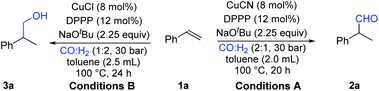Open Access Article
Open Access ArticleCopper-catalyzed hydroformylation and hydroxymethylation of styrenes†‡
Hui-Qing
Geng§
a,
Tim
Meyer§
a,
Robert
Franke
c and
Xiao-Feng
Wu
 *ab
*ab
aLeibniz-Institut für Katalyse e. V., Albert-Einstein-Straße 29a, 18059, Rostock, Germany. E-mail: Xiao-Feng.Wu@catalysis.de
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, Liaoning, China. E-mail: xwu2020@dicp.ac.cn
cEvonik Operations GmbH, Germany and Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, 44780, Bochum, Germany
First published on 29th October 2021
Abstract
Hydroformylation catalyzed by transition metals is one of the most important homogeneously catalyzed reactions in industrial organic chemistry. Millions of tons of aldehydes and related chemicals are produced by this transformation annually. However, most of the applied procedures use rhodium catalysts. In the procedure described here, a copper-catalyzed hydroformylation of alkenes has been realized. Remarkably, by using a different copper precursor, the aldehydes obtained can be further hydrogenated to give the corresponding alcohols under the same conditions, formally named as hydroxymethylation of alkenes. Under pressure of syngas, various aldehydes and alcohols can be produced from alkenes with copper as the only catalyst, in excellent regioselectivity. Additionally, an all-carbon quaternary center containing ethers and formates can be synthesized as well with the addition of unactivated alkyl halides. A possible reaction pathway is proposed based on our results.
Introduction
Hydroformylation was discovered by Otto Roelen in 1938 for the synthesis of aldehydes by the addition of syngas (a mixture of CO and H2) to olefins (Scheme 1, eqn (a)).1,2 As one of the most important homogeneously catalyzed industrial processes, millions of tons of aldehydes and related chemicals including bulky compounds and fine chemicals are produced annually by transition metal-catalyzed hydroformylation of alkenes.3,4 Although several transition metal catalysts have been explored in this transformation,5 most of the applied procedures use rhodium or cobalt catalysts.6 Rhodium catalysts are applied due to their exceptionally high catalytic reactivity, but the high cost of rhodium is considered a significant disadvantage. Hydroformylation was originally discovered by the use of a non-expensive cobalt catalyst;7 however, the connected regioselectivity issues of cobalt, which give a mixture of linear and branched products, and its inert state with ligand tuning diminished its applicability and diversity.8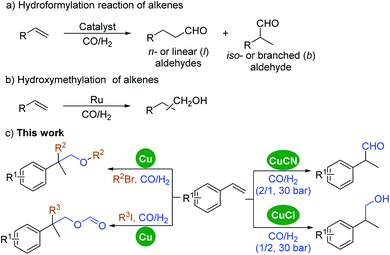 | ||
| Scheme 1 (a) Hydroformylation reaction of alkenes. (b) Hydroxymethylation of alkenes. (c) This work. | ||
On the other hand, copper is known as an environmentally benign metal catalyst that is non-expensive and abundant and has been extensively explored and applied in various organic transformations.9 Its outstanding catalytic activity in cross-coupling chemistry (with bond formation including C–N, C–O, etc.) is even better than that of noble metal catalysts such as palladium. Its powerful catalytic ability can also be proven by the known named reactions based on copper catalysts, for example the Chan–Lam reaction, Ullmann coupling, Glaser reaction, and so on.10–13 In particular, copper(I) hydride reagents have played a great role in organic synthesis as a long-established complex, especially in reduction reactions.14,15 In recent years, copper(I) hydride complex catalyzed carbonylative coupling reactions have been explored by Mankad's group,16–20 our group,21,22 and others.23–25 Notably, the groups of Yu24a and Tsuji24b have explored copper catalyzed hydroxymethylation of the C–C double bond with CO2 for the synthesis of alcohols by using silanes as the reducing agent and hydride source. Wang and co-workers developed a Ni/Cu-catalyzed carbonylation of acetylene to acrylic acid in 2009.25 Under their best reaction conditions, the selectivity and conversion can reach up to 90%. However, a copper catalyst has not yet been reported in hydroformylation reactions of alkenes with syngas.
Herein, a copper-catalyzed hydroformylation has been realized (Scheme 1, eqn (c)). Remarkably, by using a different copper precursor, the aldehydes obtained can be further hydrogenated to give the corresponding alcohols under the same conditions, formally named as hydroxymethylation of alkenes, which was usually catalyzed by ruthenium (Scheme 1, eqn (b)).26–29 Under pressure of syngas, various aldehydes and alcohols can be produced effectively from alkenes with copper as the only catalyst in excellent regioselectivity. Additionally, an all-carbon quaternary center containing ethers and formates can also be formed in a one-step manner by adding alkyl halides as the fourth reaction partner.
In order to establish this new catalyst system, styrene was chosen as the model substrate and systematic optimization studies were performed (for optimization details, see the ESI‡). Under our optimized reaction conditions, by using DPPP as the ligand and NaOtBu as the base in toluene at 100 °C, 2-phenylpropan-1-ol and 2-phenylpropanal can be produced selectively under pressure of syngas with different copper precursors (CuCl produces 3a and CuCN produces 2a). In the catalytic system with CuCN as the catalyst for 2-phenylpropanal production, the selectivity between 2a and 3a decreased at lower temperature while reaction efficiency dropped at higher temperature (Table 1, entries 2–3). Different phosphine ligands were also used; however, their use decreased both reaction efficiency and selectivity (Table 1, entries 4–6). In the testing of solvents, toluene proved to be the best reaction medium for this transformation (Table 1, entries 7–11). Interestingly, by using CuCl as the catalyst, 2-phenylpropan-1-ol 3a can be produced as the main product, which is obviously formed by the hydrogenation of the hydroformylation product. In order to facilitate the hydrogenation step, the ratio of CO and H2 was modified and 68% yield of 3a was finally achieved. Copper salts, organic solvents and phosphine ligands were screened; without exception no better results can be obtained concerning reaction selectivity and efficiency (Table 1, entries 13–19). It is important to mention that ethylbenzene can be detected as the main by-product during the optimization process. By the addition of a catalytic amount of KCN or NaCN into the CuCl catalytic system, the aldehyde can immediately become the main product. Hence, the coordinating behavior of –CN plays an important role in the reaction selectivity. The possible effects of rhodium and cobalt impurities have been considered as well, and control experiments and ICP measurements were carried out to exclude their presence and ensure that they don't act as the real catalysts here (for details, see the ESI‡).
| Entry | Variation from the standard conditions for 2a | Conv of 1a | 2a:3a | Yield |
|---|---|---|---|---|
| a Conditions A: CuCN (3.1 mg, 8 mol%), DPPP (21.3 mg, 12 mol%), NaOtBu (93 mg, 2.25 equiv), toluene (2.0 mL), styrene 1a (50 μL, 0.43 mmoL), CO (20 bar), H2 (10 bar), 100 °C, 20 h; conditions B: CuCl (3.4 mg, 8 mol%), DPPP (21.3 mg, 12 mol%), NaOtBu (93 mg, 2.25 equiv), toluene (2.5 mL), styrene 1a (50 μL, 0.43 mmoL), CO (10 bar), H2 (20 bar), 100 °C, 24 h. Conversion of styrene, the ratio of 2a and 3a, and the yields were measured by GC using hexadecane as the internal standard. b Cyclohexane (1.8 mL), toluene (0.2 mL) as solvent, 20 h. DPPE: 1,2-bis(diphenylphosphino)ethane. DPPP: 1,3-bis(diphenylphosphino)propane. DPPB: 1,4-bis(diphenylphosphino)butane. DPPF: 1,1′-bis(diphenylphosphino)ferrocene. CuBr·DMS: copper(I) bromide dimethyl sulfide complex. Xantphos: 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene. | ||||
| 1 | — | 91% | 92/8 | 68% |
| 2 | 90 °C instead of 100 °C | 92% | 73/27 | 66% |
| 3 | 110 °C instead of 100 °C | 88% | 94/6 | 57% |
| 4 | DPPE instead of DPPP | 59% | 88/12 | 33% |
| 5 | DPPB instead of DPPP | 60% | 87/17 | 32% |
| 6 | DPPF instead of DPPP | 73% | 86/14 | 57% |
| 7 | o-Xylene instead of toluene | 88% | 76/24 | 73% |
| 8 | Cyclohexane instead of toluene | 87% | 76/24 | 42% |
| 9 | Benzene instead of toluene | 84% | 74/26 | 68% |
| 10 | Ethylbenzene instead of toluene | 69% | 74/26 | 65% |
| 11 | Methoxybenzene instead of toluene | 75% | 80/20 | 69% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Variation from the standard conditions for 3a | ||||
| 12 | — | 86% | <1/99 | 68% |
| 13 | CuBr instead of CuClb | 95% | <1/99 | 54% |
| 14 | CuBr·DMS instead of CuClb | 91% | <1/99 | 48% |
| 15 | Cyclohexane (1.8 mL), toluene (0.2 mL) as solvent | 92% | <1/99 | 58% |
| 16 | Xantphos instead of DPPPb | 100% | 25/75 | 16% |
| 17 | DPPE instead of DPPPb | 61% | 75/25 | 28% |
| 18 | DPPB instead of DPPPb | 85% | 58/42 | 51% |
| 19 | DPPF instead of DPPPb | 100% | 80/20 | 51% |
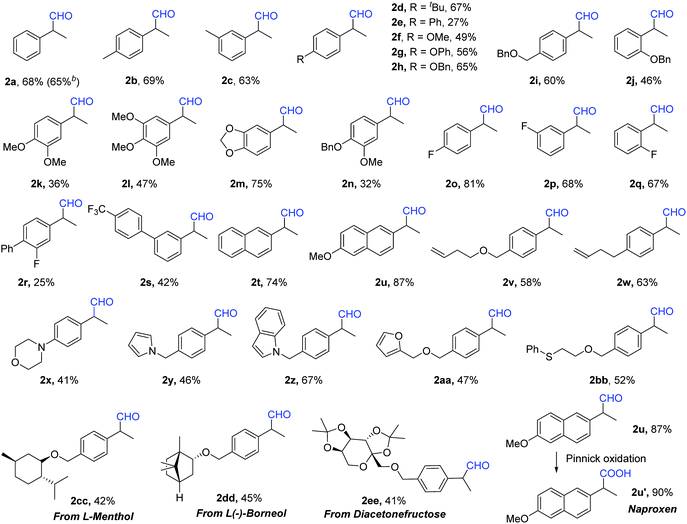
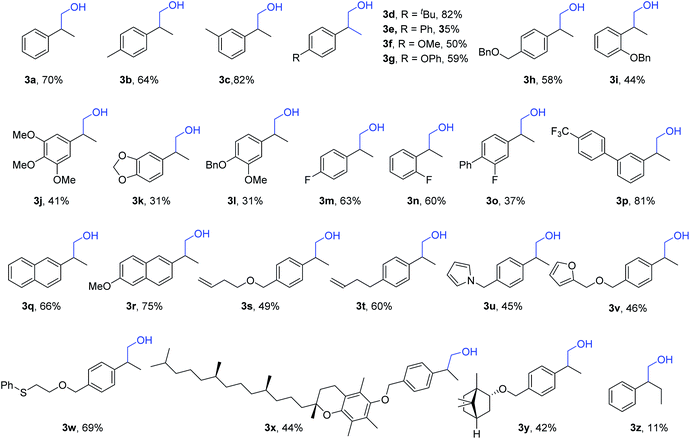
Under our best reaction conditions, the testing of different substrates was subsequently carried out. Under the reaction conditions for aldehydes production, various styrenes were tested (Table 2). Substrates with various substituents were tested and converted into the desired aldehydes with moderate to good yields. Notably, substrates containing a terminal aliphatic alkene group can also be well tolerated (2v and 2w). This reaction can be easily enlarged to the 5 mmol scale without losing reaction efficiency and selectivity. Complexed alkenes can be applied under our standard conditions without any problem. A one-pot two-step synthesis of Naproxen (2u′) via Pinnick oxidation was proven to be possible as well.
With the use of CuCl as the catalyst, the synthesis of alcohols was subsequently performed (Table 3). Moderate to good yields of the desired alcohols can be obtained successfully without further optimization. Functional groups including methoxy, phenoxy, fluoro, trifluoromethyl, pyrrole, thioether, etc. can be well tolerated. Besides the naphthyl group, even a terminal alkene group can survive under our standard conditions (3s and 3t). More complexed styrenes were also tested, and it was found that the targeted products can be isolated without any problem (3x and 3y). Without exception, the internal alkene was checked; however, only an 11% yield of the target product could be detected (3z). Additionally, several chiral phosphine ligands were checked with the target to obtain a chiral alcohol product. However, low or no enantioselectivity was obtained (For details see the ESI‡).
| a Standard conditions: 1 (0.43 mmol, 1 equiv), CuCl (8 mol%), DPPP (12 mol%), NaOtBu (2.25 equiv), toluene (2.5 mL), CO (10 bar), H2 (20 bar), 100 °C, 24 h, isolated yield. Yield of 3z was determined by NMR. |
|---|
|
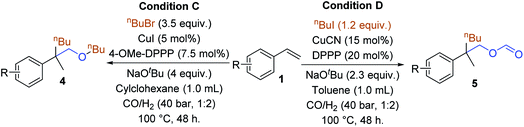
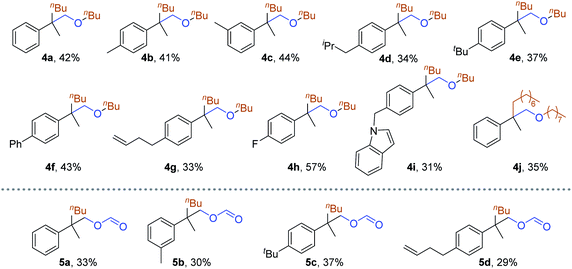
By adding unactivated halides as the fourth reaction partner together with styrene under a CO and H2 atmosphere, we can obtain different types of product depending on whether alkyl iodides or alkyl bromides was added. After optimizing the reaction conditions (for details see the ESI‡), an all-carbon quaternary center containing ethers can be synthesized by Cu-catalyzed reaction of olefins with alkyl bromides under a syngas atmosphere. We evaluated the reactivity of various alkenes (Table 4). Functionalized aryl alkenes with alkyl, phenyl, alkyl olefine, fluoro, and indole groups can be tolerated and gave the corresponding products in moderate yields (4a–4i). The use of alkyl bromide with a longer chain length has less effect on the reaction result (4j). An all-carbon quaternary center containing formates can be obtained by using alkyl iodides as the reaction partner and all the corresponding products were obtained in moderate yields (5a–5d). Several other alkyl iodides were tested as well; however, besides the low yields (15–26%), it was also difficult to obtain the products.
a Conditions C: 1 (0.2 mmol, 1 equiv), nBuBr (3.5 equiv), CuI (5 mol%), 4-OMe-DPPP (7.5 mol%), NaOtBu (4 equiv), cyclohexane (1.0 mL), CO/H2 (40 bar, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 100 °C, 48 h, isolated yield. Conditions D: 1 (0.2 mmol, 1 equiv), nBuI (1.2 equiv), CuCN (15 mol%), DPPP (20 mol%), NaOtBu (2.3 equiv), toluene (1.0 mL), CO/H2 (40 bar, 1 2), 100 °C, 48 h, isolated yield. Conditions D: 1 (0.2 mmol, 1 equiv), nBuI (1.2 equiv), CuCN (15 mol%), DPPP (20 mol%), NaOtBu (2.3 equiv), toluene (1.0 mL), CO/H2 (40 bar, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 100 °C, 48 h, isolated yield. 2), 100 °C, 48 h, isolated yield.
|
|---|
|
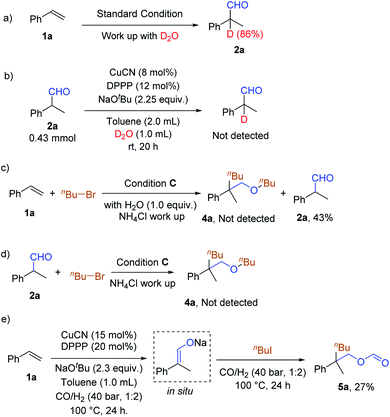
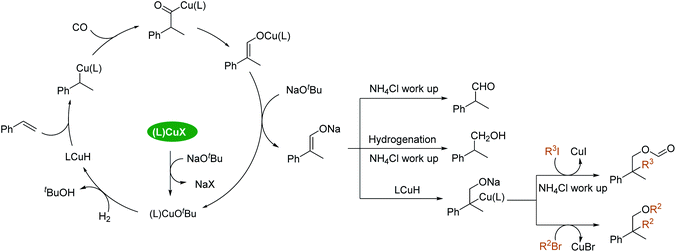
In order to understand the reaction pathway, control experiments were performed (Scheme 2). In our standard procedure, the addition of water was involved in the work-up part. Instead of water, heavy water (D2O, 1 mL) was used for the work-up and 86% of benzylic deuterated aldehyde was obtained (Scheme 2, eqn (a)). However, no deuterated aldehyde could be detected under the same conditions starting from an aldehyde (Scheme 2, eqn (b)). These results imply that a vinyloxyl intermediate was most likely involved and that it had been hydrolyzed by water during the work-up process to give the final products. Under the standard conditions of the Cu-catalyzed reaction of styrene with alkyl bromide, the addition of 1 equivalent of H2O will inhibit the reaction (Scheme 2, eqn (c)). No ether could be detected, while the aldehyde can be obtained in 43% yield. Using aldehyde 2a as the starting material under conditions C, the corresponding ester 4a cannot be detected (Scheme 2, eqn (d)). These results suggest that aldehydes are not the intermediates for producing ethers. The reaction using styrene as the starting material proceeded for 24 h under conditions D without n-butyl iodide. Then n-butyl iodide was added, and the reaction was continued for another 24 h under a CO and H2 atmosphere (Scheme 2, eqn (e)). 5a was obtained in 27% yield, showing that the sodium vinyloxyl may be the key intermediate of these transformations.
Based on our results, a possible reaction pathway is proposed (Scheme 3). The reaction starts with the formation of a LCuOtBu complex, which will produce LCuH after reaction with H2. Subsequently, the LCuH inserts into the double bond of the alkene to give an alkylcopper intermediate which will react with CO to produce an acylcopper complex. A vinyloxyl copper complex will be formed after rearrangement of the acylcopper intermediate. Finally, sodium vinyloxyl will be eliminated which will give the final product after the hydrolysis work-up. This step is different from the traditional hydroformylation pathway (via δ bond metathesis or oxidative addition between acylmetal and H2) and explains the excess amount of base needed. The key intermediate of these Cu-catalyzed tandem reactions is the sodium vinyloxyl species. After the (L)CuH inserts into the double bond of sodium vinyloxyl, alkyl halides react with the alkyl-copper complex to produce the final products.30
Conclusions
In summary, a copper-catalyzed hydroformylation of alkenes has been developed. With CuCN as the catalyst under pressure of syngas, various aldehydes can be produced with good functional group tolerance. Notably, by using CuCl as the catalyst precursor, alcohols can be formed, named as hydroxymethylation of alkenes. Derivatization reactions have also been developed; when unactivated halides were applied, an all-carbon quaternary center containing ethers and formates can be formed in a one-pot manner. A possible reaction pathway is proposed based on our results which is fundamentally different from known mechanisms.Author contributions
XFW directed this project. HQG and TM performed all the experiments. HQG, RF and XFW prepared the draft and XFW revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Chinese Scholarship Council (CSC) and LIKAT for financial support. We also thank the analytical team of LIKAT for their very kind support.Notes and references
-
O. Roelen, to Chemische Verwertungsgesellschaft Oberhausen m.b.H., German Patent DE, 849548, 1938/1952, US Pat., 2327066, 1943
.
- H. Adkins and G. Krsek, J. Am. Chem. Soc., 1949, 71, 3051–3055 CrossRef CAS
.
-
A. Börner and R. Franke, Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2016 Search PubMed
.
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675–5732 CrossRef CAS PubMed
.
-
(a)
K.-D. Wiese and D. Obst in Catalytic Carbonylation Reactions, ed. M. Beller, Springer, Berlin, 2010, pp. 1−33 Search PubMed
; (b) C. H. Tien, A. Trofimova, A. Holownia, B. S. Kwak, R. T. Larson and A. K. Yudin, Angew. Chem., Int. Ed., 2021, 60, 4342–4349 CrossRef CAS PubMed
.
-
P. W. N. M. van Leeuwen and C. Claver, Rhodium Catalyzed Hydroformylation, Kluwer Academic Publishers, Netherlands, 2000 Search PubMed
.
- M. Orchin, Acc. Chem. Res., 1981, 14, 259–266 CrossRef CAS
.
- D. M. Hood, R. A. Johnson, A. E. Carpenter, J. M. Younker, D. J. Vinyard and G. G. Stanley, Science, 2020, 367, 542–548 CrossRef CAS PubMed
.
-
G. Anilkumar and S. Saranya, Copper Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2020 Search PubMed
.
- A. Casitas and X. Ribas, Chem. Sci., 2013, 4, 2301–2318 RSC
.
- P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632–2657 CrossRef CAS PubMed
.
- R. Y. Liu and S. L. Buchwald, Acc. Chem. Res., 2020, 53, 1229–1243 CrossRef CAS PubMed
.
- C. Deutsch, N. Krause and B. H. Lipshutz, Chem. Rev., 2008, 108, 2916–2927 CrossRef CAS PubMed
.
- A. J. Jordan, G. Lalic and J. P. Sadighi, Chem. Rev., 2016, 116, 8318–8372 CrossRef CAS PubMed
.
- C. Deutsch, N. Krause and B. H. Lipshutz, Chem. Rev., 2008, 108, 2916–2927 CrossRef CAS PubMed
.
- L. J. Cheng and N. P. Mankad, J. Am. Chem. Soc., 2017, 139, 10200–10203 CrossRef CAS PubMed
.
- L. J. Cheng, S. M. Islam and N. P. Mankad, J. Am. Chem. Soc., 2018, 140(3), 1159–1164 CrossRef CAS PubMed
.
- S. Zhao and N. P. Mankad, Org. Lett., 2019, 21, 10106–10110 CrossRef CAS PubMed
.
- S. Zhao and N. P. Mankad, Angew. Chem., Int. Ed., 2018, 57, 5867–5870 CrossRef CAS PubMed
.
- L. J. Cheng and N. P. Mankad, Acc. Chem. Res., 2021, 54, 2261–2274 CrossRef CAS PubMed
.
- Y. Yuan, F. Zhao and X. F. Wu, Chem. Sci., 2021, 12, 12676–12681 RSC
.
- Y. Yuan, F. P. Wu, C. Schünemann, J. Holz, P. C. Kamer and X. F. Wu, Angew. Chem., Int. Ed., 2020, 59, 22441–22445 CrossRef CAS PubMed
.
- X. Jin, H. C. Fu, M. Y. Wang, S. Huang, Y. Wang, L. N. He and X. Ma, Org. Lett., 2021, 23, 4997–5001 CrossRef CAS PubMed
.
-
(a) Y. Y. Gui, N. Hu, X. W. Chen, L. L. Liao, T. Ju, J. H. Ye, Z. Zhang, J. Li and D. G. Yu, J. Am. Chem. Soc., 2017, 139, 17011–17014 CrossRef CAS PubMed
; (b) Y. Tani, K. Kuga, T. Fujihara, J. Terao and Y. Tsuji, Chem. Commun., 2015, 51, 13020–13023 RSC
.
- C. Tang, Y. Zeng, P. Cao, X. Yang and G. Wang, Catal. Lett., 2009, 129, 189–193 CrossRef CAS
.
- I. Fleischer, K. M. Dyballa, R. Jennerjahn, R. Jackstell, R. Franke, A. Spannenberg and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 2949–2953 CrossRef CAS PubMed
.
- L. Wu, I. Fleischer, R. Jackstell, I. Profir, R. Franke and M. Beller, J. Am. Chem. Soc., 2013, 135, 14306–14312 CrossRef CAS PubMed
.
- Y. Yuki, K. Takahashi, Y. Tanaka and K. Nozaki, J. Am. Chem. Soc., 2013, 135, 17393–17400 CrossRef CAS PubMed
.
-
(a) M.-Y. Ngai, E. Skucas and M. J. Krische, Org. Lett., 2008, 10, 2705–2708 CrossRef CAS PubMed
; (b) T. Smejkal, H. Han, B. Breit and M. J. Krische, J. Am. Chem. Soc., 2009, 131, 10366–10367 CrossRef CAS PubMed
; (c) B. Sam, T. P. Montgomery and M. J. Krische, Org. Lett., 2013, 15, 3790–3793 CrossRef CAS PubMed
; (d) A. Köpfer, B. Sam, B. Breit and M. J. Krische, Chem. Sci., 2013, 4, 1876–1880 RSC
; (e) C. C. Bausch, R. L. Patman, B. Breit and M. J. Krische, Angew. Chem., Int. Ed., 2011, 50, 5687–5690 CrossRef CAS PubMed
.
- W. Li and X.-F. Wu, Chem. – Eur. J., 2015, 21, 14943–14948 CrossRef CAS PubMed
.
Footnotes |
| † Dedicated to Professors Matthias Beller and Armin Börner of LIKAT! |
| ‡ Electronic supplementary information (ESI) available: general comments, general procedure, analytical data, and NMR spectra. See DOI: 10.1039/d1sc05474k |
| § These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |