After incubation for 16 h, the fluorescence of the solutions was read at 590 nm (λex = 560 nm). Fluorescence values were normalised to DMSO-containing controls and plotted as concentration of test compound versus % mammospheres viability. IC50 values were interpolated from the resulting dose dependent curves. The reported IC50 values are the average of two independent experiments, each consisting of two replicates per concentration level (overall n = 4). The microscope used for this study is a Nikon TE300 semi-automatic microscope with automated shutters and filter wheels and manual XY control. The imaging part of the system is automated and controlled by Improvision's Openlab software, running on a Mac (OS X).Annexin V-FITC/PI cell viability assay
MDA231 and BT549 cells were seeded at 4.5 × 104 cells in a 24 well plate for 24 h prior to treating with cisplatin, GANT61, GANT61-D, 1 and 2 at stated doses for 48 h. Apoptosis was assessed by annexin V-FITC/propidium iodide staining. The cells were collected, washed with PBS and resuspended in annexin V binding buffer (10 mM Hepes pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) and annexin V-FITX (0.25 mg ml−1 BioLegend, 640906, Sigma-Aldrich P4170) and PI (1 mg ml−1) was added to each sample. Cell viability was determined by flow cytometry on the Biosciences LSR II flow cytometer. Viability is shown normalised to DMSO control. Dose–response curves and IC50's were fitted using nonlinear regression in GraphPad Prism 9.Immunoblotting analysis
HMLER-shEcad cells (5 × 103 cells) were incubated with 2 (IC50/2 & IC50 for 72 h) at 37 °C. Cells were washed with PBS, scraped into SDS-PAGE loading buffer (64 mM Tris–HCl (pH 6.8)/9.6% glycerol/2% SDS/5% β-mercaptoethanol/0.01% bromophenol blue), and incubated at 95 °C for 10 min. Whole cell lysates were resolved by 4–20% sodium dodecylsulphate polyacylamide gel electrophoresis (SDS-PAGE; 200 V for 25 min) followed by electro transfer to polyvinylidene difluoride membrane, PVDF (350 mA for 1 h). Membranes were blocked in 5% (w/v) non-fat milk in PBST (PBS/0.1% Tween 20) and incubated with the appropriate primary antibodies (Cell Signalling Technology). After incubation with horseradish peroxidase-conjugated secondary antibodies (Cell Signalling Technology), immune complexes were detected with the ECL detection reagent (BioRad) and analysed using a chemiluminescence imager (Bio-Rad ChemiDoc Imaging System).Conclusions
In summary, we present a novel trans-Pt(II) complex of the Hh pathway inhibitor GANT61, trans-[Pt(II)Cl2(dmso)(GANT61)] 2. This complex does undergo isomerisation from trans- to cis-in solution and therefore the biological activity of 2 is also associated with the cis-configuration. This complex is a potent inhibitor of the growth of breast CSC-depleted HMLER and breast CSC-enriched HMLER-shEcad cells. Furthermore 2 markedly reduced the size and viability and significantly reduced the number of CSC-enriched HMLER-shEcad mammospheres formed. 2 also induced apoptosis with low micromolar IC50 values against two TNBC cell lines, MDA-MB-231 (MDA231) and BT549 2, which possesses the Hh pathway inhibitor GANT61 as an N donor ligand exhibits far superior anti-CSC activity including in the CSC-enriched mammosphere model and activity against TNBC cells as compared to its control analogue, the trans-Pt(II) 4-PCA complex 1.We hypothesised that the trans-Pt(II) GANT61 complex would release the bioactive Hh pathway inhibitor GANT61-D, in a similar manner to GANT61, whilst 4-PCA would remain bound to the Pt(II) centre as the stable amine carrier ligand. The trans-Pt(II) complex 2, was shown to release GANT61 over a 72 hours timepoint in solution and to cause DNA damage and inhibit the Hh pathway at the level of GLI in HMLER-shEcad cells.
