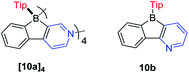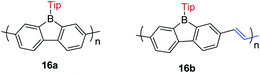Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
(Hetero)arene-fused boroles: a broad spectrum of applications
Jiang
He
,
Florian
Rauch
,
Maik
Finze
 * and
Todd B.
Marder
* and
Todd B.
Marder
 *
*
Institute for Inorganic Chemistry and Institute for Sustainable Chemistry & Catalysis with Boron (ICB), Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: maik.finze@uni-wuerzburg.de; todd.marder@uni-wuerzburg.de
First published on 24th November 2020
Abstract
(Hetero)arene-fused boroles are a class of compounds containing a 5-membered boron diene-ring. Based on their molecular framework, the (hetero)arene-fused boroles can be considered as boron-doped polycyclic antiaromatic hydrocarbons and are thus of great interest. Due to the vacant pz orbital on the 3-coordinate boron atom, the antiaromaticity and strain of the 5-membered borole ring, (hetero)arene-fused boroles possess strong electron accepting abilities and Lewis acidity. By functionalization, they can be tuned to optimize different properties for specific applications. Herein, we summarize synthetic methodologies, different strategies for their functionalization, and applications of (hetero)arene-fused boroles.
Introduction
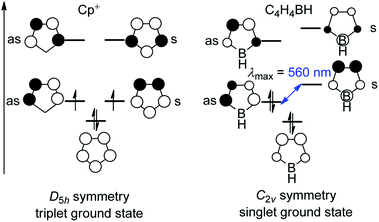
Three-coordinate boranes have been studied intensely for applications such as anion sensors,1–3 nonlinear optical materials (NLOs),4–14 live cell imaging,15–18 sensing of DNA, RNA and proteins,19,20etc.21–32 Among them, boroles are distinct, being 5-membered unsaturated 4π-electron heterocycles containing a 3-coordinate boron center. Interest in boroles originates from their being isoelectronic with the cyclopentadiene cation (Cp+) which, in terms of Hückel's rule,33–36 is antiaromatic and thus highly reactive. The isolation of “free” Cp+ has not been achieved. Cp+ has a triplet electronic ground state, which was confirmed by ESR spectroscopic measurements of the pentaphenylcyclopentadienyl cation at low temperature.37 The C2v symmetry of a borole is lower than that of Cp+ (D5h, Fig. 1), which results in a splitting of the previously degenerate half-filled molecular orbitals. The orbital with a nodal plane passing through the boron atom (“as” in Fig. 1) is lowered in energy and occupied, which leads to a singlet ground state and diamagnetic character of boroles, in contrast to the biradical character of Cp+. The small HOMO–LUMO gap in boroles leads to their intense color.Similar to Cp+, the “free” HBC4H4 is inaccessible due to its high reactivity caused by its antiaromaticity38–40 and low lying LUMO. Via steric protection, monomeric pentaphenylborole (PhBC4Ph4, PhB) was first synthesized in 1969 by Eisch and co-workers,41 but its crystal structure was only determined in 2008 by Braunschweig and co-workers.42 Steric protection of boroles was not only achieved with phenyl groups but also with other even bulkier protecting groups, e.g., FPh (pentafluorophenyl),43 Mes (2,4,6-trimethylphenyl)44 and FMes (2,4,6-tritrifluoromethylphenyl).45 However, these non-fused “free” boroles are still highly reactive compounds. The chemistry of non-fused “free” boroles was previously reviewed by Eisch,46 Marder and co-workers,47 Braunschweig and co-workers,48–50 Wakamiya,51 Martin and co-workers52 and Kinjo and co-workers.53,54
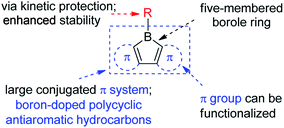
The stability of boroles is largely enhanced by annulation, and fused boroles are readily accessible and functionalizable (Scheme 1). In comparison to related triarylboranes, the electron accepting ability and Lewis acidity of the boron atom is largely enhanced in boroles, as a result of the antiaromaticity and strain of the 5-membered borole ring. Such fused boroles can be considered to be boron-doped polycyclic antiaromatic hydrocarbons.
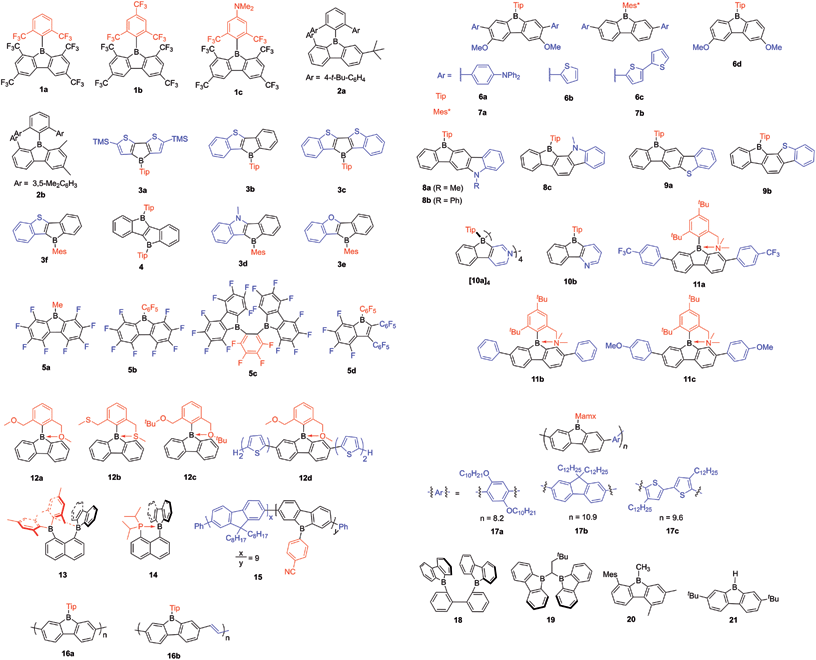
The first dibenzo-fused borole, namely the parent 9-borafluorene (Bf), was reported in the early 1960s by Köster and Benedikt,55 but only at the start of this century, has the chemistry of Bfs started to attract increasing interest. (Hetero)arene-fused boroles have been reviewed very briefly either as part of reviews on “free” boroles47,49,51,54 or on boron-doped polycyclic aromatic hydrocarbons (PAHs)56,57 or in the context of subvalent boranes.58 In this review, we address the synthesis, properties and applications of (hetero)arene-fused boroles in detail, our primary focuses are: (i) the optoelectronic behavior of the compounds; and (ii) their classification based on structure property relationships. New developments in the use of the exo-substituents on boron are also discussed. The structures of all compounds with Arabic numerals are shown in Scheme 25 at the end of the paper for the reader's convenience.
Synthetic methodology
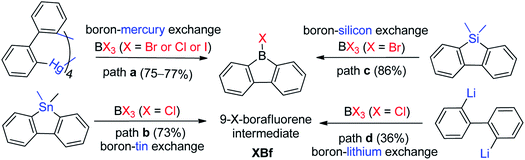
There are three main approaches for the synthesis of 9-aryl-9-borafluorenes: (1) the use of easy-to-functionalize 9-X-9-borafluorenes (XBf, X = Cl or Br or I) as key intermediate; (2) assembling 9-aryl-9-borafluorenes in one step; and (3) stepwise substitution reactions at boron.The first approach relies on readily available 9-X-9-borafluorenes (XBf, X = Cl or Br or I). Four synthetic approaches to XBf derivatives have been developed. In 1985, Nöth and co-workers applied a boron–mercury exchange reaction (Scheme 2, path a).59 Analogous to the preparation of PhB,60 a boron–tin exchange reaction can also be utilized for the synthesis of XBfs (Scheme 2, path b).61,62 To avoid using these highly toxic metals, a boron–silicon exchange reaction was developed (Scheme 2, path c).63ClBf can be obtained directly from reaction of 2,2′-dilithiobiphenyl with BCl3 in an aliphatic solvent (Scheme 2, path d).64 The advantage of this approach is that once intermediate XBf is obtained, it is useful for subsequent derivatization.
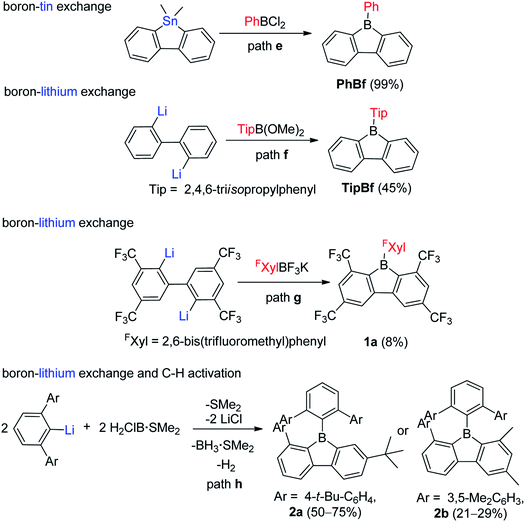
For the one-step assembly of 9-aryl-9-borafluorenes, a boron–tin exchange reaction was utilized. Instead of using BX3 (X = Cl or Br or I), PhBCl2 was used directly (Scheme 3, path e).65 The drawback of this methodology is that only sterically relatively unencumbered groups (e.g., phenyl and pentafluorophenyl) can be used;61e.g., MesBCl2 (Mes = mesityl group) is unsuitable for this methodology. By changing the boron source from PhBCl2 to dimethoxy aryl borates, and subsequent reaction with dilithiobiphenyl, 9-aryl-9-borafluorenes can be synthesized (Scheme 3, path f).66,67 There are two advantages to this approach: (1) dimethoxy aryl borates can tolerate coordinating solvents and are much more stable than their corresponding aryldihaloboranes, which makes the work up much easier; and (2) bulkier aryl groups (e.g., 2,4,6-triisopropylphenyl (Tip)) can be used. The third approach which can assemble 9-aryl-9-borafluorenes in one step was reported by Marder and co-workers,45,68 in which more stable aryltrifluoroborate salts were used as the boron source (Scheme 3, path g).69–74 The downside of this approach is the comparably low yields. Besides these three widely applied approaches, which can assemble 9-aryl-9-borafluorenes in one step, the Wehmschulte group synthesized two unsymmetric 9-borafluorenes (2a and 2b, Scheme 3, path h) in one step by using H2ClB·SMe2 as the boron source.75,76 The formation of the borole ring takes place via a facile intramolecular C–H activation process, but the requirement of bulky terphenyl precursors limits its further application.
