Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Orthogonally aligned cyclic BODIPY arrays with long-lived triplet excited states as efficient heavy-atom-free photosensitizers†
Zhaoyang
Zhu‡
a,
Xue
Zhang‡
 b,
Xing
Guo
a,
Qinghua
Wu
a,
Zhongxin
Li
a,
Changjiang
Yu
b,
Xing
Guo
a,
Qinghua
Wu
a,
Zhongxin
Li
a,
Changjiang
Yu
 a,
Erhong
Hao
a,
Erhong
Hao
 *a,
Lijuan
Jiao
*a,
Lijuan
Jiao
 *a and
Jianzhang
Zhao
*a and
Jianzhang
Zhao
 *b
*b
aLaboratory of Functionalized Molecular Solids, Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002, China. E-mail: jiao421@ahnu.edu.cn; haoehong@ahnu.edu.cn
bState Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: zhaojzh@dlut.edu.cn
First published on 29th October 2021
Abstract
In photosensitizers, long triplet excited state lifetimes are key to their efficient electron transfer or energy transfer processes. Herein, we report a novel class of cyclic trimeric BODIPY arrays which were efficiently generated from easily accessible meso-mesityldipyrrinone and arylboronic acids in one pot. Arylboronic acid, for the first time, was used to provide a boron source for BODIPY derivatives. Due to the well-defined and orthogonally aligned BODIPY cores as verified by X-ray crystallography, these BODIPY arrays show strong exciton coupling effects and efficient intersystem crossings, and are novel heavy-atom-free photosensitizers with a long-lived triplet excited state (lifetime up to 257.5 μs) and good reactive oxygen species generation efficiency (up to 0.72) contributed by both 1O2 and O2−˙ under light irradiation.
Introduction
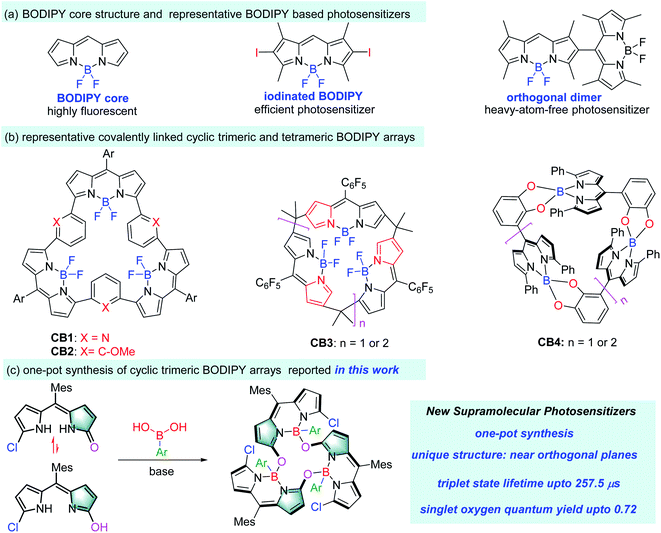
Chromophore arrays with well-defined architectures possess unique photophysical properties and have attracted wide research interest in a variety of research fields.1 Among those, porphyrinoid-based chromophore arrays,2 especially the porphyrin-based dimers and trimers (Fig. 1a), have been extensively investigated as mimics of natural light-harvesting antennae, related energy transfer cassettes and photosensitizers.3 BODIPY4 (boron dipyrromethene, Fig. 1a) as “the little sister of porphyrin” can be viewed as a boron complex of a half porphyrin framework. It features a rigid structure, easy accessibility, reasonably good stability and excellent photophysical properties, including strong photon capture ability which is easily tuneable from the visible to near infrared range.4c–g It also has rich chemistry and multiple sites (six pyrrolic, one meso- and one boron) for the facile incorporation of various functionalities at different positions of the BODIPY platform to meet a set of specific requirements for different applications, from fluorescence probes (for metal ions,5 biomolecules,6 viscosity,7 polarity8 and temperature9 within living cells) to photosensitizers for photodynamic therapy (PDT).10 Therefore, BODIPY is an appealing platform for the construction of chromophore arrays.However, unlike porphyrin arrays, currently BODIPY arrays are relatively unexplored, and the most reported ones are linear BODIPY arrays with low structural rigidity.11,12 Covalently linked cyclic BODIPY arrays can fix the rigidity issue since each of the BODIPY subunits is locked by their adjacent ones. Due to the synthetic challenge, there are only a handful of cyclic BODIPY arrays (for example, CB1 to CB4, Fig. 1b)13–16 available. Their synthesis is mainly based on the boron complexation of premade porphyrin analogues. CB1 and CB2 with a bowl-shaped structure, both from BF2 complexation of expanded porphyrins,13 showed interesting exciton coupling between the BODIPY subunits and strong recognition of guest species. CB3 was prepared from BF2 complexation of expanded N-confused calix[n]phyrins,14 and exhibited unique luminescence and lasing properties. Two types of cyclic BODIPY arrays were also synthesized from BODIPY monomers through self-coupling or condensation with pentafluorobenzaldehyde.15 A sole example of elegant cyclic BODIPY array CB4 linked through boron atoms was reported by Nabeshima and co-workers16 using the reaction of catecholyldipyrrin with boron trichloride. Despite the low yields (less than 5%) of this one-pot reaction, the resultant CB4 was a nice supramolecular host for alkali metal ions. In general, these reported cyclic BODIPY arrays typically showed strong excitonic coupling of the singlet excited state due to their closely packed structures.11–17 However, such systems with fine-tuned triplet excited states have not been developed.
Due to their promising photophysical properties, BODIPY dyes have been transformed to triplet photosensitizers through improving their intersystem crossing (ISC) from the singlet state to triplet state.18,19 The majority of these BODIPY based photosensitizers relied on the installation of heavy atoms to induce ISC and the consequent singlet oxygen formation (for example, iodinated BODIPY18h in Fig. 1a). However, despite having good singlet oxygen quantum yields, these halogenated BODIPYs typically show short triplet state lifetimes due to the enhanced and undesired ISC from the T1 state to the S0 state.19 Long triplet state lifetimes of photosensitizers are key to efficient electron transfer or energy transfer processes for application in photodynamic therapy (PDT).
Herein, we report a series of calixarene-like cyclic trimeric BODIPY arrays based on the direct condensation of meso-mesityldipyrrinones with arylboronic acids, and they show efficient ISC due to the orthogonally aligned BODIPY cores, good singlet oxygen generation efficiency and long-lived triplet excited states (Fig. 1c). To the best of our knowledge, this is the first time that a cyclic BODIPY array has been used to fine-tune its triplet excited state to produce an efficient supramolecular photosensitizer with a long triplet state lifetime.
Results and discussion
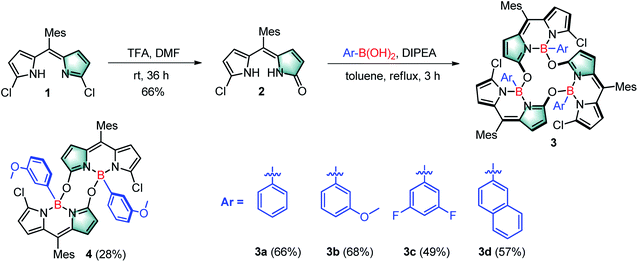
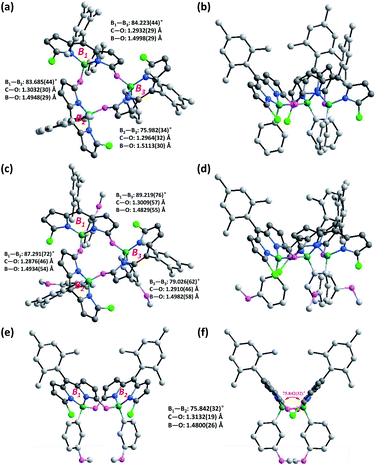
Inspired by our recent synthesis of boron-functionalized BODIPYs through organotrifluoroborate salts,20 we rationalized that arylboronic acids may also be able to complex with α-hydroxydipyrrin (or its tautomer dipyrrinone) and undergo further condensation to generate novel cyclic BODIPYs with B–O linkages (Fig. 1c). Therefore, we chose its analogue, meso-mesityldipyrrinone 2, as the starting material, which was smoothly generated as yellow powder in 66% yield within one step from the acid-catalyzed hydrolysis (using TFA, trifluoroacetic acid, as the acid) of readily available 1,9-dichlorodipyrrin 121 in DMF at room temperature (Scheme 1). Initially, commercial phenylboronic acid was chosen for the subsequent complexation with meso-mesityldipyrrinone 2. It was performed in refluxing toluene in the presence of 1 equiv. of DIPEA (diisopropylethylamine), from which a major product was isolated as an orange solid in 66% yield, and was fully characterized by 1H NMR, 13C NMR and HRMS. The 1H NMR spectrum shows sharp and simple pattern signals, indicating a symmetric structural feature. In comparison with that of the starting meso-mesityldipyrrinone 2, two NH signals (at 9.99 and 10.69 ppm) are missing and high-field shifted doublet signals are observed for the pyrrolic β-proton (Scheme 1, H1, 4.80–4.90 ppm with respect to the 6.58–6.07 ppm for 2). These results indicate the successful complexation with phenylboronic acid. HRMS-ESI mass analysis gives a strong peak at m/z 1219.3995, which indicates the formation of cyclic trimeric BODIPY 3a (calcd for C72H60B3Cl3N6O3Na+ [M + Na]+: 1219.3980). The structure of 3a was further confirmed by X-ray analysis.Crystals suitable for the X-ray analysis of 3a were obtained by slow evaporation of its dichloromethane solution. X-ray analysis results clearly show that the α-hydroxyl moiety (derived from the ketone moiety of meso-mesityldipyrrinone 2) on each BODIPY chromophore is bonded to the central boron atoms of two adjacent BODIPY chromophores (Fig. 2a and b). The dihedral angles between these BODIPY subunits approach orthogonality (76°–84.2°), similar to those of the reported orthogonal BODIPY dimers (Fig. 1a).10b Meanwhile, the two β-pyrrolic protons (H1) are located in a ring system, which leads to a shielding effect from the diatropic ring currents residing on the adjacent BODIPY units.3a This might explain the observed high-field-shift of pyrrolic protons in the 1H NMR spectra of 3a with respect to that of meso-mesityldipyrrinone 2.
To test the versatility of this strategy, we further applied some other commercial arylboronic acids, including phenylboronic acid derivatives with electron-rich methoxyl or electron-deficient fluoro-substituents, and more conjugated 2–naphthaleneboronic acid for this reaction (Scheme 1). In all the cases, the complexation smoothly proceeded and generated the desired corresponding cyclic trimeric BODIPYs 3b–d in 49–68% isolated yields (see the ESI† for their detailed synthesis). These target products were fully characterized by 1H NMR, 13C NMR and ESI. Similar to 3a, these products all show sharp and simple spectrum patterns in their 1H NMR spectra, which indicates their symmetric structural features. In addition, the missing of two NH signals (at 9.99 and 10.69 ppm) and high-field shifted doublet proton signals for the pyrrolic β-protons in the range of 4.80–4.90 ppm are observed. HRMS-ESI mass analysis shows a strong [M + Na]+ peak at m/z 1307.4303 for 3b (calculated 1307.4281), 1303.3583 for 3c (calculated 1303.3579), and 1369.4454 for 3d (calculated 1369.4455), respectively. Among those, the structure of 3b is further confirmed by X-ray analysis (Fig. 2). Similar to 3a, all the B–O bond lengths within 3b are around 1.49(1) Å (Table S1†), and the two β-pyrrolic protons (H1) are located in a ring system. This might explain the observed high-field-shift of the pyrrolic protons in the 1H NMR spectra of 3b with respect to that of dipyrrinone 2. The dihedral angles between each of the BODIPY subunits are slightly larger (around 89.2°) than those observed in 3a (84.2°) (Fig. 2c and d), which indicates that the substituents on the arylboronic acid can tune the dihedral angles between each of the BODIPY subunits.
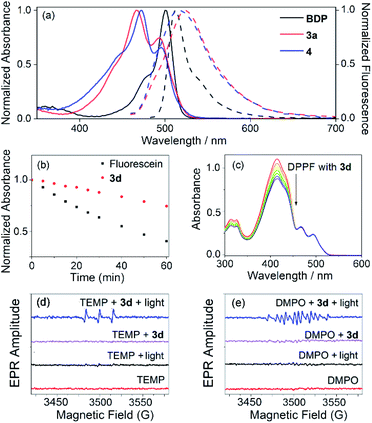
Interestingly, the reaction of 3-methoxyphenyl boronic acid with meso-mesityldipyrrinone 2 also generated a reasonably stable minor product 4 in 28% yield besides 3b (68% isolated yield). The yield of this minor product 4 can be further tuned via simple variation of the amount of the DIPEA base in the system. It was characterized by 1H NMR, ESI mass and X-ray analysis. A strong peak at m/z 857.3004 in ESI mass indicates the formation of a dimeric BODIPY framework. Its dimeric BODIPY structure was further confirmed by X-ray analysis, in which the two BODIPY subunits stay far away from each other with few overlaps of the their BODIPY chromophores. In great contrast to the cyclic trimeric BODIPY 3b, no high-field-shift of pyrrolic protons was observed in its 1H NMR spectra. In comparison with trimeric 3b, dimeric 4 shows lower photostability which can be observed with the naked eye. Initially, 4 is nonfluorescent in organic solvent. Upon strong light irradiation under vacuum, there is a dramatic turn-on of fluorescence in its solution. By contrast, the continuous strong irradiation of 3d with a 50 W white LED lamp in toluene for a period of 60 min leads to less than 25% degradation of this molecule (Fig. S12†). Similar to the photodegradation phenomena of classical BODIPY dyes,22a we did not observe any change in the shape of the longest wavelength absorption band of 3d, except for the decrease in peak heights, during the above photoexposure. It is worth noticing that 3d is even more stable than the well-known commercial fluorescein, which gives around 60% degradation under the same irradiation conditions (Fig. 3b).
Cyclic trimeric BODIPYs 3 each shows a dual intense broad absorption in the common organic solvents studied (as summarized in Fig. 3, Table S4, and Fig. S7–S10†). For example, 3a displays dual absorption bands centered at 467 and 493 nm, respectively (molar absorption coefficient ε = 8.71 × 104 M−1 cm−1 at 467 nm; Table S4†). Similarly, 4 also shows dual absorption bands centered at 472 and 497 nm (Fig. 3a), respectively. However, the absorption efficiency is slightly lower (ε = 3.09 × 104 M−1 cm−1 at 472 nm) with respect to that of 3a. These results are in great contrast to those of their monomeric meso-mesityl BODIPY (BDP, Fig. 3a),22b which shows only a maximum absorption peak at 498 nm in dichloromethane. These dual absorption bands indicate the existence of exciton coupling in these cyclic dimeric and trimeric BODIPYs.11,12
Cyclic trimeric BODIPYs 3 all show extremely low fluorescence (ca. Φ = 0.001) in the various common organic solvents studied (Table 1), which is also in agreement with the extremely low oscillator strengths for their electronic transitions from theoretical calculations (Table S7†). Inspired by the orthogonally aligned BODIPY cores observed in our cyclic trimeric BODIPYs, we envisaged that ISC would be possible for these molecules since the other nonradiative decay pathways of the S1 state would be less efficient for these rigid cyclic molecules. The calculated S1 energy for the parent trimeric BODIPY 3 (meso-mesityl group and the boron substituted aryl group were removed for simplicity) was 2.48 eV, while the calculated T1, T2, T3 and T4 energies were 1.53, 1.53, 1.53 and 2.48 eV, respectively. Since T1, T2 and T3 are below the S1 state, and T4 is the same as S1, S1 → T1, S1 → T2, S1 → T3 and S1 → T4 are considered to be the possible pathways of ISC. To investigate the underlying reason for the enhanced ISC in these trimeric BODIPYs, the spin–orbit coupling (SOC) matrix elements between the singlet state and the triplet excited states for 3 were further calculated as 0.74, 0.83 and 1.58 cm−1 for T1, T2 and T3 states, respectively (Table S10†), which were considered large enough to induce ISC.10b–e Furthermore, the 〈S1|ĤSO|T3〉 value (Table S12†) found for 3 (1.58 cm−1) is almost 1 order of magnitude higher than the 〈S1|ĤSO|T4〉 value (0.19 cm−1). Then, natural transition orbital (NTO) analysis was conducted to further investigate the excited state properties. The spin–orbit charge transfer ISC (SOCT-ISC) mechanism23 is found to be preferred for 3, and charge transfer occurs more efficiently from a singlet CT state (S1) to a triplet LE state (T3) due to a relatively large SOC value (Fig. S22–S24†).
| Dyes | λ abs (nm) | λ em (nm) | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmaxc εmaxc |
Stokes shift (cm−1) | Φ F | Φ Δ | τ T |
|---|---|---|---|---|---|---|---|
| a Maximum absorption peak. b Shoulder peak. c Molar absorption coefficients correspond to the maximum absorption peak. d Fluorescence quantum yields were obtained by using fluorescein (ΦF = 0.90 in 0.1 M NaOH) as the reference. The standard errors are less than 10% based on three measurements. e Singlet oxygen quantum yield with RB (ΦΔ = 0.80 in methanol) as the standard; determination error = ±0.03. f Triplet state lifetime in deaerated dichloromethane, λex = 490 nm. RB was used as the standard compound, ΦΔ = 80% in methanol. g Not measured. | |||||||
| 3a | 467a/493b | 523 | 4.94 | 1164 | <0.001 | 0.35 | 257.5 |
| 3b | 468a/494b | 523 | 4.91 | 2247 | <0.001 | 0.72 | —g |
| 3c | 471a/496b | 526 | 4.82 | 2220 | <0.001 | 0.34 | — |
| 3d | 468a/494b | 521 | 5.16 | 1049 | <0.001 | 0.41 | 188.9 |
| 4 | 472a/497b | 515 | 4.49 | 1769 | 0.002 | — | — |
The singlet oxygen generation efficiency of these trimeric BODIPYs was tested under broadband light (ca. 490 nm) irradiation conditions by using 1,3-diphenylisobenzofuran (DPBF) as the trap molecule and Rose Bengal (RB, ΦΔ = 0.80 under the investigated conditions) as the reference compound, respectively (Fig. 3c, and Table S5 in the ESI†). As expected, the cyclic trimeric BODIPYs 3 show reasonably good to high singlet oxygen generation efficiencies (ΦΔ = 0.35, 0.72, 0.34 and 0.41 for 3a, 3b, 3c and 3d in dichloromethane, respectively). Among those, the singlet oxygen quantum yield for 3b is much higher than those of nonhalogenated BODIPY dyes and many other organic chromophores and photosensitizers under comparable conditions. Notably, both HOMOs and LUMOs for 3a and 3c are distributed over only the BODIPY cores. However, for 3b, only the LUMO is distributed on the BODIPY cores, while the HOMO is located on the 3-methoxylbenzene group (Fig. S25†), indicating a possible intramolecular charge transfer (ICT) process24 in this trimer. The efficient ICT in 3b may facilitate the SOCT ISC process via efficient photoinduced charge separation,23 providing its high singlet oxygen generation efficiency. The singlet oxygen quantum yield values in toluene and THF solutions are also satisfactory for those trimeric BODIPYs (Table S6†).
Next, EPR spectra were used to track the reactive oxygen species formed during light irradiation of our cyclic trimer 3d. TEMP, an 1O2 specific spin-trap agent, was added to the solution of 3d, and a strong EPR signal was observed (Fig. 3d) after light irradiation of the solution mixture. Without light or addition of TEMP, no EPR signal was found. This characteristic EPR signal of the paramagnetic adduct from 1O2 and TEMP25 indicated that cyclic trimer 3d generated 1O2 efficiently under light irradiation. Furthermore, when DMPO, an O2−˙ specific spin-trap agent, was added to the solution of 3d, a characteristic EPR signal of the paramagnetic adduct from O2−˙ and DMPO25 was also observed upon light irradiation (Fig. 3e), indicating the production of O2−˙ by cyclic trimer 3d under light irradiation. Thus, the ΦΔ values of our cyclic trimers should be contributed by both 1O2 and O2−˙ through energy transfer and electron transfer pathways, respectively.
BODIPYs 3a and 3d were then selected to study their triplet-state properties with nanosecond transient absorption (ns TA) spectroscopy (Fig. 4). Upon pulsed laser excitation at 490 nm, a ground-state bleaching (GSB) band at ca. 470 nm was observed along with excited-state absorption (ESA) bands in the range of 300–800 nm (superimposed with the GSB band). These ESA bands are different from the typical BDP triplet-state ESA bands centered at ca. 420 and 675 nm. By monitoring the decay of the GSB signal at 470 nm, the triplet excited states for 3a and 3d were found to be long-lived (hundreds of microseconds, 257.5 and 188.9 μs) in deaerated solution (Fig. 4c and d). These triplet state lifetimes are much longer than those of typical heavy atom-containing triplet photosensitizers with comparably long-wavelength absorption (tT = 1.7 μs for 2,6-diiodo-bisstyrylBODIPY).26 This long-lived triplet state lifetime is essential for their applications in photocatalysis and PDT since the triplet state lifetimes of photosensitizers would greatly affect their intermolecular diffusion-controlled triplet energy transfer or electron transfer efficiency.18,19
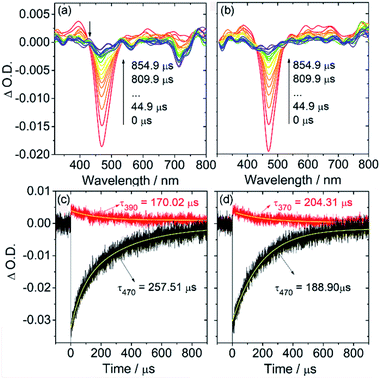 | ||
| Fig. 4 Nanosecond transient absorption spectra of (a) 3a and (c) the decay trace; (b) 3d and (d) the decay trace. c = 1.0 × 10−5 M. λex = 490 nm, in deaerated dichloromethane, 20 °C. | ||
To enhance the biocompatibility and solubility of the cyclic trimeric BODIPY, classical Cremophor-EL surfactant was used to obtain the self-assembly of micelles of 3b and 3d (Fig. S26†). The absorption spectra of 3b/3d micelles in water were similar to their spectra in dichloromethane, suggesting that 3b/3d micelles could disperse in the aqueous phase well. Transmission electron microscopy (TEM) images showed that the diameter of 3d micelles was about 55 nm (Fig. S27†), which was also confirmed by the dynamic light scattering (DLS) results (Fig. S26†). Furthermore, stabilities of both 3b and 3d in water toward pH and storage were evaluated using absorption spectra. There was no significant difference in the absorptions of 3b/3d micelles in solutions with different pH values in the range of 3–10 (Fig. S28†). What's more, although these micelles were stored for 48 h in buffer solutions with different pH values, the absorbance only exhibited negligible declines (Fig. S29 and S30†). These results suggested that similar to classical BODIPY dyes,27 these cyclic BODIPY trimers have promising stabilities to cope with different physiological pH conditions in practical applications.
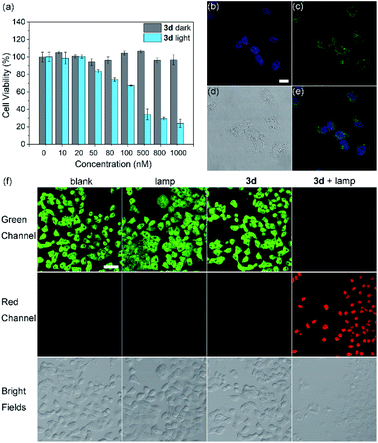
When HeLa cells were incubated with 3d micelles for 1 h, weak green fluorescence was observed under a confocal fluorescence microscope (Fig. 5c), indicating that 3d micelles could penetrate the cell membrane. Next, a CCK-8 assay was conducted to determine the dark-toxicity of 3d micelles to living HeLa cells. The results showed that 3d possesses no significant cytotoxicity even at a concentration of 30 μM, and the IC50 was 68.4 μM (Fig. S31†) indicating the good biocompatibility of 3d micelles. The photocytotoxicity of the cyclic trimeric BODIPY 3d micelles was further investigated (Fig. 5a). Even at very low concentrations of 3d micelles, a significant decrease of cell viability was observed with a remarkable IC50 of 162 nM (Fig. S34†). By contrast, there was no statistically significant change when the cells were kept in the dark in the presence of the same concentration of the photosensitizer 3d micelles (gray, Fig. 5a). Similarly, 3b micelles were also studied, which also showed an effective IC50 value of 246 nM (Fig. S33†). Furthermore, under these conditions, the photocytotoxicity of the reference photosensitizer RB was studied, which showed an IC50 of 1.2 μM (Fig. S32†). From the above results, the PDT treatment window of 3d micelles was 162 nM to 74.8 μM. Such a wide range is far superior to those of heavy atom effect type photosensitizers, which suffer from severe dark toxicity resulting from heavy atoms and a short treatment window.
To visualize the PDT effect of 3d micelles toward cancer cells, the phototoxicity of 3d micelles was also evaluated using the fluorescence images of live/dead co-stained cells (Fig. 5f). Acridine orange (AO, green channel) tends to combine with living cells and emits green fluorescence, while propidium iodide (PI, red channel) selectively combines with dead cells and glows with red fluorescence because of changes in membrane permeability. In the control groups, the cells which were treated with 3d micelles or LED lamp illumination alone or without any treatment emitted bright green fluorescence, indicating that the dark cytotoxicity of 3d micelles was low, and the thermal side effect of LED lamp irradiation could be ignored (blank, lamp only and 3d only groups in Fig. 5f). In contrast, when 3d micelles were illuminated with an LED lamp, the cells emitted brilliant red fluorescence and negligible green fluorescence, suggesting that nearly all cells were dead (Fig. 5f and S37,†3d + lamp).
Conclusions
In summary, a new type of cyclic trimeric BODIPY array was reported from easily accessible meso-mesityldipyrrinone and arylboronic acids in a one-pot reaction. These cyclic trimeric BODIPYs show a close to orthogonal arrangement of the BODIPY units in their X-ray structures. The unique and rigid structures not only provide strong excitonic coupling of dyes in the singlet excited state, but also promote a long-lived triplet excited state through an efficient ISC process. These BODIPY arrays, as novel heavy-atom-free photosensitizers, showed good reactive oxygen species generation efficiencies (up to 0.72) which were contributed by both 1O2 and O2−˙ under light irradiation, and promising in vitro PDT results with a large PDT treatment window and high photocytotoxicity of cells at 152.1 nM.Data availability
The experimental, crystallographic and computational data are available in the ESI.†Author contributions
Zhaoyang Zhu and Xue Zhang contributed equally. Z. Zhu carried out the synthesis, the steady state optical spectroscopy studies and data analysis; X. Zhang performed the nanosecond transient absorption and singlet oxygen measurements; X. Guo performed the EPR spectral measurements and biological studies. Dr Q. Wu carried out the theoretical calculations. Z. Li and Dr C. Yu obtained the X-ray structures and part of the optical spectra. Prof. E. Hao, Prof. L. Jiao and Prof. J. Zhao carried out the data analysis, devised the project and co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (Grant Nos 21871006, 21971004 and U2001222) and the Anhui Provincial Natural Science Foundation (2008085QB67 and 2108085QB81) for supporting this work. The numerical calculations in this paper were performed on the supercomputing system at the Supercomputing Center of The University of Science and Technology of China.Notes and references
-
(a) P. Frischmann, K. Mahata and F. Würthner, Chem. Soc. Rev., 2013, 42, 1847 RSC
; (b) H. Peng, L. Niu, Y. Chen, L. Wu, C. Tung and Q. Yang, Chem. Rev., 2015, 115, 7502 CrossRef CAS PubMed
; (c) T. Mirkovic, E. E. Ostroumov, J. M. Anna, R. van Grondelle, Govindjee and G. D. Scholes, Chem. Rev., 2017, 117, 249 CrossRef CAS PubMed
; (d) S. Shoji, Y. Nomura and H. Tamiaki, Photochem. Photobiol. Sci., 2019, 18, 555 CrossRef CAS PubMed
; (e) N. Taylor and L. Kassal, Chem. Sci., 2019, 10, 9576 RSC
.
-
(a) Y. Nakamura, N. Aratani and A. Osuka, Chem. Soc. Rev., 2007, 36, 831 RSC
; (b) N. Aratani, D. Kim and A. Osuka, Acc. Chem. Res., 2009, 42, 1922 CrossRef CAS PubMed
; (c) J. Yang, M. Yoon, H. Yoo, P. Kim and D. Kim, Chem. Soc. Rev., 2012, 41, 4808 RSC
; (d) S. Ooi, B. Adinarayana, D. Shimizu, T. Tanaka and A. Osuka, Angew. Chem., Int. Ed., 2020, 59, 9423 CrossRef CAS PubMed
; (e) K. Wang, A. Osuka and J. Song, ACS Cent. Sci., 2020, 6, 2159 CrossRef CAS PubMed
.
-
(a) D. Shimizu, J. Oh, K. Furukawa, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2015, 54, 6613 CrossRef CAS PubMed
; (b) L. Xu, B. Wen, G. Kim, T. Kim, F. Cheng, M. Zhou, L. Xu, T. Tanaka, B. Yin, A. Osuka and D. Kim, Angew. Chem., Int. Ed., 2017, 129, 12490 CrossRef
; (c) M. Jirásek, M. Rickhaus, L. Tejerina and H. L. Anderson, J. Am. Chem. Soc., 2021, 143, 2403 CrossRef PubMed
.
-
(a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed
; (b) G. U. R. Ziessel and A. Osuka, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef PubMed
; (c) H. Lu, J. Mack, Y. Yang and Z. Shen, Chem. Soc. Rev., 2014, 43, 4778 RSC
; (d) Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774 RSC
; (e) Y. Ge and D. F. O'Shea, Chem. Soc. Rev., 2016, 45, 3846 RSC
; (f) N. Boens, B. Verbelen, M. J. Ortiz, L. Jiao and W. Dehaen, Coord. Chem. Rev., 2019, 399, 213024 CrossRef CAS
; (g) J. Wang, N. Boens, L. Jiao and E. Hao, Org. Biomol. Chem., 2020, 18, 4135 RSC
; (h) J. Labella, G. Durán-Sampedro, M. V. Martínez-Díaz and T. Torres, Chem. Sci., 2020, 11, 10778 RSC
.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC
.
-
(a) P. Verwilst, H. Kim, S. Kim, C. Kang and J. Kim, Chem. Soc. Rev., 2018, 47, 2249 RSC
; (b) J. Franke, B. Raliski, B. Boggess, D. Natesan, E. Koretsky, P. Zhang, R. Kulkarni, P. Deal and E. Miller, J. Am. Chem. Soc., 2019, 141, 12824 CrossRef CAS PubMed
; (c) L. Niu, Y. Guan, Y. Chen, L. Wu, C. Tung and Q. Yang, J. Am. Chem. Soc., 2012, 134, 18928 CrossRef CAS PubMed
; (d) H. Liu, W. Song, S. Zhang, K. S. Chan, Z. Guo and Z. Shen, Chem. Sci., 2020, 11, 8495 RSC
.
-
(a) M. Kuimova, Phys. Chem. Chem. Phys., 2012, 14, 12671 RSC
; (b) L. Wang, Y. Xiao, W. Tian and L. Deng, J. Am. Chem. Soc., 2013, 135, 2903 CrossRef CAS PubMed
; (c) Y. Zatsikha, N. Didukh, R. Swedin, V. Yakubovskyi, T. Blesener, A. Healy, D. Herbert, D. Blank, V. Nemykin and Y. Kovtun, Org. Lett., 2019, 21, 5713 CrossRef CAS PubMed
.
-
(a) H. Sunahara, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 5597 CrossRef CAS PubMed
; (b) A. Polita, S. Toliautas, R. Žvirblis and A. Vyšniauskas, Phys. Chem. Chem. Phys., 2020, 22, 8296 RSC
.
-
(a) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953 RSC
; (b) M. Ogle, A. McWilliams, M. Ware, S. Curley, S. Corr and A. Martí, J. Phys. Chem. B, 2019, 123, 7282 CrossRef CAS PubMed
.
-
(a) Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. Yildirim, A. Dogan, D. Guc and E. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937 CrossRef CAS PubMed
; (b) Z. Wang, L. Huang, Y. Yan, A. M. El-Zohry, A. Toffoletti, J. Zhao, A. Barbon, B. Dick, O. F. Mohammed and G. Han, Angew. Chem., Int. Ed., 2020, 59, 16114 CrossRef CAS PubMed
; (c) V.-N. Nguyen, Y. Yim, S. Kim, B. Ryu, K. M. K. Swamy, G. Kim, N. Kwon, C.-Y. Kim, S. Park and J. Yoon, Angew. Chem., Int. Ed., 2020, 59, 8957 CrossRef CAS PubMed
; (d) G. Turkoglu, G. K. Koygun, M. N. Z. Yurt, S. N. Pirencioglu and S. Erbas-Cakmak, Chem. Sci., 2021, 12, 9754 RSC
; (e) R. Wang, K. Dong, G. Xu, B. Shi, T. Zhu, P. Shi, Z. Guo, W. Zhu and C. Zhao, Chem. Sci., 2019, 10, 2785 RSC
; (f) P. De Bonfils, L. Péault, P. Nun and V. Coeffard, Eur. J. Org. Chem., 2021, 1809 CrossRef CAS
; (g) R. Prieto-Montero, A. Prieto-Castañeda, R. Sola-Llano, A. R. Agarrabeitia, D. García-Fresnadillo, I. López-Arbeloa, A. Villanueva, M. J. Ortiz, S. Moya and V. Martínez-Martínez, Photochem. Photobiol., 2020, 96, 458 CrossRef CAS PubMed
.
-
(a) Y. Qin, X. Liu, P. Jia, L. Xu and H. Yang, Chem. Soc. Rev., 2020, 49, 5678 RSC
; (b) T. Köhler, M. C. Hodgson, D. Seidel, J. M. Veauthier, S. Meyer, V. Lynch, P. D. W. Boyd, P. J. Brothers and J. L. Sessler, Chem. Commun., 2004, 1060 RSC
.
-
(a) A. B. Nepomnyashchii, M. Bröring, J. Ahrens and A. Bard, J. Am. Chem. Soc., 2011, 133, 19498 CrossRef CAS PubMed
; (b) A. B. Nepomnyashchii, M. Bröring, J. Ahrens and A. Bard, J. Am. Chem. Soc., 2011, 133, 8633 CrossRef CAS PubMed
; (c) L. Patalag, L. Ho, P. Jones and D. B. Werz, J. Am. Chem. Soc., 2017, 139, 15104 CrossRef CAS PubMed
; (d) S. Ye, J. Rao, S. Qiu, J. Zhao, H. He, Z. Yan, T. Yang, Y. Deng, H. Ke, H. Yang, Y. Zhao, Z. Guo and H. Chen, Adv. Mater., 2018, 30, 1801216 CrossRef PubMed
; (e) Q. Wu, Z. Kang, Q. Gong, X. Guo, H. Wang, D. Wang, L. Jiao and E. Hao, Org. Lett., 2020, 22, 7513 CrossRef CAS PubMed
; (f) A. Patra, L. J. Patalag, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 747 CrossRef CAS PubMed
; (g) L. Patalag, J. Hoche, M. Holzapfel, A. Schmiedel, R. Mitric, C. Lambert and D. B. Werz, J. Am. Chem. Soc., 2021, 143, 7414 CrossRef CAS PubMed
; (h) K. Teng, W. Chen, L. Niu, W. Fang, G. Cui and Q. Yang, Angew. Chem., Int. Ed., 2021, 60, 19912 CrossRef CAS PubMed
; (i) M. Bröring, R. Krüger, S. Link, C. Kleeberg, S. Köhler, X. Xie, B. Ventura and L. Flamigni, Chem.–Eur. J., 2008, 14, 2976 CrossRef PubMed
.
-
(a) X. Ke, T. Kim, V. Lynch, D. Kim and J. Sessler, J. Am. Chem. Soc., 2017, 139, 13950 CrossRef CAS PubMed
; (b) T. Nakamura, G. Yamaguchi and T. Nabeshima, Angew. Chem., Int. Ed., 2016, 55, 9606 CrossRef CAS PubMed
; (c) R. Matsuoka, S. Himori, G. Yamaguchi and T. Nabeshima, Org. Lett., 2020, 22, 8764 CrossRef CAS PubMed
.
- M. Ishida, T. Omagari, R. Hirosawa, K. Jono, Y. Sung, Y. Yasutake, H. Uno, M. Toganoh, H. Nakanotani, S. Fukatsu, D. Kim and H. Furuta, Angew. Chem., Int. Ed., 2016, 55, 12045 CrossRef CAS PubMed
.
-
(a) T. Sakida, S. Yamaguchi and H. Shinokubo, Angew. Chem., Int. Ed., 2011, 50, 2280 CrossRef CAS PubMed
; (b) T. Kim, Z. Duan, S. Talukdar, C. Lei, D. Kim, J. Sessler and T. Sarma, Angew. Chem., Int. Ed., 2020, 59, 13063 CrossRef CAS PubMed
.
- C. Ikeda and T. Nabeshima, Chem. Commun., 2008, 721 RSC
.
-
(a) A. J. Musser, P. P. Neelakandan, J. M. Richter, H. Mori, R. H. Friend and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 12050 CrossRef CAS PubMed
; (b) G. Gupta, A. Das, K. C. Park, A. Tron, H. Kim, J. Mun, N. Mandal, K.-W. Chi and C. Y. Lee, Inorg. Chem., 2017, 56, 4615 CrossRef PubMed
.
-
(a) X. Xiao, W. Tian, M. Imran, H. Cao and J. Zhao, Chem. Soc. Rev., 2021, 50, 9686 RSC
; (b) E. Bassan, A. Gualandi, P. G. Cozzi and P. Ceroni, Chem. Sci., 2021, 12, 6607 RSC
; (c) J. Wang, Q. Gong, L. Wang, E. Hao and L. Jiao, J. Porphyrins Phthalocyanines, 2020, 24, 603 CrossRef CAS
; (d) J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904 RSC
; (e) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC
; (f) S. G. Awuah and Y. You, RSC Adv., 2012, 2, 11169 RSC
; (g) K. Teng, L. Niu and Q. Yang, Chem. Sci., 2020, 11, 9703 RSC
; (h) T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162 CrossRef CAS PubMed
.
-
(a) Z. Wang, A. Toffoletti, Y. Hou, J. Zhao, A. Barbon and B. Dick, Chem. Sci., 2021, 12, 2829 RSC
; (b) V.-N. Van-Nghia Nguyen, Y. Yan, J. Zhao and J. Yoon, Acc. Chem. Res., 2021, 54, 207 CrossRef PubMed
; (c) M. A. Filatov, Org. Biomol. Chem., 2020, 18, 10 RSC
; (d) Y. Dong, B. Dick and J. Zhao, Org. Lett., 2020, 22, 5535 CrossRef CAS PubMed
; (e) S. Qi, N. Kwon, Y. Yim, V. Nguyen and J. Yoon, Chem. Sci., 2020, 11, 6479 RSC
.
-
(a) N. Chen, W. Zhang, S. Chen, Q. Wu, C. Yu, Y. Wei, Y. Xu, E. Hao and L. Jiao, Org. Lett., 2017, 19, 2026 CrossRef CAS PubMed
; (b) Z. Wang, C. Cheng, Z. Kang, W. Miao, Q. Liu, H. Wang and E. Hao, J. Org. Chem., 2019, 84, 2732 CrossRef CAS PubMed
.
-
(a) W. Miao, E. Dai, W. Sheng, C. Yu, E. Hao, W. Liu, Y. Wei and L. Jiao, Org. Lett., 2017, 19, 6244 CrossRef CAS PubMed
; (b) S. Shimizu, Y. Ito, K. Oniwa, S. Hirokawa, Y. Miura, Q. Matsushita and N. Kobayashi, Chem. Commun., 2012, 48, 3851 RSC
.
-
(a) S. Mula, A. K. Ray, M. Banerjee, T. Chaudhuri, K. Dasgupta and S. Chattopadhyay, J. Org. Chem., 2008, 73, 2146 CrossRef CAS PubMed
; (b) X. Guo, B. Tang, H. Wu, Q. Wu, Z. Xie, C. Yu, E. Hao and L. Jiao, Mater. Chem. Front., 2021, 5, 3664 RSC
.
-
(a) X. Zhao, Q. Yao, S. Long, W. Chi, Y. Yang, D. Tan, X. Liu, H. Huang, W. Sun, J. Fan and X. Peng, J. Am. Chem. Soc., 2021, 143, 12345 CrossRef CAS PubMed
; (b) D. Liu, A. M. El-Zohry, M. Taddei, C. Matt, L. Bussotti, Z. Wang, J. Zhao, O. F. Mohammed, M. Di Donato and S. Weber, Angew. Chem., Int. Ed., 2020, 59, 11591 CrossRef CAS PubMed
; (c) M. Lv, Y. Yu, M. E. Sandoval-Salinas, J. Xu, Z. Lei, D. Casanova, Y. Yang and J. Chen, Angew. Chem., Int. Ed., 2020, 59, 22179 CrossRef CAS PubMed
.
- L. Gartzia-Rivero, E. M. Sánchez-Carnerero, J. Jiménez, J. Bañuelos, F. Moreno, B. L. Maroto, I. López-Arbeloa and S. de la Moya, Dalton Trans., 2017, 46, 11830 RSC
.
- Q. Gong, Q. Wu, X. Guo, W. Li, L. Wang, E. Hao and L. Jiao, Org. Lett., 2021, 23, 7220 CrossRef CAS PubMed
.
-
(a) J. Ma, X. Yuan, B. Küçüköz, S. Li, C. Zhang, P. Majumdar, A. Karatay, X. Li, H. Gul Yaglioglu, A. Elmali, J. Zhao and M. Hayvali, J. Mater. Chem. C, 2014, 2, 3900 RSC
; (b) S. Wu, F. Zhong, J. Zhao, S. Guo, W. Yang and T. Fyles, J. Phys. Chem. A, 2015, 119, 4787 CrossRef CAS PubMed
.
- E. V. Rumyantsev, S. N. Alyoshin and Y. S. Marfin, Inorg. Chim. Acta, 2013, 408, 181 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1915579, 1915582 and 1915583. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04893g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |