Synthesis of sulfide solid electrolytes from Li2S and P2S5 in anisole†
Riku
Maniwa
 a,
Marcela
Calpa
a,
Marcela
Calpa
 ab,
Nataly Carolina
Rosero-Navarro
ab,
Nataly Carolina
Rosero-Navarro
 *b,
Akira
Miura
*b,
Akira
Miura
 *b and
Kiyoharu
Tadanaga
*b and
Kiyoharu
Tadanaga
 b
b
aGraduate School of Chemical Sciences and Engineering, Hokkaido University, Sapporo, Hokkaido 060-8628, Japan
bDivision of Applied Chemistry, Faculty of Engineering, Hokkaido University, Sapporo, Hokkaido 060-8628, Japan. E-mail: amiura@eng.hokudai.ac.jp; rosero@eng.hokudai.ac.jp
First published on 9th December 2020
Abstract
We report the liquid-phase synthesis of sulfide solid electrolytes from Li2S and P2S5 using anisole at 200–300 °C under microwave irradiation, in which β-Li3PS4 and Li7P3S11 were directly precipitated in anisole in 30 min. Anisole afforded reasonable reactivity toward Li2S and P2S5 to form β-Li3PS4 and Li7P3S11 powders at 200–300 °C. Moreover, a Li6PS5Cl precursor solution was synthesized from a Li3PS4–anisole suspension by adding ethanol, Li2S, and LiCl. The proposed synthesis using anisole is advantageous as a simple, short-time process and would be applicable for the production of all-solid-state batteries.
1. Introduction
The development of next-generation Li-ion secondary batteries is desired in various fields, including mobile phones, electric vehicles, and power storage systems.1–4 One urgent target for next-generation of Li-ion secondary batteries is to improve their safety. Current Li-ion secondary batteries use organic electrolytes, which involve the risk of leakage and flammability. For this reason, all-solid-state lithium batteries, in which liquid electrolytes are replaced with flame-retardant inorganic solid electrolytes, have drawn considerable attention.Sulfide solid electrolytes are attractive because of their high ionic conductivity, which in some cases is comparable to that in liquid electrolytes.5–7 In addition, the good ductility of sulfide solid electrolytes is advantageous for producing all-solid-state batteries because the resistance between grain boundaries can be greatly reduced by only cold pressing.8 Among sulfide solid electrolytes, Li2S–P2S5 systems with different structures and compositions have been reported with a conductivity ranging between 10−6 and 10−2 S cm−1.9–11 For example, α-, β-, and γ-Li3PS4 electrolytes each comprise different arrangements of PS43−.12 Li7P3S11 is composed of Li+, PS43− and P2S74−, and shows a higher ionic conductivity (1.7 × 10−2 S cm−1) than β-Li3PS4 (3.3 × 10−4 S cm−1).6,13 In addition to a simple Li2S–P2S5 system, Li2S–P2S5–LiX (X: Cl, Br, and I) is a well-known system for producing sulfide electrolytes with a high ionic conductivity (∼10−3 S cm−1), such as Li6PS5X.14,15
Liquid-phase synthesis is a promising approach for producing sulfide electrolytes because it has the potential for large-scale synthesis and production of composite electrodes.16–19 β-Li3PS4 and Li7P3S11 are synthesized in two steps: formation of Li3PS4–solvent complexes from Li2S and P2S5 precursors in a solvent; and their decomposition via post heat treatment in an inert atmosphere.13,20–28 Synthesis of the complexes generally takes more than several hours, and thus a number of studies have focused on shortening the synthesis time of Li3PS4–solvent complexes by ultrasonic and microwave irradiation.24,25,29 Li6PS5X halide-containing argyrodite can be synthesized by adding ethanol to dissolve all ion species, followed by heat treatment to remove the ethanol.29
In liquid phase synthesis, selection of solvents is a key issue. Synthesis has been reported with solvents such as acetonitrile (ACN),22,24,25,27,28,30,31 THF20,25,32,33 and ethyl propionate.21,29 In the present study, we focused on anisole as a solvent for the synthesis of Li2S–P2S5 based solid electrolytes. There are four reasons for our selection. First, anisole is an aprotic solvent. When protons are present in the solvent, there is a risk of toxic hydrogen sulfide generation through the exchange reaction between Li+ in Li2S and H+ in the solvent. Second, P2S5 is easily dissolved in anisole.34 It can be expected that the reaction will proceed uniformly and quickly in the liquid phase. Third, the electron-donating ability, which can be quantified using the donor number, is suitable for the synthesis reaction. While a donor number of 0, such as for toluene, does not advance the reaction for the synthesis of electrolytes, a number of 14 or above, such as for acetonitrile and ethanol, decomposes the solid electrolyte.35 The donor number for anisole is 9,36 which is suitable for the synthesis reaction without decomposing the synthesized electrolytes.35 Fourth, anisole is a solvent that dissolves rubber-based binders.35 A suspension of the electrode composite, in which the binder is dissolved, can be applied. A sheet-type all-solid-state battery with good uniformity can be constructed. Thus, the proposed liquid-phase synthesis will be easy to apply in the future fabrication of electrode composites.
Here, we proposed a new approach to synthesize various sulfide solid electrolytes using anisole and microwave irradiation. By irradiating with microwaves, the suspension of anisole and sulfide electrolytes was directly obtained from Li2S and P2S5 in 30 min.
2. Experimental
2.1 Synthesis of the solid electrolyte
Fig. 1 shows the overall synthesis scheme. For the synthesis of β-Li3PS4 and Li7P3S11, the starting materials were Li2S (Mitsuwa Chemical, 99.9%) and P2S5 (Aldrich, 99%), and anisole (Fujifilm Wako Pure Chemical) was used as a solvent. After heating by microwave irradiation, a white suspension was obtained. The suspension was centrifuged, and the obtained precipitates were evaluated. The solid electrolyte powders were obtained by drying under vacuum followed by heat-treatment. For Li6PS5Cl, Li2S, LiCl (Aldrich, 99.9%) and ethanol (Fujifilm Wako Pure Chemical) were added to the suspension, producing a transparent solution. This solution was dried under vacuum and powders were sintered. Details of reagent and solvent amounts are summarized in the supplementary information (Table S1).† | ||
| Fig. 1 Synthesis process and obtained suspension, solution and powders. Precipitates from suspension, vacuumed powders and sintered Li6PS5Cl were characterized. | ||
Powders of Li2S (Mitsuwa Chemical, 99.9%) and P2S5 (Aldrich, 99%) at a molar ratio of Li2S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 75
P2S5 = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 were mixed in a mortar and pestle. The powders and anisole were placed in a 20 mL borosilicate microwave vial (Anton Paar). Microwave irradiation (2.45 GHz) was applied to the mixture using a microwave reactor (Monowave 400, Anton Paar). The microwave output power was set to 100 W, and an infrared sensor was used to control the temperature. The microwave irradiation was applied until the temperature reached 180–300 °C in 2–7 min (stirring rate was 1200 rpm). Liquid-phase synthesis above the boiling point (anisole: 153.8 °C) was performed using a closed vial. After heating to the target temperature, the temperature was maintained for 20 min by adjusting the microwave output. A portion of the suspension was centrifuged, and the precipitates were evaluated. The obtained suspension was dried at 150 °C for 3 h under vacuum to remove the solvent, and the powder was subsequently heat-treated at 200 °C for 1 h.
25 were mixed in a mortar and pestle. The powders and anisole were placed in a 20 mL borosilicate microwave vial (Anton Paar). Microwave irradiation (2.45 GHz) was applied to the mixture using a microwave reactor (Monowave 400, Anton Paar). The microwave output power was set to 100 W, and an infrared sensor was used to control the temperature. The microwave irradiation was applied until the temperature reached 180–300 °C in 2–7 min (stirring rate was 1200 rpm). Liquid-phase synthesis above the boiling point (anisole: 153.8 °C) was performed using a closed vial. After heating to the target temperature, the temperature was maintained for 20 min by adjusting the microwave output. A portion of the suspension was centrifuged, and the precipitates were evaluated. The obtained suspension was dried at 150 °C for 3 h under vacuum to remove the solvent, and the powder was subsequently heat-treated at 200 °C for 1 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 70
P2S5 = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 were mixed. The powder and anhydrous anisole were heated to 260 °C by microwave irradiation and dried under vacuum under the same conditions as for β-Li3PS4. The powder was heat-treated at 300 °C for 1 h.
