Flexible fluorinated multi-walled carbon nanotube/polyarylene ether nitrile metacomposites with negative permittivity†
Weihua
Han
ab,
Feng
Gao
a,
Lingyun
Zhou
a,
Lu
Wang
c,
Xiufu
Hua
*d,
Xinyu
Xue
a,
Zhiqiang
Li
a,
Wei
Luo
a,
Lingyun
Pang
a and
Renbo
Wei
 *a
*a
aSchool of Chemical Engineering, Northwest University, Xi’an 710069, China. E-mail: weirb10@nwu.edu.cn
bHigh-tech Institute, QingZhou 262550, China
cSichuan Institute of Piezoelectric and Acoustooptic Technology, Chongqing 400060, China
dYangtze Delta Region Institute of Tsinghua University, Jiaxing 314006, China. E-mail: hua_xiufu@163.com
First published on 19th November 2021
Abstract
Flexible metacomposites demonstrating negative permittivity have shown promising applications in flexible electronics. In this work, flexible fluorinated multi-walled carbon nanotube/polyarylene ether nitrile porous metacomposite films (FMWCNT/PEN) with negative permittivity have been fabricated via a delayed phase inversion method. FMWCNT, which demonstrates excellent dispersion in PEN, was prepared via the fluorination of MWCNT by treating it with NaOH and HF sequentially, and then characterized by XPS, XRD, FT-IR, Raman spectroscopy, SEM and TEM. SEM observations indicate the honeycomb porous structure of FMWCNT/PEN, and FMWCNT disperses homogeneously on the surface of the walls of the porous PEN matrix. The results show that a continuous conductive network is formed in the FMWCNT/PEN porous metacomposite at an FMWCNT content as low as 2 wt%, resulting in a plasma-type negative permittivity spectrum of FMWCNT/PEN via the plasma oscillation of the delocalized charge carriers in the system. In addition, the FMWCNT/PEN metacomposite reveals outstanding flexibility with an elongation at break of 80.3% and stable dielectric properties even after 50 folding and unfolding cycles. This work shows an innovative technique to realize metacomposites demonstrating regulable negative permittivity and outstanding mechanical properties.
1 Introduction
Metamaterials that can interact with electromagnetic waves under a desired mode have recently attracted growing interest due to their promising applications in invisible cloaks, sensors, waveguides, electronic devices, antennas, microwave absorbers, magnetic resonance imaging and so forth.1–8 As a kind of metamaterial, metamaterials with negative permittivity (also named as ε-negative materials) possess a negative dielectric constant at a certain frequency in comparison with the traditional positive dielectric constant.9,10 ε-negative materials could be used to fabricate capacitors showing huge permittivity and low dissipation.11–13 Therefore, material scientists are increasingly turning to the preparation of ε-negative materials with increasing applications in electronic devices.14Like other metamaterials, ε-negative materials are initially engineered with an artificially periodic array of structures.15 However, the fabrication of the periodic array of structures is too laborious to achieve their practical applications.16,17 Therefore, more and more attention have been drawn to isotropic ε-negative materials, which can be obtained via classical material design and universal fabrication techniques.18 Particularly, homogeneous metacomposites whose negative permittivity can be facilely tuned by adjusting their constituents have been extensively investigated as one of the hotspots of metamaterials.19 In these metacomposites, the synergistic effect between the matrix and the filler results in the negative permittivity rather than the periodic array of structures.20 In addition, both the additives and the matrices can be replaced by other materials demonstrating similar properties.21,22 Therefore, metacomposites have broadened the scope of the fabrication and practical applications of ε-negative materials.23–25
With their ultrahigh electron density, metals exhibit negative permittivity via electron plasma oscillation.26,27 However, their strong negative dielectric constant and high dissipation lead to an increase of the imaginary value of complex permittivity of metals, which hampers their application.18 Fortunately, metals can be used as additives in nonmetal matrices to obtain composites demonstrating negative permittivity.28,29 For example, ceramic-based metacomposites have been reported by incorporating Cu, Ag, Fe, Ni and their alloys into Al2O3 and other ceramic matrices.30–34 Ceramic metacomposites have already demonstrated exceptional functions and applications.35,36 Recently, polymeric-based metacomposites have been intensively investigated due to the development of flexible electronics.37,38 Besides metals, carbon materials with excellent electrical and mechanical properties are also used as conductive additives for the fabrication of polymeric-based metacomposites.39–42 More importantly, non-conductive fillers (including SiC, BaTiO3, and Fe3O4) can also be applied when conducting polymers such as polypyrrole (PPy) and polyaniline (PANI) are used as the polymeric matrices.43–46 This tremendously enriches the variety of the ε-negative materials.47
Polymers have been widely used as dielectric materials.48 With the incorporation of conducting additives, the dielectric constant of the obtained composite enhances when the content of the filler is lower than its percolating threshold value due to the Maxwell–Wagner effect.49,50 For example, PANI, CNT, graphene and others have been introduced into polymers matrices for the improvement of their dielectric constant.51–55 It has been reported that the negative permittivity of the composites is related to the free carrier density in the system.56,57 In order to fabricate polymeric-based metacomposites, abundant fillers whose content should be higher than its percolating threshold value are needed.58,59 This negative permittivity is obviously at the expense of other properties of the composite, especially for additives such as CNT and graphene, which will aggregate spontaneously at a higher content.60,61 As a result, composites with a low percolation threshold are highly demanded in the fabrication of ε-negative materials.62 Recently, we have prepared porous PEN via the delayed phase inversion method.63,64 The high void fraction enables the enrichment of fillers at the walls of the porous structure of PEN. Therefore, negative permittivity can be potentially obtained at a lower content of additives.
In this study, multi-walled carbon nanotubes (MWCNT) and polyarylene ether nitrile are used as raw materials for the fabrication of metacomposites with negative permittivity. In order to improve the dispersion of MWCNT in PEN, MWCNT was modified by NaOH and HF resulting in fluorinated MWCNT (FMWCNT). The FMWCNT/PEN composite demonstrating negative permittivity was obtained at a low FMWCNT content via the delayed phase inversion method. This research shows an innovative technique to realize metacomposites demonstrating regulated negative permittivity and outstanding mechanical properties.
2 Experimental
2.1 Materials
MWCNTs (95%, diameter: 20–40 nm) were supplied by the Nanjing XFNano Materials. Dichlorobenzonitrile, ethanol, acetone, sodium hydroxide, hexafluorobisphenol A, N,N-dimethylacetamide (DMAc), hydrochloric acid, potassium carbonate and N-methylpyrrolidone were purchased from Chengdu Kelong. PEN was prepared according to the method mentioned in the literature.632.2 Fabrication of fluorinated multi-walled carbon nanotube FMWCNT
The fluorinated MWCNTs were prepared according to the literature.65 Firstly, 0.2 g commercial MWCNTs were washed with 0.2 L acetonum, and then dispersed in 0.1 L sodium hydroxide (1 mol L−1) for 8 hours with magnetic stirring, resulting in hydrophilic MWCNTs. Secondly, these obtained hydrophilic MWCNTs were filtered and washed with water until neutral. Next, hydrophilic MWCNTs were fluorinated by stirring in 0.1 L hydrofluoric acid (wt = 10%) for 12 h. Finally, fluorinated MWCNTs (named FMWCNT) were obtained by sequential filtration, washing until neutral and drying at 80 °C for 12 h.2.3 Fabrication of FWCNT/PEN dense composite film
The FMWCNT/PEN dense composite film was fabricated via the casting method. The 2 wt% FMWCNT and 98 wt% PEN were added in NMP in an 80 °C bath with stirring for 2 hours and obtaining a homogeneous FWCNT/PEN dispersion. Then, the obtained dispersion was poured on a glass plate, which naturally transformed it into a liquid film. The liquid film was dried at 100 °C, 120 °C, 140 °C, 160 °C and 200 °C for 2 h each in an oven. After cooling back to room temperature naturally, the FMWCNT/PEN dense composite film was obtained. The FMWCNT/PEN dense composite film was named as FMWCNT2/PEN-DF, and the pure PEN dense film was named as PEN-DF.2.4 Fabrication of FWCNT/PEN porous metacomposite film
The FMWCNT/PEN porous metacomposite film with negative permittivity was fabricated via the delayed phase inversion method. Firstly, 2 wt% FMWCNT and 98 wt% PEN were dispersed in NMP in an 80 °C bath with stirring for 2 hours, obtaining a homogeneous FMWCNT/PEN dispersion. Secondly, the obtained FMWCNT/PEN dispersion was cast on a glass plate and then scraped into a 200 μm thickness liquid film by a scratcher. In the meantime, 2-methyl imidazole was mixed with ethanol offering a ethanol/2-methyl imidazole coagulation bath. After that, the FMWCNT/PEN liquid film, which was on the glass slide was moved into the ethanol/2-methyl imidazole coagulant bath for 12 h, during which, the delayed phase inversion approach processes produced the FMWCNT/PEN porous metacomposite film. Ultimately, FMWCNT/PEN porous metacomposite film was dried at 60 °C for 20 hours. The FMWCNT/PEN porous the metacomposite film was named FMWCNT2/PEN-PF, and the pure PEN film made from the delayed phase inversion method was named PEN-PF. For comparison, an MWCNT/PEN porous film was also prepared under the same conditions.2.5 Characterization
AST-2722 semiconductor powder resistivity tester was used to measure the electronic conductivity of the composites. A scanning electron microscope (SEM, Sirion 200 FEI) and transmission electron microscope (TEM, JEM-2100, JEOL) were used to identify microstructures in the samples. Energy dispersive spectroscopy (EDS, INCA Energy Spectrometer), X-ray photoelectron spectroscopy (XPS, Escalab 250) and Fourier transforms infrared spectroscopy (FT-IR, Nicolet 5700) were used for the characterization of chemical components in the samples. X-ray diffraction (XRD, Brucker D8 Advance diffractometer with Cu Kα radiation) was used for characterizing the crystal structure of CNTs. LabRAM HR800 (JY Ho-riba) with a laser excitation at 532 nm was applied for the collection of Raman spectra on the CNTs. Netzsch STA 449C thermogravimetric-differential scanning calorimetry (TG-DSC) was used for collecting TGA curves on samples in N2 from RT to 800 °C at a rate of 10 °C min−1. An SANSCMT6104 series desktop electromechanical universal testing machine was used for measuring the mechanical properties of the PEN-based composites. A dielectric spectrometer (Concept 50, Novocontrol) was applied to test the dielectric property of the samples from 1 Hz to 2 × 107 Hz.3 Results and discussion
In this study, a flexible fluorinated multi-walled carbon nanotube/polyarylene ether nitrile porous metacomposite film (FMWCNT/PEN-PF) with negative permittivity was fabricated. Firstly, MWCNT was treated with NaOH and HF sequentially resulting in FMWCNT, which was characterized using XPS, XRD, FT-IR, Raman spectrum and TEM. At the same time, PEN containing hexafluorobisphenol A unit was polymerized on the basis of the literature.63 Finally, FMWCNT/PEN-PF was obtained through the prepared FMWCNT and PEN via the delayed phase inversion method. The fluorination of MWCNT enabled the excellent dispersibility of FMWCNT in the PEN matrix, and the delayed phase inversion method introduces honeycomb porous structures in the composites. As a result, the low content of FMWCNTs enriches the surface of walls of porous structures forming the conducting network in composites, which leads to negative permittivity according to the Drude model.45
Fig. 1a shows the XPS spectra of MWCNT and FMWCNT, compared with the spectrum of MWCNT, which exhibits only one peak at 284.5 eV, coming from the C element, FMWCNT demonstrate three peaks at 687.0, 533.0 and 284.5 eV, indicating the existence of F, O and C elements. To further analyse the chemical states of C elements, high-resolution XPS spectra of MWCNT and FMWCNT were obtained. Fig. 1b is C1s XPS spectrum of MWCNT from which the spectrum can be fitted to four different states peaked at 284.8, 285.3, 286.5 and 290.9 eV, corresponding to C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O respectively. After the fluorination treatment, the peak from O
C–O respectively. After the fluorination treatment, the peak from O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O disappears and the intensities of peaks from C–O, C
C–O disappears and the intensities of peaks from C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O decrease, while a new peak at 290.2 eV for C–F is observed. (Fig. 1c). The chemical structure of FMWCNT was also investigated through FTIR spectra, as shown in Fig. 1d. For MWCNT, no obvious vibration peaks were observed, as there are only symmetrical C–C bonds in its structure. In comparison, vibration bands centered at 1210 and 3420 cm−1 are observed in the FT-IR spectrum of FWCNT. These vibration bands are coming from the characteristic bands of C–F and O–H bonds after fluorination treatment. Fig. 1e shows Raman spectra of MWCNT and FMWCNT. Two peaks centered at 1590 and 1330 cm−1 were detected from CNT spectra, corresponding to G and D bands. The intensity ratio of these peaks (ID/IG) increases obviously from MWCNT to FMWCNT, suggesting that the incorporation of F atoms destroys the chemical structure of MWCNT. Fig. 1f presents XRD patterns of MWCNT and FMWCNT. For the XRD pattern of MWCNT, the peak at 2θ = 26.28° corresponds to (002) plane of the graphite structure and suggests that the interlayer spacing of MWCNT is 0.33 nm. In comparison, the peak disappears and two new peaks centered at around 13° (001) and 42° (100) were observed for FMWCNT. The disappearance of the peak of 26.28° might be due to the fact that fluorination destroys the structure of MWCNT. A similar result has also been reported in the literature.66 According to the Scherrer equation, the interlayer distance of 0.34 nm is thus obtained for FMWCNT. In addition, the HRTEM micrograph shown in Fig. S1 in the ESI† also shows an interlayer distance of 0.34 nm of FMWCNT.
O decrease, while a new peak at 290.2 eV for C–F is observed. (Fig. 1c). The chemical structure of FMWCNT was also investigated through FTIR spectra, as shown in Fig. 1d. For MWCNT, no obvious vibration peaks were observed, as there are only symmetrical C–C bonds in its structure. In comparison, vibration bands centered at 1210 and 3420 cm−1 are observed in the FT-IR spectrum of FWCNT. These vibration bands are coming from the characteristic bands of C–F and O–H bonds after fluorination treatment. Fig. 1e shows Raman spectra of MWCNT and FMWCNT. Two peaks centered at 1590 and 1330 cm−1 were detected from CNT spectra, corresponding to G and D bands. The intensity ratio of these peaks (ID/IG) increases obviously from MWCNT to FMWCNT, suggesting that the incorporation of F atoms destroys the chemical structure of MWCNT. Fig. 1f presents XRD patterns of MWCNT and FMWCNT. For the XRD pattern of MWCNT, the peak at 2θ = 26.28° corresponds to (002) plane of the graphite structure and suggests that the interlayer spacing of MWCNT is 0.33 nm. In comparison, the peak disappears and two new peaks centered at around 13° (001) and 42° (100) were observed for FMWCNT. The disappearance of the peak of 26.28° might be due to the fact that fluorination destroys the structure of MWCNT. A similar result has also been reported in the literature.66 According to the Scherrer equation, the interlayer distance of 0.34 nm is thus obtained for FMWCNT. In addition, the HRTEM micrograph shown in Fig. S1 in the ESI† also shows an interlayer distance of 0.34 nm of FMWCNT.
 | ||
| Fig. 1 (a) XPS spectra of MWCNT and FMWCNT. XPS C1s spectrum of MWCNT (b) and FMWCNT (c). FTIR spectra (d), Raman spectra (e) and XRD patterns (f) of MWCNT and FMWCNT. | ||
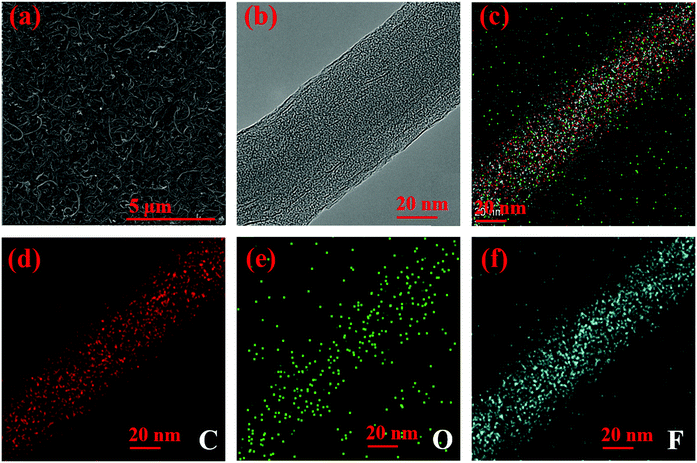
The microscopic morphology of FMWCNT is shown in Fig. 2. Fig. 2a is the SEM micrograph of FMWCNT, from which tubular-shaped nanotubes are observed. Compared with MWCNT (Fig. S2, ESI†), the diameter of FMWCNT increases while its length decreases. This is due to the introduction of F atoms in FMWCNT and C–C bonds are destroyed during fluorination. In addition, compared with MWCNTs that are heavily agglomerated, FMWCNT can be easily and uniformly dispersed in a solvent such as ethanol. Fig. 2b is the TEM micrograph of FMWCNT, from which a diameter of 60 nm can be obtained, which is identical to the result of SEM. TEM EDS mapping was also used to characterize the structure of FMWCNT. C, O and F elements are clearly observed from the TEM EDS mapping micrograph of FMWCNT (Fig. 2c–f). what is more, fluorine atoms distribute homogeneously at the surfaces of FMWCNT according to EDS mapping. According to the measurements mentioned above, FMWCNT was successfully prepared.
