Strength of attraction: pyrene-based hole-transport materials with effective π–π stacking for dopant-free perovskite solar cells†
Marina
Tepliakova
 *a,
Igor K.
Yakushenko
b,
Elena I.
Romadina
*a,
Igor K.
Yakushenko
b,
Elena I.
Romadina
 ab,
Artyom V.
Novikov
ab,
Artyom V.
Novikov
 ab,
Petr M.
Kuznetsov
b,
Keith J.
Stevenson
ab,
Petr M.
Kuznetsov
b,
Keith J.
Stevenson
 a and
Pavel A.
Troshin
a and
Pavel A.
Troshin
 ab
ab
aSkolkovo Institute of Science and Technology, Nobel Street 3, Moscow, 143026, Russia. E-mail: marina.tepliakova@skoltech.ru
bIPCP RAS, Semenov Prospect 1, Chernogolovka, 142432, Moscow Region, Russia
First published on 18th November 2020
Abstract
Currently, organic small molecules represent a widely used class of hole-transport layer (HTL) materials in perovskite solar cells (PSCs). Commonly, these materials are doped to compensate for their low hole-mobility, which leads to an additional increase of the device cost along with the stability issues. An alternative approach is to use compounds containing large aromatic moieties, which can interact within the film at the intermolecular level, improving its conductivity. To demonstrate this strategy, we present four pyrene derivatives as HTL materials for perovskite solar cells. It is shown that devices fabricated using thermally evaporated films of pyrene derivatives deliver superior performance as compared to the cells with solution-processed HTLs. The power conversion efficiency of 17.9% is achieved for dopant-free perovskite solar cells using pyrene-based HTL with α-naphthylamine substituents.
Introduction
Perovskite solar cells (PSCs) represent the emerging photovoltaic technology demonstrating rapidly growing power conversion efficiencies (PCE), which are comparable now with crystalline silicon solar cells.1 Importantly, PSCs can be produced using solution-based high-throughput roll-to-roll coating and printing techniques enabling scalability of the technology and low-cost of the produced photovoltaic panels.2In a planar PSC configuration, the active layer is sandwiched between two charge transport interlayers, which selectively extract and transport holes and electrons to the respective electrodes. The main obstacle for the PSCs commercialization is their low operational stability, which can be mitigated by designing advanced charge-transport materials featuring excellent photochemical and thermal stability (in particular, high glass transition temperatures), good film formation properties coupled with low gas permeability.3,4 Additionally, the cost of these charge transport materials should be low enough for massive industrial applications.5 Currently, state-of-the-art hole-transport layer (HTL) materials are costly and fail to provide the desired stability to the PSCs.6
The most popular HTL materials are represented by low molecular weight organic compounds, such as well-known Spiro-OMeTAD. Generally, such materials have insufficient intrinsic charge carrier mobilities7,8 (e.g. <10−4 cm2 V−1 s−1) and hence additional doping is required to improve their conductivity and enable their efficient operation as HTLs in PSCs.9 However, doping makes the processing of these devices even more costly and deteriorates the device operational stability.10–12
In this regard, the compounds incorporating large planar aromatic fragments are of great interest since they can have high enough intrinsic charge carrier mobilities making them efficient HTLs without doping.13,14 Indeed, it is well known that planar aromatic molecules tend to undergo strong intermolecular interactions through π–π stacking leading to their self-assembling in the solid-state with the formation of ordered columnar or other types of structures.15–17 Therefore, thin films of such materials usually have good charge transport properties and operate well as HTLs without additional doping.18,19
In particular, some pyrene-based HTLs materials have been successfully applied in organic light-emitting diodes and perovskite solar cells due to their good charge transport properties and outstanding thermal stability with the glass transition points going beyond 300 °C in some cases.20–25 The first report on the application of pyrene-based HTLs in PSCs presented a comparative study of pyrene derivatives bearing one, three, and four bis(4-methoxyphenyl)amine substituents providing in devices the PCEs of 3.3%, 12.3%, and 12.4%, respectively.26 The lower efficiency of devices with the pyrene derivative with a single pendant amine substituent was attributed to inappropriate energy level alignment causing insufficient hole extraction.
The influence of the position and nature of the substituents in the pyrene-based HTL materials on their performance in PSCs was analyzed in the recent literature.27,28 For example, the dopant-free PSCs assembled using as HTL the pyrene derivative with two triarylamine-type substituents at positions 1 and 6 demonstrated high efficiencies (PCE = 17.0%) and open-circuit voltages (VOC = 1.13 V), whereas the devices using similar 2,7-disubstituted pyrene derivative showed inferior characteristics: PCE of 14.7% and VOC of 1.07 V.29 The observed differences between two isomeric pyrene derivatives were attributed to the better film formation properties, higher hole mobility, and optimal energy alignment provided by the 1,6-disubstituted compound.
The highest occupied molecular orbitals (HOMO) of pyrene derivatives with Ar2N substituents perfectly match the perovskite valence band thus ensuring efficient hole extraction.30 Furthermore, it was shown that pyrene derivatives bearing arylamine or thiophene pendant groups passivate efficiently defects in the perovskite absorber layer, e.g. at grain boundaries.31,32
Pyrene derivatives without bulky solubilizing groups often exhibit insufficient solubility in organic solvents preventing processing their high-quality films using e.g. spin coating or doctor blading techniques. Therefore, some alternative film deposition methods have to be applied with thermal evaporation in vacuum being the most straightforward approach. Interestingly, the impact of the HTL processing technique on the PSCs performance and stability remains virtually unexplored.
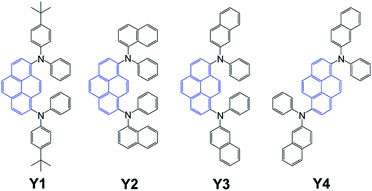
Herein, we report a systematic study of four pyrene derivatives Y1–Y4 bearing two diarylamine substituents (Fig. 1).23
Compound Y1 has diphenylamine pendant groups loaded with tert-butyl substituent ensuring decent solubility of the material in organic solvents. Compounds Y2–Y4 are isomeric species, which differ by the type of naphthyl groups at amine center (α-naphthyl for Y2 and β-naphthyl for Y3–Y4) and the arrangement of two diarylamine substituents in pyrene core: at positions 1 and 8 in case of Y2–Y3 or at 1 and 6 for Y4.23 The introduction of the bulky napththyl substituents is known to increase glass transition temperatures of arylamine-based HTL materials and improve intermolecular π–π stacking. Exploring a series of similar pyrene derivatives was aimed at revealing some important molecular structure–HTL performance relationships. Furthermore, we paid particular attention to studying the influence of the material deposition method on the characteristics of PSCs.
Results and discussion
The electrochemical properties of pyrene derivatives were investigated using cyclic voltammetry measurements performed for thin films following the previously reported procedure.33 The highest occupied molecular orbital energy levels (EHOMO) of compounds Y1–Y4 were estimated from the onset of the oxidation wave (Eonsetox) on the obtained cyclic voltammograms (Fig. S1†) and using −5.1 eV as the potential of the Fc+/Fc redox couple in the Fermi energy scale.34The optical bandgap (Eoptg) was estimated from the low-energy onset of the absorption band (Fig. S2†). The lowest unoccupied molecular orbital energy level (ELUMO) was calculated as Eoptg + EHOMO (Table 1).
| HTL | μ h, cm2 V−1 s−1 | E HOMO, eV | E LUMO, eV | E g, eV | T m, °C | T D, °C |
|---|---|---|---|---|---|---|
| Y1 | 3.2 × 10−4 | −5.5 | −2.8 | 2.6 | 310 | 455 |
| Y2 | 5.3 × 10−3 | −5.5 | −2.8 | 2.6 | 290 | 460 |
| Y3 | 3.7 × 10−3 | −5.5 | −2.7 | 2.7 | 270 | 450 |
| Y4 | 1.6 × 10−4 | −5.4 | −2.7 | 2.7 | 405 | 460 |
We investigated charge transport properties of Y1–Y4 using the space charge limited current (SCLC) method (Table 1).35 The measurements were performed for the hole-only devices with the ITO/PEDOT:PSS/Y1–Y4/VOx (25 nm)/Ag configuration. It was found that pyrene derivatives one-two orders of magnitude higher hole mobilities than the commonly used reference material spiro-OMeTAD in non-doped state. The highest mobility μh of 5 × 10−3 cm2 V−1 s−1 was obtained for Y2, which might be related to optimal molecular geometry and better supramolecular ordering in the films of this compound.
Furthermore, the surface properties of the pyrene derivatives were evaluated using contact angle measurements. The water contact angles on the thin films of Y1–Y4 were ranging from 74° to 101° thus featuring well-pronounced hydrophobicity of these compounds (Fig. S3†). Such moisture repellent properties of the studied pyrene derivatives are expected to provide good protection for the underlying photoactive perovskite layer during short exposure to ambient atmosphere (e.g. during technological roll-to-roll processing of PSCs).
It is well known that heat stress, which is unavoidable under realistic solar cell operational conditions, might induce degradation of the device stack. In particular, organic HTL materials can undergo heat-induced crystallization or even melting, resulting in the device failure.36 Therefore, it is crucial to enable good thermal stability of the used HTLs. In that context, pyrene derivatives Y1–Y4 seem to be highly promising since they showed impressively high melting points (Tm) and decomposition temperatures (TD) as revealed by thermal gravimetry analysis (TGA) coupled with differential scanning calorimetry (DSC) (Table 1, Fig. S4†).
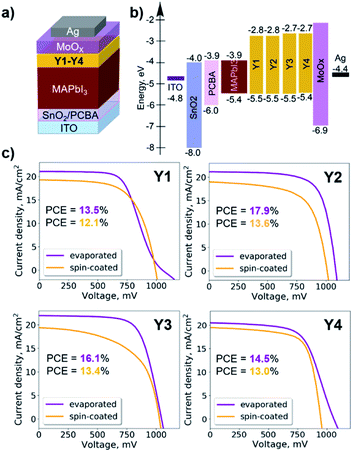
Pyrene derivatives Y1–Y4 were explored as HTL materials in PSCs with standard n–i–p architecture. The bottom indium tin oxide (ITO) electrode was covered with the electron-transport layer (ETL) of solution-processed SnO2 nanoparticles, which were additionally passivated from the surface using fullerene derivative PCBA.37,38 The standard methylammonium lead iodide perovskite absorber MAPbI3 was deposited by spin-coating from the solution of MAI and PbI2 precursors in dimethylformamide (DMF). The HTLs of pyrene derivatives Y1–Y4 were deposited in each case using two techniques: spin-coating and thermal evaporation. The schematic principles of two methods used for processing HTLs based pyrene derivatives is shown in Fig. 2a and b.
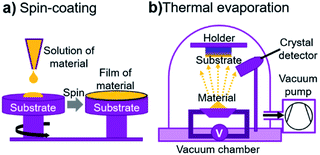 | ||
| Fig. 2 Principles of two HTL deposition methods: spin-coating (a) and thermal evaporation in vacuum using the quartz crystal detector calibrated by AFM (b). | ||
It is also known that the introduction of the metal oxide interlayer between the organic HTL and the electrode improves both efficiency and operational stability of perovskite solar cells.39,40 Therefore, we deposited a thin (10 nm) MoOx interlayer atop Y1–Y4 films. The ITO/SnO2:PCBA/MAPbI3/Y1–Y4/MoOx/Ag device architecture (Fig. 3a) was completed by the evaporation of silver electrodes in a high vacuum. The energy level diagram (Fig. 3b) shows that all pyrene derivatives Y1–Y4 have optimal HOMO energies matching the perovskite valence band, which should enable efficient hole extraction.
In the case of spin-coating, the thickness of the deposited films was controlled by the variation of the substrate rotation speed and material concentration in solution. In particular, the 4–10 mg mL−1 solutions of Y1–Y4 in chlorobenzene were spin-coated at the frequencies ranging from 1000 to 5000 rpm when optimizing the HTL film thickness (Fig. S1 and Table S1†).
The poly[bis(4-phenyl)(2,5,6-trimethylphenyl)amine] (PTAA) synthesized via Suzuki–Miyaura polycondensation reaction41 was used as a reference in all optimization experiments since it represents a well-established benchmark of HTL material for perovskite solar cells.
The J–V characteristics for the best devices assembled using solution-processed films of pyrene derivatives Y1–Y4 as HTLs are shown in Fig. 3c, whereas the device characteristics are listed in Table 2. Interestingly, all devices showed similar performances regardless of the used HTL material: the lowest efficiency of ∼12% (extracted from reverse J–V scan) was obtained in the case of using Y1, whereas the highest value of 13.6% was delivered by devices incorporating Y2.
| HTM | Depos. method | V OC, mV | J SC, mA cm−2 | FF, % | PCE, % |
|---|---|---|---|---|---|
| Y1 | Spin-coating | 994 (970 ± 20) | 20.2 (20.0 ± 1.0) | 65 (59 ± 2) | 12.1 (11.1 ± 0.6) |
| Thermal evap. | 1072 (1061 ± 7) | 22.2 (21.0 ± 1.0) | 61 (55 ± 4) | 14.2 (12.0 ± 1.0) | |
| Y2 | Spin-coating | 1020 (1000 ± 20) | 20.4 (20.2 ± 0.4) | 65 (63 ± 1) | 13.6 (12.5 ± 0.5) |
| Thermal evap. | 1103 (1090 ± 10) | 21.2 (21.1 ± 0.5) | 78 (72 ± 5) | 17.9 (16.0 ± 1.0) | |
| Y3 | Spin-coating | 1047 (990 ± 60) | 19.6 (19.3 ± 0.5) | 66 (56 ± 6) | 13.4 (10.3 ± 1.7) |
| Thermal evap. | 1062 (1050 ± 10) | 22.0 (21.0 ± 0.7) | 70 (66 ± 5) | 16.1 (14.0 ± 1.0) | |
| Y4 | Spin-coating | 959 (900 ± 200) | 20.6 (19.0 ± 0.9) | 70 (51 ± 8) | 13.7 (8.0 ± 3.0) |
| Thermal evap. | 1061 (1050 ± 10) | 21.5 (21.0 ± 0.3) | 64 (55 ± 7) | 14.5 (12.0 ± 2.0) |
Thermal evaporation under reduced pressure can be applied for processing thin films of volatile small molecules such as the pyrene derivatives Y1–Y4. While performing the device optimization, the thickness of HTL films was varied from 20 to 60 nm (Fig. S5 and Table S2†). The readings of the quartz crystal detector in the evaporation chamber were calibrated by profiling the evaporated films through scratches by atomic force microscopy.
It was demonstrated that an increase in the film thickness of pyrene derivatives Y1, Y3, and Y4 from 20 to 40 nm results in significant deterioration of the device performance along with the appearance of the s-shaped J–V curves suggesting inefficient extraction of charge carriers.42 This effect was the most severe for devices assembled using Y1 presumably due to the presence of bulky tert-butyl substituents in its structure, which negatively affect the intermolecular π–π stacking and suppress charge transport. Further increase of the HTL film thickness up to 50–60 nm resulted in a progressing s-shape effect in J–V curves of devices using Y3 or Y4.
Pyrene derivative Y2 demonstrated notably different behavior. First, an impressive power conversion efficiency of 17.9% was achieved in the case of using thin (20 nm) films of Y2. Furthermore, only a minor performance roll-off was observed with the HTL thickness increase. In particular, using 60 nm thick films of Y2 delivered still comparable efficiency of 15.4%. It is notable also that no s-shaped J–V curves were observed for devices incorporating Y2 as HTL material.
Thus, the pyrene derivative Y2 with α-naphthyl substituents appears to be the most promising HTL material. We believe that such a substitution pattern provides optimal molecular geometry leading to compact packing in the solid-state and improved charge transport characteristics.
It is also important to compare reproducibility of the characteristics of the solar cell with pyrene derivatives Y1–Y4 as HTL materials. The histograms showing the distribution of the PCE values (Fig. 4) unambiguously confirm that Y2 is indeed a highly promising material providing the most reproducible results for both types of devices with solution-processed and evaporated HTLs. On the contrary, the reproducibility of the devices assembled using Y4 was very poor, which can be explained by the extremely low solubility of this material in chlorobenzene and its tendency to crystallize and form inhomogeneous and rough films with incomplete perovskite coverage.
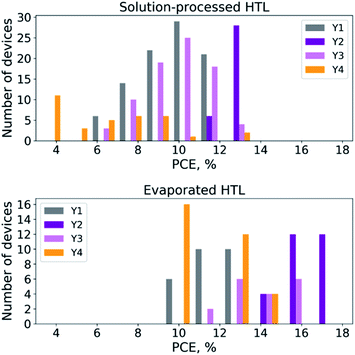 | ||
| Fig. 4 Histograms showing the power conversion efficiency (PCE) distribution for PSCs using pyrene derivatives Y1–Y4 as HTL materials. | ||
A comparison of the histograms shown in Fig. 4 leads to another important conclusion: all studied HTL materials enabled better device performance when they were processed by thermal evaporation and not by spin-coating. This result is most likely related to better uniformity and reduced density of defects in the evaporated films.
The morphology of the evaporated and spin-coated films of pyrene derivatives was studied using atomic force microscopy (Fig. S7†). It can be noticed that all evaporated films have more uniform structure and demonstrate much fewer defects as compared to the spin-coated films. The most spectacular difference is observed for compound Y4, which forms big aggregates (>1 μm) when processed from solution presumably due to the solvent-induced crystallization. On the contrary, the evaporated films of Y4 show compact structure with the small (<100 nm) grains. Another example is provided by Y3, which demonstrates multiple craters in the films processed from solution, which are missing in the evaporated films.
Indeed, some solvent molecules might be trapped in the solution-processed films and their subsequent evaporation leaves voids, which impede transport of charge carriers and might lead to incomplete coverage of perovskite absorber by organic HTL. Therefore, thermal evaporation should be considered as a more reliable technique for screening the performance of volatile organic HTL materials.
Conclusion
To summarize, we performed a systematic comparative study of four structurally similar pyrene derivatives with diarylamine substituents as hole-transport materials in n–i–p perovskite solar cells. It has been shown that the type of substituents strongly affects the device performance of the studied HTL materials. In particular, compounds with tert-butylphenyl or β-naphthyl substituents (Y1 and Y3–Y4) enable decent HTL performance only in thin films (20 nm), whereas using thicker films leads to the appearance of s-shaped J–V curves suggesting inefficient extraction of charge carriers. On the contrary, pyrene derivative Y2 bearing N-phenyl-α-naphthylamine pendant groups provided high power conversion efficiency of 17.9% and showed just a minor efficiency roll-off with the increase in the HTL film thickness. The achieved device efficiency matches current state-of-the-art in the development of dopant-free HTL materials for n–i–p perovskite solar cells.43,44Another important result was the demonstration of the superior performance of PSCs assembled using evaporated films of pyrene-based HTLs in comparison with the solution-processed ones. Thus, this study revealed that the hole-transport layer deposition technique can strongly affect the perovskite solar cell performance and this aspect was largely overlooked so far in the literature.
Finally, we believe that pyrene derivatives hold a great promise for the development of inexpensive and highly efficient dopant-free HTL materials for perovskite solar cells.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported at Skoltech by the Russian Foundation for Basic Research, project number 19-33-90294. General support was also provided by the Russian Ministry of Science and Education (project 0089-2019-0010/AAAA-A19-119071190044-3) at IPCP RAS.Notes and references
- NREL Best Research-Cell Photovoltaic Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html, accessed august, 2020 Search PubMed.
- M. Saliba, J.-P. Correa-Baena, M. Grätzel, A. Hagfeldt and A. Abate, Angew. Chem., Int. Ed., 2018, 57, 2554–2569 CrossRef CAS.
- L. Gao, T. H. Schloemer, F. Zhang, X. Chen, C. Xiao, K. Zhu and A. Sellinger, ACS Appl. Energy Mater., 2020, 3, 4492–4498 CrossRef CAS.
- L. Wang, H. Zhou, N. Li, Y. Zhang, L. Chen, X. Ke, Z. Chen, Z. Wang, M. Sui, Y. Chen, Y. Huang, L. Li, Z. Xu, Q. Chen, L.-D. Sun and C.-H. Yan, J. Mater. Chem. A, 2020, 8, 14106–14113 RSC.
- P.-H. Lin, K.-M. Lee, C.-C. Ting and C.-Y. Liu, J. Mater. Chem. A, 2019, 7, 5934–5937 RSC.
- L. Qiu, S. He, L. K. Ono, S. Liu and Y. Qi, ACS Energy Lett., 2019, 4, 2147–2167 CrossRef CAS.
- D. P. Tabor, V. A. Chiykowski, P. Friederich, Y. Cao, D. J. Dvorak, C. P. Berlinguette and A. Aspuru-Guzik, Chem. Sci., 2019, 10, 8360–8366 RSC.
- K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2015, 137, 16172–16178 CrossRef CAS.
- H. D. Pham, L. Xianqiang, W. Li, S. Manzhos, A. K. K. Kyaw and P. Sonar, Energy Environ. Sci., 2019, 12, 1177–1209 RSC.
- A. K. Jena, M. Ikegami and T. Miyasaka, ACS Energy Lett., 2017, 2, 1760–1761 CrossRef CAS.
- Q. Wang, Phys. Chem. Chem. Phys., 2018, 20, 10114–10120 RSC.
- X. Wang, J. Wu, Y. Yang, X. Liu, Q. Guo, Z. Song, G. Li, Z. Lan and M. Huang, J. Mater. Chem. A, 2019, 7, 13256–13264 RSC.
- X. Sun, F. Wu, C. Zhong, L. Zhu and Z. a. Li, Chem. Sci., 2019, 10, 6899–6907 RSC.
- X. Sun, Q. Xue, Z. Zhu, Q. Xiao, K. Jiang, H.-L. Yip, H. Yan and Z. a. Li, Chem. Sci., 2018, 9, 2698–2704 RSC.
- T. V. Dubinina, E. O. Moiseeva, D. A. Astvatsaturov, N. E. Borisova, P. A. Tarakanov, S. A. Trashin, K. De Wael and L. G. Tomilova, New J. Chem., 2020, 44, 7849–7857 RSC.
- M. M. Islam, Z. Hu, Q. Wang, C. Redshaw and X. Feng, Mater. Chem. Front., 2019, 3, 762–781 RSC.
- Y. Li, C. Zhang, P. Gu, Z. Wang, Z. Li, H. Li, J. Lu and Q. Zhang, Chem. - Eur. J., 2018, 24, 7845–7851 CrossRef CAS.
- H. D. Pham, T. T. Do, J. Kim, C. Charbonneau, S. Manzhos, K. Feron, W. C. Tsoi, J. R. Durrant, S. M. Jain and P. Sonar, Adv. Energy Mater., 2018, 8, 1703007 CrossRef.
- H. D. Pham, K. Hayasake, J. Kim, T. T. Do, H. Matsui, S. Manzhos, K. Feron, S. Tokito, T. Watson, W. C. Tsoi, N. Motta, J. R. Durrant, S. M. Jain and P. Sonar, J. Mater. Chem. C, 2018, 6, 3699–3708 RSC.
- K.-R. Wee, H.-C. Ahn, H.-J. Son, W.-S. Han, J.-E. Kim, D. W. Cho and S. O. Kang, J. Org. Chem., 2009, 74, 8472–8475 CrossRef CAS.
- F. Yang, P. Zhang, M. A. Kamarudin, G. Kapil, T. Ma and S. Hayase, Adv. Funct. Mater., 2018, 28, 1804856 CrossRef.
- J.-Y. Shao, B. Yu, Y.-D. Wang, Z.-R. Lan, D. Li, Q. Meng and Y.-W. Zhong, ACS Appl. Energy Mater., 2020, 3, 5058–5066 CrossRef CAS.
- M. G. Kaplunov, I. K. Yakushchenko, S. S. Krasnikova and S. B. Echmaev, Mendeleev Commun., 2016, 26, 437–439 CrossRef CAS.
- J. P. Mora-Fuentes, D. Cortizo-Lacalle, S. Collavini, K. Strutynski, W. R. Tress, M. Saliba, S. M. Zakeeruddin, I. Kosta, M. Melle-Franco, M. Gratzel, J. L. Delgado and A. Mateo-Alonso, J. Mater. Chem. C, 2019, 7, 4332–4335 RSC.
- J. Salunke, A. Singh, D. He, H. D. Pham, Y. Bai, L. Wang, S. Dahlström, M. Nyman, S. Manzhos, K. Feron, R. Österbacka, A. Priimagi, P. Vivo and P. Sonar, Org. Electron., 2020, 77, 105524 CrossRef CAS.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087–19090 CrossRef CAS.
- B. B. Cui, C. Zhu, S. S. Yang, Y. Han, N. Yang, L. Z. Zhang, Y. Wang, Y. F. Jia, L. Zhao and Q. Chen, ACS Omega, 2018, 3, 10791–10797 CrossRef CAS.
- W. Chen, A. A. Said, Z. Wang, Y. Zhou, W. Liu, W.-B. Gao, M. Liu and Q. Zhang, ACS Appl. Energy Mater., 2019, 2, 5716–5723 CrossRef CAS.
- J. Qiu, H. Liu, X. Li and S. Wang, Chem. Eng., 2020, 387, 123965 CrossRef CAS.
- N. Andijani, O. Al-Qurashi, N. Wazzan and A. Irfan, Sol. Energy, 2019, 188, 898–912 CrossRef CAS.
- L. Zhang, C. Liu, J. Zhang, X. Li, C. Cheng, Y. Tian, A. K.-Y. Jen and B. Xu, Adv. Mater., 2018, 30, 1804028 CrossRef.
- C. Sun, Z. Wu, H.-L. Yip, H. Zhang, X.-F. Jiang, Q. Xue, Z. Hu, Z. Hu, Y. Shen, M. Wang, F. Huang and Y. Cao, Adv. Energy Mater., 2016, 6, 1501534 CrossRef.
- A. V. Akkuratov, F. A. Prudnov, L. N. Inasaridze and P. A. Troshin, Tetrahedron Lett., 2017, 58, 97–100 CrossRef CAS.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367–2371 CrossRef CAS.
- V. Podzorov, S. E. Sysoev, E. Loginova, V. M. Pudalov and M. E. Gershenson, Appl. Phys. Lett., 2003, 83, 3504–3506 CrossRef CAS.
- T. Malinauskas, D. Tomkute-Luksiene, R. Sens, M. Daskeviciene, R. Send, H. Wonneberger, V. Jankauskas, I. Bruder and V. Getautis, ACS Appl. Mater. Interfaces, 2015, 7, 11107–11116 CrossRef CAS.
- J. C. Hummelen, B. W. Knight, F. LePeq, F. Wudl, J. Yao and C. L. Wilkins, J. Org. Chem., 1995, 60, 532–538 CrossRef CAS.
- S. Tsarev, T. S. Dubinina, S. Y. Luchkin, I. S. Zhidkov, E. Z. Kurmaev, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. C, 2020, 124, 1872–1877 CrossRef CAS.
- T. H. Schloemer, T. S. Gehan, J. A. Christians, D. G. Mitchell, A. Dixon, Z. Li, K. Zhu, J. J. Berry, J. M. Luther and A. Sellinger, ACS Energy Lett., 2019, 4, 473–482 CrossRef CAS.
- M. M. Tepliakova, A. N. Mikheeva, L. A. Frolova, A. G. Boldyreva, A. Elakshar, A. V. Novikov, S. A. Tsarev, M. I. Ustinova, O. R. Yamilova, A. G. Nasibulin, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. Lett., 2020, 11, 5563–5568 CrossRef CAS.
- M. M. Tepliakova, A. V. Akkuratov, S. A. Tsarev and P. A. Troshin, Tetrahedron Lett., 2020, 152317, DOI:10.1016/j.tetlet.2020.152317.
- R. Saive, IEEE J. Photovolt., 2019, 9, 1477–1484 Search PubMed.
- B. Cai, X. Yang, X. Jiang, Z. Yu, A. Hagfeldt and L. Sun, J. Mater. Chem. A, 2019, 7, 14835–14841 RSC.
- S. Tsarev, I. K. Yakushchenko, S. Y. Luchkin, P. M. Kuznetsov, R. S. Timerbulatov, N. N. Dremova, L. A. Frolova, K. J. Stevenson and P. A. Troshin, Sustainable Energy Fuels, 2019, 3, 2627–2632 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0se01300e |
| This journal is © The Royal Society of Chemistry 2021 |