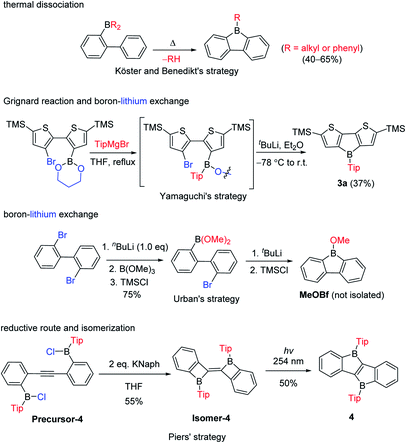
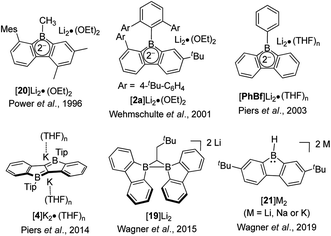
The third approach is a stepwise substitution reaction at boron, which was first reported in 1963 by Köster and Benedikt (Scheme 4).55 Thus, 9-alkyl-9-borafluorenes can be synthesized from B,B-dialkyl-2-biphenylborane intermediates by thermal dissociation of one alkyl-group at 180–200 °C. The compound 9-phenyl-9-borafluorene (PhBf) can also be synthesized from B,B-diphenyl-2-biphenylborane in the same way, but the temperature needs to be increased to 280–300 °C. The harsh conditions in this approach limit its further application. In 2011, the Yamaguchi group applied boronic esters as the boron source for the synthesis of heteroarene-fused boroles.77 Using the dithiophene-fused borole (3a) as an example, the boronic ester was introduced at the bithiophene via boron–lithium exchange in the first step, then the protecting group at the boron was introduced with a Grignard reagent. Finally, an intramolecular cyclization reaction completed the synthesis of 3a. More recently, Urban and co-workers applied a similar strategy, but used an intramolecular cyclization in the second step, obtaining 9-methoxy-9-borafluorene (MeOBf),78 a potential intermediate for synthesizing other 9-substituted-9-borafluorenes. In 2012, the Piers group used a reductive route with a haloborane (Precursor-4, obtained by three stepwise boron–metal exchange reactions) while attempting to synthesize the diborole 4 (Scheme 4, bottom). Instead, they initially obtained an isomer of 4 (Isomer-4) which, under irradiation with UV light (254 nm), isomerized to the desired diborole 4.79 Subsequently, the same group reported another, more efficient, thermal route to the diborole 4 from Isomer-4.80
Stability of 9-borafluorenes
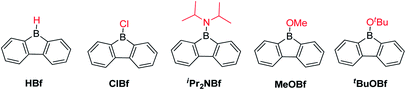
The advantage of incorporating a boron atom into a 5-membered diene-ring is the enhancement of electron accepting ability and Lewis acidity, but at the same time, stability is sacrificed. By fusing two phenyl rings onto a borole, the stability is greatly enhanced. The stability of the resulting 9-borafluorenes depends largely on the exo-substituent at boron. In this section, we compare the stability of different 9-borafluorenes.When the exo-substituent is not a bulky aryl group, 9-borafluorenes remain highly reactive (Scheme 5). In fact, HBf in an unsymmetric dimer in solution and, after some time, it forms oligomers via a ring-opening mechanism even in dry and deoxygenated C6D6.55,81–84ClBf85–87 and iPr2NBf both show more than 50% decomposition within 1 hour in solution when exposed to the atmosphere.88tBuOBf is much more stable and exhibits less than 10% decomposition after 1 hour in CDCl3 in air. This can be attributed to the steric demand of the tert-butyl group as the less bulky derivative MeOBf59 is as sensitive to air/moisture as iPr2NBf and ClBf.88
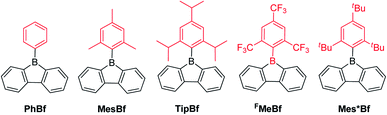
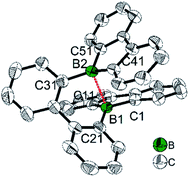
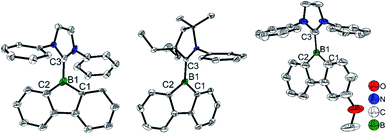
An aryl group as the exo-substituent at 9-borafluorene increases the stability drastically (Scheme 6). PhBf and MesBf decompose only slowly in the air.54PhBf nonetheless retains high reactivity, e.g., azides can insert into one of the B–C bonds of PhBf to generate 9,10-B,N-phenanthrenes.62,89 The reaction of PhBf with 1,2-dipolar substrates leads to the formation of the corresponding ring expansion adducts.90,91 By employing Tip or FMes as the protecting group at the boron atom of 9-borafluorenes, their stability is greatly enhanced, and both derivatives can be purified by column chromatography, even in air.66,88 Stability tests indicate that TipBf shows ca. 15% decomposition in solution in air after 24 hours and FMesBf shows only ca. 5% decomposition under the same conditions. Considering that the trifluoromethyl group is less bulky than the isopropyl group,92 this observation initially seems counterintuitive. In fact, the higher stability of FMesBf is likely due to the stabilizing interaction of the vacant pz-orbital of boron by lone pairs of the fluorine atoms of the two ortho-CF3 groups of the exo-FMes. This σ-donation was confirmed by the short B⋯F distances (2.682(6) and 2.577(5) Å) observed in the solid state (Fig. 2), which are much shorter than the sum of the van der Waals radii for boron and fluorine (3.39 Å).93 The σ-donation from the fluorine atom(s) of ortho-CF3 group(s) to the vacant pz-orbital of boron was also observed in other boranes94–102 and boroles.45,68 The 2,4,6-tri-tert-butylphenyl (Mes*) group is the bulkiest substituent among these protecting groups and, thus, provides the most stable 9-borafluorenes.103 Compared to TipBf, which still exhibits reactivity towards the small F− anion and can be applied as a F− sensor, Mes*Bf is inert to F−. This demonstrates the superior stability of Mes*Bf.
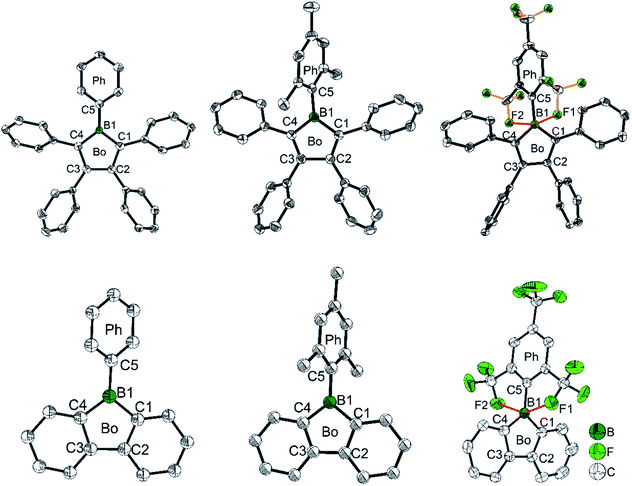
To gain a deeper understanding of the relation between the structure and the stability, selected bond parameters of PhBf,104MesBf,105 and FMesBf88 derived from single crystal X-ray studies are listed in Table 1 and the molecular structures are depicted in Fig. 2. The free non-fused boroles, PhB,42 1-mesityl-2,3,4,5-tetraphenylborole (MesB)44 and 1-(2,4,6-tritrifluoromethylphenyl)-2,3,4,5-tetraphenylborole (FMesB),45 are included for comparison. Compared to PhB, the C–B bond lengths of the other five compounds are significantly longer. The short C–B bond lengths of PhB are a result of the strong pπ(B)–π* conjugation. The C1–C2 and C3–C4 bonds in MesB (1.356(2) and 1.351(2) Å) and FMesB (1.359(3) and 1.358(3) Å) are obviously double bonds, but in the other four compounds, these bonds are significantly longer. In contrast, the remaining inner-borole C2–C3 bond is quite long in MesB (1.537(2)) and FMesB (1.526(3)) compared to those in PhB and the three fused boroles. So, similar to PhB, the Cring–Cring distances in fused boroles are indicative of some electron delocalization, which is in stark contrast to typical non-fused boroles such as MesB, resulting in significant differences in the properties of fused vs. typical non-fused boroles. The ∠C1–B1–C4 angles of the six compounds shown in Fig. 2 are quite similar. The torsion angles between the exo-aryl ring bonded to boron and the borole core are 32.71(5)° (PhB), 68.7(1)° (MesB), 82.4(3)° (FMesB), 32.8° and 39.2° (PhBf), 84.1° (MesBf), 80.9(12)° and 82.2 (2)° (FMesBf) (the unit cell of PhBf and FMesBf exhibit two distinct molecules), respectively. Apparently, the bulkier the exo-aryl group is, the larger the torsion angle becomes, resulting in more efficient kinetic protection.
| PhB | MesB | FMesB | PhBf | MesBf | FMesBf | |
|---|---|---|---|---|---|---|
| B1–C1 | 1.526(2) | 1.586(2) | 1.571(3) | 1.573(9) | 1.566(2) | 1.550(6) |
| B1–C4 | 1.539(2) | 1.575(2) | 1.576(3) | 1.59(1) | 1.567(2) | 1.556(5) |
| B1–C5 | 1.516(2) | 1.560(2) | 1.580(3) | 1.58(1) | 1.565(2) | 1.584(5) |
| C1–C2 | 1.428(2) | 1.356(2) | 1.359(3) | 1.431(9) | 1.416(2) | 1.421(5) |
| C3–C4 | 1.426(2) | 1.351(2) | 1.358(3) | 1.421(9) | 1.415(2) | 1.411(5) |
| C2–C3 | 1.470(2) | 1.537(2) | 1.526(3) | 1.51(1) | 1.485(2) | 1.485(5) |
| B1–F1 | 2.385(3) | 2.682(6) | ||||
| B1–F2 | 2.556(5) | 2.576(5) | ||||
| ∠C1–B1–C4 | 105.4(1) | 105.2(1) | 106.19(17) | 104.2(5) | 103.7(1) | 105.1(3) |
| ∠Bo–Ph | 32.71(5) | 68.7(1) | 82.4(3) | 39.2 | 84.1 | 82.2(2) |
9-Borafluorenes with a fluorinated backbone
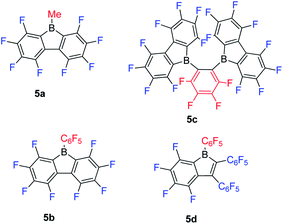
Inspired by the wide application of B(C6F5)3,106–108 and the fact that 9-borafluorenes are more Lewis acidic than their corresponding boranes, Piers and co-workers synthesized a series of 9-borafluorenes with fluorinated backbones (Scheme 7). Compounds 5a and 5b are pale yellow or orange solids which exhibit lowest energy absorption maxima at 398 nm and 440 nm, respectively, in hexane.61 To explore the effect of two fluorinated 9-borafluorene centers in a molecular framework on the Lewis acidity, 5c was synthesized.109 In 5c, two fluorinated 9-borafluorenes are situated ortho to one another, forming a chelating bidentate Lewis acid. Compound 5c is a deep orange solid with a lowest energy absorption maximum at 425 nm (ε = 590 M−1 cm−1) in hexane, comparable to that of 5b, indicating that the two chromophores of the fluorinated 9-borafluorenes are not coupled. To the best of our knowledge, 5d is the only example of mono-aryl fused borole.110 Compound 5d is a red solid with its lowest energy absorption maximum at 465 nm (ε = 900 M−1 cm−1 in toluene), and is readily soluble in most solvents.111Compared with the corresponding fluorinated perfluoroboranes (MeB(C6F5)2, B(C6F5)3 and C6F4-1,2-[B(C6F5)2]2),109,112–114 fluorinated 9-borafluorenes show stronger Lewis acidities, as demonstrated by Lewis base competition reactions, the Childs method,115 and semiempirical MNDO calculations.116 Apparently, compared with the corresponding fluorinated triarylboranes, the loss of two fluorine atoms is compensated by the antiaromaticity and strain of the 5-membered borole ring. Weak Lewis bases (LBs), e.g., THF and CH3CN, both bind to these four fluorinated 9-borafluorenes. After introduction of a Lewis base, the orange solutions of 5a and 5c, or lime green solution of 5b, become colorless,117 and the red solution of 5d turns pale yellow.111 This color change is due to the interruption of pπ(B)–π* conjugation upon coordination of the Lewis base to the boron center, which results in a higher LUMO energy.66
In a CH3CN competition reaction between 5b and B(C6F5)3·CH3CN at 25 °C, an equilibrium constant of ca. 1.3 was found indicating a preference for formation of 5b·CH3CN vs. B(C6F5)3·CH3CN.117 In another competition experiment, with the bulkier THF as the base (in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5b, B(C6F5)3 and THF in d8-toluene), only the 5b·THF adduct was observed by NMR spectroscopy. Applying the Childs method, 5a and 5b have a relative Lewis acidity value of 0.58 ± 0.02 and 0.70 ± 0.02, respectively, which is only slightly higher than that of the corresponding MeB(C6F5)2 (0.56 ± 0.02) and B(C6F5)3 (0.68 ± 0.02) obtained by Piers.61 For the smaller Lewis base CH3CN, 5b and B(C6F5)3 show comparable Lewis acidities, but for the larger Lewis base THF, 5b shows a much stronger Lewis acidity than B(C6F5)3. Based on these results, the authors concluded that the relative Lewis acidities of 5b and B(C6F5)3 are determined by steric factors, rather than the antiaromaticity of 5b.
1 for 5b, B(C6F5)3 and THF in d8-toluene), only the 5b·THF adduct was observed by NMR spectroscopy. Applying the Childs method, 5a and 5b have a relative Lewis acidity value of 0.58 ± 0.02 and 0.70 ± 0.02, respectively, which is only slightly higher than that of the corresponding MeB(C6F5)2 (0.56 ± 0.02) and B(C6F5)3 (0.68 ± 0.02) obtained by Piers.61 For the smaller Lewis base CH3CN, 5b and B(C6F5)3 show comparable Lewis acidities, but for the larger Lewis base THF, 5b shows a much stronger Lewis acidity than B(C6F5)3. Based on these results, the authors concluded that the relative Lewis acidities of 5b and B(C6F5)3 are determined by steric factors, rather than the antiaromaticity of 5b.
Addition of Cp2Zr(CH3)2 to 5a or 5b, leads to Me− abstraction, and the corresponding ion pairs are formed rapidly.61 Both of them are remarkably more stable than their corresponding borane ion pairs in toluene (the ion pairs formed from MeB(C6F5)2 and Cp2Zr(CH3)2 can exchange a C6F5 group from MeB(C6F5)2 with a methyl group from Cp2Zr(CH3)2, resulting in Me2B(C6F5) and Cp2Zr(CH3)(C6F5) under similar conditions).118 The combination 5a/Cp2Zr(CH3)2 and 5b/Cp2Zr(CH3)2 are more active and stable than their corresponding borane/Cp2Zr(CH3)2 ion pairs as activators for olefin polymerization. To investigate further the coordination chemistry of 5a and 5b, [Cp*Al]4 was used.65 Surprisingly, only the thermally robust η1 Lewis acid–base adduct was observed. Thus, the fragment of Cp*Al behaves only as a Lewis base rather than as a two-electron reducing agent. The reaction of Cp*Al with the less Lewis acidic PhBf also provides the η1 Lewis acid–base adduct. Alternative routes to η5 9-borafluorene aluminum complexes via reaction of PhBfLi2 with Cp*AlCl2(THF) were also unsuccessful. The reaction of 5b with LtBuScR2 (LtBu = ((Ar)NC(tBu)CHC(R)N(tBu)), Ar = 2,6-iPr-C6H3) produced the corresponding contact ion pairs, the structures of which were thoroughly investigated both in solution and the solid state.119
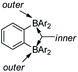
Ortho-phenylene-bridged diboranes are interesting compounds and can be applied as co-initiators for olefin polymerizations.120 Depending on the binding position of the Lewis base, ortho-phenylene-bridged diboranes (and diborole 5c) can adopt inner or outer facial coordination modes (Scheme 8).112–114,121 By adding a neutral Lewis base, e.g., CH3CN or THF, coordination to the less sterically encumbered outer face of 5c was observed exclusively.109 This is in contrast to the corresponding diborane C6F4-1,2-[B(C6F5)2]2, to which CH3CN coordinates to the inner face. The authors suggested that this is likely a result of less strain in the outer coordination mode caused by pyramidalization of the boron center in 5c; it may also be a result of the more rigid adjacent borafluorene's steric interaction which prevents the inner coordination mode in 5c. The reaction of 5c with PhCMe2X (X = Cl, OMe or N3) gives thermally stable and isolable ion pairs which feature a weakly coordinating anion (WCA). The application of these ion pairs as initiators for isobutene polymerization were studied and the results show that the combination of C6F4-1,2-[B(C6F5)2]2 with PhCMe2X is more suitable than 5c with PhCMe2X.122
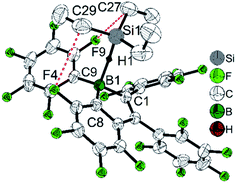
While perfluoropentaphenylborole reacts rapidly and irreversibly with dihydrogen (H2),123,1245b is inert to H2 under various conditions. Therefore, 5d, which is a structural hybrid of 5b and perfluoropentaphenylborole, was designed and synthesized.110 The reaction between 5d and H2 was investigated experimentally and by theoretical calculations. Compound 5d reacts reversibly with H2, but side reactions occur resulting in only limited turnover numbers of this metal-free H2 activation reaction. Compound 5d has a comparable Lewis acidity to that of perfluoropentaphenylborole but exhibits a much better solubility than perfluoropentaphenylborole in non-coordinating solvents. Due to the better solubility of 5d, a low temperature experiment between 5d and Et3SiH was possible.111 The borole–silane complex formation in d8-toluene was studied by variable-temperature NMR spectroscopy. The trends of the Si–H coupling constant and the infrared stretching frequency of the Si–H bond as a function of temperature, and the molecular structure of the complex determined by X-ray diffraction (Fig. 3), clearly prove that an interaction exists between the boron atom and the silicon atom through the Si–H bond. These direct observations thus confirmed the previously proposed mechanism, i.e., that perfluoroarylboranes catalyze the hydrosilylation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds via borane activation of the Si–H bond, not via a classical Lewis acid/base adduct process.125
O bonds via borane activation of the Si–H bond, not via a classical Lewis acid/base adduct process.125
Donor–acceptor 9-borafluorenes
The 9-borafluorenes exhibit a weakly allowed lowest energy absorption which extends into the visible region. This absorption was attributed to the low-lying LUMO which originates from the pπ–π* conjugation through the vacant pz orbital of boron.126 By incorporating electron donating group(s) or electron withdrawing group(s) at different positions, the photophysical properties can be modified (Scheme 9). In this section, the 9-borafluorenes are classified according to their functional groups at different positions. | ||
| Scheme 9 A depiction of the different positions on a 9-borafluorene to which functional groups are attached. | ||
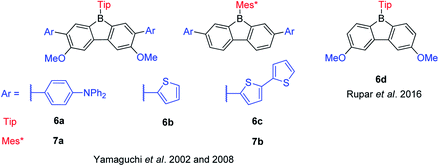
Pioneered by Yamaguchi and co-workers in 2002, three functionalized TipBfs (6a–6c) with donors (methoxy and amine groups or methoxy and thiophene groups) at the m- and p-positions were reported (Scheme 10).66 Compared with the non-functionalized TipBf, both the absorption and emission of these functionalized TipBfs are red shifted and the quantum yields decrease (Table 2). The red shifted absorption and emission of donor-functionalized TipBfs were attributed to intramolecular charge transfer (ICT). Addition of F− (or coordinating solvents) leads to a blue shift of both the absorption and emission of these functionalized TipBfs and, the quantum yields dramatically increase to ca. 0.5–0.9. Thus, these functionalized TipBfs can be applied as F− sensor. In contrast to tri(9-anthryl)borane, which loses its fluorescence properties after coordination with F− and was labeled as a “turn off” sensors,1 due to the increase of the emission intensity after adding F−, these functionalized 9-borafluorenes were termed fluorescence “turn on” sensors.
| Compound | Solvent | λ abs/nm (log(ε)) | λ em/nm | Φ F |
|---|---|---|---|---|
| a Non-emissive. b Not reported. | ||||
| 1a 68 | Hexane | 400 (2.48) | 521 | 0.37 |
| 1b 68 | Hexane | 386 (2.60) | 510 | 0.30 |
| 1c 68 | Hexane | 396 (2.48) | 627 | 0.03 |
| 3a 77 | CH2Cl2 | 552 (3.05) | ||
| 3b 77 | CH2Cl2 | 469 (2.93) | ||
| 3c 77 | CH2Cl2 | 600 (3.04) | ||
| 3d 105 | CH2Cl2 | 479 (3.09) | ||
| 3e 105 | CH2Cl2 | 468 (3.06) | ||
| 3f 105 | CH2Cl2 | 474 (3.05) | ||
| 5a 61 | Hexane | 398 (−) | ||
| 5b 61 | Hexane | 440 (−) | ||
| 5c 109 | Hexane | 425 (2.77) | ||
| 5d 110 | Hexane | 465 (2.95) | ||
| TipBf 66 | THF | 410 (2.39) | 514 | 0.09 |
| 6a 66 | THF | 480 (3.08) | 561 | 0.03 |
| 6b 66 | THF | 488 (2.95) | 550 | 0.041 |
| 6c 66 | THF | 504 (3.51) | 576 | 0.022 |
| 6d 88 | CH2Cl2 | 398 (2.80) | 499 | 0.10 |
| Mes*Bf 103 | THF | 397 (2.42) | 501 | 0.35 |
| 7a 103 | THF | 470 (4.06) | 608 | 0.24 |
| 7b 103 | THF | 457 (3.80) | 600 | 0.48 |
| 8a 127 | Cyclohexane | 430 (3.28) | 529 | 0.21 |
| 8b 127 | Cyclohexane | 430 (3.27) | 527 | 0.36 |
| 8c 127 | Cyclohexane | 451 (4.03) | 567 | 0.13 |
| 9a 128 | Cyclohexane | 380 (3.75) | 519 | 0.55 |
| 9b 128 | Cyclohexane | 411 (3.81) | 513 | 0.42 |
| [10a]4 (ref. 129) | THF | 322 (4.70) | 495 | 0.12 |
| 10b 129 | THF | 375 (2.95) | 520 | 0.27 |
Six years later, the same group synthesized another two 9-Mes*-borafluorenes (7a and 7b) with donors (amine or thiophene groups) at the m-positions (Scheme 10).103 Compared with the non-functionalized Mes*Bf, the molar extinction coefficients are much higher and a red shift was observed in both absorption and emission. Compared withtheir corresponding 9-Tip-9-borafluorenes, the fluorescence quantum yields of 9-Mes*-9-borafluorenes are higher, which is most likely due to the restricted rotation of the bulky Mes* group. The bulky Mes* group also leads to enhanced stability of these 9-Mes*-9-borafluorenes, which paves the way for their application as accepting units in organic (opto)electronics.
In 2016, Rupar and co-workers synthesized 6d with two methoxy donors at the p-positions (Scheme 10). The lowest energy absorption and emission peak of 6d appear in the same range as that of TipBf, and the quantum yields are also the same. The similar photophysical properties of these two compounds may be due to the weak donating ability of the methoxy groups.88
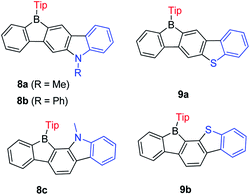
Encouraged by the wide application of carbazole as a donating group and 9-borafluorene as an accepting group, the Zhao group synthesized three ladder-type B,N-bridged p-terphenyls, with indole fused at the p-, m-positions (8a and 8b) or o-, m-positions (8c) on one side of the borafluorene (Scheme 11).127 Later, the same group replaced the indole with benzothiophene and reported another two ladder-type B,S-bridged p-terphenyls (9a and 9b).128 In these ladder-type boroles, the products of fusing at the p-, m-positions (8a, 8b and 9a), are tolerant to air and moisture, but fusing at the o-, m-positions (8c and 9b) leads to products that show slow decomposition in dilute solution in air (no specific solvent is mentioned), which is probably caused by steric congestion. Both absorption and emission show negligible solvatochromism for these ladder-type boroles which indicates only a small polarity change between the ground state and the excited state. No difference of absorption and emission was observed between N-methyl 8a and N-phenyl 8b, which may be due to the large torsion angle between the phenyl group and pyrrole. Compared to 8a, 8c shows a red shift in both absorption and emission which was attributed to the lower LUMO energy as evidenced by computational studies. Compared with carbazole-fused 9-borafluorenes, the benzothiophene fused 9-borafluorenes show only slight hypochromism of both absorption and emission but double the quantum yields. In addition to the broad use of 9-borafluorenes as F− sensors, 9a was applied as a Hg2+ sensor because of its high affinity for Hg2+ due to the S atom. Additionally, these ladder-type boroles exhibit considerable potential for application as bipolar electron-transporting materials.
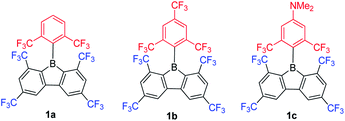
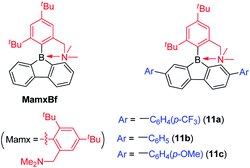
In contrast to the incorporation of donating groups at the 9-borafluorene core, more recently, Marder and co-workers reported compound 1a with four inductively withdrawing CF3 groups attached to the o- and p-positions at the biphenyl main core (Scheme 12).68 To investigate the effect of substitution at the p′-position in 1a, 1b with a CF3 electron withdrawing group at the p′-position and 1c with an NMe2 electron donating group at the p′-position were also synthesized. Although examples appear in patents,1301c is the only example of a 3-coordinate 9-borafluorene which incorporated a donor at the exo-aryl to have been reported in a paper.68
Due to the strong electron withdrawing ability of the four CF3 groups at the 9-borafluorene core, the electron accepting ability of boron was greatly enhanced, as evidenced by cyclic voltammetry (see below in the Electrochemistry section). With the extra protection of two CF3 groups at the o-positions, 1a and 1b are more stable than FMesBf. Surprisingly, although 1c has a strong donor at the p′-position, its absorption appears in the same region as those of 1a and 1b (this could also be caused by the weak absorption of 1c in the lower energy region), but the emission shows a large red shift (1a: λem = 510 nm; 1b: λem = 521 nm; 1c: λem = 627 nm in hexane). Both 1a (τF = 151 ns) and 1b (τF = 224 ns) exhibit very long fluorescence lifetimes in hexane, but behave differently; 1c exhibits two radiative processes (τp = 9.2 ns and τd = 1.6 μs), the latter resulting from thermally activated delayed fluorescence (TADF). Compound 1c is the first example of a borafluorene to exhibit TADF, but the rather low quantum yield (ΦF = 0.03 in hexane) limits its further application. In contrast to the low quantum yield of 1c in hexane, 1a (ΦF = 0.30) and 1b (ΦF = 0.37) exhibit relatively high quantum yields. Theoretical studies indicate that the LUMO of 1c is located on the biphenyl core with a large contribution from the boron atom, whereas the HOMO is located on the exo-aryl moiety. Thus, the HOMO to LUMO transition is an ICT process with a small overlap coefficient (Λ) which also fits the requirement for TADF.
Heteroarene-fused boroles
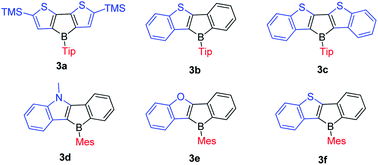
In 2011, the Yamaguchi group fused electron-rich thiophene(s) onto boroles by stepwise substitution reactions and synthesized 3a–3c (Scheme 13).77 Surprisingly, these three Tip-protected thiophene-fused boroles are air- and moisture-sensitive. Considering that TipBf is stable enough to be purified in air, this instability is in opposition to an expectation that applying electron-rich thiophene would decrease the Lewis acidity of boron to form more stable compounds. The antiaromaticity of the borole rings was evaluated by DFT calculations of the nucleus-independent chemical shifts (NICS) values (Table 3).The NICS(1)ZZ (ppm) values increase in the order TipBf < TipB < 3b < 3a < 3c. Thus, the biphenyl-fused borole, TipBf exhibits less antiaromatic character whereas the antiaromaticity of the electron-rich thiophene-fused boroles is enhanced, and is even higher than that of the non-fused “free” borole (1-Tip-1-borole, TipB), as suggested by the NICS(1)ZZ values. This result is also opposite to the conventional understanding that fusing electron-rich aromatic arenes decreases the antiaromaticity of the 5-membered borole ring.131,132
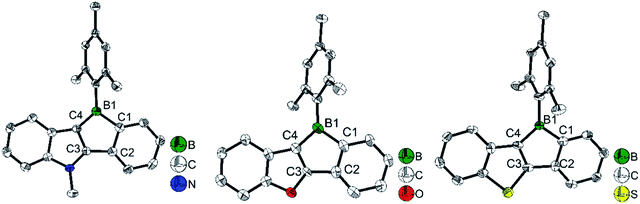
To study what governs the antiaromaticity and Lewis acidity of heteroarene-fused boroles, another three heteroarene-fused boroles, 3d–3f were synthesized (Scheme 13). NICS calculations were conducted with the geometries derived from the crystal structures of these heteroarene-fused boroles (Fig. 4). The conclusion reached was that the smaller extent of bond alternation in the 5-membered borole ring in heteroarene-fused boroles is responsible for the high degree of antiaromaticity. Theoretical and experimental studies suggest that the LUMO energy of these heteroarene-fused boroles are relevant to the antiaromaticity, which also linearly correlates with their Lewis acidities.105
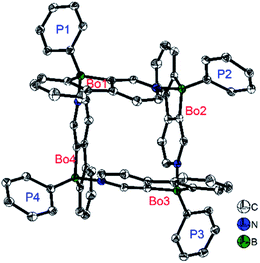
In contrast to the Yamaguchi group's fused boroles with electron-rich heteroarene(s), more recently, Marder and co-workers129 switched to the electron-poor pyridine to synthesize phenylpyridyl-fused boroles (Scheme 14). Using 4-phenylpyridine to prepare a fused borole, [10a]4 was obtained as a white solid and adopts a unique coordination mode, forming a tetramer with a central cavity in both the solid state (X-ray diffraction, Fig. 5) and solution (1H diffusion-ordered spectroscopy (1H DOSY)). The coordination mode of [10a]4 is similar to that of dimethyl(3-pyridyl)borane and diethyl(3-pyridyl)borane.133–135 The B–N bond lengths of [10a]4 (1.644(2)–1.655(2) Å) are comparable to those of pentaphenylborole·2,6-lutidine (1.6567(3) Å)136 and sterically hindered dibenzoborole·pyridine (1.638(3) Å);75 however, in contrast to both pentaphenylborole·2,6-lutidine and the sterically hindered dibenzoborole·pyridine which dissociate in solution at room temperature, [10a]4 persists as a tetramer in C6D6 even at 50 °C (1H DOSY). By switching 4-phenylpyridine to 2-phenylpyridine, 10b was prepared and isolated as a light yellow solid. The boron center of 10b is 3-coordinate in solution but 4-coordinate in the solid state, as evident from solid-state 11B{H} RSHE/MAS NMR measurements. The difference is ascribed to the steric protection of the pyridine nitrogen by the attached phenyl group at the 2-position.
Due to the inherent electron withdrawing properties of pyridine, the electron accepting ability of 10b is enhanced (see Electrochemical section below). The lowest energy absorption maximum of [10a]4 appears at 322 nm in hexane, which is blue shifted compared to those of 10b (375 nm) and other 3-coordinate 9-borafluorenes. Compared with other Tip-protected 9-borafluorenes, 10b exhibits a relatively high quantum yield (0.34 in hexane) in solution and shows an interesting dual fluorescence property. Two lifetimes are observed at the same emission wavelength of 520 nm. The authors suggested that the dual fluorescence in solution is caused by an equilibrium between the free 3-coordinate 10b and a weak intermolecular coordination adduct of 10b. This hypothesis was further supported by lifetime measurements at different concentrations, low temperature excitation spectra, low temperature 1H NMR spectra and lifetime measurements upon addition of DMAP to a solution of 10b to simulate the 4-coordinate 10b species. Thus, this dual fluorescence is different from dual fluorescence induced by B–N dissociation in the excited state.137 Interestingly, the ratios of the relative percentage of the two lifetimes shows a linear relationship with temperature.
Intramolecular dative bond in 9-borafluorenes
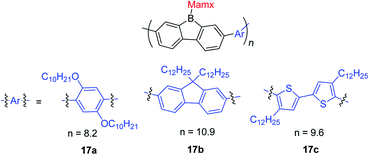
Instead of using bulky Tip or Mes* as the protecting group at boron, Chujo and co-workers used the Mamx ligand (Mamx = 2,4-di-tert-butyl-6-[(dimethylamino)methyl]phenyl) as the steric protecting group at boron in 9-borafluorenes (Scheme 15).138 The X-ray crystal structure of MamxBf indicates that the nitrogen atom coordinates to the boron atom with a B–N bond length of 1.712 Å. The 11B NMR spectrum shows a peak at 5.96 ppm, which is in the typical range for 4-coordinate boron. With the double protection of steric hindrance and nitrogen atom coordination to boron, MamxBf is stable to moisture and can be purified in air. The lowest energy absorption of MamxBf is at ca. 280 nm and it exhibits a weak emission at ca. 360 nm. Interestingly, by addition of B(C6F5)3 to a benzene solution of MamxBf, phosphorescence (τp = 8.95 μs (69%)) with a peak at 597 nm was observed at room temperature, which the authors suggest is caused by triplet exciplexes. Theoretical analysis for the excited state of MamxBf suggests that this robust B–N coordination in the ground state is cleaved in the S1 state. This B–N bond cleavage in the excited state is also suggested to be responsible for the weak emission of MamxBf.By incorporating electron withdrawing or electron donating groups at the biphenyl core, the energy of 9-Mamx-9-borafluorenes are modulated and emission from bond-cleavage-induced intramolecular charge transfer (BICT) was realized. Compound 11a has two electron withdrawing trifluoromethylphenyl groups at the biphenyl core and it shows a single emission with a peak at 373 nm, which is similar to that of MamxBf. Changing the electron withdrawing trifluoromethylphenyl groups to electron-neutral phenyl groups or electron donating methoxyphenyl groups in 11b and 11c, respectively, result in similar shaped dual emissions (ca. 330 nm and ca. 520 nm, respectively), which is in contrast to the single emission of 11a and MamxBf (Scheme 15). The authors concluded that by incorporation of electron donating groups at the biphenyl core, the boron atom exhibits a more negative charge, and a BICT process thus occurs which results in dual emission. Theoretical calculations further support the BICT transition. The short wavelength emission was assigned to a locally-excited (LE) emission from a π–π* transition and the long wavelength emission was assigned to the BICT transition. The BICT emission is highly sensitive to the solvent viscosity and, thus, 11c can be applied as a ratiometric sensor.139
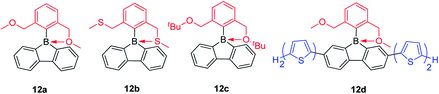
By exchanging the strongly donating dimethylamine group with the weakly donating methoxy group, Rupar and co-workers synthesized 12a (Scheme 16)140 which is a colorless powder that is air-stable in the solid state and solution. The absorption maximum appears at 284 nm and the emission maximum appears at 536 nm with a long lifetime (τF = 122 ns) in CH2Cl2. This is an extraordinary large Stokes shift (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) for a small molecule; in fact, it is the largest Stoke shifts ever reported.141 This large Stokes shift is caused by the photodissociation of the B–O dative bond in the excited state, which is further supported by theoretical studies. By changing the methoxy groups to methylthio groups or tert-butoxy groups, 12b and 12c were synthesized, respectively, which show nearly identical structural and optical properties to that of 12a. By incorporation of two bithiophene groups as donors at the biphenyl core of 12a, the photophysical properties change significantly. The lowest energy absorption of 12d red shifts to 408 nm and the compound exhibits dual emission with peaks at 446 (τF = 0.5 ns) and 639 nm (τF = 4.38 ns). DFT calculations indicate that two stable structures are present in the excited state: in one, the B–O bond remains intact (4-coordinate excited state) and in the other one, the B–O bond dissociates (3-coordinate excited state). The shorter wavelength emission exhibits the shorter lifetime and is assigned to the emission from the 4-coordinate S1 state. The long wavelength emission exhibits the longer lifetime and is assigned to the 3-coordinate S1 state.
500 cm−1) for a small molecule; in fact, it is the largest Stoke shifts ever reported.141 This large Stokes shift is caused by the photodissociation of the B–O dative bond in the excited state, which is further supported by theoretical studies. By changing the methoxy groups to methylthio groups or tert-butoxy groups, 12b and 12c were synthesized, respectively, which show nearly identical structural and optical properties to that of 12a. By incorporation of two bithiophene groups as donors at the biphenyl core of 12a, the photophysical properties change significantly. The lowest energy absorption of 12d red shifts to 408 nm and the compound exhibits dual emission with peaks at 446 (τF = 0.5 ns) and 639 nm (τF = 4.38 ns). DFT calculations indicate that two stable structures are present in the excited state: in one, the B–O bond remains intact (4-coordinate excited state) and in the other one, the B–O bond dissociates (3-coordinate excited state). The shorter wavelength emission exhibits the shorter lifetime and is assigned to the emission from the 4-coordinate S1 state. The long wavelength emission exhibits the longer lifetime and is assigned to the 3-coordinate S1 state.
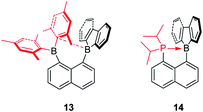
The Gabbaï group synthesized a diborane (13) with a BMes2 group and a 9-borafluorene group at the 1- and 8-positions of a naphthalene, respectively (Scheme 17).142 Interestingly, an interaction occurs between the boron atom of the 9-borafluorene and one of the Mes groups. This interaction was confirmed by a short B–C distance (2.730(3) Å) between the boron atom of 9-borafluorene and the carbon atom of the Mes group which is connected to boron. Due to this interaction, the boron atom of the 9-borafluorene is slightly pyramidalized. By changing the BMes2 group to a diisopropylphosphino group, Bourissou and co-workers synthesized the naphthyl-protected 9-borafluorene (14)143 which is only stable under an inert atmosphere, but is much more stable than 9-(2-diisopropylphosphinophenyl)-9-borafluorene.144,145 The 11B NMR signal appears at −8.5 ppm, confirming the presence of a P–B dative bond. The short P–B distance (2.011(2) Å) and the significant pyramidalization (∑C–B–C = 338.45(5)°) of the boron confirmed the strong P → B interaction. This strong P → B interaction, even with fairly bulky substituents on the phosphine, indicates the flexibility of the system. In addition to the above mentioned intramolecular dative bonds in 9-borafluorenes, another interesting topic, namely intramolecular B–B dative bonds (one and two electron σ-bonds) in 9-borafluorenes which can be formed by one or two electron reductions, is discussed in the “Chemical reduction of fused boroles” section of this review (see below).
9-Borafluorene-based main chain polymers
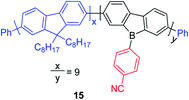
By incorporating 3-coordinate boron atoms into the main chain of conjugated polymer systems, the π-systems are extended compared to the corresponding monomers, leading to different optical properties.22 It could be envisaged that incorporation of more electron-deficient 9-borafluorenes into polymers will lead to interesting properties.146 In 2008, Scherf and co-workers reported a co-polymer incorporating polyfluorenes and 9-borafluorenes in the main chain, and applied it as an anion sensor (Scheme 18).147 Interestingly, the para-cyanophenyl group surprisingly stabilizes the 9-borafluorene, supposedly providing good environmental stability. In contrast to the bulky Tip or FMes groups, para-cyanophenyl is an “unprotected” phenyl group. Unfortunately, changing the para-cyanophenyl group to other “unprotected” phenyl groups provided unstable 9-borafluorenes. Recently, Rupar and co-workers tried to reinvestigate this compound, but although different techniques were applied, they could not reproduce the reported results.88The Rupar group adopted Yamamoto or Stille coupling reactions to synthesize two 9-Tip-9-borafluorenes based polymers (Scheme 19).148 Compared with their monomeric precursor, a red shift of the absorption and emission was observed which can be ascribed to the extended conjugation in the polymers. Quantum yields of 0.50 (16a) and 0.28 (16b) in solution were obtained. 16a and 16b have a much smaller optical bandgap (calculated from the onset of lowest energy absorption) than polyfluorenes or polycarbazoles, which is mainly attributed to the lower LUMO energies of borafluorenes, and their lower LUMO energies were confirmed by measurements of their electrochemical reduction potentials and further supported by calculations. In solution, 16a and 16b are suitable F− sensors. In a film, 16a can also be applied as a sensor for gaseous NH3.
Chujo and co-workers prepared three 9-Mamx-9-borafluorene-based conjugated polymers by Suzuki–Miyaura cross-coupling reactions (Scheme 20).149 These polymers are atmospherically stable and contain 4-coordinate boron. Compared with the corresponding gallafluorene polymers, the borafluorene polymers show stronger electron accepting abilities and lower LUMO energies.
Electrochemistry
In this section, selected fused boroles are chosen for comparison of their electron accepting abilities as determined by electrochemical measurements. Although many fused boroles can be reduced twice, here only the first reduction potentials are compared. The reduction potentials for all known aryl group protected fused boroles range from −1.1 to −2.5 V (Table 4). PhBf117 exhibits a first reversible reduction potential at −2.16 V, which is in the same range as TipBf (−2.11 V (ref. 77) and −2.31 V (ref. 88) were obtained by two different groups) and Mes*Bf (−2.28 V).103 After incorporation of donating groups (methoxy, amino, or thiophene) on the core of 9-borafluorene, the first reduction potentials of 6d,887a103 and 7b103 do not change significantly, indicating that the donating groups have only a small effect on the electron accepting ability of boron in 9-borafluorenes.| Compound | E 1/2/V (1) | E pc/V (2) | Conditions |
|---|---|---|---|
| PhBf 117 | −2.16 | — | 0.1 M [nBu4N][B(C6F5)4] in THF |
| TipBf 77 | −2.11 | −3.05 | 0.1 M [nBu4N][PF6] in THF |
| TipBf 88 | −2.31 | — | 0.1 M [nBu4N][PF6] in THF |
| Mes*Bf 103 | −2.28 | — | 0.1 M [nBu4N][ClO4] in THF |
| 6d 88 | −2.36 | — | 0.1 M [nBu4N][PF6] in THF |
| 7a 103 | −2.04 | −2.70 (r) | 0.1 M [nBu4N][ClO4] in THF |
| 7b 103 | −2.19 | −3.00 | 0.1 M [nBu4N][ClO4] in THF |
| FMesBf 88 | −1.93 | — | 0.1 M [nBu4N][PF6] in THF |
| 10b 129 | −1.94 | −2.90 | 0.1 M [nBu4N][PF6] in THF |
| 3a 88 | −1.98 | −2.79 | 0.1 M [nBu4N][PF6] in THF |
| 3b 88 | −1.96 | −2.89 | 0.1 M [nBu4N][PF6] in THF |
| 3c 77 | −1.72 | −2.61 | 0.1 M [nBu4N][PF6] in THF |
| 3d 105 | −2.25 | −3.04 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 3e 105 | −1.97 | −2.85 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 3f 105 | −1.89 | −2.78 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 4 (ref. 80) | −1.51 | −2.42 | 0.1 M [nBu4N][PF6] in THF |
| 18 (ref. 150) | −1.49 | −1.75 | 0.1 M [nBu4N][PF6] in THF |
| 1a 68 | −1.21 | −2.12 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 1b 68 | −1.13 | −2.04 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 1c 68 | −1.28 | −2.15 | 0.1 M [nBu4N][PF6] in CH2Cl2 |
| 5b·THF117 | −2.42 (ir) | — | 0.1 M [nBu4N][B(C6F5)4] in THF |
| iPr2NBf 88 | −2.95 | — | 0.1 M [nBu4N][PF6] in THF |
By employing the electron withdrawing FMes group as the exo-aryl group on a Bf, the electron accepting ability of FMesBf was enhanced and the reduction potential shifts to −1.93 V.88 The first reduction potential of phenylpyridyl-fused borole 10b (Ered1/2 = −1.94 V)129 is comparable to that of FMesBf, which suggests that the effect of fusing a pyridyl group onto boroles on their reducibility is comparable to that of the exo-FMes group in 9-borafluorenes. Benzothiophene-fused borole 3c77 exhibits a first reversible reduction potential of −1.72 V, which is less negative than the electron withdrawing group-functionalized FMesBf and 10b. The strong electron accepting property of 3c is attributed to its enhanced antiaromaticity. Diborole 4 (ref. 80) and the biphenyl-linked diborole 18 (Scheme 21)85,150 exhibit much less negative half reduction potentials of −1.51 and −1.49 V, respectively. The strong electron accepting ability of 4 and 18 is due to the two boron centers being linked to a π-conjugated unit. This enhancement is also observed in triarylboranes with two or more boron centers.100,151–153 By incorporation of four additional CF3 groups at the biphenyl core, the first half reduction potentials of 1a and 1b shift to −1.21 and −1.13 V, respectively.68 Surprisingly, although 1a and 1b exhibit extraordinarily low reduction potentials, both are stable in air.
Attempts to use noncoordinating solvents (CH2Cl2 and α,α,α-trifluorotoluene) for the cyclic voltammetry study of perfluorinated-PhBf, 5b were unsuccessful, so THF was employed.117 An irreversible process with a reduction potential at −2.42 V vs. Fc/Fc+ was observed. Such a negative reduction potential, which is even more negative than that of PhBf, was not expected. The authors suggested that the reduction process actually takes place at the fluorinated aromatic framework, not at the boron center due to the formation of 5b·THF in THF. iPr2NBf has the most negative reversible half reduction potential of −2.95 V.88 The weak electron accepting ability of iPr2NBf is caused by the electron-rich nitrogen atom π-backbonding to the vacant pz-orbital of boron.
Chemical reduction of fused boroles
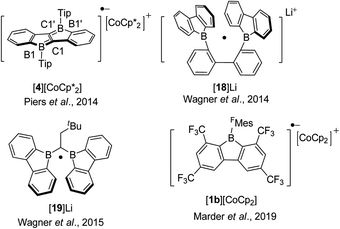
Boroles readily accept one electron to form a radical anion or two successive electrons to give a dianion. The chemical reduction of non-fused “free” boroles and some 9-borafluorenes was reviewed by the Braunschweig group in 2011 (ref. 48) and 2016.49 Here we focus only on fused boroles.One electron reduction of 9-borafluorene leads to a radical anion. In 2008, the Yamaguchi group reduced Mes*Bf with potassium in THF and the EPR signal of the reduced Mes*Bf exhibits an eleven-line signal (g = 2.002).103 According to the simulation of the spectrum, the spin density on boron is estimated to be 0.21, indicating delocalization over the biphenylene unit of the 9-borafluorene. A similar reaction was also carried out with 7b and a spin density of 0.18 on boron was estimated by simulation. The lower spin density on boron in 7b suggested that it is delocalized over the bithiophene skeleton. The Piers group synthesized the ladder-type diborole 4 which exhibits a first reversible reduction at −1.51 V. Such a small negative reduction potential makes it possible to perform a one electron reduction with bis(pentamethylcyclopentadienyl)cobalt(II) (E0′(Cp*2Co) = −1.9 V).154 Isolated [4][CoCp*2] is a deep blue solid (Scheme 21).80 The C1–C1′ distance in [4][CoCp*2] of 1.410(3) Å is significantly longer than that of its neutral form for which d(C1–C1′) = 1.367(5) Å while d(B1–C1) of [4][CoCp*2] with 1.524(3) Å is significantly shorter compared to 1.571(4) Å for 4. A detailed inspection of the structure combined with a theoretical analysis shows that there is still a high degree of delocalization of the unpaired electron throughout the whole π system.
In 2014, Wagner and co-workers linked two 9-borafluorenes by a biphenyl (18) and carried out the one electron reduction with lithium naphthalenide in toluene (Scheme 21),150 obtaining [18]Li·(THF)4 × 0.5C10H8 as black crystals. Single crystal X-ray diffraction shows that the distance between the two boron centers of [18]Li·(THF)4 × 0.5C10H8 is 2.265(4) Å, which is 0.655 Å shorter than that in its precursor 18 (2.920(6) Å) (Fig. 6),84 and lies between those of 18 (no B–B bond) and [HBf–HBf][K·Et2O]K (1.83(2) Å) or [HBf–HBf][Na·(THF)3]2 (1.822(4) Å, B–B two electron σ-bond. It is important to note that [HBf–HBf]Na2 can be prepared in quantitative yields and represents an extremely rare example of diborane(6) dianions).155–158 In [18]Li·(THF)4 × 0.5C10H8, only a moderate pyramidalization of the two boron centers was observed (∑C–B–C = 351.6° and 353.0°). The EPR spectrum of [18]Li in THF exhibits a seven-line signal. The spectrum was successfully simulated, assuming the two boron nuclei to be magnetically equivalent (a(11B) = 4.8 ± 0.1 G and a(10B) = 1.6 ± 0.1 G), and the small a(11B) value strongly supports the contribution of the unpaired electron to a 2pzσ(B·B) bond. The computed SOMO and the localization of the spin-density mainly between the two boron centers further confirm the existence of a B·B one electron σ-bond. [18]Li·(THF)4 × 0.5C10H8 is the first crystallographic characterized compound to have a B·B one electron σ-bond.
One year later, a radical anion containing two 9-borafluorenes linked by a substituted methylene group, [19]Li, was synthesized by a comproportionation reaction between neutral 19 and its dianion, [19]Li2.158 The radical anion [Li(THF)4][19]·(THF)4 × THF was characterized by single-crystal X-ray diffraction and exhibits a B⋯B distance of 2.166(4) Å. Via simulation of the EPR spectrum, comparable hyperfine coupling values of [19]˙− (a(11B) = 5.1 ± 0.1 G and a(10B) = 1.7 ± 0.2 G) to that of [18]˙− were obtained. Based on the small a(11B) value, as well as the low degree of pyramidalization of the boron atom in [Li(THF)4][19]·(THF)4 × THF, the authors concluded that there is a strong contribution from pz orbital on each boron to the SOMO. This is similar to the situation in [18]˙−, but the hyperfine coupling to protons in [19]˙− suggests that the spin density is delocalized over the π-systems.
The tetrakis-CF3-functionalized FMesBf, 1b synthesized by Marder and co-workers exhibits a first half-reduction potential at −1.13 V, and thus can be reduced by Cp2Co (E0′(Cp2Co) = −1.3 V).154 [1b][Cp2Co] was obtained as a dark purple solid with a complex EPR signal centered at giso = 2.004 in THF, consisting of hyperfine splitting to boron (a(11B) = 3.3 G), the fluorine atoms (a(19F) = 11.3 and 6.0 G) from the CF3 groups at the 9-borafluorene core, and the hydrogen atoms (a(1H) = 6.1 and 2.9 G) at the 9-borafluorene core. The relatively large proton and fluorine hyperfine couplings, in contrast to the relatively small boron hyperfine coupling, indicates that the spin density is delocalized significantly over the biphenyl core of the 9-borafluorene. Compared to the crystal structure of neutral 1b, changes in bond lengths of [1b][Cp2Co] were mainly observed on the 9-borafluorene core, indicating delocalization of the unpaired electron on the 9-borafluorene core, with no contribution from the exo-aryl FMes group.68
The discovery and study of 9-borafluorenyl dianions were reported earlier than that of the radical anions of 9-borafluorenes. In 1996, during an investigation of the reduction of sterically encumbered arylboron dihalides, the Power group isolated a bislithium-9-borafluorenyl complex [20]Li2·(Et2O)2 (Scheme 22),155 obtained directly by treatment of 2,6-Mes2C6H3BX2 (X = Br or Cl) with an excess of lithium in Et2O. [20]Li2·(Et2O)2 is a red solid with a 11B NMR (C6D6) chemical shift of 14.3 ppm (s, W1/2 ≈ 380 Hz). [20]Li2·(Et2O)2 was the first structurally characterized 9-borafluorenyl dianion, the core of which is still planar. The lithium ions are solvated by diethyl ether and adopt an η5-coordination to the 5-membered borole ring. Reduction of 2,6-Mes2C6H3BBr2 with lithium in benzene over 5 days and extraction with ether/hexane gave a dimer that has a structure analogous to that of [20]Li2·(Et2O)2. [2a]Li2 (ref. 75) and [PhBf]Li2 (ref. 65) were isolated by treatment of the corresponding 9-borafluorenes with lithium in diethyl ether or THF. [2a]Li2 is a deep red, almost black solid with a 11B NMR (C6D6) chemical shift of 13.6 ppm (s, W1/2 ≈ 430 Hz). The two lithium ions in [2a]Li2·(Et2O)2 are also solvated by diethyl ether and are situated almost symmetrically above and below of the 5-membered borole core. The 11B NMR (d8-THF) chemical shift of [PhBf]Li2 shows a sharp peak at 6.3 ppm. By treatment of diborole 4 with potassium naphthalenide (2 eq.) in THF, [4]K2 was isolated as a red solid with a 11B NMR (C6D6) chemical shift of 32.1 ppm. Its X-ray crystal structure indicates that the two potassium atoms are situated above and below the center of the dibenzo-fused-diborole core in a centrosymmetric arrangement.80 Similarly to diborole 4, compound 19 possesses two 9-borafluorene moieties and thus can easily accept two electrons.158 The dianion [19]Li2 was obtained as a red solid with a 11B NMR (d8-THF) chemical shift of −6.7 ppm (h1/2 = 270 Hz). The distance between the two boron atoms in [19][Li(Et2O)2][Li(Et2O)] is 1.906(3) Å, which is shorter than that in its radical anion ([Li(THF)4][19]·(THF)4 × THF, 2.166(4) Å) and neutral form (19, 2.534(2) Å). The ∠B–C–B angles decrease from 105.5(2)° ([19][Li(Et2O)2][Li(Et2O)]) to 86.9(2)° (radical anion [Li(THF)4][19]·(THF)4 × THF), and to 73.2(1)° (neutral form 19). The spectroscopic and structural parameters clearly suggest the presence of a covalent two center-two electron (2c-2e−) B–B bond in [19]Li2, which was further supported by theoretical studies.
The Wagner group also investigated the redox chemistry of 9-H-9-borafluorene, 21 (Scheme 22).159,160 Upon reduction, adducts arising from extensive structural rearrangement were formed and the corresponding mechanism was studied. Besides rearrangement adducts, recently, the dianion [21]2− was isolated upon reduction of monomeric 21·THF.157 Treatment of 21 with lithium, sodium or potassium in THF led to the isolation of the respective dianions. [21]Li2·(THF)3 and [21][Na·(THF)3][Na·THF] were obtained as greenish-brown solids and [21]K2·(THF) is a brown solid. The 11B NMR (d8-THF) chemical shifts are 3.7 ppm (s, W1/2 ≈ 290 Hz, [21]Li2), 3.9 ppm (s, W1/2 ≈ 290 Hz, [21]Na2) and 8.0 ppm (s, W1/2 ≈ 360 Hz, 21K2). From a comparison of the bond lengths of the computed structure of neutral 21 and of the reduced form [21][Na·(THF)3][Na·THF], the authors draw the conclusion that the two added electrons are delocalized over the 9-borafluorene core, rather than being localized at the pz-orbital of boron. [21]2− is the first example of a hydride ligand-stabilized 9-borafluorene anion. Due to the easy abstraction of the hydride, [21]2− is a surrogate of a nucleophilic 9-borafluorene anion. The reaction of [21]2− with MeI and Et3SiCl further proves that [21]2− can be applied as a 9-borafluorene anion. Similarly, reaction of B–B dianions with MeI led to the formation of 9-methyl-borafluorenes.161,162 At the same time, radical reactivity of [21]2− was found, e.g., by the reaction of [21]2− with Me3SnCl or (bromomethyl)cyclopropane.
Three-coordinate borafluorenium cations
Instead of adding electrons to Bfs in a reduction process, another interesting topic is extracting an anion from 3-coordinate Bf to generate a 3-coordinate borafluorenium cation. In 1985, Nöth and co-workers applied GaCl3 and AlCl3 as a Cl− acceptor for the pyridine adduct of ClBf and the acridine adduct of ClBf (Scheme 23).163 Both [PyBf][GaCl4] and [AcrBf][GaCl4] are red solids. Due to the insolubility of [PyBf][GaCl4] in most solvents, the only direct proof of the formation of [PyBf][GaCl4] is its IR spectrum which exhibits a strong band at 373 cm−1. This band is in the typical range for νabs (GaCl4).164 The structure of [AcrBf][GaCl4] was confirmed by single crystal X-ray diffraction, but without further characterization or study.Interested in the properties of borafluorenium cations, recently, Gilliard Jr and co-workers extracted a bromide ion from carbene-stabilized BrBfs with AgSbF6 and synthesized [IPrBf][SbF6] and [Et2CAACBf][SbF6] (Scheme 24).165 The 11B NMR spectra of [IPrBf][SbF6] and [Et2CAACBf][SbF6] show signals at 63.6 and 65.5 ppm, respectively, confirming that these two borafluorenium cations are 3-coordinate. Inspired by the decolorization upon addition of THF, they designed [IPrMeOBf][SbF6] to tune the color.
The single-crystal X-ray diffraction analyses indicate that the two C–B bond lengths in the borole ring are the same for [IPrBf][SbF6] (C1–B1 1.615(11) and C2–B1 1.57(2) Å, Fig. 7) and [Et2CAACBf][SbF6] (C1–B1 1.553(10) and C2–B1 1.567(10) Å). However, in [IPrMeOBf][SbF6], the C1–B1 bond (1.509(7) Å) is much shorter than the C2–B1 bond (1.602(7) Å). The authors suggested that this is caused by the conjugation of the lone pair on oxygen with the cationic boron center. Interestingly, compared to [IPrBf][SbF6] and [Et2CAACBf][SbF6] which exhibit a very weak absorbance in the 400–600 nm range in CH2Cl2, a strong absorbance at 430–600 nm in CH2Cl2 was found for [IPrMeOBf][SbF6]. Another interesting finding is that [IPrMeOBf][SbF6] shows thermochromic behavior; the red color of [IPrMeOBf][SbF6] faded to colorless in non-coordinating solvents upon cooling. The authors suggested that this phenomenon is caused by an intermolecular O⋯B interaction of [IPrMeOBf][SbF6] which is favored at low temperature. Attempts to grow colorless crystals of [IPrMeOBf][SbF6] which feature an O⋯B interaction at low temperature were unsuccessful. Furthermore, to support their hypothesis, THF was added to simulate the intermolecular coordination. After addition of 50 equivalents of THF to a red CH2Cl2 solution of [IPrMeOBf][SbF6], the solution became colorless. When this solution was heated to 40 °C, the red color was recovered. After cooling, this solution became colorless again. These phenomena further support the hypothesis of an intermolecular O⋯B interaction at low temperature.
Conclusions and outlook
This review begins with the fundamental synthetic strategies for preparing (hetero)arene-fused boroles and the stability of different 9-substituent-9-borafluorenes, and then discusses functionalized (hetero)arene-fused boroles which can be applied as Lewis acids, activators of H2, fluorescent materials, electron accepting units, etc. For functionalized (hetero)arene-fused boroles, the chemistry of reported 9-borafluorenes is classified, and a guide for the design of novel (hetero)arene-fused boroles to achieve different properties is provided.Compared to the corresponding triarylboranes, (hetero)arene-fused boroles exhibit an enhanced electron accepting ability, which is attributed to the antiaromaticity and strain of the 5-membered borole ring. Triarylboranes have found wide application, e.g., as acceptors in TADF materials. The exo-aryl group of 9-aryl-9-borafluorene adopts a twisted arrangement with respect to the 9-borafluorene core and thus, by functionalization, may also generate good candidates for TADF materials. Surprisingly, thus far, only one such example was reported outside of patents. More studies on the functionalization of the exo-aryl moiety will be of particular interest.
Compared to non-fused “free” boroles, arene-fused boroles exhibit higher stability and potential for functionalization. Depending on the fused aryl groups, enhanced electron accepting ability and enhanced antiaromaticity, even greater than that of non-fused “free” boroles, unique coordination modes, and dual fluorescence can be realized. Heteroarene-fused boroles are interesting compounds which require further study, (e.g., other electron-rich or -poor heteroarene-fused boroles), as they have many potential applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. H. thanks the China Scholarship Council for a PhD scholarship. We thank the Julius-Maximilians-Universität Würzburg, the Deutsche Forschungsgemeinschaft (DFG), (GRK 2112 and MA 4471/8-1) and the Bavarian State Ministry of Science, Research, and the Arts under the Collaborative Research Network “Solar Technologies go Hybrid” for financial support.References
- S. Yamaguchi, S. Akiyama and K. Tamao, J. Am. Chem. Soc., 2001, 123, 11372–11375 CrossRef CAS.
- K. Parab, K. Venkatasubbaiah and F. Jäkle, J. Am. Chem. Soc., 2006, 128, 12879–12885 CrossRef CAS.
- T. W. Hudnall, C.-W. Chiu and F. P. Gabbaï, Acc. Chem. Res., 2009, 42, 388–397 CrossRef CAS.
- Z. Yuan, N. J. Taylor, T. B. Marder, I. D. Williams, S. K. Kurtz and L.-T. Cheng, J. Chem. Soc., Chem. Commun., 1990, 1489–1492 RSC.
- M. Lequan, R. M. Lequan and K. C. Ching, J. Mater. Chem., 1991, 1, 997–999 RSC.
- M. Lequan, R. M. Lequan, K. C. Ching, A.-C. Callier, M. Barzoukas and A. Fort, Adv. Mater. Opt. Electron., 1992, 1, 243–247 CrossRef CAS.
- Z. Yuan, N. J. Taylor, Y. Sun, T. B. Marder, I. D. Williams and L.-T. Cheng, J. Organomet. Chem., 1993, 449, 27–37 CrossRef CAS.
- Z. Yuan, N. J. Taylor, R. Ramachandran and T. B. Marder, Appl. Organomet. Chem., 1996, 10, 305–316 CrossRef CAS.
- C. Branger, M. Lequan, R. M. Lequan, M. Barzoukas and A. Fort, J. Mater. Chem., 1996, 6, 555–558 RSC.
- Z. Yuan, J. C. Collings, N. J. Taylor, T. B. Marder, C. Jardin and J.-F. Halet, J. Solid State Chem., 2000, 154, 5–12 CrossRef CAS.
- Z. Yuan, C. D. Entwistle, J. C. Collings, D. Albesa-Jové, A. S. Batsanov, J. A. K. Howard, N. J. Taylor, H. M. Kaiser, D. E. Kaufmann, S.-Y. Poon, W.-Y. Wong, C. Jardin, S. Fathallah, A. Boucekkine, J.-F. Halet and T. B. Marder, Chem.–Eur. J., 2006, 12, 2758–2771 CrossRef CAS.
- J. C. Collings, S.-Y. Poon, C. Le Droumaguet, M. Charlot, C. Katan, L.-O. Pålsson, A. Beeby, J. A. Mosely, H. M. Kaiser, D. Kaufmann, W.-Y. Wong, M. Blanchard-Desce and T. B. Marder, Chem.–Eur. J., 2009, 15, 198–208 CrossRef CAS.
- C. D. Entwistle, J. C. Collings, A. Steffen, L.-O. Pålsson, A. Beeby, D. Albesa-Jové, J. M. Burke, A. S. Batsanov, J. A. K. Howard, J. A. Mosely, W.-Y. Wong, F. Ibersiene, S. Fathallah, A. Boucekkine, J.-F. Halet and T. B. Marder, J. Mater. Chem., 2009, 19, 7532–7544 RSC.
- L. Ji, R. M. Edkins, L. J. Sewell, A. Beeby, A. S. Batsanov, K. Fucke, M. Drafz, J. A. K. Howard, O. Moutounet, F. Ibersiene, A. Boucekkine, E. Furet, Z. Q. Liu, J.-F. Halet, C. Katan and T. B. Marder, Chem.–Eur. J., 2014, 20, 13618–13635 CrossRef CAS.
- S. Griesbeck, Z. Zhang, M. Gutmann, T. Lühmann, R. M. Edkins, G. Clermont, A. N. Lazar, M. Haehnel, K. Edkins, A. Eichhorn, M. Blanchard-Desce, L. Meinel and T. B. Marder, Chem.–Eur. J., 2016, 22, 14701–14706 CrossRef CAS.
- S. Griesbeck, E. Michail, C. Wang, H. Ogasawara, S. Lorenzen, L. Gerstner, T. Zang, J. Nitsch, Y. Sato, R. Bertermann, A. Friedrich, M. Haehnel, D. Sieh, M. Taki, S. Yamaguchi and T. B. Marder, Chem. Sci., 2019, 10, 5405–5422 RSC.
- S. Griesbeck, M. Ferger, C. Czernetzi, C. Wang, R. Bertermann, A. Friedrich, M. Haehnel, D. Sieh, M. Taki, S. Yamaguchi and T. B. Marder, Chem.–Eur. J., 2019, 25, 7679–7688 CrossRef CAS.
- S. Griesbeck, E. Michail, F. Rauch, H. Ogasawara, C. Wang, Y. Sato, R. M. Edkins, Z. Zhang, M. Taki, C. Lambert, S. Yamaguchi and T. B. Marder, Chem.–Eur. J., 2019, 25, 13164–13175 CrossRef CAS.
- Ž. Ban, S. Griesbeck, S. Tomić, J. Nitsch, T. B. Marder and I. Piantanida, Chem.–Eur. J., 2020, 26, 2195–2203 CrossRef.
- H. Amini, Ž. Ban, M. Ferger, S. Lorenzen, F. Rauch, A. Friedrich, I. Crnolatac, A. Kenđel, S. Miljanić, I. Piantanida and T. B. Marder, Chem.–Eur. J., 2020, 26, 6017–6028 CrossRef CAS.
- C. D. Entwistle and T. B. Marder, Angew. Chem., Int. Ed., 2002, 41, 2927–2931 CrossRef CAS.
- C. D. Entwistle and T. B. Marder, Chem. Mater., 2004, 16, 4574–4585 CrossRef CAS.
- S. Yamaguchi and A. Wakamiya, Pure Appl. Chem., 2006, 78, 1413–1424 CAS.
- F. Jäkle, Coord. Chem. Rev., 2006, 250, 1107–1121 CrossRef.
- Z. M. Hudson and S. Wang, Acc. Chem. Res., 2009, 42, 1584–1596 CrossRef CAS.
- C. R. Wade, A. E. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS.
- Z. M. Hudson and S. Wang, Dalton Trans., 2011, 40, 7805–7816 RSC.
- A. Wakamiya and S. Yamaguchi, Bull. Chem. Soc. Jpn., 2015, 88, 1357–1377 CrossRef CAS.
- L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846–863 RSC.
- S.-Y. Li, Z.-B. Sun and C.-H. Zhao, Inorg. Chem., 2017, 56, 8705–8717 CrossRef CAS.
- G. Turkoglu, M. E. Cinar and T. Ozturk, Molecules, 2017, 22, 1522 CrossRef.
- X. Yin, J. Liu and F. Jäkle, Chem.–Eur. J., 2020 DOI:10.1002/chem.202003481.
- R. Hoffmann, J. Chem. Phys., 1963, 39, 1397–1412 CrossRef CAS.
- R. Breslow, Chem. Eng. News, 1965, 43, 90–100 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 1973, 6, 393–398 CrossRef CAS.
- M. Saunders, R. Berger, A. Jaffe, J. M. McBride, J. O'Neill, R. Breslow, J. M. Hoffmann, C. Perchonock and E. Wasserman, J. Am. Chem. Soc., 1973, 95, 3017–3018 CrossRef CAS.
- W. A. Yager, J. Am. Chem. Soc., 1963, 85, 2033–2034 CrossRef.
- E. J. P. Malar and K. Jug, Tetrahedron, 1986, 42, 417–426 CrossRef CAS.
- P. v. R. Schleyer, P. K. Freeman, H. Jiao and B. Goldfuss, Angew. Chem., Int. Ed., 1995, 34, 337–340 CrossRef CAS.
- M. K. Cyranski, T. M. Krygowski, A. R. Katritzky and P. v. R. Schleyer, J. Org. Chem., 2002, 67, 1333–1338 CrossRef CAS.
- J. J. Eisch, N. K. Hota and S. Kozima, J. Am. Chem. Soc., 1969, 91, 4575–4577 CrossRef CAS.
- H. Braunschweig, I. Fernández, G. Frenking and T. Kupfer, Angew. Chem., Int. Ed., 2008, 47, 1951–1954 CrossRef CAS.
- C. Fan, W. E. Piers and M. Parvez, Angew. Chem., Int. Ed., 2009, 48, 2955–2958 CrossRef CAS.
- H. Braunschweig, V. Dyakonov, J. O. C. Jimenez-Halla, K. Kraft, I. Krummenacher, K. Radacki, A. Sperlich and J. Wahler, Angew. Chem., Int. Ed., 2012, 51, 2977–2980 CrossRef CAS.
- Z. Zhang, R. M. Edkins, M. Haehnel, M. Wehner, A. Eichhorn, L. Mailänder, M. Meier, J. Brand, F. Brede, K. Müller-Buschbaum, H. Braunschweig and T. B. Marder, Chem. Sci., 2015, 6, 5922–5927 RSC.
- J. J. Eisch, Adv. Organomet. Chem., 1996, 39, 355–392 CrossRef CAS.
- A. Steffen, R. M. Ward, W. D. Jones and T. B. Marder, Coord. Chem. Rev., 2010, 254, 1950–1976 CrossRef CAS.
- H. Braunschweig and T. Kupfer, Chem. Commun., 2011, 47, 10903–10914 RSC.
- H. Braunschweig and I. Krummenacher, Organic Redox Systems: Synthesis, Properties, and Applications, ed. T. Nishinaga, John Wiley & Sons, Hoboken (New Jersey), 2016, pp. 503–522 Search PubMed.
- H. Braunschweig, I. Krummenacher and J. Wahler, in Adv. Organomet. Chem., 2013, vol. 61, pp. 1–53 Search PubMed.
- A. Wakamiya, in Main Group Strategies towards Functional Hybrid Materials, ed. T. Baumgartner and F. Jäkle, John Wiley & Sons Ltd., Hoboken (New Jersey), 2018, vol. 1, pp. 1–26 Search PubMed.
- J. H. Barnard, S. Yruegas, K. Huang and C. D. Martin, Chem. Commun., 2016, 52, 9985–9991 RSC.
- B. Su and R. Kinjo, Synthesis, 2017, 2985–3034 CAS.
- Y. Su and R. Kinjo, Chem. Soc. Rev., 2019, 48, 3613–3659 RSC.
- R. Köster and G. Benedikt, Angew. Chem., Int. Ed., 1963, 6, 323–324 CrossRef.
- A. Escande and M. J. Ingleson, Chem. Commun., 2015, 51, 6257–6274 RSC.
- E. v. Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef.
- H. Budy, J. Gilmer, T. Trageser and M. Wagner, Eur. J. Inorg. Chem., 2020 DOI:10.1002/ejic.202000786.
- C. K. Narula and H. Nöth, J. Organomet. Chem., 1985, 281, 131–134 CrossRef CAS.
- J. J. Eisch, J. E. Galle and S. Kozima, J. Am. Chem. Soc., 1986, 108, 379–385 CrossRef CAS.
- P. A. Chase, W. E. Piers and B. O. Patrick, J. Am. Chem. Soc., 2000, 122, 12911–12912 CrossRef CAS.
- S. Yruegas, J. J. Martinez and C. D. Martin, Chem. Commun., 2018, 54, 6808–6811 RSC.
- U. Gross and D. Kaufmann, Chem. Ber., 1987, 120, 991–994 CrossRef CAS.
- H. Hong and T. Chung, J. Organomet. Chem., 2004, 689, 58–64 CrossRef CAS.
- P. E. Romero, W. E. Piers, S. A. Decker, D. Chau, T. K. Woo and M. Parvez, Organometallics, 2003, 22, 1266–1274 CrossRef CAS.
- S. Yamaguchi, T. Shirasaka, S. Akiyama and K. Tamao, J. Am. Chem. Soc., 2002, 124, 8816–8817 CrossRef CAS.
- R.-F. Chen, Q.-L. Fan, C. Zheng and W. Huang, Org. Lett., 2006, 8, 203–205 CrossRef CAS.
- F. Rauch, S. Fuchs, A. Friedrich, D. Sieh, I. Krummenacher, H. Braunschweig, M. Finze and T. B. Marder, Chem.–Eur. J., 2020, 26, 12794–12808 CrossRef CAS.
- K. Schickedanz, T. Trageser, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2015, 51, 15808–15810 RSC.
- K. Schickedanz, J. Radtke, M. Bolte, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2017, 139, 2842–2851 CrossRef CAS.
- D. J. Morrison, W. E. Piers and M. Parvez, Synlett, 2004, 2429–2433 CAS.
- Á. Gyömöre, M. Bakos, T. Földes, I. Pápai, A. Domján and T. Soós, ACS Catal., 2015, 5, 5366–5372 CrossRef.
- J. Merz, J. Fink, A. Friedrich, I. Krummenacher, H. H. Al Mamari, S. Lorenzen, M. Haehnel, A. Eichhorn, M. Moos, M. Holzapfel, H. Braunschweig, C. Lambert, A. Steffen, L. Ji and T. B. Marder, Chem.–Eur. J., 2017, 23, 13164–13180 CrossRef CAS.
- J. Merz, A. Steffen, J. Nitsch, J. Fink, C. B. Schürger, A. Friedrich, I. Krummenacher, H. Braunschweig, M. Moos, D. Mims, C. Lambert and T. B. Marder, Chem. Sci., 2019, 10, 7516–7534 RSC.
- R. J. Wehmschulte, M. A. Khan, B. Twamley and B. Schiemenz, Organometallics, 2001, 20, 844–849 CrossRef CAS.
- R. J. Wehmschulte, A. A. Diaz and M. A. Khan, Organometallics, 2003, 22, 83–92 CrossRef CAS.
- A. Iida and S. Yamaguchi, J. Am. Chem. Soc., 2011, 133, 6952–6955 CrossRef CAS.
- M. Urban, K. Durka, P. Górka, G. Wiosna-Sałyga, K. Nawara, P. Jankowski and S. Luliński, Dalton Trans., 2019, 48, 8642–8663 RSC.
- J. F. Araneda, B. Neue, W. E. Piers and M. Parvez, Angew. Chem., Int. Ed., 2012, 51, 8546–8550 CrossRef CAS.
- J. F. Araneda, W. E. Piers, M. J. Sgro and M. Parvez, Chem. Sci., 2014, 5, 3189–3196 RSC.
- A. Hübner, Z.-W. Qu, U. Englert, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, J. Am. Chem. Soc., 2011, 133, 4596–4609 CrossRef.
- A. Das, A. Hübner, M. Weber, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2011, 47, 11339–11341 RSC.
- A. Hübner, M. Diefenbach, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, Angew. Chem., Int. Ed., 2012, 51, 12514–12518 CrossRef.
- A. Hübner, A. M. Diehl, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2013, 32, 6827–6833 CrossRef.
- S. Biswas, I. M. Oppel and H. F. Bettinger, Inorg. Chem., 2010, 49, 4499–4506 CrossRef CAS.
- C. J. Berger, G. He, C. Merten, R. McDonald, M. J. Ferguson and E. Rivard, Inorg. Chem., 2014, 53, 1475–1486 CrossRef CAS.
- Y. Shoji, N. Tanaka, S. Muranaka, N. Shigeno, H. Sugiyama, K. Takenouchi, F. Hajjaj and T. Fukushima, Nat. Commun., 2016, 7, 1–7 Search PubMed.
- M. F. Smith, S. J. Cassidy, I. A. Adams, M. Vasiliu, D. L. Gerlach, D. A. Dixon and P. A. Rupar, Organometallics, 2016, 35, 3182–3191 CrossRef CAS.
- W. Zhang, G. Li, L. Xu, Y. Zhuo, W. Wan, N. Yan and G. He, Chem. Sci., 2018, 9, 4444–4450 RSC.
- S. Yruegas, J. H. Barnard, K. Al-Furaiji, J. L. Dutton, D. J. D. Wilson and C. D. Martin, Organometallics, 2018, 37, 1515–1518 CrossRef CAS.
- K. R. Bluer, L. E. Laperriere, A. Pujol, S. Yruegas, V. A. K. Adiraju and C. D. Martin, Organometallics, 2018, 37, 2917–2927 CrossRef CAS.
- F. Leroux, ChemBioChem, 2004, 5, 644–649 CrossRef CAS.
- M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806–5812 CrossRef CAS.
- S. M. Cornet, K. B. Dillon, C. D. Entwistle, M. A. Fox, A. E. Goeta, H. P. Goodwin, T. B. Marder and A. L. Thompson, Dalton Trans., 2003, 4395–4405 RSC.
- X. Yin, J. Chen, R. A. Lalancette, T. B. Marder and F. Jäkle, Angew. Chem., Int. Ed., 2014, 53, 9761–9765 CrossRef CAS.
- R. J. Blagg, E. J. Lawrence, K. Resner, V. S. Oganesyan, T. J. Herrington, A. E. Ashley and G. G. Wildgoose, Dalton Trans., 2016, 45, 6023–6031 RSC.
- R. J. Blagg, T. R. Simmons, G. R. Hatton, J. M. Courtney, E. L. Bennett, E. J. Lawrence and G. G. Wildgoose, Dalton Trans., 2016, 45, 6032–6043 RSC.
- X. Yin, F. Guo, R. A. Lalancette and F. Jäkle, Macromolecules, 2016, 49, 537–546 CrossRef CAS.
- S. Toyota, M. Asakura, M. Oki and F. Toda, Bull. Chem. Soc. Jpn., 2000, 73, 2357–2362 CrossRef CAS.
- F. Rauch, P. Endres, A. Friedrich, D. Sieh, M. Hähnel, I. Krummenacher, H. Braunschweig, M. Finze, L. Ji and T. B. Marder, Chem.–Eur. J., 2020, 26, 12951–12963 CrossRef CAS.
- A. K. Narsaria, F. Rauch, J. Krebs, P. Endres, A. Friedrich, I. Krummenacher, H. Braunschweig, M. Finze, J. Nitsch, F. M. Bickelhaupt and T. B. Marder, Adv. Funct. Mater., 2020, 30, 2002064 CrossRef CAS.
- F. Rauch, J. Krebs, J. Günther, A. Friedrich, M. Hähnel, I. Krummenacher, H. Braunschweig, M. Finze and T. B. Marder, Chem.–Eur. J., 2020, 26, 10626–10633 CrossRef CAS.
- A. Wakamiya, K. Mishima, K. Ekawa and S. Yamaguchi, Chem. Commun., 2008, 579–581 RSC.
- L. Kaufmann, H. Vitze, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2008, 27, 6215–6221 CrossRef CAS.
- A. Iida, A. Sekioka and S. Yamaguchi, Chem. Sci., 2012, 3, 1461–1466 RSC.
- W. E. Piers, Adv. Organomet. Chem., 2005, 52, 1–76 CAS.
- G. Erker, Dalton Trans., 2005, 1883–1890 RSC.
- W. E. Piers and T. Chivers, Chem. Soc. Rev., 1997, 26, 345–354 RSC.
- P. A. Chase, L. D. Henderson, W. E. Piers, M. Parvez, W. Clegg and M. R. Elsegood, Organometallics, 2006, 25, 349–357 CrossRef CAS.
- A. Y. Houghton, V. A. Karttunen, W. E. Piers and H. M. Tuononen, Chem. Commun., 2014, 50, 1295–1298 RSC.
- A. Y. Houghton, J. Hurmalainen, A. Mansikkamäki, W. E. Piers and H. M. Tuononen, Nat. Chem., 2014, 6, 983–988 CrossRef CAS.
- V. C. Williams, W. E. Piers, W. Clegg, M. R. Elsegood, S. Collins and T. B. Marder, J. Am. Chem. Soc., 1999, 121, 3244–3245 CrossRef CAS.
- V. C. Williams, G. J. Irvine, W. E. Piers, Z. Li, S. Collins, W. Clegg, M. R. Elsegood and T. B. Marder, Organometallics, 2000, 19, 1619–1621 CrossRef CAS.
- V. C. Williams, C. Dai, Z. Li, S. Collins, W. E. Piers, W. Clegg, M. R. Elsegood and T. B. Marder, Angew. Chem., Int. Ed., 1999, 38, 3695–3698 CrossRef CAS.
- R. F. Childs, D. L. Mulholland and A. Nixon, Can. J. Chem., 1982, 60, 801–808 CrossRef CAS.
- P. Laszlo and M. Teston, J. Am. Chem. Soc., 1990, 112, 8750–8754 CrossRef CAS.
- P. A. Chase, P. E. Romero, W. E. Piers, M. Parvez and B. O. Patrick, Can. J. Chem., 2005, 83, 2098–2105 CrossRef CAS.
- K. Köhler, W. E. Piers, A. P. Jarvis, S. Xin, Y. Feng, A. M. Bravakis, S. Collins, W. Clegg, G. P. A. Yap and T. B. Marder, Organometallics, 1998, 17, 3557–3566 CrossRef.
- P. G. Hayes, W. E. Piers and M. Parvez, Organometallics, 2005, 24, 1173–1183 CrossRef CAS.
- S. P. Lewis, N. J. Taylor, W. E. Piers and S. Collins, J. Am. Chem. Soc., 2003, 125, 14686–14687 CrossRef CAS.
- L. D. Henderson, W. E. Piers, G. J. Irvine and R. McDonald, Organometallics, 2002, 21, 340–345 CrossRef CAS.
- J. Chai, S. P. Lewis, S. Collins, T. J. Sciarone, L. D. Henderson, P. A. Chase, G. J. Irvine, W. E. Piers, M. R. Elsegood and W. Clegg, Organometallics, 2007, 26, 5667–5679 CrossRef CAS.
- C. Fan, L. G. Mercier, W. E. Piers, H. M. Tuononen and M. Parvez, J. Am. Chem. Soc., 2010, 132, 9604–9606 CrossRef CAS.
- A. Y. Houghton, V. A. Karttunen, C. Fan, W. E. Piers and H. M. Tuononen, J. Am. Chem. Soc., 2013, 135, 941–947 CrossRef CAS.
- D. J. Parks and W. E. Piers, J. Am. Chem. Soc., 1996, 118, 9440–9441 CrossRef CAS.
- N. L. Allinger and J. H. Siefert, J. Am. Chem. Soc., 1975, 97, 752–760 CrossRef CAS.
- D.-M. Chen, Q. Qin, Z.-B. Sun, Q. Peng and C.-H. Zhao, Chem. Commun., 2014, 50, 782–784 RSC.
- D.-M. Chen, S. Wang, H.-X. Li, X.-Z. Zhu and C.-H. Zhao, Inorg. Chem., 2014, 53, 12532–12539 CrossRef CAS.
- J. He, F. Rauch, A. Friedrich, J. Krebs, I. Krummenacher, R. Bertermann, J. Nitsch, H. Braunschweig, M. Finze and T. B. Marder, Angew. Chem., Int. Ed. Search PubMed , submitted.
- Selected patents: (a) H. J. Lee, Y. J. Cho, H. J. Kwon, B. O. Kim, S. M. Kim and S. S. Yoon, CN Pat., 102574871A, 2010; (b) C. S. Kim, Y. J. Cho, H. J. Kwon, B. O. Kim and S. M. Kim, KR Pat., 20110049012A, 2011; (c) Z. F. Xie, CN Pat., 108409762A, 2018; (d) Y. L. Wang, Z. Xue, H. Y. Li, J. P. Wang, C. X. Gao, Z. W. Chen, L. G. Li and W. J. Wang, CN Pat., 109293690A, 2018; (e) W. Gao, L. Zhang, W. P. Dai, J. H. Niu and H. Y. Zhu, CN Pat., 110003258A, 2019.
- A. D. Allen and T. T. Tidwell, Chem. Rev., 2001, 101, 1333–1348 CrossRef CAS.
- T. B. Tai, V. T. T. Huong and M. T. Nguyen, Chem. Commun., 2013, 49, 11548–11550 RSC.
- Y. Sugihara, R. Miyatake, K. Takakura and S. Yano, J. Chem. Soc., Chem. Commun., 1994, 1925–1926 RSC.
- Y. Sugihara, K. Takakura, T. Murafuji, R. Miyatake, K. Nakasuji, M. Kato and S. Yano, J. Org. Chem., 1996, 61, 6829–6834 CrossRef CAS.
- S. Wakabayashi, S. Imamura, Y. Sugihara, M. Shimizu, T. Kitagawa, Y. Ohki and K. Tatsumi, J. Org. Chem., 2008, 73, 81–87 CrossRef CAS.
- K. Ansorg, H. Braunschweig, C.-W. Chiu, B. Engels, D. Gamon, M. Hügel, T. Kupfer and K. Radacki, Angew. Chem., Int. Ed., 2011, 50, 2833–2836 CrossRef CAS.
- K. Matsuo, S. Saito and S. Yamaguchi, J. Am. Chem. Soc., 2014, 136, 12580–12583 CrossRef CAS.
- T. Matsumoto, K. Tanaka, K. Tanaka and Y. Chujo, Dalton Trans., 2015, 44, 8697–8707 RSC.
- T. Matsumoto, H. Takamine, K. Tanaka and Y. Chujo, Mater. Chem. Front., 2017, 1, 2368–2375 RSC.
- S. J. Cassidy, I. Brettell-Adams, L. E. McNamara, M. F. Smith, M. Bautista, H. Cao, M. Vasiliu, D. L. Gerlach, F. Qu, N. I. Hammer, D. A. Dixon and P. A. Rupar, Organometallics, 2018, 37, 3732–3741 CrossRef CAS.
- F. Vollmer, W. Rettig and E. Birckner, J. Fluoresc., 1994, 4, 65–69 CrossRef CAS.
- J. D. Hoefelmeyer, S. Solé and F. P. Gabbaï, Dalton Trans., 2004, 1254–1258 RSC.
- S. Bontemps, M. Devillard, S. Mallet-Ladeira, G. Bouhadir, K. Miqueu and D. Bourissou, Inorg. Chem., 2013, 52, 4714–4720 CrossRef CAS.
- S. Bontemps, G. Bouhadir, K. Miqueu and D. Bourissou, J. Am. Chem. Soc., 2006, 128, 12056–12057 CrossRef CAS.
- L. A. Essex, J. W. Taylor and W. H. Harman, Tetrahedron, 2019, 75, 2255–2260 CrossRef CAS.
- Y. Tokoro, A. Nagai, K. Tanaka and Y. Chujo, Macromol. Rapid Commun., 2012, 33, 550–555 CrossRef CAS.
- V. D. B. Bonifácio, J. Morgado and U. Scherf, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2878–2883 CrossRef.
- I. A. Adams and P. A. Rupar, Macromol. Rapid Commun., 2015, 36, 1336–1340 CrossRef CAS.
- T. Matsumoto, S. Ito, K. Tanaka and Y. Chujo, Polym. J., 2018, 50, 197–202 CrossRef CAS.
- A. Hübner, A. M. Diehl, M. Diefenbach, B. Endeward, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, Angew. Chem., Int. Ed., 2014, 53, 4832–4835 CrossRef.
- K. Okada, T. Sugawa and M. Oda, J. Chem. Soc., Chem. Commun., 1992, 74–75 RSC.
- T. Noda and Y. Shirota, J. Am. Chem. Soc., 1998, 120, 9714–9715 CrossRef CAS.
- Y. Sun, N. Ross, S.-B. Zhao, K. Huszarik, W.-L. Jia, R.-Y. Wang, D. Macartney and S. Wang, J. Am. Chem. Soc., 2007, 129, 7510–7511 CrossRef CAS.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS.
- W. J. Grigsby and P. P. Power, J. Am. Chem. Soc., 1996, 118, 7981–7988 CrossRef CAS.
- Y. Shoji, T. Matsuo, D. Hashizume, M. J. Gutmann, H. Fueno, K. Tanaka and K. Tamao, J. Am. Chem. Soc., 2011, 133, 11058–11061 CrossRef CAS.
- J. Gilmer, H. Budy, T. Kaese, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2020, 59, 5621–5625 CrossRef CAS.
- A. Hübner, T. Kaese, M. Diefenbach, B. Endeward, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, J. Am. Chem. Soc., 2015, 137, 3705–3714 CrossRef.
- A. Hübner, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2014, 53, 10408–10411 CrossRef.
- T. Kaese, A. Hübner, M. Bolte, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2016, 138, 6224–6233 CrossRef CAS.
- T. Kaese, T. Trageser, H. Budy, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Sci., 2018, 9, 3881–3891 RSC.
- T. Trageser, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2020, 132, 7726–7731 CrossRef.
- C. K. Narula and H. Nöth, Inorg. Chem., 1985, 24, 2532–2539 CrossRef CAS.
- D. M. Adams, J. Chatt, J. M. Davidson and J. Gerratt, J. Chem. Soc., 1963, 2189–2194 RSC.
- W. Yang, K. E. Krantz, L. A. Freeman, D. A. Dickie, A. Molino, A. Kaur, D. J. Wilson and R. J. Gilliard Jr, Chem.–Eur. J., 2019, 25, 12512–12516 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |