Open Access Article
Open Access ArticleSimultaneous detection of vesicular content and exocytotic release with two electrodes in and at a single cell†
Chaoyi
Gu
 and
Andrew G.
Ewing
and
Andrew G.
Ewing
 *
*
Department of Chemistry and Molecular Biology, University of Gothenburg, Kemivägen 10, 412 96 Gothenburg, Sweden. E-mail: andrewe@chem.gu.se
First published on 20th April 2021
Abstract
We developed a technique employing two electrodes to simultaneously and dynamically monitor vesicular neurotransmitter storage and vesicular transmitter release in and at the same cell. To do this, two electrochemical techniques, single-cell amperometry (SCA) and intracellular vesicle impact electrochemical cytometry (IVIEC), were applied using two nanotip electrodes. With one electrode being placed on top of a cell measuring exocytotic release and the other electrode being inserted into the cytoplasm measuring vesicular transmitter storage, upon chemical stimulation, exocytosis is triggered and the amount of release and storage can be quantified simultaneously and compared. By using this technique, we made direct comparison between exocytotic release and vesicular storage, and investigated the dynamic changes of vesicular transmitter content before, during, and after chemical stimulation of PC12 cells, a neuroendocrine cell line. While confirming that exocytosis is partial, we suggest that chemical stimulation either induces a replenishment of the releasable pool with a subpool of vesicles having higher amount of transmitter storage, or triggers the vesicles within the same subpool to load more transiently at approximately 10–20 s. Thus, a time scale for vesicle reloading is determined. The effect of L-3,4-dihydroxyphenylalanine (L-DOPA), the precursor to dopamine, on the dynamic alteration of vesicular storage upon chemical stimulation for exocytosis was also studied. We found that L-DOPA incubation reduces the observed changes of vesicular storage in regular PC12 cells, which might be due to an increased capacity of vesicular transmitter loading caused by L-DOPA. Our data provide another mechanism for plasticity after stimulation via quantitative and dynamic changes in the exocytotic machinery.
Introduction
Chemical communication, which is achieved through the process of exocytosis, offers the ability to transmit and exchange information within a network of neuronal cells and is fundamental, crucial and tightly regulated.1 The organelles that execute chemical communication are termed secretory vesicles, including small synaptic vesicles, inside which small-molecule transmitters are present, and large dense-core vesicles (LDCVs), which are packed with monoamines and neuropeptides.2,3 Prior to exocytosis, secretory vesicles are loaded with certain neurotransmitters and trafficked to the active zones on the cellular membrane, where exocytosis will take place. In response to an external stimulus, a transient fusion pore is generated by means of merging between the plasma membrane and the membrane of the transmitter-loaded vesicle, leading to the discharge of neurotransmitters to the extracellular space.4 Upon binding to receptors, the discharged transmitters can subsequently stimulate or depress another cell, enabling the propagation of chemical signals among neurons.Research attempting to understand exocytosis has been ongoing for decades, but the mechanism of exocytosis remains controversial. Traditionally, exocytotic release is thought to be a process of full release, or quantal release.5 During full release, the fusion pore expands continuously after its formation till that the vesicle merges completely into the plasma membrane, releasing all its stored content, including neurotransmitters and proteins. However, accumulating studies have emphasized the importance of another mode of exocytosis, called partial release, or “subquantal” release, during which the fusion pore closes after its expansion, allowing only part of the transmitter content to be discharged, and the vesicles are then directly recycled and reused.6–13 The fraction of release, calculated by the amount of transmitter release over the amount of transmitter storage, is not fixed and can be adjusted via drug treatment or repeated stimulation, suggesting more possible pathways for a cell to respond to external stimulus or stress brought by its surrounding environment.14–17 Since on average, a single vesicle does not release all its stored transmitters during partial release, defining the dynamic alteration of the averaged vesicular content before, during, and after stimulated exocytosis may improve our understanding considering the mechanism of exocytosis as well as pre- and post-exocytotic loading of transmitters into vesicles.
Neuroendocrine cells, such as pheochromocytoma (PC12) cells, have LDCVs which are enriched with catecholaminergic transmitters and are favoured by studies concerning exocytosis and vesicular loading. The PC12 cell, first isolated from a rat tumour by Greene and Tischler, is a well-established dopaminergic cell line which synthesizes and packs dopamine into its LDCVs.18,19 These cells possess the ability to undergo exocytotic release as well as induced differentiation.20 During partial release, regulation of exocytosis can be achieved not only by affecting the release process itself, but also via promoting or inhibiting vesicular loading to change vesicular storage before exocytosis occurs. Vesicular monoamine transporter (VMAT), a transport protein localized in the vesicular membrane, utilizes the proton gradient between the interior and exterior of the vesicle to pump monoamine transmitters from cytosol into the vesicle.21,22 For PC12 LDCVs, the presence of VMAT1, one isoform of VMAT, enables the loading of dopamine, and in small part norepinephrine.23 A commonly used drug to manipulate vesicular dopamine load is L-3,4-dihydroxyphenylalanine (L-DOPA), which is the precursor of dopamine.24,25
A variety of techniques are capable of visualizing or measuring exocytotic release, among which is single-cell amperometry (SCA).26 SCA offers ideal temporal resolution, down to sub-millisecond level, to resolve individual release events and meanwhile is the only approach that allows the quantification of the number of released molecules.27 In addition, SCA is a relatively simple method, especially when it comes to measuring the release of electroactive neurotransmitters, such as dopamine, norepinephrine and serotonin. In addition to quantifying release, it is also of great importance to measure the vesicular transmitter storage, as the storage is adjustable when considering partial release. A technique called IVIEC, intracellular vesicle impact electrochemical cytometry, was introduced in 2015 to quantify storage in single vesicles.8 An electrode with a nano-sized tip is fabricated and inserted directly into the cytosol of a cell through penetrating the plasma membrane. Vesicles in the cytosol adsorb and rupture on the electrode tip to expose the transmitters to the active surface of the electrode and thus, the number of transmitter storage can be counted. The combination of these two electrochemical techniques has already added a wealth of information to the understanding of chemical communication.8,10,13
In this paper, we developed a technique combining SCA and IVIEC with two electrodes, and utilized it to simultaneously measure exocytotic release and vesicular content from a single PC12 cell. We observed significant alterations in the average vesicular content at different time points after the cell was stimulated for exocytosis, and this effect was removed by pre-incubating the cell with L-DOPA. These results suggest a cellular change or stimulation-induced plasticity via the loading of transmitter into vesicles, and this plasticity is diminished with drug treatment.
Results and discussion
Studying transmitter release and storage at the same time with two electrodes
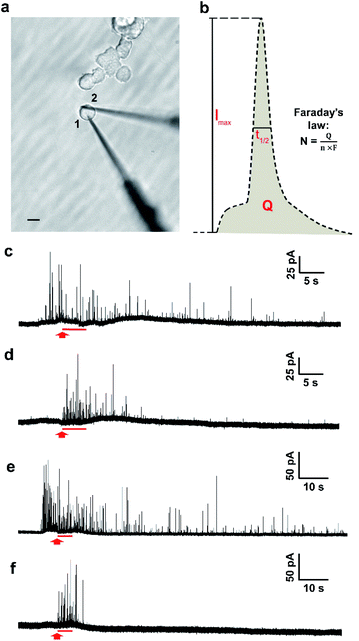
The two-electrode system is depicted in Fig. 1a, in which electrode 1 was employed to quantify transmitter storage with IVIEC and electrode 2 was utilized to perform SCA to measure release. Both electrodes were nanotip electrodes. Exocytosis is typically measured by placing a carbon-fibre disk microelectrode in close contact to the surface of a single cell.26 However, as the tip of a disk electrode (diameter > 5 μm) occupies a relatively large surface area when it is being put on a PC12 cell (diameter around 10–15 μm), it interferes with placement of the other electrode. Since the quantification of exocytotic release shows no significant difference between disk and nanotip electrodes,28 we chose to use nanotip electrodes to measure both release and storage.The measurement was started by inserting electrode 1 through the edge of a single non-treated PC12 cell into the cytosol of the cell. A train of amperometric spikes from IVIEC was evoked (Fig. 1c, before the red arrow) corresponding to numerous non-stimulated vesicles bursting on the nanotip electrode and their entire transmitter content being expelled and oxidized on the electrode surface. Secretion was triggered by a 5 s pressure injection of 100 mM K+ stimulation solution 5–10 s after the insertion of the first electrode and the cellular response was recorded by electrode 2. No obvious electrochemical signals were detected by the second electrode before applying the chemical stimulus (Fig. 1d), indicating that the effect caused on electrode 2 by the placement of electrode 1 was negligible. Upon the delivery of the stimulus, a cluster of amperometric current transients was observed, each resulting from the oxidation of exocytosed molecules during each release event. Meanwhile, the recording of IVIEC signals at electrode 1 continued during as well as after the 5 s period of chemical stimulation (Fig. 1c, after the red arrow). Another set of experiments was carried out in PC12 cells which were pre-incubated with 100 μM L-DOPA for 2 h to investigate the effect of increased vesicular loading on the stimulation-induced alteration of vesicular storage. Fig. 1e and f show examples of the amperometric traces obtained by IVIEC and SCA, respectively, from an L-DOPA-treated cell.
Quantitative analysis of exocytotic release and transmitter storage simultaneously detected at two electrodes
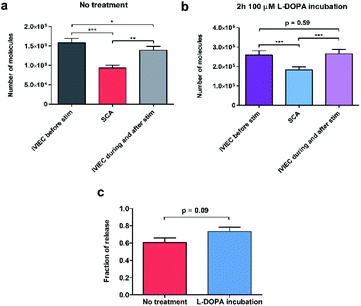
To calculate the number of molecules measured from individual SCA or IVIEC current spikes, Faraday's law, N = Q/nF, was applied. Q is the charge of the spike (Fig. 1b), n is the number of electrons transferred in the oxidation reaction (2 electrons for the oxidation of catecholamines), and F is the Faraday constant. Fig. 2a shows the number of catecholamine molecules counted for SCA measurements and IVIEC before, and during, and immediately after stimulated release from untreated PC12 cells. Fig. 2b shows the same for L-DOPA incubated cells. Here, we show that for both groups of cells, the number of molecules released, which was examined by SCA using the electrode 2 in Fig. 1a, is significantly lower than the transmitter storage in non-stimulated vesicles, which was quantified with IVIEC on the electrode 1. This is consistent with previous findings and the theory of partial release.6,8–10,29,30 When comparing the results between non-treated and L-DOPA pre-treated cells, it is interesting to find that the main difference between the two groups lies in the vesicular content quantified by IVIEC right after the exocytotic stimulation. For control PC12 cells, the stimulation induces a significant depletion in the transmitter storage (Fig. 2a). However, when incubated with L-DOPA, this depletion is abolished. As shown in Fig. 2b, no significant difference is observed between vesicular content quantified before and after the stimulation upon L-DOPA incubation. One possible reason is that L-DOPA incubation accelerates the process of dopamine reuptake by vesicles, whereas in control cells, this process is slower. Since normally only a fraction of vesicles undergo exocytosis, it might be that this fraction is different between control and L-DOPA treated cells.We subsequently divided the results from SCA over IVIEC before stimulation to calculate and compare the fraction of release between control and L-DOPA treated cells, and the data are plotted in Fig. 2c. The L-DOPA group has an average release fraction of 0.73 (±0.05), and the control group is 0.61 (±0.05). It appears that L-DOPA treatment increases the fraction of release by 0.12, which varies from a previous study showing that the two release fractions are nearly the same,8 and this might be explained by the different ways of calculation. Using two electrodes, a release fraction from a single cell can be calculated by dividing the amount of transmitter release (measured by one electrode) over the transmitter content (measured by the other electrode) of that cell, and the average release fraction from a cell population can thus be calculated. Whereas average release amount from a cell population divided by average transmitter storage from a similar cell population is used to obtain the release fraction when using one electrode. Therefore, it is understandable that this value might vary a little. Typically, release fraction is calculated using the transmitter content before stimulation. However, to provide greater insights into the alteration of release fraction, we also compared release fractions before stimulation, during, and after stimulation for both the control and L-DOPA treated groups (Fig. S1†). Release fraction from control cells increases upon stimulation, whereas it remains the same for the L-DOPA-treated cells.
Knowing that for control cells, the average release fraction is 0.61, it is possible to estimate the percentage of vesicles that being released. If all vesicles release a fraction of 0.61 of their content, the remaining transmitter storage should be a fraction of 0.39, which is an average of 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules per vesicle. Compared to the actual value of IVIEC measured during and after stimulation, we estimate that 21% of vesicles undergo exocytosis in control cells. For L-DOPA treated cells, on the other hand, the percentage is much lower. However, as the post-exocytotic process is complicated, this might involve vesicle movement and reloading (discussed in the following text), these estimations are preliminary.
000 molecules per vesicle. Compared to the actual value of IVIEC measured during and after stimulation, we estimate that 21% of vesicles undergo exocytosis in control cells. For L-DOPA treated cells, on the other hand, the percentage is much lower. However, as the post-exocytotic process is complicated, this might involve vesicle movement and reloading (discussed in the following text), these estimations are preliminary.
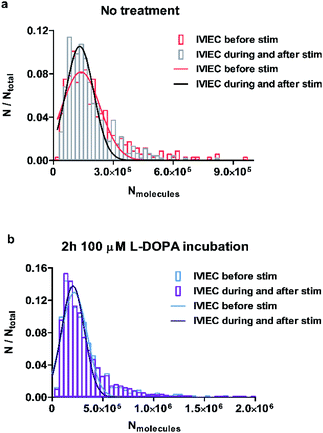
To examine whether the observations regarding the change of average vesicular storage is consistent for all the vesicles quantified, we pooled the number of molecules for all IVIEC events before stimulation, as well as during and after the stimulation, and the frequency distributions are plotted in Fig. 3a and b for control and L-DOPA treated groups, respectively. Upon chemical stimulation, the distribution of the vesicular content for the non-treated cells tends to be narrower and concentrates more within the range of less than 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules (Fig. 3a). This change of distribution demonstrates the depletion of vesicular storage within some vesicles, but not all vesicles release in response to a stimulus. However, if the cells are incubated with L-DOPA, the distribution of the vesicular content seems to be unaffected by the stimulation and the two distributions overlap completely (Fig. 3b). This is in agreement with the result regarding the unchanged average transmitter content observed for the L-DOPA group.
000 molecules (Fig. 3a). This change of distribution demonstrates the depletion of vesicular storage within some vesicles, but not all vesicles release in response to a stimulus. However, if the cells are incubated with L-DOPA, the distribution of the vesicular content seems to be unaffected by the stimulation and the two distributions overlap completely (Fig. 3b). This is in agreement with the result regarding the unchanged average transmitter content observed for the L-DOPA group.
To achieve a better understanding regarding how chemical stimulation affects the dynamic change of vesicular storage and how L-DOPA incubation eliminates the depletion of average transmitter storage that is observed in control cells, we dissected the post-stimulation IVIEC results into shorter time-intervals, including every 10 s and 5 s. When the IVIEC data were divided into a time interval of 10 s, no significant variations are observed for both groups of cells (Fig. S2†), though a small drop in the average vesicular storage beginning at 20 s after the stimulation is indicated for the control group (Fig. S2a†).
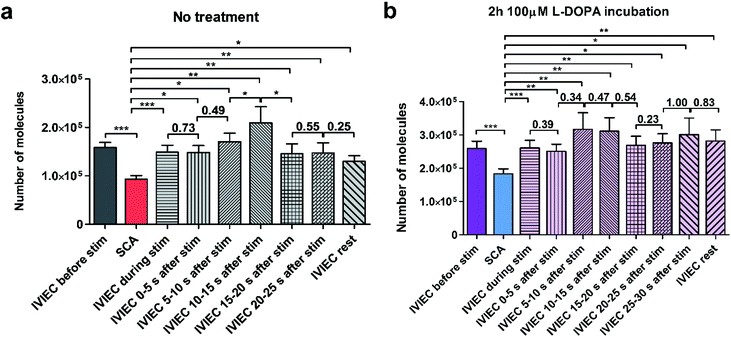
We further divided the IVIEC results and Fig. 4a demonstrates the number of molecules measured by IVIEC from non-treated PC12 cells analysed with a time-interval of 5 s. Vesicular content remains constant during the 5 s stimulation and 0–5 s after the stimulation, and increases, but not significantly, during 5–10 s time interval after the stimulation. Interestingly, 10–15 s after the cell has been stimulated, the average transmitter storage inside the vesicles increases significantly, followed by another significant decline during the 15–20 s interval. The Imax and t1/2 (illustrated in Fig. 1b) of the IVIEC events measured during 5–10 s, 10–15 s, and 15–20 s after stimulation were also examined and plotted as Fig. S3.†Imax reflects the maximum efflux of transmitters during IVIEC and shows the same trend as the number of molecules quantified, whereas t1/2 is the opposite.
The average amount of transmitter content detected during the time interval of 20–25 s is nearly the same as the previous 5 s and slightly higher than the average IVIEC measured afterwards. Therefore, an augmentation followed by a reduction is observed for the alteration of vesicular storage in response to a chemical stimulus in the control cells. However, for cells pre-treated with L-DOPA, the change of transmitter storage is not as obvious. As shown in Fig. 4b, there are also an increase in the average vesicular storage during 5–15 s after the stimulation and a decrease at 15–20 s, but both changes are insignificant. When comparing these post-stimulation IVIEC results to the average number of molecules quantified per vesicle before the stimulation, we found that for the non-treated cells, IVIEC data every 5 s after the stimulation are slightly lower than the average IVIEC value without stimulation, except for the values detected during 5–15 s period of post-stimulation. For L-DOPA incubated cells, all the IVIEC values measured, starting from 5 s after the stimulation, are slightly higher than the average IVIEC value before the stimulation. In addition, we also compared the IVIEC results measured during each 5 s time-interval to the exocytotic release quantified by SCA, shown in Fig. 4. For both treatment groups, IVIEC values after stimulation are significantly higher than the SCA value, which is consistent with the results in Fig. 2a and b.
Possible mechanisms regarding the dynamic alteration of transmitter storage induced by chemical stimulation
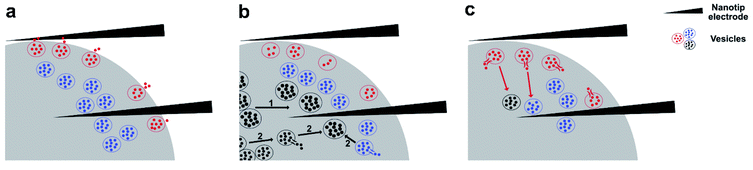
The total amount of neurotransmitters stored inside individual vesicles is clearly variable during cell function. PC12 cells treated with cocaine or methylphenidate, drugs that block the dopamine transporter, have vesicles with decreased amount of transmitter storage.15 In contrast, ATP, the cellular energy source, assists vesicular loading. The co-incubation of ATP and norepinephrine (NE) promotes the loading of NE into chromaffin vesicles, increasing storage of NE.31 However, pharmacologically induced alteration of vesicular storage is typically slow, occurring within a few hours. By repeated stimulation of PC12 cells to trigger exocytosis, it was found that within several minutes, the vesicular transmitter storage can be reduced by natural cell function.17In untreated PC12 cells, vesicular storage has no obvious change between the time during the stimulation, and 0–5 s right after the stimulation, suggesting that the vesicles being measured during this period of time come from the same population or the same subpool with their sizes and transmitter concentrations being analogous. It is understood that vesicles reside at different positions in the cytoplasm, including those that are docked closely on the membrane and ready to undergo exocytosis, as well as those that are undocked and reside away from the membrane. These are measured together on the surface of the nanotip electrode when being inserted inside a cell during IVIEC. This is depicted in a proposed mechanism in Fig. 5a. Therefore, after exocytosis is triggered, especially during the time period when exocytotic spikes are observed (IVIEC during stim and IVIEC 0–5 s after stim), a fraction of vesicles undergoing partial exocytosis and recapture might also burst on the electrode, leading to only part of the transmitter storage being detected. This might explain the slight decrease in vesicular transmitter storage right after applying the stimulation (Fig. 4a and b). However, this difference is insignificant, indicating that this effect is minor.
Three mechanisms might help to explain the increased amount of vesicular storage observed during 5–15 s of post-stimulation (Fig. 4a) in control PC12 cells. First is the reloading of exocytosed vesicles. Assuming this to be the main reason leading to the observed increase and knowing that only a fraction of vesicles normally undergo exocytosis, the average IVIEC measured during and after stimulation would be higher than, or at least at the same level as, the IVIEC measured before stimulation. However, as can be seen in Fig. 2a, this is not the case. Thus, reloading is not the main reason for this observation.
A second possibility is presented by several studies that have suggested the existence of multiple subpools of DCVs in neuroendocrine cells and neurons.32–34 Based on whether the vesicles are readily releasable or not, the DCVs in mouse chromaffin cells are divided into two subpools, one being readily releasable and the other one being slowly releasable.35 In bovine chromaffin cells, DCVs are located at different positions and can be grouped according to how long time they have been generated. Younger or freshly formed vesicles locate closely to the cellular membrane, forming the readily releasable pool. The older or more mature vesicles, on the other hand, reside further inside the cell.36 Furthermore, using electron microscopy and amperometry, two sizes of DCVs, small and large DCVs, were identified in differentiated PC12 cells and reported by Adams et al.37 According to this theory of the two subpools of DCVs, it seems reasonable that in non-treated PC12 cells, the increased transmitter storage at 5–15 s after the stimulation (Fig. 4a) is due to replenishing the releasable pool or the release sites to some extent. More mature or larger-sized vesicles might replace used smaller ones, with these replacement vesicles storing a higher number of neurotransmitters. In L-DOPA treated cells (Fig. 4b), an increase is observed, but not a significant increase, in the transmitter storage at 5–15 s after the stimulation. Pre-incubation of L-DOPA can promote vesicular loading of catecholamines in PC12 cells.38 This increased loading ability might reduce the heterogeneity existed between the younger and the older DCVs, particularly regarding the amount of transmitter storage and therefore, no significant increase in transmitter storage is shown at 5–15 s after the stimulation.
As the theory of the existence of two DCV-subpools in neuroendocrine cells is not generally accepted, we propose an alternative, third, mechanism where stimulation-induced transient vesicular loading of neurotransmitters takes place in a population of vesicles that is more homogeneous and all vesicles store similar amount of neurotransmitters inside. In response to a chemical stimulus, vesicles remaining in the releasable pool might be transiently filled with more transmitters. Alternatively, vesicles in the reserve pool might be transiently loaded with more transmitters and translocated to replenish the releasable pool. Thereby, the increase of vesicular storage quantified at 5–15 s after the stimulation in non-treated PC12 cells (Fig. 4a) might come from bursting of these transiently loaded vesicles on the electrode. In L-DOPA treated cells, as the vesicular loading is already enhanced to a relatively high level, the effect of transient loading is not as significant (Fig. 4b). It should be noted that in the case of PC12 cells, replenishing the releasable pool to a great extent after stimulated exocytosis is unlikely to happen within a short time span. By depolarizing a PC12 cell repetitively for six times, with the duration of each depolarization being 2 min and the recovery periods being 5 min, it was suggested that the depletion of vesicles in the releasable pool caused by depolarization is long-term, and the replenishment and recovery of the releasable pool does not occur rapidly.39 Moreover, a PC12 cell that is repetitively stimulated six times, with the duration of each stimulation being 5 s and the time-interval being 2 min, was also shown to possess a continuous decrease in the number of exocytotic events, starting from the second stimulation.17 This, again, suggests that the full recovery of the releasable pool of vesicles in PC12 cells might happen slowly, even when the duration of a stimulus is relatively short (5 s). Both mechanisms, including the existence of a subpool of more mature vesicles and the stimulation-induced transient vesicular loading, might function to compensate the loss of number of vesicles in the releasable pool and the illustration of these two mechanisms is demonstrated in Fig. 5b.
After the increase of transmitter storage from 5 to 15 s detected by IVIEC in control PC12 cells, a significant reduction is observed from 15–20 s and the rest of the IVIEC result is either similar or lower than that (Fig. 4a). Previous research in bovine chromaffin cells has indicated the possible coupling of rapid endocytosis to secretion.40 Using capacitance measurements, the process of rapid endocytosis is revealed to be completed with a maximum of 20 s after exocytosis of chromaffin vesicles.41 PC12 cells being differentiated by glucocorticoid have also been shown to have a rapid endocytosis of less than 20 s.42 However, as the secretory capability and secretory cycle of vesicles in glucocorticoid-differentiated PC12 cells are promoted, the endocytic process in regular undifferentiated PC12 cells would be expected to be longer. Therefore, we speculate that the significant decrease of vesicular storage observed from 15 s after chemical stimulation results from the endocytosed vesicles, which have previously released a part of their transmitter storage during the stimulation. These vesicles might be refilled, either partly or fully, with transmitters and trafficked back to the releasable pool or to the reserved pool, during which some of them hit on the electrode surface and are quantified, as illustrated in Fig. 5c. As for the L-DOPA incubated cells, on the other hand, no significant decrease in the vesicular storage is observed from 15 s after the stimulation to the end of the amperometric recording (Fig. 4b), which can be explained by the increased loading ability incurred by L-DOPA incubation and thus, the endocytosed vesicles are refilled to a higher degree than the control vesicles.
Although the destiny of the rapidly endocytosed vesicles in neuroendocrine cells is not certain, based on previous observations regarding the depletion of vesicles in the releasable pool by repetitive stimulation,17,39 it is quite possible that a large fraction of these endocytosed vesicles are not involved again in the exocytotic machinery within a short period of time.
Conclusions
We have combined simultaneous measurements of exocytotic release and vesicular neurotransmitter storage with two electrodes one at and one in a single cell. This has allowed us to compare exocytotic release and vesicular storage at the same cell in real time. By doing this, we have verified that the average exocytotic release is significantly lower than both the average vesicular storage before, and during and after chemical stimulation is applied to trigger exocytosis, demonstrating partial release as the dominant mode of exocytosis. Increased vesicular loading of dopamine by L-DOPA incubation increases the release fraction by 0.12. By comparing the average vesicular storage before the stimulation to the one after the stimulation, we have discovered that storage significantly decreases but this decrease is not uniform among the vesicles inside the cell. The real-time nature of the combined measurement allowed us to analyse the IVIEC data at specific time intervals after stimulation and compare to SCA data. Here, we discovered significant enhancement in vesicular storage from 10 to 20 s after the stimulation followed by a significant reduction. This short-time cell-activity-driven increase in storage, a parameter usually taking hours to manipulate pharmacologically, might be from transient loading of transmitters into vesicles within the same subpool as the released vesicles or from another subpool. It might also be due to replenishing the releasable pool with more mature vesicles, which store larger amount of transmitters inside. The subsequent reduction we speculate to be the rapidly endocytosed vesicles which have previously undergone exocytosis and have not been completely reloaded with transmitters. Upon L-DOPA incubation, however, all significant changes are diminished which suggests that enhanced vesicular loading may eliminate the difference among the vesicles in the two subpools or that it induces a less prominent transient-loading effect, and meanwhile promotes the process of post-exocytotic reloading of neurotransmitters. This is important as it creates a basis for a complex hierarchy of events for plasticity in exocytosis.The two-electrode approach enables a direct and dynamic comparison between exocytosis and vesicular transmitter storage to study the effect of cellular-driven activity on these processes in real-time. The dynamic and quantitative aspects of exocytosis and vesicular loading are important in understanding the adaptation of cells to their environment, stimuli, and rapid plasticity.
Author contributions
All authors have approved the final version of the manuscript. C. G. and A. E. conceived the idea. C. G. performed the experiments, analysed and interpreted the data, and wrote and edited the manuscript. A. E. was involved in funding acquisition, supervising, discussions of data interpretation, and outlining and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The European Research Council (ERC Advanced Grant Project No. 787534 NanoBioNext), Knut and Alice Wallenberg Foundation, and the Swedish Research Council (VR Grant No. 2017-04366) are acknowledged for financial support.Notes and references
- R. D. Burgoyne and A. Morgan, Physiol. Rev., 2003, 83, 581–632 CrossRef CAS PubMed.
- R. B. Kelly, Cell, 1993, 72(suppl), 43–53 CrossRef PubMed.
- Y. Park and K. T. Kim, Cell. Signalling, 2009, 21, 1465–1470 CrossRef CAS.
- J. E. Heuser, T. S. Reese, M. J. Dennis, Y. Jan, L. Jan and L. Evans, J. Cell Biol., 1979, 81, 275–300 CrossRef CAS PubMed.
- J. Del Castillo and B. Katz, J. Physiol., 1954, 124, 560–573 CrossRef CAS PubMed.
- D. M. Omiatek, Y. Dong, M. L. Heien and A. G. Ewing, ACS Chem. Neurosci., 2010, 1, 234–245 CrossRef CAS PubMed.
- C. Amatore, A. I. Oleinick and I. Svir, ChemPhysChem, 2010, 11, 159–174 CrossRef CAS PubMed.
- X. Li, S. Majdi, J. Dunevall, H. Fathali and A. G. Ewing, Angew. Chem., Int. Ed., 2015, 54, 11978–11982 CrossRef CAS PubMed.
- L. Ren, L. J. Mellander, J. Keighron, A. S. Cans, M. E. Kurczy, I. Svir, A. Oleinick, C. Amatore and A. G. Ewing, Q. Rev. Biophys., 2016, 49, e12 CrossRef PubMed.
- N. T. N. Phan, X. Li and A. G. Ewing, Nat. Rev. Chem., 2017, 1, 0048 CrossRef CAS.
- W. Shin, L. Ge, G. Arpino, S. A. Villarreal, E. Hamid, H. Liu, W. D. Zhao, P. J. Wen, H. C. Chiang and L. G. Wu, Cell, 2018, 173, 934–945 e912 CrossRef CAS PubMed.
- Q. H. Wu, Q. F. Zhang, B. Liu, Y. L. Li, X. Wu, S. T. Kuo, L. H. Zheng, C. H. Wang, F. P. Zhu and Z. Zhou, J. Neurosci., 2019, 39, 199–211 CrossRef CAS PubMed.
- A. Larsson, S. Majdi, A. Oleinick, I. Svir, J. Dunevall, C. Amatore and A. G. Ewing, Angew. Chem., Int. Ed. Engl., 2020, 59, 6711–6714 CrossRef CAS PubMed.
- L. Ren, M. D. Pour, P. Malmberg and A. G. Ewing, Chem.–Eur. J., 2019, 25, 5406–5411 CrossRef CAS PubMed.
- W. Zhu, C. Gu, J. Dunevall, L. Ren, X. Zhou and A. G. Ewing, Angew. Chem., Int. Ed., 2019, 58, 4238–4242 CrossRef CAS PubMed.
- S. Majdi, A. Larsson, N. Najafinobar, R. Borges and A. G. Ewing, ACS Chem. Neurosci., 2019, 10, 2459–2466 CrossRef CAS PubMed.
- C. Y. Gu, A. Larsson and A. G. Ewing, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 21409–21415 CrossRef CAS PubMed.
- L. A. Greene and A. S. Tischler, Proc. Natl. Acad. Sci. U. S. A., 1976, 73, 2424–2428 CrossRef CAS PubMed.
- L. A. Greene and G. Rein, Brain Res., 1977, 129, 247–263 CrossRef CAS PubMed.
- R. H. Westerink and A. G. Ewing, Acta Physiol., 2008, 192, 273–285 CrossRef CAS PubMed.
- H. Varoqui and J. D. Erickson, Mol. Neurobiol., 1997, 15, 165–191 CrossRef CAS PubMed.
- K. Wimalasena, Med. Res. Rev., 2011, 31, 483–519 CrossRef CAS PubMed.
- Y. Liu, E. S. Schweitzer, M. J. Nirenberg, V. M. Pickel, C. J. Evans and R. H. Edwards, J. Cell Biol., 1994, 127, 1419–1433 CrossRef CAS PubMed.
- E. Pothos, M. Desmond and D. Sulzer, J. Neurochem., 1996, 66, 629–636 CrossRef CAS PubMed.
- T. L. Colliver, S. J. Pyott, M. Achalabun and A. G. Ewing, J. Neurosci., 2000, 20, 5276–5282 CrossRef CAS PubMed.
- D. J. Leszczyszyn, J. A. Jankowski, O. H. Viveros, E. J. Diliberto, J. A. Near and R. M. Wightman, J. Biol. Chem., 1990, 265, 14736–14737 CrossRef CAS.
- R. M. Wightman, J. A. Jankowski, R. T. Kennedy, K. T. Kawagoe, T. J. Schroeder, D. J. Leszczyszyn, J. A. Near, E. J. Diliberto Jr and O. H. Viveros, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10754–10758 CrossRef CAS PubMed.
- C. Gu, X. Zhang and A. G. Ewing, Anal. Chem., 2020, 92, 10268–10273 CrossRef CAS PubMed.
- X. C. Li, J. Dunevall and A. G. Ewing, Acc. Chem. Res., 2016, 49, 2347–2354 CrossRef CAS PubMed.
- E. M. Ranjbari and A. G. Ewing, Trends Chem., 2019, 1, 440–451 CrossRef CAS.
- A. Larsson, S. Majdi, R. Borges and A. Ewing, ACS Chem. Neurosci., 2019, 10, 4735–4740 CrossRef CAS PubMed.
- D. Bruns, D. Riedel, J. Klingauf and R. Jahn, Neuron, 2000, 28, 205–220 CrossRef CAS PubMed.
- L. M. Koval, E. N. Yavorskaya and E. A. Lukyanetz, Gen. Comp. Endocrinol., 2001, 121, 261–277 CrossRef CAS PubMed.
- C. P. Grabner, S. D. Price, A. Lysakowski and A. P. Fox, J. Neurophysiol., 2005, 94, 2093–2104 CrossRef PubMed.
- T. Voets, T. Moser, P. E. Lund, R. H. Chow, M. Geppert, T. C. Sudhof and E. Neher, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11680–11685 CrossRef CAS PubMed.
- R. R. Duncan, J. Greaves, U. K. Wiegand, I. Matskovich, G. Bodammer, D. K. Apps, M. J. Shipston and R. H. Chow, Nature, 2003, 422, 176–180 CrossRef CAS PubMed.
- R. D. Adams and A. B. Harkins, Biophys. J., 2014, 107, 2838–2849 CrossRef CAS PubMed.
- K. D. Kozminski, D. A. Gutman, V. Davila, D. Sulzer and A. G. Ewing, Anal. Chem., 1998, 70, 3123–3130 CrossRef CAS PubMed.
- R. H. Westerink, A. de Groot and H. P. Vijverberg, Biochem. Biophys. Res. Commun., 2000, 270, 625–630 CrossRef CAS PubMed.
- H. von Grafenstein and D. E. Knight, J. Physiol., 1992, 453, 15–31 CrossRef CAS PubMed.
- C. R. Artalejo, J. R. Henley, M. A. McNiven and H. C. Palfrey, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 8328–8332 CrossRef CAS PubMed.
- A. Elhamdani, M. E. Brown, C. R. Artalejo and H. C. Palfrey, J. Neurosci., 2000, 20, 2495–2503 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals and solutions, fabrication of nanotip electrodes, cell culture, two-electrode experiments, data processing and statistics, Fig. S1, Fig. S2, Fig. S3. See DOI: 10.1039/d1sc01190a |
| This journal is © The Royal Society of Chemistry 2021 |