Open Access Article
Open Access ArticlePreparation and reactions of polyfunctional magnesium and zinc organometallics in organic synthesis†
Alexander
Kremsmair
,
Johannes H.
Harenberg
,
Kuno
Schwärzer
,
Andreas
Hess
and
Paul
Knochel
 *
*
Department of Chemistry, Ludwig-Maximilans-Universität München, Butenandtstraße 5-13, 81377 München, Germany. E-mail: paul.knochel@cup.uni-muenchen.de
First published on 16th March 2021
Abstract
Polyfunctional organometallics of magnesium and zinc are readily prepared from organic halides via a direct metal insertion in the presence of LiCl or a Br/Mg-exchange using iPrMgCl·LiCl (turbo-Grignard) or related reagents. Alternatively, such functionalized organometallics are prepared by metalations with TMP-bases (TMP = 2,2,6,6-tetramethylpiperidyl). The scope of these methods is described as well as applications in new Co- or Fe-catalyzed cross-couplings or aminations. It is shown that the use of a continous flow set-up considerably expands the field of applications of these methods and further allows the preparation of highly reactive organosodium reagents.
1. Introduction
The compatibility of functional groups with a carbon–metal bond in an organometallic reagent is essential for broad synthetic applications in modern organic synthesis. In this perspective article, we will show that Mg and Zn organometallics are unique for combining an excellent functional group tolerance with a high reactivity toward various classes of electrophiles. Furthermore, magnesium and zinc derivatives are non-toxic and the moderate price of these two elements makes them ideal candidates for industrial applications. Although, some innovative chemistry of lithium, sodium and potassium will be presented considering some remarkable properties of these species, the main part of this article will concern the preparation and reactivity of polyfunctional Mg and Zn reagents. The behaviour of aryl- and heteroaryl organometallics will be especially emphasized because of their importance in material, agrochemical and pharmaceutical research. We will also show that continuous flow set-ups involving such organometallic reactions further expands the application scope, especially by allowing some new Barbier-type procedures.2. Preparation of polyfunctional Zn and Mg organometallics
2.1 Direct reaction of magnesium or zinc with organic halides
The carbon–zinc bond is a covalent carbon–metal bond with moderate intrinsic reactivity. Metallic zinc is a weaker reducing agent compared to magnesium, and therefore a mixed metal synthesis using magnesium dust in the presence of LiCl and ZnCl2 is an advantageous procedure for the preparation of aryl and heteroaryl zinc reagents bearing sensitive functional groups.1 Under these conditions, methyl 4-bromobenzoate (1) underwent a smooth conversion to the corresponding zinc reagent within 3 h at 25 °C (see Scheme 1).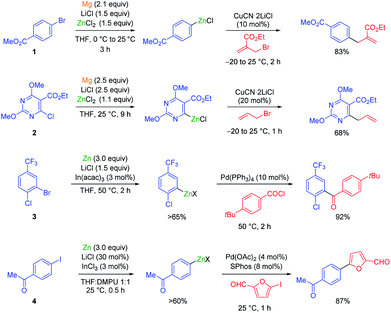 | ||
| Scheme 1 Magnesium and zinc insertions to functionalized (hetero)aryl halides mediated by LiCl and indium salts. | ||
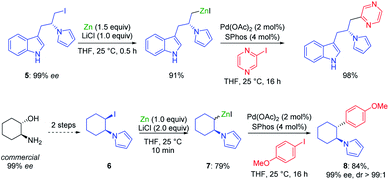
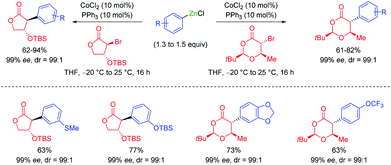
The addition of LiCl was essential for removing the organometallic species on the metal surface by forming a mixed magnesium–lithium complex of the type RMgX·LiCl.2 Fast magnesium insertion rates were observed with electron-deficient substrates like electron-poor aromatics or heterocyclic chlorides such as 2.1 The direct zinc insertion may require the addition of a Lewis-acid catalyst whose role is to facilitate electron transfer steps from the metal surface to the organic halide. Thus, in the presence of In(acac)3 (3 mol%), 2-bromo-1-chloro-4-(trifluoromethyl)-benzene (3) was converted to the corresponding zinc reagent at 50 °C within 2 h.3 The use of a polar co-solvent such as DMPU proved to be helpful.4 Under these conditions, a sensitive functional group like an acetyl group which is prone to enolization, like in 4-iodoacetophenone (4), was perfectly tolerated. The mild conditions required for these insertion reactions are also compatible with the presence of acidic NH-groups.5 Thus, the iodo-indole derivative 5 was converted to the corresponding zinc reagent at 25 °C.6 In the presence of a palladium catalyst (2 mol% Pd(OAc)2; 4 mol% SPhos), a Negishi cross-coupling with an N-heterocyclic iodide readily took place at 25 °C. Secondary alkyl iodides usually react faster in these direct insertion reactions. Thus, cis-iodo-pyrrole 6, which was readily available from trans-2-aminocyclohexanol, underwent a zinc insertion within 10 min at 25 °C leading to a cis, trans-mixture of zinc reagent 7. This loss of stereochemistry is characteristic for Mg or Zn insertions which proceed through radical intermediates. However, in the presence of a palladium catalyst, a diastereoselective cross-coupling7 took place leading exclusively to the trans-cyclohexane derivative 8 (84%; 99% ee; dr >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; see Scheme 2).6
1; see Scheme 2).6
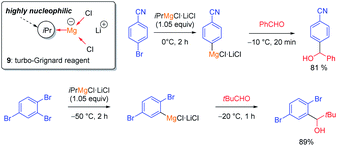
2.2 The halogen/metal exchange
The halogen/lithium exchange (Hal = I, Br) is a fast reaction, independently discovered in 1939 by Gilman and Wittig.8 In comparison, the halogen/magnesium-exchange is a much slower reaction, which had only found applications in the preparation of some heterocyclic Grignard reagents9 and magnesium carbenoids.10 However, by using organomagnesium halides complexed by LiCl such as iPrMgCl·LiCl (9, turbo-Grignard reagent) fast I/Mg- and Br/Mg-exchanges took place producing functionalized aryl and heteroaryl magnesium reagents under mild conditions (see Scheme 3).11The kinetics of the Br/Mg-exchange12 as well as the mechanism of the reaction have been well studied.13 It was postulated that the rate of a halogen/metal exchange depends on the ionic character of the carbon–metal bond: the more electro-positive the metal is, the faster the halogen/metal exchange takes place. This hypothesis led to the discovery of halogen/lanthanide exchange reactions.14 The replacement of LiCl in the turbo-Grignard reagent (9) with lithium alkoxides (LiOR) led to even more powerful exchange reagents (sBuMgOR1·LiOR1 and sBu2Mg·2LiOR1; R1 = 2-ethylhexyl) soluble in toluene. These reagents allowed the performance of some Cl/Mg-exchanges15 as well as regioselective exchanges on various dibromopyridines such as 10.16 Furthermore, the use of the corresponding zinc reagents (sBu2Zn·2LiOR2 or ptol2Zn·2LiOR211) allowed the performance of an I/Zn-exchange on functionalized aryl iodides in toluene (see Scheme 4).17
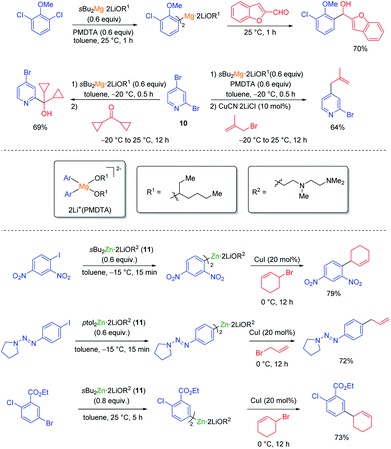 | ||
| Scheme 4 Halogen/magnesium and zinc exchanges using the exchange reagents sBu2Mg·2LiOR1, sBu2Zn·2LiOR2 or pTol2Zn·2LiOR2 (11). | ||
2.3 Directed magnesiation and zincation with TMP-bases complexed with LiCl
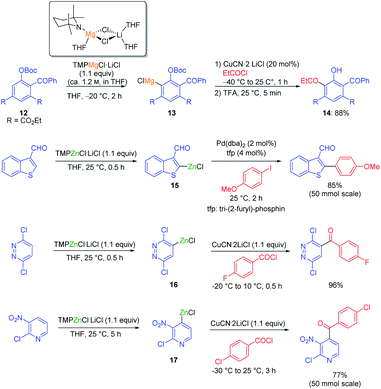
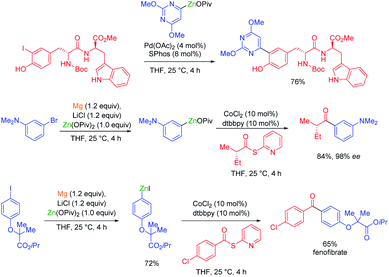
In general, magnesium amides (R2NMgX or (R2N)2Mg) are poorly soluble in THF and display moderate kinetic basicity.18 However, by using a sterically hindered amine (2,2,6,6-tetramethylpiperidine, TMP-H), it was possible to prepare a series of metallic amides complexed with LiCl (TMPMgCl·LiCl, TMP2Mg·2LiCl, TMPZnCl·LiCl and TMP2Zn·2LiCl) with high solubility in THF (1.2–1.4 M) and exceptional kinetic basicity.19 The preparation of polyfunctional magnesium reagents became now possible from halide-free precursors. Thus, the highly functionalized arene 12 was magnesiated with TMPMgCl·LiCl at −20 °C leading to an arylmagnesium species 13 bearing several sensitive functional groups (OBoc, CO2Et, COPh).20 A copper-mediated acylation afforded the penta-substituted arene 14 in 88% yield. By using TMPZnCl·LiCl, aryl and heteroaryl zinc organometallics 15–17 were produced.21 Since a carbon–zinc bond is much more covalent than a carbon–magnesium bond, the inherent reactivity of the carbon–zinc bond is much lower and therefore it becomes possible to prepare highly functionalized organozinc derivatives. Due to the presence of low lying p-orbitals at the zinc centre, various transmetalations with transition metal salts proceeded readily, providing transition metal intermediates which underwent new reaction pathways not possible for main-group organometallics (oxidative addition, reductive elimination, insertion reaction). This behaviour allowed an efficient reaction with numerous electrophiles (see Scheme 5).In contrast to TMPMgCl·LiCl, TMPZnCl·LiCl is less prone to undergo kinetic metalations and thermodynamic considerations are relevant for predicting the zincation regioselectivity. Thus, the site of metalation can be readily determined by calculation of the pKa-values of various unsaturated substrates. The zincation of new heterocyclic systems such as 18–20 were predicted by this model and subsequent functionalizations were performed successfully (see Scheme 6).22 In general, TMPMgCl·LiCl and TMPZnCl·LiCl are valuable reagents for the metalation of various heterocycles.23 Remarkably, the compatibility of these bases with various Lewis acids including BF3·OEt2 has also been observed.24
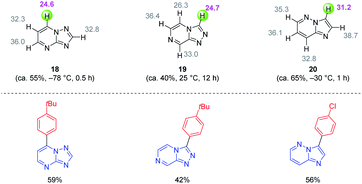 | ||
| Scheme 6 Calculation of the pKa-values of condensed N-heterocycles to predict their reactivity with TMPZnCl·LiCl and subsequent quenching with electrophiles. | ||
For example, this possibility of forming frustrated Lewis pairs has been exploited for the regioselective functionalization of uridines such as 21. By using TMPMgCl·LiCl in THF a complexation occurred at the heterocyclic amide function directing the magnesiation at the adjacent position leading to products of type 22a–b. On the other hand, using TMP2Zn·2LiCl in the presence of MgCl2 similarly led to a complexation of MgCl2 at the amide function and hampered the approach of the zinc base which eventually deprotonated at position 6 leading to products of type 22c–d (see Scheme 7).25 The performance of kinetically controlled metalations (usually triggered by a pre-complexation of the base to a Lewis-basic centre of the substrate)26 is often amplified by the use of a low polarity solvent such as toluene. Thus, designing a new toluene soluble base (BuMgTMP) allowed a regioselective kinetic metalation of various aryl azoles at the ortho-position of the aryl ring resulting in products of great interest for pharmaceutical research (Scheme 7).27
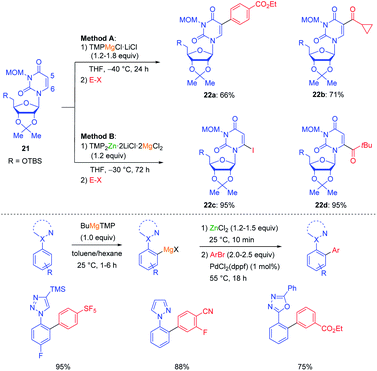 | ||
| Scheme 7 Regioselective magnesiations and zincations of uridines with TMP-bases and magnesiations of aryl azoles in toluene with BuMgTMP. | ||
3. Reactions of polyfunctional organozinc reagents
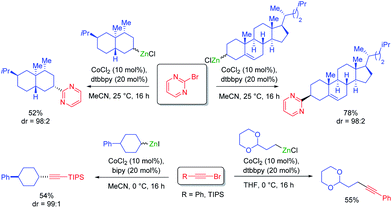
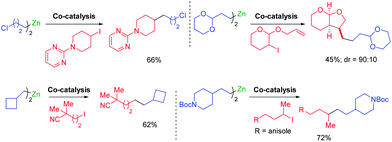
Cobalt salts are ca. 1000 times cheaper than palladium salts and many cobalt-catalyzed cross-couplings require only inexpensive N-ligands such as 2,2′bipyridine (bipy) or 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbbpy). Thus, 2-bromopyrimidine underwent diastereoselective cross-couplings with secondary alkylzinc reagents furnishing products in high diastereomeric ratio (see Scheme 8). These cross-couplings were also extended to 1-bromoalkynes.28Furthermore, a range of cobalt catalyzed cross-couplings29 proceeded well with functionalized organozinc and organomagnesium reagents.30 For example, highly diastereoselective cross-couplings were achieved with α-bromo lactones (see Scheme 9).31
Novel Csp3–Csp3 cross-couplings catalyzed by CoCl2 (20 mol%) in the presence of trans-1,2-bis-dimethylaminocyclohexane or neocuproine (20 mol%) allowed the preparation of polyfunctional products. Furthermore, ring closures and openings indicated the occurance of radical intermediates in the course of such cross-couplings (see Scheme 10).32
3.1 Preparation of organozinc pivalates with enhanced air and water stability
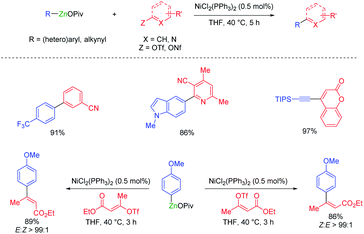
Organozinc pivalates are mixed zinc–magnesium organometallics bearing a carbon–zinc bond and magnesium or zinc pivalate (OCOtBu) units.33 These organometallic reagents are readily prepared by various methods and produce, after solvent evaporation, solid organozinc species with enhanced air and water stability.34 Polyfunctional organozinc pivalates underwent Pd-catalyzed cross-couplings with peptidic aryl halides bearing various acidic protons.35 They proved also very advantageous for performing other transition metal catalyzed cross-couplings. Thus, a cobalt-catalyzed acylation of thiopyridyl esters allowed the preparation of α-chiral ketones as well as a short synthesis of the pharmaceutical fenofibrate (see Scheme 11).36Organozinc pivalates often undergo challenging cross-couplings better than analogous organozinc halides. Therefore, the nickel-catalyzed cross-couplings of various aryl, heteroaryl and alkenyl triflates or nonaflates with aryl- and heteroarylzinc pivalates were achieved in good yields and with high stereoretention (see Scheme 12).37
3.2 Transition-metal catalyzed electrophilic aminations using organozinc reagents
Organozinc pivalates38 and halides39 are also useful for the performance of cobalt-catalyzed electrophilic aminations. Drugs like paroxetine 24 or sertraline 25 were functionalized by this method and converted into the new valuable derivatives 26 and 27 (see Scheme 13).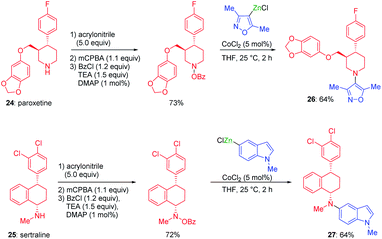 | ||
| Scheme 13 Cobalt-catalyzed electrophilic aminations for the functionalization of secondary amine pharmaceuticals. | ||
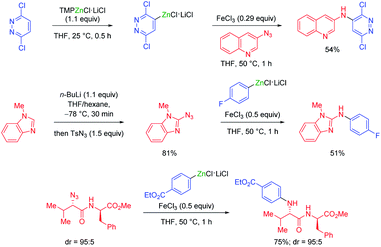
Furthermore, organozinc chlorides were converted to secondary amines by iron(III) chloride mediated reactions with various organic azides.40 For this amination procedure both the organozinc reagent and the aryl azide41 could be generated in situ. In addition, chiral azides with peptidic structures led to the corresponding arylated, chiral secondary amines (see Scheme 14).40
4. Lewis pairs involving organozinc and organomagnesium reagents; new Barbier-reactions
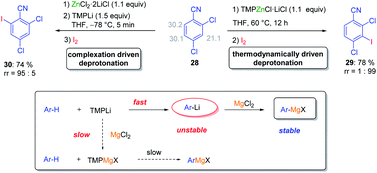
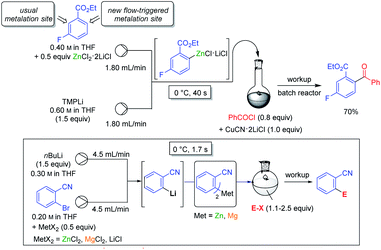
Various magnesium and zinc organometallics are compatible with strong Lewis acids such as BF3·OEt2 and this behaviour has already been exploited for performing selective metalations.24 The field of Barbier reactions remained largely unexplored although remarkable selectivities were achieved.42 A recent example concerned the regioselective metalation of 2,4-dichlorobenzonitrile 28 (see Scheme 15).43In accordance with the pKa-values of the ring hydrogens, the most acidic 3-position of benzonitrile derivative 28 was readily zincated by TMPZnCl·LiCl. As indicated above, this base is especially prone to undergo thermodynamically driven metalations.22 After 12 h at 60 °C and subsequent iodolysis, nitrile 29 was obtained as the only regioisomer. However, with the strong lithium base TMPLi, a complexation driven deprotonation was triggered by coordination of this base to the cyano group inducing an ortho-deprotonation. Performing this lithiation only with TMPLi led to extensive decomposition due to the high reactivity of the resulting aryllithium species. However, mixing 29 with the THF-soluble salt ZnCl2·2LiCl and adding TMPLi at −78 °C led to a fast kinetic deprotonation followed by a transmetalation with the zinc(II)-salt, providing a stable arylzinc reagent which after iodolysis produced regioselectively the iodonitrile 30. This behaviour proved to be quite general and MgCl2 or CuCN·2LiCl allowed similar reactions. However, the scale-up of these reactions proved to be difficult. This problem was solved by performing these metalations in continuous flow using micro-reactors (see Scheme 16).44
It was possible to perform a short-cut in such Barbier-reactions by mixing the electrophile (E–X) directly with the substrate bearing an acidic proton avoiding the need of transmetalations. Thus, the treatment of mixtures of various formamides (HCONR1R2) and electrophiles such as ketones, aldehydes, allylic bromides, disulfides, morpholino- and Weinreb amides in continuous flow provided a very convenient and readily scalable synthesis of functionalized amides (see Scheme 17).45
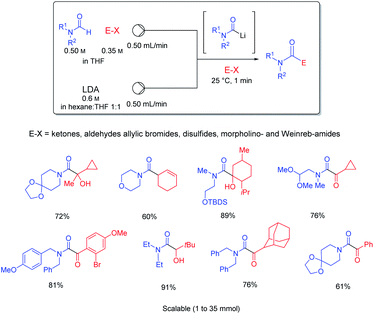 | ||
| Scheme 17 Barbier reactions in continuous flow involving an in situ generation of carbamoyllithium derivatives. | ||
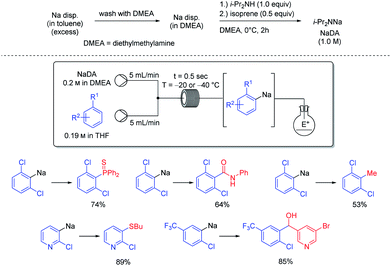
Organosodium chemistry has been mostly of academic interest, despite the low costs and toxicity of metallic sodium as well as its easy handling and recycling.46 The use of a continuous flow set-up allowed the sodiation of various unsaturated heterocyclic and aromatic substrates (see Scheme 18).47
5. Preparative aspects
All reactions were performed using standard Schlenk-line techniques under argon atmosphere.48 TMPMgCl·LiCl and iPrMgCl·LiCl are commercially available (Sigma-Aldrich, Acros Organics, Albemarle, etc.) and were as all other organometallic reagents used after titration.49 No glove-box or special apparatus were required. Only dry solvents and analytically pure starting materials were used.6. Conclusions
Broadly applicable preparations of functionalized Zn- and Mg-organometallics are now available and described in this perspective article. A range of transition metal catalyzed reactions including Negishi cross-couplings and acylation reactions allow a straightforward functionalization of these polyfunctional organometallics.50 The use of continuous flow further broadens the scope of these organometallics. Also, the performance of new Barbier-type reactions opens up new synthetic applications. Thus, the chemistry of these functionalized main-group organometallics should pave the way for further discoveries and give an even more prominent place in organic synthesis to these ecologically friendly metals.Author contributions
A. K. developed and assembled the section on the preparation of polyfunctional organometallics and reactions of polyfunctional organozinc reagents. J. H. H. developed and assembled the section on continuous flow set-ups. K. S. developed and assembled the section on reactions of polyfunctional organozinc reagents. A. H. developed and assembled the section on directed magnesiations and zincations. A. K., J. H. H., K. S., A. H. and P. K. wrote and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) and the Ludwig-Maximilians-Universität München for financial support.References
- A. Krasovskiy, V. Malakhov, A. Gavryushin and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 6040–6044 CrossRef CAS PubMed; F. M. Piller, A. Metzger, M. A. Schade, B. A. Haag, A. Gavryushin and P. Knochel, Chem.–Eur. J., 2009, 15, 7192–7202 CrossRef.
- C. Feng, D. W. Cunningham, Q. T. Easter and S. A. Blum, J. Am. Chem. Soc., 2016, 138, 11156–11159 CrossRef CAS.
- A. D. Benischke, G. Le Corre and P. Knochel, Chem.–Eur. J., 2017, 23, 778–782 CrossRef CAS PubMed.
- T. Mukhopadhyay and D. Seebach, Helv. Chim. Acta, 1982, 65, 385–391 CrossRef CAS.
- G. Manolikakes, M. A. Schade, C. M. Hernandez, H. Mayr and P. Knochel, Org. Lett., 2008, 10, 2765–2768 CrossRef CAS PubMed; G. Manolikakes, C. Muñoz Hernandez, M. A. Schade, A. Metzger and P. Knochel, J. Org. Chem., 2008, 73, 8422–8436 CrossRef PubMed; Z. Dong, G. Manolikakes, J. Li and P. Knochel, Synthesis, 2009, 2009, 681–686 CrossRef; G. Manolikakes, M. S. Z. Dong, H. Mayr, J. Li and P. Knochel, Chem.–Eur. J., 2009, 15, 1324–1328 CrossRef PubMed.
- M. Leroux, W.-Y. Huang, Y. Lemke, T. J. Koller, K. Karaghiosoff and P. Knochel, Chem.–Eur. J., 2020, 26, 8951–8957 CrossRef CAS PubMed.
- T. Thaler, B. Haag, A. Gavryushin, K. Schober, E. Hartmann, R. M. Gschwind, H. Zipse, P. Mayer and P. Knochel, Nat. Chem., 2010, 2, 125–130 CrossRef CAS PubMed.
- H. Gilman, W. Langham and A. L. Jacoby, J. Am. Chem. Soc., 1939, 61, 106–109 CrossRef CAS; G. Wittig and U. Pockels, Ber. Dtsch. Chem. Ges., 1939, 72, 884–886 CrossRef.
- M. Rottländer, L. Boymond, L. Bérillon, A. Leprêtre, G. Varchi, S. Avolio, H. Laaziri, G. Quéguiner, A. Ricci, G. Cahiez and P. Knochel, Chem.–Eur. J., 2000, 6, 767–770 CrossRef CAS; A. Boudier, L. O. Bromm, M. Lotz and P. Knochel, Angew. Chem., Int. Ed., 2000, 39, 4414–4435 CrossRef PubMed; P. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis and V. A. Vu, Angew. Chem., Int. Ed., 2003, 42, 4302–4320 CrossRef PubMed.
- J. Villiéras, Bull. Soc. Chim. Fr., 1967, 5, 1520 Search PubMed; J. Villiéras, B. Kirschleger, R. Tarhouni and M. Rambaud, Bull. Soc. Chim. Fr., 1986, 470–478 Search PubMed; S. Avolio, C. Malan, I. Marek and P. Knochel, Synlett, 1999, 1820–1822 CrossRef CAS; L. Boymond, M. Rottländer, G. Cahiez and P. Knochel, Angew. Chem., Int. Ed., 1998, 37, 1701–1703 CrossRef PubMed.
- A. Krasovskiy and P. Knochel, Angew. Chem., Int. Ed., 2004, 43, 3333–3336 CrossRef CAS PubMed.
- I. Hiriyakkanavar, O. Baron, A. J. Wagner and P. Knochel, Chem. Lett., 2006, 35, 2–7 CrossRef CAS; G. Dagousset, C. François, T. León, R. Blanc, E. Sansiaume-Dagousset and P. Knochel, Synthesis, 2014, 46, 3133–3171 CrossRef; N. M. Barl, V. Werner, C. Samann and P. Knochel, Heterocycles, 2014, 88, 827–844 CrossRef.
- A. Krasovskiy, B. F. Straub and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 159–162 CrossRef CAS PubMed; L. Shi, Y. Chu, P. Knochel and H. Mayr, Angew. Chem., Int. Ed., 2008, 47, 202–204 CrossRef PubMed; L. Shi, Y. Chu, P. Knochel and H. Mayr, Org. Lett., 2009, 11, 3502–3505 CrossRef PubMed; L. Shi, Y. Chu, P. Knochel and H. Mayr, Org. Lett., 2012, 14, 2602–2605 CrossRef PubMed.
- A. D. Benischke, L. Anthore-Dalion, G. Berionni and P. Knochel, Angew. Chem., Int. Ed., 2017, 56, 16390–16394 CrossRef CAS PubMed; A. D. Benischke, L. Anthore-Dalion, F. Kohl and P. Knochel, Chem.–Eur. J., 2018, 24, 11103–11109 CrossRef PubMed; L. Anthore-Dalion, A. D. Benischke, B. Wei, G. Berionni and P. Knochel, Angew. Chem., Int. Ed., 2019, 58, 4046–4050 CrossRef PubMed.
- D. S. Ziegler, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2018, 57, 6701–6704 CrossRef CAS PubMed.
- A. Desaintjean, T. Haupt, L. J. Bole, N. R. Judge, E. Hevia and P. Knochel, Angew. Chem., Int. Ed., 2021, 60, 1513–1518 CrossRef CAS PubMed.
- M. Balkenhohl, D. S. Ziegler, A. Desaintjean, L. J. Bole, A. R. Kennedy, E. Hevia and P. Knochel, Angew. Chem., Int. Ed., 2019, 58, 12898–12902 CrossRef CAS PubMed.
- C. R. Hauser and H. G. Walker, J. Am. Chem. Soc., 1947, 69, 295–297 CrossRef CAS.
- A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 2958–2961 CrossRef CAS PubMed; S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7685–7688 CrossRef PubMed; B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794–9824 CrossRef PubMed; S. M. Manolikakes, N. M. Barl, C. Sämann and P. Knochel, Z. Naturforsch., B: J. Chem. Sci., 2013, 68, 411–422 CrossRef.
- W. Lin, O. Baron and P. Knochel, Org. Lett., 2006, 8, 5673–5676 CrossRef CAS PubMed.
- M. Mosrin and P. Knochel, Org. Lett., 2009, 11, 1837–1840 CrossRef CAS PubMed; T. Bresser, G. Monzon, M. Mosrin and P. Knochel, Org. Process Res. Dev., 2010, 14, 1299–1303 CrossRef.
- M. Balkenhohl, H. Jangra, I. S. Makarov, S.-M. Yang, H. Zipse and P. Knochel, Angew. Chem., Int. Ed., 2020, 59, 14992–14999 CrossRef CAS PubMed.
- K. Schwärzer, C. P. Tüllmann, S. Graßl, B. Górski, C. E. Brocklehurst and P. Knochel, Org. Lett., 2020, 22, 1899–1902 CrossRef PubMed.
- M. Jaric, B. A. Haag, A. Unsinn, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 5451–5455 CrossRef CAS PubMed; K. Groll, S. M. Manolikakes, X. M. du Jourdin, M. Jaric, A. Bredihhin, K. Karaghiosoff, T. Carell and P. Knochel, Angew. Chem., Int. Ed., 2013, 52, 6776–6780 CrossRef PubMed; S. M. Manolikakes, M. Jaric, K. Karaghiosoff and P. Knochel, Chem. Commun., 2013, 49, 2124–2126 RSC.
- L. Klier, E. Aranzamendi, D. Ziegler, J. Nickel, K. Karaghiosoff, T. Carell and P. Knochel, Org. Lett., 2016, 18, 1068–1071 CrossRef CAS PubMed.
- M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., Int. Ed., 2004, 43, 2206–2225 CrossRef CAS PubMed.
- F. H. Lutter, L. Grokenberger, L. A. Perego, D. Broggini, S. Lemaire, S. Wagschal and P. Knochel, Nat. Commun., 2020, 11, 4443 CrossRef CAS PubMed.
- F. H. Lutter, L. Grokenberger, P. Spieß, J. M. Hammann, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2020, 59, 5546–5550 CrossRef CAS PubMed.
- G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435–1462 CrossRef CAS PubMed.
- A. Guérinot and J. Cossy, Acc. Chem. Res., 2020, 53, 1351–1363 CrossRef PubMed.
- M. S. Hofmayer, A. Sunagatullina, D. Brösamlen, P. Mauker and P. Knochel, Org. Lett., 2020, 22, 1286–1289 CrossRef CAS PubMed.
- F. H. Lutter, L. Grokenberger, M. Benz and P. Knochel, Org. Lett., 2020, 22, 3028–3032 CrossRef CAS PubMed.
- A. Hernán-Gómez, E. Herd, E. Hevia, A. R. Kennedy, P. Knochel, K. Koszinowski, S. M. Manolikakes, R. E. Mulvey and C. Schnegelsberg, Angew. Chem., Int. Ed., 2014, 53, 2706–2710 CrossRef PubMed.
- C. I. Stathakis, S. Bernhardt, V. Quint and P. Knochel, Angew. Chem., Int. Ed., 2012, 51, 9428–9432 CrossRef CAS PubMed; C. I. Stathakis, S. M. Manolikakes and P. Knochel, Org. Lett., 2013, 15, 1302–1305 CrossRef PubMed; S. M. Manolikakes, M. Ellwart, C. I. Stathakis and P. Knochel, Chem.–Eur. J., 2014, 20, 12289–12297 CrossRef PubMed; T. J. Greshock, K. P. Moore, R. T. McClain, A. Bellomo, C. K. Chung, S. D. Dreher, P. S. Kutchukian, Z. Peng, I. W. Davies, P. Vachal, M. Ellwart, S. M. Manolikakes, P. Knochel and P. G. Nantermet, Angew. Chem., Int. Ed., 2016, 55, 13714–13718 CrossRef PubMed; Y.-H. Chen, M. Ellwart, V. Malakhov and P. Knochel, Synthesis, 2017, 49, 3215–3223 CrossRef.
- M. Leroux, T. Vorherr, I. Lewis, M. Schaefer, G. Koch, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2019, 58, 8231–8234 CrossRef CAS PubMed.
- F. H. Lutter, L. Grokenberger, M. S. Hofmayer and P. Knochel, Chem. Sci., 2019, 10, 8241–8245 RSC.
- M. S. Hofmayer, F. H. Lutter, L. Grokenberger, J. M. Hammann and P. Knochel, Org. Lett., 2019, 21, 36–39 CrossRef CAS.
- Y.-H. Chen, S. Graßl and P. Knochel, Angew. Chem., Int. Ed., 2018, 57, 1108–1111 CrossRef CAS PubMed.
- S. Graßl, Y.-H. Chen, C. Hamze, C. P. Tüllmann and P. Knochel, Org. Lett., 2019, 21, 494–497 CrossRef PubMed; S. Graßl and P. Knochel, Org. Lett., 2020, 22, 1947–1950 CrossRef PubMed.
- S. Graßl, J. Singer and P. Knochel, Angew. Chem., Int. Ed., 2020, 59, 335–338 CrossRef PubMed.
- S. Ohta, N. Tsuno and S. Nakamura, Heterocycles, 2000, 53, 1939–1955 CrossRef CAS.
- C. Blomberg, The Barbier reaction and related one-step processes, Springer Science & Business Media, 2012, vol. 31 Search PubMed.
- A. Frischmuth, M. Fernández, N. M. Barl, F. Achrainer, H. Zipse, G. Berionni, H. Mayr, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2014, 53, 7928–7932 CrossRef CAS PubMed.
- M. R. Becker and P. Knochel, Angew. Chem., Int. Ed., 2015, 54, 12501–12505 CrossRef CAS PubMed; M. Ketels, M. A. Ganiek, N. Weidmann and P. Knochel, Angew. Chem., Int. Ed., 2017, 56, 12770–12773 CrossRef PubMed.
- M. A. Ganiek, M. R. Becker, G. Berionni, H. Zipse and P. Knochel, Chem.–Eur. J., 2017, 23, 10280–10284 CrossRef CAS PubMed.
- A. Gissot, J.-M. Becht, J. R. Desmurs, V. Pévère, A. Wagner and C. Mioskowski, Angew. Chem., Int. Ed., 2002, 41, 340–343 CrossRef CAS PubMed; P. C. K. Vesborg and T. F. Jaramillo, RSC Adv., 2012, 2, 7933–7947 RSC; Y. Ma, R. F. Algera and D. B. Collum, J. Org. Chem., 2016, 81, 11312–11315 CrossRef PubMed; R. F. Algera, Y. Ma and D. B. Collum, J. Am. Chem. Soc., 2017, 139, 7921–7930 CrossRef PubMed; R. F. Algera, Y. Ma and D. B. Collum, J. Am. Chem. Soc., 2017, 139, 11544–11549 CrossRef PubMed; R. F. Algera, Y. Ma and D. B. Collum, J. Am. Chem. Soc., 2017, 139, 15197–15204 CrossRef PubMed; R. A. Woltornist, Y. Ma, R. F. Algera, Y. Zhou, Z. Zhang and D. B. Collum, Synthesis, 2020, 52, 1478–1497 CrossRef.
- N. Weidmann, M. Ketels and P. Knochel, Angew. Chem., Int. Ed., 2018, 57, 10748–10751 CrossRef CAS PubMed; J. H. Harenberg, N. Weidmann and P. Knochel, Angew. Chem., Int. Ed., 2020, 59, 12321–12325 CrossRef PubMed; J. H. Harenberg, N. Weidmann, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2021, 60, 731–735 CrossRef PubMed.
- P. Knochel and P. Jones, Organozinc reagents: a practical approach, Oxford University Press, 1999 Search PubMed; F. H. Lutter, M. S. Hofmayer, J. M. Hammann, V. Malakhov and P. Knochel, Organic Reactions, 2020, pp. 63–120 Search PubMed.
- A. Krasovskiy and P. Knochel, Synthesis, 2006, 2006, 0890–0891 CrossRef.
- T. Noël and S. L. Buchwald, Chem. Soc. Rev., 2011, 40, 5010–5029 RSC; D. Cantillo and C. O. Kappe, ChemCatChem, 2014, 6, 3286–3305 CrossRef CAS; D. Haas, J. M. Hammann, R. Greiner and P. Knochel, ACS Catal., 2016, 6, 1540–1552 CrossRef; M. Markovič, P. Lopatka, T. Gracza and P. Koóš, Synthesis, 2020, 52, 3511–3529 CrossRef; B. Heinz, D. Djukanovic, M. A. Ganiek, B. Martin, B. Schenkel and P. Knochel, Org. Lett., 2020, 22, 493–496 CrossRef PubMed.
Footnote |
| † In memory of Victor Snieckus. |
| This journal is © The Royal Society of Chemistry 2021 |