The Hh pathway inhibitor GANT61 enhances the cytotoxicity of the trans-[Pt(II)Cl2(dmso)L] class of Pt complex towards breast cancer stem cells and triple negative breast cancer cells. Ultimately hedgehog pathway inhibition may ameliorate the anticancer activity of the many different classes of Pt(II) complexes.
Author contributions
Aisling L. Ryan: conceptualisation, methodology, investigation, writing – original draft preparation, funding acquisition. Joshua Northcote-Smith: methodology, investigation. Aoife McKeon: conceptualisation, methodology, investigation. Andrew Roe: methodology, investigation, writing – review and editing. Paul O'Dowd: methodology, investigation. Brendan Twamley: methodology, resources, writing – original draft preparation. Triona Ní Chonghaile: methodology, resources, writing – review and editing. Kogularamanan Suntharalingam: conceptualisation, resources, methodology, writing – original draft preparation. Darren M. Griffith: conceptualisation, resources, methodology, writing – original draft preparation, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
ALR sincerely thanks the Irish Research Council (GOIPG/2017/1384) for financial support. DMG and POD gratefully acknowledges funding received from the Synthesis and Solid State Pharmaceutical Centre (SSPC), financed by a research grant from Science Foundation Ireland (SFI) and co-funded under the European Regional Development Fund under Grant Number 12/RC/2275_P2. AM and DG are also funded by a research grant from SFI under Grant Number 12/IP/1305. KS and JNS thank EPSRC (EP/S005544/1) and the University of Leicester for funding. TNC and AR are funded by SFI under grant number 19/FFP/6461.References
- S. Rottenberg, C. Disler and P. Perego, Nat. Rev. Cancer, 2021, 21, 37–50 CrossRef CAS PubMed.
- S. Alassadi, M. J. Pisani and N. J. Wheate, Dalton Trans., 2022, 51, 10835–10846 RSC.
- R. Oun, Y. E. Moussa and N. J. Wheate, Dalton Trans., 2018, 47, 6645–6653 RSC.
- J. Zhou, Y. Kang, L. Chen, H. Wang, J. Liu, S. Zeng and L. Yu, Front. Pharmacol., 2020, 11, 343 CrossRef CAS PubMed.
- L. Yang, H.-J. Xie, Y.-Y. Li, X. Wang, X.-X. Liu and J. Mai, Oncol. Rep., 2022, 47, 82 CrossRef CAS PubMed.
- C. Correia, T. M. Weiskittel, C. Y. Ung, J. C. Villasboas Bisneto, D. D. Billadeau, S. H. Kaufmann and H. Li, Front. Cell Dev. Biol., 2022, 10, 752326 CrossRef PubMed.
- F. Tomao, A. Papa, L. Rossi, M. Strudel, P. Vici, G. Lo Russo and S. Tomao, J. Exp. Clin. Cancer Res., 2013, 32, 48 CrossRef.
- S. Muñoz-Galván and A. Carnero, Cells, 2020, 9(6), 1402 CrossRef PubMed.
- N. M. Nguyen and J. Cho, Int. J. Mol. Sci., 2022, 23, 1733 CrossRef CAS PubMed.
- B. Aramini, V. Masciale, G. Grisendi, F. Bertolini, M. Maur, G. Guaitoli, I. Chrystel, U. Morandi, F. Stella, M. Dominici and K. H. Haider, Cancers, 2022, 14(4), 976 CrossRef CAS PubMed.
- K. Song and M. Farzaneh, Stem Cell Res. Ther., 2021, 12, 245 CrossRef PubMed.
- T. Zhang, H. Zhou, K. Wang, X. Wang, M. Wang, W. Zhao, X. Xi, Y. Li, M. Cai, W. Zhao, Y. Xu and R. Shao, Biomed. Pharmacother., 2022, 147, 112616 CrossRef CAS PubMed.
- M. Lauth, Å. Bergström, T. Shimokawa and R. Toftgård, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8455–8460 CrossRef CAS PubMed.
- A. Agyeman, B. K. Jha, T. Mazumdar and J. A. Houghton, Oncotarget, 2014, 5, 4492 CrossRef PubMed.
- T. Mazumdar, J. DeVecchio, A. Agyeman, T. Shi and J. A. Houghton, Cancer Res., 2011, 71, 5904–5914 CrossRef CAS PubMed.
- T. Mazumdar, J. DeVecchio, T. Shi, J. Jones, A. Agyeman and J. A. Houghton, Cancer Res., 2011, 71, 1092–1102 CrossRef CAS PubMed.
- P. Bhateja, M. Cherian, S. Majumder and B. Ramaswamy, Cancers, 2019, 11, 1126 CrossRef CAS PubMed.
- M. Lauth, V. Rohnalter, A. Bergstrom, M. Kooshesh, P. Svenningsson and R. Toftgard, Mol. Pharmacol., 2010, 78(3), 486–496 CrossRef CAS PubMed.
- A. Calcaterra, V. Iovine, B. Botta, D. Quaglio, I. D'Acquarica, A. Ciogli, A. Iazzetti, R. Alfonsi, L. Lospinoso Severini and P. Infante, J. Enzyme Inhib. Med. Chem., 2018, 33, 349–358 CrossRef CAS PubMed.
- A. L. Ryan, M.-C. Fitzgerald, A. Ozsváth, B. Twamley, P. Buglyó, B. M. Murphy and D. M. Griffith, Inorg. Chem., 2019, 58, 16075–16086 CrossRef CAS PubMed.
- J. J. Wilson and S. J. Lippard, Chem. Rev., 2014, 114, 4470–4495 CrossRef CAS PubMed.
- M. Van Beusichem and N. Farrell, Inorg. Chem., 1992, 31, 634–639 CrossRef CAS.
- G. Giannikopoulos, C.-L. Teo, M. D. Hall, R. R. Fenton and T. W. Hambley, Aust. J. Chem., 2003, 56, 685–689 CrossRef CAS.
- N. Farrell, L. R. Kelland, J. D. Roberts and M. Van Beusichem, Cancer Res., 1992, 52, 5065–5072 CAS.
- P.-C. Kong, D. Iyamuremye and F. D. Rochon, Bioinorg. Chem., 1976, 6, 83–89 CrossRef CAS PubMed.
- L. G. Marzilli, Y. Hayden and M. D. Reily, Inorg. Chem., 1986, 25, 974–978 CrossRef CAS.
- A. McKeon, PhD thesis, Royal College of Surgeons in Irelands, 2017 Search PubMed.
- F. Caruso, R. Spagna and L. Zambonelli, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 713–715 CrossRef.
- P. B. Gupta, T. T. Onder, G. Jiang, K. Tao, C. Kuperwasser, R. A. Weinberg and E. S. Lander, Cell, 2009, 138, 645–659 CrossRef CAS PubMed.
- A. Eskandari, A. Kundu, S. Ghosh and K. Suntharalingam, Angew. Chem., Int. Ed., 2019, 58, 12059–12064 CrossRef CAS PubMed.
- C. M. Perou, T. Sørlie, M. B. Eisen, M. van de Rijn, S. S. Jeffrey, C. A. Rees, J. R. Pollack, D. T. Ross, H. Johnsen, L. A. Akslen, Ø. Fluge, A. Pergamenschikov, C. Williams, S. X. Zhu, P. E. Lønning, A.-L. Børresen-Dale, P. O. Brown and D. Botstein, Nature, 2000, 406, 747–752 CrossRef CAS PubMed.
- V. Chenna, C. Hu and S. R. Khan, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, 49, 641–647 CrossRef CAS PubMed.
- V. Y. Kukushkin, A. J. L. Pombeiro, C. M. P. Ferreira and L. I. Elding, Inorg. Synth., 2002, 33, 189–196 CAS.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- Bruker, APEX3 v2017.3-0, Bruker AXS Inc., Madison, WI, USA, 2017 Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2196198 and 2196199. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt02865d |
| This journal is © The Royal Society of Chemistry 2022 |