Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Circularly polarized luminescence from Tb(III) interacting with chiral polyether macrocycles†‡
Alexandre
Homberg
a,
Federica
Navazio
ab,
Antoine
Le Tellier
a,
Francesco
Zinna
 c,
Alexandre
Fürstenberg
c,
Alexandre
Fürstenberg
 de,
Céline
Besnard
de,
Céline
Besnard
 f,
Lorenzo
Di Bari
f,
Lorenzo
Di Bari
 c and
Jérôme
Lacour
c and
Jérôme
Lacour
 *a
*a
aDepartment of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland. E-mail: Jerome.lacour@unige.ch
bSchool of Science and Technology, Chemistry Division, University of Camerino, via S. Agostino n. 1, 62032 Camerino, Italy
cDipartimento di Chimica e Chimica Industriale, Università di Pisa, Via Moruzzi 13, 56124 Pisa, Italy
dDepartment of Inorganic and Analytical Chemistry, University of Geneva, 1211 Geneva, Switzerland
eDepartment of Physical Chemistry, University of Geneva, 1211 Geneva, Switzerland
fLaboratory of Crystallography, University of Geneva, Quai Ernest Ansermet 24, 1211 Geneva 4, Switzerland
First published on 4th October 2022
Abstract
A straightforward two-step synthesis protocol affords a series of chiral amide-based bis-pyridine substituted polyether macrocycles. One ligand is particularly able to complex terbium(III) ions spontaneously. Upon complexation, interesting chiroptical properties are observed both in absorbance (ECD) and in fluorescence (CPL). In ligand-centered electronic circular dichroism, a sign inversion coupled with a signal enhancement is measured; while an easily detectable metal-centered circularly polarized luminescence with a glum of 0.05 is obtained for the main 5D4 → 7F5 terbium transition. The coordination mode and structure of the complex was studied using different analysis methods (NMR analysis, spectrophotometric titration and solid-state elucidation).
Introduction
The study of chiral lanthanide complexes is a strong and developing field of research.1–26 These complexes often display interesting circularly polarized luminescence (CPL) properties and,27 for this reason, applications can be found in key research areas such as in bioanalytical assays and imaging28–39 or circularly polarized OLEDs.40–44 To promote efficient chiroptical properties, polydentate monoligands, such as triazacyclononane or tetraazacyclododecane, are usually employed.2 However, fully oxygenated ligands remain scarce in the design of chiral lanthanide complexes,45 possibly due to limited examples of efficient syntheses of such chiral functionalized compounds. Recently, the direct synthesis of a new family of highly functionalized chiral polyether macrocycles of type 1, made in only two steps from cyclic ethers, was reported.46–48 These molecules find applications in a variety of fields49,50 from analytical51 and supramolecular52 sciences to catalysis,53 luminescence54–57 and photophysics.58–60 Herein, in a new development, we report that the complex formed by ligand 1a (X = O, Fig. 1 and 2) and terbium(III) triflate display strong electronic circular dichroism (ECD) but also CPL properties. Upon Tb complexation, in ECD, transitions sign inversion and signal enhancement are observed while in CPL a glum of 0.05 is measured for the main 5D4 → 7F5 terbium transition. In addition, 1H-NMR analysis, spectrophotometric titrations and solid-state elucidation of parent complexes were performed to confirm the proposed binding mode of the metal ion and the ligand macrocycle.Results and discussion
Two-step straightforward synthesis of ligands
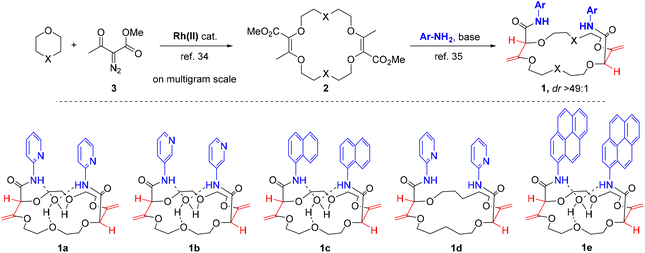
Practically, ligands of type 1 were synthetized in two steps from 1,4-dioxane or tetrahydropyran (THP) (Fig. 2). First, unsaturated macrocycles 2 were synthesized by [3 + 6 + 3 + 6] Rh(II)-catalyzed condensations of 1,4-dioxane or THP with α-diazo-β-ketoester 3 on multigram scale (e.g., 72% for X = O).47,61,62 Then, with compounds 2 in hand (X = O or CH2) and using α and β amino-pyridines or 1-naphthylamine as nucleophiles, a series of chiral macrocycles was prepared following a double tandem [amidation + olefin transposition] protocol to afford bisamides 1a to 1d as single diastereoisomers (Fig. 2, 43–60%, dr > 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).48
1).48
For each compound, the enantiomers could be efficiently separated by chiral stationary phase (CSP) HPLC on a semi-preparative scale using CHIRALPAK® IG column and a mixture of CH2Cl2–MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1% diethanolamine) as mobile phase (see ESI† for details).56
1, 0.1% diethanolamine) as mobile phase (see ESI† for details).56
Optical properties of the terbium complex
With the four candidates 1a–1d in hand, the ability of these macrocycles to act as antennas for lanthanide emission (ligand-to-terbium energy transfer) was assessed qualitatively. Of the four moieties, 2-pyridine substituted 1a exhibited, in the presence of an excess of terbium(III) triflate and upon ligand excitation (366 nm), the most intense characteristic green emission (see ESI† for details). Consequently, the absorption and emission spectra of 1a and of a mixture of 1a with 3.0 equivalents of Tb(III) were recorded in acetonitrile (Fig. 3, bottom). The four characteristic major and sharp emission bands of the 5D4 → 7Fj (j = 6 − 3) terbium transitions were observed at 485 nm, 545 nm, 580 nm and 620 nm respectively with a total quantum yield of 5% (relative to 9,10-diphenylanthracene).The formation of the complex between 1a and terbium(III) was then monitored through spectrophotometric titration, firstly using UV-Vis absorption spectroscopy. In the titration experiment, aliquots of a Tb(OTf)3 solution were added to a macrocycle solution (ca. 0.5 × 10−5 M) in acetonitrile. The free ligand spectrum (red line) displays two strong absorption bands with maxima at 238 nm and 270 nm which can be assigned to the π → π* transitions of pyridine. Upon Tb(OTf)3 addition, the two main absorption bands behave differently: the most red-shifted one displayed a small hyperchromic effect while the more blue-shifted experienced a small hypochromic effect. Furthermore, as the equivalents of terbium(III) increased, a new weak band around 300 nm appeared signaling the coordination of the lanthanide with macrocycle. Above 1.5 equivalents of terbium salt, no further evolution of the spectra was observed (Fig. S6†). To better understand the coordination behaviour of terbium inside the ligand, an analogous titration of 1a was performed with Ba(ClO4)2, a salt for which a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and coordination geometry have been already previously established with related macrocycles.55,56 The results are reported in Fig. S8† in which the two main transitions evolve, similarly to the terbium titration, with the related hypo-hyperchromic effect. This indicates analogous binding modes for the two metal salts and hence a 1
1 stoichiometry and coordination geometry have been already previously established with related macrocycles.55,56 The results are reported in Fig. S8† in which the two main transitions evolve, similarly to the terbium titration, with the related hypo-hyperchromic effect. This indicates analogous binding modes for the two metal salts and hence a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between the terbium and macrocycle 1a. More precisely, we propose that the terbium replaces the coordinated water molecule. The coordination of the metal occurs with the six oxygen atoms of the polyether ring while the two carbonyl groups of the amides rotate inward to reach an eightfold coordination pattern (Fig. 3, top). The interaction of 1a with terbium(III) is clearly appreciated in emission. The luminescence spectrum displays the characteristic sharp bands associated to f–f Tb(III) transitions, which reach an intensity maximum upon the addition of 1.5 equivalents of lanthanide.63 Also, the luminescence lifetime of complex [1a·Tb][ClO4]3 at 545 nm at a concentration of 0.1 mM upon excitation at 305 nm was determined to be 1.9 ± 0.1 ms both in acetonitrile and acetonitrile-d3 solutions (Fig. S17†). This finding indicates that either no labile solvent molecule is present in the first coordination sphere of Tb(III) in the complex or that C–H vibrations in acetonitrile do not lead to efficient deactivation of the excited complex. The lifetime of Tb(III) in dry acetonitrile was reported to be 1.87 ms for Tb(NO3)3, similar to the lifetime of complex [1a·Tb][ClO4]3, and 2.42 ms for Tb(ClO4)3.64 It seems therefore highly unlikely that a water molecule is present in the first coordination sphere of the complex in acetonitrile, unless this water molecule cannot be exchanged for an acetonitrile molecule in solution. The solubility of 1a in water was too low to be able to induce complex formation in aqueous solution, otherwise the lifetime of Tb(III) would be significantly shorter.65,66
1 complexation between the terbium and macrocycle 1a. More precisely, we propose that the terbium replaces the coordinated water molecule. The coordination of the metal occurs with the six oxygen atoms of the polyether ring while the two carbonyl groups of the amides rotate inward to reach an eightfold coordination pattern (Fig. 3, top). The interaction of 1a with terbium(III) is clearly appreciated in emission. The luminescence spectrum displays the characteristic sharp bands associated to f–f Tb(III) transitions, which reach an intensity maximum upon the addition of 1.5 equivalents of lanthanide.63 Also, the luminescence lifetime of complex [1a·Tb][ClO4]3 at 545 nm at a concentration of 0.1 mM upon excitation at 305 nm was determined to be 1.9 ± 0.1 ms both in acetonitrile and acetonitrile-d3 solutions (Fig. S17†). This finding indicates that either no labile solvent molecule is present in the first coordination sphere of Tb(III) in the complex or that C–H vibrations in acetonitrile do not lead to efficient deactivation of the excited complex. The lifetime of Tb(III) in dry acetonitrile was reported to be 1.87 ms for Tb(NO3)3, similar to the lifetime of complex [1a·Tb][ClO4]3, and 2.42 ms for Tb(ClO4)3.64 It seems therefore highly unlikely that a water molecule is present in the first coordination sphere of the complex in acetonitrile, unless this water molecule cannot be exchanged for an acetonitrile molecule in solution. The solubility of 1a in water was too low to be able to induce complex formation in aqueous solution, otherwise the lifetime of Tb(III) would be significantly shorter.65,66
Coordination mode of the terbium complexes
To rationalize the emission (chir)optical properties, care was taken to demonstrate that terbium coordination occurs within the macrocycle ring (and not with the pyridine moieties). Changing the antennas to regioisomeric aminopyridines (ligand 1b), or different extended aromatic moieties (1c) gave similar results in binding studies. Ligands 1b and 1c also allowed ligand-to-terbium energy transfer, resulting in the characteristic green emission (Fig. S9 and S10†). However, with ligand 1d, which lacks two oxygen atoms in the macrocyclic platform in comparison to 1a, only a very weak emission pattern of Tb3+ was observed (Fig. S11†).This corroborates the hypothesis that the terbium ion coordinates to the polyether ring and requires six ether and two carbonyl oxygen atoms to complete its coordination sphere. However, to ascertain this coordination mode, further analyses were performed to determine more precisely the nature of the lanthanide ion/macrocycle interaction. First, 1H-NMR spectroscopic analyses were carried out. For the complexation study, diamagnetic lutetium(III) cation was selected as the paramagnetic character of the Tb(III) does not permit direct analysis in the present case. Spectra were recorded in the presence of different equivalents of LuCl3 (0, 0.5, 1 and 2 equivalents) with ligand 1a in CD3CN (c = 15 mM, 600 MHz, 298 K, Fig. S15†). Upon titration, the following was noted: (i) only a broadening of the aromatic protons signal occurs while the amide protons are shifted downfield (Δδ = +0.8 ppm); (ii) the remainder of the signals, for instance of the polyether ring, undergoes negligible changes after Lu(III) addition; (iii) the structure conserves a local symmetry, most probably C2. This data, and the deshielding of the N–H signals particularly, corroborates the postulated rotation of the amide moieties to provide two carbonyl groups for metal binding. Also, on the NMR time scale, only one global set of signals are observed upon titration, which tends to indicate a fast host–guest exchange.8 Such a kinetic lability of the lanthanide ion might be a reason for the difficulty experienced in the attempts to isolate the targeted metal complex.
In fact, in our hands and despite many efforts, it was not possible to either crystalize nor precipitate complexes [1a·Tb][ClO4]3 or [1a·Lu][Cl]3. Looking for an a work-around solution, we briefly turned our attention to other ligand structures such as pyrene macrocycle 1e, that has furnished many evidences for solution and solid state complexation with metal ions. As desired, single crystals could be formed between 1e and La(ClO4)3, by slow evaporation of a CH3CN solution. The resulting complex was analyzed by X-ray diffraction (Fig. 4). Globally, the structure is C2-symmetric and organized around a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction of the M3+ ion and the ligand structure. More precisely, a coordination mode of 10 is observed around the lanthanum ion. A bicapped square antiprismatic polyhedral is formed with the 6 oxygen atoms of the polyether ring, the two carbonyls from the amides and 2 extra water molecules.67 This structure for [1e·La]3+ fits rather well the postulated conformation in Fig. 3 for [1a·Tb]3+ considering nevertheless with caution the exact number of water molecules bound to the lanthanide ion that might be the result of the metal-at-play and of the solid-state geometry. In particular, we recall that the ionic radius of La(III) is around 10% bigger than the one of Tb(III), and therefore, La can better accommodate coordination water molecules. Moreover, Tb falls in the second half of the lanthanide transition, where smaller coordination numbers are usually reported.68
1 interaction of the M3+ ion and the ligand structure. More precisely, a coordination mode of 10 is observed around the lanthanum ion. A bicapped square antiprismatic polyhedral is formed with the 6 oxygen atoms of the polyether ring, the two carbonyls from the amides and 2 extra water molecules.67 This structure for [1e·La]3+ fits rather well the postulated conformation in Fig. 3 for [1a·Tb]3+ considering nevertheless with caution the exact number of water molecules bound to the lanthanide ion that might be the result of the metal-at-play and of the solid-state geometry. In particular, we recall that the ionic radius of La(III) is around 10% bigger than the one of Tb(III), and therefore, La can better accommodate coordination water molecules. Moreover, Tb falls in the second half of the lanthanide transition, where smaller coordination numbers are usually reported.68
 | ||
| Fig. 4 Stick view of the crystal structure of [1e·La·(H2O)2]3+ (A: side view; B: top view). Counterions (perchlorate) and solvent molecules are omitted for clarity. | ||
Chiroptical studies (ECD and CPL)
Chiroptical properties of the ligand and the complex were then studied. First the ECD spectra were recorded for each single enantiomer of ligand 1a, previously separated by CSP-HPLC. As expected, the spectra presented a perfect mirror image shape and, globally, relative weak signal intensities (Fig. 5, red curves, Δε < 5 M−1 cm−1). Then, the spectra of the complexes were recorded after addition either of Tb(OTf)3 (Fig. 5, green curves) or of Ba(ClO4)2 (Fig. S13†).69 Upon the addition of the metal ions, a remarkable ECD sign reversal was observed for the two main transitions at 238 nm and 270 nm. The ECD spectra displayed mainly monosignate signals for the absorption band around 238 nm for both Tb3+ and Ba2+. Furthermore, the intensity of the ECD signals increased significantly. This (+/−)-ECD switch behavior probably originates from the conformational changes induced by the rotation of carbonyl groups (upon complexation) and the subsequent decrease of the number of conformations as computed previously on related systems.70 The change in intensity can be quantified using δΔε, which is the difference in normalized ECD intensity in the presence and the absence of tested metal ions (eqn (1)):| δΔε = |Δε(cation) − Δε(without)| | (1) |
At the maximum absorption (225 nm), a δΔε of 20 M−1 cm−1 is calculated for the terbium complex and 29 M−1 cm−1 for the barium one (235 nm). As the ECD is ligand-centered, the similarity between the spectra of [1a·Ba]2+ and [1a·Tb]3+ indicates that the two cations induce an overall similar effect onto the ligand, calling again for a similar coordination mode in the two cases for the two metals.
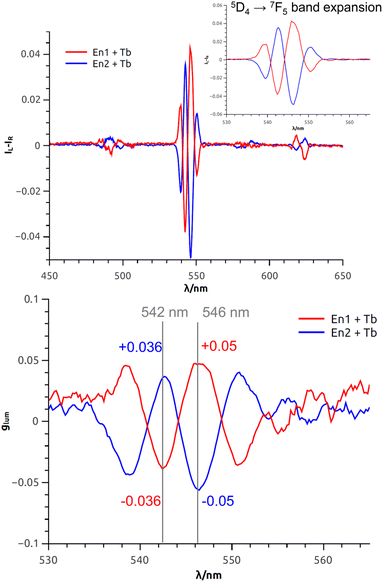
Finally, but most importantly, the CPL properties of the complex [1a·Tb]3+ were recorded. Again, 3.0 equivalents of Tb(OTf)3 were added to a solution of (+) and (−)-1a in acetonitrile. The results are reported in Fig. 6. Upon excitation of the ligand (254 nm), sharp and intense CPL signals associated to 5D4 → 7FJ (J = 6 − 3) terbium transitions were recorded. The mere presence of such signal is an indication of the coordination of Tb3+ in a dissymmetric environment, which in this case is provided by the chiral macrocycle. In fact, the CPL signal being Tb-centered, it is possible to monitor the interaction from the point of view of the guest rather than the host (as it is the case with ECD). Thus, Tb acts as a CPL-active probe for the chirality of the macrocycle ring cavity. Such possibility is obviously precluded when Ba2+ or other non-luminescent cations are employed.
The fine structure observed for the 5D4 → 7F5 transition originates from the different non-degenerate MJ sublevels of the transition. To quantify the circular polarization degree of the emission, the luminescence dissymmetry factor glum was used as defined by eqn (2) where IL and IR correspond to left and right circularly polarized component of the emission respectively:
 | (2) |
In this particular case, the glum factor for the 5D4 → 7F5 transition is 0.05 at 546 nm (Fig. 6, bottom). This value is in accordance with that observed for other chiral terbium complexes reported in the literature.8,9,11,15,71–75 As expected, the three other terbium main transitions (5D4 → 7FJ with J = 6,4,3 at 485 nm, 580 nm and 620 nm respectively) display visible but weaker CPL.
Conclusions
In conclusion, the formation of a terbium complex with a polyether macrocyclic ligand in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was achieved. Macrocycle 1a was readily accessed in enantiopure form (CSP HPLC) after a two-step synthesis from simple 1,4-dioxane. Upon Tb(III) complexation, the compound exhibited a characteristic (+/−)-ECD switch behavior and an easily detectable CPL emission with a glum value of 0.05. In this manner, the Tb-macrocycle interaction can be observed not only from the effects on the ligand (through ECD), but also from the effects on the metal guest itself thanks to terbium CPL. The structure of the complex is also confirmed by combining data obtained from different type of analysis (1H-NMR analysis, spectrophotometric titrations and solid-state elucidation).
1 stoichiometry was achieved. Macrocycle 1a was readily accessed in enantiopure form (CSP HPLC) after a two-step synthesis from simple 1,4-dioxane. Upon Tb(III) complexation, the compound exhibited a characteristic (+/−)-ECD switch behavior and an easily detectable CPL emission with a glum value of 0.05. In this manner, the Tb-macrocycle interaction can be observed not only from the effects on the ligand (through ECD), but also from the effects on the metal guest itself thanks to terbium CPL. The structure of the complex is also confirmed by combining data obtained from different type of analysis (1H-NMR analysis, spectrophotometric titrations and solid-state elucidation).
Author contributions
A. H., F. N., A. L. T., A. F. and F. Z. performed the experiments, the characterizations and analyzed the data. C. B. performed the crystallographic measurements. A. H., F. N., A. L. T., A. F., C. B., F. Z., L. D. B. and J. L. wrote the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. N. thanks the University of Camerino (FAR2018), and Prof. Enrico Marcantoni and Dr Cristina Cimarelli in particular, for promoting her internship in Geneva and their financial support. A. H., F. N., A. L. T. and J. L. thank the University of Geneva and the Swiss National Science Foundation for financial support (Grant 200020-184843). F. Z. and L. D. B. thank the Uni Pisa for financial support (PRA 2020_21). This project received funding from the European Commission Research Executive Agency (Grant Agreement Number: 859752 HEL4CHIROLED H2020-MSCA-ITN-2019). We thank Dr Pilar Franco and Mrs Assunta Green (Chiral Technologies, Illkirch, France) for their help and advices.References
- C. Lincheneau, C. Destribats, D. E. Barry, J. A. Kitchen, R. D. Peacock and T. Gunnlaugsson, Dalton Trans., 2011, 40, 12056–12059 RSC.
- R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673–7686 RSC.
- X.-L. Li, Y.-L. Gao, X.-L. Feng, Y.-X. Zheng, C.-L. Chen, J.-L. Zuo and S.-M. Fang, Dalton Trans., 2012, 41, 11829–11835 RSC.
- F. Zinna and L. Di Bari, Chirality, 2015, 27, 1–13 CrossRef CAS PubMed.
- J. P. Byrne, J. A. Kitchen, J. E. O'Brien, R. D. Peacock and T. Gunnlaugsson, Inorg. Chem., 2015, 54, 1426–1439 CrossRef CAS PubMed.
- D. E. Barry, D. F. Caffrey and T. Gunnlaugsson, Chem. Soc. Rev., 2016, 45, 3244–3274 RSC.
- J. P. Byrne, M. Martínez-Calvo, R. D. Peacock and T. Gunnlaugsson, Chem. – Eur. J., 2016, 22, 486–490 CrossRef CAS PubMed.
- M. Leonzio, A. Melchior, G. Faura, M. Tolazzi, F. Zinna, L. Di Bari and F. Piccinelli, Inorg. Chem., 2017, 56, 4413–4421 CrossRef CAS PubMed.
- B. Casanovas, F. Zinna, L. Di Bari, M. S. El Fallah, M. Font-Bardía and R. Vicente, Dalton Trans., 2017, 46, 6349–6357 RSC.
- S. Shuvaev, M. Starck and D. Parker, Chem. – Eur. J., 2017, 23, 9974–9989 CrossRef CAS PubMed.
- M. Leonzio, A. Melchior, G. Faura, M. Tolazzi, M. Bettinelli, F. Zinna, L. Arrico, L. Di Bari and F. Piccinelli, New J. Chem., 2018, 42, 7931–7939 RSC.
- E. Kreidt, L. Arrico, F. Zinna, L. Di Bari and M. Seitz, Chem. – Eur. J., 2018, 24, 13556–13564 CrossRef CAS PubMed.
- M. Górecki, L. Carpita, L. Arrico, F. Zinna and L. Di Bari, Dalton Trans., 2018, 47, 7166–7177 RSC.
- F. Zinna, L. Arrico and L. Di Bari, Chem. Commun., 2019, 55, 6607–6609 RSC.
- B. Casanovas, S. Speed, M. S. El Fallah, R. Vicente, M. Font-Bardía, F. Zinna and L. Di Bari, Dalton Trans., 2019, 48, 2059–2067 RSC.
- M. Starck, L. E. MacKenzie, A. S. Batsanov, D. Parker and R. Pal, Chem. Commun., 2019, 55, 14115–14118 RSC.
- D. E. Barry, J. A. Kitchen, L. Mercs, R. D. Peacock, M. Albrecht and T. Gunnlaugsson, Dalton Trans., 2019, 48, 11317–11325 RSC.
- H.-Y. Wong, W.-S. Lo, K.-H. Yim and G.-L. Law, Chem, 2019, 5, 3058–3095 CAS.
- M. Deng, N. D. Schley and G. Ung, Chem. Commun., 2020, 56, 14813–14816 RSC.
- B. Lefeuvre, C. A. Mattei, J. F. Gonzalez, F. Gendron, V. Dorcet, F. Riobé, C. Lalli, B. Le Guennic, O. Cador, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard and F. Pointillart, Chem. – Eur. J., 2021, 27, 7362–7366 CrossRef CAS PubMed.
- L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109–124 CrossRef CAS.
- M. Cui, A.-L. Wang, C.-L. Gao, L. Zhou, T. Wu, S. Fang, H.-P. Xiao, F.-C. Li and X.-L. Li, Dalton Trans., 2021, 50, 1007–1018 RSC.
- E. Cavalli, C. Nardon, O. G. Willis, F. Zinna, L. Di Bari, S. Mizzoni, S. Ruggieri, S. C. Gaglio, M. Perduca, C. Zaccone, A. Romeo and F. Piccinelli, Chem. – Eur. J., 2022, 28, e202200574 CrossRef CAS PubMed.
- C. A. Mattei, K. Dhbaibi, B. Lefeuvre, V. Dorcet, G. Argouarch, O. Cador, B. Le Guennic, O. Maury, C. Lalli, S. Guy, A. Bensalah-Ledoux, F. Riobé, B. Baguenard and F. Pointillart, Chirality, 2022, 34, 34–47 CrossRef CAS PubMed.
- N. F. M. Mukthar, N. D. Schley and G. Ung, J. Am. Chem. Soc., 2022, 144, 6148–6153 CrossRef CAS PubMed.
- O. G. Willis, F. Petri, G. Pescitelli, A. Pucci, E. Cavalli, A. Mandoli, F. Zinna and L. Di Bari, Angew. Chem., Int. Ed., 2022, 61, e202208326 CrossRef CAS PubMed.
- T. Mori, Frontiers of Circularly Polarized Luminescence Chemistry of Isolated Small Organic Molecules, Springer Singapore, Singapore, 2020 Search PubMed.
- J.-C. G. Bünzli and S. V. Eliseeva, Chem. Sci., 2013, 4, 1939–1949 RSC.
- J. Yuasa, T. Ohno, H. Tsumatori, R. Shiba, H. Kamikubo, M. Kataoka, Y. Hasegawa and T. Kawai, Chem. Commun., 2013, 49, 4604–4606 RSC.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Chem. Rev., 2014, 114, 4496–4539 CrossRef CAS PubMed.
- J.-C. G. Bünzli, J. Lumin., 2016, 170, 866–878 CrossRef.
- E. Brichtová, J. Hudecová, N. Vršková, J. Šebestík, P. Bouř and T. Wu, Chem. – Eur. J., 2018, 24, 8664–8669 CrossRef PubMed.
- T. Wu and P. Bouř, Chem. Commun., 2018, 54, 1790–1792 RSC.
- T. Wu, J. Kessler, J. Kaminský and P. Bouř, Chem. – Asian J., 2018, 13, 3865–3870 CrossRef CAS PubMed.
- M. Krupová, J. Kapitán and P. Bouř, ACS Omega, 2019, 4, 1265–1271 CrossRef PubMed.
- L. VandenElzen and T. A. Hopkins, ACS Sustainable Chem. Eng., 2019, 7, 16690–16697 CrossRef CAS.
- T. Wu, P. Bouř and V. Andrushchenko, Sci. Rep., 2019, 9, 1068 CrossRef PubMed.
- L. E. MacKenzie, L.-O. Pålsson, D. Parker, A. Beeby and R. Pal, Nat. Commun., 2020, 11, 1676 CrossRef CAS PubMed.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. Di Bari and U. Giovanella, Adv. Funct. Mater., 2017, 27, 1603719 CrossRef.
- J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef CAS.
- J. R. Brandt, X. Wang, Y. Yang, A. J. Campbell and M. J. Fuchter, J. Am. Chem. Soc., 2016, 138, 9743–9746 CrossRef CAS PubMed.
- F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS PubMed.
- Y. Yang, R. C. da Costa, D.-M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624–2628 CrossRef CAS PubMed.
- D. H. Metcalf, R. G. Ghirardelli and R. A. Palmer, Inorg. Chem., 1985, 24, 634–636 CrossRef CAS.
- A. Homberg, D. Poggiali, M. Vishe, C. Besnard, L. Guénée and J. Lacour, Org. Lett., 2019, 21, 687–691 CrossRef CAS PubMed.
- D. Poggiali, A. Homberg, T. Lathion, C. Piguet and J. Lacour, ACS Catal., 2016, 6, 4877–4881 CrossRef CAS.
- M. Vishe, R. Hrdina, A. I. Poblador-Bahamonde, C. Besnard, L. Guénée, T. Bürgi and J. Lacour, Chem. Sci., 2015, 6, 4923–4928 RSC.
- A. Homberg and J. Lacour, Chem. Sci., 2020, 11, 6362–6369 RSC.
- E. Brun, K.-F. Zhang, L. Guénée and J. Lacour, Org. Biomol. Chem., 2020, 18, 250–254 RSC.
- Z. Jarolímová, M. Vishe, J. Lacour and E. Bakker, Chem. Sci., 2016, 7, 525–533 RSC.
- S. K. Ray, A. Homberg, M. Vishe, C. Besnard and J. Lacour, Chem. – Eur. J., 2018, 24, 2944–2951 CrossRef CAS PubMed.
- A. Homberg, R. Hrdina, M. Vishe, L. Guénée and J. Lacour, Org. Biomol. Chem., 2019, 17, 6905–6910 RSC.
- F. Zinna, S. Voci, L. Arrico, E. Brun, A. Homberg, L. Bouffier, T. Funaioli, J. Lacour, N. Sojic and L. Di Bari, Angew. Chem., Int. Ed., 2019, 58, 6952–6956 CrossRef CAS PubMed.
- M. Vishe, T. Lathion, S. Pascal, O. Yushchenko, A. Homberg, E. Brun, E. Vauthey, C. Piguet and J. Lacour, Helv. Chim. Acta, 2018, 101, e1700265 CrossRef.
- A. Homberg, E. Brun, F. Zinna, S. Pascal, M. Górecki, L. Monnier, C. Besnard, G. Pescitelli, L. Di Bari and J. Lacour, Chem. Sci., 2018, 9, 7043–7052 RSC.
- S. Sinn, F. Biedermann, M. Vishe, A. Aliprandi, C. Besnard, J. Lacour and L. De Cola, ChemPhysChem, 2016, 17, 1829–1834 CrossRef CAS PubMed.
- A. Aster, G. Licari, F. Zinna, E. Brun, T. Kumpulainen, E. Tajkhorshid, J. Lacour and E. Vauthey, Chem. Sci., 2019, 10, 10629–10639 RSC.
- A. Baghdasaryan, E. Brun, Y. Wang, G. Salassa, J. Lacour and T. Bürgi, Chem. Sci., 2021, 12, 7419–7427 RSC.
- A. Aster, F. Zinna, C. Rumble, J. Lacour and E. Vauthey, J. Am. Chem. Soc., 2021, 143, 2361–2371 CrossRef CAS PubMed.
- M. Vishe, R. Hrdina, L. Guénée, C. Besnard and J. Lacour, Adv. Synth. Catal., 2013, 355, 3161–3169 CrossRef CAS.
- W. Zeghida, C. Besnard and J. Lacour, Angew. Chem., Int. Ed., 2010, 49, 7253–7256 CrossRef CAS PubMed.
- Calculation of the binding constant for the [1a·Tb]3+ complex was attempted both using absorption and emission data but failed to give unambiguously values. Notably, the reliability of the determination is poor as only weak change in the absorbance signals are observed in absorbance and that multiple sub-species could also compete and skew the calculations.
- J.-C. G. Bünzli, J.-R. Yersin and M. Vuckovic, in The Rare Earths in Modern Science and Technology: Volume 2, ed. G. J. McCarthy, J. J. Rhyne and H. B. Silber, Springer, US, Boston, MA, 1980, pp. 133–138 Search PubMed.
- I. Billard, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner, J. C. G. Bünzli and V. K. Pecharsky, Elsevier, 2003, vol. 33, pp. 465–514 Search PubMed.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. Gareth Williams and M. Woods, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- Analysis of the polyhedron has revealed the geometry is slighly closer to a sphenocorona but agreement with a more classical bicapped square antiprism is also excellent.
- S. A. Cotton and P. R. Raithby, Coord. Chem. Rev., 2017, 340, 220–231 CrossRef CAS.
- Additions of 3.0 equivalents of each metal salts were selected to ensure the full formation of the complex [1a·M]n+ in acetonitrile.
- It was shown previously that this type of macrocycles possesses many different conformations with opposite CD bands that can partly cancel the signal observed. See ref. 56 in particular.
- A. P. S. Samuel, J. L. Lunkley, G. Muller and K. N. Raymond, Eur. J. Inorg. Chem., 2010, 2010, 3343–3347 CrossRef PubMed.
- M. Seitz, K. Do, A. J. Ingram, E. G. Moore, G. Muller and K. N. Raymond, Inorg. Chem., 2009, 48, 8469–8479 CrossRef CAS PubMed.
- M. Seitz, E. G. Moore, A. J. Ingram, G. Muller and K. N. Raymond, J. Am. Chem. Soc., 2007, 129, 15468–15470 CrossRef CAS PubMed.
- R. S. Dickins, J. A. K. Howard, C. L. Maupin, J. M. Moloney, D. Parker, J. P. Riehl, G. Siligardi and J. A. G. Williams, Chem. – Eur. J., 1999, 5, 1095–1105 CrossRef CAS.
- H. G. Brittain and K. H. Pearson, Inorg. Chem., 1983, 22, 78–82 CrossRef CAS.
Footnotes |
| † In addition, the dataset for this article can be found at the following https://doi.org/10.26037/yareta:rj5oxgsdxrhqdm7g6p6gnu4iiy It will be preserved for 10 years. |
| ‡ Electronic supplementary information (ESI) available: Experimental conditions; 1H NMR, CSP-HPLC traces; UV-Vis, ECD, fluorescence and CPL spectra. CCDC 2189306. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt02627a |
| This journal is © The Royal Society of Chemistry 2022 |