Preparation and characterization of nanofunctionalized alginate/methacrylated gelatin hybrid hydrogels†
R. Kadri‡
a,
G. Ben Messaoud‡a,
A. Tamayolbc,
B. Aliakbariande,
H. Y. Zhangf,
M. Hasana,
L. Sánchez-Gonzáleza and
E. Arab-Tehrany*a
aLaboratoire d'ingénierie des biomolécules (LIBio), ENSAIA-Université de Lorraine, 2 avenue de la forêt de Haye, TSA 40602, 54518 Vandoeuvre-lès-Nancy Cedex, France. E-mail: elmira.arab-tehrany@univ-lorraine.fr; Fax: +33 3 83 58 57 72; Tel: +33 3 83 58 5 77
bBiomaterials Innovation Research Center, Division of Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston 02139, MA, USA
cHarvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge 02139, MA, USA
dDepartment of Civil, Chemical and Environmental Engineering (DICCA), University of Genoa, Genoa, Italy
eResearch Center for Biologically Inspired Engineering in Vascular Medicine and Longevity (BELONG), Genoa, Italy
fUniversité de Lorraine, Institut Jean Lamour UMR 7198 CNRS, Ecole des Mines, Parc de Saurupt, CS 14234, 54042 Nancy, France
First published on 10th March 2016
Abstract
Curcumin has been shown to be effective in many biomedical processes. However, the functionalization of hydrogel constructs with curcumin has been challenging due to its low water solubility. Here, we developed the preparation of alginate/methacrylated gelatin (GelMA) hybrid hydrogels functionalized with nanoliposomes encapsulating curcumin. Physicochemical properties of the polymer solutions and resulting hydrogels were studied. The engineered systems showed great rheological and swelling properties. The release behavior of curcumin-loaded nanoliposomes demonstrated their suitability as a drug delivery system. These novel biomaterial systems could be an excellent candidate for tissue engineering applications.
1. Introduction
Hydrogels are three-dimensional polymeric networks with the capability of immobilizing high amounts of water or aqueous solutions.1 These biomaterials possess interesting characteristics like mechanical flexibility and softness, the ability to transport small compounds and molecules, ease of functionalization, biocompatibility, and facile controllability of their microstructure.Due to these attractive properties, hydrogels have been widely used in a wide spectrum of applications in food,2 nutraceutical systems,3 environment and water treatment,4 energy storage5,6 and biomedical applications.7
The latter category covers several domains such as tissue engineering,8,9 drug delivery,10 and microfluidics.11
Hydrogels could be prepared from natural and synthetic polymers or their mixtures. Synthetic polymers offer stronger mechanical properties as well as controllable in term of gelation, structure, and degradation. In contrast, natural polymers offer better biological properties and biocompatibility.12
Among natural polysaccharides, alginate a natural polysaccharide extracted from algae that has been widely used in the literature for fabrication of hydrogel constructs.13 It is found to be mucoadhesive and non-immunogenic with having low toxicity.14 Alginate is composed of two uronic acid monomers attached by glycosidic bonds: (1–4)-linked β-D-mannuronic acid (M unit) and C-5 epimer α-L-guluronic acid (G unit). These uronic acid units are distributed along the polymer chain in a pattern of blocks, consisting of homopolymeric blocks (G and M-blocks) and heteropolymeric blocks with alternating sequences of M and G residues (MG-blocks).14 The proportion and the sequential distribution of the M and G residues is closely related to the origin source of alginate.
Alginate can be reversibly crosslinked by the complexation of the G subunits with divalent cations such as calcium. MG blocks also participate by forming weaker junctions.14 Alginate hydrogels involve electrostatic, van der Waals forces and hydrogen interactions.15 However, alginate lacks cell binding sites, which makes it use for 3D cell encapsulation challenging.
