The microwave synthesis of porous CoSe2 nanosheets for super cycling performance supercapacitors
Received
5th October 2020
, Accepted 17th November 2020
First published on 18th November 2020
Abstract
Porous CoSe2 nanosheets prepared via a one-step microwave method can be considered an excellent electrode material and the associated electrochemical properties were evaluated. The porous CoSe2 nanosheets revealed admirable electrochemical properties in terms of high specific capacitance (333 F g−1 when the current density is 1 A g−1) and excellent enduring stability (100.97% of the initial specific capacity over 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1). An asymmetric supercapacitor (CoSe2-anode, AC-cathode) was assembled, offering good properties accompanied by an energy density of 18.9 W h kg−1 when the power density reached 387 W kg−1. These impressive results are obtained largely on account of the porous structure of CoSe2, which provides a path for ion transport, and the introduction of Se into CoSe2, which offers high electrical conductivity, further enhancing the electrochemical performance.
000 cycles at 5 A g−1). An asymmetric supercapacitor (CoSe2-anode, AC-cathode) was assembled, offering good properties accompanied by an energy density of 18.9 W h kg−1 when the power density reached 387 W kg−1. These impressive results are obtained largely on account of the porous structure of CoSe2, which provides a path for ion transport, and the introduction of Se into CoSe2, which offers high electrical conductivity, further enhancing the electrochemical performance.
1. Introduction
In recent years, many researchers have focused on exploring new and efficient energy storage devices in order to tackle the energy crisis.1–3 Supercapacitors, as novel energy storage devices, have achieved noteworthy interest on account of their unique electrochemical properties like high power density, environmental friendliness, being maintenance free, with a long cycling life as well as high safety performance.4–11 As prospective candidates for supercapacitors, transition metal selenides (NiSe2,12,13 CoSe,14 MoSe2,15–17 CoSe2,18etc.) have attracted considerable attention owing to the higher electroconductibility of selenium (1 × 10−3 S m−1) than that of S (5 × 10−28 S m−1) or O (1 × 10−5 S m−1).19–22 In particular, cobalt-based selenides are widely researched due to their outstanding electrochemical activities.23,24 For example, Song et al. synthesized a CoSe2@rGO nanohybrid with superior energy storage performance, with the specific capacitance (Cs) of 219 F g−1 at a current density of 0.5 A g−1 and better durability (91.3% retention over 5000 cycles).23 Zhu et al. prepared CoSe with a decent Cs of 440 F g−1 (1 A g−1) and stability with 96.3% conservation for 5000 cycles (10 A g−1).25 However, the practical application of cobalt-based selenides in the energy storage field remains a problem on account of their unsatisfactory cycling durability.
It is comprehensively known that the construction of porous structures is one of the major characteristics of outstanding electrode materials for improving electrochemical performance. Significantly, electrode materials with mesoporous structures are attracting great interest. This is mainly because the appropriate aperture distribution of mesoporous materials can shorten the diffusion pathway and facilitate electrolytic ion transport.26–28 Meanwhile, a mesoporous structure is beneficial for accommodating the possible volumetric change in the long-term cycling process.29 For instance, Huang et al. reported that Mn3O4 nanoflakes/rGO (reduced graphene oxide) with a moderate pore size (5–10 nm) delivered a high Cs (351 F g−1, 0.5 A g−1) and desirable cyclic durability (80.1% of maximum Cs over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).30 Zhang et al. manufactured mesoporous (∼16–50 nm) V2O5 and macroporous (∼60 nm) V2O5. The mesoporous V2O5 displayed an excellent Cs (680 F g−1, 1 A g−1) and impressive cyclic retention (70% over 10
000 cycles).30 Zhang et al. manufactured mesoporous (∼16–50 nm) V2O5 and macroporous (∼60 nm) V2O5. The mesoporous V2O5 displayed an excellent Cs (680 F g−1, 1 A g−1) and impressive cyclic retention (70% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 3 A g−1), while macroporous (∼60 nm) V2O5 showed relatively poor performance with a Cs of 406 F g−1 and cyclic conservation of 40% during 4000 cycles at 3 A g−1.29 In addition, Zhou et al. designed Co(OH)2/BAC (bamboo activated carbon) containing mesoporous (∼20 nm) or macroporous (∼85 nm) structures. Mesoporous Co(OH)2/BAC expressed a more outstanding specific capacitance (345 F g−1 at 0.1 A g−1) than that of macroporous Co(OH)2/BAC with 289 F g−1 at 0.1 A g−1, and showed satisfactory stability with 84% of Cs conservation over 15
000 cycles at 3 A g−1), while macroporous (∼60 nm) V2O5 showed relatively poor performance with a Cs of 406 F g−1 and cyclic conservation of 40% during 4000 cycles at 3 A g−1.29 In addition, Zhou et al. designed Co(OH)2/BAC (bamboo activated carbon) containing mesoporous (∼20 nm) or macroporous (∼85 nm) structures. Mesoporous Co(OH)2/BAC expressed a more outstanding specific capacitance (345 F g−1 at 0.1 A g−1) than that of macroporous Co(OH)2/BAC with 289 F g−1 at 0.1 A g−1, and showed satisfactory stability with 84% of Cs conservation over 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.31 Consequently, the mesoporous morphology shows impressive advantages in increasing the electrochemical properties, especially in maintaining cyclic stability.
000 cycles.31 Consequently, the mesoporous morphology shows impressive advantages in increasing the electrochemical properties, especially in maintaining cyclic stability.
The successful synthesis of a specific structure is subject to the preparation strategy. Recently, a microwave process has been rapidly developed in the acquisition of inorganic materials synthesis. Compared to traditional synthesis tactics (hydrothermal, solvothermal, electrodeposition etc.), the microwave process has some advantages such as convenience, no post-treatment and savings in synthesis time. Meanwhile, the microwave process can control the size distribution and morphology.32–36 In this work, we offer a one-step microwave synthesis of a porous CoSe2 nanosheet as well as researching its electrochemical performance in a three-electrode system. In the meantime, an asymmetric supercapacitor (ASC) was prepared employing the porous CoSe2 nanosheet and AC as the anode and cathode, respectively.
2. Experimental
2.1. Materials
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O) and potassium hydroxide (KOH) were acquired from Tianjin Damao Co., Ltd. Selenium powder (Se) was obtained from Aladdin Reagent Co. Ltd. Copper(II) oxide powder was purchased from Wuxi Yatai United Chemical Co. Ltd. Ethylenediamine (C2H8N2) and ethylene glycol (C2H6O2) were supplied by Tianjin Tianli Co., Ltd.
2.2. Preparation of CoSe2
CoSe2 was synthesized in a single step via a microwave irradiation method and the synthesis procedure is explained in detail below. Co(NO3)2·6H2O (40 mg) and Se (20 mg) powders were mixed and ground homogeneously. The obtained mixture was put into an alumina crucible (10 mL) and then ethylenediamine (400 μL) and ethylene glycol (200 μL) were added to the mixture. All ingredients in the alumina crucible (10 mL) were stirred evenly. Then the alumina crucible (10 mL) was placed into a 50 mL alumina crucible. In particular, the inside of the 50 mL alumina crucible contained a suitable amount of CuO powder. CuO powder was added to the large alumina crucible (50 mL) for heating purposes by absorbing microwave energy, not for the reaction. The horizontal height of the CuO powder is slightly higher than that of the reactive materials, which makes the heating temperature uniform. Finally, the alumina crucible matryoshka was placed in a microwave oven (Panasonic NN-GF352 M) and irradiated with microwave energy. The powder (without CuO) obtained from the alumina crucible (10 mL) was used to perform a series of characterizations. And various reaction conditions in term of different microwave power and time were explored so as to investigate the optimum synthesis conditions of CoSe2, as seen in Table 1.
Table 1 Experimental conditions to be explored with different microwave times and powers
| Sample |
Co(NO3)2·6H2O/mg |
Se powder/mg |
Microwave power/W |
Microwave time/s |
| A: different microwave powers. B: different microwave times. |
| A1 |
40 |
20 |
600 |
90 |
| A2 |
40 |
20 |
800 |
90 |
| A3 |
40 |
20 |
1000 |
90 |
| B1 |
40 |
20 |
1000 |
60 |
| B2 |
40 |
20 |
1000 |
120 |
2.3. Material characterization
For the X-ray diffraction analysis, the shortened form of XRD was aimed to probe the crystallinity of the resultant materials with Cu Kα radiation using a Rigaku instrument, model D/Max-2500. The microtopography and architecture were investigated via scanning electron microscopy (SEM) with a TSCAN Mira3 LMH instrument and energy dispersive spectroscopy (EDS) with an INCA MAX-50 instrument. The elaborate crystal structure was further inspected via high-resolution electron microscopy (HRTEM) with a JEM-2100F microscope. The X-ray photoelectron spectroscopy (XPS) tests were performed with a Thermo Scientific Escalab 250Xi to detect the surface compositions of the as-synthesized electrode materials. In addition, the nitrogen adsorption/desorption isotherm was deciphered via a specific surface and pore size analyzer (JW-BK122W).
2.4. Electrochemical characterization
The working electrode was manufactured based on the steps below: a mixture of CoSe2 (8/10), acetylene black (1/10) and polyvinylidene fluoride (1/10) was mixed uniformly to form a slurry. Then, the resultant slurry was coated on nickel foam with an active area of 1.0 cm2. Ultimately, the nickel foam, which contained 1.0 mg of the active material, was dried at 80 °C for 24 h. Using a three-electrode system, the CV (cyclic voltammetry), the galvanostatic charge/discharge (GCD) and the electrochemical impedance spectroscopy (EIS) measurements were carried out in an electrochemical workstation (CHI660E, Shanghai). In addition, the counter and reference electrodes were a platinum sheet and Hg/HgO, respectively. And 6 M KOH solution was applied as the electrolyte. Furthermore, the cyclic stability of the CoSe2 electrode was studied on a LAND battery test system (CT2001A). Cs was calculated with the following equation:37,38| | | Cs (F g−1) = IΔt/(mΔV) | (1) |
where Cs represents the specific capacitance, and Δt (s) and ΔV (V) are the time of discharge and the potential voltage window, respectively. The constant current is revealed using I (A). The loading mass of active material is indicated by m (g).
The ASC was assembled with a working electrode (anode) and active carbon (AC, cathode). The ratio between the mass of the anode and the mass of the cathode was determined with the following equation:39,40
| | | m+/m+ = Cs−ΔV−/(Cs+ΔV+) | (2) |
where the parameters are the same as in
eqn (1).
E (energy density) is obtained from
eqn (3).
P (power density) is revealed by
eqn (4).
41| | | E (W h kg−1) = Cs × (ΔV)2/7.2 | (3) |
| | | P (W kg−1) = 3600 × E/Δt | (4) |
3. Results and discussion
3.1. Characterization
XRD was applied to identify the microstructures of CoSe2 under various reaction conditions. The XRD patterns of CoSe2 under all reaction conditions (except 1000 W, 60 s) consisted of orthorhombic as well as cubic CoSe2 and the relevant PDF standard cards are JCPDS # 53-0449 and JCPDS # 89-2002, respectively.23 As displayed in Fig. 1, the diffraction peaks corresponding to 28.97°, 30.76°, 34.50°, 37.09°, 47.80°, 53.56°, 57.02° and 63.35° could be indexed to the (011), (101), (120), (200), (211), (031), (131) and (122) crystal planes of orthorhombic CoSe2, respectively. And the diffraction peaks at 34.27°, 37.61° and 51.72° correspond to the lattice planes of (210), (211) and (311), respectively, which belong to cubic CoSe2. Besides the diffraction peaks of CoSe2, there were other peaks present at 23.50°, 29.72°, 41.29°, 43.66°, 45.33°, 61.22°, 61.68° and 65.25° that could be assigned to the (100), (101), (110), (102), (111), (103), (202) and (210) crystal planes of Se (JCPDS # 06-0362), respectively, at a short microwave time (1000 W, 60 s). This is mostly because the microwave time is too short for the Se powder to react properly. The diffraction peaks of CoSe2 are broad and weak, indicating deficient crystallinity.
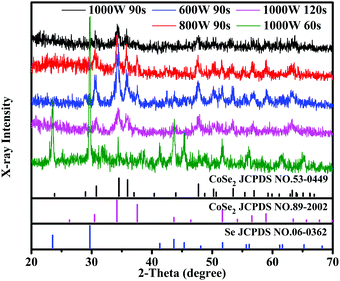 |
| | Fig. 1 XRD patterns of CoSe2 obtained under different reaction conditions. | |
The chemical bonding states on the surface of CoSe2 (sample A3) were evaluated via XPS. The existence of Co, C (as reference) and Se elements appear in the survey spectrum in Fig. 2a. The Co 2p spectrum (Fig. 2b) consisted of 2p3/2 (780.9 eV) and 2p1/2 (796.6 eV) and was accompanied by two satellite peaks (785.8 eV and 802.4 eV), which can be ascribed to the Co2+ state. From Fig. 2c, the binding energies at 54.2 eV and 54.8 eV are consistent with Se 3d5/2 and 3d3/2, respectively, which can be ascribed to the Se22− state. In addition, the peak of SeOx is shown at 58.6 eV. The results from XRD and XPS indicated the synthesis of CoSe2 by a one-step microwave irradiation method.
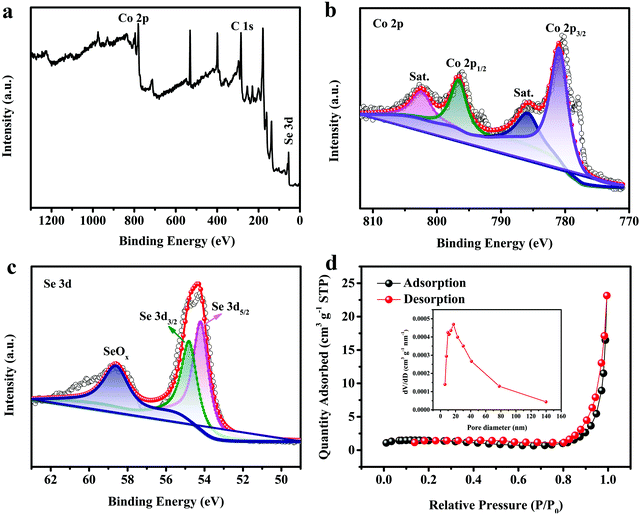 |
| | Fig. 2 (a) XPS survey spectrum; (b) Co 2p and (c) Se 3d XPS elemental spectra; and (d) N2 adsorption–desorption isotherm and pore size distribution of CoSe2. (Reaction conditions: 1000 W and 90 s.) | |
From Fig. 2d, we can see that the N2 adsorption–desorption isotherm of sample A3 has an obvious type-IV isotherm with an H3 hysteresis loop over a relative pressure range above 0.4, indicating that the sample expresses mesoporosity and has a wide pore-size distribution. The surface area of sample A3 is confirmed as 4.8 m2 g−1via the Brunauer–Emmett–Teller (BET) method. The inset to Fig. 2d shows the pore size distribution plot tested via the Barrett–Joyner–Halenda (BJH) method, which shows that the pore diameter distribution range runs mainly from 2 to 50 nm and the average pore size is 34.32 nm, indicating that the resultant sample is a mesoporous material. The mesopores can be used as “ion-buffering storage”, which shorten the ion transport path and promote rapid ion transport. Meanwhile, the mesopores can also increase the stability by changing the buffer volume in a long-term cyclic test.
SEM and TEM images and EDS analysis are shown in Fig. 3. Fig. 3a–e display the microstructures of the as-gained materials for different microwave powers and times. From Fig. 3c, the electrode material (1000 W, 90 s) had a porous sheet structure, which was conducive to the electrolyte moving into the electrode material interior, and thus accelerating the ion transfer. However, when the microwave power was reduced (800 W, 90 s), a stacked non-porous lamellar structure appeared in Fig. 3b. Reducing the performance of microwave power even further resulted in the structure of a continuous piece. The non-porous structure is not conducive to an improvement in electrochemical performance. On the other hand, when the microwave time is shortened (1000 W, 60 s), the material displays an uneven structure in which small pieces and particles are mixed, as expressed in Fig. 3d. Fig. 3e shows the structure of the obtained electrode material when the microwave time was too long (1000 W, 120 s) and the excessive microwave energy caused the material to agglomerate. The crystal structure of sample A3 (1000 W, 90 s) was measured by transmission electron microscopy (TEM). From Fig. 3f and g, nanosheets containing mesopores can be clearly observed. The elaborate crystal structure was further inspected by high-resolution electron microscopy (HRTEM). As shown in Fig. 3h, adjacent lattice fringes were observed with a lattice spacing of 0.249 nm, which corresponds to the (120) plane of CoSe2 (JCPDS # 53-0449). And the lack of obvious diffraction spots in the selected-area electron diffraction (SAED) pattern (shown in the inset to Fig. 3h), consistent with the XRD, confirmed the deficient crystalline structure of sample A3. The Co/Se atomic ratio and elemental mapping images of sample A3 (1000 W, 90 s) are shown in the EDS diagram (Fig. 3i). The atomic ratio of Se and Co elements was approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, further demonstrating the successful synthesis of CoSe2.
1, further demonstrating the successful synthesis of CoSe2.
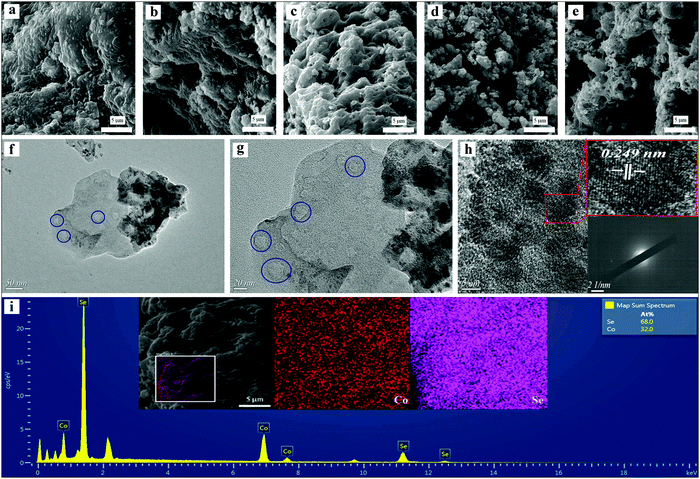 |
| | Fig. 3 (a)–(e) SEM images of CoSe2 obtained under different microwave conditions; (f)–(h) TEM and HRTEM images, and SAED patterns of CoSe2 (1000 W, 90 s); (i) EDS spectrum and elemental mapping images of CoSe2 (1000 W, 90 s). | |
3.2. Electrochemical measurements
3.2.1. Effects of experimental conditions on specific capacitance.
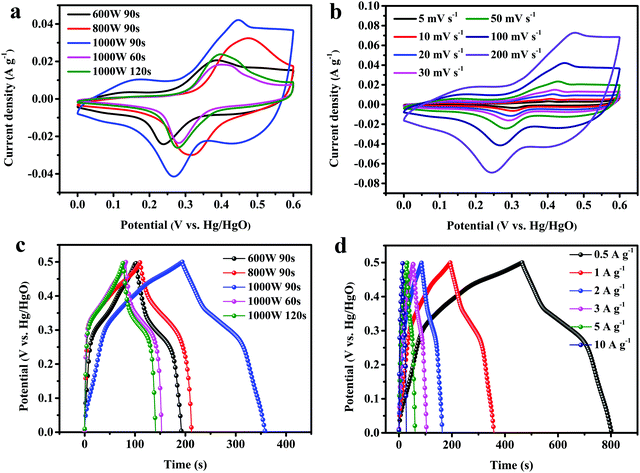
From Fig. 4a, it can be clearly seen that the Cs of the as-prepared electrode material increased with an increase in microwave power. Sample A3 achieved an average Cs of 327.0 F g−1 (1 A g−1) at 1000 W microwave power and 90 s heating time, which is higher than that of sample A2 at low microwave power (220.3 F g−1, 800 W) or that of sample A1 (179.3 F g−1, 600 W). Inadequate microwave energy resulted in unsatisfactory morphology, which affected the specific capacitance. Additionally, in Fig. 4b, the Cs of the as-prepared electrode material first increased and then declined as the microwave time increased. At a short microwave time (60 s), sample B1 had a low average Cs of 172.0 F g−1 (1 A g−1) derived from some of the Se powder not having fully reacted. However, at long microwave time (120 s), an excess of microwave energy may lead to product aggregation and sample B2 has a low average Cs of 143.3 F g−1. Therefore, the most appropriate reaction conditions for the fabricated CoSe2 were 1000 W microwave power with a 90 s heating period.
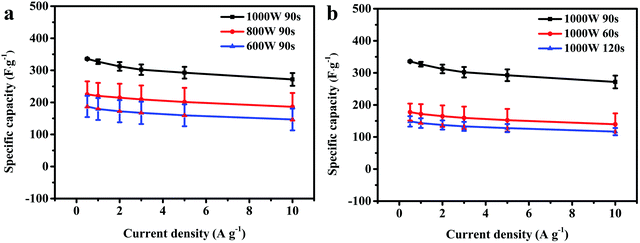 |
| | Fig. 4 Specific capacitance values of CoSe2 obtained using different (a) microwave powers and (b) microwave times. | |
3.2.2. Electrochemical performance of CoSe2 electrode materials.
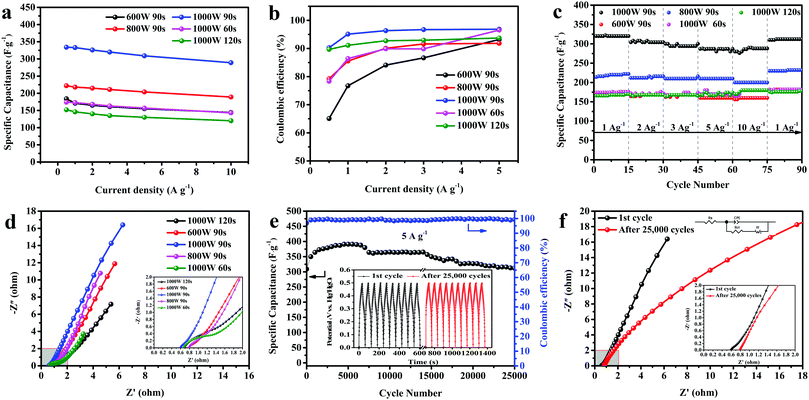
Fig. 5a compares the CV curves of the samples under various reaction conditions at 100 mV s−1. The range of voltage is between 0 and 0.6 V. All the CV curves showed symmetric redox peaks and the possible reactions can be explained by the following equations:42| | 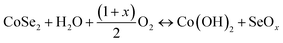 | (5) |
| | | Co(OH)2 + OH− ↔ CoOOH + H2O + e− | (6) |
| | | CoOOH + OH− ↔ CoO2 + H2O + e− | (7) |
 |
| | Fig. 5 CV curves of CoSe2 samples obtained under (a) all reaction conditions and (b) at 1000 W for 90 s. GCD curves of CoSe2 samples obtained under (c) all reaction conditions and (d) at 1000 W for 90 s. | |
The maximum enclosed area was observed for sample A3 (1000 W, 90 s) among these, implying the highest specific capacitance. The CV curves of sample A3, as shown in Fig. 5b, were measured under multiple scanning rates (5–100 mV s−1) and the CV curves showed no obvious change in shape as the scan rate increased. The oxidation peaks shifted toward the higher potential direction and the reduction peaks shifted toward the lower potential direction with an increase in scan rates, which may result from the rise in internal diffusion resistance for the electrode material.43 A comparison of the charge/discharge curves of different samples at 1 A g−1 is shown in Fig. 5c. The potential window of the samples is 0–0.5 V. Under the same conditions, sample A3 had a longer discharge time than the others and it possessed the largest Cs, which was consistent with the CV curve (Fig. 5a). In Fig. 5d, the GCD curves of sample A3 are shown from 0.5 to 10 A g−1. These curves are nearly symmetrical, suggesting that the as-prepared electrode had ideal reversibility in the charge/discharge process. The Cs at 0.5, 1, 2, 3, 5, 10 A g−1, were as high as 334, 333, 326, 320, 309 and 289 F g−1, respectively, and were higher than those of other similar materials (Table 2), revealing the good capacitance of the as-prepared CoSe2 electrode.
Table 2 A comparison of the electrochemical performances of Se-based electrode materials reported previously and in the current work
| Material |
Structure |
Method |
Specific capacitance |
Retention |
Ref. |
| MoSe2/rGO |
Nanosheets |
Hydrothermal |
211 F g−1 at 5 mV s−1 |
180% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
44
|
| MoSe2/NF |
Nanoflakes |
Spraying chemical vapor deposition |
205 F g−1 at 1 A g−1 |
73.6% after 5000 cycles at 1 A g−1 |
45
|
| NiSe |
Microspheres |
Solvothermal |
492 F g−1 at 0.5 A g−1 |
84.6% after 200 cycles at 0.5 A g−1 |
46
|
| CoSe2@rGO |
Nanoparticles |
Hydrothermal |
219 F g−1 at 0.5 A g−1 |
91.3% after 5000 cycles at 5 A g−1 |
23
|
| CoSe2 |
Nanoparticles |
Hydrothermal |
99 F g−1 at 0.5 A g−1 |
— |
23
|
| NiSe2 |
Hollow spheres |
Hydrothermal |
341 F g−1 at 1 A g−1 |
78.8% after 1000 cycles at 1 A g−1 |
47
|
| NiSe2 |
Nanoparticles |
Hydrothermal |
75 F g−1 at 1 mA cm−2 |
94% after 5000 cycles at 1 mA cm−2 |
48
|
| CoSe2 |
Porous nanosheets |
One-step microwave |
333 F g−1 at 1 A g−1 |
100.97% after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
This work |
In Fig. 6a, the Cs of sample A3 are much higher than for other samples at different current densities. For sample A3, the specific capacitance at 10 A g−1 reaches 86.8% of that at 1 A g−1, which is better than those of sample B2 (82.1%), sample A2 (86.7%), sample A1 (84.2%), or sample B1 (82.7%). The Coulombic efficiency (CE), the ratio of discharging time to charging time, is an important parameter of electrochemical performance. The relevant calculation formula is as follows:49
where subscript d represents discharging and subscript c represents charging. As can be seen in
Fig. 6b, the CE values of all the samples were obtained at different current densities. For sample A3, the CE value was 95.70% at 1 A g
−1, while the values of samples A1, A2, B1 and B2 were 76.73%, 85.54%, 86.42% and 91.14%, implying higher reversibility for sample A3 than for the others. The rate performance of all samples is revealed in
Fig. 6c. When the ampere density returned to 1 A g
−1 again, the
Cs of sample A3 remained at 312 F g
−1 which was higher than for the others, demonstrating steady as well as good electrochemical properties. The excellent electrochemical property expressed by the electrode materials is attributed to their good electrical conductivity and fast charge transport ability. The EIS was illustrated in the frequency range between 1 Hz and 100 KHz. In
Fig. 6d, a semicircle and a diagonal line form the Nyquist plots of all samples in high- and low-frequency ranges. Among them, the
Rs, the intercept of the
X-axis, corresponds to the internal resistance. And the
Rct revealed from the semicircle, explores the charge transfer kinetics. The diffusive resistance corresponding to the Warburg impedance (
W) was represented
via a sloping line in the low-frequency range. From
Fig. 6d, the
Rs and
Rct of sample A3 were smaller than those of the others, demonstrating an optimal conductivity and fast charge transfer behavior. At low frequency, the more vertical slope of the straight line for sample A3 suggested rapid electrolyte ion diffusion. The long-term cycle ability of sample A3 at 5 A g
−1 is expressed in
Fig. 6e. Firstly, the
Cs went through a process of growth due to the activation of the electrode. During this process, the electrolyte gradually infiltrated into the electrode material, increasing the contact area between them and improving ion transport and diffusion to the inner layer. Then, the curve showed a steady state and the specific capacitance was maintained as 100.97% of the original specific capacitance over 25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
000 cycles, which was 79.8% of the highest specific capacitance (391 F g
−1 at 4500 cycles), implying superior cyclic durability. Meanwhile, from
Fig. 6e, the CE of sample A3 reached 99.3% after 25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
000 cycles at 5 A g
−1. The inset of
Fig. 6e shows that the first and last ten GCD curves were almost symmetric as well as being similar, confirming remarkable reversibility and structural stability.
50,51 The Nyquist plots (sample A3) before and after 25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
000 cycles are revealed in
Fig. 6f. The
Rs and
Rct were 0.73 Ω and 0.39 Ω, respectively, before cycling and increased to 0.83 Ω and 0.49 Ω, respectively, after 25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
000 cycles. The slight change proved that the as-obtained electrode material had fast ion transfer and a stable structure in the long-term charge–discharge process.
52,53 Based on the above discussion of electrochemistry, the excellent properties of the porous CoSe
2 nanosheet come mainly from the following two aspects: (1) the mesoporous structure shortens the ion transport pathway and has high mechanical properties, which can maintain the steady microstructure over the long period of the cycling test; (2) Se has higher electrical conductivity and can provide greater capacitive activity.
 |
| | Fig. 6 (a) Specific capacitances at various current densities, (b) coulombic efficiencies at different current densities, (c) rate performances, and (d) Nyquist plots of CoSe2 obtained under all reaction conditions. (e) Cycling stability and variation in Coulombic efficiency with charge–discharge cycles of CoSe2 (1000 W, 90 s) at 5 A g−1. (f) Nyquist plots of CoSe2 (1000 W, 90 s) before and after cycling. | |
The sweep voltammetry was further explored in order to understand the kinetic information about sample A3. The two processes that contribute to global charge storage are the surface capacitive and the diffusion-controlled capacity (battery process). As can be seen in Fig. 7a, the slopes of the fitted lines (b) reflected the charge-storage mechanism of the electrode materials. The calculation formula is shown below:54
where
i and
v reveal the peak current and corresponding scanning rate, respectively. From
Fig. 7a, the
b values belonging to the positive pole and the negative pole are 0.85255 and 0.83014, respectively, demonstrating the pseudocapacitive course among capacitors and batteries. And the contribution of the two process can be revealed in the equation below:
55–57
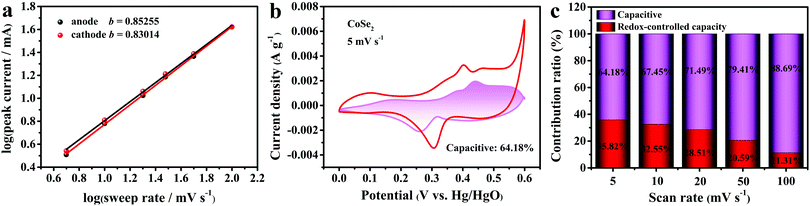 |
| | Fig. 7 (a) Charts of log(peak current) versus log(sweep rate) calculated from CV curves. (b) A CV plot indicating the capacitive contribution of CoSe2 at 5 mV s−1. (c) A bar diagram of capacitive and diffusive contributions to the outright capacity at different scan rates. | |
Fig. 7b displays the capacitive contribution of CoSe2 at 5 mV s−1 and accounts for 64.18% of the total charge. From Fig. 7c, it was found that the percentage of diffusion-controlled capacity decreased with the rise in scan rate, implying that capacitive capacity was a major contribution to the high rate capability.
3.2.3. Electrochemical performance of CoSe2//AC.
With the purpose of investigating the electrochemical performance of CoSe2 for realistic applications, an asymmetric supercapacitor (ASC) with an anode (sample A3) and cathode (active carbon) was assembled. The mass ratio of the two electrodes was determined as 1.07, according to eqn (2). The CV curves of CoSe2 and AC measured at 100 mV s−1 are expressed in Fig. 8a. The voltage window of CoSe2 is 0 V to 0.6 V and that of AC started at −1 V and ended at 0 V. Fig. 8b displays the CV curves of CoSe2//AC tested at multiple scan rates (5–200 mV s−1) and the potential voltage started at 0 V and ended at 1.6 V, implying incorporation with double-layer and pseudocapacitive contributions. In Fig. 8c, the CV curves, which were tested in various potential windows, did not deform appreciably, demonstrating a good current response. From Fig. 8d the ASC obtained specific capacitance values of 56.7, 48.8, 40.3, 36.3, 31.6 and 28.0 F g−1 corresponding to 0.5–10 A g−1, respectively and the values of CE at multiple ampere densities were 84.16%, 92.34%, 94.89%, 96.08%, 96.27% and 96.35%, respectively. From Fig. 8e, the perfect reversibility of the GCD curves is displayed in a potential window of 1.55 V on account of their high symmetry. In addition in Fig. 8f, the GCD curves at 1 A g−1 are shown with a voltage window of 1.0 V to 1.55 V. In Fig. 8g, the Nyquist impedance plot is revealed. The Rct and Rs are 1.245 Ω and 0.819 Ω, respectively, implying the rapid transfer rate of ions. In Fig. 8h, the long-term cycling process and corresponding CE at 5 A g−1 are revealed. The conservation of Cs of CoSe2//AC reached 93.3% of the original value over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, indicating excellent cycling durability. Meanwhile, the CE was maintained at 99.12% after cycling. In the inset to Fig. 8h, the last ten charge/discharge cycles show no obvious deformation compared to the first ten cycles, which implies good electrochemical stability. The E and P of the ASC were acquired according to eqn (3) and (4). The Ragone plot of CoSe2//AC can be observed in Fig. 8i. The maximum E of 18.9 W h kg−1 was achieved when P was 387 W kg−1 and the maximum P (7750 W kg−1) was acquired at an E of 9.34 W h kg−1, which is obviously higher than those of NiFe-LDH@CoS2@Ni//AC,58 Ni3Se2//AC,59 NiCo2S4@CoS2//AC,60 or Ni0.9Co1.92Se4//AC.61 In addition, five LEDs can be lit when two identical ASC devices are connected in series, as seen in the inset to Fig. 8i, demonstrating its good practical applicability.
000 cycles, indicating excellent cycling durability. Meanwhile, the CE was maintained at 99.12% after cycling. In the inset to Fig. 8h, the last ten charge/discharge cycles show no obvious deformation compared to the first ten cycles, which implies good electrochemical stability. The E and P of the ASC were acquired according to eqn (3) and (4). The Ragone plot of CoSe2//AC can be observed in Fig. 8i. The maximum E of 18.9 W h kg−1 was achieved when P was 387 W kg−1 and the maximum P (7750 W kg−1) was acquired at an E of 9.34 W h kg−1, which is obviously higher than those of NiFe-LDH@CoS2@Ni//AC,58 Ni3Se2//AC,59 NiCo2S4@CoS2//AC,60 or Ni0.9Co1.92Se4//AC.61 In addition, five LEDs can be lit when two identical ASC devices are connected in series, as seen in the inset to Fig. 8i, demonstrating its good practical applicability.
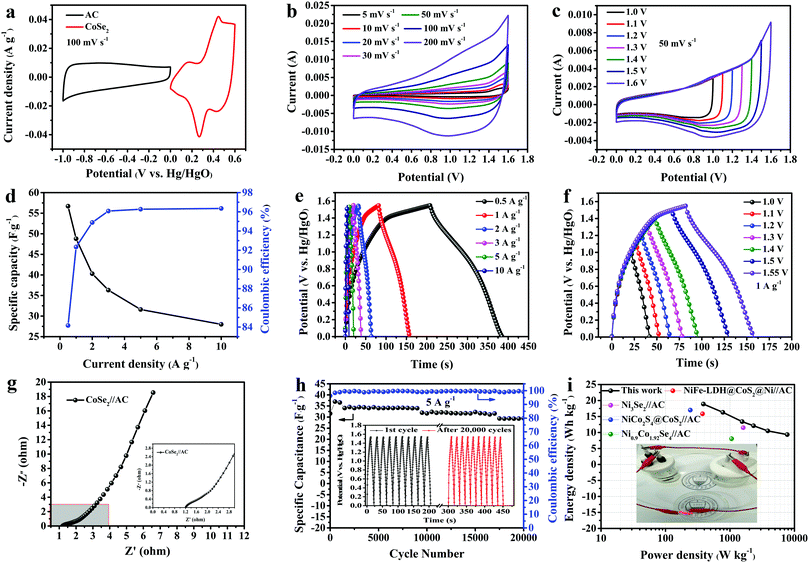 |
| | Fig. 8 (a) CV curves of AC and CoSe2 electrodes at a scan rate of 50 mV s−1; (b) CV curves of CoSe2//AC ASC at different scan rates; (c) CV curves of CoSe2//AC ASC in different potential windows at a scan rate of 50 mV s−1; (d) specific capacitances and Coulombic efficiencies at different current densities; (e) GCD curves of CoSe2//AC at different current densities; (f) GCD curves of CoSe2//AC in different voltage windows at 1 A g−1; (g) a Nyquist plot of CoSe2//AC; (h) the cycling stability and variation in Coulombic efficiency with charge–discharge cycle number of CoSe2//AC at 5 A g−1; and (i) comparisons of power and energy densities of asymmetric supercapacitors. | |
4. Conclusions
Here, porous CoSe2 nanosheets were synthesized in a single step via a microwave method and applied in a supercapacitor. The microstructure and electrochemical properties were revealed via XRD, XPS, BET, SEM, EDS, CV, GCD, etc. The results reveal that the porous CoSe2 nanosheets have superior cycling durability, with 100.97% retention over 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 and a good Cs value of 333 F g−1 (1 A g−1). The assembled asymmetric supercapacitor also showed impressive energy storage performance. The results prove that the porous CoSe2 nanosheets prepared via one-step microwave synthesis are suitable as an electrode material for practical applications in supercapacitors.
000 cycles at 5 A g−1 and a good Cs value of 333 F g−1 (1 A g−1). The assembled asymmetric supercapacitor also showed impressive energy storage performance. The results prove that the porous CoSe2 nanosheets prepared via one-step microwave synthesis are suitable as an electrode material for practical applications in supercapacitors.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors acknowledge financial support via the National Natural Science Foundation of China (51672185).
References
- M. A. A. M. Abdah, N. H. N. Azman, S. Kulandaivalu, N. A. Rahman, A. H. Abdullah and Y. Sulaiman, J. Power Sources, 2019, 444, 227324 CrossRef.
- K. Tao, S. Lv, Y. Hai and Y. Gong, CrystEngComm, 2019, 21, 7424–7436 RSC.
- Y. Sun, J. Zhang, X. Sun and N. Huang, CrystEngComm, 2019, 21, 7468–7475 RSC.
- S. L. Patil, S. S. Raut and B. R. Sankapal, Thin Solid Films, 2019, 692, 137584 CrossRef.
- G. Yang, T. Takei, S. Yanagida and N. Kumada, Appl. Surf. Sci., 2019, 498, 143872 CrossRef.
- X. Yu, J. Yu, L. Hou, A. Gagnoud, Y. Fautrelle, Z. Ren and X. Li, J. Power Sources, 2018, 408, 65–73 CrossRef CAS.
- M. Guo, J. Balamurugan, N. H. Kim and J. H. Lee, Appl. Catal., B, 2018, 239, 290–299 CrossRef CAS.
- G. Yang and S.-J. Park, J. Alloys Compd., 2020, 835, 155270 CrossRef CAS.
- A. Bello, O. O. Fashedemi, F. Barzegar, M. J. Madito, D. Y. Momodu, T. M. Masikhwa, J. K. Dangbegnon and N. Manyala, J. Alloys Compd., 2016, 681, 293–300 CrossRef CAS.
- W. Zheng, P. Zhang, W. Tian, Y. Wang, Y. Zhang, J. Chen and Z. Sun, Mater. Lett., 2017, 209, 122–125 CrossRef CAS.
- J. Zhao, J. Gong, G. Wang, K. Zhu, K. Ye, J. Yan and D. Cao, Chem. Eng. J., 2020, 401, 125456 CrossRef CAS.
- N. S. Arul and J. I. Han, Mater. Lett., 2019, 234, 87–91 CrossRef CAS.
- Y. Gu, L.-Q. Fan, J.-L. Huang, C.-L. Geng, J.-M. Lin, M.-L. Huang, Y.-F. Huang and J.-H. Wu, J. Power Sources, 2019, 425, 60–68 CrossRef CAS.
- A. Alam and S. Lim, J. Korean Soc. Imaging Sci. Technol., 2019, 25, 94–100 CrossRef.
- C. Li, Y. Zhou, P. Huo and X. Wang, J. Alloys Compd., 2020, 826, 154175 CrossRef CAS.
- X. Liu, J.-Z. Zhang, K.-J. Huang and P. Hao, Chem. Eng. J., 2016, 302, 437–445 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang and J.-L. Cai, Electrochim. Acta, 2015, 180, 770–777 CrossRef CAS.
- J. Lin, H. Wang, Y. Yan, X. Zheng, H. Jia, J. Qi, J. Cao, J. Tu, W. Fei and J. Feng, J. Mater. Chem. A, 2018, 6, 19151–19158 RSC.
- Q. Cai, Y. Li, L. Wang, Q. Li, J. Xu, B. Gao, X. Zhang, K. Huo and P. K. Chu, Nano Energy, 2017, 32, 1–9 CrossRef CAS.
- F. Ma, J. Lu, L. Pu, W. Wang and Y. Dai, J. Colloid Interface Sci., 2020, 563, 435–446 CrossRef.
- X. Xie, K. Huang, X. Wu, N. Wu, Y. Xu, S. Zhang and C. Zhang, Carbon, 2020, 169, 1–8 CrossRef CAS.
- Z.-B. Zhai, K.-J. Huang and X. Wu, Nano Energy, 2018, 47, 89–95 CrossRef CAS.
- Y. Song, A. Ran, Z. Peng, W. Huang, B. Huang, X. Jian and C. Mu, Composites, Part B, 2019, 174, 107001 CrossRef CAS.
- Q. Wang, Y. Ma, X. Liang, D. Zhang and M. Miao, J. Mater. Chem. A, 2018, 6, 10361–10369 RSC.
- Y. Zhu, X. Chen, H. Chen, X. Ji and Y. Liu, J. Electrochem. Soc., 2017, 164, A2341–A2347 CrossRef CAS.
- H. Lu, Q. Li, J. Guo, A. Song, C. Gong, J. Zhang and J. Zhang, Appl. Surf. Sci., 2018, 427, 992–999 CrossRef CAS.
- N. Padmanathan and S. Selladurai, Ionics, 2014, 20, 479–487 CrossRef CAS.
- Q. Lu, Y. Chen, W. Li, J. G. Chen, J. Q. Xiao and F. Jiao, J. Mater. Chem. A, 2013, 1, 2331–2336 RSC.
- H. Zhang, X. Han, R. Gan, Z. Guo, Y. Ni and L. Zhang, Appl. Surf. Sci., 2020, 511, 145527 CrossRef.
- Z. Huang, S. Li, Z. Li, J. Li, G. Zhang, L. Cao and H. Liu, J. Alloys Compd., 2020, 830, 154637 CrossRef.
- F. Zhou, Q. Liu, J. Gu, W. Zhang and D. Zhang, Electrochim. Acta, 2015, 170, 328–336 CrossRef.
- K. Seevakan, A. Manikandan, P. Devendran, Y. Slimani, A. Baykal and T. Alagesan, J. Magn. Magn. Mater., 2019, 486, 165254 CrossRef.
- K. Seevakan, A. Manikandan, P. Devendran, A. Shameem and T. Alagesan, Ceram. Int., 2018, 44, 13879–13887 CrossRef.
- K. Seevakan, A. Manikandan, P. Devendran, Y. Slimani, A. Baykal and T. Alagesan, Ceram. Int., 2018, 44, 20075–20083 CrossRef CAS.
- S. Faraji and F. N. Ani, J. Power Sources, 2014, 263, 338–360 CrossRef CAS.
- J. Yan, Z. Fan, W. Sun, G. Ning, T. Wei, Q. Zhang, R. Zhang, L. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS.
- D. Liu, X. Wang, X. Wang, W. Tian, J. Liu, C. Zhi, D. He, Y. Bando and D. Golberg, J. Mater. Chem. A, 2013, 1, 1952–1955 RSC.
- M. Shao, F. Ning, Y. Zhao, J. Zhao, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2012, 24, 1192–1197 CrossRef CAS.
- M. Sakthivel, S. Ramaraj, S.-M. Chen and K.-C. Ho, J. Mater. Chem. A, 2019, 7, 12565–12581 RSC.
- C. Miao, X. Xiao, Y. Gong, K. Zhu, K. Cheng, K. Ye, J. Yan, D. Cao, G. Wang and P. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 9365–9375 CrossRef CAS.
- L. Fang, Y. Qiu, W. Li, F. Wang, M. Lan, K. Huang and Q. Jing, J. Colloid Interface Sci., 2018, 512, 282–290 CrossRef CAS.
- T. Chen, S. Li, J. Wen, P. Gui and G. Fang, ACS Appl. Mater. Interfaces, 2017, 9, 35927–35935 CrossRef CAS.
- Y. Zheng, Y. Tian, S. Sarwar, J. Luo and X. Zhang, J. Power Sources, 2020, 452, 227793 CrossRef CAS.
- S. K. Balasingam, J. S. Lee and Y. Jun, Dalton Trans., 2016, 45, 9646–9653 RSC.
- Y. Liu, W. Li, X. Chang, H. Chen, X. Zheng, J. Bai and Z. Ren, J. Colloid Interface Sci., 2020, 562, 483–492 CrossRef CAS.
- K. Guo, F. Yang, S. Cui, W. Chen and L. Mi, RSC Adv., 2016, 6, 46523–46530 RSC.
- M. Lu, X.-P. Yuan, X.-H. Guan and G.-S. Wang, J. Mater. Chem. A, 2017, 5, 3621–3627 RSC.
- N. S. Arul and J. I. Han, Mater. Lett., 2016, 181, 345–349 CrossRef CAS.
- A. K. Thakur, M. Majumder, R. B. Choudhary and S. B. Singh, J. Power Sources, 2018, 402, 163–173 CrossRef CAS.
- J. Zhao, B. Guan, B. Hu, Z. Xu, D. Wang and H. Zhang, Electrochim. Acta, 2017, 230, 428–437 CrossRef.
- D. Du, R. Lan, J. Humphreys, W. Xu, K. Xie, H. Wang and S. Tao, J. Electrochem. Soc., 2017, 164, A2881–A2888 CrossRef.
- G. S. R. Raju, E. Pavitra, G. Nagaraju, S. C. Sekhar, S. M. Ghoreishian, C. H. Kwak, J. S. Yu, Y. S. Huh and Y.-K. Han, J. Mater. Chem. A, 2018, 6, 13178–13190 RSC.
- B. Ye, C. Gong, M. Huang, J. Ge, L. Fan, J. Lin and J. Wu, New J. Chem., 2019, 43, 2389–2399 RSC.
- S. Goel, G. Singh and R. K. Sharma, Mater. Chem. Phys., 2020, 244, 122694 CrossRef.
- K. A. S. Raj, A. S. Shajahan, B. Chakraborty and C. S. Rout, Chem. – Eur. J., 2020, 26, 6662–6669 CrossRef.
- P. Xue, N. Wang, Z. Fang, Z. Lu, X. Xu, L. Wang, Y. Du, X. Ren, Z. Bai, S. Dou and G. Yu, Nano Lett., 2019, 19, 1998–2004 CrossRef CAS.
- X. Mao, X. He, W. Yang, H. Liu, Y. Zhou, J. Xu and Y. Yang, Electrochim. Acta, 2019, 328, 135078 CrossRef.
- J. Tian, A. Zhang, R. Liu, W. Huang, Z. Yuan, R. Zheng, D. Wei and J. Liu, J. Colloid Interface Sci., 2020, 579, 607–618 CrossRef.
- L. Zhao, P. Zhang, Y. Zhang, Z. Zhang, L. Yang and Z.-G. Chen, J. Mater. Sci. Technol., 2020, 54, 69–76 CrossRef.
- M. Govindasamy, S. Shanthi, E. Elaiyappillai, S.-F. Wang, P. M. Johnson, H. Ikeda, Y. Hayakawa, S. Ponnusamy and C. Muthamizhchelvan, Electrochim. Acta, 2019, 293, 328–337 CrossRef.
- W. An, L. Liu, Y. Gao, Y. Liu and J. Liu, RSC Adv., 2016, 6, 75251–75257 RSC.
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a and
Xinyu
Zhang
*a and
Xinyu
Zhang
 *b
*b
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1). An asymmetric supercapacitor (CoSe2-anode, AC-cathode) was assembled, offering good properties accompanied by an energy density of 18.9 W h kg−1 when the power density reached 387 W kg−1. These impressive results are obtained largely on account of the porous structure of CoSe2, which provides a path for ion transport, and the introduction of Se into CoSe2, which offers high electrical conductivity, further enhancing the electrochemical performance.
000 cycles at 5 A g−1). An asymmetric supercapacitor (CoSe2-anode, AC-cathode) was assembled, offering good properties accompanied by an energy density of 18.9 W h kg−1 when the power density reached 387 W kg−1. These impressive results are obtained largely on account of the porous structure of CoSe2, which provides a path for ion transport, and the introduction of Se into CoSe2, which offers high electrical conductivity, further enhancing the electrochemical performance.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).30 Zhang et al. manufactured mesoporous (∼16–50 nm) V2O5 and macroporous (∼60 nm) V2O5. The mesoporous V2O5 displayed an excellent Cs (680 F g−1, 1 A g−1) and impressive cyclic retention (70% over 10
000 cycles).30 Zhang et al. manufactured mesoporous (∼16–50 nm) V2O5 and macroporous (∼60 nm) V2O5. The mesoporous V2O5 displayed an excellent Cs (680 F g−1, 1 A g−1) and impressive cyclic retention (70% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 3 A g−1), while macroporous (∼60 nm) V2O5 showed relatively poor performance with a Cs of 406 F g−1 and cyclic conservation of 40% during 4000 cycles at 3 A g−1.29 In addition, Zhou et al. designed Co(OH)2/BAC (bamboo activated carbon) containing mesoporous (∼20 nm) or macroporous (∼85 nm) structures. Mesoporous Co(OH)2/BAC expressed a more outstanding specific capacitance (345 F g−1 at 0.1 A g−1) than that of macroporous Co(OH)2/BAC with 289 F g−1 at 0.1 A g−1, and showed satisfactory stability with 84% of Cs conservation over 15
000 cycles at 3 A g−1), while macroporous (∼60 nm) V2O5 showed relatively poor performance with a Cs of 406 F g−1 and cyclic conservation of 40% during 4000 cycles at 3 A g−1.29 In addition, Zhou et al. designed Co(OH)2/BAC (bamboo activated carbon) containing mesoporous (∼20 nm) or macroporous (∼85 nm) structures. Mesoporous Co(OH)2/BAC expressed a more outstanding specific capacitance (345 F g−1 at 0.1 A g−1) than that of macroporous Co(OH)2/BAC with 289 F g−1 at 0.1 A g−1, and showed satisfactory stability with 84% of Cs conservation over 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.31 Consequently, the mesoporous morphology shows impressive advantages in increasing the electrochemical properties, especially in maintaining cyclic stability.
000 cycles.31 Consequently, the mesoporous morphology shows impressive advantages in increasing the electrochemical properties, especially in maintaining cyclic stability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, further demonstrating the successful synthesis of CoSe2.
1, further demonstrating the successful synthesis of CoSe2.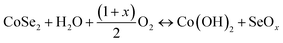
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1
000 cycles at 5 A g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1
000 cycles at 5 A g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which was 79.8% of the highest specific capacitance (391 F g−1 at 4500 cycles), implying superior cyclic durability. Meanwhile, from Fig. 6e, the CE of sample A3 reached 99.3% after 25
000 cycles, which was 79.8% of the highest specific capacitance (391 F g−1 at 4500 cycles), implying superior cyclic durability. Meanwhile, from Fig. 6e, the CE of sample A3 reached 99.3% after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1. The inset of Fig. 6e shows that the first and last ten GCD curves were almost symmetric as well as being similar, confirming remarkable reversibility and structural stability.50,51 The Nyquist plots (sample A3) before and after 25
000 cycles at 5 A g−1. The inset of Fig. 6e shows that the first and last ten GCD curves were almost symmetric as well as being similar, confirming remarkable reversibility and structural stability.50,51 The Nyquist plots (sample A3) before and after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles are revealed in Fig. 6f. The Rs and Rct were 0.73 Ω and 0.39 Ω, respectively, before cycling and increased to 0.83 Ω and 0.49 Ω, respectively, after 25
000 cycles are revealed in Fig. 6f. The Rs and Rct were 0.73 Ω and 0.39 Ω, respectively, before cycling and increased to 0.83 Ω and 0.49 Ω, respectively, after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The slight change proved that the as-obtained electrode material had fast ion transfer and a stable structure in the long-term charge–discharge process.52,53 Based on the above discussion of electrochemistry, the excellent properties of the porous CoSe2 nanosheet come mainly from the following two aspects: (1) the mesoporous structure shortens the ion transport pathway and has high mechanical properties, which can maintain the steady microstructure over the long period of the cycling test; (2) Se has higher electrical conductivity and can provide greater capacitive activity.
000 cycles. The slight change proved that the as-obtained electrode material had fast ion transfer and a stable structure in the long-term charge–discharge process.52,53 Based on the above discussion of electrochemistry, the excellent properties of the porous CoSe2 nanosheet come mainly from the following two aspects: (1) the mesoporous structure shortens the ion transport pathway and has high mechanical properties, which can maintain the steady microstructure over the long period of the cycling test; (2) Se has higher electrical conductivity and can provide greater capacitive activity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, indicating excellent cycling durability. Meanwhile, the CE was maintained at 99.12% after cycling. In the inset to Fig. 8h, the last ten charge/discharge cycles show no obvious deformation compared to the first ten cycles, which implies good electrochemical stability. The E and P of the ASC were acquired according to eqn (3) and (4). The Ragone plot of CoSe2//AC can be observed in Fig. 8i. The maximum E of 18.9 W h kg−1 was achieved when P was 387 W kg−1 and the maximum P (7750 W kg−1) was acquired at an E of 9.34 W h kg−1, which is obviously higher than those of NiFe-LDH@CoS2@Ni//AC,58 Ni3Se2//AC,59 NiCo2S4@CoS2//AC,60 or Ni0.9Co1.92Se4//AC.61 In addition, five LEDs can be lit when two identical ASC devices are connected in series, as seen in the inset to Fig. 8i, demonstrating its good practical applicability.
000 cycles, indicating excellent cycling durability. Meanwhile, the CE was maintained at 99.12% after cycling. In the inset to Fig. 8h, the last ten charge/discharge cycles show no obvious deformation compared to the first ten cycles, which implies good electrochemical stability. The E and P of the ASC were acquired according to eqn (3) and (4). The Ragone plot of CoSe2//AC can be observed in Fig. 8i. The maximum E of 18.9 W h kg−1 was achieved when P was 387 W kg−1 and the maximum P (7750 W kg−1) was acquired at an E of 9.34 W h kg−1, which is obviously higher than those of NiFe-LDH@CoS2@Ni//AC,58 Ni3Se2//AC,59 NiCo2S4@CoS2//AC,60 or Ni0.9Co1.92Se4//AC.61 In addition, five LEDs can be lit when two identical ASC devices are connected in series, as seen in the inset to Fig. 8i, demonstrating its good practical applicability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 and a good Cs value of 333 F g−1 (1 A g−1). The assembled asymmetric supercapacitor also showed impressive energy storage performance. The results prove that the porous CoSe2 nanosheets prepared via one-step microwave synthesis are suitable as an electrode material for practical applications in supercapacitors.
000 cycles at 5 A g−1 and a good Cs value of 333 F g−1 (1 A g−1). The assembled asymmetric supercapacitor also showed impressive energy storage performance. The results prove that the porous CoSe2 nanosheets prepared via one-step microwave synthesis are suitable as an electrode material for practical applications in supercapacitors.