With the prepared FMWCNT and PEN, the FMWCNT/PEN porous metacomposite film was fabricated via the delayed phase inversion method. Fig. 3 depicts the real permittivity of the PEN-based composites within the frequency range of 1–2 × 107 Hz. For the pristine PEN, the dense film made from the solution casting method (PEN-DF) demonstrates a dielectric constant of 3.8 at 1 kHz (Fig. 3a), the result has been reported in the literature.63 The porous film made from the delayed phase inversion method of PEN (PEN-PF) exhibits a low permittivity of 1.7 at 1 kHz (Fig. 3b). This phenomenon has resulted from the incorporation of pores into PEN-PF during the delayed phase inversion method, and these porous structures lower the permittivity of the obtained film.63 With the addition of fillers, it has been widely reported that the permittivity of polymeric dielectrics can be easily enhanced by adding conducting fillers and/or additives with high permittivity due to the Maxwell Wagner Sillars effect.67 As a result, the dielectric constant of the dense composite film FMWCNT2/PEN-DF increases to 50 at 1 kHz (Fig. 3a) when 2 wt% of FMWCNT is incorporated into the PEN matrix. However, an interesting negative dielectric phenomenon was observed for FMWCNT2/PEN-PF with 2 wt% of FMWCNT incorporated into the porous PEN matrix (Fig. 3b and Fig. S3, S4, ESI†). In addition, the dielectric constant of FMWCNT2/PEN-PF changes from minus to positive with the increase in frequency, demonstrating a plasma-type negative permittivity spectrum.68,69
Generally, negative permittivity results from the damping of free electrons.69 Therefore, metals exhibit negative permittivity easily due to the plasma oscillation of free electrons under alternating electric fields. As for the nonmetal dielectric, the formation of a continuous conducting network, which can form plasma oscillation via the delocalized charge carriers can also lead to negative permittivity.68–71 The Drude model is usually applied to explain plasma-type negative permittivity spectrum.45,68–70 Based on the Drude model the dielectric constant of dielectrics could be described according to eqn (1):71
| ε(ω) = 1−ωp2/[ω(ω–iγ)] | (1) |
In which ω represents the angular frequency, γ means damping constant, ωp expresses the plasma frequency. The fitting curve of the permittivity of FMWCNT2/PEN-PF obtained using the Origin software is shown in Fig. 3b. The fitted curve is in accordance with the measured result with a coefficient (R2) of 0.99566. What is more, according to the equation, the dielectric constant would be minus at the condition of ω < ωp, and it switches to positive when ω > ωp. Based on the Drude model for the fitted curve of FMWCNT2/PEN-PF, the ωp is 18 Hz. The imaginary permittivity of FMWCNT/PEN-PF composites is shown in Fig. S3e (ESI†). It is obvious that the imaginary permittivity of the composites increases with the increase of FMWCNT content. For FMWCNT2/PEN-PF, the imaginary permittivity decreases exponentially from 1–30 Hz due to the conduction loss.69 Furthermore, a peak around 20–28 Hz, which is corresponding to ωp was observed from the dielectric loss tangent of FMWCNT2/PEN-PF (Fig. S3f, ESI†).69 The conduction loss and loss peak indicate the formation of the conducting network in FMWCNT2/PEN-PF, which leads to negative permittivity.
Fig. 4 shows cross-sectional SEM micrographs of the PEN-based materials. The micromorphology of pure dense PEN film (PEN-DF) exhibits a compact structure, as shown in Fig. 4a. In comparison, a honeycomb porous structure with a size of about 2–4 μm was observed for the porous PEN film (PEN-PF) (Fig. 4b and e). This porous structure explains the decrement of dielectric constant from PEN-DF to PEN-PF. With the incorporation of FMWCNT into the PEN matrix, the cross-sectional SEM micrograph of FMWCNT2/PEN-DF is shown in Fig. 4d. As the FMWCNT are modified by fluorine atoms, the FMWCNT disperses homogeneously in the bulk PEN matrix. In addition, as only 2 wt% of FMWCNT is incorporated into FMWCNT2/PEN-DF, which is lower than the percolating threshold value (2.6–3.2 wt%, Fig. S5a, ESI†) of the FMWCNT/PEN-DF composite, FMWCNT cannot form a conducting network in the FMWCNT2/PEN-DF composite (Fig. S6a, ESI†).72,73 As a result, the dielectric constant of FMWCNT2/PEN-DF is enhanced to 50 from 3.8 of PEN-DF. As for FMWCNT2/PEN-PF, the cellular porous morphology was also observed from SEM micrographs (Fig. 4c and f). What is more, FMWCNT also disperses homogeneously in FMWCNT/PEN-PF without aggregation. However, it can be seen obviously that the FMWCNT is at the surface of PEN rather than penetrating the bulk of PEN. As a result, FMWCNT is enriched at the surface of walls of FMWCNT2/PEN-PF and thus can form a continuous conducting network at FMWCNT content of 2 wt% (Fig. S6b, ESI†). The plasma oscillation of the delocalized charge carriers in the continuous conducting network contributes to the negative permittivity property of FMWCNT2/PEN-PF.
 | ||
| Fig. 4 SEM micrographs of PEN-DF (a), PEN-PF (b and e), FMWCNT2/PEN-PF (c and f), and FMWCNT2/PEN-DF (d). | ||
To further investigate the reason for the negative permittivity of porous FMWCNT2/PEN-PF composite film, 1 wt% of FMWCNT was incorporated into the porous PEN matrix to prepare FMWCNT1/PEN-PF. Fig. S7 (ESI†) shows that FMWCNT is dispersed at the surface of PEN walls of the cellular porous structures of FMWCNT1/PEN-PF. As less FMWCNT was applied, FMWCNT cannot form a conducting network in the FMWCNT1/PEN-PF composite (Fig. S6c, ESI†). Consequently, the dielectric constant of FMWCNT1/PEN-PF was enhanced compared to PEN-PF and no negative permittivity was observed at the measured frequency (Fig. S3d and S4c, ESI†). Furthermore, a higher content of FMWCNT (10 wt%) was incorporated into the dense PEN matrix during the preparation of FMWCNT10/PEN-DF. A conducting network can be observed in the composite as shown in Fig S6d and Fig S8 (ESI†). As a result, a plasma-type negative permittivity spectrum as shown in Fig. S9 (ESI†) was obtained for FMWCNT10/PEN-DF. These results indicate that the formation of conduction networks with delocalized charge carriers in the system leads to the negative permittivity of composites. Besides, 10 wt% of MWCNT was incorporated into the dense and porous PNE matrix during the preparation of the composites MWCNT10/PEN-DF and MWCNT10/PEN-PF. The results show that the dielectric constants of MWCNT10/PEN-DF and MWCNT10/PEN-PF are enhanced to higher positive values rather than negative permittivity (Fig. 3c). This is because MWCNT will aggregate spontaneously in the PEN matrix through the van der Waals force (Fig. S10, ESI†). Although the content of MWCNT is higher than that in FMWCNT2/PEN-PF, no continuous conducting network was formed in MWCNT10/PEN-DF nor in MWCNT10/PEN-PF (Fig. S6e, ESI†), thus no negative permittivity was observed in these systems. Therefore, excellent dispersion and high enough content of FMWCNT are necessary for the formation of conductive networks, which contributes to the negative permittivity of the composites. As FWMCNT can be enriched at the surface of the PEN walls of the honeycomb pores of FMWCNT/PEN porous composites, the negative permittivity of the composite can be obtained at a lower content of 1.8 wt% of FMWCNT (Fig. S5b, ESI†). This FMWCNT content is lower than most of the reported filler's content in composites showing negative permittivity (Table 1), which avoids the sacrifice of other properties of the polymer matrix.
| Sample | Filler | Content | Ref. |
|---|---|---|---|
| Polyurethane sponge/AgNWs | AgNWs | 6.3 wt% | 2 |
| Polyurethanes/MWCNTs | MWCNTs | 20 wt% | 9 |
| Polyvinylidene fluoride/Ti3AlC2 | Ti3AlC2 | 77 vol% | 11 |
| Polyvinylidene fluoride/graphite | Graphite | 20.3 vol% | 13 |
| Graphene/CaCu3Ti4O12 | Graphene | 4 wt% | 16 |
| Polydimethylsiloxane/graphene | Graphene | 20 wt% | 18 |
| Polyimide/TiN | TiN | 5 wt% | 47 |
| Polyvinylidene fluoride/Ni | Ni | 6 wt% | 47 |
| Polyimide/Carbon nanotubes | CNTs | 8.1 vol% | 74 |
| PEN/FMWCNT | FMWCNT | 1.8 wt% | This work |
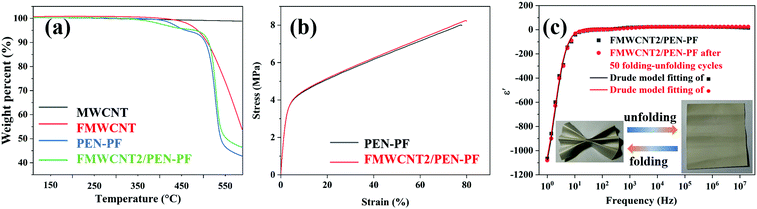
Fig. 5a is the thermogravimetric analysis results of MWCNT, FMWCNT, PEN-PF and FMWCNT2/PEN-PF. Although the structure of FMWCNT was destroyed to a certain degree during the fluorination process, it did not disintegrate until 402 °C. In addition, the decomposition temperature T10% of PEN-PF is 504.5 °C. Therefore, the decomposition temperature T10% of FMWCNT2/PEN-PF was as high as 511.9 °C. Fig. 5b and Fig. S11 (ESI†) show the stress–strain curves of porous PEN-PF, PEN-DF and their composites. The tensile modulus FMWCNT2/PEN-PF was calculated to be 172.7 MPa. The tensile modulus of FMWCNT2/PEN-PF was much lower than that of PEN-DF and FMWCNT2/PEN-DF (Fig. S11, ESI†) due to its high void fraction. However, as the density of FMWCNT2/PEN-PF, which can be measured through a solid densitometer directly was 0.216 g cm−3, FMWCNT2/PEN-PF exhibited a higher specific modulus (1171 MPa cm3 g−1) than most of the used polypropylene.63 Another result that can be obtained from the stress–strain curve of FMWCNT2/PEN-PF is that it demonstrates excellent flexibility with the elongation at a break of 80.3%. This excellent flexibility can also be confirmed by the repeated folding and unfolding of FMWCNT2/PEN-PF as shown in Fig. 5c. The result indicates that FMWCNT2/PEN-PF demonstrates stable dielectric properties even after 50 folding and unfolding processes. The excellent mechanical properties of FMWCNT2/PEN-PF enable it to be used as a component in flexible electronics.
4 Conclusions
In this work, flexible porous FMWCNT/PEN metacomposite demonstrating negative permittivity was successfully fabricated via the delayed phase inversion method. The results of XPS, FT-IR, Raman, XRD and TEM EDS mapping micrographs confirmed the successful preparation of FMWCNT. SEM observations indicated that honeycomb porous structures of FMWCNT/PEN-PF composites could be obtained through the delayed phase inversion method, and FMWCNT dispersed homogeneously in the PEN matrix. Compared with the MWCNT/PEN-PF, MWCNT/PEN-DF and FMWCNT/PEN-DF composites, a continuous conducting network of FMWCNT/PEN-PF was formed at an FMWCNT content of 2 wt%, which is lower than the percolating threshold value of FMWCNT/PEN-DF composite, indicating the enrichment of FMWCNT at the surface of the walls of the honeycomb porous structures of FMWCNT/PEN-PF metacomposite. Deriving from the formed continuous conducting network, FMWCNT2/PEN-PF demonstrated a plasma-type negative permittivity via the plasma oscillation of the delocalized charge carriers in the system. In addition, the FMWCNT2/PEN-PF metacomposite exhibited an elongation at a break of 80.3%, which is much higher than that of FMWCNT/PEN-DF composites, and stable dielectric property even after 50 folding and unfolding cycles. This FMWCNT2/PEN-PF metacomposite with negative permittivity demonstrated promising applications in flexible electronics and broadened the fabrication approaches of flexible metacomposites.Authorship contribution
Weihua Han: writing – original draft, writing – review and editing, methodology, resources, formal analysis. Feng Gao: writing – review and editing, formal analysis. Lingyun Zhou: writing – review and editing, forma analysis. Lu Wang: methodology. Xiufu Hua: review and editing, supervision. Xinyu Xue: methodology, resources, formal analysis. Zhiqiang Li: methodology, resources, formal analysis. Wei Luo: methodology, resources. Lingyun Pang: formal analysis, resources. Renbo Wei: writing – review and editing, funding acquisition, project administration, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from Northwest University (No. 363042005106) is gratefully acknowledged.Notes and references
- Z. Wang, K. Sun, P. Xie, R. Fan, Y. Liu, Q. Gu and J. Wang, J. Eur. Ceram. Soc., 2020, 40, 1917–1921 CrossRef CAS.
- Z. Wang, K. Sun, H. Wu, P. Xie, Z. Wang, X. Li and R. Fan, J. Mater. Sci., 2020, 55, 15481–15492 CrossRef.
- J. Ni, R. Zhan and J. Qiu, Org. Electron., 2020, 82, 105706 CrossRef CAS.
- F. C. Yao, W. H. Xie, M. Yang, H. Zhang, H. B. Gu, A. Du, N. Naik, D. P. Young, J. Lin and Z. H. Guo, Mater. Today Phys., 2021, 21, 100502 CrossRef CAS.
- J. C. Xu, J. Q. Cao, M. H. Guo, S. L. Yang, H. M. Yao, M. Lei, Y. N. Hao and K. Bi, Adv. Compos. Hybrid Mater., 2021, 4, 761–767 CrossRef.
- X. H. Wu, C. J. Fu and Z. M. Zhang, ES Energy Environ., 2020, 8, 5–14 Search PubMed.
- T. C. Han, W. Luo, G. Yang and L. J. Deng, ES Energy Environ., 2020, 10, 45–49 Search PubMed.
- J. P. Huang., ES Energy Environ., 2019, 6, 1–3 Search PubMed.
- Z. Wang, K. Sun, Q. Jiang, K. Yin, L. Xie, S. Cao, Y. Zhang, X. Li and R. Fan, Mater. Today Commun., 2020, 24, 101230 CrossRef CAS.
- M. Rajnak, Z. Spitalsky, B. Dolnik, J. Kurimsky, L. Tomco, R. Cimbala, P. Kopcansky and M. Timko, Phys. Rev. Appl., 2019, 11, 024032 CrossRef CAS.
- Z. Wang, J. Fan, X. Guo, J. Ji and Z. Sun, RSC Adv., 2020, 10, 27025–27032 RSC.
- Z. Wang, J. Fan, Y. Li, X. Wang and H. Chen, Ceram. Int., 2019, 7, 160–167 Search PubMed.
- J. Wang, Z. Shi, F. Mao, S. Chen and X. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 1793–1800 CrossRef CAS PubMed.
- A. K. Yadav, K. X. Nguyen, Z. Hong, P. García-Fernández, P. Aguado-Puente, C. T. Nelson, S. Das, B. Prasad, D. Kwon and S. Cheema, Nature, 2019, 565, 468 CrossRef CAS PubMed.
- H. Luo and J. Qiu, Ceram. Int., 2019, 45, 843–848 CrossRef CAS.
- Y. Qu, Y. Du, G. Fan, J. Xin, Y. Liu, P. Xie, S. You, Z. Zhang, K. Sun and R. Fan, J. Alloys Compd., 2019, 771, 699–710 CrossRef CAS.
- C. Cheng, R. Fan, G. Fan, H. Liu, J. Zhang, J. Shen, Q. Ma, R. Wei and Z. Guo, J. Mater. Chem. C, 2019, 7, 3160–3167 RSC.
- K. Sun, J. Dong, Z. Wang, Z. Wang, G. Fan, Q. Hou, L. An, M. Dong, R. Fan and Z. Guo, J. Phys. Chem. C, 2019, 123, 23635–23642 CrossRef CAS.
- H. Gu, X. Xu, M. Dong, P. Xie, Q. Shao, R. Fan, C. Liu, S. Wu, R. Wei and Z. Guo, Carbon, 2019, 147, 550–558 CrossRef CAS.
- C. Yang, Z. Shi, C. Zhang and R. Fan, Mater. Lett., 2018, 231, 87–90 CrossRef CAS.
- G. Fan, G. Shi, H. Ren, Y. Liu and R. Fan, Mater. Lett., 2019, 257, 126683 CrossRef CAS.
- K. Sun, J. Xin, Z. Wang, S. Feng, Z. Wang, R. Fan, H. Liu and Z. Guo, J. Mater. Sci.: Mater. Electron., 2019, 30, 14745–14754 CrossRef CAS.
- P. Xie, Z. Wang, Z. Zhang, R. Fan, C. Cheng, H. Liu, Y. Liu, T. Li, C. Yan and N. Wang, J. Mater. Chem. C, 2018, 6, 5239–5249 RSC.
- T. C. Lim, Adv. Compos. Hybrid Mater., 2019, 2, 657–669 CrossRef.
- Y. L. Jia, P. W. Ren, J. Q. Wang, C. Z. Fan and E. J. Liang, ES Energy Environ., 2020, 7, 4–11 CAS.
- X. Zhang, X. Yan, Q. He, H. Wei, J. Long, J. Guo, H. Gu, J. Yu, J. Liu and D. Ding, ACS Appl. Mater. Interfaces, 2015, 7, 6125–6138 CrossRef CAS.
- K. Sun, Z. Wang, J. Xin, Z. Wang, P. Xie, G. Fan, V. Murugadoss, R. Fan, J. Fan and Z. Guo, Macromol. Mater. Eng., 2020, 305, 1900709 CrossRef CAS.
- Q. Hou, K. Yan, R. Fan, K. Zhang, Z. Zhang, S. Pan and M. Yu, Mater. Chem. Phys., 2016, 170, 113–117 CrossRef CAS.
- K. Sun, J. Xin, Y. Li, Z. Wang, Q. Hou, X. Li, X. Wu, R. Fan and K. L. Choy, J. Mater. Sci. Technol., 2019, 35, 2463–2469 CrossRef.
- Z. C. Shi, R. H. Fan, Z. D. Zhang, L. Qian, M. Gao, M. Zhang, L. T. Zheng, X. H. Zhang and L. W. Yin, Adv. Mater., 2012, 24, 2349–2352 CrossRef CAS PubMed.
- K. Sun, R. Fan, Z. Zhang, K. Yan, X. Zhang, P. Xie, M. Yu and S. Pan, Appl. Phys. Lett., 2015, 106, 172902 CrossRef.
- Z. Shi, R. Fan, Z. Zhang, H. Gong, J. Ouyang, Y. Bai, X. Zhang and L. Yin, Appl. Phys. Lett., 2011, 99, 032903 CrossRef.
- K. Sun, Z. Zhang, L. Qian, F. Dang, X. Zhang and R. Fan, Appl. Phys. Lett., 2016, 108, 061903 CrossRef.
- M. Chen, R. H. Fan, M. Gao, S. B. Pan, M. X. Yu and Z. D. Zhang, J. Magn. Magn. Mater., 2015, 381, 105–108 CrossRef CAS.
- Z. Wang, K. Sun, P. Xie, Y. Liu, Q. Gu, R. Fan and J. Wang, J. Materiomics, 2020, 6, 145–151 CrossRef.
- M. Chen, X. Hu, K. Sun, Y. Qu and R. Fan, ECS J. Solid State Sci. Technol., 2021, 10, 043002 CrossRef CAS.
- X. Yao, X. Kou and J. Qiu, Carbon, 2016, 107, 261–267 CrossRef CAS.
- R. Aepuru, B. B. Rao, S. Kale and H. Panda, Mater. Chem. Phys., 2015, 167, 61–69 CrossRef CAS.
- L. Sun, N. Wu and R. Peng, J. Appl. Polym. Sci., 2020, 137, 49582 CrossRef CAS.
- X. Kou, X. Yao and J. Qiu, Org. Electron., 2016, 38, 42–47 CrossRef CAS.
- H. Wu, R. Yin, L. Qian and Z. Zhang, Mater. Des., 2017, 117, 18–23 CrossRef CAS.
- D. Estevez, F. Qin, Y. Luo, L. Quan, Y.-W. Mai, L. Panina and H.-X. Peng, Compos. Sci. Technol., 2019, 171, 206–217 CrossRef CAS.
- H. Gu, Y. Huang, X. Zhang, Q. Wang, J. Zhu, L. Shao, N. Haldolaarachchige, D. P. Young, S. Wei and Z. Guo, Polymer, 2012, 53, 801–809 CrossRef CAS.
- H. Kavas, M. Günay, A. Baykal, M. S. Toprak, H. Sozeri and B. Aktaş, J. Inorg. Organomet. Polym. Mater., 2013, 23, 306–314 CrossRef CAS.
- H. Gu, J. Guo, M. A. Khan, D. P. Young, T. Shen, S. Wei and Z. Guo, Phys. Chem. Chem. Phys., 2016, 18, 19536–19543 RSC.
- C. Xu, G. Fan, Y. Qu, Y. Liu, Z. Zhang and R. Fan, Polymer, 2020, 188, 122125 CrossRef CAS.
- B. Zhao and C. B. Park, J. Mater. Chem. C, 2017, 5, 6954–6961 RSC.
- P. Xie, Z. Wang, K. Sun, C. Cheng, Y. Liu and R. Fan, Appl. Phys. Lett., 2017, 111, 112903 CrossRef.
- F. Rogti and M. Ferhat, J. Electrostat., 2014, 72, 91–97 CrossRef CAS.
- Z. Wang, X. Li, L. Wang, Y. Li, J. Qin, P. Xie, Y. Qu, K. Sun and R. Fan, Adv. Compos. Hybrid Mater., 2020, 3, 1–7 CrossRef.
- H. Gu, J. Guo, S. Wei and Z. Guo, J. Appl. Polym. Sci., 2013, 130, 2238–2244 CrossRef CAS.
- X. Yao, X. Kou and J. Qiu, Org. Electron., 2016, 39, 133–137 CrossRef CAS.
- H. Luo and J. Qiu, Adv. Electron. Mater., 2019, 5, 1900011 CrossRef.
- H. Wu, Y. Zhang, R. Yin, W. Zhao, X. Li and L. Qian, Adv. Compos. Hybrid Mater., 2018, 1, 168–176 CrossRef CAS.
- Y. Liu, Y. Qu, J. Xin, Z. Wang, G. Fan, P. Xie and K. Sun, J. Inorg. Organomet. Polym. Mater., 2019, 29, 248–257 CrossRef CAS.
- C. Cheng, R. Fan, Z. Wang, Q. Shao, X. Guo, P. Xie, Y. Yin, Y. Zhang, L. An and Y. Lei, Carbon, 2017, 125, 103–112 CrossRef CAS.
- M. Bodea, G. Sbarcea, G. V. Naik, A. Boltasseva, T. Klar and J. Pedarnig, Appl. Phys. A: Mater. Sci. Process., 2013, 110, 929–934 CrossRef CAS.
- T. Tsutaoka, T. Kasagi, S. Yamamoto and K. Hatakeyama, Appl. Phys. Lett., 2013, 102, 181904 CrossRef.
- C. Cheng, R. Fan, Y. Ren, T. Ding, L. Qian, J. Guo, X. Li, L. An, Y. Lei and Y. Yin, Nanoscale, 2017, 9, 5779–5787 RSC.
- H. Liu, Q. Li, S. Zhang, R. Yin, X. Liu, Y. He, K. Dai, C. Shan, J. Guo and C. Liu, J. Mater. Chem. C, 2018, 6, 12121–12141 RSC.
- J. Ni, R. Zhan, J. Qiu, J. Fan, B. Dong and Z. Guo, J. Mater. Chem. C, 2020, 8, 11748–11759 RSC.
- H. Wu, Y. Qi, Z. Wang, W. Zhao, X. Li and L. Qian, Compos. Sci. Technol., 2017, 151, 79–84 CrossRef CAS.
- L. Wang, X. Liu, C. Liu, X. Zhou, C. Liu, M. Cheng, R. Wei and X. Liu, Chem. Eng. J., 2020, 384, 123231 CrossRef CAS.
- L. Wang, Z. X. Bai, C. Liu, R. Wei and X. Liu, J. Mater. Chem. C, 2020, 9, 860–868 RSC.
- Z. Jin, J. Ji, Q. He, X. Yang, Y. Zhang and M. Xu, Catal. Lett., 2018, 148, 3281–3291 CrossRef CAS.
- Y. Li, X. Wu, C. Liu, S. Wang, P. Zhou, T. Zhou, Z. Miao, W. Xing, S. Zhuo and J. Zhou, J. Mater. Chem. A, 2019, 7, 7128–7137 RSC.
- C. Wu, X. Huang, X. Wu, J. Yu, L. Xie and P. Jiang, Compos. Sci. Technol., 2012, 72, 521–527 CrossRef CAS.
- P. T. Xie, W. Sun, Y. Liu, A. Du, Z. D. Zhang, G. M. Wu and R. H. Fan, Carbon, 2018, 129, 598–606 CrossRef CAS.
- P. T. Xie, Y. F. Liu, Q. Hou, K. Y. Sui, C. Z. Liu, X. Y. Fu, J. Zhang, V. Murugadoss, J. C. Fan, Y. P. Wang, R. H. Fan and Z. H. Guo, J. Mater. Chem. C, 2020, 8(9), 3029–3039 RSC.
- Y. N. Shi, K. C. Pan, M. G. Moloney and J. Qiu, Composites, Part A, 2021, 144, 106351 CrossRef CAS.
- X. Xu, Q. Fu, H. Gu, Y. Guo, H. Zhou, J. Zhang, D. Pan, S. Wu, M. Dong and Z. Guo, Polymer, 2020, 188, 122129 CrossRef CAS.
- K. Murugappan and M. R. Castell, Electrochem. Commun., 2018, 87, 40–43 CrossRef CAS.
- N. Nikfar, Y. Zare and K. Y. Rhee, Phys. B, 2018, 533, 69–75 CrossRef CAS.
- Y. Sun, J. Wang, S. Qi, G. Tian and D. Wu, Appl. Phys. Lett., 2015, 107, 012905 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc03831a |
| This journal is © The Royal Society of Chemistry 2022 |