Gelatin is a denatured form of collagen, which is cytocompatible and possesses bioactive sequences such as Arg-Gly-Asp (RGD).16,17 GelMA is a gelatin derivative produced by the substitution of the free amino groups with methacrylate anhydride.18 It is photocrosslinkable and has been widely used for various tissue engineering applications.17 It has been shown that the preparation of interpenetrative polymer networks (IPN) of alginate and gelatin-based materials allows the fabrication of sophisticated architectures with tunable mechanical characteristics and excellent cellular response.19
In fact semi-IPN hydrogels were developed by mixing alginate and gelatin followed by alginate reticulation with calcium cations.20 Full IPN hydrogels were also prepared by mixing the two polymers followed by alginate gelation with divalent cations and gelatin chemical reticulation using crosslinking agent such as carbodiimide.21
Moreover, composite materials were also synthetized by improving the chemical affinity of alginate to gelatin by chemical oxidation of the polysaccharide.22–24
However in the best of our knowledge, there is no enough information about the preparation of semi light-polymerizable IPN hydrogels based on GelMA and alginate.
A key challenge in utilization of hydrogels is the inflammatory response of host immune cells. Thus, the functionalization of hydrogels with immune modulating compounds could be a key step towards their in vivo utilization. Curcumin is a natural compound that has been extensively studied and possesses immune modulatory properties. However, its hydrophobic nature limits its use in functionalization of hydrogel constructs.25,26
Nanoliposomes are soft natural nanoparticles well known for their efficiency in the delivery of drugs and genes and in biomedical applications.27 They are negatively charged spherical vesicles, composed of one or more lipid bilayer. Their amphiphilic characteristic give them the ability of self-sealing, isolating an intravesicular medium in the center and preventing its contact with the external environment.28 Thus, liposomes has been emerged as an excellent carrier for increasing the bioavailability of hydrophobic compounds including curcumin. The aim of this work is to functionalize alginate/GelMA hydrogels with curcumin-loaded liposomes and to investigate their effect on the physicochemical properties of the polymers solutions as well as the obtained hydrogels.
2. Results and discussion
2.1. Solutions characterization
Zeta potential and surface tension of alginate, GelMA and alginate/GelMA mixture were determined before and after their functionalization with liposomes (Table 1). Zeta potential measurements were carried out at body temperature of 37 °C. Alginate is known to be negatively charged due to its carboxylic groups (−82.4 mV). After the addition of liposomes, the charge value of the polymer was still negative (−73.9 mV) because the added soft nanoparticles are negatively charged (−3.4 mV). Zeta potential of GelMA was around −1.6 mV at pH 5. At this pH value, the zeta potential of gelatin is normally positive, however the methacrylation of the protein, which is achieved by the reaction of the free amino groups of gelatin with the methacrylate anhydride, could reduce the number of free amino groups and decrease therefore the charge of GelMA. The small quantity of the added liposomes did not have a significant effect on the total charge of GelMA (−1.3 mV). This observation could be related to the low quantity of liposomes, which did not affect the total charge of the solution or to potential interactions between the liposomes and NH3+ free amino-groups of the GelMA leading to the neutralization of Lip charge. Alginate–GelMA mixture showed a less negative zeta potential compared to alginate solution (−21.9 mV). This variation was expected as GelMA's charge is less negative than alginate and the electrostatic interaction between the positive parts of the amphoteric GelMA with the negatively charged alginate can further reduce the solution charge. The addition of liposomes did not affect significantly the zeta potential of the mixture.| Solutions | Zeta potential (mV) | Surface tension (mN m−1) |
|---|---|---|
| a a,b,c,dDifferent letters in the same column indicate significant differences among polymer solutions (p < 0.05). | ||
| Alginate | −82.4 ± 9.3a | 53.1 ± 0.6a |
| Alginate + liposomes | −73.9 ± 3.9a | 35.9 ± 0.4b |
| GelMA | −1.6 ± 0.5b | 44.4 ± 0.9c |
| GelMA + liposomes | −1.3 ± 0.1b | 45.1 ± 0.9c |
| Alginate/GelMA | −21.9 ± 2.5c | 45.0 ± 1.4c |
| Alginate/GelMA + liposomes | −21.1 ± 1.7c | 40.2 ± 0.6d |
The surface tension is an important parameter in the response of cells encapsulated within hydrogels. Determination of the surface tension using the Wilhelmy plate remains the most reliable because it reduces the errors due to the viscous forces effects.29 The pure alginate showed the highest surface tension of 53.1 ± 0.6 mN m−1.
