β-BaGa4Se7: a promising IR nonlinear optical crystal designed by predictable structural rearrangement†
Zhen
Qian
a,
Qiang
Bian
b,
Hongping
Wu
 a,
Hongwei
Yu
a,
Hongwei
Yu
 *a,
Zheshuai
Lin
*a,
Zheshuai
Lin
 *c,
Zhanggui
Hu
a,
Jiyang
Wang
a and
Yicheng
Wu
a
*c,
Zhanggui
Hu
a,
Jiyang
Wang
a and
Yicheng
Wu
a
aTianjin Key Laboratory of Functional Crystal Materials, Institute of Functional Crystal, Tianjin University of Technology, Tianjin 300384, China. E-mail: hwyu15@gmail.com
bSchool of Material and Energy, Guangdong University of Technology, Guangzhou, 510006, China
cBeijing Center of Crystal R&D, Key Lab of Functional Crystals and Laser Technology, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: zslin@mail.ipc.ac.cn
First published on 29th November 2021
Abstract
Rationally designing new inorganic materials with specific functional properties is a fascinating challenge. This is particularly true with the nonlinear optical (NLO) crystals, which can expand the spectral ranges of solid-state lasers from ultraviolet (UV) to infrared (IR) through a second harmonic generation (SHG) or optical parametric oscillation (OPO) process. Herein, a high-performance IR NLO crystal, β-BaGa4Se7, has been successfully predicted and synthesized by the optimal rearrangement of the NLO-active basic structure units. The stability and functional properties of the material were simulated by first-principles calculations, and eventually it was also successfully synthesized via a high-temperature solid-state reaction in a sealed silica tube. More importantly, this material can exhibit exceedingly excellent NLO properties, including strong SHG response (5 × AgGaS2), wide transmission range (0.44–20 μm) and high laser damage threshold (680 MW cm−2, 20 × AgGaS2), as well as stable physicochemical properties. These indicate that β-BaGa4Se7 is a promising IR NLO crystal. The discovery of β-BaGa4Se7 can also provide new insights into the rational design of IR NLO crystals based on the predictable structural rearrangement.
Mid-infrared (mid-IR, 3 μm < λ < 20 μm) coherent radiation covering the atmospheric windows of 3–5 μm and 8–12 μm is urgently desired by a variety of important scientific and industrial applications, such as remote chemical sensing, free-space communication and high-resolution molecular fingerprint-region spectroscopy.1–9 Relying on the frequency down-conversion of nonlinear optical (NLO) crystals, including difference-frequency generation (DFG), optical parametric oscillation (OPO) and optical parametric amplification (OPA), is the most effective way for solid-state lasers to achieve high-power output of the mid-IR coherent radiations.10 The developments of the above technologies strongly depend on the discoveries of high-performance IR NLO crystals. Currently, although a series of commercialized NLO crystals, such as β-BaB2O4 (β-BBO), LiB3O5 (LBO) and CsLiB6O10 (CLBO) as well as KTiOPO4 (KTP) and LiNbO3 (LN), have been used in the UV and visible (vis) regions,11–14 the available NLO crystals in the IR region are still suffering from some intrinsic drawbacks, e.g. the low laser damaged threshold (LDT) of AgGaS2 (AGS) and AgGaSe2 (AGSe) (<30 MW cm−2) and the harmful two-photon absorption (TPA) of ZnGeP2 (ZGP), which hamper their further practical applications under high power conditions.15–17 As such, exploring new high-performance IR NLO crystals with large SHG coefficients (>0.5 × AGS), wide IR transmission window (3–12 μm) and high LDT (>1 × AGS or more than 30 MW cm−2 for 10 ns pulse laser) that usually corresponds to a large energy band gap are urgently needed.18–21
It is well known that the functional properties of a material are mainly determined by its structure, which involves not only the composition of materials, i.e. the types of the basic building units (BBUs), but also the arrangement of the BBUs.22,23 In particular, the arrangement of the BBUs often has a critical effect on the properties of materials. A typical example is the polymorphism of BBO crystals (an α-phase with the group space of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c vs. β-phase with R3). Although both phases have the same BBUs of [B3O6]3− groups, they exhibit very different functional properties owing to the different BBU arrangements.10,24 α-BBO is composed of oppositely aligned [B3O6]3− groups and does not have an SHG response but exhibits a quite large birefringence, so it has been widely used as an excellent birefringent crystal. For β-BBO the parallel arranged [B3O6]3− groups result in a large SHG response, and thus this crystal has been widely used in the frequency conversion of UV coherent light. Therefore, the optimization of the spatial arrangement of BBUs would be significant for the design of a high-performance NLO crystal.
c vs. β-phase with R3). Although both phases have the same BBUs of [B3O6]3− groups, they exhibit very different functional properties owing to the different BBU arrangements.10,24 α-BBO is composed of oppositely aligned [B3O6]3− groups and does not have an SHG response but exhibits a quite large birefringence, so it has been widely used as an excellent birefringent crystal. For β-BBO the parallel arranged [B3O6]3− groups result in a large SHG response, and thus this crystal has been widely used in the frequency conversion of UV coherent light. Therefore, the optimization of the spatial arrangement of BBUs would be significant for the design of a high-performance NLO crystal.
The metal chalcogenides are often chosen as the IR NLO candidates because they can exhibit a wide IR transmission region and large SHG responses, originating from their weaker interatomic bonds and stronger polarizability of chalcogen atoms than oxygen.25–28 The reported metal chalcogenides with excellent NLO properties consist of BaGa4Q7 (Q = S, Se),29,30 BaGa2GeQ6 (Q = S, Se),31,32 LiGaQ2 (Q = S, Se),33 Na2BaMQ4 (M = Ge, Sn; Q = S, Se),24 Li2Ga2GeQ6 (Q = S, Se),34,35 Ba3AGa5Se10Cl2 (A = Cs, Rb, K),36 and [A3X][Ga3PS8] (A = K, Rb; X = Cl, Br).37 Among them, BaGa4Se7 has caught particular attention owing to its outstanding comprehensive performances, including strong SHG response (2–3 × AGS),30 wide transparency range (0.47–18 μm) and high LDT (557 MW cm−2).38 Based on the OPO technique, the BaGa4Se7 crystal has achieved the output of IR coherent radiation at the wavelengths of 3–17 μm with a high conversion efficiency.39 It should be noted, however, that BaGa4Se7 crystallizes in a low symmetric monoclinic space group Pc (named as α-BaGa4Se7 phase) resulting from the arrangement of the [GaSe4] BBUs, which makes the properties’ measurements and applications difficult because the optical axes, crystallographic axes and dielectric axes do not coincide with each other.40,41
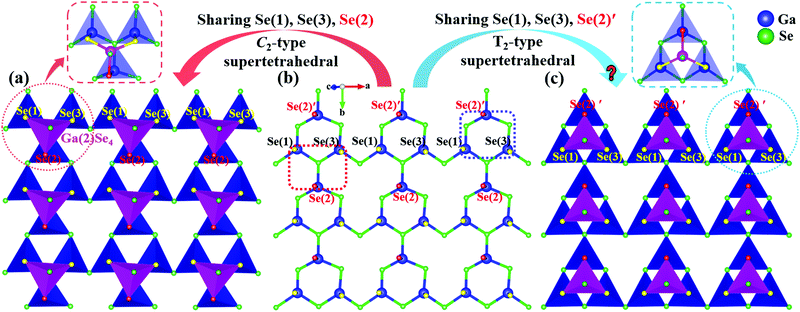
Analyzing the crystal structure of α-BaGa4Se7, one may find that its structure is composed of [GaSe4] tetrahedra (Fig. 1a and b). Clearly, the [GaSe4] BBUs connect with one another in the a–b plane to form [Ga3Se10]∞ layers (Fig. 1b), which stack along the c-axis and are linked together with Ga(2)Se4 tetrahedra through sharing the Se(1), Se(2) and Se(3), as shown in Fig. 1a. The arrangement of BBUs produces the [Ga4Se11] C2-type supertetrahedra structures with a C3v local symmetry in α-BaGa4Se7 (see the red dashed boxes in the upper left corner in Fig. 1a and the left side in Fig. 1b). By analyzing the packing pattern of the [Ga3Se10]∞ layers, we propose that actually there is another layer-to-layer connection manner, i.e., the adjacent [Ga3Se10]∞ layers can be shifted by 1/2 cell parameter along the b-axis with respect to each other and linked by sharing Se(1), Se(2)′ and Se(3) atoms (see the blue dashed box at the right side in Fig. 1b), rather than by sharing the Se(1), Se(2) and Se(3) atoms. The newly constructed structure is shown in Fig. 1c. More importantly, in this structure the [GaSe4] BBUs are arranged to form supertetrahedral [Ga4Se10] groups (see the blue dashes boxes in the upper right corner in Fig. 1c). Clearly, compared with [Ga4Se11] C2-type supertetrahedra, [Ga4Se10] supertetrahedra have a higher symmetric Td local symmetry, which would be beneficial for the structure to crystallize in the higher symmetric space group. Moreover, the supertetrahedral configuration is favorable for generating large SHG effects and band gaps due to the balanced structural feature.23
By adopting the supertetrahedral [Ga4Se10] groups, we theoretically constructed a new structure of BaGa4Se7, and the constructed structure was relaxed by first-principles geometry optimizations, which generates a new phase, i.e. β-BaGa4Se7, with a higher symmetric structure, Pna21. The first-principles phonon vibration calculation demonstrates that there is no presence of an imaginary phonon mode in the whole Brillouin zone (Fig. S1, ESI†), indicating the dynamic stability of this designed structure. The total energy calculations reveal that the total energy of β-BaGa4Se7 is very close to that of α-BaGa4Se7, with an energy difference of only ∼3 meV/atom. Both calculated results strongly suggest that the designed β-BaGa4Se7 would truly exist. The further calculations show that the three independent nonzero SHG coefficients d31, d32 and d33 in β-BaGa4Se7 are 37.91, −35.78 and 8.35 pm V−1,42,43 respectively, which are larger than d36 (12.6 pm V−1) of AGS and d11 (24.3 pm V−1) of α-BaGa4Se7.40 The first-principles calculations have proven to be one of the most accurate methods for the computation of the electronic structure of solids.44–46 This means that β-BaGa4Se7 would be a promising IR NLO crystal if it could be obtained in experiments.
In order to synthesize the designed β-BaGa4Se7 phase in experiments, a series of solid-state reactions with different calcination temperatures were performed in a sealing silica tube. After many attempts, it is very exciting that the β-BaGa4Se7 phase was successfully obtained when the calcination temperature is lower than 900 °C (Fig. S2, ESI†). When the calcination temperature is higher than 900 °C, β-BaGa4Se7 starts to transfer into α-BaGa4Se7 (Fig. S3, ESI†). These show that the β-BaGa4Se7 is a low-temperature phase. However, in contrast, the phase-transition from α-BaGa4Se7 to β-BaGa4Se7 did not occur when α-BaGa4Se7 was calcined at 850 °C for 72 h (Fig. S4, ESI†). This suggests that the phase-transition from β-BaGa4Se7 to α-BaGa4Se7 is irreversible. In addition, when β-BaGa4Se7 was soaked in water for one week, no obvious weight loss was observed and its powder X-ray diffraction pattern is also in good agreement with that before soaking. These results indicate that β-BaGa4Se7 has good thermal and environmental stabilities.
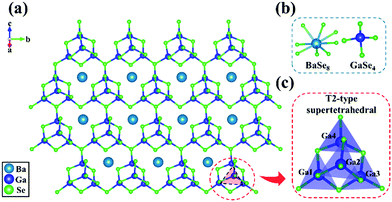
With Ga2Se3–Se as the flux, the millimeter-sized single crystals of β-BaGa4Se7 can also be successfully grown and used for single-crystal X-ray diffraction. This shows that β-BaGa4Se7 indeed crystallizes in the higher symmetric space group, Pna21, exactly the same as predicted. The crystal structure of β-BaGa4Se7 is shown in Fig. 2. In the asymmetric unit, there is one unique Ba, four unique Ga, and seven unique Se atom(s). The [GaSe4] tetrahedra are connected to each other by corner-sharing to form a 3D framework with the Ba filled in the space to balance the residual changes (Fig. 2a). Thus, all the Ga atoms are four-coordinated with the Ga–Se bonds ranging from 2.356(2) to 2.500(2) Å, and the Ba atoms are eight-coordinated with the Ba–Se bonds ranging from 3.4893(17) to 3.7742(18) Å (Fig. 2b). The calculation results of the bond valence sums (BVSs) of each atom are Ba: 1.73, Ga: 3.04–3.13, Se: 1.91–2.21, which are all consistent with the results in other compounds, indicating that the structure of β-BaGa4Se7 is reasonable. In addition, as predicted above, the [GaSe4] tetrahedra in β-BaGa4Se7 are interconnected to form the [Ga4Se10] T2-type supertetrahedral configuration (Fig. 2c). The [Ga4Se10] supertetrahedra may also be interesting for the catalyst material.47
The UV-vis-NIR spectrum in the range from 300 to 2500 nm for β-BaGa4Se7 is shown in Fig. S5 (ESI†). The cut-off edge of β-BaGa4Se7 is about 440 nm. Correspondingly, its band-gap can be calculated as 2.82 eV, which is larger than commercialized AGS (2.70 eV), AGSe (1.83 eV) and ZGP (1.75 eV). The relatively large band-gap is generally helpful for generating a large LDT. Furthermore, we also measured the LDT of β-BaGa4Se7 on a powder sample with AGS and α-BaGa4Se7 as a reference by a 1.064 μm pulse laser. The results show that β-BaGa4Se7 has a high LDT, ∼680 MW cm−2, which is ∼20 × AGS and is comparable with α-BaGa4Se7 (557 MW cm−2).
The IR spectrum of β-BaGa4Se7 is shown in Fig. S6 (ESI†). It shows that β-BaGa4Se7 has no absorption in a wide range from 4000 to 500 cm−1 (i.e. 2.5–20 μm), implying that β-BaGa4Se7 may have a wide IR transmission region and covering two important atmospheric windows, 3–5 and 8–14 μm. Furthermore, the Raman spectrum of β-BaGa4Se7 was measured (Fig. S7, ESI†). The absorption between 180 and 240 cm−1 can be assigned to the characteristic vibration of the Ga–Se mode, and other Raman peaks below 180 cm−1 are due to the Ba–Se vibrations. These are consistent with those of other related chalcogenides, such as Ba4CuGa5Se12 and Na6Zn3Ga2Se9.48,49
The birefringence is also important for the application of NLO crystals. So the birefringence of β-BaGa4Se7 was measured by a cross-polarizing microscope.50 The observed interference colors in cross-polarized light were first-order yellow for β-BaGa4Se7 (Fig. S8a, ESI†). On the basis of the Michel-Levy chart, the retardation (R values) is 350 nm.51 And the crystal thickness was measured as 7.8 μm (Fig. S8b, ESI†). Thus, the birefringence in the visible region can be calculated as 0.040 for β-BaGa4Se7, which is consistent with our calculated values (0.057–0.031@500–2000 nm) (Fig. S9, ESI†). This birefringence is also comparable with AGS, and ZGP. Additionally, we also calculated the phase matching (PM) wavelength ranges of β-BaGa4Se7 in the IR wavelength based on the calculated birefringence and added the results to Fig. S10 (ESI†). The results show that β-BaGa4Se7 is able to achieve PM in the whole Mid-IR wavelengths and cover two important atmospheric windows, 3–5 μm and 8–12 μm.
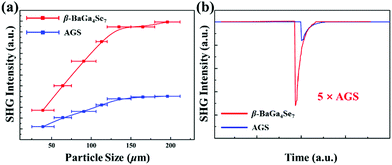
The powder SHG response of β-BaGa4Se7 was studied through the Kurtz and Perry method with 2.09 μm radiation.52 The data reveal that β-BaGa4Se7 can achieve type I PM (Fig. 3a), and the SHG response is around 5 × AGS (Fig. 3b). These results are in very good consistency with our first-principles calculations. The large SHG response of β-BaGa4Se7 will be favorable for generating high conversion efficiency in applications. In order to better understand the origin of the large SHG response in β-BaGa4Se7, the dipole moments of the [GaSe4] polyhedra were calculated based on the bond valence method.53 As listed in Table S4 (ESI†), the average dipole moments of the [GaSe4] polyhedra in α- and β-BaGa4Se7 are 9.60 and 16.99 D respectively, thus the [GaSe4] polyhedra in β-BaGa4Se7 exhibit stronger distortion. Additionally, the Ga–Se bond lengths, polarization directions and dipole moments in the [GaSe4] polyhedra of the two compounds are also given in Fig. S11 (ESI†). Clearly, the range of dipole moments for [GaSe4] polyhedra in β-BaGa4Se7 is from 5.02 D to 27.95 D, which is larger than that for [GaSe4] polyhedra in α-BaGa4Se7, 6.82 D to 12.58 D. That indicates that the larger SHG response of polarity of β-BaGa4Se7 may be attributed to the larger distortion of the [GaSe4] polyhedra in the structure. Furthermore, the dipole moments of α- and β-BaGa4Se7 in a unit cell are 15.64 and 45.07 D, respectively. This indicates that β-BaGa4Se7 can exhibit stronger polarity in the unit cell than that of α-BaGa4Se7. Generally, for the polar materials, a larger net dipole moment in the unit cell often means a larger SHG response,54–56 and the SHG signal of β-BaGa4Se7 is around 1.5 times that of α-BaGa4Se7 under the same conditions (Fig. S12, ESI†). Therefore, the larger SHG response of β-BaGa4Se7 is attributed to its stronger structural polarity.
Comparing β-BaGa4Se7 with the commercialized IR NLO crystals (Table 1), one can find that β-BaGa4Se7 exhibits excellent NLO properties and would be a promising IR NLO crystal. Firstly, β-BaGa4Se7 possesses a comparably large SHG response, suitable birefringence, and a wide IR transmission range compared with the commercialized AGS, AGSe and ZGP. These will enable the high laser conversion efficiency of β-BaGa4Se7 in applications. More importantly, β-BaGa4Se7 has a larger band gap than AGS, AGSe and ZGP, which results in its larger LDT (680 MW cm−2, 20 times higher than that of AGS). Therefore, β-BaGa4Se7 is suitable for high-power applications, which are often limited for AGS, AGSe and ZGP. In addition, just like AGS, AGSe and ZGP, β-BaGa4Se7 also crystallizes in the higher symmetric space group of Pna21 than the monoclinic Pc of α-BaGa4Se7. This will also be favorable for its processing and practical applications.
| Crystal | Space group | Power SHG | d ij (pm V−1) | Trans. rang (μm) | E g (eV) | Δn | LDT (MW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Measured SHG coefficients. b Calculated SHG coefficients. c Estimated from powder SHG data. d Powder test under the same conditions. | ||||||||
| AgGaS2 |
I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
1 × AGSa | d 36 = 12.6a | 0.47–13 | 2.73 | 0.039 | 34 | 57 |
| AgGaSe2 |
I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
3 × AGSa | d 36 = 39.5a | 0.76–18 | 1.83 (TPA) | 0.02 | 13 | 58 |
| β-BaGa4Se7 | Pna21 | 5 × AGSc | d 36 = 37.97b | 0.44–20 | 2.82 | 0.04 | 680d | This work |
| ZnGeP2 |
I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
6 × AGSa | d 36 = 75a | 0.7–13 | 2.00 (TPA) | 0.04 | 55.6 | 59 |
Clearly, the excellent NLO properties of β-BaGa4Se7 are related to its T2-type [Ga4Se10] supertetrahedra in the structure. Generally, the Tn-type supertetrahedra are often observed in organic–inorganic hybrid materials, and they have achieved many interesting photochemical and electrochemical functional properties in organic–inorganic hybrid materials.60,61 But for inorganic chalcogenides, the Tn-type supertetrahedra are still rare. Only several compounds are reported to contain the T2-type supertetrahedra, such as Ba6Zn7Ga2S16,62 Pb6ZnGa5S1563 and the halide-containing chalcogenides Ba3AGa5Se10Cl2 (A = K, Rb, Cs),36 Ba4MGa4Se10Cl2 (M = Zn, Cd, Mn, Cu/Ga, Co, Fe)64 and [A3X][Ga3PS8](A = K, Rb; X = Cl, Br).37 Remarkably, most of these materials exhibit excellent NLO properties. This indicates that the Tn-type supertetrahedra can be seen as an ideal structural gene for the design of new IR NLO crystals. The structural and property superiorities of Tn-type supertetrahedra are proved in β-BaGa4Se7. Structurally, the Tn-type supertetrahedra have the same local symmetry as the simple tetrahedra. Considering Tn-type supertetrahedra as four-connected nets, the Tn-type chalcogenides can exhibit a similar structural topology to the simple tetrahedral chalcogenides. For example, considering T2-type [Ga4Se10] supertetrahedra as the four-connected nets, we can find that β-BaGa4Se7 exhibits a similar structural topology to that of AGSe (Fig. S13, ESI†). As for properties, the Tn-type supertetrahedra can also make the structure possess a higher tetrahedral packing number in the unit cell, and larger “flexibility index” in β-BaGa4Se7 (Table S5, ESI†), which is generally helpful for materials to exhibit large SHG responses and birefringence, just as we have observed in β-BaGa4Se7.
Conclusions
In conclusion, a new polymorph of BaGa4Se7, β-BaGa4Se7 has been successfully predicted and synthesized. It features a typical supertetrahedral configuration. More importantly, it exhibits a large SHG response (5 × AGS), a wide IR transmission region (2.5–20 μm), a wide band gap (2.82 eV) and high LDT (680 MW cm−2) as well as moderate birefringence (∼0.04@visible region). Comparing these properties of β-BaGa4Se7 with some other excellent IR NLO crystals (Table 1), one can conclude that β-BaGa4Se7 will also be an excellent candidate for IR NLO crystals. In addition, this work will provide an effective strategy for exploring new NLO crystals through predictable structural rearrangement. Further research on the crystal growth of β-BaGa4Se7 is ongoing.Author contributions
Z. Q. performed the experiments, data analysis, and paper writing. Q. B. developed the theoretical calculations. H. W. designed and supervised the experiments. H. Y. and Z. L. provided major revisions of the manuscript. Z. H. supervised the optical experiments. J. W. and Y. W. helped with the analyses of the crystallization process and the data. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51802217, 51972230, 61835014, 51890864, and 51890865), the Natural Science Foundation of Tianjin (19JCZDJC38200), the National Key R&D Program (Grant No. 2016YFB0402103) and Tianjin Science and technology plan Program (Grant No. 19ZYPTJC00070).Notes and references
- D. F. Eaton, Science, 1991, 253, 281 CrossRef CAS PubMed.
- C. Chen, Y. Wang, B. Wu, K. Wu, W. Zeng and L. Yu, Nature, 1995, 373, 322 CrossRef CAS.
- P. S. Halasyamani and K. R. Poeppelmeier, Chem. Mater., 1998, 10, 2753 CrossRef CAS.
- K. M. Ok, Acc. Chem. Res., 2016, 49, 2774 CrossRef CAS PubMed.
- L. Kang, F. Liang, X. X. Jiang, Z. S. Lin and C. T. Chen, Acc. Chem. Res., 2020, 53, 209 CrossRef CAS PubMed.
- M. Mutailipu, K. R. Poeppelmeier and S. L. Pan, Chem. Rev., 2021, 121, 1130 CrossRef CAS PubMed.
- A. H. Reshak, N. M. Abbass, J. Bila, M. R. Johan and I. Kityk, J. Phys. Chem. C, 2019, 123, 27172 CrossRef CAS.
- A. H. Reshak, Sci. Rep., 2017, 7, 46415 CrossRef CAS PubMed.
- A. H. Reshak, J. Alloys Compd., 2017, 722, 438 CrossRef CAS.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 28, 235 Search PubMed.
- C. Chen, Y. Wu, A. Jiang, B. Wu, G. You, R. Li and S. Lin, J. Opt. Soc. Am. B, 1989, 6, 616 CrossRef CAS.
- Y. Mori, I. Kuroda, S. Nakajima, T. Sasaki and S. Nakai, Appl. Phys. Lett., 1995, 67, 1818 CrossRef CAS.
- J. D. Bierlein and H. Vanherzeele, J. Opt. Soc. Am. B, 1989, 6, 622 CrossRef CAS.
- G. D. Boyd, K. Nassau, R. C. Miller, W. L. Bond and A. Savage, Appl. Phys. Lett., 1964, 5, 234 CrossRef CAS.
- F. Liang, L. Kang, Z. S. Lin and Y. C. Wu, Cryst. Growth Des., 2017, 17, 2254 CrossRef CAS.
- A. Abudurusuli, J. Huang, P. Wang, Z. Yang, S. Pan and J. Li, Angew. Chem., Int. Ed., 2021, 60, 2 CrossRef.
- P. G. Schunemann, AIP Conf. Proc., 2007, 916, 541 CrossRef CAS.
- K. Wu, Z. H. Yang and S. L. Pan, Angew. Chem., 2016, 128, 6825 CrossRef.
- G. M. Li, K. Wu, Q. Liu, Z. H. Yang and S. L. Pan, J. Am. Chem. Soc., 2016, 138, 7422 CrossRef CAS PubMed.
- K. Wu, B. B. Zhang, Z. H. Yang and S. L. Pan, J. Am. Chem. Soc., 2017, 139, 14885 CrossRef CAS PubMed.
- X. L. Chen, H. Jo and K. M. Ok, Angew. Chem., Int. Ed., 2020, 59, 7514 CrossRef CAS PubMed.
- M. D. Donakowski, R. Gautier, J. Yeon, D. T. Moore, J. C. Nino, P. S. Halasyamani and K. R. Poeppelmeier, J. Am. Chem. Soc., 2012, 134, 7679 CrossRef CAS PubMed.
- L. Kang, F. Liang, X. X. Jiang, Z. S. Lin and C. T. Chen, Acc. Chem. Res., 2020, 53, 209 CrossRef CAS PubMed.
- R. Appel, C. Dyer and J. Lockwood, Appl. Opt., 2002, 41, 2470 CrossRef CAS PubMed.
- I. Chung and M. G. Kanatzidis, Chem. Mater., 2014, 26, 849 CrossRef CAS.
- F. Liang, L. Kang, Z. S. Lin, Y. C. Wu and C. T. Chen, Coord. Chem. Rev., 2017, 333, 57 CrossRef CAS.
- S. P. Guo, Y. Chi and G. C. Guo, Coord. Chem. Rev., 2017, 335, 44 CrossRef CAS.
- K. Wu and S. Pan, Coord. Chem. Rev., 2018, 377, 191–208 CrossRef CAS.
- X. S. Lin, G. Zhang and N. Ye, Cryst. Growth Des., 2009, 9, 1186 CrossRef CAS.
- J. Y. Yao, D. J. Mei, L. Bai, Z. S. Lin, W. L. Yin, P. Z. Fu and Y. C. Wu, Inorg. Chem., 2010, 49, 9212 CrossRef CAS PubMed.
- W. L. Yin, K. Feng, R. He, D. J. Mei, Z. S. Lin, J. Y. Yao and Y. C. Wu, Dalton Trans., 2012, 41, 5653 RSC.
- X. S. Lin, Y. F. Guo and N. Ye, J. Solid State Chem., 2012, 195, 172 CrossRef CAS.
- V. Petrov, A. Yelisseyev, L. Isaenko, S. Lobanov, A. Titov and J. J. Zondy, Appl. Phys. B: Lasers Opt., 2004, 78, 543 CrossRef CAS.
- Y. Kim, I. Seo, S. W. Martin, J. Baek, P. S. Halasyamani, N. Arumugam and H. Steinfink, Chem. Mater., 2008, 20, 6048 CrossRef CAS.
- D. J. Mei, W. L. Yin, K. Feng, Z. S. Lin, L. Bai, J. Y. Yao and Y. C. Wu, Inorg. Chem., 2012, 51, 1035 CrossRef CAS PubMed.
- P. Yu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227 CrossRef CAS PubMed.
- B. W. Liu, H. Y. Zeng, X. M. Jiang, G. E. Wang, S. F. Li, L. Xu and G. C. Guo, Chem. Sci., 2016, 7, 6273 RSC.
- J. Y. Yao, W. L. Yin, K. Feng, X. M. Li, D. J. Mei, Q. M. Lu, Y. B. Ni, Z. W. Zhang, Z. G. Hu and Y. C. Wu, J. Cryst. Growth, 2012, 346, 1 CrossRef CAS.
- N. Kostyukova, A. Boyko, V. Badikov, D. Badikov, G. Shevyrdyaeva, V. Panyutin, G. Marchev, D. Kolker and V. Petrov, Opt. Lett., 2016, 41, 3667 CrossRef CAS PubMed.
- X. Zhang, J. Yao, W. Yin, Y. Zhu, Y. Wu and C. Chen, Opt. Express, 2015, 23, 552 CrossRef PubMed.
- H. Yu, H. Huang, A. H. Reshak, S. Auluck, L. Liu, T. Ma and Y. Zhang, Appl. Catal., B, 2021, 284, 119709 CrossRef CAS.
- R. Ullah, A. H. Reshak, M. A. Ali, A. Khan, G. Murtaza, M. AL-Anazy, H. Althib and T. H. Flemban, Int. J. Energy Res., 2021, 45, 8711 CrossRef CAS.
- R. Singla, S. Kumar, T. A. Hackett, A. H. Reshak and M. K. Kashyap, J. Alloys Compd., 2021, 859, 157776 CrossRef CAS.
- E. Boursier, P. Segonds, J. Debray, P. Inácio, V. Panyutin, V. Badikov, D. Badikov, V. Petrov and B. Boulanger, Opt. Lett., 2015, 40, 4591 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- J. M. Rondinelli and E. Kioupakis, Annu. Rev. Mater. Res., 2015, 45, 491 CrossRef CAS.
- J. Zhang, X. Bu, P. Feng and T. Wu, Acc. Chem. Res., 2020, 53, 2261 CrossRef CAS PubMed.
- S. M. Kuo, Y. M. Chang, I. Chung, J. I. Jang, B. H. Her, S. H. Yang, J. B. Ketterson, M. G. Kanatzidis and K. F. Hsu, Chem. Mater., 2013, 25, 2427 CrossRef CAS.
- A. Abudurusuli, K. Wu, Y. Rouzhahong, Z. Yang and S. Pan, Inorg. Chem. Front., 2018, 5, 1415 RSC.
- X. Wang, F. Zhang, L. Gao, Z. Yang and S. Pan, Adv. Sci., 2019, 6, 1901679 CrossRef CAS PubMed.
- B. E. Sorensen, Eur. J. Mineral., 2013, 25, 5 CrossRef.
- S. Kurtz and T. A. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS.
- K. M. Ok and P. S. Halasyamani, Inorg. Chem., 2005, 44, 3919 CrossRef CAS PubMed.
- H. W. Yu, H. P. Wu, S. L. Pan, Z. H. Yang, X. L. Hou, X. Su, Q. Jing, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2014, 136, 1264 CrossRef CAS PubMed.
- H. P. Wu, S. L. Pan, K. R. Poeppelmeier, H. Y. Li, D. Z. Jia, Z. H. Chen, X. Y. Fan, Y. Yang, J. M. Rondinelli and H. S. Luo, J. Am. Chem. Soc., 2011, 133, 7786 CrossRef CAS PubMed.
- J. J. Zhou, H. P. Wu, H. W. Yu, S. T. Jiang, Z. G. Hu, J. Y. Wang, Y. C. Wu and P. S. Halasyamani, J. Am. Chem. Soc., 2020, 142, 4616 CrossRef CAS PubMed.
- L. I. Isaenko and I. G. Vasilyeva, J. Cryst. Growth, 2008, 310, 1954 CrossRef CAS.
- G. D. Boyd, F. G. Storz, J. H. Mcfee and F. G. Storz, IEEE J. Quantum Electron., 1972, 8, 900 CrossRef CAS.
- G. D. Boyd, E. Buehler and F. G. Storz, Appl. Phys. Lett., 1971, 18, 301 CrossRef CAS.
- N. Zheng, X. Bu, B. Wang and P. Feng, Science, 2002, 298, 2366 CrossRef CAS PubMed.
- N. Zheng, X. Bu and P. Feng, Nature, 2003, 426, 428–432 CrossRef CAS PubMed.
- Y. Y. Li, P. F. Liu and L. M. Wu, Chem. Mater., 2017, 29, 5259 CrossRef CAS.
- R. H. Duan, J. S. Yu, H. Lin, Y. J. Zheng, H. J. Zhao, S. X. Huang-Fu, M. A. Khan, L. Chena and L. M. Wu, Dalton Trans., 2016, 45, 12288 RSC.
- Y. Y. Li, P. F. Liu and L. Hu, Adv. Opt. Mater., 2015, 3, 957 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2048470. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1tc05436h |
| This journal is © The Royal Society of Chemistry 2022 |