Microfluidic paper analytical device for the chromatographic separation of ascorbic acid and dopamine†
A. Murphy,
B. Gorey,
K. de Guzman,
N. Kelly,
E. P. Nesterenko and
A. Morrin*
National Centre for Sensor Research, School of Chemical Sciences, Dublin City University, Dublin 9, Ireland. E-mail: aoife.morrin@dcu.ie
First published on 23rd October 2015
Abstract
Cellulose-based filter papers were used as base materials to construct microfluidic paper-based analytical devices (μPADs) coupling a separation channel with electrochemical detection. Channel widths were defined by hydrophobic wax, and gold-sputtering through a mask was used to pattern an electrochemical cell at the end of the channel. The physical properties and surface chemistries of various filter papers were studied with respect to the separation of ascorbic acid (AA) and dopamine (DA). Both porosity as well as the ion-exchange capacity of the filter papers were found to influence the separation. Under the conditions used, Whatman grade P81 strong cation exchange paper based on cellulose phosphate was found to fully retain DA. Detection of both AA and DA was achieved on the other filter papers, however, different behaviours were observed. Whatman 4 could not resolve AA from DA while VWR 413 could achieve baseline separation under the conditions used. Depending on the level of oxidative treatment that they undergo, cellulose papers can have carboxyl groups present on the fibres that can act as sources of ion-exchange sites, thus making these types of papers potentially useful for ion-exchange separations. The ion-exchange capacities of the filter papers were investigated and quantified. It was shown that the ion-exchange properties of the papers evaluated varied dramatically. Furthermore, eluent ionic strength and pH were optimised to achieve a baseline resolution of AA and DA. The limit of detection of DA was 3.41 μM when analysed in the presence of 1 mM AA showing the potential of this μPAD for the detection of catecholamines in biological samples containing high concentrations of AA.
Introduction
Cellulose-based paper is a ubiquitous material that is produced by compacting moist fibres, often in the form of cellulose pulp derived from wood and dried into flexible sheets. The established large-scale paper manufacturing processes, coupled with paper's inherent wicking capability and versatility in terms of chemical and physical functionalisations, places it in an excellent position as a material for creating low-cost microfluidic-based analytical platforms. Since the discovery of paper chromatography in 1940s, cellulose-based supports became very popular in separation science for the field analysis, and to date they still are widely used. More recently, paper has been exploited as an excellent substrate for low-cost analytical devices such as sensors.1–3 As a microfluidic platform, paper obviates the need for a pump, and has been shown as a suitable substrate upon which to build an electrochemical sensor or detector. Today, these devices, known as microfluidic paper-based analytical devices (μPADs) utilise colorimetric,4 electrochemical,5 immunoassays and potentiometric ion sensing6 for the detection of analytes of interest. For example, one such μPAD integrated a paper-based immunoassay with a rapid visible-light-induced polymerisation to provide signal amplification for detection of plasmodium falciparum histidine-rich protein 2.4Research efforts in μPADs have recently expanded to focus on developing independent and multiplex separation functions, which will ultimately lead to the development of total analysis μPAD systems.7 Thus, Carvalhal et al. (2010) demonstrated chromatographic separation of uric and ascorbic acid on a Whatman grade 1 chromatography paper using differences in the solubilities of the two molecules.8 This group also demonstrated the separation of paracetamol and p-aminophenol on Whatman P81 strong cation exchange paper.9 Separation and enrichment of three metal complexes via an electrochromatographic separation has also been achieved using Whatman #1 filter paper.10 These μPADs were constructed by integrating paper with leading electrode wires attached onto the sampling areas, two fixed centrifuge tubes as reservoirs and direct current power. Moving chelation boundary (MCB) electrophoresis, partition chromatography and electroosmotic flow (EOF) were assigned as the main separation principles exploited here. Abbas et al.11 designed a novel, star-shaped multi-channel type configuration, again based on Whatman #1 filter paper. In this instance, each paper channel was coated with selected polyelectrolytes of different charges and concentrations to form a gradient of charges that controlled the direction and distance of migration of the analytes depending on their electrostatic interactions with the charged substrate. A paper-based device for electrophoretic separation of proteins was reported by Luo et al. (2014) who used a 3-dimensional μPAD based on Whatman grade 1 chromatography paper, characterised by high protein binding capability. Whatman grade 1 chromatography paper, functionalised with agglutinating antibodies has also been used for the separation of plasma from whole blood.12 Paper-based devices have also been integrated into electrochemical detection systems which utilize flow injection analysis. Lankelma et al.13 designed a device that detected glucose in urine and continuous flow of buffer in the device was achieved by capillary forces. This study was able to resolve interfering redox species by strategically immobilizing glucose oxidase on a working electrode downstream on a nitrocellulose pad.23 Another study by Kwong et al.14 modified paper-based microfluidic devices by depositing functional polymers onto the paper through vapor phase polymerization. This technique provided the device with the ability to separate analytes while maintaining the porosity necessary for capillary-force driven flow. It was demonstrated that a mixture could be separated into its anionic and cationic components. This study also demonstrated the ability of a UV-responsive polymer deposited on the paper to act as a “switch” to control the path of fluid flow.
Many of the devices reported use Whatman #1 filter or grade 1 chromatography papers (cellulose-based papers) that have specific flow rates and particle retention capabilities. However, there are many other products with various flow rates, as well as different manufacturers of papers such as VWR and Machery Nagel. The surface chemistries on different celluloses can differ greatly from manufacturer to manufacturer. For example, during the production and processing of cellulose materials, a range of chemical procedures (oxidation and hydrolysis) and structural modifications (degree of crystallinity and form of the unit cell) are applied in order to control reactivity and minimise degradation. The methods for processing cellulose can have a direct influence on its surface chemistry, as well as on fibrillar structure, i.e., if the cellulose structure is loosened, it can cause swelling of the polymer and increased accessibility of active groups.15 Modifications of the cellulose surface, arising during a manufacturing process or subsequent to it, will dramatically alter the interaction ability of the cellulose fibres with components of the liquid phase. These modifications can be targeted, but can also arise from treatments such as oxidation that can be used to strengthen fibres. It is necessary to have a good understanding of the chemistries of different cellulose papers, in order to judge the polymers' interaction ability in terms of for example, hydrophobic nature, or ion exchange capacity. This is vital for developing future analytical applications on paper.
This article reports a μPAD device fabricated via wax-printing. Here, various filter papers were investigated as μPAD substrates for the separation and detection of dopamine (DA) in the presence of ascorbic acid (AA). The concentration of DA in the brain extracellular fluid of healthy individuals is in the submicromolar range, but is lower for individuals suffering from certain neurological disorders. Moreover, DA and AA co-exist in extra-cellular fluid and have similar oxidation potentials, which can results in an overlap of their respective voltammetric responses.16,17 AA is also typically present in extracellular fluid at concentrations 1000 times higher than DA.20 In this regard, strategies such as that on the μPAD proposed here, that can quickly and selectively detect DA in the presence of high concentrations of AA are of considerable interest.
Materials and methods
Materials
L-Ascorbic acid (AA), dopamine hydrochloride (DA), potassium ferricyanide, murexide, EDTA, potassium chloride, sodium acetate, acetic acid, potassium phosphate monobasic and dibasic, potassium chloride, sodium acetate and calcium acetate were all purchased from Sigma Aldrich. Whatman 4 (Cat. no. 1004-110, 205 μm thickness, 92 g m−2 density) and Whatman 5 (Cat. no. 1005-110, 200 μm thickness, 98 g m−2 density) filter papers were purchased from GE Healthcare (UK). VWR 413 (Cat. no. 516-0816, 73 g m−2 density) was purchased from VWR International. P81 Whatman paper (typical ion-exchange capacity is 18.0 μeq cm−2, 230 μm thickness) was supplied by Prof. Kubota (Institute of Chemistry-UNICAMP, Campinas, Brazil).Instrumentation
Xerox ColorQube 8570 was used for all wax patterning. A Quorum Q150TS Sputter Coater was used for all gold deposition. All electrochemical measurements were performed with a CH Instrument electrochemical analyser (CH Instrument Model: CHI600C).Design and construction of the paper-based microfluidic device
Various standard cellulose filter papers from Whatman and VWR were used to construct the μPADs. Fluidic separation channels were fabricated by printing wax-based hydrophobic block patterns onto the filter paper with a spacing of 4 mm. Gold electrodes were sputtered at the end of the paper channel using a mask to define the areas of the working, reference and counter electrodes. The length of the working and reference electrodes were 2 mm and the length of the counter electrode was 8 mm. Once the electrodes were deposited, the paper device was then placed flat on a hotplate set at 100 °C for 60 s until the wax melted through the filter paper, defining the channel and electrode widths. After melting, the overall channel width and hence electrode widths decreased from 4 mm to approx. 3 mm due to the lateral melting of the wax from the channel sides.Electrochemical measurements
A voltammetric study was carried out using potassium ferricyanide (3 mM) in KCl (0.1 M). The base of the channel was placed into the Fe3+ solution. The solution was allowed to flow up the channel and over the electrodes. Once the solution passed the electrodes, a scan rate study was undertaken in the range of 0.01–0.5 V s−1 from +0.3 to −0.4 V vs. on-paper Au reference.Amperometric detection of AA and DA was carried out in buffer solutions (acetate/acetic acid pH 3.8 buffer unless otherwise specified) where the base of the channel (0.5 cm approx.) was placed in buffer solution. The buffer solution wicked up the channel and a potential of +0.3 V vs. on-paper Au reference was applied to the working electrode. Once the current at the working electrode had reached steady state, 0.5 μL of a solution containing AA and DA was applied 30 mm from the working electrode and the response over time was monitored. All analyses were performed in triplicate. The concentrations of both components of the sodium acetate/acetic acid buffer were 0.1 M for analysis at pH 3.8. Using an ionic strength formula, ionic strength was calculated to be 0.2 for the mixed pH 3.8 buffer. For the ionic strength study, the concentrations of the sodium acetate and acetic acid were increased to 0.25 M and decreased 0.05 M, leading to ionic strengths of 0.5 and 0.1 respectively while keeping pH constant.
Determination of the carboxyl group content of filter paper by complexometric titration
Approximately 1.0 g of VWR 413 and Whatman 4 filter papers were separately weighed into 250 mL glass-stopper flasks. 100 mL of a calcium acetate solution (0.1 M) was added to each flask. The flasks were shaken overnight and the filter paper fibres were removed from solution by filtration. 10 mL of each solution were transferred to a beaker where the indicator murexide was added. The pH of the solutions was adjusted to 12 using a solution of 0.1 M sodium hydroxide. 0.1 M EDTA was used as the titrating agent and a calcium acetate solution without filter paper was used as a blank. The end-point of the titration was observed visually by a colour change. The carboxyl group content of the two filter papers was calculated according to the following equation:| COOH (mmol kg−1) = (Veqb − Veqa) × C × 1000/m | (1) |
Results and discussion
Electrochemical characterisation of the μPAD
The constructed μPAD comprised a fluidic separation channel coupled with an electrochemical cell patterned at the end of the channel for detection of redox-active species eluting off the channel (Scheme 1). The μPAD is pump-free and therefore relies on capillary forces to induce fluid wicking up the channel. The electrochemical detector was positioned at the end of the channel and comprised of three Au electrodes. The first electrode along the channel is the working electrode. The pseudo reference and auxillary electrodes were positioned up-stream from the working electrode according to Scheme 1. Beyond the channel, excess paper served as a reservoir for waste eluent.In order to characterise the electrochemical cell, a voltammetric study was undertaken where a solution of K3[Fe(CN)6] was allowed to flow up the 30 mm channel and over the electrodes. The working electrode, comprising an Au-sputtered thin film on the cellulose fibres can be thought of as a porous, flow-through electrode, whose depth dimension is approximately the depth of the electrochemical cell. Reversible Fe2+/Fe3+ electrochemistry was observed in the region from −0.4 to +0.3 V vs. Au, where the ratio of anodic to cathodic peak current (ipa/ipc) ≈ 1 over scan rates from 0.001–0.5 V s−1. As expected, the paper-based gold reference electrode resulted in a negative shift of formal potential (approx. 0.165 V) when compared to use of a standard Ag/AgCl electrode18 (data not shown). Note: the formal potential is defined as the average potential of the redox peaks.
At higher scan rates (0.1–0.5 V s−1), ΔEp values were close to zero and the peak current was found to be proportional to υ (r2 = 0.998), indicating that the voltammetry is thin layer in behaviour and thus charge transfer limited (Fig. 1). At these higher scan rates, the diffusion layer thickness is small and is approximately equal to the depth of the porous flow-through electrode. At scan rates slower than 0.1 V s−1, ΔEp was at least 0.059 V and maximum peak current height was no longer linear with respect to υ. This change in behaviour at slower scan rates can be attributed to the build-up of a thicker diffusion layer. A thin adsorbed aqueous layer above the cellulose fibres may facilitate this. Alternatively, swelling of the cellulose polymer may cause complex effects that allow for the build-up of a diffusion layer over the Au electrode surface.
Characterisation of filter papers
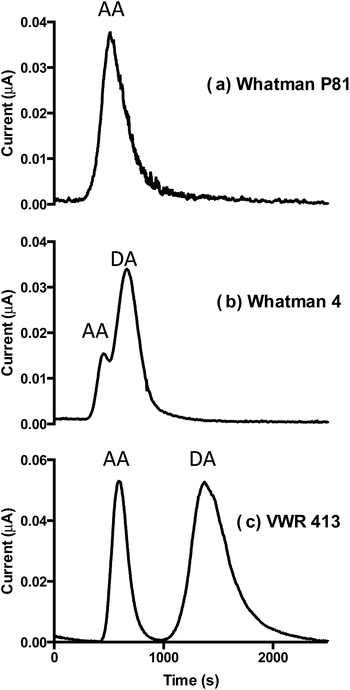
Various commercial cellulose filter papers were used as the base material to fabricate the microfluidic device. The cellulose paper device comprises the microfluidic channel (4 mm printed diameter) where fluid flow is driven by capillary forces. The cellulose filter papers selected were tested for their ability to separate AA (pKa 4.7) and DA (pKa 8.9). The physical properties of the different cellulose materials, as well as their ion-exchange capacities were used to explain the different retention properties on the different papers. Whatman 5 filter paper, specified to have a slow flow rate was tested first but the flow rate was prohibitively slow and did not reach the top of the channel (30 mm path). However, good flow was observed for Whatman P81, Whatman 4 and VWR 413. Fig. 2 shows chromatograms generated for AA and DA on the three different filter papers with acetic acid buffer pH 3.8 as a mobile phase. Whatman P81 is a strong cation-exchanger and under the conditions used was found to fully retain DA and thus only elute AA (average retention time 519 ± 6 s). This was evidenced by the single peak of AA observed on the chromatogram (Fig. 2a) and was confirmed by running the appropriate controls (data not shown). Whatman 4 (Fig. 2b), specified as having a fast flow rate and a large pore size (20–25 μm) eluted two poorly resolved peaks with average retention times of 458 ± 19 s and 666 ± 14 s. These peaks were assigned to AA and DA, respectively, confirmed by analysis of the AA and DA separately (data not shown). Analysis on VWR 413 filter paper (medium flow rate) is shown in Fig. 2c. It can be seen that in this case, a baseline separation of AA and DA was obtained with average peak retention times of 598 ± 6 s and 1344 ± 28 s, respectively. The separation of these analytes, in both instances, is attributed to an ion-exchange mechanism, given the differences in the pKa values and hence their overall charges at low pH.As detection was carried out in a fixed spot (30 mm from the starting point), for calculation of chromatographic parameters, such as retention factor, efficiency and selectivity, it is important to know how the flow rate changes with time for each type of support. As sample was introduced onto the already fully wetted paper, the flow rate in such a system known as the fully wetted flow, significantly differs from that on dry paper, known as the wetting-out flow. In the case of wetting-out flow, the visualisation of fluid movement can be rather simple.19 Investigation into fully wetted-out flow behaviour was previously described by Kauffman et al. (2011)20 where methods developed by this group allowed for the effective visualisation of fully wetted flow, but were quite complex in experimental setup. Here, for identification of the fully-wetted flow rates, a two-step experiment was carried out. First, the position of the wetting-out mobile phase front on each paper was identified and recorded every 60 s (see Fig. ESI-1†). From this data, it was possible to estimate the time at which the systems approached equilibrium (e.g. time at which steady state was reached, approx. 20 min for all papers). Hence, for all analyses, eluent was allowed to flow through the support for at least 30 min before injecting sample to ensure flow rate stabilisation. This time lag also ensured the working electrode current stabilisation as well as the removal of any loosely bound impurities in the paper support.
Secondly, for the measurement of the fully wetted flow rate, deionised water was passed through the paper for at least 30 min to ensure steady state flow rate. Following this, water was switched to the eluent used for the separation, namely 0.1 M acetate buffer, pH 3.8. This resulted in a visible increase in non-faradaic current signal and corresponds to the elution time of an unretained compound, t0. Using this method, the t0 value for the VWR 413 paper was 296 ± 9 s (n = 4), for the Whatman 4 paper it was 189 ± 4 s (n = 3) and for the Whatman P81 it was 458 ± 50 s (n = 4). For the Whatman P81 paper, the current response to the 0.1 M eluent was weak and therefore acetate buffer with increased ionic strength was used (0.25 M) to determine t0. Based on the findings of Fu et al. (2011),19 who showed that the fully wetted flow in a narrow channel of constant width is linear and constant, and knowing the paper length and t0 values, it was possible to establish that linear fully wetted flow rates in the current study were 6.08 ± 0.09 mm min−1 for VWR 413 paper, 9.52 ± 0.06 mm min−1 for Whatman 4 paper, and 3.93 ± 0.32 mm min−1 for Whatman P81.
Although the described system represents a planar mode of chromatography, an approximation to column liquid chromatography can be made. Firstly, the system operates under constant flow and equilibrium conditions. Fully wetting-out the paper eliminates the possibility of the formation of the gradient of impurities that may be present in the paper. Moreover, introduction of the sample into the flow and detection at a fixed point past the separation media is also an attribute of column LC mode. Thus, it is suggested that chromatographic parameters for this system can be calculated as those in column chromatography (Table 1).
| Paper | k | Rs | α(DA/AA) | |
|---|---|---|---|---|
| AA | DA | |||
| VWR 413 | 1.02 ± 0.02 | 3.54 ± 0.09 | 1.10 ± 0.07 | 3.47 ± 0.13 |
| Whatman 4 | 1.35 ± 0.09 | 2.45 ± 0.08 | 0.51 ± 0.01 | 1.82 ± 0.07 |
| Whatman 81 | 0.11 ± 0.03 | |||
From the resultant data in Table 1, it can be seen that only VWR 413 paper is suitable for quantitative separation of the analytes, as Rs > 1. Ascorbic acid was retained on Whatman 4 and VWR 413, while in the chosen conditions it was almost unretained on Whatman P81.
As separation was carried out in equilibrium conditions, it was possible to estimate peak efficiency for both AA and DA, separated on VWR 413 paper using the following equation:
 | (2) |
In order to further investigate the difference in chromatographic behaviours between Whatman 4 and VWR 413 (Fig. 4b and c), the flow rates and the ion-exchange capacities of these two papers were considered. At pH 3.8, AA would be almost neutral and elutes earlier on the Whatman 4 than on the VWR by approx. 200 s. This can be partially explained by the faster mobile phase flow rate for the Whatman 4 paper in comparison to VWR 413 filter paper. The elution of DA also happens quicker on the Whatman 4 paper but it cannot alone be attributed to the faster flow rate, as DA elutes approx. 700 s earlier on the Whatman 4 when compared to the VWR paper. Therefore, the retention of DA which is protonated at low pH, is significantly stronger on the VWR paper, which can be explained by a difference in ion-exchange capacities of these two papers. Such difference can arise from differing procedures used in production of these materials. Typically, during processing, the fibres are bleached by oxidative reagents to remove residual lignin. This process is known to result in the formation of carboxyl groups on the surface of the cellulose fibres.21 The observed ion-exchange behaviour can be explained by the presence of these carboxyl groups. Depending on the oxidative treatment used, the amount of carboxyl groups present, and hence the ion-exchange capacity in a particular cellulose material can vary. Thus, it is essential to quantify the amount of carboxylic acid groups on the surface of papers of interest and identify their ion-exchange capacities. In order to do so, a complexometric titration of these materials was carried out. The method is based on the ion exchange reaction between calcium ions and carboxyl groups15 (see Methods section). It was found that the carboxyl group content of the Whatman 4 paper was just 32 ± 18.55 mmol kg−1, while that of the VWR 413 paper was 134 ± 23.87 mmol kg−1. A t-test was performed (p < 0.0002; n = 5; two-tailed) and was found to be significant. It is important to note that the these results may not represent absolute amounts of carboxyl group residues. Interactions between carboxyl groups and calcium ions are not limited to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and so it is likely that higher stiochiometries, including chelate-like calcium binding by multiple spatially close carboxyl groups will also be present. However, this titration represents a basis for relative comparison of the amount of carboxyl groups between filter papers. As such, there is evidence to suggest that the oxidative treatment used in the VWR 413 manufacturing process results in a significantly greater carboxyl group content when compared to that of Whatman 4 paper. Indeed, the titration data supports the conclusion that the VWR 413 paper has an ion-exchange capacity approximately 4 times greater than Whatman 4, and so protonated DA is expected to be retained more strongly on this type of paper. On account of this, the VWR 413 filter paper was selected as the substrate for the further development of this μPAD for catecholamine analysis.
1 stoichiometry and so it is likely that higher stiochiometries, including chelate-like calcium binding by multiple spatially close carboxyl groups will also be present. However, this titration represents a basis for relative comparison of the amount of carboxyl groups between filter papers. As such, there is evidence to suggest that the oxidative treatment used in the VWR 413 manufacturing process results in a significantly greater carboxyl group content when compared to that of Whatman 4 paper. Indeed, the titration data supports the conclusion that the VWR 413 paper has an ion-exchange capacity approximately 4 times greater than Whatman 4, and so protonated DA is expected to be retained more strongly on this type of paper. On account of this, the VWR 413 filter paper was selected as the substrate for the further development of this μPAD for catecholamine analysis.
Ion-exchange separation optimisation and characterisation
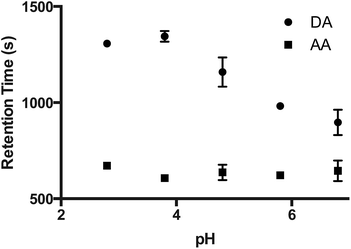
The effect of pH and ionic strength of the eluent on the separation of AA and DA was studied on VWR 413 filter paper. Fig. 3 shows the effect of eluent pH on the retention times of both AA and DA. The effect of pH was studied in the range of 2.8–6.8. DA becomes less stable with increasing pH due to deprotonation of hydroxyl groups in physiological or alkaline conditions,22 therefore, basic pHs were not investigated. As can be seen from Fig. 3, the retention time of AA was practically independent of pH, resulting in it being unretained under chosen conditions. The retention of DA was seen to increase with decreasing pH. As DA is weakly basic, this is what would be expected. From this graph, pH 3.8 was selected, as in these conditions the best analyte resolution is expected, and on practice a baseline separation of AA from DA was achieved.The effect of ionic strength (IS) was also studied (Fig. 4). It can be seen that the retention of DA decreases with increasing ionic strength on the VWR 413 paper, while the retention time of the AA remains unaffected. This provides further evidence for the ion-exchange retention mechanism of DA on the cellulose paper. The optimum ionic strength providing an adequate baseline resolution in minimum time was found to be 0.2 M (Fig. 4b).
 | ||
| Fig. 4 Normalised chromatograms for AA and DA where the ionic strength was varied from (a) 0.1 M (b) 0.2 M and (c) 0.5 M (concentrations of individual buffer components are 0.05 M, 0.1 M and 0.25 M respectively). All other conditions were the same as in Fig. 3. Chromatograms were normalised by the injection time (i.e. where the time of injection is taken as t = 0). | ||
AA is well known for its anti-oxidant properties and typically co-exists with catecholamines in biological samples. Catecholamines and AA are known to oxidise at similar electrochemical potentials at bare electrodes making catecholamines difficult to analyse in the presence of AA. As the concentration of AA is generally much higher than that of catecholamines (100–1000 times) in biological samples, it was of interest to use this new μPAD device to quantify the selected catecholamine, DA, in the presence of high concentrations of AA. Thus, a calibration curve for DA was generated where the measurements' of DA were made in the presence of 1 mM AA. The calibration curve for DA was found to be linear over the range 5–100 μM (r2 = 0.998; n = 3) (Fig. 5). The LOD was found to be 3.41 μM based on three times the standard deviation of the response (Sy) divided by the slope. This LOD for DA is within the concentration range typically seen for DA analyses in biological fluids (0.01–10 μM23) and is approximately 300 times lower than the concentration of AA present in the buffer. While the LOD reported here for this μPAD only allows for detection of DA across the upper clinical concentration range, it is envisaged that by optimisation of various parameters, e.g. applied potential, injection volume and electrode geometry, a significantly lower LOD could be achieved. Response reproducibility for repeated measurements was assessed using a 5 μM DA solution where repeated injections (n = 10) of this concentration resulted in an RSD value of 8.2%. The total analysis time was 1800 s, and while a shorter analysis time would be desirable in some cases this is a trade-off in terms of device simplicity, e.g., pump-free and disposable and low cost.
 | ||
| Fig. 5 (a) Overlaid chromatograms for solutions containing 1 mM AA and 20, 60, 100 and 500 μM DA. (b) Calibration curve for DA from 5–100 μM (r2 = 0.998, n = 3). All conditions same as in Fig. 4b. | ||
Conclusions
These results demonstrate the potential for μPADs to sensitively detect particular electroactive compounds in the presence of common interferents that undergo redox processes at similar potentials to that of the target compound(s). It was shown that some cellulose filter papers possess surface carboxyl groups that can behave as weak ion-exchangers and their counter-ions can be exploited for ion-exchange chromatography in μPADs. The concentrations of carboxyl groups24,25 on the different filter papers was measured via complexometric titration. It was discovered that the ion-exchange capacities of the filter papers investigated vary, which is likely attributed to the different manufacturers using different oxidation treatments during processing. Optimal baseline resolution of DA was achieved using a standard filter paper from VWR (VWR 413). The application demonstrated in this paper could be applicable for DA, or even total catecholamines analysis in the presence of AA, as these species typically co-exist in biological samples. A detection limit of 3.41 μM was demonstrated for DA demonstrating that these types of systems show promise for real applications. Future work will be to develop a μPAD method for the separation of a set of structurally similar analytes such as catecholamines or other phenolics. Functionalisation of the cellulose in order to tune the retention properties will be required. This will help realize the next generation of μPAD devices where the ability to quantitate structurally similar analytes in a single analysis step on a disposable platform will advance the exciting field of μPADs considerably.Acknowledgements
The authors wish to thank Science Foundation Ireland for financial assistance provided under the TIDA Programme (13/TIDA/B2598). The authors also gratefully acknowledge Prof. Kubota (Institute of Chemistry-UNICAMP, Campinas, Brazil) for supplying the P81 Whatman paper used in the experiments.References
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3–10 CrossRef CAS PubMed.
- E. J. Maxwell, A. D. Mazzeo and G. M. Whitesides, MRS Bull., 2013, 38, 309–314 CrossRef CAS.
- A. K. Badu-Tawiah, S. Lathwal, K. Kaastrup, M. Al-Sayah, D. C. Christodouleas, B. S. Smith, G. M. Whitesides and H. D. Sikes, Lab Chip, 2015, 15, 655–659 RSC.
- A. C. Glavan, D. C. Christodouleas, B. Mosadegh, H. D. Yu, B. S. Smith, J. Lessing, M. T. Fernandez-Abedul and G. M. Whitesides, Anal. Chem., 2014, 86, 11999–12007 CrossRef CAS PubMed.
- W. J. Lan, X. U. Zou, M. M. Hamedi, J. B. Hu, C. Parolo, E. J. Maxwell, P. Buhlmann and G. M. Whitesides, Anal. Chem., 2014, 86, 9548–9553 CrossRef CAS PubMed.
- L. Ge, S. W. Wang, S. G. Ge, J. H. Yu, M. Yan, N. Q. Li and J. D. Huang, Chem. Commun., 2014, 50, 5699–5702 RSC.
- R. F. Carvalhal, M. S. Kfouri, M. H. D. Piazetta, A. L. Gobbi and L. T. Kubota, Anal. Chem., 2010, 82, 1162–1165 CrossRef CAS PubMed.
- L. Y. Shiroma, M. Santhiago, A. L. Gobbi and L. T. Kubota, Anal. Chim. Acta, 2012, 725, 44–50 CrossRef CAS PubMed.
- L. F. OuYang, C. H. Wang, F. Du, T. F. Zheng and H. Liang, RSC Adv., 2014, 4, 1093–1101 RSC.
- A. Abbas, A. Brimer, J. M. Slocik, L. M. Tian, R. R. Naik and S. Singamaneni, Anal. Chem., 2013, 85, 3977–3983 CrossRef CAS PubMed.
- X. X. Yang, O. Forouzan, T. P. Brown and S. S. Shevkoplyas, Lab Chip, 2012, 12, 274–280 RSC.
- J. Lankelma, Z. Nie, E. Carrilho and G. M. Whitesides, Anal. Chem., 2012, 84, 4147–4152 CrossRef CAS PubMed.
- P. Kwong and M. Gupta, Anal. Chem., 2012, 84, 10129–10135 CrossRef CAS PubMed.
- K. Stana-Kleinschek, L. Fras, V. Ribitsch, M. Sfiligoj-Smole and T. Kreze, Lenzinger Ber., 2002, 81, 80–88 Search PubMed.
- D. N. Oko, S. Garbarino, J. M. Zhang, Z. M. Xu, M. H. Chaker, D. L. Ma, D. Guay and A. C. Tavares, Electrochim. Acta, 2015, 159, 174–183 CrossRef CAS.
- J. Y. Sun, L. Li, X. P. Zhang, D. Liu, S. M. Lv, D. R. Zhu, T. Wu and T. A. You, RSC Adv., 2015, 5, 11925–11932 RSC.
- C. O. Parker, Y. H. Lanyon, M. Manning, D. W. M. Arrigan and I. E. Tothill, Anal. Chem., 2009, 81, 5291–5298 CrossRef CAS PubMed.
- E. L. Fu, S. Ramsey, P. Kauffman, B. Lutx and P. Yager, Microfluid. Nanofluid., 2011, 10, 29–35 CrossRef CAS PubMed.
- P. Kauffman, E. Fu, B. Lutz and P. Yager, Lab Chip, 2010, 10, 2614–2617 RSC.
- L. C. A. Barbosa, C. R. A. Maltha, A. J. Demuner, C. M. Cazal, E. L. Reis and J. L. Colodette, Bioresources, 2013, 8, 1043–1054 CrossRef.
- Y. Shen and M. Y. Yen, J. Liq. Chromatogr., 1994, 17, 1557–1565 CrossRef CAS.
- C. C. Harley, A. D. Rooney and C. B. Breslin, Sens. Actuators, B, 2010, 150, 498–504 CrossRef CAS.
- L. Fras, K. Stana-Kleinschek, V. Ribitsch, M. Sfiligoj-Smole and T. Kreze, Mater. Res. Innovations, 2004, 8, 145–146 CAS.
- L. F. Zemljic, Z. Persin, P. Stenius and K. Stana-Kleinschek, Cellulose, 2008, 15, 681–690 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16272f |
| This journal is © The Royal Society of Chemistry 2015 |