The addition of liposomes to the alginate solution decreased the surface tension 1.4 times. In fact, liposomes are rigid due to the low quantity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds as well as the presence of saturated fatty acids and fatty acid mono unsaturated. Moreover, liposomes are amphiphilic surfactants, which lower the surface tension.30 Although added in small amount in the polymer, they decreased the surface tension of the solution. Contrarily, the addition of liposomes to the GelMA solution did not have significant effect on its surface tension. GelMA contains polar and nonpolar regions which allowed a better diffusion of the amphiphilic liposomes in the solution.31,32 The mixture of the two polymers showed values tending towards the GelMA surface tension. Moreover, the impact of the liposomes on the surface of the mixture is significant and a diminution from 45 mN m−1 to 40 mN m−1 is observed after Lip incorporation.
C bonds as well as the presence of saturated fatty acids and fatty acid mono unsaturated. Moreover, liposomes are amphiphilic surfactants, which lower the surface tension.30 Although added in small amount in the polymer, they decreased the surface tension of the solution. Contrarily, the addition of liposomes to the GelMA solution did not have significant effect on its surface tension. GelMA contains polar and nonpolar regions which allowed a better diffusion of the amphiphilic liposomes in the solution.31,32 The mixture of the two polymers showed values tending towards the GelMA surface tension. Moreover, the impact of the liposomes on the surface of the mixture is significant and a diminution from 45 mN m−1 to 40 mN m−1 is observed after Lip incorporation.
2.2. Hydrogels characterization
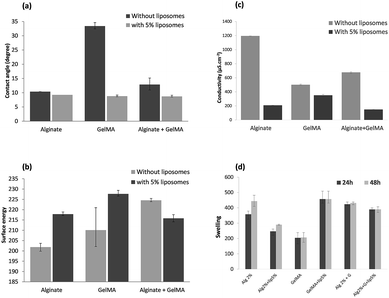
The physicochemical properties of the alginate, GelMA, and alginate/GelMA solutions with or without liposomes were also investigated. Fig. 1a shows the measured contact angle of alginate, GelMA and alginate/GelMA composite hydrogels.The obtained static water contact angle values were in the range of 8.7–33.4°, demonstrating the hydrophilicity the formed hydrogels.
Alginate showed a significantly lower contact angle (10°) than GelMA (33.4°) illustrating the high affinity of the polysaccharide to water molecules. In fact, alginate hydrogels can absorb high water content into the polymer network due to the formation of hydrogen bonds between the free carboxylic groups and water molecules. These free carboxylate groups were part of the alginate M-Blocks which did not interact with calcium cations during the gelation process.14 The difference of the contact angle between alginate and GelMA is related to the chemical nature of GelMA, which is an amphiphilic polymer with a lower affinity to water molecules than alginate. The negatively charged alginate ensured a more favorable surface for water molecules than the GelMA polymer.33,34 The wettability of GelMA hydrogel was improved by blending the polymer with alginate. The addition of liposomes affected the surface properties of the hydrogels. The presence of liposomes reduced the contact angle of the water droplet indicating an increase of the surface hydrophilic character of hydrogels. Liposomes are organized in bilayers with non-polar hydrophobic tails in the center to minimize the interaction with water molecules and polar hydrophilic heads exposed to the interior and exterior aqueous phases.35,36 This configuration could explain the decrease of the contact angle value after liposomes addition.
Although, the concentration of Lip in the hydrogel was low, their effect on the wettability of the hydrogels was significant.
The surface energy of the different hydrogels is shown in Fig. 1b. It is related to the contact angle as well as the chemical composition of the hydrogel surfaces. Pure GelMA showed a high surface energy in comparison to alginate due to the presence of amino groups. These groups increased the polarity of the hydrogel and thus enhanced the total surface energy. The surface energy of the functionalized hydrogels varied significantly compared to the pristine hydrogels. The polar head of liposomes increased the polar component on the surface of the hydrogel and improved its surface energy. It is remarkable that before the addition of liposomes, the IPN alginate/GelMA hydrogel had the highest surface energy showing that the mixture of the two polymers has improved the characteristics of the hydrogels. Contrarily, the addition of liposomes decreased the surface energy of the hydrogel. It may be due to the arbitrary position of liposomes in the matrix.
The electrical conductivity of the hydrogels is an important factor especially in cardiac, neural, and muscle tissue engineering applications. It improves the cell–cell signalling, which in turn affects cellular growth, viability, and function.37 The obtained results (Fig. 1c) showed an electrical conductivity in the range of 200 to 1200 μS cm−1. Even if the addition of liposomes decreased the conductivity of the hydrogels, the values remained interesting.
The swelling properties of the hydrogels were also measured and their capacity of swelling was determined. As shown in Fig. 1d the saturation of the hydrogels before and after functionalization occurs over a 24 h incubation period. The addition of liposomes increased the swelling of hydrogels, which is speculate to be caused by the formation of weak points within the polymeric network and exposing their hydrophilic structures. The swelling of alginate hydrogel causes an expansion of the chain leading to a release of the entrapped liposomes and a decrease of the hydrogel weight. In GelMA, the interactions between the negatively charged liposomes and positively charged amino groups of GelMA decreased the release of the hydrophilic liposomes. The IPN hydrogels had a higher swelling ratio than pristine GelMA due to the presence of the carboxylate groups in alginate which enhanced the hydrophilic character of the hydrogel. The functionalization of the IPN hydrogels showed a low release of liposomes due to potential electrostatic interaction between GelMA and nanoparticles.
In order to evaluate the microstructure of the hydrogels before and after liposomes addition, SEM images were captured and are shown in Fig. 2. The SEM images demonstrated a significant differences of the hydrogels surface microstructure before and after liposomes incorporation. In fact, the addition of nanoparticles converted the rough surface of alginate into a surface dominated by porous microstructures. The appearance of pores confirmed the presence of liposomes on the surface after gelation of alginate. While for GelMA which initially showed heterogeneous pores, the addition of liposomes increased their size and enhanced their distribution on the surface area of the hydrogel. This confirmed the important role of liposomes on tuning and control of the pore size and distribution in GelMA hydrogel. For alginate/GelMA mixture, the addition of liposomes in hydrogels resulted in a highly porous surface with more regular and well defined pores on the surface, giving more advantages in term of porosity to the hydrogel.
It is interesting to note that pore sizes were higher for alginate and IPN alginate/GelMA than for GelMA hydrogels after nanofunctionalization. This observation could be related to the preparation process. In fact, during the preparation of alginate and IPN hydrogels the addition of divalent calcium cations may cause the aggregation of liposomes and their potential fusion which results in large pore size.
The properties of the prepared hydrogels for drug delivery were tested following the release profiles in water of drug model molecules entrapped within the hydrogels.
Relative amounts of released curcumin-loaded liposomes from alginate, GelMA and alginate–GelMA hydrogels versus time elapsed after biomaterials preparation in water at 37 °C is plotted in Fig. 2b.
Curcumin release from the different hydrogels showed significant differences. In fact, the total amount of curcumin released after 48 hours as well as the rate of release varied significantly. For alginate hydrogel, the release rate of curcumin increased quickly and achieved an equilibrium value of 40% after a time slot of 8 hours. However for GelMA hydrogel, the equilibrium was achieved between 8 and 24 hours. Concerning the IPN hydrogel, the diffusion equilibrium was not achieved after 48 hours.
