Open Access Article
Open Access ArticleGas-phase identification of (Z)-1,2-ethenediol, a key prebiotic intermediate in the formose reaction†
Mattia
Melosso
 *ab,
Luca
Bizzocchi
ac,
Houda
Gazzeh
de,
Francesca
Tonolo
*ab,
Luca
Bizzocchi
ac,
Houda
Gazzeh
de,
Francesca
Tonolo
 ac,
Jean-Claude
Guillemin
ac,
Jean-Claude
Guillemin
 d,
Silvia
Alessandrini
d,
Silvia
Alessandrini
 ac,
Víctor M.
Rivilla
fg,
Luca
Dore
ac,
Víctor M.
Rivilla
fg,
Luca
Dore
 a,
Vincenzo
Barone
a,
Vincenzo
Barone
 c and
Cristina
Puzzarini
c and
Cristina
Puzzarini
 *a
*a
aDipartimento di Chimica “Giacomo Ciamician”, Università di Bologna, Via F. Selmi 2, 40126 Bologna, Italy. E-mail: mattia.melosso2@unibo.it; cristina.puzzarini@unibo.it
bScuola Superiore Meridionale, Università di Napoli Federico II, Largo San Marcellino 10, 80138 Naples, Italy
cScuola Normale Superiore, Piazza dei Cavalieri 7, 56126 Pisa, Italy
dUniv Rennes, Ecole Nationale Supérieure de Chimie de Rennes, CNRS, ISCR – UMR6226, F-35000 Rennes, France
eUniversité de Monastir, Avenue Taher Hadded B. P 56, Monastir 5000, Tunisia
fCentro de Astrobiología (CSIC-INTA), Ctra. de Ajalvir Km. 4, Torrejón de Ardoz, 28850 Madrid, Spain
gINAF – Osservatorio Astrofisico di Arcetri, Largo E. Fermi 5, 50125 Florence, Italy
First published on 4th February 2022
Abstract
Prebiotic sugars are thought to be formed on primitive Earth by the formose reaction. However, their formation is not fully understood and it is plausible that key intermediates could have formed in extraterrestrial environments and subsequently delivered on early Earth by cometary bodies. 1,2-Ethenediol, the enol form of glycolaldehyde, represents a highly reactive intermediate of the formose reaction and is likely detectable in the interstellar medium. Here, we report the identification and first characterization of (Z)-1,2-ethenediol by means of rotational spectroscopy. The title compound has been produced in the gas phase by flash vacuum pyrolysis of bis-exo-5-norbornene-2,3-diol at 750 °C, through a retro-Diels–Alder reaction. The spectral analysis was guided by high-level quantum-chemical calculations, which predicted spectroscopic parameters in very good agreement with the experiment. Our study provides accurate spectral data to be used for searches of (Z)-1,2-ethenediol in the interstellar space.
The origin of life from simple raw materials, i.e. abiogenesis, is still a hot, highly debated topic involving many research fields. While a general and robust solution to this problem is still sought, it is nowadays widely accepted that abiogenesis was not a smooth process, but more likely a dynamic scenario involving a number of successive steps and occurring across different geological eras and environments.1 Indeed, none of the main accredited theories for the origin of life, i.e. primordial soup, hydrothermal vents, and extraterrestrial origin of biological building blocks, is able to fully explain how bio-relevant molecules could have formed and survived in hostile conditions, although each of them provides unique pieces to complete the overall puzzle. In this context, the extraterrestrial formation (and the subsequent delivery on Earth) of the main building blocks of life, such as amino acids and sugars, is increasingly recognized as an important pathway.2
One of the most important routes towards the synthesis of prebiotic species starting from simple organic material is the formose reaction, which is thought to be responsible for the formation of simple carbohydrates, such as ribose, on early Earth environments.3 The formose reaction uses formaldehyde (H2CO, 1) as starting material and proceeds through aldol, reverse-aldol, and isomerization reactions, eventually forming glycolaldehyde (HOCH2CHO, 2), glyceraldehyde, and a number of four, five, and six-carbon atoms sugars.4–6 This process, firstly discovered by Butlerov in 1861,6 leads to the formation of fundamental biological units found in RNA and cofactors (e.g., ATP and NADH). However, the formose reaction exhibits some limits in aqueous solution,1 thereby reducing its possible role for the sugar formation on early Earth conditions.
Conversely, it is conceivable that formose-like reactions might have played an important role in the gas-phase and dust-grain surface chemistry occurring in the interstellar medium (ISM).7 The discovery of an enantiomeric excess of L-amino acids in the Murchison meteorite8 and then in many carbonaceous chondrites further supports this hypothesis, as L-amino acids are found to act as catalyst in the formose reaction, thus promoting the formation of sugars belonging to the D-series.9 These findings seem to suggest that an enantiomeric imbalance has possibly emerged elsewhere and could have then been transferred to Earth by meteoritic and cometary bombardment. Therefore, it is not unrealistic to think that prebiotic molecules formed in the ISM could have played a role in setting the stage of life on early Earth. This hypothesis is also supported by the detection of interstellar glycolaldehyde10 and many other complex, bio-relevant species in molecular clouds, star-forming regions, and Solar-type protostars.11–15 Such observations demonstrate the existence of formation mechanisms of organic molecules compatible with the harsh conditions of the ISM (T = 10–100 K, number densities ≈ 104 cm−3), whose detailed comprehension still requires a huge effort and, in particular, the identification of key intermediate species linking small organic species to bio-relevant molecules. Hydroxymethylene (HCOH, 3) is a prototypical short-lived species involved in sugar formation in non-aqueous conditions that escaped detection for a long time.16 It is thought to be responsible for an efficient initiation of formose-like reactions,17 especially at low temperatures such as those found in the ISM. In a similar manner, 1,2-ethenediol (HO–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–OH, 4) has been suggested being deeply involved in the process of sugar formation in the ISM, e.g. by producing glyceraldehyde through its reaction with formaldehyde.18
CH–OH, 4) has been suggested being deeply involved in the process of sugar formation in the ISM, e.g. by producing glyceraldehyde through its reaction with formaldehyde.18
Diol 4, the higher energy and highly reactive enol of 2, has been recently identified in an experiment simulating methanol-bearing interstellar ice-analogues exposed to ionizing radiation.18 Similarly to other prebiotic species,19–214 can be formed on the icy mantles of interstellar dust grains before being released into the gas phase through thermal desorption or energetic processes, such as strong UV irradiation. This suggests that 4 is likely present in the interstellar medium. However, its detection is currently not possible, because its rotational spectrum is unknown to date. Indeed, it should be noted that the 90% of our astrochemical inventory has been discovered through the observation of rotational transitions.22
Compared to other techniques, e.g., electronic or vibrational spectroscopy, which are more suitable for the identification of functional groups, rotational spectroscopy allows for distinguishing among different isotopologues, structural isomers, and even conformers. In this context, it is noteworthy that both 1 and 2, as well as two additional C2H4O2 isomers (acetic acid and methyl formate), have been identified in space thanks to the observation of their spectroscopic signatures,10,23,24 as it has been the case for halogenated,25 aromatic,26,27 or chiral molecules.28
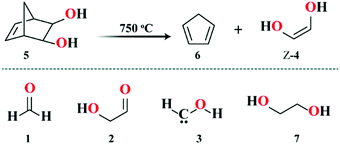
Here, in order to enable a step forward in the understanding of sugar formation in the ISM, we report the gas-phase synthesis of the Z isomer of 4via flash vacuum pyrolysis (FVP) of bis-exo-5-norbornene-2,3-diol (C7H10O2, 5, see Scheme 1) and its spectroscopic characterization. The synthesis is based on the general retro-Diels–Alder reaction proposed by Turecek.29 The Z isomer of 4 (hereafter Z-4) has been characterized via measurements of its spectrum using a frequency-modulation submillimeter-spectrometer and its comparison with simulations based on high-level quantum-chemical calculations (see ESI† for details).
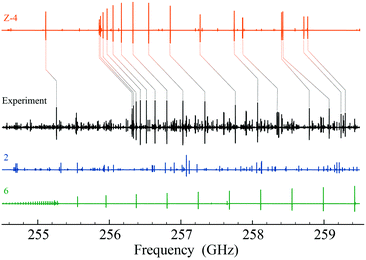
The spectrum, recorded between 80–125 GHz and 240–375 GHz during the FVP of 5 at 750 °C, revealed absorptions due to the by-product cyclopentadiene (c-C5H6, 6) and the more stable tautomeric form 2.30 However, the most intense features detected in the spectrum (Fig. 1) do not belong to any of these or other known molecules, while they match very well the spectral patterns predicted for the Z-(syn,anti) form of 4.‡
With the help of theoretical predictions (based on coupled-cluster theory§) and using the SPCAT31 subroutine of the CALPGM suite, the signals around 257 GHz could be unambiguously assigned to the strongest μa-allowed transitions of Z-4 (μa = 1.96 D), shifted with respect to predictions by only 0.1%. This first assignment has been used to refine the spectral predictions, in order to reduce the discrepancy between the computed and observed spectra and to facilitate the interpretation of the subsequent measurements. In addition, we found that a mild heating of 5 decreases the intensity of the interfering lines produced by 2. Under the best conditions, the Z-4 to 2 abundance ratio is about 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The identification of Z-4 was further confirmed by the presence of a tiny splitting in most of the absorption features. These splittings are a clear evidence of a tunneling motion occurring within the molecular frame, as expected in view of the similarity of Z-4 with ethylene glycol (HO–(CH2)2–OH, 7).32 In particular, the –OH moieties undergo a concerted large amplitude tunneling motion between two equivalent positions of Z-(syn,anti)-4, thus splitting each rotational energy level into two. As a consequence, the transitions allowed by the dipole moment component oriented along the exchange axis must occur between the two inversion states and show a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 intensity ratio obeying to spin-statistics (Fig. 2, see also Section 2 in the ESI†). On the other hand, moderately intense b-type transitions (μb = 0.62 D) take place within the inversion states.
6 intensity ratio obeying to spin-statistics (Fig. 2, see also Section 2 in the ESI†). On the other hand, moderately intense b-type transitions (μb = 0.62 D) take place within the inversion states.
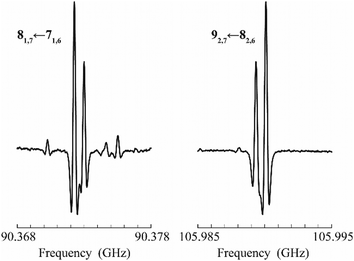 | ||
Fig. 2 Tunneling splittings recorded for two low-frequency transitions of Z-4. The observed ratio is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in accordance with spin-statistics. 6 in accordance with spin-statistics. | ||
In agreement with the results of Kleimeier et al.,18 our computed equilibrium geometry of Z-4 indicates a non-planar structure (the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–H dihedral angle being about 25°), which should result in a moderate dipole moment component along the c-axis of the molecule (μc = 0.97 D). However, no μc-allowed transitions could be observed in the spectrum. While this fact may appear puzzling at first, the experimental evidence is explained by the very low barrier to planarity (≈ 0.2 kJ mol−1 at the B2PLYP-D3/aug-cc-pVTZ level of theory; see ESI† for details), ruled by the motion of a single H atom. Hence, in the ground vibrational state the molecule is effectively planar, as confirmed by its inertia defect, whose value is close to zero and slightly negative (see Table 1), a clear sign of near planarity together with the low frequency out-of-plane motion.33
C–O–H dihedral angle being about 25°), which should result in a moderate dipole moment component along the c-axis of the molecule (μc = 0.97 D). However, no μc-allowed transitions could be observed in the spectrum. While this fact may appear puzzling at first, the experimental evidence is explained by the very low barrier to planarity (≈ 0.2 kJ mol−1 at the B2PLYP-D3/aug-cc-pVTZ level of theory; see ESI† for details), ruled by the motion of a single H atom. Hence, in the ground vibrational state the molecule is effectively planar, as confirmed by its inertia defect, whose value is close to zero and slightly negative (see Table 1), a clear sign of near planarity together with the low frequency out-of-plane motion.33
| Constant | Unit | Valuea | Theoryb | Constant | Unit | Valuea |
|---|---|---|---|---|---|---|
| a Numbers in parentheses represent the error in the unit of the last quoted digit. When the error is not reported, the parameter is kept fixed to the corresponding computed value. b See ESI for details about the level of theory employed. | ||||||
| A | MHz | 19507.2(2) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 597.9 597.9 |
E* | MHz | 0.361(3) |
| B | MHz | 6312.1(2) | 6298.6 | E* J | kHz | 0.12(1) |
| C | MHz | 4772.4533(2) | 4766.8 | E* K | kHz | 0.75(5) |
| D J | kHz | 6.9479(4) | 7.21 | E* JJ | Hz | 0.10(1) |
| D JK | kHz | −36.977(6) | −39.15 | E* JK | Hz | 0.7(1) |
| D K | kHz | 103.10(1) | 107.88 | E* KK | Hz | 1.6(2) |
| d 1 | kHz | −2.2929(2) | −2.35 | F ab | MHz | −218(6) |
| d 2 | kHz | −0.15145(4) | −0.15 | Δ | u Å2 | −0.078 |
| H J | mHz | 3.1(1) | −0.26 | |||
| H JK | mHz | 112.7(5) | 171 | |||
| H KJ | mHz | −1378.(5) | −1660 | |||
| H K | mHz | 3490 | 3490 | |||
| h 1 | mHz | 2.9(1) | 1.74 | |||
| h 2 | mHz | 1.080 | 1.080 | |||
| h 3 | mHz | 0.243 | 0.243 | |||
| L KKJ | mHz | 0.074(6) | ||||
From the analysis of the spectrum, more than 1100 transitions have been assigned to Z-4 and analysed with an effective Hamiltonian that accounts for centrifugal distortion as well as tunneling effects. The optimized spectroscopic constants are in excellent agreement with the corresponding computed values (Table 1). This, together with the observed tunneling splittings, provides strong evidence for the correct identification of Z-4 and for its structure and internal dynamics. The small energy difference between the two inversion substates is a clear hint of a high-barrier separating the two identical conformations of Z-(syn,anti)-4. In the aGg′ form of 7, this energy difference is four orders of magnitude larger,34 because the single C–C bond lowers the barrier height considerably.
The accurate spectroscopic characterization of Z-4 now permits its search in the ISM. The observation of 4 would shed light on how formose-like reactions can proceed under interstellar conditions, whether following the mechanism proposed by Ricardo et al.35 or a different pathway,5 and can provide additional insights on sugar formation in extraterrestrial environments.
Being the highly energetic tautomeric form of 2, the diol 4 can promote chemical reactions when no external energetic sources are available, as it is the case at very low temperatures. This opens up reaction channels that otherwise would be closed and expands the horizon of sugars formation in the ISM. With both 1 and 2 already detected in different astronomical environments,10,23,36,37 the presence of 4 in the interstellar medium appears more than plausible. The interest in 4 is, however, not limited to prebiotic and interstellar chemistry; enols are also key intermediates of tropospheric acids via keto-enol photo-tautomerization mechanisms38 and of hydrocarbon oxidation processes,39 thus widening the number of applications for which our gas-phase spectroscopic identification is invaluable.
From another point of view, this work demonstrates the feasibility of producing 4 directly in the gas phase through the retro-Diels–Alder reaction of 5, thereby allowing its spectroscopic characterization. Although the coupling of FVP with high-resolution spectroscopic techniques has been widely employed in the past for the study of enols,40,41 carbenes,42,43 and other unstable species,44–46 this is the first time that an enediol is observed directly in the gas phase by rotational spectroscopy. The analysis of the spectrum, supported by quantum-chemical calculations, has revealed a vibrationally-averaged planar structure of Z-(syn,anti)-4, which undergoes a large amplitude tunneling motion that exchanges the position of the two hydroxyl groups. The experimental findings are in excellent agreement with results issuing from high-level quantum-chemical calculations. In view of the important information that can be retrieved from the spectroscopic characterization in an isolated environment, and the concrete possibility to search for the title molecule in the ISM, we are currently extending the approach presented here to a number of highly reactive species not yet observed in the gas phase.
This study was supported by Bologna University (RFO funds) and the Italian Space Agency (ASI; ‘Life in Space’ project, N. 2019-3-U.0). The SMART@SNS Laboratory (http://smart.sns.it) is acknowledged for providing high-performance computing facilities. J.-C. G. thanks the Centre National d'Etudes Spatiales (CNES) and the “Programme National Physique et Chimie du Milieu Interstellaire” (PCMI) of CNRS/INSU with INC/INP co-funded by CEA and CNES for a grant. V. M. R. acknowledges support from the Comunidad de Madrid through the Atracción de Talento Investigador Modalidad 1 (Doctores con experiencia) Grant (COOL: Cosmic Origins Of Life; 2019-T1/TIC-15379).
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Kitadai and S. Maruyama, Geosci. Front., 2018, 9, 1117–1153 CrossRef CAS
.
- K. Ruiz-Mirazo, C. Briones and A. de la Escosura, Chem. Rev., 2014, 114, 285–366 CrossRef CAS PubMed
.
- R. Shapiro, Origins Life Evol. Biospheres, 1988, 18, 71–85 CrossRef CAS PubMed
.
- R. Breslow, Tetrahedron Lett., 1959, 1, 22–26 CrossRef
.
- C. Appayee and R. Breslow, J. Am. Chem. Soc., 2014, 136, 3720–3723 CrossRef CAS PubMed
.
- A. Butlerow, Justus Liebigs Ann. Chem., 1861, 120, 295–298 CrossRef
.
- A. F. Jalbout, L. Abrell, L. Adamowicz, R. Polt, A. J. Apponi and L. M. Ziurys, Astrobiology, 2007, 7, 433–442 CrossRef CAS PubMed
.
- J. R. Cronin and S. Pizzarello, Science, 1997, 275, 951–955 CrossRef CAS PubMed
.
- S. Pizzarello and A. L. Weber, Origins Life Evol. Biospheres, 2010, 40, 3–10 CrossRef CAS PubMed
.
- J. M. Hollis, F. J. Lovas and P. R. Jewell, Astrophys. J., 2000, 540, L107–L110 CrossRef CAS
.
- V. M. Rivilla, I. Jimenez-Serra, J. Martin-Pintado, C. Briones, L. F. Rodriguez-Almeida, F. Rico-Villas, B. Tercero, S. S. Zeng, L. Colzi, P. de Vicente, S. Martin and M. A. Requena-Torres, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(22), e2101314118 CrossRef CAS PubMed
.
- S. Manigand, J. K. Jorgensen, H. Calcutt, H. S. P. Muller, N. F. W. Ligterink, A. Coutens, M. N. Drozdovskaya, E. F. van Dishoeck and S. F. Wampfler, Astron. Astrophys., 2020, 635, A48 CrossRef CAS
.
- A. Belloche, R. T. Garrod, H. S. P. Muller, K. M. Menten, I. Medvedev, J. Thomas and Z. Kisiel, Astron. Astrophys., 2019, 628, A10 CrossRef CAS
.
- S. Zeng, D. Quenard, I. Jimenez-Serra, J. Martin-Pintado, V. M. Rivilla, L. Testi and R. Martin-Domenech, Mon. Not. R. Astron. Soc., 2019, 484, L43–L48 CrossRef CAS
.
- A. Belloche, K. M. Menten, C. Comito, H. S. P. Muller, P. Schilke, J. Ott, S. Thorwirth and C. Hieret, Astron. Astrophys., 2008, 482, 179–U137 CrossRef CAS
.
- P. R. Schreiner, H. P. Reisenauer, F. C. Pickard, A. C. Simmonett, W. D. Allen, E. Matyus and A. G. Csaszar, Nature, 2008, 453, 906–U942 CrossRef CAS PubMed
.
- A. K. Eckhardt, M. M. Linden, R. C. Wende, B. Bernhardt and P. R. Schreiner, Nat. Chem., 2018, 10, 1141–1147 CrossRef CAS PubMed
.
- N. F. Kleimeier, A. K. Eckhardt and R. I. Kaiser, J. Am. Chem. Soc., 2021, 143, 14009–14018 CrossRef CAS PubMed
.
- F. Dulieu, T. Nguyen, E. Congiu, S. Baouche and V. Taquet, Mon. Not. R. Astron. Soc., 2019, 484, L119–L123 CrossRef CAS
.
- M. Melosso, A. Belloche, M. A. Martin-Drumel, O. Pirali, F. Tamassia, L. Bizzocchi, R. T. Garrod, H. S. P. Muller, K. M. Menten, L. Dore and C. Puzzarini, Astron. Astrophys., 2020, 641, L160 CrossRef
.
- V. M. Rivilla, J. Martin-Pintado, I. Jimenez-Serra, S. Martin, L. F. Rodriguez-Almeida, M. A. Requena-Torres, F. Rico-Villas, S. S. Zeng and C. Briones, Astrophys. J., Lett., 2020, 899, L28 CrossRef CAS
.
- B. A. McGuire, Astrophys. J., Suppl. Ser., 2018, 239, 17 CrossRef CAS
.
- L. E. Snyder, D. Buhl, B. Zuckerman and P. Palmer, Phys. Rev. Lett., 1969, 22, 679–681 CrossRef CAS
.
- S. J. El-Abd, C. L. Brogan, T. R. Hunter, E. R. Willis, R. T. Garrod and B. A. McGuire, Astrophys. J., 2019, 883, 129 CrossRef CAS
.
- E. C. Fayolle, K. I. Oberg, J. K. Jorgensen, K. Altwegg, H. Calcutt, H. S. P. Muller, M. Rubin, M. H. D. van der Wiel, P. Bjerkeli, T. L. Bourke, A. Coutens, E. F. van Dishoeck, M. N. Drozdovskaya, R. T. Garrod, N. F. W. Ligterink, M. V. Persson, S. F. Wampfler and R. Team, Nat. Astron., 2017, 1, 703–708 CrossRef
.
- B. A. McGuire, A. M. Burkhardt, S. Kalenskii, C. N. Shingledecker, A. J. Remijan, E. Herbst and M. C. McCarthy, Science, 2018, 359, 202–205 CrossRef CAS
.
- B. A. McGuire, R. A. Loomis, A. M. Burkhardt, K. L. K. Lee, C. N. Shingledecker, S. B. Charnley, I. R. Cooke, M. A. Cordiner, E. Herbst, S. Kalenskii, M. A. Siebert, E. R. Willis, C. Xue, A. J. Remijan and M. C. McCarthy, Science, 2021, 371, 1265–1269 CrossRef CAS PubMed
.
- B. A. McGuire, P. B. Carroll, R. A. Loomis, I. A. Finneran, P. R. Jewell, A. J. Remijan and G. A. Blake, Science, 2016, 352, 1449–1452 CrossRef CAS PubMed
.
- F. Tureček and V. Hanuš, Mass Spectrom. Rev., 1984, 3, 85–152 CrossRef
.
- H. S. P. Muller, F. Schloder, J. Stutzki and G. Winnewisser, J. Mol. Struct., 2005, 742, 215–227 CrossRef
.
- H. M. Pickett, J. Mol. Spectrosc., 1991, 148, 371–377 CrossRef CAS
.
- D. Christen, L. H. Coudert, R. D. Suenram and F. J. Lovas, J. Mol. Spectrosc., 1995, 172, 57–77 CrossRef CAS
.
- T. Oka, J. Mol. Struct., 1995, 352-353, 225–233 CrossRef
.
- M. Melosso, L. Dore, F. Tarnassia, C. L. Brogan, T. R. Hunter and B. A. McGuire, J. Phys. Chem. A, 2020, 124, 240–246 CrossRef CAS PubMed
.
- A. Ricardo, F. Frye, M. A. Carrigan, J. D. Tipton, D. H. Powell and S. A. Benner, J. Org. Chem., 2006, 71, 9503–9505 CrossRef CAS PubMed
.
- J. K. Jørgensen, A. Belloche and R. T. Garrod, Annu. Rev. Astron. Astrophys., 2020, 58, 727–778 CrossRef
.
- S. K. Blair, L. Magnani, J. Brand and J. G. A. Wouterloot, Astrobiology, 2008, 8, 59–73 CrossRef CAS PubMed
.
- D. U. Andrews, B. R. Heazlewood, A. T. Maccarone, T. Conroy, R. J. Payne, M. J. T. Jordan and S. H. Kable, Science, 2012, 337, 1203–1206 CrossRef CAS PubMed
.
- C. A. Taatjes, N. Hansen, A. McIlroy, J. A. Miller, J. P. Senosiain, S. J. Klippenstein, F. Qi, L. S. Sheng, Y. W. Zhang, T. A. Cool, J. Wang, P. R. Westmoreland, M. E. Law, T. Kasper and K. Kohse-Hoinghaus, Science, 2005, 308, 1887–1889 CrossRef CAS PubMed
.
- A. Mardyukov, A. K. Eckhardt and P. R. Schreiner, Angew. Chem., Int. Ed., 2020, 59, 5577–5580 CrossRef CAS PubMed
.
- A. Mardyukov, F. Keul and P. R. Schreiner, Angew. Chem., Int. Ed., 2021, 60, 15313–15316 CrossRef CAS PubMed
.
- C. C. Womack, K. N. Crabtree, L. McCaslin, O. Martinez, R. W. Field, J. F. Stanton and M. C. McCarthy, Angew. Chem., Int. Ed., 2014, 53, 4089–4092 CrossRef CAS PubMed
.
- A. K. Eckhardt and P. R. Schreiner, Angew. Chem., Int. Ed., 2018, 57, 5248–5252 CrossRef CAS
.
- P. Neuhaus and W. Sander, Angew. Chem., Int. Ed., 2010, 49, 7277–7280 CrossRef CAS PubMed
.
- K. Edel, S. A. Brough, A. N. Lamm, S. Y. Liu and H. F. Bettinger, Angew. Chem., Int. Ed., 2015, 54, 7819–7822 CrossRef CAS PubMed
.
- N. M. Kidwell, V. Vaquero-Vara, T. K. Ormond, G. T. Buckingham, D. Zhang, D. N. Mehta-Hurt, L. McCaslin, M. R. Nimlos, J. W. Daily, B. C. Dian, J. F. Stanton, G. B. Ellison and T. S. Zwier, J. Phys. Chem. Lett., 2014, 5, 2201–2207 CrossRef CAS PubMed
.
- A. Karton and D. Talbi, Chem. Phys., 2014, 436, 22–28 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc06919e |
| ‡ Diol 4 can exist in two different stereoisomeric forms, namely E and Z, with the latter being more stable by about 20 kJ mol−1.47 The Z isomer possesses two configurations, the preferred (syn, anti) form and the higher energetic (anti, anti) conformer. |
| § Equilibrium rotational constants obtained using the CCSD(T)/CBS+CV composite scheme (see ESI†) and augmented by vibrational corrections computed at the fc-MP2/cc-pVTZ level of theory; quartic and sextic centrifugal distortion constants obtained from anharmonic force field calculations at the fc-MP2/cc-pVTZ level. |
| This journal is © The Royal Society of Chemistry 2022 |