Novel H2S multifunctional sensing materials: Cu or Ag-decorated (4,4)SWSiC nanotubes
R.
Safaiee
 *a,
M. M.
Golshan
b and
M.
Khalifeh
b
*a,
M. M.
Golshan
b and
M.
Khalifeh
b
aFaculty of Advanced Technologies, Shiraz University, Shiraz, Iran. E-mail: safaiee@shirazu.ac.ir; Fax: +98-71-36460839; Tel: +98-71-36139661
bPhysics Department, College of Sciences, Shiraz University, Shiraz, Iran
First published on 19th November 2021
Abstract
Density functional theory employing Grimme's D3 method was used to study the H2S sensing ability of pristine, as well as Cu or Ag decorated, (4,4)SWSiC nanotubes (NTs). The adsorption energy, most stable geometry and electronic (spin-distinct) structures of the compounds were calculated. The results show that the H2S adsorption process is exothermic, with energies of −0.391 eV (physical), −0.965 eV (chemical) and −0.618 eV (chemical) for pristine, Cu or Ag-decorated (4,4)SWSiCNTs, respectively. The range of adsorption energies points to the possible use of Cu-decorated (4,4)SWSiCNTs as an effective thermopower-based H2S sensing material. From a study on electronic structures, moreover, it was concluded that the adsorption of H2S leads to a considerable change in the corresponding energy band gaps and the carrier effective masses. The compounds under study were then treated as suitable candidates for incorporation into resistance-based schemes. Among the three compounds, the adsorption of H2S onto a Cu-decorated (4,4)SWSiCNT showed largest change (16.09%) in the true band gap and a change of 57.49% (33.36%) in the effective spin-up (spin-down) electron mass, meaning that this compound may be used with more efficiency in such schemes. Furthermore, the spin-distinct band structures of the compounds, influenced by the presence of H2S, indicate that the decorated (in contrast to the pristine) (4,4)SWSiCNTs remain magnetically bipolar. It then naturally follows that external potentials (gate potentials), needed to generate spin-polarized current, undergo an observable change when H2S is adsorbed onto the decorated (4,4)SWSiCNTs. Therefore, the decorated (4,4)SWSiCNTs can be employed in spin-current-based sensing devices. Last but not least, a complete discussion of the magnetization and the nature of the current carriers (electrons or holes), reveals that some of the compounds under investigation can potentially be used in more advanced and accurate schemes, such as magneto-based or Seebeck-effect-based H2S detection. The recovery time for the detection of H2S by any of the three compounds and the suggestions to make it shorter are also fully discussed. For completion, the physical reasons behind the electronic behavior in the compounds are given in detail. The materials presented in this article, therefore, provide new insights into the quest for designing high-performance H2S sensing devices.
Introduction
The quest for high-performance means of detecting hydrogen sulfide (H2S) has in recent years attracted increasing interest.1–12 This momentum is due to the fact that H2S is proven to be an extremely hazardous pollutant, mostly produced in petroleum and natural gas extraction,1,3,11–13 petrochemical plants,3,14,15 and paper mills,3,16,17 among other industries. The increased interest in the detection of H2S is a result of the expansion of such H2S producing facilities.1–5 Moreover, H2S is colorless, highly flammable, highly corrosive and highly irritative.2,5–8,11,13 In fact, H2S causes irritation to eyes, fluid retention in the lungs, sore throat, shortness of breath, nervous system damage, loss of consciousness and possible death, depending on its concentration.2,3,5,7,11,13 It is then obvious why one has to explore novel materials for effectively sensing this dangerous gas. Along these lines, the present article aims to propose materials to sense H2S gas with high sensitivity, relatively short recovery time and cost effectiveness.1,2,4,6 It may also be added that the sensing material should be miniature in size so that it can be integrated into practical kits.18,19 To this end, nanomaterials are the most promising candidates.6,7,18,20–25In general, an appropriate candidate for any analyte sensor should fulfill some basic criteria, which include, easy synthesis, uncostly fabrication, robustness against environment conditions and easy integration into a device. Moreover, such materials should efficiently respond to the presence of the analyte. The efficiency of the material, in turn, is determined through the surface-to-volume ratio,22–26 reasonable recovery time6,18,21,27 and operating temperature.2,5,6,18,21,27 In addition, the presence of analytes should induce measurable changes in the electronic properties of the sensing material.28–30 To this end, one may exploit the changes in the electronic energy structure to design resistance-based,2,5,6,18,20,24,27 magneto-based,23,31 spin-current-based32,33 and Seebeck-effect-based34,35 schemes. It is thus of great importance to look for substances that fall into the aforementioned categories, for the detection of H2S.
With the technological advances in manufacturing nanostructures, much research has been focused on a variety of nanostructures, as analytes sensors.20–25 Among these proposals, in particular for H2S sensing, carbon-based ones have attracted more attention.1,26,36 As such, we may mention that the use of graphene (sheets),7,11 graphene nanoribbons,1,37 doped graphene,31,38 functionalized graphene,12,39etc., has been extensively investigated. Needless to say that the ongoing interest in carbonaceous structures stems from their extraordinary physicochemical properties,36 as well as them being environmentally-friendly substances. However, pristine graphene has been shown to exhibit poor response in the presence of H2S.11,12,36,38 This shortcoming, to some degree, has been remedied by cutting1,11,38,40 and rolling graphene.8–10,40,41 More recently, however, carbonaceous nanostructures in which some of the carbon atoms in graphene are replaced by other elements, in a variety of shapes (sheets, tubes, etc.) have emerged as useful materials for a vast range of applications.18,24,36 To this end, the replacement of silicon atoms in the position of carbon atoms, towards so-called siligraphene, has been synthesized and used.42,43 According to the Si–C ratios (the unit ratio corresponds to the most stable configuration)44 in the resulting substance, the corresponding electronic structures can be made to change according to applicable requirements. Since the valence electrons in Si atoms are more loosely attached to the nucleus, it is anticipated that siligraphene-based nanostructures (sheets, tubes) exhibit more reactivity towards H2S, which is essential for the task in hand. We shall, therefore, attempt to explore the electronic properties of a particular siligraphene-based single-wall nanotube; widely known as single-wall silicon-carbide nanotubes (SWSiCNTs).45–47
The synthesis of SWSiCNTs, to the best of our knowledge, has been around for the past two decades.48,49 To put it in the simplest way, a SWSiCNT is formed by rolling a siligraphene sheet about a chiral vector. Depending on the selected chiral vector, the electrons in the generated tubes behave much differently.50–52 In fact, it is well established that all (with very few exceptions) SWSiCNTs behave as semiconductors, as opposed to graphene-based nanotubes (CNTs), which fall into the class of conductors or semiconductors.50,53 In practice, the final product of the synthesized CNTs contains both types, while for SWSiCNTs only semiconductor-type is observed. This pureness of the product then makes it more efficient to use SWSiCNTs as an analyte sensing material. Moreover, the Si–C bonds in SWSiCNTs are of polar nature,45,47 so it may be conjectured that they can operate at a relatively higher temperature compared to CNTs.45,46 We might add that due to the larger size of the valence orbitals in Si, the π electrons in SWSiCNTs tend to react with external agents more easily. This fact allows one to functionalize SWSiCNTs by/or sense external agents. Having established the advantages of SWSiCNTs over CNTs, in what follows we examine (4,4)SWSiCNTs (the notation shall be described soon), pristine or otherwise, as an H2S sensing material.