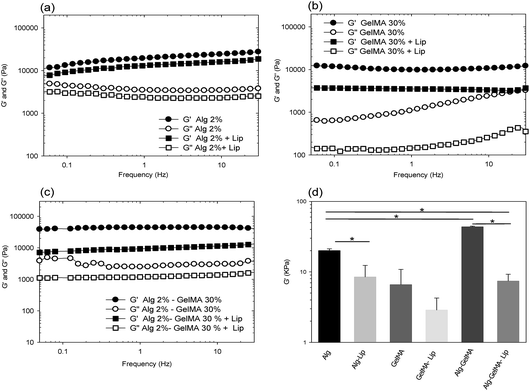
Dynamic shear oscillation measurements were used to characterize the mechanical properties of the crosslinked hydrogels and the influence of liposomes incorporation on the viscoelastic properties of alginate, GelMA and IPN hydrogels at 37 °C.
Fig. 3a–c displayed the average elastic and viscous moduli of the prepared hydrogels over a frequency range of 0.05–30 Hz. The elastic modulus G′ represents the elastic part, the loss modulus G′′ represents the viscous part of a material.
One distinctive feature of all mechanical spectra is that G′ ≫ G′′, confirming that alginate, GelMA and IPN materials have predominantly elastic rather than viscous character. This criterion distinguishes gels from viscous liquids and specifies that the deformation energy is recovered in the elastic stretching of chemical bonds.38
The small increase of alginate gels' storage modulus with frequency (Fig. 3a) indicated the existence of relaxation processes, which could be induced by intermolecular junctions opening.39 These junctions resulted from the coordination of Ca2+ cations to the alginate's interchain cavities, resulting in development of a so-called ‘egg-box’.40 In general, the storage modulus of an alginate hydrogel is related to the number of cross-links and length and stiffness of the chains between cross-links.
The addition of liposomes reduced significantly the viscoelasticity of the alginate hydrogels. In fact, the presence of Lip could affect the activity of Ca2+ during alginate gelation due to potential electrostatic interaction between the divalent cations and the negatively charged phospholipids.41 The presence of liposomes and potentially fused or aggregated liposomes (due to calcium cations) with a large size could occasion spatial hindrance during crosslinking and consequently decreases G′.42
For GelMA hydrogels, the storage modulus was higher than the loss modulus (Fig. 3b). This demonstrated that they were elastomeric materials at 37 °C. G′ was reasonably constant with increasing frequency. In general, the mechanical stability of GelMa gels results from both chemical cross-linking and physical structuring.18
However, at 37 °C GelMA hydrogels structure is maintained essentially by the chemical crosslinking via C–C bond between GelMA macromolecules.
The addition of liposomes decreased but not significantly the elastic modulus of GelMA hydrogels which could be associated to the soft nature of the liposomes membrane.43,44
The mechanical spectrum of alginate/GelMA IPN hydrogel (Fig. 3c) showed the highest G′ and G′′ with no significant frequency dependence. G′ of the IPN gel is higher than G′ of pure alginate and GelMA hydrogels.
However, the incorporation of Lip results in a significant decrease of the G′ and G′′. This observation could be directly related to the preparation process of the alginate/GelMA hydrogel.
In fact, the gelation procedure of the mixture starts by the preparation of a semi-IPN by alginate gelation with calcium followed by UV exposure. Therefore, during alginate reticulation, the calcium cations could interact with liposomes and resulted in fused liposomes with larger size which will interfere not only with the formation of alginate gel (as shown in Fig. 2a) but also with GelMA gelation. Therefore, the control of gelation conditions and liposomes amount is necessary for a better modulation of IPN-Lip final mechanical properties. For a better comparison between the different systems, the elastic modulus G′ at 1 Hz of the studied hydrogels is plotted in Fig. 3d. The IPN alginate/GelMA hydrogel showed the highest mechanical stability compared to alginate and GelMA hydrogel, however the incorporation of liposomes in the IPN hydrogels decreased significantly the mechanical strength of the final matrix. Therefore, a study of the influence of liposomes concentration is essential to modulate the final physicochemical properties of the IPN hydrogels.
3. Conclusions
In summary, we successfully synthesized IPN hydrogels based on alginate and GelMA polymers. The obtained matrix was functionalized with liposomes which were used as nanovehicles to encapsulate and transport curcumin. Finally, the association between GelMA and alginate might be a promising matrix in tissue engineering. Nevertheless, the efficiency of the obtained IPN hydrogels should be evaluated for mouse or human cells and properties like cells attachment, adhesion and proliferation must be investigated.Acknowledgements
We are grateful to the European Commission for Erasmus Mundus Grant to R. Kadri (Erasmus Mundus External Window “ELEMENT” Program). The ANRT French agency (project No. 312/2012) is also acknowledged for financial support of G. Ben Messaoud.Notes and references
- M. Hamidi, A. Azadi and P. Rafiei, Adv. Drug Delivery Rev., 2008, 60, 1638–1649 CrossRef CAS PubMed.
- S. Farris, K. M. Schaich, L. Liu, L. Piergiovanni and K. L. Yam, Trends Food Sci. Technol., 2009, 20, 316–332 CrossRef CAS.
- L. Chen, G. E. Remondetto and M. Subirade, Trends Food Sci. Technol., 2006, 17, 272–283 CrossRef CAS.
- G. Crini, Prog. Polym. Sci., 2005, 30, 38–70 CrossRef CAS.
- Y. Xu, Z. Lin, X. Huang, Y. Wang, Y. Huang and X. Duan, Adv. Mater., 2013, 25, 5779–5784 CrossRef CAS PubMed.
- X.-L. Wu and A.-W. Xu, J. Mater. Chem. A, 2014, 2, 4852–4864 CAS.
- R. Langer and D. A. Tirrell, Nature, 2004, 428, 487–492 CrossRef CAS PubMed.
- M. P. Lutolf and J. A. Hubbell, Nat. Biotechnol., 2005, 23, 47–55 CrossRef CAS PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- N. A. Peppas, Curr. Opin. Colloid Interface Sci., 1997, 2, 531–537 CrossRef CAS.
- D. J. Beebe, J. S. Moore, J. M. Bauer, Q. Yu, R. H. Liu, C. Devadoss and B.-H. Jo, Nature, 2000, 404, 588–590 CrossRef CAS PubMed.
- N. Annabi, A. Tamayol, J. A. Uquillas, M. Akbari, L. E. Bertassoni, C. Cha, G. Camci-Unal, M. R. Dokmeci, N. A. Peppas and A. Khademhosseini, Adv. Mater., 2014, 26, 85–124 CrossRef CAS PubMed.
- S. Dumitriu, Polymeric Biomaterials, Revised and Expanded, CRC Press, 2001 Search PubMed.
- S. N. Pawar and K. J. Edgar, Biomaterials, 2012, 33, 3279–3305 CrossRef CAS PubMed.
- I. Braccini and S. Pérez, Biomacromolecules, 2001, 2, 1089–1096 CrossRef CAS PubMed.
- A. Barbetta, M. Dentini, E. M. Zannoni and M. E. De Stefano, Langmuir, 2005, 21, 12333–12341 CrossRef CAS PubMed.
- J. W. Nichol, S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar and A. Khademhosseini, Biomaterials, 2010, 31, 5536–5544 CrossRef CAS PubMed.
- A. I. Van Den Bulcke, B. Bogdanov, N. De Rooze, E. H. Schacht, M. Cornelissen and H. Berghmans, Biomacromolecules, 2000, 1, 31–38 CrossRef CAS PubMed.
- A. Tamayol, A. H. Najafabadi, B. Aliakbarian, E. Arab-Tehrany, M. Akbari, N. Annabi, D. Juncker and A. Khademhosseini, Adv. Healthcare Mater., 2015, 4, 2146–2153 CrossRef CAS PubMed.
- B. Duan, L. A. Hockaday, K. H. Kang and J. T. Butcher, J. Biomed. Mater. Res., Part A, 2013, 101, 1255–1264 CrossRef PubMed.
- Y. Luo, A. Lode, A. R. Akkineni and M. Gelinsky, RSC Adv., 2015, 5, 43480–43488 RSC.
- B. Sarker, D. G. Papageorgiou, R. Silva, T. Zehnder, F. Gul-E-Noor, M. Bertmer, J. Kaschta, K. Chrissafis, R. Detsch and A. R. Boccaccini, J. Mater. Chem. B, 2014, 2, 1470–1482 RSC.
- B. Balakrishnan, M. Mohanty, P. R. Umashankar and A. Jayakrishnan, Biomaterials, 2005, 26, 6335–6342 CrossRef CAS PubMed.
- S. Sakai, S. Yamaguchi, T. Takei and K. Kawakami, Biomacromolecules, 2008, 9, 2036–2041 CrossRef CAS PubMed.
- C. Wang, N. T. Flynn and R. Langer, Adv. Mater., 2004, 16, 1074–1079 CrossRef CAS.
- N. S. Satarkar, D. Biswal and J. Z. Hilt, Soft Matter, 2010, 6, 2364–2371 RSC.
- M. R. Díaz and P. E. Vivas-Mejia, Pharmaceuticals, 2013, 6, 1361–1380 CrossRef PubMed.
- M. Hasan, N. Belhaj, H. Benachour, M. Barberi-Heyob, C. J. F. Kahn, E. Jabbari, M. Linder and E. Arab-Tehrany, Int. J. Pharm., 2014, 461, 519–528 CrossRef CAS PubMed.
- B.-B. Lee, E.-S. Chan, P. Ravindra and T. A. Khan, Polym. Bull., 2012, 69, 471–489 CrossRef CAS.
- M. Kaci, S. Meziani, E. Arab-Tehrany, G. Gillet, I. Desjardins-Lavisse and S. Desobry, Ultrason. Sonochem., 2014, 21, 1010–1017 CrossRef CAS PubMed.
- G. O. Phillips and P. A. Williams, Handbook of Hydrocolloids, Elsevier, 2009 Search PubMed.
- M. Sachithanadam and S. C. Joshi, J. Mater. Sci., 2013, 49, 163–179 CrossRef.
- S. Yamanlar, S. Sant, T. Boudou, C. Picart and A. Khademhosseini, Biomaterials, 2011, 32, 5590–5599 CrossRef CAS PubMed.
- M. Kolasińska and P. Warszyński, Appl. Surf. Sci., 2005, 252, 759–765 CrossRef.
- A. D. Bangham, Nature, 1961, 192, 1197–1198 CrossRef CAS PubMed.
- L. Bouarab, B. Maherani, A. Kheirolomoom, M. Hasan, B. Aliakbarian, M. Linder and E. Arab-Tehrany, Colloids Surf., B, 2014, 115, 197–204 CrossRef CAS PubMed.
- A. Shahini, M. Yazdimamaghani, K. J. Walker, M. A. Eastman, H. Hatami-Marbini, B. J. Smith, J. L. Ricci, S. V. Madihally, D. Vashaee and L. Tayebi, Int. J. Nanomed., 2013, 9, 167–181 Search PubMed.
- J. C. Stendahl, M. S. Rao, M. O. Guler and S. I. Stupp, Adv. Funct. Mater., 2006, 16, 499–508 CrossRef CAS.
- G. Ben Messaoud, L. Sánchez-González, L. Probst and S. Desobry, J. Colloid Interface Sci., 2016, 469, 120–128 CrossRef CAS PubMed.
- G. T. Grant, E. R. Morris, D. A. Rees, P. J. Smith and D. Thom, FEBS Lett., 1973, 32, 195–198 CrossRef CAS.
- D. Papahadjopoulos, S. Nir and N. Düzgünes, J. Bioenerg. Biomembr., 1990, 22, 157–179 CrossRef CAS PubMed.
- Y. Liu, Z. Li and D. Liang, Soft Matter, 2012, 8, 4517–4523 RSC.
- G. Grassi, A. Crevatin, R. Farra, G. Guarnieri, A. Pascotto, B. Rehimers, R. Lapasin and M. Grassi, J. Colloid Interface Sci., 2006, 301, 282–290 CrossRef CAS PubMed.
- S. Mourtas, S. Fotopoulou, S. Duraj, V. Sfika, C. Tsakiroglou and S. G. Antimisiaris, Colloids Surf., B, 2007, 55, 212–221 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03699f |
| ‡ R. Kadri and G. Ben Messaoud contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |