Preparation and characterization of enzyme-responsive emamectin benzoate microcapsules based on a copolymer matrix of silica–epichlorohydrin–carboxymethylcellulose
Mingcheng Guo,
Wenbing Zhang,
Guanglong Ding,
Dong Guo,
Juanli Zhu,
Baitao Wang,
Darunee Punyapitak and
Yongsong Cao*
College of Agriculture and Biotechnology, China Agricultural University, No. 2 Yuanmingyuan West Road, Beijing, China 100193. E-mail: caoysong@126.com; caoys@cau.edu.cn; Fax: +86-10-62734302; Tel: +86-10-62734302
First published on 16th October 2015
Abstract
Controlled release formulations of pesticides is highly desirable for maximising the utilization of the pesticide, as well as remarkably reducing environmental pollution. A stimuli-responsive controlled release formulation can intelligently respond to the stimuli produced by pests and trigger the release of the active ingredients to control pests effectively. In this work, a novel enzyme-responsive emamectin benzoate microcapsule was prepared using silica cross-linked with carboxymethylcellulose using epichlorohydrin. The results showed that the obtained microcapsules had a remarkable loading ability for emamectin benzoate (about 35% w/w) and could protect emamectin benzoate against photo- and thermal degradation effectively. The silica–epichlorohydrin–carboxymethylcellulose microcapsules displayed excellent cellulase stimuli-responsive properties and a sustained insecticidal efficacy against Myzus persicae. Allium cepa chromosome aberration assays demonstrated that the microcapsules had less genotoxicity than the technical grade emamectin benzoate (referred to throughout the manuscript as the technical). Given these advantages, the enzyme-responsive emamectin benzoate microcapsules are worth extending as a novel safe strategy for sustainable crop protection.
1. Introduction
Controlled release technology is defined as a system in which the concentration of an active ingredient is preset, has sustained release properties and thus achieves the desired results.1–5 It has increasingly gained scientific and commercial interest worldwide over the last few decades and has been identified as an emerging technology which is widely applied in the fields of medicine, coatings, pesticides, cosmetics, environmental engineering and food.6–11 The controlled release formulation of pesticides is highly desirable for maximising the utilization of the pesticide, as well as remarkably reducing environmental pollution.12–15 Due to the conservation and selective permeation properties of semi-permeable membranes, the use of pesticide microcapsules has been widely applied.16–20 However, the active ingredient release of the existing pesticide microcapsules occurs typically through passive diffusion release, erosion of the capsule wall, or active diffusion via osmotic pressure, which results in poor control of the release of the core materials. Therefore, the development of new and advanced stimuli-responsive pesticide microcapsules with intelligently controlled release by internal and external stimuli has broad prospects.21–26The wall materials of microcapsules often include organic polymers and inorganic molecules. Mesoporous silica materials are always ideal candidates as drug carriers because of their large surface area, high drug loading capability, easy preparation and surface modification, good stability and biocompatibility.27–31 Carboxymethylcellulose is an anionic linear polysaccharide derived from natural cellulose that has been widely used as a shell material for microcapsules due to its high biocompatibility, biofunctionality, and versatile chemical and physical properties.32–34 Currently, many new stimuli-responsive systems for drug release combining the advantages of silica nanoparticles and polymers have been built. Different kinds of stimuli-responsive polymers have been functionalized on the surfaces of mesoporous silica materials via direct covalent bonds35–38 or the method of assembly.39–42
Stimuli-responsive systems for “on-demand” release respond to a range of stimuli, including enzymes, pH, temperature, photoirradiation, redox, competitive binding, magnetic fields and electric fields.43–45 Among these stimuli, reduced pH and an increased level of cellulase are achieved when plants suffer from an insect pest which is already present or about to invade.23,46 An alkaline condition is obtained in insects with an alkaline gut.24 Despite many burgeoning achievements in human medicine, the application of stimuli-responsive systems in agriculture is still in its infancy. Therefore, the search for effective stimuli-responsive systems for pesticides with an “on-demand” release that, in particular, respond to internal biological stimuli still remains a big challenge in this field.
Emamectin benzoate is a broad-spectrum, high-efficiency, and low-toxicity macrocyclic lactone insecticide derived from avermectins, produced by Streptomyces avermitilis, that has been applied for the control of pests on a variety of crops worldwide.47 It is 18–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400-fold more potent against Plutella xylostella, Trichoplusia ni and Spodoptera exigua, than other traditional insecticides such as fipronil, chlorfenapyr, and tebufenozide.48 However, it is sensitive to light and strongly alkaline or strongly acidic conditions, so its biological activity is limited greatly in application.49
400-fold more potent against Plutella xylostella, Trichoplusia ni and Spodoptera exigua, than other traditional insecticides such as fipronil, chlorfenapyr, and tebufenozide.48 However, it is sensitive to light and strongly alkaline or strongly acidic conditions, so its biological activity is limited greatly in application.49
In the present work, novel functionalized emamectin benzoate microcapsules were prepared using silica cross-linked with carboxymethylcellulose using epichlorohydrin. Initially, amino-functionalized silica microcapsules were synthesized by an emulsion polymerization method using alkoxysilane as a precursor, while epichlorohydrin modified carboxymethylcellulose was synthesized. Finally, the epichlorohydrin modified carboxymethylcellulose was conjugated on the surface of the silica microcapsules, resulting in emamectin benzoate microcapsules based on a copolymer matrix of silica–epichlorohydrin–carboxymethylcellulose. The preparation conditions of the emamectin benzoate microcapsules, the effects of pH, temperature and cellulase on the sustained-release performance, stability of emamectin benzoate in the microcapsules, and the bioactivity and genotoxicity of the emamectin benzoate microcapsules were investigated.
2. Experimental
2.1 Materials
The model pesticide, emamectin benzoate (95%), was supplied by Hebei Veyong Bio-Chemical Co., Ltd. (Hebei, China). Emamectin benzoate 1% emulsifiable concentrate (EC) was purchased from Syngenta crop protection Co., Ltd. (Suzhou, China). Sodium carboxymethylcellulose (CMC), tetraethyl orthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES), ammonium hydroxide, hydrochloric acid, acetic acid, ethanol, ethyl acetate, epichlorohydrin, and hexadecyl trimethyl ammonium chloride (CTAC) were analytical chemicals purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Carbol fuchsin and cellulase from Trichoderma viride with an activity of 3–10 units per mg of solid were obtained from Sigma Aldrich (St. Louis, USA). Acetonitrile and methanol were of HPLC grade and purchased from J. T. Baker (USA). Deionized water was used for all reactions and treatment processes. Myzus persicae were supplied by the insect laboratory at China Agricultural University.2.2 Preparation of emamectin benzoate microcapsules
2.3 Characterization
2.4 High performance liquid chromatography (HPLC) analysis
The concentration of emamectin benzoate was determined by HPLC with an ultraviolet detector (Shimadzu, Japan). The HPLC separation of emamectin benzoate was carried out on a Kromasil ODS C18 column (250 mm × 4.6 mm, 5 μm; DIKMA, USA), which was equipped with a guard column (4 mm × 3 mm; Kromasil EasyGuard II C18) and pre-equilibrated with a mobile phase composition of acetonitrile and water with 0.1% acetic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) for 30 min. The flow rate was constant at 1 mL min−1 and the column temperature was at room temperature. The injection volume was 20 μL and the detection wavelength was 245 nm. All the solvents were filtered with a 0.45 μm membrane filter.
20, v/v) for 30 min. The flow rate was constant at 1 mL min−1 and the column temperature was at room temperature. The injection volume was 20 μL and the detection wavelength was 245 nm. All the solvents were filtered with a 0.45 μm membrane filter.
2.5 Stability test
20 g of the prepared emamectin benzoate microcapsule solution (2%, w/v) was packed in glass tubes and stored at 40, 50, and 60 °C for a period of 60 days, and then the changes of the emamectin benzoate were analyzed. The stability of the emamectin benzoate microcapsules against UV radiation was tested by exposing the samples to a 36 W germicidal lamp (254 nm) at a distance of 20 cm and keeping the temperature at room temperature, and then analyzing the changes of the emamectin benzoate. The methanol solution of the technical grade emamectin benzoate was used as the control sample at the same time.2.6 Controlled release kinetics
The resulting emamectin benzoate microcapsules were weighed and suspended in 500 mL of the methanol–water mixture (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v), which was used as a release medium in order to dissolve the emamectin benzoate, and incubated at a stirring speed of 100 rpm for a given time at room temperature. The mixture, collected at different intervals, was then centrifuged, and the supernatant was determined by using HPLC and the cumulative release rate of the emamectin benzoate from the microcapsules was calculated to evaluate the sustained release properties. The release behaviors of the emamectin benzoate microcapsules at different pH values, temperatures and cellulase conditions were investigated. In an enzyme experiment, 10 mg of emamectin benzoate microcapsules were suspended in 18.75 mL of the methanol–water mixture (5
70, v/v), which was used as a release medium in order to dissolve the emamectin benzoate, and incubated at a stirring speed of 100 rpm for a given time at room temperature. The mixture, collected at different intervals, was then centrifuged, and the supernatant was determined by using HPLC and the cumulative release rate of the emamectin benzoate from the microcapsules was calculated to evaluate the sustained release properties. The release behaviors of the emamectin benzoate microcapsules at different pH values, temperatures and cellulase conditions were investigated. In an enzyme experiment, 10 mg of emamectin benzoate microcapsules were suspended in 18.75 mL of the methanol–water mixture (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) at pH 7.0 and then 6.25 mL of the enzyme solution (0.4 g of enzyme in 100 mL of water at pH 7.0) was added. For the release studies of the microcapsules without enzyme, 10 mg of the microcapsules were placed in 25 mL of the methanol–water mixture (5
95, v/v) at pH 7.0 and then 6.25 mL of the enzyme solution (0.4 g of enzyme in 100 mL of water at pH 7.0) was added. For the release studies of the microcapsules without enzyme, 10 mg of the microcapsules were placed in 25 mL of the methanol–water mixture (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) at pH 7.0 and after a certain time an aliquot was separated, filtered and examined.
95, v/v) at pH 7.0 and after a certain time an aliquot was separated, filtered and examined.
2.7 Bioactivity
The bioactivity of the emamectin benzoate microcapsules against M. persicae was assayed using a modification of the pot test described by Lowery et al.50 An insecticide-susceptible strain of the green peach aphid (M. persicae) was maintained in the laboratory on Chinese cabbage (Brassica pekinensis (Lour) Rupr) at 25 °C, 60% relative humidity and an L16:D8 photoperiod. Groups of 100 adult aphids were inoculated on the leaves of each Chinese cabbage at the four-leaf stage in a cage (60 × 60 × 60 cm) covered with gauze on all sides. 10 mL of different concentrations of the microcapsules and emamectin benzoate 1% EC (3, 6, and 12 mg L−1 of emamectin benzoate) were sprayed on each Chinese cabbage with a microaerosol sprayer, and with water as the control. Each concentration was performed in triplicate. The decline rate of the insect density and control efficiency were calculated at 1, 7, 14, and 21 days after spraying.2.8 Allium cepa chromosome aberration assays
Allium cepa assays were carried out based on the protocol to evaluate the genotoxicity of chemicals proposed by Rank and Nielsen.51 A. cepa seeds were germinated in distilled water until the roots had reached a length of about 2 cm, then they were subjected to different concentrations of emamectin benzoate (10, 50, and 100 mg L−1), either free or encapsulated in the microcapsules, for periods of 24 h. Distilled water was used as the control. The roots were fixed using the ethanol–acetic acid mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After fixing, the samples were washed in distilled water and then immersed in a solution of 1 M HCl at 60 °C for 9 min. The meristematic region of the root tip was cut, placed on a slide together with a drop of carbol fuchsin, and covered with a cover slip which was used to gently squash and spread the cells. About 500 cells were observed on each slide under an optical microscope to determine the mitotic index, which considers the proportion of cells in division, and the chromosomal aberration index, which considers the proportion of divisions with chromosomal aberrations. Each treatment was performed in triplicate.
1, v/v). After fixing, the samples were washed in distilled water and then immersed in a solution of 1 M HCl at 60 °C for 9 min. The meristematic region of the root tip was cut, placed on a slide together with a drop of carbol fuchsin, and covered with a cover slip which was used to gently squash and spread the cells. About 500 cells were observed on each slide under an optical microscope to determine the mitotic index, which considers the proportion of cells in division, and the chromosomal aberration index, which considers the proportion of divisions with chromosomal aberrations. Each treatment was performed in triplicate.
3. Results and discussion
3.1 Preparation of emamectin benzoate microcapsules
In this work, novel functionalized emamectin benzoate microcapsules were prepared using silica cross-linked with carboxymethylcellulose using epichlorohydrin. Emamectin benzoate was inserted into the silica shell formed by the hydrolysis of TEOS using the emulsion polymerization method. Silica shells were modified with APTES to obtain amino-functionalized silica microcapsules. Meanwhile, EMC was synthesized. Finally, EMC was mixed and cross-linked with the resulting amino-functionalized silica microcapsules. The formation mechanism of the emamectin benzoate microcapsules is presented in Scheme 1. | ||
| Scheme 1 The possible mechanism for the preparation of the silica–epichlorohydrin–carboxymethylcellulose microcapsules. | ||
3.2 Characterization of emamectin benzoate microcapsules
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the ester at about 1748 cm−1 compared with sodium carboxymethylcellulose, demonstrating that the oxirane group of epichlorohydrin successfully reacted with the carboxyl of the carboxymethylcellulose (Fig. 3c). The silica–epichlorohydrin–carboxymethylcellulose exhibited the absorption band of the methylene group (–CH2–) at 2985 cm−1 and the carbonyl group (C
O) of the ester at about 1748 cm−1 compared with sodium carboxymethylcellulose, demonstrating that the oxirane group of epichlorohydrin successfully reacted with the carboxyl of the carboxymethylcellulose (Fig. 3c). The silica–epichlorohydrin–carboxymethylcellulose exhibited the absorption band of the methylene group (–CH2–) at 2985 cm−1 and the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the ester at about 1748 cm−1. The absorption band of the amine (–NH2) at 3401 cm−1 was also replaced by a broad band of the carboxymethylcellulose at 3416 cm−1, and the intensity decrease of the absorption band at 1550 cm−1 (N–H bending of the primary amine salt) demonstrated that EMC was linked with the amino-functionalized silica (Fig. 3d).
O) of the ester at about 1748 cm−1. The absorption band of the amine (–NH2) at 3401 cm−1 was also replaced by a broad band of the carboxymethylcellulose at 3416 cm−1, and the intensity decrease of the absorption band at 1550 cm−1 (N–H bending of the primary amine salt) demonstrated that EMC was linked with the amino-functionalized silica (Fig. 3d).
3.3 Thermal and light stability
The prepared emamectin benzoate microcapsule solution was stored at 40, 50 and 60 °C for a period of 60 days to determine the effects of temperature variation on the stability of microcapsules. Fig. 5a shows that emamectin benzoate microcapsules are more stable than the technical under high temperatures. The decomposition rate of the technical at 40, 50 and 60 °C over 60 days was higher than that of the microcapsules which exhibited less than 3% decomposition. Fig. 5b shows the effects of UV radiation on the stability of the emamectin benzoate microcapsules at pH 7 and 25 °C. Emamectin benzoate was sensitive to light, and the irradiated technical samples were degraded completely within 48 h. Nevertheless, the decomposition rate of the emamectin benzoate wrapped in microcapsules was found to be less than 25% after 72 h of UV radiation. These results evidently showed that emamectin benzoate could be protected by the microcapsule wall, demonstrating the excellent UV-shielding properties of the resulting microcapsules for emamectin benzoate.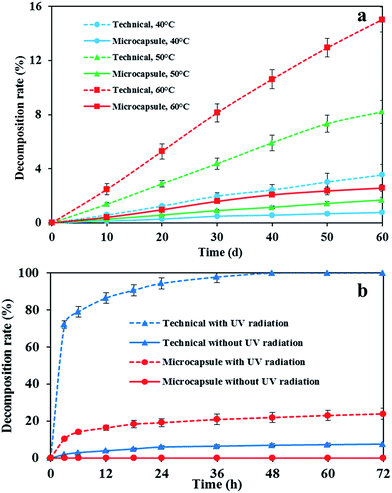 | ||
| Fig. 5 Stability of the resulting emamectin benzoate microcapsules and the technical affected by (a) temperature and (b) UV radiation . | ||
3.4 Controlled release kinetics
In order to develop the intelligent, self-regulated controlled release formulation of pesticides, the novel stimuli-responsive systems that respond to internal biological stimuli produced by pests were prepared using mesoporous silica materials cross-linked with natural polymers via direct covalent bonds. The stimuli-responsive microcapsules respond to the stimuli produced by pests and trigger the release of the active ingredients to control the pests effectively. The possible mechanism of formation of the enzyme-responsive microcapsules and triggered release by cellulase is schematically presented in Scheme 2. | ||
| Scheme 2 The possible mechanism of the formation of the enzyme-responsive microcapsules and triggered release by cellulase. | ||
3.5 Bioactivity
Fig. 7 shows the bioactivity of the emamectin benzoate microcapsules against M. persicae in concentrations ranging from 3–12 mg L−1. The results indicated that the control efficiency of emamectin benzoate 1% EC 1 day after spraying against M. persicae was better than that of the microcapsules at the same concentrations, while the control efficiency of the microcapsules against M. persicae was better than that of the emamectin benzoate 1% EC at the same concentrations since 7 days after spraying. That was probably because the stimuli, including pH and the cellulase produced by M. persicae, were weak at 1 day after spraying, and then the stimuli-responsive microcapsules, responding to the intensifying stimuli, released the active ingredient more and faster over time. The stimuli-responsive emamectin benzoate microcapsules exhibited a better and more sustained insecticidal efficacy against M. persicae than emamectin benzoate 1% EC.3.6 Allium cepa chromosome aberration assays
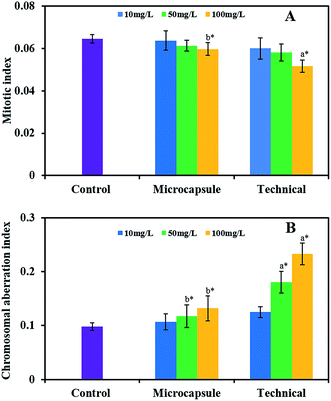
Allium cepa assays are widely used in the analysis of general genotoxicity and are sufficiently sensitive for monitoring purposes.51 Genotoxic compounds reduce the mitotic index of the A. cepa cells, as indicated by chromosome abnormalities that increase as a function of the concentration of the chemical. Fig. 8 shows the genotoxicity of the emamectin benzoate microcapsules against A. cepa at the concentrations of 10, 50, and 100 mg L−1.There was a decreased mitotic index for the microcapsules and the technical compared with the control. The mitotic index diminished as the concentration of the microcapsules and the technical increased, and the difference between the technical at a concentration of 100 mg L−1 and the control was statistically significant. The mitotic index of the microcapsules at a concentration of 100 mg L−1 was significantly higher than that of the technical at the same concentration, demonstrating that the microcapsules reduced the cytotoxicity of the technical (Fig. 8A). The chromosomal aberration index of the microcapsules and the technical was higher than that of the control. Higher concentrations of the microcapsules and the technical increased the chromosomal aberration index. Significantly greater index values were obtained for the treatments using the technical at concentrations of 50 and 100 mg L−1, compared to the control and the microcapsules at the same concentration, while the index values between the microcapsules and the control were similar, suggesting that the microcapsule wall provided a degree of protection against genotoxic effects (Fig. 8B).
4. Conclusions
In the present work, we prepared a novel enzyme-responsive emamectin benzoate microcapsule using silica cross-linked with carboxymethylcellulose using epichlorohydrin. The results showed that the resulting microcapsules had a remarkable loading ability for emamectin benzoate (about 35% w/w) and could protect emamectin benzoate against photo- and thermal degradation effectively. The silica–epichlorohydrin–carboxymethylcellulose microcapsules displayed excellent cellulase stimuli-responsive properties and a sustained insecticidal efficacy against M. persicae. A. cepa chromosome aberration assays demonstrated that the microcapsules caused less chromosome damage compared to the technical. It was concluded that the enzyme-responsive controlled release formulation provided a useful means of controlling agricultural pests, simultaneously reducing the risk of harm to the environment and human health.Acknowledgements
This work was supported by the National Natural Science Foundation of China (31471799, 31171888) and the National Department Public Benefit Research Foundation of China (201303031).Notes and references
- D. Davidson and F. X. Gu, J. Agric. Food Chem., 2012, 60, 870–876 CrossRef CAS PubMed.
- S. H. Kim, J. W. Kim, D. H. Kim, S. H. Han and D. A. Weitz, Small, 2013, 9, 124–131 CrossRef CAS PubMed.
- S. J. Holder, G. Woodward, B. McKenzie and N. A. Sommerdijk, RSC Adv., 2014, 4, 26354–26358 RSC.
- W. Zhang, S. He, Y. Liu, Q. Geng, G. Ding, M. Guo, Y. Deng, J. Zhu, J. Li and Y. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 11783–11790 CAS.
- O. I. Parisi, C. Morelli, L. Scrivano, M. S. Sinicropi, M. G. Cesario, S. Candamano, F. Puoci and D. Sisci, RSC Adv., 2015, 5, 65308–65315 RSC.
- U. R. Pothakamury and G. V. Barbosa-Cánovas, Trends Food Sci. Technol., 1995, 6, 397–406 CrossRef CAS.
- F. Siepmann, J. Siepmann, M. Walther, R. J. MacRae and R. Bodmeier, J. Controlled Release, 2008, 125, 1–15 CrossRef CAS PubMed.
- A. Popat, J. Liu, Q. Hu, M. Kennedy, B. Peters, G. Q. M. Lu and S. Z. Qiao, Nanoscale, 2012, 4, 970–975 RSC.
- Y. Rosiaux, S. Muschert, R. Chokshi, B. Leclercq, F. Siepmann and J. Siepmann, J. Controlled Release, 2013, 169, 1–9 CrossRef CAS PubMed.
- I. M. Martins, M. F. Barreiro, M. Coelho and A. E. Rodrigues, Chem. Eng. J., 2014, 245, 191–200 CrossRef CAS.
- E. Reguera-Nuñez, C. Roca, E. Hardy, M. de la Fuente, N. Csaba and M. Garcia-Fuentes, Biomaterials, 2014, 35, 2859–2867 CrossRef PubMed.
- N. F. Cardarelli, Controlled Release Pesticide Formulations, CRC Press, Cleveland, OH, 1976 Search PubMed.
- Y. Liu, Z. Tong and R. K. Prud’homme, Pest Manage. Sci., 2008, 64, 808–812 CrossRef CAS PubMed.
- F. J. Garrido-Herrera, I. Daza-Fernández, E. González-Pradas and M. Fernández-Pérez, J. Hazard. Mater., 2009, 168, 220–225 CrossRef CAS PubMed.
- H. Wanyika, J. Nanopart. Res., 2013, 15, 1–9 CrossRef.
- M. Gimeno, J. Environ. Sci. Health, Part B, 1996, 31, 407–420 CrossRef.
- S. A. Cryer and S. L. Wilson, J. Agric. Food Chem., 2009, 57, 5443–5451 CrossRef CAS PubMed.
- D. Q. Cai, L. H. Wang, G. L. Zhang, X. Zhang and Z. Y. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 9212–9216 CAS.
- D. B. Yang, N. Wang, X. J. Yan, J. Shi, M. Zhang, Z. Y. Wang and H. Z. Yuan, Colloids Surf., B, 2014, 114, 241–246 CrossRef CAS PubMed.
- H. Wu, N. Xue, C. L. Hou, J. T. Feng and X. Zhang, Food Chem., 2015, 175, 344–349 CrossRef CAS PubMed.
- D. K. Rodham, Curr. Opin. Colloid Interface Sci., 2000, 5, 280–287 CrossRef CAS.
- R. W. Dexter and B. E. Benoff, US Patent 6500447, 2002.
- J. E. van Koppenhagen, H. B. Scher, K. S. Lee, I. M. Shirley, P. Wade and R. Follows, US Patent 6514439, 2003.
- J. E. van Koppenhagen, H. B. Scher, K. S. Lee, I. M. Shirley, P. Wade and R. Follows, US Patent 6544540, 2003.
- C. Thies and S. Louis, US Patent 7192603, 2007.
- S. F. Peteu, F. Oancea, O. A. Sicuia, F. Constantinescu and S. Dinu, Polymer, 2010, 2, 229–251 CAS.
- Y. W. Yang, MedChemComm, 2011, 2, 1033–1049 RSC.
- Z. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart and J. I. Zink, Chem. Soc. Rev., 2012, 41, 2590–2605 RSC.
- N. Ž. Knežević, E. Ruiz-Hernández, W. E. Hennink and M. Vallet-Regí, RSC Adv., 2013, 3, 9584–9593 RSC.
- D. Tarn, D. P. Ferris, J. C. Barnes, M. W. Ambrogio, J. F. Stoddart and J. I. Zink, Nanoscale, 2014, 6, 3335–3343 RSC.
- S. K. Natarajan and S. Selvaraj, RSC Adv., 2014, 4, 14328–14334 RSC.
- D. Roy, M. Semsarilar, J. T. Guthrie and S. Perrier, Chem. Soc. Rev., 2009, 38, 2046–2064 RSC.
- J. Tripathy and A. M. Raichur, Colloids Surf., B, 2013, 101, 487–492 CrossRef CAS PubMed.
- P. Chitprasert and P. Sutaphanit, J. Agric. Food Chem., 2014, 62, 12641–12648 CrossRef CAS PubMed.
- A. Bernardos, L. Mondragon, E. Aznar, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, J. M. Barat, E. Pérez-Payá, C. Guillem and P. Amorós, ACS Nano, 2010, 4, 6353–6368 CrossRef CAS PubMed.
- W. R. Zhao, H. T. Zhang, Q. J. He, Y. S. Li, J. L. Gu, L. Li, H. Li and J. L. Shi, Chem. Commun., 2011, 47, 9459–9461 RSC.
- Y. Kotsuchibashi, M. Ebara, T. Aoyagi and R. Narain, Polym. Chem., 2012, 3, 2545–2550 RSC.
- B. S. Chang, D. Chen, Y. Wang, Y. Z. Chen, Y. F. Jiao, X. Y. Sha and W. L. Yang, Chem. Mater., 2013, 25, 574–585 CrossRef CAS.
- X. Mei, S. Yang, D. Y. Chen, N. J. Li, H. Li, Q. F. Xu, J. F. Ge and J. M. Lu, Chem. Commun., 2012, 48, 10010–10012 RSC.
- L. Xing, H. Q. Zheng, Y. Y. Cao and S. N. Che, Adv. Mater., 2012, 24, 6433–6437 CrossRef CAS PubMed.
- Q. Zhang, F. Liu, K. T. Nguyen, X. Ma, X. J. Wang, B. G. Xing and Y. L. Zhao, Adv. Funct. Mater., 2012, 22, 5144–5156 CrossRef CAS.
- Y. F. Sun, Y. L. Sun, L. Z. Wang, J. B. Ma, Y. W. Yang and H. Gao, Microporous Mesoporous Mater., 2014, 185, 245–253 CrossRef CAS.
- G. Sukhorukov, A. Fery and H. Möhwald, Prog. Polym. Sci., 2005, 30, 885–897 CrossRef CAS.
- A. P. Esser-Kahn, S. A. Odom, N. R. Sottos, S. R. White and J. S. Moore, Macromolecules, 2011, 44, 5539–5553 CrossRef CAS.
- J. Kost and R. Langer, Adv. Drug Delivery Rev., 2012, 64, 327–341 CrossRef.
- H. Watanabe and G. Tokuda, Annu. Rev. Entomol., 2010, 55, 609–632 CrossRef CAS PubMed.
- C. D. S. Tomlin, The Pesticides Manual: A World Compendium, British Crop Protection Council, Hampshire, U. K., 15th edn, 2009 Search PubMed.
- R. K. Jansson, R. Brown, B. Cartwright, D. Cox, D. M. Dunbar, R. A. Dybas, C. Eckel, J. A. Lasota, P. K. Mookerjee, J. A. Norton, R. F. Peterson, V. R. Starner and S. White, Emamectin benzoate: a novel avermectin derivative for control of lepidopterous pests, in Proceedings of the Third International Workshop, Kuala Lumpur, Malaysia, The management of diamondback moth and other crucifer pests, Cornell University, Ithaca, NY, USA, 1996 Search PubMed.
- S. F. Zhang, P. H. Chen, F. Zhang, Y. F. Yang, D. K. Liu and G. Wu, J. Agric. Food Chem., 2013, 61, 12219–12225 CrossRef CAS PubMed.
- D. T. Lowery, M. B. Isman and N. L. Brard, J. Econ. Entomol., 1993, 86, 864–870 CrossRef CAS.
- J. Rank and M. H. Nielsen, Hereditas, 1993, 118, 49–53 CrossRef CAS.
- K. P. Schneider, E. Wehrschuetz-Sigl, S. J. Eichhorn, A. Hasmann, T. Flock, F. Kaufmann, Y. Shyng and G. M. Guebitz, Process Biochem., 2012, 47, 305–311 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |