Facet-engineering palladium nanocrystals for remarkable photocatalytic dechlorination of polychlorinated biphenyls†
Wei
Guo
,
Binbin
Guo
,
Huiling
Chen
,
Cheng
Liu
and
Ling
Wu
 *
*
State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, 350116, China. E-mail: wuling@fzu.edu.cn
First published on 1st November 2021
Abstract
Rationally constructing functionalized cocatalysts for removing chemically inert polychlorinated biphenyls is significant and challenging. In this study, we have synthesized uniform Pd nanocrystals (Pd NCs) with cube (CUB) and octahedron (OCT) geometries, corresponding to the exposed (100) and (111) facets, and they were incorporated with ultrathin TiO2(B) nanosheets (TBs) for the photocatalytic dechlorination of polychlorinated biphenyls under visible light illumination (λ ≥ 400 nm). Photocatalytic performance experiments revealed that CUB/TBs is more efficient with a 200% enhancement higher than OCT/TBs. Physicochemical characterizations reveal that the CUB possesses higher surface charge density than OCT, which promotes the activation of the C–Cl bond of polychlorinated biphenyls, leading to higher dechlorination performances of CUB/TBs than OCT/TBs. This study not only fundamentally elucidates the effect of facet-engineering palladium nanocrystals on the photocatalytic dechlorination of polychlorinated biphenyls, but also paves the way for rationally fabricating superior photocatalysts for other advanced applications.
1. Introduction
Polychlorinated biphenyls (PCBs) consisting of 209 congeners are applied in capacitors, transformers and adhesives owing to their thermal stability and low conductivity.1–5 However, PCBs with toxicity, high persistence and bioaccumulation severely affect human health and ecological balance.6–9 PCBs are ubiquitously spread throughout the global biogeospheres and are one of the most significant pollutant groups.10–12 Therefore, an effective method is urgently needed to remove the contamination of PCBs. In particular, photocatalytic reduction is a proven and effective, green and sustainable method to eliminate polyhalogenated organic contaminants.13,14 In general, bare semiconductors (such as TiO2) are rather ineffective to low-halogen compounds, and the less halogen products are much more resistant to elimination.15 To achieve deep dehalogenation, a metal (such as Au,16 Ag,17 Pd,14,18,19 Cu (ref. 20) and Pt (ref. 21)) cocatalyst is introduced to serve as active sites and spatially separate the photoexcited charge carriers. Among these metals, Pd may possess the best performance for photocatalytic dehalogenation due to its strong affinity interaction with halogen atoms and moderate hydrogen over-voltage.We have studied the photocatalytic dehalogenation of halogenated biphenyls via Pd nanoclusters/TiO2(B) nanosheets under visible light in our previous study.22 Although we have made a great progress, and the mechanism has been deeply studied at the molecular level, and the photocatalytic dehalogenation performance still needs to be further improved. In addition, the size of Pd is as small as nanoclusters, but Pd is deposited on the surface of photocatalysts by photodeposition, leading to the formation of an irregular spherical shape with mixed crystal facets.19,20,22 The arrangement of atoms on the metal surface influences the adsorption of substrate molecules and the transfer of interfacial charges.23–26 The inhomogeneous mixing surface, the irregular surface atoms and the undefined shape of Pd can affect the catalytic performance. Furthermore, it is difficult to identify the active structure of Pd and explore the structure–performance relationship, which is disadvantageous for revealing the internal catalytic mechanism. More importantly, it also impedes the optimization of the material. A large number of recent studies have shown that the synthesis of samples with specific crystal facets can solve the above problem, and the catalytic performance can be adjusted to achieve the maximum utilization of metal co-catalysts.27–34 As such, it is significant and challenging to synthesize well-defined Pd nanocrystals. Moreover, searching for the optimal facet of Pd nanocrystals to achieve superior performance for the photocatalytic dechlorination of PCBs has not been systematically studied, and the fundamental reason for the facet-dependent photocatalytic dehalogenation activities is unknown.
Ultra-thin two-dimensional (2D) nanosheets can provide a suitable platform for exploring the dependence of photocatalytic activity on facets and the undiscovered mechanisms.34 Moreover, the unique surface property and large specific surface area of 2D nanomaterials may enhance the interaction between metal–semiconductors.35 The charges excited in 2D nanomaterials can easily move to the metal–semiconductor interface and reduce the loss of carrier while migrating to reactive sites in comparison to the conventional bulk material.36 In particular, TiO2 is considered as an important semiconductor photocatalyst with good stability and high performance. In addition, the unique surface structure of ultra-thin TiO2(B) nanosheets is easy to produce surface defects to regulate its light adsorption performance.37 These features enable the ultra-thin TiO2(B) nanosheets to be an excellent candidate for photocatalysts.
Herein, uniform Pd nanocrystals (Pd NCs) with cube (CUB) and octahedron (OCT) geometry (corresponding to the exposed (100) and (111) facet, respectively) were synthesized, and incorporated with ultrathin TiO2(B) nanosheets (TBs) to fundamentally understand how the facets of Pd NCs promote the photocatalytic dechlorination of polychlorinated biphenyls. The photocatalytic performances of the CUB/TBs and OCT/TBs were evaluated by the dechlorination of 3,3′,4,4′-tetrachlorobiphenyl (PCB77), which is considered to be one of the most toxic dioxin-like PCBs congeners.38–41 A lot of physicochemical characterizations were conducted to reveal the fundamental mechanisms. The results showed that the photocatalytic dechlorination efficiency of PCB77 over Pd-based semiconductor materials could be modulated by the facet of Pd NCs. This work advances fundamental elucidating of the effect of facet-controlled palladium nanocrystals on the photocatalytic dechlorination of polychlorinated biphenyls and demonstrates engineering of the metal facet as an effective strategy to design high-performance photocatalysts.
2. Experimental section
Details of materials, preparation method, characterization and theoretical section are attached in the ESI.†3. Results and discussion
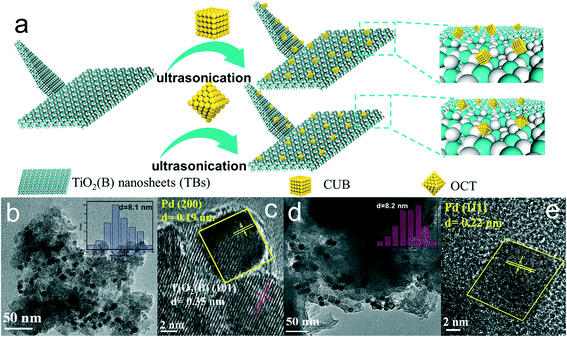
Pd NCs with cube and octahedron geometry were synthesized by a facile approach in the reported literature, as illustrated in Fig. S1.†42–44 Transmission electron microscopy (TEM) was probed to examine the morphology and size of the Pd NCs. Fig. 1a and b display a TEM image of the as-prepared monodisperse CUB with almost 100% selectivity and a mean width size of 8 nm. A HRTEM image of an individual Pd cube shown in Fig. 1c suggests that it was a single crystal with well-defined fringes and the high crystalline nature of the CUB. The intervals between two lattice fringes along with the two edges of a cube displayed in the HRTEM image were 0.19 nm, close to that of the (200) lattice spacing (0.19 nm) of the face-centered cubic (fcc) Pd, which indicates that the exposed facets of the as-prepared CUB are (100) facets (Fig. 1c and d).45,46 A TEM image of OCT (Fig. 1e) shows that the mean size was 8.1 nm (Fig. 1f). Most of monodisperse OCT are octahedrons. The lattice spacing of the octahedron was 0.22 nm, which was in accordance with the (111) crystal facet of Pd lattice spacing (0.226 nm) (Fig. 1g and h), proving that the as-prepared OCT consists of (111) facets.40,45,46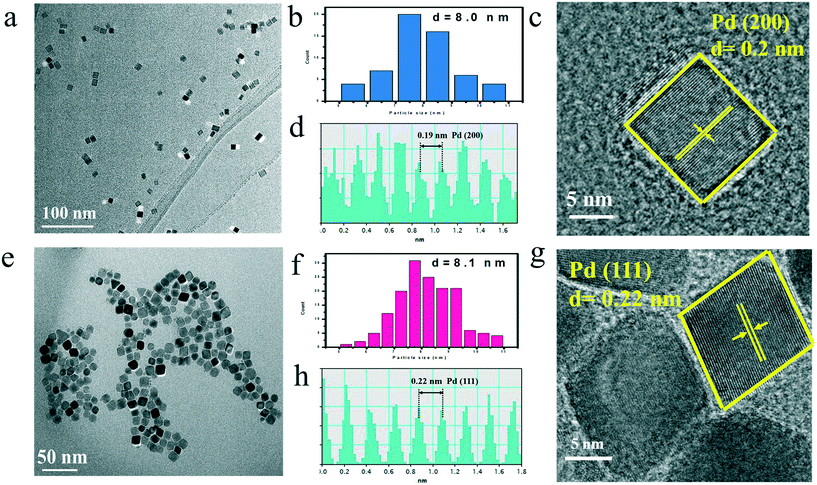 | ||
| Fig. 1 TEM image (a), size distribution (b), HRTEM image (c), intensity profiles (d) of CUB; TEM image (e) size distribution (f), HRTEM image (g), intensity profiles (h) of OCT. | ||
Ultra-thin TiO2(B) nanosheets (TBs) were synthesized via a hydrothermal process (Fig. S2†). Atomic force microscopy (AFM) was conducted to measure the thickness of the TBs. As shown in Fig. S3,† the height was about 1.6 nm, demonstrating that the TBs were ultra-thin nanosheets. CUB and OCT have been anchored onto TBs by a wet-chemical impregnation approach,40 as shown in Fig. 2a. The morphology information and microscopic structure of Pd–TB composites (denoted as CUB/TBs and OCT/TBs, respectively) were examined by TEM. Fig. 2 shows the TEM and HRTEM images of Pd–TB composites, confirming the immobilization of Pd NCs onto the TBs. The sizes of the Pd NCs loaded on CUB/TBs and OCT/TBs were measured to be 8.1 nm and 8.2 nm (Fig. 2b and d), respectively, which agreed with the size calculated in Fig. 1. Fig. 2c and e display the lattice fringes of the Pd NCs, and the spaces of which were calculated to be 0.19 nm and 0.22 nm, corresponding to the (200) and (111) crystal planes of metallic Pd. The above-mentioned results suggested that no significant changes could be found in the size and exposed facet of Pd NCs after loading. In addition, the Pd NC content of CUB/TBs and OCT/TBs have been measured by inductively coupled plasma (ICP), and their contents were very close at 1.064 ppm and 0.954 ppm, respectively (Table S1 and Fig. S4†).
The phase compositions of TBs and Pd–TB composites were verified by X-ray diffraction (XRD) (Fig. S5†). The identified peaks agreed well with TiO2(B) (JCPDS No. 74-1940). In comparison with TBs, both CUB/TBs and OCT/TBs showed additional peaks, which can be indexed to face-centered cubic (fcc) Pd (JCPDS No. 46-1043). To discover the optical properties of the samples, UV-vis diffuse reflectance spectra of TBs, CUB/TBs and OCT/TBs were collected, as illustrated in Fig. S6.† For all samples, a light absorption fingerprint centered at 350 nm, which can be attributed to the intrinsic bandgap absorption of TBs, and primarily assigned to the electron promotion of TBs from the valence band (VB) to the conduction band (CB). Moreover, the enhanced light absorption for the CUB/TBs and OCT/TBs mainly originates from the introduced Pd NCs and surface defects of TBs.37,47,48 It could also be observed that the optical properties of the as-prepared Pd–TB materials in both the UV and visible region were analogous. The similarity in DRS results implied that the light absorption properties for CUB/TBs and OCT/TBs were close.
The photocatalytic performance of CUB/TBs and OCT/TBs for the dechlorination of PCB77 was evaluated under visible light (λ ≥ 400 nm). Fig. 3a displays the temporal concentration changes (C/C0) and dechlorination efficiency (%) of PCB77 over CUB/TBs and OCT/TBs. PCB77 disappeared after irradiating for only 20 min in using CUB/TBs as the photocatalyst, and the dechlorination efficiency reached 100% in 40 min. However, it took 40 min and 60 min to remove and completely dechlorinate PCB77 in case of OCT/TBs. Furthermore, the dechlorination of PCB77 can be fitted by a zero-order reaction. The apparent rate constants (k) of the zero-order reaction were 0.054 mol L−1 min−1 and 0.027 mol L−1 min−1 for CUB/TBs and OCT/TBs, respectively, as shown in Fig. 3b and c. The results show that the photocatalytic dechlorination efficiency of PCB77 on CUB/TBs was higher than that of OCT/TBs with the same mass of Pd, which indicates that the exposed facets of Pd can influence the photocatalytic performance of the Pd–TBs composites, and CUB/TBs was more beneficial than OCT/TBs for promoting the photocatalytic activity towards the dechlorination of PCB77. It was noted that the photocatalyst containing TBs alone did not promote dechlorination, indicating that Pd was the active site of dechlorination. (Fig. S7†).
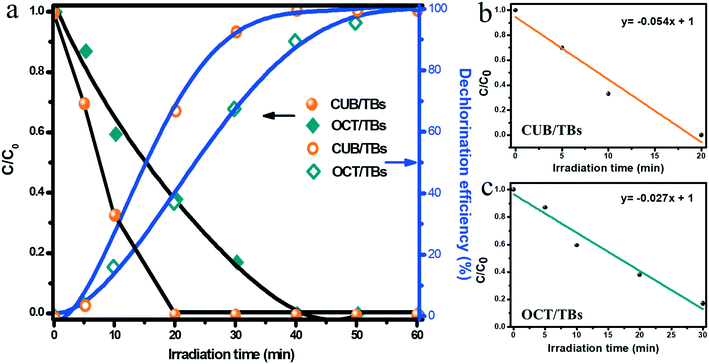 | ||
| Fig. 3 Temporal concentration changes (C/C0) and dechlorination efficiency (%) of PCB77 on CUB/TBs and OCT/TBs under visible light (a); the apparent rate constant (k) of CUB/TBs (b) and OCT/TBs (c). | ||
The effect of PVP, which was applied in the shape control synthesis of Pd NCs, on the dechlorination efficiency of PCB77 has been investigated. As is displayed in FT-IR spectra (Fig. S8a and S8b†), a sharp peak at 1648 cm−1 was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching vibrations of PVP. We observed that the peak intensity of the C
O bond stretching vibrations of PVP. We observed that the peak intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond for the treated samples significantly reduced than that of the untreated samples, indicating that much PVP has been removed. Moreover, N element was also used as an indicator to estimate residual PVP on the Pd NCs in the XPS spectra of N 1s (Fig. S8c and S8d†). The peak area of N 1s for the treated samples was smaller than that of untreated samples, suggesting that most of the PVP was washed away, which was in agreement with the FT-IR analysis. The photocatalytic dechlorination efficiency of PCB77 for the untreated and treated samples was carried out under the same conditions. The dechlorination efficiency of the treated samples was significantly higher than that of the untreated samples, as presented in Fig. S8e and S8f,† which indicates that the residual PVP of the sample will affect the dechlorination efficiency of PCB77 because the residual PVP can hinder the migration of photogenerated electrons on Pd NCs, as shown in Fig. S9.† Treated samples had a higher photocurrent response than that of the untreated samples, which suggested the enhanced mobility of charge carriers and the fast interfacial charge transfer of the treated samples. Furthermore, residual PVP partially blocked the reactive sites of Pd and reduced the dechlorination efficiency of PCB77. Moreover, recycling experiments for the photocatalytic dechlorination of PCB77 were conducted to confirm the stability of CUB/TBs and OCT/TBs. After three successive recycles, CUB/TBs and OCT/TBs still kept high activities (Fig. S10†). To learn whether there was a change in the composition and morphology of the sample after the reaction, the fresh and used catalysts have been characterized by XRD (Fig. S11†) and TEM (Fig. S12†). The used catalysts had identical XRD patterns compared to the fresh photocatalyst, suggesting that no significant changes occurred in the phase composition. Moreover, the morphology of Pd NCs and TiO2(B) nanosheets did not significantly change after being reacted in three recycles, suggesting the photostability of CUB/TBs and OCT/TBs.
O bond for the treated samples significantly reduced than that of the untreated samples, indicating that much PVP has been removed. Moreover, N element was also used as an indicator to estimate residual PVP on the Pd NCs in the XPS spectra of N 1s (Fig. S8c and S8d†). The peak area of N 1s for the treated samples was smaller than that of untreated samples, suggesting that most of the PVP was washed away, which was in agreement with the FT-IR analysis. The photocatalytic dechlorination efficiency of PCB77 for the untreated and treated samples was carried out under the same conditions. The dechlorination efficiency of the treated samples was significantly higher than that of the untreated samples, as presented in Fig. S8e and S8f,† which indicates that the residual PVP of the sample will affect the dechlorination efficiency of PCB77 because the residual PVP can hinder the migration of photogenerated electrons on Pd NCs, as shown in Fig. S9.† Treated samples had a higher photocurrent response than that of the untreated samples, which suggested the enhanced mobility of charge carriers and the fast interfacial charge transfer of the treated samples. Furthermore, residual PVP partially blocked the reactive sites of Pd and reduced the dechlorination efficiency of PCB77. Moreover, recycling experiments for the photocatalytic dechlorination of PCB77 were conducted to confirm the stability of CUB/TBs and OCT/TBs. After three successive recycles, CUB/TBs and OCT/TBs still kept high activities (Fig. S10†). To learn whether there was a change in the composition and morphology of the sample after the reaction, the fresh and used catalysts have been characterized by XRD (Fig. S11†) and TEM (Fig. S12†). The used catalysts had identical XRD patterns compared to the fresh photocatalyst, suggesting that no significant changes occurred in the phase composition. Moreover, the morphology of Pd NCs and TiO2(B) nanosheets did not significantly change after being reacted in three recycles, suggesting the photostability of CUB/TBs and OCT/TBs.
In addition, the dechlorination process of PCB77 was explored on CUB/TBs and OCT/TBs. Fig. 4a and c exhibit the concentration changes of intermediates and relative changes in distribution, respectively. 3,3′,4-Trichlorobiphenyl (PCB35) and 3,4,4′-trichlorobiphenyl (PCB37) were detected after irradiation for 5 min. Further, their concentration increased within 10 min, and then declined. di-PCBs and a small amount of 3,4′-dichlorobiphenyl (PCB13) were produced after irradiating for 10 min. The further dechlorination of di-PCBs produced mono-PCBs (PCB2 and PCB3) and biphenyl (DE). After duration for 40 min, no PCB intermediates were detected, which indicates that PCB77 dechlorination efficiency reached 100%. On the basis of the produced intermediates, the pathway of the photocatalytic dechlorination for PCB77 was put forward, as depicted in Fig. S13.† It can be seen that PCB77 is rapidly dechlorinated in a stepwise way. The dechlorination process of PCB77 on OCT/TBs was the same as on CUB/TBs, but it took a longer time for PCB77 to reach 100% dechlorination efficiency (60 min), as shown in Fig. 4b and d. The values of turnover number (TON) and turnover frequently (TOF) for biphenyl were calculated on CUB/TBs and OCT/TBs.49–51 As observed in Table S2,† the TON and TOF values of CUB/TBs were much higher than those of OCT/TBs. The difference in the contact area between Pd NCs with TBs may influence the photocatalytic performances. The contact area of CUB was larger than that of OCT with the same edge length. Therefore, CUB could accept more electrons to participate in the photocatalytic dechlorination for PCB77. The TON and TOF values of CUB/TBs were higher than those of OCT/TBs.
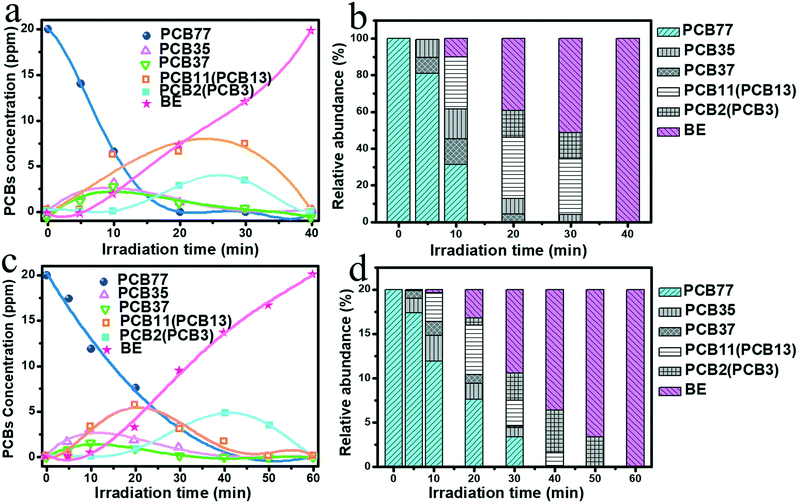 | ||
| Fig. 4 Time profiles of the concentration of PCBs for CUB/TBs (a) and OCT/TBs (c), changes in the distribution of PCBs for CUB/TBs (b) and OCT/TBs (d). | ||
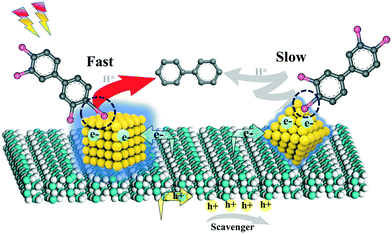
To comprehend the fundamental of the dechlorination efficiency increase of CUB/TBs over that of OCT/TBs, we probed the possible reasons based on the combined experimental analyses. Since the photocatalytic performance largely relies on the separation and transfer of photoexcited charge carriers, photoelectrochemical measurements were examined to figure out the possible mechanisms for the higher dechlorination efficiency of CUB/TBs compared to OCT/TBs. Fig. 5a displays the transient photocurrent response of CUB/TBs and OCT/TBs by several light on/off cycles of intermittently visible light illumination. The measurement shows that the addition of Pd significantly promotes their photocurrents, implying that the Pd–TB Schottky junction was beneficial for separating photogenerated electron–hole pairs. However, the transient photocurrent responses of CUB/TBs and OCT/TBs were not significantly different, indicating that the separation of photogenerated charge carriers was not the main factor affecting the photocatalytic dechlorination efficiency of PCB77 over CUB/TBs and OCT/TBs. To gain more information about charge transfer, the electrochemical impedance spectroscopy (EIS) Nyquist plots of CUB/TBs and OCT/TBs have been measured, as exhibited in Fig. 5b. It can be obviously observed that the two electrodes both showed semicircles at high frequencies. Pd–TB composites had a smaller arc at high frequencies in the EIS than that of TBs. The arc radius of the EIS Nyquist plots over CUB/TBs being slightly larger than the OCT/TB electrode implies the structure-independent ability of transfer for charge carriers on metal Pd, in line with the results in the photocurrent measurement.52 Furthermore, the photoluminescence (PL) spectrum was measured to probe the recombination of photogenerated electron–hole pairs. Fig. 5c shows the PL curves of TB and Pd–TB composites, and the PL intensity of Pd–TBs hybrids weakened as compared to that of TBs. Furthermore, it can be seen that the signal intensities of CUB/TBs and OCT/TBs were similar, suggesting the recombinational capacity of the electron–hole was equivalent.53 The above results showed that the higher photocatalytic dechlorination efficiency of CUB/TBs was not attributed to the separation and transportation of photoexcited electron–hole pairs. As shown in Fig. 5d, Fig. S14 and Table S4,† the BET surface areas of CUB/TBs surpassed that of OCT/TBs, and both of them were larger than that of TBs, while the pore volume values of CUB/TBs and OCT/TBs were comparable to TBs. A large specific surface area is more conducive to the adsorption of substrates.
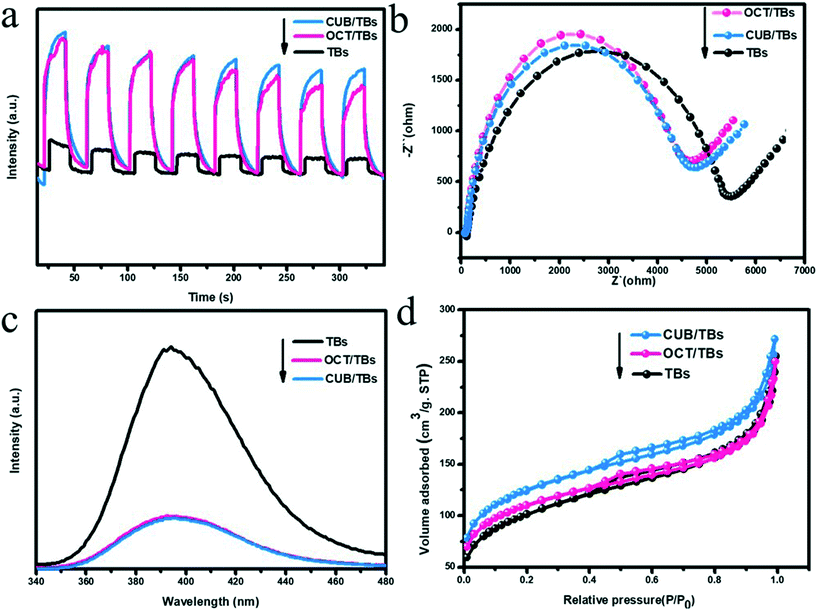 | ||
| Fig. 5 Photocurrent response (a), Nyquist plots (b), PL emission spectra (c), nitrogen adsorption/desorption isotherm (d) of TBs, OCT/TBs and CUB/TBs. | ||
Furthermore, the facet-dependent dechlorination efficiency of Pd NPs in the photocatalytic dechlorination of PCB77 was further investigated. We have already proved that Pd is the active site of photocatalytic dechlorination. The statistics of the surface and edge atoms of Pd NCs were investigated to further determine the active sites (Table S5†).35 The dechlorination efficiency of CUB/TBs was significantly higher than that of CUB/TBs, but the Xedge value of CUB was lower than that of OCT. The X100 value of CUB was higher than the X111 of OCT. Therefore, edge atoms were considered to be inactive, while surface atoms may be the main active site. Furthermore, the surface atoms between CUB and OCT were measured by the Fourier transform infrared spectroscopy (FT-IR) adsorption mode of CO (e.g., linear, bridge or hollow mode) and X-ray photoelectron spectroscopy (XPS). As shown in Fig. 6a, two new CO adsorbed peaks are observed for the samples at 1952 cm−1 (but 1907 cm−1 for OCT/TBs) and 2076 cm−1, corresponding to the bridge and linear adsorption, respectively.54–57 The band of CO bridge adsorption for CUB/TBs (1952 cm−1) was higher than that of OCT/TBs (1907 cm−1), which is due to the different arrangement of surface atoms for CUB and OCT. Furthermore, FT-IR wavenumbers and adsorption energy of CO adsorbed on CUB and OCT by bridge and linear adsorption were calculated by DFT calculations (Table S6†). The FT-IR wavenumber of CO bridge adsorption on CUB is higher than that of OCT. It can also be seen from the adsorption model diagrams that the adsorption of CO on CUB was standard, while the adsorption of CO on OCT was distorted (Fig. S15 and S16†), indicating that CUB and OCT showed different arrangement of the surface atoms. Moreover, the local charge density difference of Pd NCs with exposed (100) and (111) facets was further revealed via DFT calculations. As shown in Fig. S17,† the surface electrostatic potentials of Pd NCs with exposed (100) and (111) facets were significantly different. It was suggested that Pd NCs with exposed (100) and (111) facets exhibited different local charge density values due to the diverse arrangement of surface atoms. Fig. 6b displays the high-resolution XPS spectra of Pd 3d for CUB/TBs and OCT/TBs. Two typical peaks of Pd 3d are allocated to Pd 3d5/2 and Pd 3d3/2, respectively.31 For OCT/TBs, the peaks of Pd 3d slightly moved to higher binding energies (334.7 eV for Pd 3d5/2 and 339.9 eV for Pd 3d3/2) comparing with CUB/TBs (334.3 eV for Pd 3d5/2 and 339.5 eV for Pd 3d3/2). It implies that the Pd in CUB/TBs possesses higher local electron density than Pd in OCT/TBs, which agreed well with the FT-IR result. Pd NPs have affinity interaction with chlorine atoms to activate the C–Cl bond of PCB77. The d orbital electrons of Pd NPs would be transferred to the antibonding orbital of PCB77 to lengthen the C–Cl bond and reduce the bond energy of the C–Cl bond. CUB with higher local electron density was more conducive to activating the C–Cl bond. Therefore, the higher surface charge density of CUB for CUB/TBs resulted in higher photocatalytic dechlorination efficiency than OCT/TBs. Fig. 6c displays the XPS spectra of O 1s, O 1s of TBs is convoluted into the lattice oxygen of Ti–O (O1), surface hydroxyl oxygen (–OH) (O2) and the O-atoms near the oxygen vacancies (Vo) (O3).58,59 In XPS spectra of Ti 2p, two typical peaks centered at 458.5 eV and 464.2 eV were attributed to Ti 2p3/2 and Ti 2p1/2 for Ti 2p, respectively (Fig. 6d).60
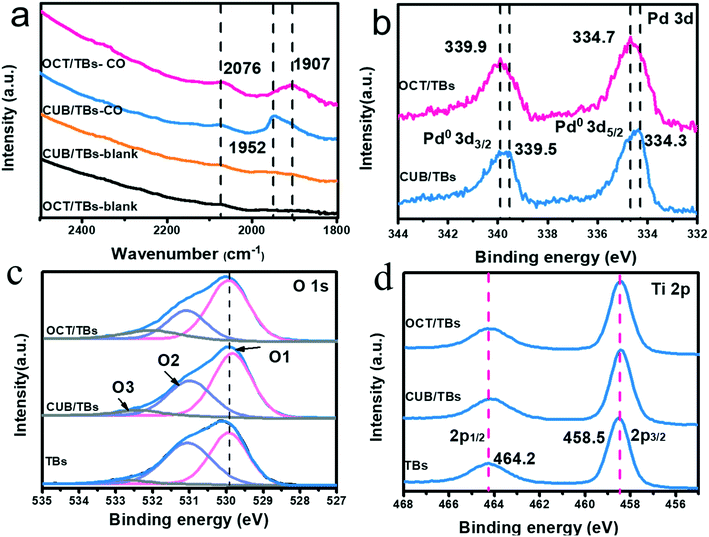 | ||
| Fig. 6 In situ FTIR spectra before and after adsorbed CO for CUB/TBs and OCT/TBs (a), XPS spectra of Pd 3d (b), O 1s (c), Ti 2p (d) for TBs, CUB/TBs and OCT/TBs. | ||
The active hydrogen species of photocatalytic dechlorination for PCB77 on CUB/TBs were proved by electron paramagnetic resonance (EPR) spectrum with 2,2′,6,6′-tetramethylpiperidine N-oxyl (TEMPO), which is a good hydrogen abstractor.61,62 Fig. S18a† describes that the signals of TEMPO gradually decayed when the light irradiation time was increased from starting to 8 min. This decay was attributed to the abstraction of a hydrogen atom from system by TEMPO, leading to the formation of hydroxylamine (TEMPOH), suggesting that active hydrogen species were H−, which were generated by H+ and e−, as illustrated in Fig. S18b† and eqn (1).
| H+ + 2e− → H− | (1) |
4. Conclusion
In summary, Pd NCs with CUB and OCT geometry in a similar size (∼8 nm) were synthesized and incorporated with TBs by a wet-chemical impregnation method, without altering sizes and exposed facets of Pd NCs. PCB77 disappeared completely after only 20 min of irradiation on the CUB/TBs, whereas only about 40% of PCB77 was eliminated in the OCT/TBs under visible light illumination (>400 nm). CUB was a more efficient cocatalyst with a 200% enhancement higher than OCT for the photocatalytic dechlorination of polychlorinated biphenyls. This improvement was predominantly attributed to the fact that the higher surface charge density of CUB promoted the activation of the C–Cl bond, leading to higher dechlorination performance of CUB/TBs than OCT/TBs. Although the dechlorination efficiencies of CUB/TBs and OCT/TBs were quite different, their dechlorination pathways were almost the same. Moreover, the residual PVP surfactant in Pd NPs influenced dechlorination efficiency by restraining interfacial charge transfer and hindering the contact between the reactant molecules and Pd NCs. This work not only fundamentally elucidates the effect of facet-engineering palladium nanocrystals on the photocatalytic dechlorination of polychlorinated biphenyls, but also paves the way for rationally fabricating superior photocatalysts for other advanced applications.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was kindly supported by the National Natural Science Foundation of China (21872032). We thank Mr. Jiwu Zhao for his help.References
- J. F. Heilier, J. Donnez and D. Lison, Chemosphere, 2008, 71, 203–210 CrossRef CAS.
- A. Galiszka, Z. M. Migaszewski and N. L. Rose, The Anthropocene Review, 2020, DOI:10.1177/2053019620916488.
- B. B. Mughal, J.-B. Fini and B. A. Demeneix, Endocr. Connect., 2018, 7, R160–R186 CAS.
- E. S. Marsan and C. A. Bayse, Chem. – Eur. J., 2020, 26, 5200–5207 CrossRef CAS PubMed.
- M. Rossbery, W. Lendle, G. Pfleiderer, A. Togel, E.-L. Dreher, E. Langer, H. Rassaerts, P. Kleinschmidt, H. Strack, R. Cook, U. Beck, K.-A. Lipper, T. R. Torkelson, E. Loser and K. K. Beutel, Mann in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed.
- I. N. Pessah, P. J. Lein, R. F. Seegal and S. K. Sagiv, Acta Neuropathol., 2019, 138, 363–387 CrossRef CAS PubMed.
- M. Y. Yao, T. T. Hu, Y. F. Wang, Y. J. Du, C. C. Hu and R. J. Wu, Environ. Pollut., 2017, 229, 837–845 CrossRef CAS.
- L. Leng, J. Li, X. M. Luo, J. K. Kim, Y. M. Li, X. M. Guo, X. Chen, Q. Y. Yang, G. Li and N. J. Tang, Environ. Int., 2016, 88, 133–141 CrossRef CAS.
- D. Zhang, P. Saktrakulkla, K. Tuttle, R. F. Marek, H. J. Lehmler, K. Wang, K. C. Hornbuckle and M. W. Duffel, Environ. Sci. Technol., 2021, 55, 2473–2481 CrossRef CAS PubMed.
- P. Saktrakulkla, T. Lan, J. Hua, R. F. Marek, P. S. Thorne and K. Hornbuckle, Environ. Sci. Technol., 2020, 54, 11443–11452 CrossRef CAS.
- M. Stancheva, S. Georgieva and L. Makedonski, Food Control, 2017, 72, 205–210 CrossRef CAS.
- K. D. Prince, S. D. Taylor and C. Angelini, Environ. Sci. Technol., 2020, 54, 10989–11001 CrossRef CAS PubMed.
- P. Zhang, R. Scrudato, J. Pagano and R. Roberts, Chemosphere, 1993, 26, 1213–1223 CrossRef CAS.
- Q. Huang and C. Hong, Chemosphere, 2000, 41, 871–879 CrossRef CAS.
- E. Zahran, N. Bedford, M. Nguyen, Y. Chang, B. Guiton, R. Naik, L. Bachas and M. Knecht, J. Am. Chem. Soc., 2014, 136, 32–35 CrossRef CAS PubMed.
- C. Y. Sun, D. Zhao, C. C. Chen, W. H. Ma and J. C. Zhao, Environ. Sci. Technol., 2009, 43, 157–162 CrossRef CAS PubMed.
- Y. Y. Wang, Q. Zhu, Y. Wei, Y. J. Gong, C. C. Chen, W. J. Song and J. C. Zhao, Appl. Catal., B, 2018, 231, 262–268 CrossRef CAS.
- M. Lei, N. Wang, L. H. Zhu and H. Q. Tang, Chemosphere, 2016, 150, 536–544 CrossRef CAS PubMed.
- L. Li, W. Chang, Y. Wang, H. Ji, C. C. Chen, W. H. Ma and J. C. Zhao, Chem. – Eur. J., 2014, 20, 150–156 Search PubMed.
- M. Lei, Z. Wang, L. Zhu, W. Nie and H. Tang, Appl. Catal., B, 2020, 261, 118236 CrossRef CAS.
- R. Narayanan and M. A. EI-Sayed, J. Phys. Chem. B, 2004, 108, 5726–5733 CrossRef CAS.
- W. Guo, Y. Qin, C. Liu, B. B. Guo, J. H. Zou, Z. H. Xie and L. Wu, Appl. Catal., B, 2021, 298, 120526 CrossRef CAS.
- B. Weng, Q. Quan and Y. J. Xu, J. Mater. Chem. A, 2016, 4, 18366–18377 RSC.
- D. M. Shuai, D. C. McCalman, J. K. Choe, J. R. Shapley, W. F. Schneider and C. J. Werth, ACS Catal., 2003, 3, 453–463 CrossRef.
- M. Laskar and S. E. Skrabalak, ACS Catal., 2014, 4, 1120–1128 CrossRef CAS.
- S. Fodor, G. Kovacs, K. Hernadi, V. Danciu, L. Baia and Z. Pap, Catal. Today, 2017, 284, 137–145 CrossRef CAS.
- M. Crespo-Quesada, A. Yarulin, M. S. Jin, Y. N. Xia and L. Kiwi-Minsker, J. Am. Chem. Soc., 2011, 133, 12787–12794 CrossRef CAS PubMed.
- S. Bai, X. J. Wang, C. Y. Hu, M. L. Xie, J. Jiang and Y. J. Xiong, Chem. Commun., 2014, 50, 6094–6097 RSC.
- S. W. Cao, Y. Li, B. C. Zhu, M. Jaroniec and J. G. Yu, J. Catal., 2017, 349, 208–217 CrossRef CAS.
- S. W. Lu, B. Weng, A. Z. Chen, X. W. Li, H. W. Huang, X. M. Sun, W. H. Feng, Y. H. Lei, Q. G. Qian and M. Q. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 13044–13054 CrossRef CAS PubMed.
- Y.-Y. Cai, X.-H. Li, Y.-N. Zhang, X. Wei, K.-X. Wang and J.-S. Chen, Angew. Chem., Int. Ed., 2013, 52, 11822–11825 CrossRef CAS PubMed.
- R. Narayanan and M. A. EI-Sayed, J. Am. Chem. Soc., 2004, 126, 7194–7195 CrossRef CAS.
- Z. N. Xu, J. Sun, C. S. Lin, X. M. Jiang, Q. S. Chen, S. Y. Peng, M. S. Wang and G. C. Guo, ACS Catal., 2013, 3, 118–122 CrossRef CAS.
- S. Bai, L. L. Wang, Z. Q. Li and Y. J. Xiong, Adv. Sci., 2017, 4, 1600216 CrossRef PubMed.
- H. Wang, S. G. Luo, Y. J. Song, Y. Z. Shi, Z. W. Wang, B. B. Guo and L. Wu, Appl. Surf. Sci., 2020, 511, 145501 CrossRef CAS.
- S. J. Liang, L. R. Wen, S. Lin, J. H. Bi, P. J. Feng, X. Z. Fu and L. Wu, Angew. Chem., Int. Ed., 2014, 53, 2951–2955 CrossRef CAS PubMed.
- W. Guo, J. H. Zou, B. B. Guo, J. H. Xiong, C. Liu, Z. H. Xie and L. Wu, Appl. Catal., B, 2020, 277, 119255 CrossRef CAS.
- S. Safe and C. Rev, Toxicology, 1994, 24, 87–149 CAS.
- N. Shaikh, S. Parkin, G. Luthe and H. Lehmler, Chemosphere, 2008, 70, 1694–1698 CrossRef CAS PubMed.
- T. Roy, J. Toxicol. Environ. Health, Part A, 2009, 72, 350–357 CrossRef CAS.
- C. Ronnback, Toxicology, 1991, 68, 340–345 Search PubMed.
- Y. J. Xiong, J. Y. Chen, B. J. M. Wiley and Y. N. Xia, J. Am. Chem. Soc., 2005, 127, 7332–7333 CrossRef CAS.
- Y. J. Xiong, H. G. Cai, B. J. M. Wiley, J. G. Wang, M. J. Kim and Y. N. Xia, J. Am. Chem. Soc., 2007, 129, 3665–3675 CrossRef CAS.
- B. B. Lim, M. J. Jiang, J. Tao, P. H. C. Camargo, Y. M. Zhu and Y. N. Xia, Adv. Funct. Mater., 2009, 19, 189–200 CrossRef CAS.
- M. S. Jin, H. Zhang, Z. X. Xie and Y. N. Xia, Energy Environ. Sci., 2012, 5, 6352–6357 RSC.
- M. S. Jin, H. Y. Liu, H. Zhang, Z. X. Xie, J. Y. Liu and Y. N. Xia, Nano Res., 2011, 4, 83–91 CrossRef CAS.
- N. Zhang and Y. J. Xu, Chem. Mater., 2013, 25, 1979–1988 CrossRef CAS.
- P. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, D. Chevrier, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu and N. F. Zheng, Science, 2016, 352, 797–801 CrossRef CAS.
- K. Takanabe, J. Catal., 2018, 370, 480–484 CrossRef.
- M. Qureshi and K. Takanabe, Chem. Mater., 2017, 20, 158–167 CrossRef.
- S. Braslavsky, A. Braun, A. Cassano, A. Emeline, M. Litter, L. Palmisano, V. Parmon and N. Serpone, Pure Appl. Chem., 2011, 83, 931–1014 CAS.
- J. H. Xiong, L. R. Wen, F. Jiang, Y. H. Liu, S. J. Liang and L. Wu, J. Mater. Chem. A, 2015, 3, 20627 RSC.
- L. Yuan, B. Weng, J. C. Colmenares, Y. G. Sun and Y. J. Xu, Small, 2017, 3, 1702253–1702263 CrossRef.
- J. L. Liu, F. R. Lucci, M. Yang, S. Lee, M. D. Marcinkowski, A. J. Therrien, C. T. Williams, C. H. Sykes and M. Flytzani-Stephanopoulos, J. Am. Chem. Soc., 2016, 138, 6396–6399 CrossRef CAS.
- Y. Y. Zhao, K. R. Yang, Z. C. Wang, X. X. Yan, S. F. Cao, Y. F. Ye, Q. Dong, X. Z. Zhang, J. E. Thorne, L. M. Kelly, T. Antonios, H. Y. Bai, S. C. Fakra, X. Y. Zhong, P. Wang, X. Q. Pan, J. H. Guo, M. Flytzani-Stephanopoulos, G. W. Brudvig, V. S. Batista and D. W. Wang, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 2902–2907 CrossRef CAS PubMed.
- Z. T. Wang, Y. J. Song, J. H. Zou, L. Y. Li, Y. Yu and L. Wu, Catal. Sci. Technol., 2018, 8, 268–275 RSC.
- O. Rosseler, A. Louvet, V. Keller and N. Keller, Chem. Commun., 2011, 47, 5331–5333 RSC.
- Y. H. Ren, J. H. Zou, K. Q. Jing, Y. Y. Liu, B. B. Guo, Y. J. Song, Y. Yu and L. Wu, J. Catal., 2019, 380, 123–131 CrossRef CAS.
- J. H. Zou, Z. T. Wang, W. Guo, B. B. Guo, Y. Yu and L. Wu, Appl. Catal., B, 2020, 260, 119255 CrossRef.
- G. L. Xiang, Y. Tang, Z. G. Liu, W. Zhu, H. T. Liu, J. O. Wang, G. M. Zhong, J. Li and X. Wang, Nano Lett., 2018, 18, 7809–7815 CrossRef CAS.
- M. Conte, H. Miyamura, S. Kobayashi and V. Chechik, J. Am. Chem. Soc., 2009, 131, 7189–7196 CrossRef CAS PubMed.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657–10666 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cy01752g |
| This journal is © The Royal Society of Chemistry 2022 |