30 were mixed. The powder and anhydrous anisole were heated to 260 °C by microwave irradiation and dried under vacuum under the same conditions as for β-Li3PS4. The powder was heat-treated at 300 °C for 1 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 75
P2S5 = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 were mixed. The powder and anhydrous anisole were heated to 220 °C by microwave irradiation under the same conditions as for β-Li3PS4. Then, Li2S, LiCl, and super anhydrous ethanol were mixed in the obtained suspension (total molar ratio Li2S
25 were mixed. The powder and anhydrous anisole were heated to 220 °C by microwave irradiation under the same conditions as for β-Li3PS4. Then, Li2S, LiCl, and super anhydrous ethanol were mixed in the obtained suspension (total molar ratio Li2S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5
P2S5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCl = 5
LiCl = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). This addition changed the suspension to a transparent solution. The solution was dried at 180 °C for 4 h under vacuum to remove the solvent to produce the sulfide electrolyte powder. A portion of the electrolyte powder was pressed under 360 MPa and sintered at 550 °C for 7 h in a sealed glass tube.
2). This addition changed the suspension to a transparent solution. The solution was dried at 180 °C for 4 h under vacuum to remove the solvent to produce the sulfide electrolyte powder. A portion of the electrolyte powder was pressed under 360 MPa and sintered at 550 °C for 7 h in a sealed glass tube.
2.2 Characterization
X-ray diffraction (XRD) patterns were measured using an X-ray diffractometer (Miniflex 600, Rigaku). Diffraction data were collected in the 2θ range between 10° and 40° at a step size of 0.02°.Raman spectra were measured using a Raman spectrometer (XploRA PLUS, Horiba Scientific) to identify structural units of the solid electrolyte samples. The excitation and intensity of the laser beam were 532 nm and 17 mW, respectively. The ionic conductivity of the pelletized samples was evaluated by electrochemical impedance spectroscopy (EIS). The solid electrolyte powders (30 or 80 mg) were pressed under 360 MPa (at room temperature) and two stainless steel (SS) disks were used as the current collectors. EIS was performed using an impedance analyzer (SI 1260, Solartron) in the frequency range of 0.1 Hz to 1 MHz at an amplitude of 10–30 mV.
3. Results and discussion
3.1 Reaction requirements under microwave irradiation
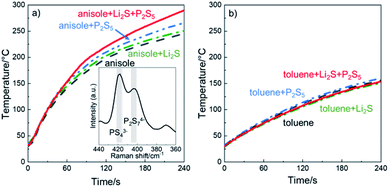
To examine which chemical species absorb microwaves, we investigated the change in the temperature of the starting materials under microwave irradiation. Fig. 2a shows the temperature change during microwave irradiation. The temperature of all the samples, including anisole without Li2S and P2S5, increased under microwave irradiation, indicating that anisole absorbs microwaves. While the temperature increase in anisole with Li2S was comparable to that of anisole alone, the temperature increase in anisole with P2S5 was enhanced. Furthermore, the sample with both Li2S and P2S5 in anisole showed the highest temperature. The temperature increase due to microwave irradiation was also confirmed in ACN and THF (Fig. S1a and b†), which have been reported as useful solvents in the liquid-phase synthesis of Li2S–P2S5 electrolytes. In contrast, no temperature difference was observed in toluene (Fig. 2b). The number of donors in toluene is 0, and the synthesis reaction cannot proceed in toluene.35 Even though microwave absorption is also affected by the dielectric constant of each solvent, the product of the chemical reaction between Li2S and P2S5 would further absorb microwaves.The inset of Fig. 2a shows the Raman spectra of the precipitate after microwave irradiation of Li2S and P2S5 in the anisole suspension and subsequent centrifugation at 8000 rpm for 10 min. Two Raman bands were centered at 419 and 405 cm−1, attributed to PS43− and P2S74− units,37 and the precipitates were β-Li3PS4 or Li7P3S11, as described in the next section. These ion species can enhance the temperature by Joule heating under microwave irradiation, as suggested earlier. Furthermore, we examined the reactivity between Li2S and Lawesson's reagents, which have similar components to P2S4–anisole (Scheme 1) and are soluble in anisole. In a similar experiment heated at 300 °C, the only product was Li2S (Fig. S2†).
To investigate how microwave irradiation affects the products, the same synthesis was performed using a SiC ampule instead of a glass ampule. Because the SiC vial absorbs almost all the microwaves to generate Joule heat, the microwave cannot reach inside the sample. Thus, the reactions proceeded only by the thermal effect. Li2S and P2S5 prepared at a molar ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and anisole as a solvent were added to a 6 mL SiC vial, and microwave irradiation was performed at an output of 100 W for 4 min. No significant difference was observed in the Raman spectra of the products synthesized with glass and SiC (Fig. S3†). Given that the P–S unit was confirmed, as shown in Fig. 2a, it can be seen that Li2S and P2S5 reacted even when a SiC vial was used. Therefore, the reaction of Li2S and P2S5 would dominantly proceed by the thermal effect, and microwave irradiation was effective for quick heating.
25 and anisole as a solvent were added to a 6 mL SiC vial, and microwave irradiation was performed at an output of 100 W for 4 min. No significant difference was observed in the Raman spectra of the products synthesized with glass and SiC (Fig. S3†). Given that the P–S unit was confirmed, as shown in Fig. 2a, it can be seen that Li2S and P2S5 reacted even when a SiC vial was used. Therefore, the reaction of Li2S and P2S5 would dominantly proceed by the thermal effect, and microwave irradiation was effective for quick heating.
3.2 Synthesis of solid electrolytes: suspension and solution
Fig. 3a shows the XRD patterns of the precipitates obtained by microwave irradiation at different temperatures. The precipitates were collected by centrifugation; thus, no further heat treatment was performed after microwave irradiation. The XRD pattern of the sample heated at 100 °C exhibited peaks assigned to Li2S as well as additional unknown XRD peaks. The XRD pattern of the sample heated at 180 °C showed Li2S and β-Li3PS4 peaks. The temperature range between 200 and 300 °C showed β-Li3PS4 as the main phase, while Li7P3S11 was detected as a minor phase between 240 and 280 °C. The increase in the ratio of starting materials, Li2S and P2S5, to anisole heated at 220 °C increased the amount of Li7P3S11 and Li2S impurities (Fig. S4†). | ||
Fig. 3 (a) XRD patterns and (b) Raman spectra of the precipitate (Li2S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 75 P2S5 = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) after microwave irradiation. 25) after microwave irradiation. | ||
Fig. 3b shows the Raman spectra of the obtained precipitates after centrifugation, corresponding to the conditions shown in Fig. 3a. The Raman spectrum of the sample heated at 100 °C exhibited a Raman band centered at 393 cm−1. This band is not attributed to PS43− (420 cm−1) or P2S74− (405 cm−1) units.37 The Raman spectra of all samples heated above 100 °C exhibited a Raman band at approximately 420 cm−1, attributed to PS43− units, and an additional band at approximately 405 cm−1, attributed to P2S74− units in the heating temperature range of 240–280 °C.
Unknown peaks in the XRD pattern and the Raman shift to 393 cm−1 of the sample heated at 100 °C can be attributed to the complex of Li3PS4 and anisole. The formation of β-Li3PS4 in the samples heated at temperatures above 100 °C was confirmed by X-ray diffraction and Raman spectroscopy. The intensity of the Raman band attributed to P2S74− units increased in the heating temperature range of 240–280 °C, which explains the additional formation of Li7P3S11, as observed by X-ray diffraction.
The Li7P3S11 suspension was synthesized in the same way as the β-Li3PS4 suspension (Fig. 3) using a heating temperature of 260 °C and a stoichiometric molar ratio of Li2S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 70
P2S5 = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Fig. 4 shows the XRD pattern and Raman spectrum of the Li7P3S11 suspension. The XRD pattern (Fig. 4a) exhibited the formation of the Li7P3S11 crystal phase; only minor additional XRD peaks corresponding to Li2S were observed. The Raman spectrum (Fig. 4b) exhibited two main bands at 420 and 405 cm−1, assigned to PS43− and P2S74− units.
30. Fig. 4 shows the XRD pattern and Raman spectrum of the Li7P3S11 suspension. The XRD pattern (Fig. 4a) exhibited the formation of the Li7P3S11 crystal phase; only minor additional XRD peaks corresponding to Li2S were observed. The Raman spectrum (Fig. 4b) exhibited two main bands at 420 and 405 cm−1, assigned to PS43− and P2S74− units.
 | ||
Fig. 4 (a) XRD pattern and (b) Raman spectra of the solid electrolyte (Li2S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2S5 = 70 P2S5 = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). The suspension was heated at 260 °C by microwave irradiation. 30). The suspension was heated at 260 °C by microwave irradiation. | ||
Fig. 5 shows the XRD patterns of the solid electrolytes synthesized from anisole in the present study. After microwave irradiation, the solid electrolytes were dried under vacuum at 150 or 180 °C and subsequently heat-treated at a specific temperature, as described in Fig. 1. The synthesis conditions are summarized in Table S1.† β-Li3PS4 was synthesized from Li2S with and without ball milling treatment. While ball-milled Li2S produced single-phase β-Li3PS4, Li2S without ball milling treatment brought about β-Li3PS4 with Li2S impurity. Because ball milling treatment would decrease the particle size of Li2S, smaller particles with a larger surface area enhance the reaction between Li2S and P2S5 in anisole. Li7P3S11 was precipitated as a major phase with a smaller amount of Li2S. Li6PS5Cl was obtained as a single phase and sintering at 550 °C enhanced the crystallinity. Various sulfide solid electrolytes can be synthesized by a simple process using anisole for liquid-phase synthesis.
 | ||
| Fig. 5 XRD patterns of the solid electrolytes after heat treatment in Ar or a vacuum. BM: ball-milled Li2S. | ||
Fig. 6 shows the Nyquist plots and Table 1 shows the ionic conductivity at room temperature of the prepared solid electrolytes (β-Li3PS4, Li7P3S11, and Li6PS5Cl). The ionic conductivities at 180–300 °C were in the range of 0.05–0.13 mS cm−1. Even though further heat-treatment was necessary, the Li6PS5Cl pellet heated at 550 °C reached a higher conductivity of 2.1 mS cm−1.18 Furthermore, we proved the lithium-ion conducting nature of the synthesized Li7P3S11 electrolyte using the discharge–charge properties of the all-solid-state battery with the cathode composite composed of this synthesized Li7P3S11 (Fig. S5†).
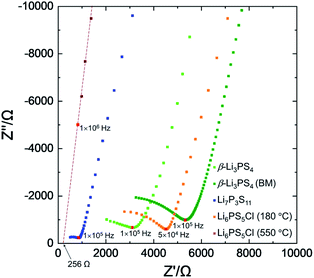 | ||
| Fig. 6 Room-temperature Nyquist plot of the prepared solid electrolyte after heat treatment in Ar or a vacuum. | ||
| Product | Microwave/°C | Heat treatment/°C | σ/mS cm−1 |
|---|---|---|---|
| β-Li3PS4 | 220 | 200 | 0.087 |
| β-Li3PS4 (BM) | 220 | 200 | 0.051 |
| Li7P3S11 | 260 | 300 | 0.13 |
| Li6PS5Cl | 220 | 180 | 0.070 |
| Li6PS5Cl | 220 | 550 | 2.1 |
Compared with those of previous reports,20,24,33 the conductivities shown in Table 1 tend to be slightly lower. The conductivity may be improved by completely removing any residual water, anisole, and hydrogen in the final product considering that Li6PS5Cl electrolyte heated at 550 °C showed one-order higher conductivity than others. A recent study suggests that residual Li2S which cannot be detected by XRD decreases the ionic conductivity of synthesized electrolytes.38 Thus, further improvement of the conductivity may be achieved by completing the synthesis reaction.
As P2S5 dissolves in anisole at high temperatures (Fig. S6†), it is considered that dissolved P2S5 is involved in the reaction. In contrast, β-Li3PS4 was not obtained from Li2S and Lawesson's reagent (which can be synthesized from P2S5 and anisole) instead of P2S5, indicating that the reaction intermediate should not be Lawesson's reagent. Because unreacted Li2S remained after the reaction to produce β-Li3PS4 or Li7P3S11 under microwave irradiation (Fig. 3 and 4), the reaction proceeded from the interface between the dissolved P2S5 species (but not Lawesson's reagent) and Li2S particles. This is supported by an enhanced reaction by using ball-milled Li2S (Fig. 5).
In the present study, we synthesized sulfide-based solid electrolytes by taking advantage of anisole with moderate nucleophilic aggression, having a donor number of 9. Because a reaction did not occur in toluene, having a donor number of 0, as a solvent,35 it is clear that the solvent is involved in the reaction of Li2S and P2S5. The synthesis reaction proceeds in ACN (donor number is 14) to form β-Li3PS4 at 200 °C, but further heat treatment above 220 °C resulted in the decomposition of β-Li3PS4 electrolytes (Fig. S7†). Therefore, anisole has the advantage of high-temperature and short-time synthesis, where the reaction kinetics increase without an unfavorable decomposition reaction of β-Li3PS4.
4. Conclusions
We succeeded in synthesizing sulfide solid electrolytes, Li3PS4, Li7P3S11, and Li6PS5Cl by rapid heating under microwave irradiation using anisole as a solvent. The advantage of this process is the direct precipitation of solid electrolytes in a solvent, in contrast to other techniques that require a post-heating process to decompose complexes composed of Li3PS4 and solvents under an inert atmosphere. Moderate reactivity in anisole allows the synthesis of solid electrolytes at 200–300 °C in a short time. This proposed approach can simplify and shorten the synthesis process, and is promising for producing the components of all-solid-state batteries. These features are advantageous not only for industrial scale-up but also for producing composite materials with electrode particles and binders. Research on the production of sheet-type batteries using this approach is currently ongoing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by KAKENHI Grant Number JP19H04682. This work was partially supported by the New Energy and Industrial Technology Development Organization (NEDO), Japan.References
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1–24 Search PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS.
- Y. Seino, T. Ota, K. Takada, A. Hayashi and M. Tatsumisago, Energy Environ. Sci., 2014, 7, 627–631 RSC.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- A. Sakuda, A. Hayashi and M. Tatsumisago, Sci. Rep., 2013, 3, 2–6 Search PubMed.
- F. Mizuno, A. Hayashi, K. Tadanaga and M. Tatsumisago, Solid State Ionics, 2006, 177, 2721–2725 CrossRef CAS.
- K. Ohara, A. Mitsui, M. Mori, Y. Onodera, S. Shiotani, Y. Koyama, Y. Orikasa, M. Murakami, K. Shimoda, K. Mori, T. Fukunaga, H. Arai, Y. Uchimoto and Z. Ogumi, Sci. Rep., 2016, 6, 1–9 CrossRef.
- A. Hayashi, K. Minami and M. Tatsumisago, J. Solid State Electrochem., 2010, 14, 1761–1767 CrossRef CAS.
- K. Homma, M. Yonemura, T. Kobayashi, M. Nagao, M. Hirayama and R. Kanno, Solid State Ionics, 2011, 182, 53–58 CrossRef CAS.
- N. H. H. Phuc, M. Totani, K. Morikawa, H. Muto and A. Matsuda, Solid State Ionics, 2016, 288, 240–243 CrossRef CAS.
- S. Boulineau, M. Courty, J. M. Tarascon and V. Viallet, Solid State Ionics, 2012, 221, 1–5 CrossRef CAS.
- H. J. Deiseroth, S. T. Kong, H. Eckert, J. Vannahme, C. Reiner, T. Zaiß and M. Schlosser, Angew. Chem., Int. Ed., 2008, 47, 755–758 CrossRef CAS.
- S. P. Culver, R. Koerver, W. G. Zeier and J. Janek, Adv. Energy Mater., 2019, 9, 1–14 Search PubMed.
- A. Sakuda, H. Kitaura, A. Hayashi, K. Tadanaga and M. Tatsumisago, Electrochem. Solid-State Lett., 2008, 11, 11–14 CrossRef.
- A. Miura, N. C. Rosero-Navarro, A. Sakuda, K. Tadanaga, N. H. H. Phuc, A. Matsuda, N. Machida, A. Hayashi and M. Tatsumisago, Nat. Rev. Chem., 2019, 3, 189–198 CrossRef CAS.
- M. Ghidiu, J. Ruhl, S. P. Culver and W. G. Zeier, J. Mater. Chem. A, 2019, 7, 17735–17753 RSC.
- Z. Liu, W. Fu, E. A. Payzant, X. Yu, Z. Wu, N. J. Dudney, J. Kiggans, K. Hong, A. J. Rondinone and C. Liang, J. Am. Chem. Soc., 2013, 135, 975–978 CrossRef CAS.
- N. H. H. Phuc, K. Morikawa, T. Mitsuhiro, H. Muto and A. Matsuda, Ionics, 2017, 23, 2061–2067 CrossRef.
- R. C. Xu, X. H. Xia, Z. J. Yao, X. L. Wang, C. D. Gu and J. P. Tu, Electrochim. Acta, 2016, 219, 235–240 CrossRef CAS.
- Y. Wang, D. Lu, M. Bowden, P. Z. El Khoury, K. S. Han, Z. D. Deng, J. Xiao, J. G. Zhang and J. Liu, Chem. Mater., 2018, 30, 990–997 CrossRef CAS.
- M. Calpa, N. C. Rosero-Navarro, A. Miura and K. Tadanaga, RSC Adv., 2017, 7, 46499–46504 RSC.
- K. Suto, P. Bonnick, E. Nagai, K. Niitani, T. S. Arthur and J. Muldoon, J. Mater. Chem. A, 2018, 6, 21261–21265 RSC.
- N. H. H. Phuc, K. Morikawa, M. Totani, H. Muto and A. Matsuda, Solid State Ionics, 2016, 285, 2–5 CrossRef CAS.
- H. Wang, Z. D. Hood, Y. Xia and C. Liang, J. Mater. Chem. A, 2016, 4, 8091–8096 RSC.
- E. Rangasamy, Z. Liu, M. Gobet, K. Pilar, G. Sahu, W. Zhou, H. Wu, S. Greenbaum and C. Liang, J. Am. Chem. Soc., 2015, 137, 1384–1387 CrossRef CAS.
- S. Chida, A. Miura, N. C. Rosero-Navarro, M. Higuchi, N. H. H. Phuc, H. Muto, A. Matsuda and K. Tadanaga, Ceram. Int., 2018, 44, 742–746 CrossRef CAS.
- M. Cuisinier, P. E. Cabelguen, B. D. Adams, A. Garsuch, M. Balasubramanian and L. F. Nazar, Energy Environ. Sci., 2014, 7, 2697–2705 RSC.
- N. C. Rosero-Navarro, A. Miura and K. Tadanaga, J. Power Sources, 2018, 396, 33–40 CrossRef CAS.
- H. D. Lim, X. Yue, X. Xing, V. Petrova, M. Gonzalez, H. Liu and P. Liu, J. Mater. Chem. A, 2018, 6, 7370–7374 RSC.
- L. Zhou, K. H. Park, X. Sun, F. Lalère, T. Adermann, P. Hartmann and L. F. Nazar, ACS Energy Lett., 2019, 4, 265–270 CrossRef CAS.
- I. Thomsen, K. Clausen, S. Scheibye and S.-O. Lawesson, Org. Synth., 1984, 62, 158 CrossRef CAS.
- M. Yamamoto, Y. Terauchi, A. Sakuda and M. Takahashi, Sci. Rep., 2018, 8, 2–11 CrossRef.
- Y. Marcus, Chem. Soc. Rev., 1993, 22, 409 RSC.
- F. Mizuno, A. Hayashi, K. Tadanaga and M. Tatsumisago, Adv. Mater., 2005, 17, 918–921 CrossRef CAS.
- K. Yamamoto, M. Takahashi, K. Ohara, N. H. H. Phuc, S. Yang, T. Watanabe, T. Uchiyama, A. Sakuda, A. Hayashi, M. Tatsumisago, H. Muto, A. Matsuda and Y. Uchimoto, ACS Omega, 2020, 5, 26287–26294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta08658d |
| This journal is © The Royal Society of Chemistry 2021 |