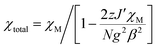Multifunctional properties existing in Ln–nitronyl nitroxide single-chain magnets†
Hongdao
Li
 *a,
Lu
Xi
b,
Pei
Jing
b,
Jianke
Tang
a,
Qi
Wang
*a,
Lu
Xi
b,
Pei
Jing
b,
Jianke
Tang
a,
Qi
Wang
 a,
Hong
Yang
a,
Lijun
Zhai
a,
Yulan
Niu
a,
Lifeng
Ding
a and
Zhenjun
Song
a,
Hong
Yang
a,
Lijun
Zhai
a,
Yulan
Niu
a,
Lifeng
Ding
a and
Zhenjun
Song
 *bc
*bc
aDepartment of Chemistry and Chemical Engineering, Taiyuan Institute of Technology, Taiyuan 030008, China. E-mail: lihong.dao@163.com
bDepartment of Chemistry, Key Laboratory of Advanced Energy Materials Chemistry and Tianjin Key Laboratory of Metal and Molecule-based Material Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: songzj@mail.nankai.edu.cn
cSchool of Pharmaceutical and Materials Engineering, Taizhou University, Taizhou 318000, China
First published on 18th November 2020
Abstract
Taking advantage of a nitronyl nitroxide radical ligand 8-QNNIT (1) (8-quinolyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide) and Ln(hfac)3·2H2O (LnIII = Tb 2 and Dy 3; hfac = hexafluoroacetylacetonate), two Ln–radical one-dimensional chains [Ln(hfac)3(8-QNNIT)]n were constructed. The magnetic properties, thermodynamics and optical behavior of both compounds were observed, exhibiting polyfunctionality of the 2p–4f system. Complexes 2 and 3 show typical single-chain magnet (SCM) properties with temperature-dependent relaxation peaks giving an effective energy barrier (Ueff/kB) of 47.4 K (2) and 64.6 K (3). Luminescence spectra and ultravioletvisible (UV-vis) absorption spectra of the Tb compound, and UV-vis absorption spectra and thermodynamics properties of the Dy complex were investigated. As far as we know, the Dy complex is the first SCM with thermodynamics behavior and optical properties comparable to those of multifunctional magnetic materials. This study aims to provide a new way and field of vision for the construction of 2p–4f heterospin multi-functional materials.
Introduction
Single-chain magnets (SCMs) are one-dimensional compounds with strong Ising-type magnetic anisotropy, significant intra-chain magnetic coupling between spin carriers and negligible inter-chain interaction, displaying slow magnetic relaxation behavior.1 Based on the above factors, the incorporation of anisotropic metal ions and short bridges such as hydrone (H2O), hydroxyl (OH−) and Br−, which can strengthen the magnetic interaction between adjacent spin carriers, is one of the effective chemical routes to synthesize single-chain magnets.2–4 Recently, Yang et al. have obtained water and hydroxyl-extended lanthanide-based SCMs with a relatively high relaxation energy barrier.2 On the other hand, conjunction with anisotropic 4f/3d ions and radical ligands, which will be able to transmit effectively magnetic interactions, can also produce SCMs successfully. One of the representative achievements is that Gatteschi and co-workers obtained the first single-chain magnet [Co(hfac)2(NITPhOMe)]n.5 Since then, several remarkable single-chain magnets involving a radical and 3d/4f/3d–4f metal ions have been reported.6 Lately, Cheng et al. employed an air-stable nitronyl nitroxide radical and Co2+ ions to achieve successfully a quasi-1D spin organization, with giant coercive fields up to 65 kOe.7On the other hand, compared to an intensive study of the magnetic properties, thermodynamics behavior and luminescence properties of lanthanide–radical complexes have rarely been explored. Moreover, in recent years, molecules with multifunctional functionalities are currently of great interest in coordination chemistry. For example, Deun et al. prepared a series of 4f-based nanosheets for optical luminescence thermometry.8 And the dual functionality of an Yb compound for slow magnetic relaxation and luminescence thermometry was explored.9 Quite lately, tetranuclear lanthanide complexes based on a functionalized nitronyl nitroxide biradical, displaying slow magnetic relaxation behavior and luminescence performance, were obtained successfully.10 In addition, Vaz et al. constructed lanthanide compounds with a dppnTEMPO radical displaying two relaxation processes and the presence of two distinct luminescent centers.11 However, to date, lanthanide–nitronyl nitroxide complexes with SCM propertyies, thermodynamics or/and optical behavior coexisting in single molecules have not been reported.
In this regard, to investigate multifunctional properties of 2p–4f heterospin SCMs, herein, we synthesize a new nitronyl nitroxide radical 8-QNNIT (1) (8-quinolyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide) and utilize the radical ligand to achieve two heterospin 1D compounds, namely, [Ln(hfac)3(8-QNNIT)]n (LnIII = Tb 2, Dy 3; hfac = hexafluoroacetylacetonate). Additionally, the magnetic, optical and thermodynamics properties of the 2p–4f system have been systematically investigated. Tb and Dy compounds display frequency-dependent out-of-phase signals indicating SCM behavior. Moreover, the Tb–radical chain shows the characteristic emission peaks of metal TbIII ions. The heat capacity of the Dy compound is also studied in the paper. To our knowledge, this is the first report on nitronyl nitroxide-based LnIII compounds with SCM properties and optical or/and thermodynamics behavior coexisting in a molecular entity.
Experimental section
General materials and physical measurements
All the reagents were used directly without any further refinement. Elemental analyses (for C, H, and N) were implemented on a PerkinElmer 240 elemental analyzer. FT-IR spectra were recorded in the region of 4000–400 cm−1 on a Bruker TENOR 27 spectrometer. Magnetic data were recorded for samples 1–3 employing a Quantum Design SQUID VSM magnetometer, corrected for the diamagnetic 1–3 with Pascal's constants and these sample holders by measurement.12 Employing an F-4500 fluorescence spectrophotometer, the excitation and emission spectra of the Tb complex were measured. Ultraviolet-visible (UV-vis) spectra were recorded through a TU-1901 spectrophotometer. The heat capacities were obtained using a Netzsch DSC 204 F1 analyzer in an N2 atmosphere.Preparation of [8-QNNIT] (1)
Radical 8-QNNIT (8-quinolyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide) was synthesized according to published literature.13 The 8-QNNIT radical was recrystallized in CH2Cl2/heptane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) mixed solution to obtain purple strip crystals. Yield 83%. C16H18N3O2 (284.33 g mol−1): calcd C 67.58, H 6.38, N 14.78; found C 67.32, H 6.39, N 14.50. FT-IR (KBr): 3411 (s), 2348 (m), 1625 (s), 1613 (s), 1221 (s), 1053 (s), 945 (m), 806 (m), 701 (m), 527(m), 506 (m) cm−1.
5) mixed solution to obtain purple strip crystals. Yield 83%. C16H18N3O2 (284.33 g mol−1): calcd C 67.58, H 6.38, N 14.78; found C 67.32, H 6.39, N 14.50. FT-IR (KBr): 3411 (s), 2348 (m), 1625 (s), 1613 (s), 1221 (s), 1053 (s), 945 (m), 806 (m), 701 (m), 527(m), 506 (m) cm−1.
Preparation of compounds 2 and 3
The synthetic reaction of both compounds was based on the following process. Ln(hfac)3·2H2O (0.01 mmol) (Ln = Tb 2 and Dy 3) in 16 mL of n-heptane was allowed to reflux for 3.5 hours, to which a solution of 8-QNNIT (0.0028 g, 0.01 mmol) in dichloromethane (4 mL) was introduced. The resulting solution was stirred for about 25 min to give a pink reaction mixture, and then the above solution was cooled to room temperature. A pink filtrate was left standing for 48 hours at ambient temperature, and pink strip crystals were generated.X-ray crystallography
Diffraction data of compounds 1–3 were gathered on a Rigaku Saturn diffractometer with Mo-Kα radiation (λ = 0.71073 Å) at 293 K for 1 and at 150 K for 2 and 3. Absorption correction was carried out via CrystalClear. The structures of the radical ligand and Tb and Dy compounds were solved by direct methods with the Olex2 program, and refined by F2 by a full-matrix least-squares procedure using SHELXL-2014.14 H atoms attached to C atoms were placed in reasonable positions, and then refined with a riding model. Anisotropic refinement was imposed on other non-H atoms. CCDC 2024373–2024375 (1–3) contain the supplementary crystallographic data.† The crystal data and structure refinement of three compounds are listed in Table 1. The important bond lengths and angles of complexes 2 and 3 are summarized in Table 2 and Tables S1 and S2 (ESI†).| Complex | 1 | 2 | 3 |
| Empirical formula | C16H18N3O2 | C31H21F18N3O8Tb | C31H21F18N3O8Dy |
| M r | 284.33 | 1064.42 | 1067.98 |
| T (K) | 293(2) | 150(2) | 150(2) |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic |
| Space group | Pca21 | P21/c | P21/c |
| a/Å | 15.2203(3) | 22.868(5) | 22.7736(11) |
| b/Å | 8.09480(16) | 16.664(3) | 16.6481(8) |
| c/Å | 11.9026(3) | 22.826(5) | 22.7247(11) |
| α/° | 90 | 90 | 90 |
| β/° | 90 | 117.20(3) | 117.2050(10) |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 1466.46(5) | 7736(3) | 7662.7(6) |
| Z | 4 | 4 | 4 |
| D calcd/g cm−3 | 1.288 | 1.828 | 1.852 |
| μ/mm−1 | 0.703 | 1.966 | 2.090 |
| θ/° | 5.46–67.23 | 2.890–25.906 | 2.015–26.420 |
| F(000) | 604 | 4152 | 4160 |
| Reflections collected | 8594 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 446 446 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997 |
| Unique reflns/Rint | 2494/0.0235 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336/0.0789 336/0.0789 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709/0.0607 709/0.0607 |
| GOF (F2) | 1.055 | 1.058 | 1.086 |
| R 1, wR2 (I > 2σ(I)) | 0.0308, 0.0829 | 0.0729, 0.1822 | 0.0613, 0.1656 |
| R 1, wR2 (all data) | 0.0323, 0.0837 | 0.1075, 0.2062 | 0.0837/0.1829 |
| Complex | 2 | 3 |
|---|---|---|
| Ln–O(hfac) | 2.345(6)–2.393(7) | 2.322(5)–2.384(5) |
| Ln–O(rad) | 2.333(6)–2.389(7) | 2.316(5)–2.384(5) |
| Ln–O–N | 137.2(6)–149.2(6) | 137.5(4)–148.7(4) |
Results and discussion
Molecular structures
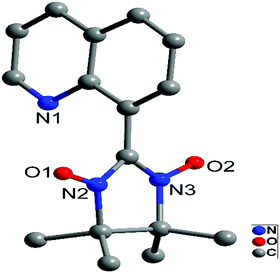
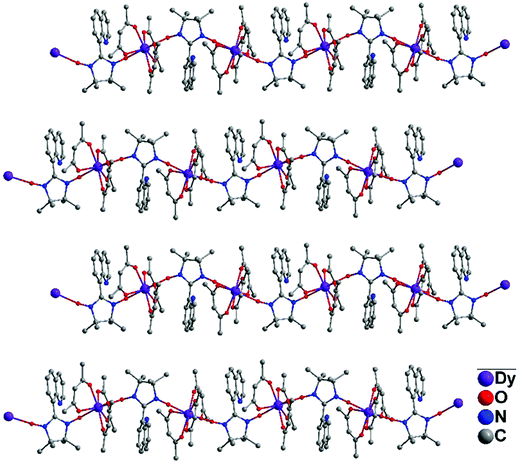
Complex 1 belongs to the orthorhombic crystal system. The molecular structure of the 8-QNNIT radical is given in Fig. 1. Two O(1)–N(2) and O(2)–N(3) distances are 1.2749(17) Å and 1.2772(16) Å, respectively, which are in line with the N–O contact in the radical (1.25 to 1.32 Å).15 The dihedral angle between the quinoline ring and five-membered heterocyclic rings of the radical is 78.86(1)°. In the intermolecular, the nearest NO–NO contact with the O⋯O distance is 4.5627(25) Å (Fig. S1, ESI†).Crystallographic studies manifest that isostructural 2 and 3 are crystallized in monoclinic space group P21/c (Fig. 2 and Fig. S2, ESI†). Taking compound 3 for example, the compound displays a one-dimensional 4f-system, which is supported by 8-QNNIT radicals. Each DyIII ion ligates two oxygen atoms of NO groups, and the rest of the coordination sphere is completed with six oxygen atoms by using three hfac− coligands. Both crystallographically independent Dy(hfac)3(8-QNNIT) moieties belong to different 1D chains. The SHAPE measure analysis16 gives these values (0.372 (Dy1) and 0.246 (Dy2)) for a triangular dodecahedron geometry (D2d symmetry) (Fig. S5 and Table S3, ESI†). The DyIII–Ohfac bond lengths are in the range of 2.322(5)–2.384(5) Å, while DyIII–Orad distances vary in the range of 2.316(5)–2.384(5) Å.
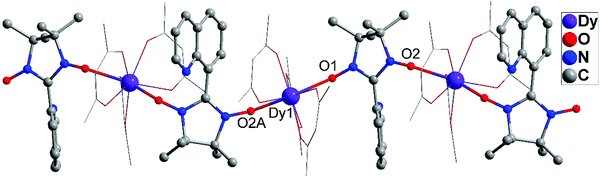 | ||
| Fig. 2 Single-crystal X-ray diffraction structure of complex 3, in which H and F atoms are omitted for clarity. | ||
As can be found, Dy–radical chains are well separated (Fig. 3), with the nearest Dy⋯Dy distance being 11.65 Å for 3, which can avert significant intermolecular interactions.
Magnetic properties
Direct current magnetic susceptibility measurements for complex 1 (Fig. 4) display that the experimental χMT value of 0.374 cm3 K mol−1 (at 300 K) agrees with the theoretical value (S = 1/2, g = 2.0, C = 0.375 cm3 K mol−1) for an 8-QNNIT radical. The χMT product remains almost constant as the temperature decreases to 30 K. Then, the value of χMT rises quickly upon further cooling, attaining 0.408 cm3 K mol−1 at 2 K. Assuming isotropic Heisenberg interactions, the reported equation of the magnetic susceptibility in terms of ferromagnetically17 coupled (J) linear arrays of 1/2 spins is| χM = (C/T)[(1 + 5.7979916K + 16.902653K2 + 29.376885K3 + 14.036981K5)/(1 + 2.7979916K + 7.0086780K2 + 8.6538644K3 + 4.5743114K4)]2/3 |
where K = J/2kT and zJ′ represents the magnetic interaction between uncoordinated NO groups.
The best fit values are J = 0.11 cm−1, zJ′ = −0.009 cm−1, C = 0.3755 emu K mol−l, and g = 2.0. In addition, the reciprocal susceptibility vs. the temperature abides by the Curie–Weiss law (2–300 K) with Weiss constant θ = 0.152 K and Curie constant C = 0.40 cm3 K mol−1, implying the existence of ferromagnetic interactions.
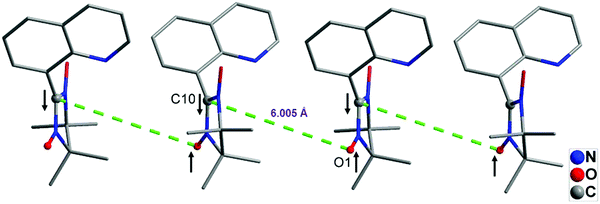
The observed positive values of J and θ may result from dominant ferromagnetic coupling, derived from the pseudo 1D chain (Fig. 5), in which the bridging sp2 carbon atoms (C10) and the oxygen atoms (O1) from the NO groups of the neighboring 8-QNNIT radicals carry the opposite sign spin densities, following McConnell's law.17 The negative value of zJ′ arises from short NO–ON contacts and π⋯π interactions.
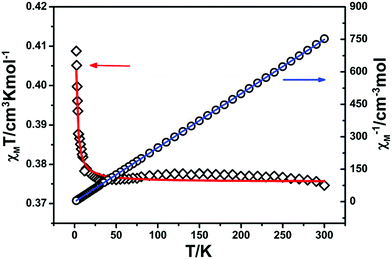
The static direct-current (dc) magnetic susceptibility properties for compounds 2 and 3 were measured under a dc field of 1 kOe from 300 to 2 K (Fig. 6). At 300 K, the χMT values are 12.27 cm3 K mol−1 for 2 and 14.94 cm3 K mol−1 for 3, which are slightly more than the theoretical values (12.19 cm3 K mol−1 for 2 and 14.54 cm3 K mol−1 for 3) for one non-interacting LnIII ion (TbIII: 7F6, S = 3, L = 3, g = 3/2, C = 11.82 cm3 K mol−1; DyIII: 6H15/2, S = 5/2, L = 5, g = 4/3, C = 14.17 cm3 K mol−1) and one 8-QNNIT radical (S = 1/2, C = 0.375 cm3 K mol−1).
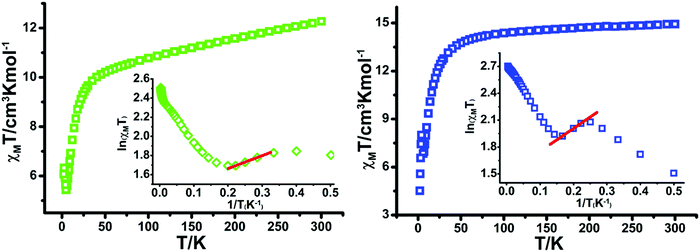 | ||
| Fig. 6 Plots of χMT–T for 2 (left) and 3 (right). Insets: ln(χMT)–1/T plots. The red solid lines represent the best fits. | ||
Upon cooling, the χMT value of compound 2 decreases steadily to 7 K and the value of 3 remains almost constant above 100 K. Then χMT values go down, followed by an abrupt increase for both complexes, and finally drop to 6.07 cm3 K mol−1 for 2 and 4.52 cm3 K mol−1 for 3 at 2.0 K. At low temperature, the χMT values of both compounds display a degree of rise, suggesting the presence of nearest-neighbor heavy lanthanide metal–radical ferromagnetic coupling (Fig. 6).
For the Ising-like or anisotropic Heisenberg 1D chain, the value of χMT could follow an exponential behavior (χMT ≈ Ceff![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Δξ/T)), in which the energy needed to create a domain wall along the chain is represented by Δξ.1a For 2 and 3, the ln(χMT) vs. 1/T plot displays a linear region between 3.5 and 4.5 K with Δξ = 1.35 K for 2 and between 4.5 and 6 K with Δξ = 2.54 K for 3, verifying the 1D Ising-like character (Fig. 6, inset).
exp(Δξ/T)), in which the energy needed to create a domain wall along the chain is represented by Δξ.1a For 2 and 3, the ln(χMT) vs. 1/T plot displays a linear region between 3.5 and 4.5 K with Δξ = 1.35 K for 2 and between 4.5 and 6 K with Δξ = 2.54 K for 3, verifying the 1D Ising-like character (Fig. 6, inset).
The isothermal field-dependent magnetization data for Tb and Dy compounds display continuous increases up to 4.86 and 6.66 Nβ at 2 K and 70 kOe (Fig. 7 and Fig. S6, ESI†) with the lack of high-field saturation, illustrating the crystal-field effects and low-lying excited states. Besides, the non-superposition of the M vs. H/T plots at different temperatures (2 K, 3 K and 5 K) is indicative of the presence of significant magnetic anisotropy (Fig. 7 and Fig. S7, ESI†).
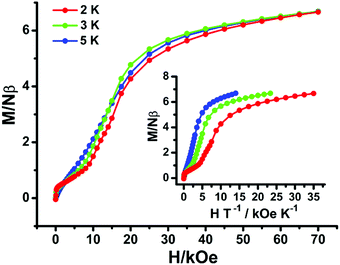 | ||
| Fig. 7 Field dependences of magnetization for compound 3 at temperatures of 2, 3, and 5 K. Insets: Plots of the reduced magnetization M versus HT−1. | ||
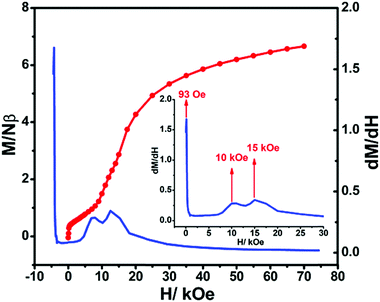
It is worth mentioning that the M vs. H curve of the Dy complex displays a sigmoid, suggesting a metamagnetic system. The dM/dH curve shows that a three-step field induced transition at critical fields of H1 = 93 Oe, H2 = 10 kOe, and H3 = 15 kOe (Fig. 8).
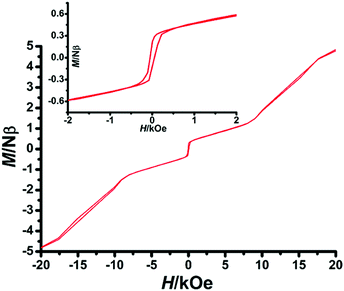
At around 93 Oe, a transition is to surmount relatively weak interchain antiferromagnetic couplings. The second metamagnetic step is related to the transformation of the system from the spin-canted phase to the spin-flop state.18 The third step shows that when the magnetic field is larger than the critical field, the intrachain antiferromagnetic interactions can be induced to transform to ferromagnetic couplings.19 Moreover, there is a narrow hysteresis at 2 K for the Dy complex (Fig. 9), indicating that a dramatic slowing down of the relaxation time does not occur as the temperature is lowered below critical temperature.
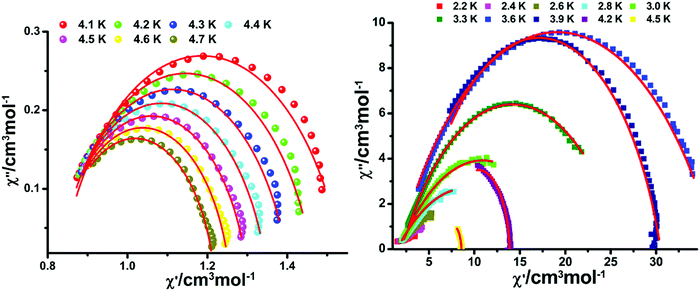
Toward further understanding the spin dynamic behaviors of compounds 2 and 3, alternating current magnetic susceptibilities in the variable-temperature and variable-frequency patterns were implemented. In the absence of a dc field, out-of-phase (χ′′) signals display temperature- and frequency-dependence obviously, revealing the slow magnetization relaxation (Fig. 10 and Fig. S8–S13, ESI†). To rule out the possibility of glassiness, the parameter φ was evaluated (φ = (ΔTp/Tp)/Δ(log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f)).20 The results show that the φ value is 0.191 for 2 and 0.090 for 3 to exclude the possibility of a spin-glass (0.01 < φ < 0.08).21
f)).20 The results show that the φ value is 0.191 for 2 and 0.090 for 3 to exclude the possibility of a spin-glass (0.01 < φ < 0.08).21
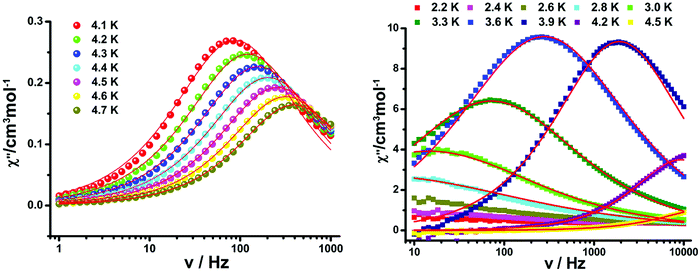 | ||
| Fig. 10 Frequency dependence of the out-of-phase (χ′′) for 2 (left) and 3 (right) under zero dc field. The solid lines represent the best fitting. | ||
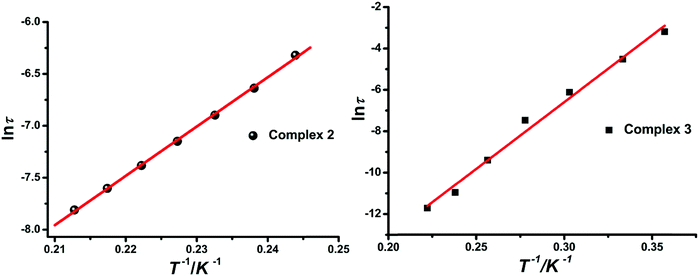
The equation τ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp
exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Δτ/kBT) was employed to analyze the relaxation time under 0 Oe DC fields (Fig. 11), giving τ0 = 1.63(5) × 10−8 s, Δτ/kB = 47.4(8) for compound 2 and τ0 = 5.26(8) × 10−12 s, Δτ/kB = 64.6(1) K for compound 3, which correspond to the values reported for rad-4f SCMs.6e,22 This energy barrier (Δτ) is made up of two parts, namely the correlation energy (Δξ) and the blocking energy (ΔA).23 And the latter is about 46.05 K for 2 and 62.06 K for 3, which are related to the magnetic anisotropy of the 4f ion unit.
(Δτ/kBT) was employed to analyze the relaxation time under 0 Oe DC fields (Fig. 11), giving τ0 = 1.63(5) × 10−8 s, Δτ/kB = 47.4(8) for compound 2 and τ0 = 5.26(8) × 10−12 s, Δτ/kB = 64.6(1) K for compound 3, which correspond to the values reported for rad-4f SCMs.6e,22 This energy barrier (Δτ) is made up of two parts, namely the correlation energy (Δξ) and the blocking energy (ΔA).23 And the latter is about 46.05 K for 2 and 62.06 K for 3, which are related to the magnetic anisotropy of the 4f ion unit.
The Cole–Cole plots (Fig. 12) can be fitted by the Debye model to afford α factors in the range of 0.14–0.19 for compound 2 with a middle distribution and 0.050–0.601 for compound 3 with a wide distribution.
Luminescence properties
The solution fluorescence of the Tb compound is recorded by using an excitation wavelength of 320 nm at room temperature in CH2Cl2 with an approximate concentration of 10−6 mol L−1, showing four groups of characteristic emission peaks of the TbIII ion at about 491, 546, 584 and 614 nm, being related to the 5D4 → 7F6,5,4,3 transitions, respectively.24 The intensity of the 5D4–7F5 transition is stronger than those of other transitions, which reveals that the 8-QNNIT radical is propitious to sensitize green luminescence of TbIII (Fig. 13). Furthermore, the green emission peak possesses a narrow full-width at half-maximum (FWHM) of about 10 nm during the 5D4–7F5 transition. The luminescence decay profile for the 5D4–7F5 emission is shown in Fig. S14 (ESI†). The lifetime value is 18.04 μs, manifesting a short-lived excited state.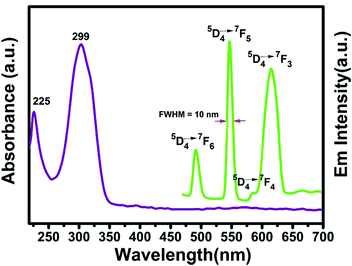 | ||
| Fig. 13 UV-vis absorption (purple line) and luminescence (green line, emission) spectra of compound 2 in dichloromethane. | ||
Ultraviolet-visible (UV-vis) spectra
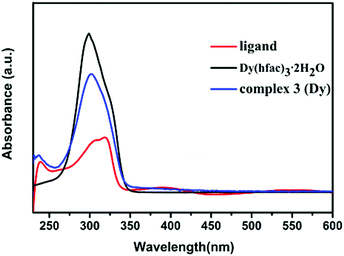
UV-vis spectra of the 8-QNNIT radical ligand, Dy(hfac)3·2H2O, and compound 3 in CH2Cl2 (1 × 10−7 mol L−1) were investigated in the range of 230–600 nm at room temperature. In addition, the UV-vis spectroscopy test of complex 2 was performed under the same conditions.As depicted in Fig. 14, the 8-QNNIT radical ligand shows three absorption bands around 238, 318 and 390 nm, on account of the π–π* and n–π* transitions of the aromatic nucleus of the radical. For Dy(hfac)3·2H2O, an intense absorption around 299 nm is observed. Complex 3 exhibits weak absorption bands at 237, assigned to the radical ligand, which is blue shifted slightly compared with the 8-QNNIT radical due to the influence of DyIII ion coordination. And, the strong absorption of compound 3 around 299 nm is related to the hfac− coligand. Besides, the wavelengths of the UV-vis absorption peaks of the Tb complex are 225 and 299 nm, respectively (Fig. 13).
Heat capacity properties
The molar heat capacity measurement of complex 3 was carried out by DSC from 263.01 to 346.71 K. The average molar heat capacities are plotted in Fig. 15. The heat capacity (Cp,m) of compound 3 gradually increases with the increase of temperature, and there is no obvious thermal anomaly. The average values of Cp,m (the molar heat capacity) were analyzed by a polynomial equation at reduced temperature (X) via the least-squares method:25,26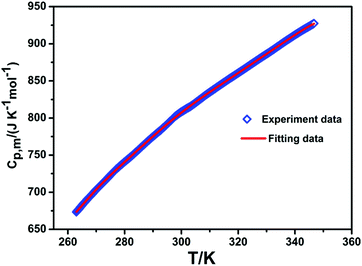 | ||
| Fig. 15 Temperature dependence of molar heat capacities for compound 3. The solid line represents the best fitting. | ||
in which X denotes the reduced temperature (X = [T − (Tmax + Tmin)/2]/[(Tmax − Tmin)/2]), T represents the experimental temperature, and Tmax and Tmin are the maximum and minimum temperatures (346.71 and 263.01 K).
Discussion and perspectives
To restrain interchain dipolar interactions of one-dimensional compounds and improve slow magnetic relaxation behavior, increasing the distance between interchain metal ions via the bulkier substituents of nitronyl nitroxide radicals is a productive way. Structural and magnetic parameters are shown in Table 3 for some reported 4f-nitronyl nitroxide 1D compounds with a similar structure. As illustrated in the table, compound {[DyCu(hfac)5(NIT-Ph-p-OCH2trz)]·0.5C6H14} displays a lower effective energy barrier (29.0 K) on account of the shorter distance between interchain DyIII ions (9.75 Å). Moreover, with the distance between interchain 4f ions increasing, the energy barrier tends to increase gradually. For the current work, the nearest distance between interchain DyIII ions is the longest with 11.98 Å, therefore interchain dipolar interactions can be ignored, exhibiting a relatively higher effective energy barrier (64.6 K). The bulkier quinoline substituent of the 8-QNNIT radical is conducive to increase the distance between interchains, and then cut down interchain dipolar interactions for optimizing magnetic behavior.| 4f-Based 1D complex | The minimum distance between interchain 4f ions (Å) | Energy barrier (K) | Relaxation time (s) | Ref. |
|---|---|---|---|---|
| {[DyCu(hfac)5(NIT-Ph-p-OCH2trz)]·0.5C6H14} | 9.75 | 29.0 | 6.1 × 10−10 | 6d |
| [Dy(hfac)3NIT3BrPhOMe]n | 10.13 | 39.8 | 2.43 × 10−10 | 27 |
| [Dy(hfac)3NITPh(OMe)2]n | 11.53 | 70.3 | 8.68 × 10−15 | 6c |
| [Dy(hfac)3(8-QNNIT)]n | 11.98 | 64.6 | 5.26 × 10−12 | This work |
On the other hand, in the field of 4f-based hetero-spin complexes involving nitronyl nitroxide, compared with well-studied SMM and SCM behaviors, the thermodynamics behavior and optical properties of lanthanide complexes have rarely been documented and multifunctional molecular materials are still in their infancy (Table 4). As far as we know, [Ln(hfac)3(8-QNNIT)]n (LnIII = Tb 2, Dy 3) is the first SCMs with thermodynamics behavior as multifunctional magnetic materials.
| Multifunctional molecular materials | Diverse properties | Ref. | ||
|---|---|---|---|---|
| [Tb(acac)3NIT2Py·0.5H2O] | Luminescence | SMM behavior | 28 | |
| [Ln2(hfac)6(H2O)2(dppnTEMPO)] (LnIII = Tb, Dy) | Luminescence | Field induced SMM behavior | 9 | |
| Ln(hfac)3(NITPhOCF3)2 (LnIII = Tb, Dy) | Thermodynamics | Slow magnetic relaxation | 25 | |
| [{Ln(hfac)3}3{Cu(hfac)2}{NIT-Ph(OMe)2}4]n (LnIII = Gd, Tb) | Magnetocaloric effect | Slow magnetic relaxation | 29 | |
| [Ln(hfac)3(8-QNNIT)]n (LnIII = Tb, Dy) | Optical property | SCM behavior | Thermodynamics | This work |
Lanthanide SCMs/SMMs as multifunctional molecular magnetic materials, such as luminescent magnets, magnetoelectric materials, conducting magnets, chiral magnets, magnetic refrigeration materials, etc., displaying specific functionalities, have gradually caused extensive concern in the molecular material field on account of their potential applications in magneto-luminescence sensing, luminescence thermometry,8,9 photocatalysis,30 single molecule detection, magnetic cooling technology, etc. Among them, photomagnets act as a kind of multifunctional magnetic material, in which the magnetic properties can be regulated or/and controlled by photons, arousing great interest of scientific research. From the point of view of practical application, photomagnets can be extended to the Ln–radical system for designing multifunctional materials involving nitronyl nitroxide, which may become the research focal point in the future.
Conclusions
In summary, we have successfully constructed a new nitronyl nitroxide radical and two 2p–4f heterospin one dimensional compounds with the radical ligand bridging LnIII ions exhibiting obvious slow magnetic relaxation behavior under zero dc field. Interestingly, the thermodynamics behavior, optical properties and SCM behavior are characteristic of those exhibited by multifunctional materials, and the Dy complex is the first SCM with thermodynamics behavior. To our knowledge, this kind of multifunctional material has rarely been reported in the radical–LnIII system. This work still sheds light on a new way to generate radical-based molecular multifunctional materials. Unremitting efforts are well underway for designing fascinating families of molecular-based multifunctional materials with interesting topological structures via a multidentate nitronyl nitroxide radical ligand.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Science and Technology Innovation Project of Shanxi Province (No. 2020L0650).References
- (a) C. Coulon, H. Miyasaka and R. Clérac, Struct. Bonding, 2006, 122, 163 CrossRef CAS; (b) R. Clérac, H. Miyasaka, M. Yamashita and C. Coulon, J. Am. Chem. Soc., 2002, 124, 12837 CrossRef.
- Y. Li, P. Zhao, S. Zhang, R. Li, Y. Q. Zhang, E. C. Yang and X. J. Zhao, Inorg. Chem., 2017, 56, 9594 CrossRef CAS.
- (a) H. Miyasaka, K. Takayama, A. Saitoh, S. Furukawa, M. Yamashita and R. Clérac, Chem. – Eur. J., 2010, 16, 3656 CrossRef CAS; (b) S. J. Liu, J. P. Zhao, W. C. Song, S. D. Han, Z. Y. Liu and X. H. Bu, Inorg. Chem., 2013, 52, 2103 CrossRef CAS; (c) T. Han, J. D. Leng, Y. S. Ding, Y. Wang, Z. Zheng and Y. Z. Zheng, Dalton Trans., 2015, 44, 13480 RSC; (d) D. Shao, X. H. Zhao, S. L. Zhang, D. Q. Wu, X. Q. Wei and X. Y. Wang, Inorg. Chem. Front., 2015, 2, 846 RSC.
- (a) J. Yang, Y. F. Deng and Y. Z. Zhang, Dalton Trans., 2020, 49, 4805 RSC; (b) M. M. Wang, X. S. Gou, W. Shi and P. Cheng, Chem. Commun., 2019, 55, 11000 RSC; (c) L. L. Lv, Y. X. Sun, C. X. Ji, S. Ma, J. W. Ren, W. W. Wang, J. P. Zhao, Z. Y. Liu, Q. Lin, K. M. Su, Y. He and F. C. Liu, Inorg. Chem. Front., 2020, 7, 186 RSC.
- A. Caneschi, D. Gatteschi, N. Lalioti, C. Sangregorio, R. Sessoli, G. Venturi, A. Vindigni, A. Rettori, M. G. Pini and M. A. Novak, Angew. Chem., Int. Ed., 2001, 40, 1760 CrossRef CAS.
- (a) M. G. F. Vaz, R. A. A. Cassaro, H. Akpinar, J. A. Schlueter, P. M. Lahti and M. A. Novak, Chem. – Eur. J., 2014, 20, 5460 CrossRef CAS; (b) Z. X. Wang, X. Zhang, Y. Z. Zhang, M. X. Li, H. H. Zhao, M. Andruh and K. R. Dunbar, Angew. Chem., Int. Ed., 2014, 53, 11567 CrossRef CAS; (c) X. F. Wang, Y. G. Li, P. Hu, J. J. Wang and L. C. Li, Dalton Trans., 2015, 44, 4560 RSC; (d) M. Yang, J. Xie, Z. Sun, L. C. Li and J. P. Sutter, Inorg. Chem., 2017, 56, 13482 CrossRef CAS; (e) J. Xie, H. D. Li, M. Yang, J. Sun, L. C. Li and J. P. Sutter, Chem. Commun., 2019, 55, 3398 RSC.
- X. Q. Liu, X. W. Feng, K. R. Meihaus, X. X. Meng, Y. Zhang, L. Li, J. L. Liu, W. Shi, Y. Q. Zhang, P. Cheng and J. R. Long, Angew. Chem., Int. Ed., 2020, 59, 10610 CrossRef CAS.
- D. Maraa, F. Artizzua, B. Laforcec, L. Vinczec, K. V. Hecke, R. Van Deun and A. M. Kaczmarek, J. Lumin., 2019, 213, 343 CrossRef.
- M. Fondo, J. C. Vázquez, A. M. G. Deibe, J. S. Matalobos, M. Amoza, A. M. P. Botas, R. A. S. Ferreira, L. D. Carlos and E. Colacioc, Inorg. Chem. Front., 2020, 7, 3019 RSC.
- L. Xi, H. D. Li, J. Sun, Y. Ma, J. K. Tang and L. C. Li, Inorg. Chem., 2020, 59(1), 443 CrossRef CAS.
- S. G. Reis, M. Briganti, S. Soriano, G. P. Guedes, S. Calancea, C. Tiseanu, M. A. Novak, M. A. Sánchez, F. Totti, F. L. Ortiz, M. Andruh and M. G. F. Vaz, Inorg. Chem., 2016, 55, 11676 CrossRef CAS.
- O. Kahn, Molecular Magnetism, VCH, Weinheim, 1993 Search PubMed.
- M. S. Davis, K. Morokuma and R. W. Kreilick, J. Am. Chem. Soc., 1972, 94, 5588 CrossRef CAS.
- (a) G. M. Sheldrick, SHELXS-2014, Program for structure solution, Universitat of Gottingen Germany, 2014 Search PubMed; (b) G. M. Sheldrick, SHELXL-2014, Program for structure refinement, Universitat of Gottingen, Gottingen, Germany, 2014 Search PubMed.
- (a) Z. X. Xiao, H. Miao, D. Shao, H. Y. Wei, Y. Q. Zhang and X. Y. Wang, Chem. Commun., 2018, 54, 9726 RSC; (b) J. Y. Shi, M. Z. Wu, P. Y. Chen, T. Li, L. Tian and Y. Q. Zhang, Inorg. Chem., 2019, 58, 14285 CrossRef CAS; (c) K. Wang, J. Sun, L. Xi, J. Lu, P. Jing and L. C. Li, Dalton Trans., 2019, 48, 14383 RSC.
- (a) M. Lluncll, D. Casanova, J. Circra, J. M. Bofill, P. Alcmany, S. Alvarez, M. Pinsky and D. Avnir, SHAPE, version 2.1, University of Barcelona: Barcelona and Hebrew University of Jerusalem, Jerusalem, Spain, Israel, 2005 Search PubMed; (b) D. Casanova, M. Llunell, P. Alemany and S. Alvarez, Chem. – Eur. J., 2005, 11, 1479 CrossRef CAS.
- (a) H. M. McConnell, J. Chem. Phys., 1963, 39, 1910 CrossRef CAS; (b) F. L. Panthou, D. Luneau, J. Laugier and P. Rey, J. Am. Chem. Soc., 1993, 115, 9095 CrossRef.
- T. F. Liu, D. Fu, S. Gao, Y. Z. Zhang, H. L. Sun, G. Su and Y. J. Liu, J. Am. Chem. Soc., 2003, 125, 13976 CrossRef CAS.
- K. Bernot, J. Luzon, A. Caneschi, D. Gatteschi, R. Sessoli, L. Bogani, A. Vindigni, A. Rettori and M. G. Pini, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 134419 CrossRef.
- J. A. Mydosh, Spin glasses: An Experimental Introduction, Taylor & Francis, London, 1993 Search PubMed.
- E. Pardo, C. Train, R. Lescouezec, Y. Journaux, J. Pasan, C. Ruiz-Perez, F. S. Delgado, R. Ruiz-Garcia, F. Lloret and C. Paulsen, Chem. Commun., 2010, 46, 2322 RSC.
- (a) Z. X. Wang, X. Zhang, Y. Z. Zhang, M. X. Li, H. H. Zhao, M. Andruh and K. R. Dunbar, Angew. Chem., Int. Ed., 2014, 53, 11567 CrossRef CAS; (b) R. N. Liu, L. C. Li, X. L. Wang, P. P. Yang, C. Wang, D. Z. Liao and P. J. Sutter, Chem. Commun., 2010, 46, 2566 RSC; (c) X. Q. Liu, Y. X. Wang, Z. S. Han, T. Han, W. Shi and P. Cheng, Dalton Trans., 2019, 48, 8989 RSC.
- K. Bernot, L. Bogani, A. Caneschi, D. Gatteschi and R. Sessoli, J. Am. Chem. Soc., 2006, 128, 7947 CrossRef CAS.
- K. R. Johnson, S. B. Vittardi, M. A. Gracia-Nava, J. J. Rack and A. Bettencourt-Dias, Dalton Trans., 2020, 49, 6661 RSC.
- M. Y. Song, L. M. Wen, S. P. Wang, S. T. Yang, J. J. Zhang, L. N. Geng and S. K. Shi, Inorg. Chim. Acta, 2015, 430, 1 CrossRef CAS.
- J. R. Zheng, N. Ren, J. J. Zhang, D. H. Zhang, L. Z. Yan and K. Z. Wu, Thermochim. Acta, 2012, 547, 31 CrossRef CAS.
- P. Hu, X. F. Wang, Y. Ma, Q. L. Wang, L. C. Li and D. Z. Liao, Dalton Trans., 2014, 43, 2234 RSC.
- A. Lannes, M. Intissar, Y. Suffren, C. Reber and D. Luneau, Inorg. Chem., 2014, 53, 9548 CrossRef CAS.
- X. F. Wang, C. Li, J. Sun and L. C. Li, Dalton Trans., 2015, 44, 18411 RSC.
- Y. Luo, S. Wang, K. Ren, J. P. Chou, J. Yu, Z. M. Sun and M. L. Sun, Phys. Chem. Chem. Phys., 2019, 21, 1791 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2024373–2024375. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0tc04266h |
| This journal is © The Royal Society of Chemistry 2021 |