As was already mentioned, when a SiC sheet is rolled about a chiral vector, na1 + ma2, where a1 and a2 define the primitive vectors in the sheet, the result is a tube, identified in the literature as an (n,m)SWSiCNT.44 The selected chirality vector, in turn, specifies the radius of the tube, its boundary conditions and curvature.50 These factors then determine the corresponding electronic behavior. In fact, it is well known that the tube radii are given by 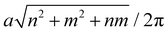 ,54 which can be made small (high curvature) or large (low curvature), depending on the chiral vector. In this expression a is the lattice constat of SiC sheet. To this end, for extremely high curvatures (extremely small radii) strong hybridization of the π and σ orbitals occurs, reducing the reactivity of the tubes.51 The weakness of tubes with extremely large radii, on the other hand, is the widening of the energy gaps,51 which limits the applicability of such tubes. In regard to their electronic behavior, the (n,m)SWSiCNTs can be divided into two categories: n = m, m ≠ 0 and n > m, m = 0,1,2,… (the case of n < m is irrelevant because of the symmetry). A survey of the literature reveals that extensive investigations on armchair SWSiCNTs (m = n = 3–8, 12, 15),50,51 zigzag SWSiCNTs (m = 0, n = 3–6, 8, 9, 12, 16, 20, 24)50,52 and chiral SWSiCNTs ((n,m) = (4,2), (6,2), (8,4), (10,4))51 have been carried out. From the information extracted from ref. 50–52, we have judged that singular (4,4)SWSiCNTs, with a radius of ≅3.37–3.72 Å and a gap of ≅1.60–1.80 eV, are most suited for practical H2S detection. This judgment is verified in the body of the present report. Another important point addressed in the text is that functionalization of (4,4)SWSiCNTs with light noble metals, namely copper and silver atoms, indeed improves the H2S sensing ability. The physical reasons for such improvements are also discussed. All the calculations were performed within density functional theory (DFT), using the Quantum-ESPRESSO computational package. The remaining part of this section is devoted to the organization of the present work, along with a brief outline of our novel results. After the introductory section, we give a detailed account of the manner with which DFT and the Quantum-ESPRESSO package55 are employed in the second section. In this section we also make a comparison between our calculated results for pristine (4,4)SWSiCNTs, with the corresponding data available in the literature, thus validating our method. A thorough investigation on the characteristics of the tubes, as a sensing material, when H2S is adsorbed onto them, forms the subject of the third section. In this section it is demonstrated that the material in question can be employed in resistance-based and Seebeck-effect-based, but not magneto-based or spin-conduction based, schemes. In the fourth section, we proceed to study the effect of decorating (4,4)SWSiCNTs using Cu or Ag for the detection of H2S. The main reasons for choosing these two noble metals are as follows. Firstly, the intrinsic magnetic dipoles of the adsorbed Cu or Ag atoms can, and in fact do, break the magnetic (spin) degeneracy of the siligraphene tubes. Secondly, the choice of Cu or Ag is also due to the fact that they are robust against corrosion and relatively cheap. The high affinity of these elements is also encouraging for use in H2S sensing. These points are crucial for the design of devices and thus further motivate the use of Cu or Ag atoms as the adsorbents. More importantly, perhaps, is the fact that among transition metals, copper and silver are of low toxicity and are thus more biocompatible. In addition, substances decorated with Cu or Ag have also been proposed for the effective sensing of other analytes.56,57
,54 which can be made small (high curvature) or large (low curvature), depending on the chiral vector. In this expression a is the lattice constat of SiC sheet. To this end, for extremely high curvatures (extremely small radii) strong hybridization of the π and σ orbitals occurs, reducing the reactivity of the tubes.51 The weakness of tubes with extremely large radii, on the other hand, is the widening of the energy gaps,51 which limits the applicability of such tubes. In regard to their electronic behavior, the (n,m)SWSiCNTs can be divided into two categories: n = m, m ≠ 0 and n > m, m = 0,1,2,… (the case of n < m is irrelevant because of the symmetry). A survey of the literature reveals that extensive investigations on armchair SWSiCNTs (m = n = 3–8, 12, 15),50,51 zigzag SWSiCNTs (m = 0, n = 3–6, 8, 9, 12, 16, 20, 24)50,52 and chiral SWSiCNTs ((n,m) = (4,2), (6,2), (8,4), (10,4))51 have been carried out. From the information extracted from ref. 50–52, we have judged that singular (4,4)SWSiCNTs, with a radius of ≅3.37–3.72 Å and a gap of ≅1.60–1.80 eV, are most suited for practical H2S detection. This judgment is verified in the body of the present report. Another important point addressed in the text is that functionalization of (4,4)SWSiCNTs with light noble metals, namely copper and silver atoms, indeed improves the H2S sensing ability. The physical reasons for such improvements are also discussed. All the calculations were performed within density functional theory (DFT), using the Quantum-ESPRESSO computational package. The remaining part of this section is devoted to the organization of the present work, along with a brief outline of our novel results. After the introductory section, we give a detailed account of the manner with which DFT and the Quantum-ESPRESSO package55 are employed in the second section. In this section we also make a comparison between our calculated results for pristine (4,4)SWSiCNTs, with the corresponding data available in the literature, thus validating our method. A thorough investigation on the characteristics of the tubes, as a sensing material, when H2S is adsorbed onto them, forms the subject of the third section. In this section it is demonstrated that the material in question can be employed in resistance-based and Seebeck-effect-based, but not magneto-based or spin-conduction based, schemes. In the fourth section, we proceed to study the effect of decorating (4,4)SWSiCNTs using Cu or Ag for the detection of H2S. The main reasons for choosing these two noble metals are as follows. Firstly, the intrinsic magnetic dipoles of the adsorbed Cu or Ag atoms can, and in fact do, break the magnetic (spin) degeneracy of the siligraphene tubes. Secondly, the choice of Cu or Ag is also due to the fact that they are robust against corrosion and relatively cheap. The high affinity of these elements is also encouraging for use in H2S sensing. These points are crucial for the design of devices and thus further motivate the use of Cu or Ag atoms as the adsorbents. More importantly, perhaps, is the fact that among transition metals, copper and silver are of low toxicity and are thus more biocompatible. In addition, substances decorated with Cu or Ag have also been proposed for the effective sensing of other analytes.56,57
From the electronic structures of Cu- or Ag-decorated (4,4)SWSiCNTs plus H2S, it can be concluded that the former decorated tubes can participate, with high efficiency, in any of measuring schemes outlined in the above. The latter decorated tube, on the other hand, turns out to be useless in the Seebeck-effect-based measurements. To this end, the magnetic nature of the three suggested sensing materials is also discussed in these two sections. In the fourth section, moreover, a comparison between the electronic behavior of the three substances is also made. Finally, the more important points of our findings are outlined in the concluding section. In this section we also compare the sensing ability of the proposed materials with some other nanotubes suggested for H2S detection.
Computational preliminaries
In our investigation, use is made of DFT, within the framework of Kohn–Sham equations, adopted for the adsorption of H2S molecules upon decorated single-walled (4,4)SiCNTs. The calculations were performed using the Quantum-ESPRESSO package,55 in spin-polarized mode. As is well known,58 this mode enables one to segregate the computed electronic properties into two spin components. Moreover, the Riemannian integrals over the first Brillouin zone were carried out using an optimized 1 × 1 × 9 Monkhorst–Pack Γ-centered grid of the k-points. For the decomposition of the total wavefunction in terms of plane-wave basis, a cut-off energy of 54 Ry, again optimized, was used. However, the two sets of plane-waves participating in the expansion of the charge density were cut at 540 Ry. The exchange–correlation energy was taken within the Generalized Gradient Approximation (GGA) of Perdew–Burke–Ernzerhof (PBE),59 while the actual crystalline potential was approximated using the well-accepted ultra-soft (Vanderbilt) pseudopotential.60 It is worth mentioning that energy band structures, in particular band gaps, can be more accurately calculated if hybrid, as a better approximation to semi-local, functionals lead to more accurate results for such quantities.61–63 The crucial factor in a sensing mechanism, however, is the actual changes that occur in the physical properties of the sensing material, as a result of gas (or otherwise) being present. In our opinion, our results are accurate enough to employ the proposed substances in H2S sensing devices. The effect of van der Waals interactions is included explicitly, using the semi-empirical scheme of Grimme's (DFT-D3) method for periodic systems64 with the corresponding default values already defined in the package. Moreover, we allocated a single adsorbate entity (copper, silver atoms and/or H2S molecules) to each supercell, which is assumed to consist of 3 unit-cells of (4,4)SWSiC nanotubes. This manner of allocation then prevents the overlap of the adsorbate electronic wavefunctions and thus we can ignore the Cu–Cu or Ag–Ag, and H2S–H2S interactions in the periodic images of the supercells. As the computational package operates in three dimensions, we extended the axes normal to the (4,4)SWSiCNT to a value of 35 Å, preventing interaction between periodic images of the (4,4)SWSiCNT. For the occupation of electronic levels, Marzari–Vanderbilt smearing, with a standard deviation of 0.003 Ry, was used. The above suggested parameters are, consequently, employed in the Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm65 for the geometrical optimization of the system when different compositions of the (4,4)SWSiCNT and the adsorbates are formed. The algorithm was operated until interatomic forces were less than 0.001 Ry a0−1, with a0 being the Bohr radius. It is worth emphasizing that in our use of the computational package, the variation in the positions of the constituents is allowed in all three dimensions. For the validation of our procedure, it is suffice to mention that our calculated geometrical characteristics (bond lengths, bond angles, etc.),66–68 as well as energy band gaps, are in excellent agreement with those reported in ref. 68.In the following, the procedure outlined in the above is used to extract geometrical configurations, adsorption energies, work functions, band structures, charge density redistributions and spin-polarizations for the compounds in hand.
Electronic properties of the combination of (4,4)SWSiCNT plus H2S
The present section is devoted to a study of the changes in the electronic properties of a (4,4)SWSiCNT, when molecules of hydrogen sulfide (H2S) are adsorbed onto it. This section also provides the basis for a comparison of the sensing abilities with the cases of H2S adsorbed on decorated (4,4)SWSiCNTs. To this end, the computational procedure of the previous section was used. Our study begins by specifying the adsorption sites for initiating the package, which we take at highly symmetric ones; above the center of the hexagons (designated by TH), on top of a C atom (designated by TC) or on top of a Si atom (designated by Tsoi). Moreover, it is assumed that the vector normal to the H2S molecular plane makes a specific angle with the tube axis. To complete the initializing orientation of the adsorbate molecules, we considered two cases: (I) the hydrogen atoms are located away from the tube and (II) the hydrogen atoms are located towards the tube. In this manner, the indices I or II are added to the above designations of each adsorption site (THI(II), for instance).We recapitulated the results of our calculation for the determination of the most stable adsorption configuration in Table 1. From the table, it is clear that the most stable site (the lowest adsorption energy, EAds) results from the initial positions TSiI and TSiII. When relaxation is completed, however, the H2S molecular plane becomes parallel to the tube's circumference, along its axis. This point is further discussed shortly (see Fig. 3). It is also clear from the sign and value of the adsorption energy for the most stable configuration, that the corresponding process is exothermic and physical. Although the remaining discussion is concentrated on the behavior of the most stable configuration, some other geometrical aspects, such as adsorption distance, hAds, and bulging distances of Si (dSi) and C (dC), all measured as the distance of the center of the corresponding atoms from the tube's axis, for other trial positions are also recorded in Table 1.
| Initial configuration | E Ads(eV) | h Ads (Å) | d Si (Å) | d C (Å) |
|---|---|---|---|---|
| THI | −0.346 | 2.813 | 0.179 | 0.034 |
| THII | −0.387 | 2.738 | 0.186 | 0.021 |
| TCI | −0.082 | 3.499 | 0.011 | 0.039 |
| TCII | −0.133 | 3.673 | 0.014 | 0.017 |
| TSiI | −0.391 | 2.726 | 0.185 | 0.017 |
| TSiII | −0.390 | 2.743 | 0.183 | 0.015 |
An important factor in sensing applications is the recovery time, with the approximate expression (in standard notations), τ ≅ 10−12exp(−EAds/KBT).25,69,70 To this end, the recovery time, for which the gas molecules break away from pristine (4,4)SWSiCNT at room temperature, was calculated to be 3.56 × 10−6 s. This estimated recovery time is used, in due time, for comparison with that of decorated (4,4)SWSiCNTs.
We now turn our attention to the question of how charge is redistributed when full ionic relaxation is achieved. For this purpose, a schematic representation of the orbitals (states) of sulfur and hydrogen atoms (right side), along with the projected densities of states for H2S (left side), is illustrated in Fig. 1. The figure is the result of our calculations and, to the best of our knowledge, has not been presented elsewhere. The left side of Fig. 1 clearly indicates that the corresponding lowest unoccupied molecular orbital (LUMO) is the result of the hybridization of S orbitals (3s,3p) and hydrogen 1s orbitals, giving a σ* orbital for hydrogen sulfide. On the other hand, the highest occupied molecular orbital (HOMO) is formed by the p orbitals of sulfur alone.
We take advantage of Fig. 1 to illustrate the manner of charge redistribution in Fig. 2. Although the representation of energy levels in this figure (left panel) is schematic, the energy values are those extracted from our DFT calculations. In the left panel, ECBM, EVBM and Eref denote the minimum of the conduction band, the maximum of the valence band and the vacuum energy, respectively. Here, the phrase vacuum energy (reference energy) is used for the places at which electrons experience no forces. The routes of charge (electron) transfer are also indicated by curved arrows in this panel. From a glance at the energy levels in the left panel of Fig. 2 one can conclude that a net migration of electrons, from H2S to (4,4)SWSiCNT takes place. This migration, moreover, is equally likely for spin-up or spin-down electrons. As a result, one expects to see a negative charge accumulation in the vicinity of the tube. From the corresponding values in the left panel, the electron affinity, defined as EEA = Eref − ECBM (ELUMO) for semiconductors (molecules) is calculated to be 3.005 eV (0.829 eV) for the (4,4)SWSiCNT (H2S molecule). Such calculated affinities strongly confirm our earlier conclusions regarding the manner of charge transfer. To this end, in the right panel of Fig. 2 we represent the isosurfaces for the densities of charges. In this panel and hereafter, different atoms are represented in pink (Si atoms), black (C atoms), light gray (S atoms) and dark gray (H atoms) colors. The color green (red) in the right panel indicates accumulation (depletion) of the electronic density. Wherever appropriate, moreover, the yellow (blue) color is an indication of the spin-up (down) accumulation of electrons. An important point is clear from the right panel: separation of positive and negative charges around H2S induces an electric dipole normal to the tube and the accumulation of spin-up and spin-down electrons is the same, so that the nonmagnetic nature of the (4,4)SWSiCNT remains intact, even in the presence of H2S.
For completion, our calculations reveal that a total charge, ΔQ = −0.26 e, consisting of; ΔQ↑ = −0.13 e and ΔQ↓ = −0.13 e is accumulated near to the (4,4)SWSiCNT. In the process of adsorption, the total charge of the H2S molecules decreases, resulting in a weaker bond between H and S. Notably, this conclusion indicates an elongation of H–S bonds (see also the discussion surrounding Fig. 3). The mechanism of charge transfer, as described in the above, then causes a deformation of the geometrical characteristics of the entities participating in the combinations. To see such changes, we illustrate the geometrical aspects of the combination of (4,4)SWSiCNT plus H2S in Fig. 3. As both panels of this figure clearly show, after relaxation the H2S molecular plane becomes parallel to the surface of the (4,4)SWSiCNT. Moreover, it is also clear that after relaxation is achieved, H2S is adsorbed by the (4,4)SWSiCNT on top of the Si atoms of the tube. We further note that in the combination, the length of the H–S bonds in H2S is increased by 0.30%. In addition, close-ups of two hexagons, most affected by the presence of H2S, are also included in the right part of Fig. 3. In comparison with the pristine (4,4)SWSiCNT, it is seen that near the adsorption sites the bond lengths of Si–C bonds increase, while the corresponding bond angles decrease. The physical reason behind this observation is attributed to hybridization of the HOMO orbitals of H2S (describing the non-bonded pair of electrons in sulfur, see also Fig. 1) and π electrons of Si, causing the formation of sp3 orbitals instead of sp2 ones at the adsorption sites. Since sp3 orbitals are geometrically tetrahedral, the Si atoms bulge away from the tube. Because of the bonds between Si and C atoms, the latter also bulges as well. The bulging of Si and C, measured as the distance of the center of the corresponding marbles from the tube's axis, increases by 1.01% for Si and 0.42% for C (for details, see the left part of Fig. 3).
To complete our discussion on the characterization of the adsorption process, we now focus on the electronic band structure of the combination of (4,4)SWSiCNT plus H2S. To this end, the spin-distinct energy bands (SDEB), the projected density of states (PDOS), as well as the total density of states (TDOS), are presented in Fig. 4. A comparison of the side panels in which the SDEBs are illustrated, reveals that the behavior of the two spin states is identical, so that the combination is still nonmagnetic. Moreover, the energy band gap is increased by 0.95% relative to that of the pristine (4,4)SWSiCNT. The minute widening of the gap is caused by the negligibly small bulging of the tube. As another result of the SDEBs presented in Fig. 4 (note the convexities), one expects a decrease in the electronic effective mass. Our calculations reveal that the electronic effective mass in the (4,4)SWSiCNT plus H2S, extracted from the second derivatives of the conduction (valence) band, decreases by 58.87%. Since the resistance of a compound is directly related to the effective mass, the resistance of the compound decreases tangibly. This point, although not practical, may be used for a resistance-based sensor. From a comparison with the band structure in Fig. 4 and that presented in,57 we conclude that the presence of H2S causes the tube to change from a p-type semiconductor to an n-type one. The latter point particularly suggests that (4,4)SWSiCNTs can potentially be used as a Seebeck-effect34,35 H2S sensor.
Moreover, the energies of the two spin states are extremely close to each other because the bulging of the tube is negligibly small. From the PDOS illustration in the middle panel of Fig. 4, the symmetry of the density of states for spin-up and spin-down can also be observed. This observation confirms our earlier conclusion on the nonmagnetic nature of the compound. The middle panel also clearly demonstrates the hybridization of participating orbitals, as was described earlier.
Electronic properties of the combination; Cu- or Ag-decorated (4,4)SWSiCNT Plus H2S
Although the mechanism of decorating a (4,4)SWSiCNT with Cu or Ag is fully discussed in ref. 57, we just state the quantities essential to the main subject of the present work. In this regard, our DFT calculations show that Cu relaxes on the side-wise bridges, inclined towards C atoms. The Ag atoms also relax on the midpoint of the same bridge. Accordingly, we initialized the computational package by locating H2S molecules on top of Cu (Ag) or on the non-identical hollows, adjacent to the adsorption sites (a total of four trial sites) of the two elements. For each initial location, we also considered two orientations for the H2S molecules, as in the previous section. In this manner, the computational package was initialized by eight different configurations. Since the decorating elements in our study intrinsically possess magnetization, we used the computational package in ferromagnetic (FM), antiferromagnetic (AFM) and nonmagnetic (NM) spin-polarized modes. Our calculations then indicate that the relaxed orientation of H2S molecules is such that their molecular plane becomes parallel to the circumference of both decorated tubes. The relaxed position of the gas molecules, determined by the location of S, turns out to be on top of Cu for Cu–(4,4)SWSiCNT and on top of Si, furthest from the Ag adsorption sites for the other compound (see also Fig. 7). The numerical results for the adsorption energies (FM, AFM and NM modes), adsorption distances and bulging distances are recapitulated in Table 2. For the purpose of comparison, such quantities for the adsorption of H2S on the pristine (4,4)SWSiCNT are also included in the table.| Compound | Quantity | |||
|---|---|---|---|---|
| E Ads FM; AFM; NM (eV) | h Ads (Å) | d Si (Å) | d C (Å) | |
| (4,4)SWSiCNT + H2S | −0.391; −0.391; −0.391 | 2.726 | 0.185 | 0.017 |
| Cu–(4,4)SWSiCNT + H2S | −0.965; −0.963; −0.842 | 2.200 | 0.328 | 0.021 |
| Ag–(4,4)SWSiCNT + H2S | −0.618; −0.617; −0.483 | 2.691 | 0.261 | 0.013 |
It is noted from Table 2 that the adsorption of gas molecules onto either of the two decorated tubes is chemical and again exothermic. The range of adsorption energies in the table points to the possibility of employing the decorated tubes in thermopower-based27 sensing schemes for the detection of H2S. Moreover, we can see from the table that the combination of a decorated (4,4)SWSiCNT plus H2S is ferromagnetic. According to our calculations, the magnetization of the two decorated (4,4)SWSiCNTs plus H2S, turn out to be 0.88 μB per cell for Cu and 0.95 μB per cell for Ag decorations.
The bulging effect of the Cu-decorated tube, upon the adsorption of H2S, can also be seen from Table 2 to be much greater than its counterparts. As a result, one expects a wider energy gap for Cu-decorated (4,4)SWSiCNT substance plus H2S, relative to the other two combinations. This point shall be discussed further in a short while. As in the previous section, the recovery times for the two decorated cases, at room temperature, were also calculated. The average time for H2S to leave Cu-decorated (4,4)SWSiCNT turns out to be 1.54 × 104 s while it is 2.34 × 10−2 s for Ag-decorated (4,4)SWSiCNT (note the value of the corresponding adsorption energies). It is thus concluded that for the former substance, the application of external agents, such as UV or heat sources, are needed to speed up the recovery process.24,71
We now embark upon investigating the manner of charge transfer when H2S molecules are adsorbed onto either Cu-decorated (4,4)SWSiCNT or Ag-decorated (4,4)SWSiCNT. To this end, a schematic illustration of the mechanism for charge transfer is given in Fig. 5. In both parts, the donation and back-donation paths are presented as green and red curves, respectively. In addition, the dominant path is identified by the solidity of the curves. A glance at the position of the energy levels (LUMOs, HOMOs, valence and conduction bands) in Fig. 5 indicates that the electronic charge of distinct spin polarization migrates from the gas to the two decorated (4,4)SWSiCNTs. To be more specific on the details, we point out that spin-up electrons fill the lowest conduction band, while the spin-down electrons occupy the highest valence band of the decorated (4,4)SWSiCNT, in the process of electron migration. Our calculations for Cu–(4,4)SWSiCNT plus H2S show that a total charge, ΔQ = −0.11 e (ΔQ↑ = −0.02 e and ΔQ↓ = −0.09 e), is transferred to Cu–(4,4)SWSiCNT. For the (4,4)SWSiCNT decorated with Ag, plus H2S, a total charge of ΔQ = −0.16 e (consisting of ΔQ↑ = −0.01 e and ΔQ↓ = −0.15 e), is transferred from H2S to Ag–(4,4)SWSiCNT, according to our calculations. These values are in accordance with larger electron affinities of Cu–(4,4)SWSiCNT (≅3.258 eV) and Ag–(4,4)SWSiCNT (3.572 eV), in comparison with that of a H2S molecule (≅0.829 eV). Consequently, a larger amount of charge is transferred from H2S to the latter substance. We physically attribute these facts (the stronger H2S adsorption onto Cu–(4,4)SWSiCNT, in spite of a smaller amount of charge transfer) to the interaction of intrinsic H2S electric dipoles and the ones in the decorated tubes.57 In fact, our calculations show that the molecular dipole moment reduces from 1.102 D to 0.874 D upon adsorption onto Cu–(4,4)SWSiCNT. For the case of adsorption onto Ag–(4,4)SWSiCNT, on the other hand, the molecular dipole moment decreases to 0.579 D. As a result, in the case of Cu–(4,4)SWSiCNT, the corresponding dipole–dipole interaction is larger (note also the smaller adsorption distance) so that the adsorption of the molecules becomes tighter. The dipole–dipole interaction resulting from the adsorption of H2S onto the pristine tube is, however, negligibly small (note the adsorption distances in Table 2).
To further verify the mechanism of charge transfer, as described in the above, the isosurfaces of charge density redistributions and spin-distinct charge densities were calculated and the results are shown in Fig. 6. Since we have already discussed how the total charge is redistributed as a result of gas adsorption, we call attention to part (a) of the two panels of Fig. 6. This part clearly shows that negative charge migrates from H2S to the decorated tubes, as already discussed. More importantly, perhaps, is the separation of electronic spin states in such a migration, illustrated in parts (b) to (d) of the figure. This separation is, of course, due to the bulging effect. From a comparison between parts (b) and (c) of both panels, it is observed that more spin-down electrons, relative to spin-up ones, transfer from the gas molecules to the decorated (4,4)SWSiCNTs. The balance of spin-distinct migration then causes a reduction in the magnetization of the decorated tubes. Accumulation of spin-down electrons around the decorated (4,4)SWSiCNTs can also be clearly observed in part (c) of Fig. 6. In fact, our calculations indicate that the magnetization of suspended Cu–(4,4)SWSiCNT (the sensing material) reduces from 0.935 μB per cell to 0.801 μB per cell, a reduction of 14.33%, when H2S is adsorbed. The magnetization of suspended Ag–(4,4)SWSiCNT is also reduced from 0.965 μB per cell to 0.781 μB per cell, a reduction of 19.07%, in the presence of H2S. These numerical values are in agreement with part (d) of Fig. 6. The variation in the magnetization of the sensing material, here Cu–(4,4)SWSiCNT or Ag–(4,4)SWSiCNT, may be potentially used in magneto-based sensing devices23,31 for H2S detection.
Due to the manner of charge exchange, as described in the above, one expects a deformation of the geometrical characteristics of the constituents participating in the combinations. To this end, the geometrical aspects of the combinations are illustrated in Fig. 7. It can be clearly seen from this figure that the H2S molecular plane becomes parallel to the surface of the decorated tubes. The molecular plane, however, relaxes closer to the tube for the adsorption of H2S by Ag–(4,4)SWSiCNT, as compared with the case of adsorption by Cu–(4,4)SWSiCNT. Moreover, it is calculated that the length of H–S bonds in H2S is increased by 0.80% for adsorption onto Cu–(4,4)SWSiCNT and 4.05% for adsorption on Ag–(4,4)SWSiCNT. The angle between these bonds, on the other hand, decreases by a factor of 0.64% (0.91%) for the adsorption of H2S on Cu(Ag)–(4,4)SWSiCNT. Our calculations also indicate that near the adsorption sites (it is recalled that H2S relaxes on top of Cu when adsorbed onto Cu–(4,4)SWSiCNT), the Si–C bond lengths increase by a factor of 1.74%, while the corresponding bond angles decrease by a factor of 2.33%. For the other case these factors turn out to be 1.67% and 3.36%, respectively. It is also worth mentioning that the distance between Cu and the tube is decreased by a factor of 2.57%, when H2S is adsorbed. This fact points to a stronger bond between Cu and the tube, arising from enhanced charge-density forming this bond. As a result, Si atoms in the compound undergo greater bulging (about 43.86%), compared to the suspended Cu–(4,4)SWSiCNT. Since H2S relaxes on top of Si, which is further away from Ag in the other combination, two types of bulging of the tube are observed: at the adsorption site and under the Ag atom. The two bulges are 0.261 Å and 0.131 Å, respectively. The latter shows a decrease of 28.57% (the former bulge is completely absent before gas adsorption) in comparison with the suspended Ag–(4,4)SWSiCNT. Such increases (decreases) are again in agreement with the mechanism of charge transfer.
 | ||
| Fig. 7 The relaxed geometries of the combinations of Cu- (top panel) or Ag- (bottom panel) decorated (4,4)SWSiCNT plus H2S. | ||
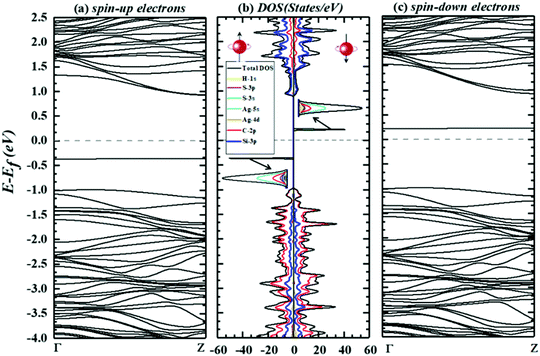
To conclude this section, an illustration of SDEBS (side panels), along with TDOS and PDOS (both in the middle panels), for the combinations of decorated tubes plus H2S molecules is presented in Fig. 8 and 9. Fig. 8 is devoted to these characteristics for Cu–(4,4)SWSiCNT, while Fig. 9 presents the same for Ag–(4,4)SWSiCNT, in the presence of gas. The TDOS and PDOS in the middle panels of the two figures indicate the manner of orbital hybridizations, which in turn confirms the mechanism of charge transfer. In the remainder of this section we focus on the characteristics directly related to the gas sensing ability of the two materials. The magnetic bipolarity, in the sense that by application of gate potentials (side-wise) one can generate a completely spin-polarized current, is clear from the side panels of both figures. In fact, the side panels of both figures show that (note the Fermi energy level) for the spin-up channel both decorated substances behave as p-type semiconductors, while for the spin-down channel they are n-type in nature. It is then customary to address such materials as bipolar. The TDOS in the middle panel strongly support the latter claim. To this end, we took the gate potentials to supply energy to the system, so that the Fermi level touches the minimum of the conduction band (spin-down channel) or the maximum of the valence band (spin-up channel). The former then defines a negative gate, while the latter corresponds to a positive one. Our calculations indicate that in the substance Cu–(4,4)SWSiCNT plus H2S, a gate potential equivalent to an energy of ≅ +0.344 eV (≅−0.237 eV), with sharp tunability, can generate spin-up (spin-down) currents. Relative to bare Cu–(4,4)SWSiCNT,57 the gate potential of the spin-up (spin-down) channel has increased (decreased) by a factor of 7.6 (0.39). It is thus seen that by dialing the gate potentials, the presence of H2S can be sensed.
The side panels of Fig. 9 also indicate the magnetic bipolarity of the substance Ag–(4,4)SWSiCNT plus H2S. However, according to our calculations, a gate potential of ≅ +0.377 eV (≅−0.208 eV) generates spin-up (spin-down) currents. This shows an increase (decrease) by a factor of 1.15 (0.97), relative to bare Ag–(4,4)SWSiCNT.57 It then appears that one can, in principle, employ this feature of the two decorated tubes for the spin-current-based (spintronic sensing)32,33 detection of H2S. To this end, however, Cu–(4,4)SWSiCNT is a more suitable candidate for such a task.
For applications in resistance-based sensing, we also performed calculations on electronic effective masses, which were extracted from our calculations on the conduction (valence) band convexity (also observed in the right (left) panels of Fig. 8 and 9). It was found that in the spin-up (spin-down) channel, the electronic effective mass in the compound Cu–(4,4)SWSiCNT plus H2S is decreased by 57.49% (33.36%). This result indicates that the resistivity of the suspended Cu–(4,4)SWSiCNT decreases substantially in the presence of H2S. It is therefore conceivable to construct an H2S resistance-based24,27,72 sensing device using Cu–(4,4)SWSiCNTs. Using the same manner of calculations, we find that the spin-up effective mass decreases by 11.04%, while the spin-down electrons experience an increase of 21.48% in the compound Ag–(4,4)SWSiCNT plus H2S. Moreover, from the position of the lowest conduction band (right panel) and the highest valence band (left panel), it is observed that a transition to the conduction band can occur only via a spin–flip process. As a comparison between the left and right panels of Fig. 8 and 9 clearly shows, if a current is established in the two decorated cases, the carriers exhibit spin-down polarization. To establish such a current, it is then necessary to feed the decorated tubes an energy equal to the energy difference between the maximum of the valence band (filled by spin-up electrons) and the minimum of the conduction band (to be filled by spin-down electrons). We term this energy as the true energy gap. The terminology we use stems from the fact that, in practical applications, this much energy is needed to establish a spin polarized current. From Fig. 8, it can be deduced that the true energy gap (spin–flip gap), is reduced by 16.09%, for the compound Cu–(4,4)SWSiCNT after H2S adsorption. However, in the compound Ag–(4,4)SWSiCNT, as Fig. 9 implies, the true gap is increased by only 7.72% as a result of gas adsorption. With regards to the latter two points, we conclude that Cu–decorated (4,4)SWSiCNTs are a more efficient candidate for H2S resistance-based detecting. In addition, Cu–(4,4)SWSiCNT transforms from a p-type semiconductor57 to an n-type one, as a result of H2S adsorption. The feasibility of employing Cu–(4,4)SWSiCNTs as a Seebeck-effect sensing material is again apparent. The more important points of the material just discussed are concisely presented in Table 4 in the concluding section.
Conclusion
The present article proposes novel materials for the purpose of hydrogen sulfide (H2S) gas sensing. To this end, we performed a first-principles study on pristine (4,4)SWSiCNT and Cu- or Ag-decorated ones onto which H2S was adsorbed. As the tool of our investigation, dispersion-corrected density functional theory (DFT-D3), with spin-polarization, was employed. To begin the study, several initial configurations for the adsorption sites were examined. By means of the calculated adsorption energies, the most stable sites and orientations were distinguished and used for the rest of the calculations. We then proceed by reporting the geometrical characteristics, accompanied by illustrations, of the stable configurations for the adsorption of H2S onto (4,4)SWSiCNT, Cu–(4,4)SWSiCNT and Ag–(4,4)SWSiCNT. This was fully discussed in the third and fourth sections. A detailed account of the manner with which charge is transferred and/or redistributed in the aforementioned adsorption processes, along with appropriate diagrams, was also presented. To complete the investigation, the spin-distinct electronic band structures (SDEBS), the total densities of states (TDOS) and the projected density of states (PDOS) were calculated and discussed. Along these lines, the electronic properties most essential in designing H2S sensors, were emphatically described. Although a complete elaboration on our results is made in the text, the novel points of the present article, closely related to the sensing ability of the compounds, are recapitulated in the following tables. To this end, the spin-distinct (first and second columns) and the net (third column) charge transfers, resulting from the adsorption of H2S onto the compounds, are quantitatively presented in Table 3. It is emphasized that in the adsorption process, H2S loses charge, while the sensing materials gain charge. A comparison of the first and second columns shows that the compounds acquire a net magnetization that forms the subject of the fourth column in Table 3.| Compound | Quantity | ||||||
|---|---|---|---|---|---|---|---|
| ΔQ↑ (e) | ΔQ↓ (e) | ΔQ (e) | Sensor Magnetization (μB per cell) | Adsorption Energy (eV) | Recovery Time (s) | ||
| Before H2S | After H2S | ||||||
| (4,4)SWSiCNT + H2S | 0.13 | 0.13 | 0.26 | 0 | 0 | −0.391 | 3.56 × 10−6 |
| Cu–(4,4)SWSiCNT + H2S | 0.02 | 0.09 | 0.11 | 0.935 | 0.801 | −0.965 | 1.54 × 104 |
| Ag–(4,4)SWSiCNT + H2S | 0.01 | 0.15 | 0.16 | 0.965 | 0.781 | −0.618 | 2.34 × 10−2 |
The change in magnetization, as a consequence of H2S adsorption, is evident from this column. The fourth column of this table clearly indicates that the present compounds may indeed be employed in magneto-based sensing schemes.31,32 From the adsorption energies, given in the fifth column, one can calculate the well-known recovery time30 which is the subject of the last column. A glance at the recovery times shows that Ag-decorated (4,4)SWSiCNT is suited for any type of sensing mechanism, except Seebeck-effect ones. On the other hand, in spite of its long recovery time, Cu-decorated (4,4)SWSiCNT may well be used in thermopower-based sensing schemes (note the large adsorption energy).27,73 However, it is feasible that by application of UV radiation, for instance, one can overcome this deficiency. Moreover, for potential use of the proposed materials in spin-current-based detecting, as well as resistance-based ones or Seebeck schemes, electronic characteristics directly related to these topics are outlined in Table 4. For completeness, the bipolar nature of the compounds is also included in the first column of the table. From an observation of the gate potentials (a side-wise potential to generate spin-polarized current) and its tunability, the subject of the second and third columns, respectively, one concludes that Cu-decorated (4,4)SWSiCNTs are more suitable for H2S spin-current-based detecting. Moreover, from the carriers’ effective masses and true gaps, given in the fourth and fifth columns, respectively, it is seen that both quantities undergo drastic changes when H2S is adsorbed onto Cu–(4,4)SWSiCNT. It is thus concluded that the latter substance is also a suitable candidate for use in resistance-based detection devices. If, on the other hand, the proposed materials are to be used in Seebeck-effect detecting schemes, Cu–(4,4)SWSiCNT again does meet the necessary criteria.
| Compound | Quantity | |||||
|---|---|---|---|---|---|---|
| Magnetic bipolarity | Gate potential Spin-up (down) (eV) | Tunability spin-up (down) (eV) | Spin-up (down) Effective mass, (m0) | True gap (eV) | Type of semiconductor | |
| (4,4)SWSiCNT | — | — | — | 1.000 | 1.892 | p |
| (4,4)SWSiCNT + H2S | — | — | — | 0.411 (0.411) | 1.910 | n |
| Cu–(4,4)SWSiCNT | ✓ | +0.040 (−0.615) | ±0.008 (±0.038) | 18.381 (3.408) | 0.609 | p |
| Cu–(4,4)SWSiCNT + H2S | ✓ | +0.344 (−0.237) | ±0.020 (± 0.050) | 7.814 (2.271) | 0.511 | n |
| Ag–(4,4)SWSiCNT | ✓ | +0.328 (−0.215) | ±0.004 (±0.009) | 40.547 (13.573) | 0.531 | n |
| Ag–(4,4)SWSiCNT + H2S | ✓ | +0.376 (−0.209) | ±0.005 (±0.008) | 36.071 (16.489) | 0.572 | n |
In fact, we can observe from the last column of Table 4 that the latter substance changes from a p-type semiconductor to an n-type one, as a result of H2S adsorption. The pristine (4,4)SWSiCNT also exhibits potential for being employed in such a scheme.
For comparison purposes, characteristics of the more compatible honeycomb based nanotubes proposed for the detection of H2S, are summarized in Table 5. In order to make such a comparison, the first three rows of Table 5 are dedicated to our results. All the entries in the table correspond to the sensing material in the presence of H2S (we have omitted the phrase “plus H2S” for conciseness). It is evident from Table 5 that Cu-decorated (4,4)SWSiCNT can be used in any of the presently available detecting schemes. For this substance, however, the recovery time is relatively large (note also the two rows before the last) which can be reduced by application of external energy sources, such as UV radiation or heat. Although the recovery time for Ag-decorated (4,4)SWSiCNT is quite short, its use is limited to thermopower-based, magneto-based or resistance-based measurements.
| Compound | Quantity | |||||||
|---|---|---|---|---|---|---|---|---|
| E Ads (eV) | Recovery Time (s) | Suitability for different H2S sensing mechanisms | Ref. | |||||
| Thermopower-based | Magneto-based | Seebeck-effect-based | Spin conduction-based | Resistance-based | ||||
| Pristine (4,4)SWSiCNT | −0.391 | 3.56 × 10−6 | Weak | ✗ | ✓ | ✗ | ✓ | This work |
| Cu-decorated (4,4)SWSiCNT | −0.965 | 1.54 × 104 | ✓ | ✓ | ✓ | ✓ | ✓ | This work |
| Ag-decorated (4,4)SWSiCNT | −0.618 | 2.34 × 10−2 | ✓ | ✓ | ✗ | Weak | ✓ | This work |
| Pristine (10,0)CNT | +0.80 | NA | ✗ | NR | ✗ | NR | ✓ | ref. 9, 2019 |
| B-doped (10,0)CNT | +0.61 | NA | ✗ | NR | ✗ | NR | ✓ | ref. 9, 2019 |
| N-doped (10,0)CNT | +0.79 | NA | ✗ | NR | ✗ | NR | ✓ | ref. 9, 2019 |
| Pt3-decorated (8,0)CNT | −2.61 | 6.41 × 1032 * | ✓ | NR | ✓ | NR | ✓ | ref. 41, 2018 |
| Au-doped (8,0)CNT | −1.25 | 9.56 × 108 * | ✓ | NR | ✗ | NR | ✓ | ref. 8, 2014 |
| (5,5)CNT | −0.002 | 1.08 × 10−12 * | ✗ | NR | ✗ | NR | ✓ | ref. 10, 2013 |
In short, the material presented in this article paves the way towards the development of practical and efficient devices as H2S sensors.
Author contributions
R. Safaiee: conceptualization, methodology, formal analysis. M. M. Golshan: methodology, supervision, writing-review and editing. M. Khalifeh: conceptualization, software.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was in part supported by grants from the Research Council of Shiraz University, under the contracts 99GRD1M240190 (R. Safaiee) and 99GRD1M1110 (M. M. Golshan).References
- E. Salih and A. I. Ayesh, Superlattices Microstruct., 2020, 146, 106650 CrossRef CAS.
- G. Liu, Y. Wang, J. Li, Y. Liu and M. Salehabadi, J. Sulfur Chem., 2020, 1–11 Search PubMed.
- M. Bagherizadeh, M. Arabieh and N. Gilani, Phys. E, 2019, 110, 81 CrossRef CAS.
- S. Bagherzadeh-Nobari, K. H. Istadeh and R. Kalantarinejad, Phys. E, 2020, 115, 113691 CrossRef CAS.
- X. Tong, W. Shen, X. Zhang, J. P. Corriou and H. Xi, J. Alloys Compd., 2020, 832, 155015 CrossRef CAS.
- S. Yang, J. Sun, L. Xu, Q. Zhou, X. Chen, S. Zhu and H. Song, Sens. Actuators, B, 2020, 324, 128725 CrossRef CAS.
- X. Jia, H. Zhang, Z. Zhang and L. An, Superlattices Microstruct., 2019, 134, 106235 CrossRef CAS.
- X. Zhang, Z. Dai, Q. Chen and J. Tang, Phys. Scr., 2014, 89, 065803 CrossRef CAS.
- R. Srivastava, H. Suman, S. Shrivastava and A. Srivastava, Chem. Phys. Lett., 2019, 731, 136575 CrossRef CAS.
- M. Oftadeh, M. Gholamian and H. H. Abdallah, Int. Nano Lett., 2013, 3, 7 CrossRef.
- X. Gao, Q. Zhou, J. Wang, L. Xu and W. Zeng, Nanomaterials, 2020, 10, 299 CrossRef CAS.
- E. Mohammadi-Manesh, M. Vaezzadeh and M. Saeidi, Surf. Sci., 2015, 636, 36 CrossRef CAS.
- S. Kundu and P. Sahoo, New J. Chem., 2019, 43, 12369 RSC.
- Y. Boutillara, J. L. Tombeur, G. De Weireld and P. Lodewyckx, Chem. Eng. J., 2019, 372, 631 CrossRef CAS.
- A. Sultangazin, J. Kusmangaliyev, A. Aitkulov, D. Akilbekova, M. Olivero and D. Tosi, IEEE Sens. J., 2017, 17, 6935 CAS.
- S. Li, L. Xie, M. He, X. Hu, G. Luo, C. Chen and Z. Zhu, Sens. Actuators, B, 2020, 310, 127828 CrossRef CAS.
- I. Urriza-Arsuaga, M. Bedoya and G. Orellana, Anal. Chem., 2018, 91, 2231 CrossRef.
- R. Malik, V. K. Tomer, Y. K. Mishra and L. Lin, Appl. Phys. Rev., 2020, 7, 021301 CAS.
- N. Dasgupta, S. Ranjan and E. Lichtfous, Environmental Nanotechnology, Springer, Switzerland, 2020, vol. 4 Search PubMed.
- H. Xun, Z. Zhang, A. Yu and J. Yi, Sens. Actuators, B, 2018, 273, 983 CrossRef CAS.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795 CrossRef CAS PubMed.
- G. Jimenez-Cadena, J. Riu and F. X. Rius, Analyst, 2007, 132, 1083 RSC.
- Y. T. Azar, M. S. Lakmehsari, S. M. K. Manzoorolajdad, V. Sokhanvaran, Z. Ahadi, A. Shahsavani and P. K. Hopke, Mater. Chem. Phys., 2020, 239, 122105 CrossRef CAS.
- H. Cui, C. Yan, P. Jia and W. Cao, Appl. Surf. Sci., 2020, 512, 145759 CrossRef CAS.
- I. Torres, S. Mehdi Aghaei, A. Rabiei Baboukani, C. Wang and S. Bhansali, C–Open Access Carbon Res. J., 2018, 4, 44 CrossRef.
- S. W. Lee, W. Lee, Y. Hong, G. Lee and D. S. Yoon, Sens. Actuators, B, 2018, 255, 1788 CrossRef CAS.
- S. Nikmanesh, R. Safaiee and M. H. Sheikhi, Mater. Chem. Phys., 2021, 257, 123808 CrossRef CAS.
- S. Nasresfahani, R. Safaiee and M. H. Sheikhi, Surf. Sci., 2017, 662, 93 CrossRef CAS.
- R. Ghanbari, R. Safaiee and M. M. Golshan, Appl. Surf. Sci., 2018, 457, 303 CrossRef CAS.
- N. Ershadi, R. Safaiee and M. M. Golshan, Appl. Surf. Sci., 2021, 536, 147718 CrossRef CAS.
- S. Impeng, A. Junkaew, P. Maitarad, N. Kungwan, D. Zhang, L. Shi and S. Namuangruk, Appl. Surf. Sci., 2019, 473, 820 CrossRef CAS.
- D. Matatagui, O. V. Kolokoltsev, N. Qureshi, E. V. Mejía-Uriarte and J. M. Saniger, Nanoscale, 2015, 7, 9607 RSC.
- C. Chakravarty, B. Mandal and P. Sarkar, J. Phys. Chem. C, 2016, 120, 28307 CrossRef CAS.
- A. Bellucci, M. Girolami and D. M. Trucchi, Ultra-High Temperature Thermal Energy Storage, Transfer and Conversion, Woodhead Publishing Series in Energy, 2021, p. 253–284 Search PubMed.
- C. A. Papadopoulos, D. S. Vlachos and J. N. Avaritsiotis, Sens. Actuators, B, 1996, 34, 524 CrossRef CAS.
- E. Ashori, F. Nazari and F. Illas, Int. J. Hydrogen Energy, 2014, 39, 6610 CrossRef CAS.
- H. Suman, R. Srivastava, S. Shrivastava, A. Srivastava, A. P. Jacob and C. S. Malvi, Chem. Phys. Lett., 2020, 745, 137280 CrossRef.
- Z. Khodadadi, Phys. E, 2018, 99, 261 CrossRef CAS.
- Z. Bo, X. Guo, X. Wei, H. Yang, J. Yan and K. Cen, Phys. E, 2019, 109, 156 CrossRef CAS.
- D. Muliyati, S. A. Wella, T. D. K. Wungu and Suprijadi, AIP Conf. Proc., 2015, 1677, 080009 CrossRef.
- X. Zhang, H. Cui, D. Chen, X. Dong and J. Tang, Appl. Surf. Sci., 2018, 428, 82 CrossRef CAS.
- S. Lin, S. Zhang, X. Li, W. Xu, X. Pi, X. Liu and H. Chen, J. Phys. Chem. C, 2015, 119, 19772 CrossRef CAS.
- S. S. Lin, J. Phys. Chem. C, 2012, 116, 3951 CrossRef CAS.
- G. Alfieri and T. Kimoto, J. Comput. Theor. Nanosci., 2012, 9, 1850 CrossRef CAS.
- P. Nematollahi and M. D. Esrafili, RSC Adv., 2016, 6, 59091 RSC.
- J. A. Talla, Comput. Condens. Matter, 2019, 19, e00378 CrossRef.
- M. D. Esrafili and N. Saeidi, Chem. Phys. Lett., 2017, 671, 49 CrossRef CAS.
- W. Shi, S. Wu and Z. Wang, Phys. E, 2016, 81, 192 CrossRef CAS.
- C. Pham-Huu, N. Keller, G. Ehret and M. J. Ledoux, J. Catal., 2001, 200, 400 CrossRef CAS.
- I. J. Wu and G. Y. Guo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 035343 CrossRef.
- S. P. Huang, D. S. Wu, J. M. Hu, H. Zhang, Z. Xie, H. Hu and W. D. Cheng, Opt. Express, 2007, 15, 10947 CrossRef CAS PubMed.
- B. Baumeier, P. Krüger and J. Pollmann, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085407 CrossRef.
- J. Kürti, V. Zólyomi, M. Kertesz, G. Sun, R. H. Baughman and H. Kuzmany, Carbon, 2004, 42, 971–978 CrossRef.
- J. W. Ding, X. H. Yan and J. X. Cao, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 073401 CrossRef.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni and A. Dal Corso, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- R. Ghanbari, R. Safaiee, M. H. Sheikhi, M. M. Golshan and Z. K. Horastani, ACS Appl. Mater. Interfaces, 2019, 11, 21795 CrossRef CAS and references therein.
- M. Khalifeh, R. Safaiee and M. M. Golshan, Appl. Surf. Sci., 2021, 540, 148323 CrossRef CAS and references therein.
- M. Arabieh and Y. T. Azar, Appl. Surf. Sci., 2018, 434, 604 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- K. Laasonen, R. Car, C. Lee and D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 6796 CrossRef CAS.
- K. M. Alam and A. K. Ray, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 035436 CrossRef.
- S. I. Allec and B. M. Wong, J. Phys. Chem. Lett., 2016, 7, 4340 CrossRef CAS.
- D. Shiojiri, T. Iida, T. Kadono, M. Yamaguchi, T. Kodama, S. Yamaguchi, S. Takahashi, Y. Kayama, K. Hiratsuka, M. Imai, N. Hirayama and Y. Imai, J. Appl. Phys., 2021, 129, 115101 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- J. D. Head and M. C. Zerner, Phys. Lett., 1985, 122, 264 CAS.
- Y. Zhang, H. Qin, E. Cao, F. Gao, H. Liu and J. Hu, J. Phys. Chem. A, 2011, 115, 9987 CrossRef CAS PubMed.
- B. Xu, J. Ouyang, Y. Xu, M. S. Wu, G. Liu and C. Y. Ouyang, Comput. Mater. Sci., 2013, 68, 367 CrossRef CAS.
- J. S. Khaira, R. N. Jain, B. Chakraborty and L. M. Ramaniah, AIP Conf. Proc., 2015, 1665, 130061 CrossRef.
- H. Cui, P. Jia and X. Peng, Appl. Surf. Sci., 2020, 513, 145863 CrossRef CAS.
- H. Cui, G. Zhang, X. Zhang and J. Tang, Nanoscale Adv., 2019, 1, 772 RSC.
- X. Hao, C. Ma, X. Yang, T. Liu, B. Wang, F. Liu and G. Lu, Sens. Actuators, B, 2018, 255, 3033 CrossRef CAS.
- H. Cui, P. Jia, X. Peng and P. Li, Mater. Chem. Phys., 2020, 249, 123006 CrossRef CAS.
- G. Korotcenkov, Catalysts used in calorimetric (Combustion-Type) gas sensors, Handbook of Gas Sensor Materials. Integrated Analytical Systems, Springer, New York, 2013 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |