Creating and testing an activity with interdisciplinary connections: entropy to osmosis
Brianna L.
Martinez
 ab,
Alex T.
Kararo
ab,
Alex T.
Kararo
 b,
Kristin N.
Parent
b,
Kristin N.
Parent
 c,
Sonia M.
Underwood
c,
Sonia M.
Underwood
 *b and
Rebecca L.
Matz†
*b and
Rebecca L.
Matz†
 *a
*a
aHub for Innovation in Learning and Technology, Michigan State University, East Lansing, Michigan, USA. E-mail: rlmatz@umich.edu
bDepartment of Chemistry & Biochemistry and STEM Transformation Institute, Florida International University, Miami, Florida, USA. E-mail: sonia.underwood@fiu.edu
cDepartment of Biochemistry & Molecular Biology, Michigan State University, East Lansing, Michigan, USA
First published on 1st April 2021
Abstract
Students often struggle to make interdisciplinary connections and cite a lack of opportunity to make such connections. To address this issue, we are developing activities aligned with the framework of three-dimensional learning that provide students with opportunities to make connections between chemistry concepts and biological phenomena. Here, we focus on an activity that asks students to incorporate the concept of entropy in explaining the biological phenomenon of osmosis across a cell membrane. This activity was administered in both introductory cell and molecular biology and second-semester general chemistry courses. We found that after completing carefully scaffolded questions within the activity, students were better able to correctly use the concept of entropy in explaining osmosis than they were before the scaffolding questions. Additionally, we found that students’ course history appeared to impact their explanations of this phenomenon in that students who had taken second-semester general chemistry (i.e., the semester in which entropy is discussed for these students) provided more sophisticated responses and were less likely to include scientifically inaccurate ideas than their peers who had not taken second-semester general chemistry.
Introduction
Challenges in our world, such as healthcare and environmental issues, require scientists and non-scientists alike to be able to integrate seemingly disconnected information across disciplines. As described by Tripp and Shortlidge (2019), there are several components relevant to supporting students’ well-rounded understanding of the interdisciplinary nature of science, such as disciplinary humility, disciplinary grounding, and different research methods. The work described in this paper fits most closely with the criterion called “advancement through integration”, an idea first articulated by Boix Mansilla and Duraising (2007) that underscores the furthering of understanding through the combination of two or more disciplinary perspectives. Interdisciplinary studies like this one are important for building understanding about how to support students in transferring knowledge across disciplines (National Research Council, 2012b).As chemistry concepts like thermodynamics govern many biological processes, it is particularly useful to provide opportunities for students to make connections between chemistry and biology (American Association for the Advancement of Science, 2011). Additionally, general chemistry and introductory biology courses are often related as pre- or corequisite courses (Sorensen, 2000; Bialek and Botstein, 2004; Freeman et al., 2011). However, few assessments at the college level, especially in introductory courses, encourage students to cross this disciplinary boundary and bring their understanding of chemistry to bear on explanations of biological phenomena (Haudek et al., 2012). Further, introductory biology courses have been shown to largely target recall of factual information and procedural skills (Momsen et al., 2010, 2013).
The Framework for K-12 Science Education (Framework) (National Research Council, 2012a) aims to address such issues with a vision for science education known as “three-dimensional learning” (3DL) which integrates disciplinary core ideas, crosscutting concepts, and scientific practices. To encourage alignment with 3DL instead of simply factual recall, assessments should probe students’ abilities to use scientific practices in the context of disciplinary core ideas and crosscutting concepts to make sense of phenomena. However, writing valid assessments that support 3DL is difficult (Underwood et al., 2018). Towards this end, our team is developing and testing cross-disciplinary assessments that incorporate the principles of 3DL, expecting that these activities will help us understand how students connect their chemistry and biology knowledge (Matz et al., 2019). As part of this larger project, here we describe our approach to developing, testing, and evaluating the effectiveness of one such activity.
Activity development process
Selecting the topic of interest
Given that numerous areas of connection exist between general chemistry and introductory biology curricula (Schwartz and Serie, 2001), the first step was identifying and prioritizing these topics. Over several iterations of reviewing the curricula in the courses of interest (i.e., general chemistry and cell and molecular biology) at two different institutions, observing class meetings, and discussing with research team members (including those who teach these courses of interest), we developed a list of 11 potential biology topics that rely on chemistry principles to be fully explained.To prioritize which topics were more valuable to biology faculty, we developed a survey. Each of the 11 topics was unpacked into three components: description of the biological phenomenon, description of the underlying chemistry core ideas, and the explanation of the connecting idea between biology and chemistry. We then asked faculty how valued (highly, a little, or not at all) each of these 11 areas were for their section of introductory cell and molecular biology course (Bio I) and why. Since all of the topics were covered in the relevant Bio I at one institution (a large, public, research-intensive university located in the Midwest region of the United States), this was the only institution solicited to complete the survey. The survey was given to 15 instructors, 11 of whom responded. The survey results guided which connection areas were prioritized for activity development. Here, we report on one of the first activities developed following this process which focuses on using the chemistry core idea of change and stability in chemical systems (Cooper et al., 2017) within the context of entropy to explain the biological phenomenon of osmosis across a cell membrane. This connection area was rated as of high value by all 11 responding instructors.
Why osmosis is an important biological phenomenon
Osmosis is a biochemical process referring to “water movement across a semipermeable membrane driven by differences in osmotic pressure” (Nelson and Cox, 2017). Osmosis is frequently covered in introductory science courses and is an important factor in the life of cells. Students may first be exposed to osmosis in high school (Friedler et al., 1987) while taking a biology course, again in a chemistry course when learning about osmotic pressure, and perhaps even in a physics course (Redish et al., 2014).Osmosis is a highly interdisciplinary phenomenon with concepts from biology, chemistry, and physics at play (Shen et al., 2014), therefore it is not surprising that students often struggle to provide fully correct, detailed explanations about osmosis. Indeed, research has shown that students at all levels often hold incorrect ideas even if they can predict the direction that water will flow (Odom and Barrow, 1995; Fisher et al., 2011). Friedler et al. (1987) found that some high school students cite osmosis as the result of a desire to equalize concentrations. This explanation can predict the natural direction of water flow, however, it offers no mechanism for why osmosis occurs and instead relies on anthropomorphism and the cell “wanting” to equalize concentrations.
A thermodynamic explanation of osmosis in terms of the chemical potential of solvent and solute (Gibbs, 1897) is correct and has been available for more than a century; however, it does not provide much insight into the mechanism of osmosis at the molecular level. Kramer and Myers (2012) use the molecular explanation of osmosis from Joos and Freeman (1951) to further emphasize the involvement of interactions and forces surrounding the solute, solvent, and semipermeable membrane which together result in osmosis. Here, we elected to focus on an entropy perspective to explain osmosis since the mechanistic explanation is uncommon in introductory chemistry and biology textbooks (e.g., Kramer and Boyer, 1995; Graham et al., 2003; Moore et al., 2009; Taiz and Zeiger, 2010; Jones et al., 2012; Brown et al., 2018), though it is becoming more common in biophysics textbooks (Benedek and Villars, 2000; Nelson, 2003). Our aim was to help undergraduate students build toward a thermodynamic explanation for osmosis using an interdisciplinary activity that reflects three-dimensional learning.
Using three-dimensional learning as the theoretical framework
Three-dimensional learning (3DL) is an approach to science education that incorporates scientific practices, crosscutting concepts, and disciplinary core ideas with the goal of students building and integrating their knowledge to explain phenomena that they encounter in their studies, careers, and everyday life (National Research Council, 2012a; Krajcik and Delen, 2017). The scientific practices described by the Framework together summarize what scientists do with their knowledge, such as analyzing and interpreting data and developing arguments based on evidence. The practices describe how scientists authentically behave and should be a central component of what students experience in college courses. Crosscutting concepts are somewhat less familiar than the practices but have been conceptualized as productive lenses, tools, and rules for problems (Rivet et al., 2016; Cooper, 2020); examples include patterns, cause and effect, and structure and function. Core ideas refer to touchstone concepts in each discipline that both explain much of what is already known and can be used to generate hypotheses about new situations (Cooper et al., 2017). Change and stability in chemical systems, for example, is a core idea in chemistry that explains existing phenomena and provides a framework for investigating new problems. While the Framework was designed for the K-12 levels, it is also relevant at the college level as the goal of helping students learn how to think about and do science like disciplinary experts cuts across grades (National Research Council, 2000; Cooper et al., 2015). A similar vision for teaching and learning is described in Vision and Change (American Association for the Advancement of Science, 2011) and the National Research Council (2012b) report on discipline-based education research, both of which are targeted at the college level.Drafting the activity
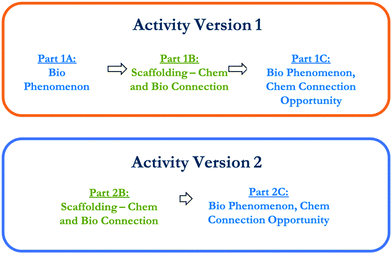
The osmosis activity was designed and developed following a similar process to our corresponding DNA (Roche Allred et al., 2021) and ATP (Green et al., 2021) activities in that it solicited students’ knowledge from both Bio I and general chemistry and incorporated a biological phenomenon, chemistry scaffolding questions, and an opportunity for students to connect a chemistry core idea with the biological phenomenon. Using a simplified evidence-centered design approach (Harris et al., 2016; Stowe and Cooper, 2019) we designed the activity by first working backwards from the biological phenomenon of osmosis across a cell membrane to identify the relevant core idea.Two versions of the activity were developed (Fig. 1). Throughout this paper we refer to specific sets of questions with shorthand, as the versions have different numbers of questions. For example, Part 1A-Q4 refers to Question 4 from Part A of Version 1 of the activity (shown in Appendix 1). As discussed previously, a complete explanation of osmosis requires discussion of the interactions and forces between solute, solvent, and the membrane, but since this activity is designed for introductory level courses in biology and chemistry, students may not have the physics background required and we, therefore, focused on entropy alone.
In Version 1 of the activity (Appendix 1), given through on online system described below, the introductory questions (Part 1A) were developed to elicit student ideas regarding osmosis without prompting for chemistry ideas. We asked students for their initial prediction about whether water would move in or out of an animal cell to achieve osmotic balance if it was put into pure water. We purposefully chose to present students with an animal cell instead of a plant cell because the cell wall of a plant cell makes it less likely to rupture in a hypotonic environment.
In Part 1B, drawing on the core idea of change and stability in chemical systems, students were presented with chemistry and biology connection questions that asked them to use entropy to predict changes in solutions of dye and water. This chemistry context was specifically selected because the students responding to this activity were enrolled in a general chemistry course with a transformed curriculum known as Chemistry, Life, the Universe and Everything (CLUE) (Cooper and Klymkowsky, 2013). At the end of the first semester and beginning of the second semester of CLUE general chemistry, students learn about entropy in terms of possible arrangements using two key examples within the context of the change and stability core idea. In the first example, students learn about the permutations in arrangement of a solution of dye and water molecules and why dye cannot unmix out of the solution. In the second example, students learn about the possible arrangements of quanta as a means to explain why heat transfers from hot to cold objects. Part 1B of the activity built on the dye and water example in that Q1 and Q2 asked students to predict which way water molecules would transfer through a membrane selectively permeable to water but not dye molecules. Entropy was introduced to assist students with their explanation about how the water molecules would move to reflect the core idea of change and stability in chemical systems.
In Part 1C, students were provided a second opportunity to explain the same biological phenomenon presented in Part 1A regarding osmosis across a cell membrane, however, this time they were explicitly asked to incorporate their understanding of entropy and solutions to predict and explain what would happen. Lastly, students were asked to rank their familiarity and confidence regarding osmosis and entropy concepts.
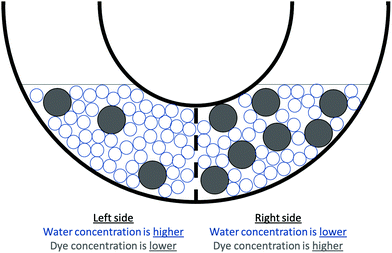
The activity was modified to create Version 2 (Appendix 2) before a second administration. In Version 2, Part A was removed because the activity was given as homework on a physical worksheet which meant students could modify their initial answers while completing the questions without our knowledge; therefore, this version began with chemistry scaffolding questions (Part 2B) nearly identical to those in Part 1B. These questions were similarly followed by an opportunity to apply the chemistry concepts of entropy and change and stability in chemical systems to explain the biological phenomenon (Part 2C). Additionally, we updated the figure for the dye and water solution to minimize confusion and misconceptions that could be introduced from trying to represent some but not all of the water molecules (see the figures in Appendices 1 and 2). Version 2 of this figure maximizes the space with water molecules to avoid the misinterpretation that there is empty space within the solution.
Since the activity was designed with 3DL as a guiding framework (National Research Council, 2012a), we used the Three-Dimensional Learning Assessment Protocol (3D-LAP) (Laverty et al., 2016) to verify that the activity had the potential to elicit student ideas regarding each of the three dimensions—core ideas, scientific practices, and crosscutting concepts (Appendix 3).
Research questions
In this paper we investigate the following research questions: (Study 1) How does chemistry scaffolding impact students’ explanations about the biological phenomenon of osmosis? and (Study 2) How do students’ prior chemistry and biology course backgrounds impact their explanations about osmosis? These studies were approved as exempt research and all students were provided with appropriate information as required by the Institutional Review Board.Methods
Participants and data collection
Of the 931 students enrolled in total across the three sections, a subset of 245 students were solicited to complete this activity, while the remaining students in the course completed other activities. This strategy was purposeful and prevents survey fatigue within a single student population as, in this context, multiple researchers are typically studying the impacts of course reform and have projects relevant to this same population at any given time. Since our study investigated how students connect chemistry and biology course content, we focused on including students who were co-enrolled in Bio I and GC II in our subsample, and, indeed, these students were largely familiar with the concepts of osmosis and entropy (Appendix 4). Within this subset of 245 students, 108 students were co-enrolled in Bio I, ensuring responses from a population that would support investigation of Study 2. We note that 216 total students were co-enrolled in Bio I; we randomly selected half of these students to receive this activity and half to receive another activity that is described elsewhere (Roche Allred et al., 2021). The remaining students included in the subsample were randomly chosen so that all activities were administered to similar numbers of students.
Of the 245 students solicited to complete the activity, 202 returned the activity. Two students did not complete the entire activity and were removed from these analyses, leaving a final sample of 200 responses for analysis. The students who returned the activity earned final course grades (M = 3.17, SD = 0.98) no different from another sub-population of students (M = 3.18, SD = 0.93) with similar biology course background that completed a different interdisciplinary activity (t(402) = −0.18, p = 0.86). These sub-populations also had similar proportions of students (∼70%) enrolled in biology and biology-related degree programs. That is, the students who completed the activity are reasonably representative of similar student populations at this institution.
Students’ course history information regarding concurrent and prior biology and chemistry courses at the introductory level was obtained from the Office of the Registrar. Students in GC II were classified as either concurrently taking Bio I, having previously taken Bio I or received transfer credit, or having never taken Bio I. Similarly, students in Bio I were classified as either concurrently taking GC II, having previously taken GC II or received transfer credit, or having never taken GC II. Based on these course histories, groups for analysis were defined as shown in Fig. 2.
Data analyses
| Activity Version 1a | Activity Version 2a |
|---|---|
| a See Appendices 1 and 2 for the complete versions of the activity including images. | |
| Part A: Biological Phenomenon | |
| Q4: Again consider a scenario where an animal cell is placed in pure water. Some solutes (molecules or ions) are dissolved in water inside the cell, but no solutes are dissolved in the pure water outside the cell. The net flow of water would go from outside to inside the cell. Explain why the water flows from outside to inside the cell. | |
| Part B: Scaffolding – Chemistry and Biology Connection | |
| Q5: The final state of the container is shown in the figure. The final state would be favored in terms of entropy. Explain why the container in the final state is more favored in terms of entropy. | |
| Part C: Biological Phenomenon | |
| Q1: Let's once more consider the scenario where an animal cell is placed in pure water. Recall that some solutes (molecules and ions) are dissolved in water inside the cell, but no solutes are dissolved in pure water outside the cell. The net flow of water would go from the outside to the inside of the cell. Incorporating your understanding of entropy and solutions, explain why water would go from the outside to the inside of the cell. | Q3: Now incorporating your understanding of entropy and solutions, explain what happens to the volume of a cell that is placed in a container of pure water. |
Initially, responses to the questions of interest (Part 1A-Q4 from before the chemistry and biology connection scaffolding and Part 1C-Q1 from after scaffolding) were compiled to support the development of a coding scheme using an open coding approach (Strauss, 1987; Corbin and Strauss, 2015). We looked for how students did or did not apply chemistry ideas to explain the biological phenomenon of osmosis, particularly in relation to our ideal student response. Ideally, students would have responded that when the cell is placed in pure water, the cell volume increases due to more water entering the system of the cell from the surroundings. This movement occurs because the surroundings consist of pure water while the system inside of the cell consists of solutes (molecules and ions) in solution, meaning that more possible arrangements would occur among the system and surroundings if water molecules were added to the cell versus water leaving the cell. Interactions among the solvent, solute and membrane were ignored based on the content covered in the course by the time this activity was administered.
Trends in the sophistication of student responses emerged in the following three overall categories: not scientifically accurate (non-normative), relying on the idea of concentration difference between the inside and outside of the cell, and explaining the phenomenon using the change in entropy (Table 2). The levels of sophistication related to concentration and entropy were further separated into two categories each. That is, for the concentration category, we separated students by whether they discussed concentration changes explicitly (e.g., Marshall, Table 2) or more broadly and implicitly (e.g., Barney, Table 2). With respect to the entropy category, we found that students used entropy in terms of increased favorability (e.g., Ted, Table 2) or in terms of probability (e.g., Robin, Table 2), the highest level of sophistication observed in student responses.
Some students included both concentration and entropy changes in their explanations of why water would transfer into the cell. For example, Stella wrote, “The free water molecules would move from high concentration (outside) to low concentration (inside) and there would be more possible arrangements of the solute molecules/ions with the water molecules once osmosis has occurred.” In cases such as this in which both ideas of concentration and entropy, or both entropy codes, were incorporated, the student response was “coded up” as ordered in Table 2 to capture the highest level of sophistication in their response. Two authors (BLM and RLM) iteratively coded identical subsets of student responses and reconciled their codes resulting in further refinement of each code description. Once substantial inter-rater agreement was achieved using a subset of 52 responses (κ = 0.80 (95% CI, 0.68 to 0.93), p < 0.01) the coding scheme was used by one researcher to code the remaining responses.
After coding student responses to the pre-scaffolding and post-scaffolding questions of interest, we expanded our analyses and used the same coding scheme to code student responses to a similar question in the scaffolding chem-bio connection section (Part 1B-Q5). A Wilcoxon Signed-Ranks test was used to compare how student responses changed from Part 1A-Q4 to Part 1B-Q5 and from Part 1B-Q5 to Part 1C-Q1 since these analyses consisted of a paired sample of students before and after scaffolding. The codes were ordered in increasing sophistication according to Table 2, with the categories “Other” and “Non-normative” grouped for simplicity; responses coded with either of these least sophisticated categories were not relevant to the question and thus seen as having equivalent value for the purpose of this particular analysis.
For both Study 1 and Study 2, the resultant coding of students’ responses was exported to Excel for analysis and statistical tests were conducted using the Statistical Package for the Social Sciences (IBM SPSS Statistics 26).
Validity
As a measure of construct validity, a subset of undergraduate students (n = 7) who completed the activity during the Bio I course of interest were recruited for a 30 to 45 minute interview at the end of the semester and provided a $25 gift card incentive for their participation. The students were asked to talk through their responses to all the questions on Version 2 of the activity in a think-aloud interview with a member of the research team, revealing that students were interpreting the questions as intended.Results and discussion
Study 1
The activity was designed to provide students the opportunity to use their understanding of a chemistry core idea (change and stability in chemical systems) in the context of entropy to explain a biological phenomenon (osmosis across a cell membrane). As discussed previously, students ideally would incorporate ideas of entropy and how the system would change to explain the phenomenon of osmosis. Here, we first present how students responded to the three relevant questions independently (pre-scaffolding, during scaffolding, and post-scaffolding) followed by a discussion of how student reasoning changed throughout the whole activity (pre-scaffolding to during scaffolding and during scaffolding to post-scaffolding).While this heuristic might be useful for predicting the movement of water across a semipermeable membrane for osmosis, it does not explain the underlying mechanism of why water moves from high to low concentration across the cell membrane. In addition, students must be aware of what is moving (solvent or solute) and whether the “high to low” concentration change is referring to the concentration of solute or solvent. As shown in the following student example from Quinn, some students repeated the heuristic without specifying what concentration they were referring to or where the “high concentration” was relative to the “low concentration”. Additionally, some students appeared to confound the solute and solvent terms. The following student responses given by Zoey and Nora both indicate that they believe there are solutes outside of the cell even though the question prompts stated that no such solutes were present outside the cell. These responses suggest that the students are confusing the terms “solute” and “solvent”, though we cannot know for sure without further questioning.
“High concentration to low concentration” – Quinn
“There are more solutes on the outside of the cell than the inside of the cell” – Zoey
“The solutes outside would go to where there [are] less solutes which is the inside of the cell” – Nora
Only a few students (2%, n = 3) brought in mechanistic ideas about entropy initially to explain why water would move from outside to inside the cell. The remaining students discussed either scientifically inaccurate (non-normative) ideas (19%, n = 38) or responded in a way that was not connected to the coding scheme (other; 15%, n = 30), including restatements of the prompt and correct but irrelevant information, such as information about aquaporins (membrane proteins that allow a transfer of water molecules into and out of cells).
In this scaffolding section, a majority of students (63%, n = 125) used entropy to explain osmosis (Fig. 3). It should be noted that 93 of these 125 students (74%) used entropy from a probabilistic viewpoint, indicating that most of these students had the ability to successfully explain osmosis within the chemistry example of dye and water molecules using ideas of entropy when prompted. However, about 38% (n = 75) of students overall had difficulty with the concept of entropy and relied on ideas about concentration (15%, n = 30), gave a non-normative explanation (15%, n = 30), or were coded as other (8%, n = 15).
Comparing student responses throughout the entire activity. The coding scheme was condensed to three categories (i.e., other/non-normative, concentration-based, and entropy-based) to simplify the process of comparing how student reasoning changed with each question. Table 3 presents how individual students' level of sophistication in their reasoning changed throughout the activity, showing whether their response increased in sophistication, decreased in sophistication, or showed no change between each set of question prompts. For example, the 85 students in the “increase, same” group increased their level of sophistication from the pre-scaffolding to during scaffolding question and then maintained that level of sophistication for the post-scaffolding response. This pattern could reflect a student who increased from the other/non-normative to concentration level or from the concentration to entropy level, for example.
| During to post | ||||
|---|---|---|---|---|
| Increase | Same | Decrease | ||
| Pre to during | Increase | 1 (1%) | 85 (43%) | 47 (24%) |
| Same | 21 (11%) | 20 (10%) | 2 (1%) | |
| Decrease | 13 (7%) | 11 (6%) | 0 (%) | |
Table 3 shows that 67% of the students (n = 133) increased the sophistication of their response from the pre-scaffolding to during scaffolding question. Of these 133 students, 85 (43%) maintained that level of sophistication in responding to the post-scaffolding prompt. Of these students that increased and maintained their level of sophistication, almost all of them increased to, and maintained, an entropy-based argument (n = 81). This pattern corroborates the findings shown in Fig. 3 that students appear to have incorporated entropy-based reasoning in response to the during scaffolding question and then maintained that reasoning for the post-scaffolding prompt.
Some students who initially increased the sophistication of their response, however, did not carry this demonstrated level of understanding through to the post-scaffolding prompt and, instead, decreased the sophistication of their response (24%, n = 47). Of these students who increased and then decreased, the plurality (n = 17) began at a concentration code, increased to an entropy code, and then decreased to the other/non-normative code. As shown in the following example from Brad, these students appeared to have had difficulty applying what they demonstrated in the during scaffolding prompt to the new system in the post-scaffolding prompt, showing some fragility in transferring knowledge from one context to another.
Brad's response:
Pre-scaffolding: “The water flows from outside to inside the cell because of the concentration.” (coded as level 3 from Table 2 – Concentration – Explicit)
During scaffolding: “Because there are more possible arrangements for the molecules and its more disorder.” (coded as level 5 from Table 2 – Entropy – Probability)
Post-scaffolding: “I'm not sure” (coded as level 1A from Table 2 – Other)
Of the students who maintained the same reasoning level between the first set of questions (pre-scaffolding to during scaffolding; 22%, n = 43), about half (n = 21) increased their sophistication for the last question while the other half (n = 20) maintained the same reasoning sophistication level throughout the activity. That is, no matter how the question was framed, these students remained consistent in their responses throughout the whole activity. The majority (60% of 20, n = 12) of these students who were consistent in their sophistication throughout remained at the other/non-normative level while six students remained at the concentration-based argument level. Of the students that stayed the same and then increased, 19 (90%) improved to an entropy-based argument.
Some students (12%, n = 24) decreased their level of sophistication in reasoning across the first set of prompts. All but one of these students began at the concentration-based level and decreased to the other/non-normative code, perhaps resulting from confusion at the introduction of the dye and water molecule scenario. Of these students who decreased, nine increased to an entropy-based argument in the post-scaffolding prompt while 11 remained at the other/non-normative level.
A Wilcoxon Signed-Ranks test confirmed that student responses within the chemistry scaffolding section (Part 1B-Q5) were more sophisticated (mean rank = 96.29) than their responses before the scaffolding (Part 1A-Q4; mean rank = 64.50, Z = −8.087, p < 0.001, effect size = 0.40) with a medium to large effect size (Cohen, 2013). Additionally, a Wilcoxon Signed-Ranks test showed that student responses after chemistry scaffolding (Part 1C-Q1) decreased in sophistication (mean rank = 64.85) from their responses within the chemistry scaffolding section (Part 1B-Q5; mean rank = 57.98, Z = −2.049, p = 0.040, effect size = 0.10) with a small effect size.
Study 2
Here, we investigated how students’ course history in GC II and Bio I related to their explanations of the biological phenomenon, that is, why water would transfer into the cell. Since the two activity versions differ slightly, only the final biological phenomenon question common to both versions was used for the purpose of comparison (i.e., Parts 1C-Q1 and 2C-Q3). Specifically, both versions asked students to integrate their understanding of entropy in their explanation, but Version 2 did not explicitly state which direction water would move across the cell membrane. This difference was intentional because Version 1 was administered in beSocratic, meaning students could not go back to previous questions, while Version 2 was administered as a worksheet, meaning students could see and potentially modify all their responses. The responses from Study 1 students were combined with responses from students in the Bio I course to ensure a range of student course backgrounds. Students from Study 1 who completed the activity as part of GC II were grouped based on their Bio I course experience while students who completed the activity as part of their Bio I course were grouped based on their GC II course experience (Fig. 2).Several observations can be made in separating student responses by their course history (Fig. 4). First, the GC II students (Groups 1–3), regardless of their biology background, appeared to have a similar trend in sophistication of their responses. That is, students in GC II generally provided more sophisticated explanations than students in Bio I overall by incorporating the concept of entropy into their response at either the probabilistic or favorability levels (Group 1, 60%, n = 58; Group 2, 38%, n = 11; Group 3, 58%, n = 43). Correspondingly, fewer students in these groups incorporated other information or non-scientifically accurate ideas (Group 1, 25%, n = 25; Group 2, 45%, n = 13; Group 3, 25%, n = 19). For the students enrolled in Bio I, however, it appears that their chemistry course history is related to the level of sophistication of their response. Here, only 35% of students (n = 15) who had previously taken GC II (Group 5) and 14% (n = 9) of students who had no GC II experience (Group 6) included entropy into their response. It appears that Bio I Group 5 students (prior GC II) were more apt to incorporate entropy into their response, perhaps due to having focused on the concept previously compared to students in Group 6 who had not yet been exposed to this material. In general, Group 5 and 6 students tended to provide non-normative reasoning (Group 5, 35%, n = 15 and Group 6, 46%, n = 29). This trend in lower sophistication of responses and more non-normative responses was most apparent for students in Group 6. Indeed, Group 5 and 6 students were almost three times more likely (odds ratio = 2.93, 95%CI = 1.74–4.93) to include non-normative reasoning within their response compared to students currently enrolled in the GC II course, regardless of their Bio I background.
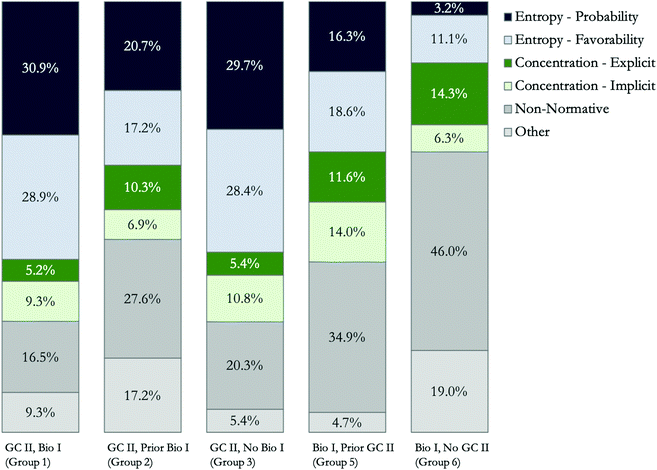 | ||
| Fig. 4 Coding of students’ explanations of osmosis from the post-scaffolding questions (either Part 1C-Q1 or Part 2C-Q3, as appropriate by course history group). | ||
In the CLUE curriculum, entropy is typically introduced in the last few weeks of GC I in terms of the thermodynamics of pure substances undergoing a phase change (e.g., water molecules in the gas phase have more possible arrangements than water molecules in the liquid phase). These concepts are expanded during the first two months of GC II in terms of solution chemistry and acid–base chemistry along with other thermodynamic terms such as enthalpy and Gibbs free energy. For Group 5 students, at least one semester had passed since they had taken GC II; therefore, it may be that these students were unsure about how to incorporate entropy correctly into their response, that they had not understood entropy while taking GC II in the first place, or that they lost some of their prior understanding of the topic due to the lapse in time between the two courses. Indeed, enhancing retention of knowledge from course to course is a keystone issue (Rubin and Wenzel, 1996), and students may have been particularly prone to losing this knowledge if they had only a surface level of understanding of entropy in GC II (Bacon and Stewart, 2006). Group 6 had the largest proportion of students with non-normative responses, most likely because these students had not yet been introduced to entropy, meaning the prompts about the chemistry phenomenon of the solution of dye and water were not helpful in connecting to prior knowledge as this knowledge did not exist in order to incorporate it into their response.
Limitations
This study is limited in that all students in Study 1 and the majority of students in Study 2 who had previously enrolled in GC II (74%, n = 32) had taken a GC II course that uses the transformed general chemistry CLUE curriculum. CLUE emphasizes four chemistry core ideas as well as scientific practices and, specifically relevant to this study, addresses entropy from a distinguishable arrangements perspective rather than a disorder approach. Therefore, the student responses analyzed here may not be representative of how students in a traditional general chemistry course think about entropy. Administration to students outside of the CLUE curriculum may require different or additional scaffolding to assist in connecting the biological phenomenon to a relevant chemistry phenomenon. That is, if students were not familiar with the dye and water solution example for entropy then this scaffolding phenomenon example may not assist students with connecting their chemistry and biology knowledge. We also recognize that administering a single assessment to a student cannot fully capture their ideas about entropy or osmosis (or diffusion more generally, for that matter). Entropy and osmosis are ideas present across the introductory science curriculum (Shen et al., 2014), and the results from these activities, having been offered a single point in time, do not capture all the nuanced disciplinary perspectives that students have or the stability of their ideas over time.Conclusion and implications
The key takeaways from this work overall are that (a) directly connecting chemistry and biology ideas in the activity supported students in learning to use a chemistry core idea to explain the biological phenomenon of osmosis across a cell membrane, (b) the activity was structured in a way that supported more than half of students in improving their explanations over the course of the activity, and (c) the activity may be more appropriate for general chemistry students (regardless of whether they have introductory biology experience) compared to introductory biology students (regardless of whether they have general chemistry experience). That is, overall, it appears that students’ course background is related to the level of sophistication that students provided in their reasoning about why water would net flow into the cell when placed in pure water. In general, carefully analyzing the background experiences that students have in pre- and co-requisite courses is important work for interdisciplinary assessment design.Prior work shows the importance of providing opportunities for students to make interdisciplinary connections (Geller et al., 2014; Roche Allred et al., 2021) and here, we highlight the importance of supporting students with scaffolds to help them make those connections. In this specific case, integrating the activity with appropriate supports in an introductory biology course may take more time and require more support than doing the same in a general chemistry course, however in either case the activity provides students an opportunity to connect their knowledge across disciplines. Students have reported that their courses generally do not provide opportunities for them to make interdisciplinary connections, and they are left to make such connections on their own (Shen et al., 2014). The activity could be modified as well in service of instructors’ specific teaching goals. For example, a parallel question could be added that asks students what they know about semipermeable membranes as well as about the affordances and constraints of representing such a membrane as a simple, dashed line.
Together, these studies support the importance of giving students the opportunity to explain biological phenomena in chemistry courses where the chemistry scaffolding is already built in. We saw an increase in sophistication in students’ explanations of the biological phenomenon after chemistry scaffolding, bringing in more chemistry knowledge. Students who did not have GC II struggled to bring in chemistry ideas. We contend that this activity can be adapted for use in other courses and may be particularly appropriate for interdisciplinary courses that explicitly integrate general chemistry and introductory biology (Schwartz and Serie, 2001).
Author contributions
SMU and RLM jointly contributed to conceptualization, acquiring funding, project administration, and supervision of students. KNP supported the investigation with expertise in biochemistry and course access. BLM led data curation, formal analysis, and visualization with support from ATK. BLM, SMU, and RLM wrote the original draft. All authors contributed to the study design and reviewed and edited the paper.Conflicts of interest
There are no conflicts to declare.Appendix 1: osmosis activity version 1
We want to know what you think about these questions. Please answer them to the best of your ability and do not consult outside sources (e.g., classmates, textbooks, websites, class notes, etc.). Receiving credit for this assignment is based on participation and effort, not on correctness. You will not be able to move backwards through the slides, so make sure you are satisfied with your answers before moving on.Part 1A: Bio Phenomenon
Q1. In 1-2 sentences, what do you know about osmosis?
Q2. It is important for animal cells to maintain osmotic balance across the cell membrane, ensuring that water moves into the cell and outside the cell at the same rate. Note that throughout this assessment, we are referring to animal cells, not plant cells. What do you think would happen to a cell that is placed in pure water?
Q3. Now, think specifically about cell volume. What do you think would happen to the volume of a cell that is placed in pure water?
i. The cell volume would decrease
ii. The cell volume would stay the same
iii. The cell volume would increase
iv. Not enough information to predict
Explain why you selected your response.
Q4. Again consider a scenario where an animal cell is placed in pure water. Some solutes (molecules or ions) are dissolved in water inside the cell, but no solutes are dissolved in the pure water outside the cell. The net flow of water would go from outside to inside the cell. Explain why the water flows from outside to inside the cell.
Part 1B: Scaffolding – Chem and Bio Connection
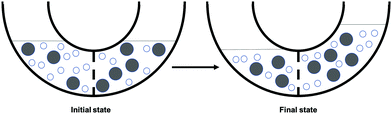
Q1. Now consider a container of water molecules (small, unshaded) and dye molecules (large, shaded) (Fig. 5). The container has a selectively permeable membrane in the middle of it that allows for passive transport of water. The dye molecules are too big to cross the membrane. What do you expect would happen to the water molecules in the container over time?
i. The net flow of water would be from left to right
ii. There would be no net flow of water
iii. The net flow of water would be from right to left
iv. Not enough information to predict
Explain why you selected your response.
Q2. In the empty tube (Fig. 6), draw a picture of what you would expect the molecules in the container to look like after some time has passed. Include (1) all 16 water molecules (small, white), (2) all 8 dye molecules (large, gray), and (3) the solution levels (two horizontal lines).
Q3. In 1–2 sentences, what do you know about entropy? In 1–2 sentences, what role does entropy have in mixing solutions?
Q4. Between (1) the initial state of the container and (2) the state of the container you just drew, which is more favored in terms of entropy?
i. The container in the initial state
ii. The container in the final state
iii. The entropy is the same for both
iv. Not enough information to predict
Explain why you selected your response.
Q5. The final state of the container is shown in Fig. 7. The final state would be favored in terms of entropy. Explain why the container in the final state is more favored in terms of entropy.
Part 1C: Bio Phenomenon, Chem Connection Opportunity
Q1. Let's once more consider the scenario where an animal cell is placed in pure water. Recall that some solutes (molecules and ions) are dissolved in water inside the cell, but no solutes are dissolved in pure water outside the cell. The net flow of water would go from the outside to the inside of the cell. Incorporating your understanding of entropy and solutions, explain why water would go from the outside to the inside of the cell.
Q2. Indicate how familiar you are with each of the topics listed below.
Osmosis
Very Familiar
Mostly Familiar
Moderately Familiar
Slightly Familiar
Not at All Familiar
Entropy
Very Familiar
Mostly Familiar
Moderately Familiar
Slightly Familiar
Not at All Familiar
Q3. Indicate how confident you were in answering questions related to each of the topics listed below.
Osmosis
Very Confident
Mostly Confident
Moderately Confident
Slightly Confident
Not at All Confident
Entropy
Very Confident
Mostly Confident
Moderately Confident
Slightly Confident
Not at All Confident
Q4. Please provide any feedback you have about how this activity could be improved. If something was confusing or unclear, please let us know.
Appendix 2: osmosis activity version 2
The following questions are intended to collect your ideas about an application of entropy in a biological system. You will earn credit for completing this activity even if your answers are not correct so long as you answer to the best of your ability. Work individually and do not consult textbooks, the internet, etc. The activity draws on some chemistry ideas; if you are currently taking general chemistry I (or equivalent), just do your best.Part 2B: Scaffolding – Chem and Bio Connection
Q1. What do you know about entropy and its role in mixing solutions?
Q2. Consider a container of water molecules (small, unshaded) and dye molecules (large, shaded) (Fig. 8). The container has a selectively permeable membrane in the middle that allows for passive transport of water. The dye molecules are too big to cross the membrane. What would happen to the water molecules after time has passed? Circle your choice.
i. The net flow of water would go from left to right
ii. There would be no net flow of water
iii. The net flow of water would go from right to left
iv. Not enough information to predict
Explain your reasoning for this selection.
Q3. In the empty tube (Fig. 9), draw a picture of what you expect the molecules would look like after some time has passed. Be sure to include water molecules (small, unshaded), dye molecules (large, shaded), and the solution levels (horizontal lines).
Q4. Compare the drawings of the initial state of the container (Question 2) and the final state of the container (Question 3).
Which state of the container is more favored in terms of entropy? Circle your choice.
i. The container in the initial state (Question 2)
ii. The container in the final state (Question 3)
iii. The entropy is the same for both (Questions 2 and 3)
iv. Not enough information to predict
Explain your reasoning for this selection.
Part 2C: Bio Phenomenon, Chem Connection Opportunity
Q1. Now, consider the following biological system. Animal cells are each surrounded by a plasma membrane. In addition to the nucleus and organelles, the inside of the cell contains solutes (molecules and ions) in addition to water. It is important for cells to maintain osmotic balance across the membrane, meaning that water moves passively into the cell and out of the cell at the same rate. Solutes do not passively move across the membrane. What would happen to the volume of a cell that is placed in a container of pure water? Circle your choice.
i. The cell volume would decrease
ii. The cell volume would stay the same
iii. The cell volume would increase
iv. Not enough information to predict
Explain your reasoning for this selection.
Q2. What is happening to the number of distinguishable arrangements for each of the following (Table 4)?
| Change in number of distinguishable arrangements (+, 0, or −) | Explain your reasoning for this selection | |
|---|---|---|
| System (the cell) | ||
| Surroundings (the container) | ||
| System + surroundings (the cell + container) |
Q3. Now incorporating your understanding of entropy and solutions, explain what happens to the volume of a cell that is placed in a container of pure water.
Q4. Could the volume of a cell change in this way indefinitely? What do you think would happen eventually?
Q5. Write any feedback you have about this activity here.
Appendix 3: characterization of the osmosis activity using the 3D-LAP
| Dimension | 3D-LAP Criteriaa | Part B: Scaffolding – Chemistry and Biology Connection | Part C: Biological Phenomenon |
|---|---|---|---|
| Core ideas | Change and Stability in Chemical Systems: Energy and entropy changes, the rates of competing processes, and the balance between opposing forces govern the fate of chemical systems | Students are given an initial state of a U-manometer filled with dye and water molecules, asked to draw the final state, and use entropy to explain which state (final or initial) is favored | Students are asked to use entropy to explain the biological phenomenon of osmosis (a cell expanding when placed in pure water) |
| Scientific practices | Constructing Explanations and Engaging in Argument from Evidence: Students are asked to provide reasoning based on evidence to support a claim | Students are asked to draw a picture of what they would expect a U-manometer to look like after some time has passed, showing both the water and dye molecules, and use it to explain why the container in the final state is favored in terms of entropy | Students are asked to describe how the volume of a cell changes in a solution of pure water and explain why using their understanding of entropy and solutions |
| Developing and Using Models: Students are given or asked to construct a graphical, computational, symbolic, mathematical, or pictorial representation and use it to explain or predict an event, observation or phenomenon | |||
| Crosscutting concepts | Cause and Effect: Mechanism and Explanation: The question provides at most two of the following: (1) a cause, (2) an effect, and (3) the mechanism that links the cause and effect, and the student is asked to provide the other(s) | Students are asked to draw an arrangement of water and dye molecules in the final state of the U-manometer and asked to explain | Students are asked how the volume of a cell changes when placed in pure water and explain using their understanding of entropy and solutions |
| Stability and Change: Students are asked to determine (1) if a system is stable and provide the evidence for this, or (2) what forces, rates, or processes make a system stable (static, dynamic, or steady state), or (3) under what conditions a system remains stable, or (4) under what conditions a system is destabilized and the resulting state |
Appendix 4: student familiarity and confidence with osmosis and entropy
Fig. 10.Acknowledgements
We thank the students who completed this activity and the instructors for allowing us access to their courses. We also thank Zahilyn D. Roche Allred, Laura Santiago-Caobi, and Brittney Pardinas (FIU) and Abigail I. Green and Caitlyn Q. Herr (MSU) for their input and support during the development of this activity. This material is based upon work supported by the National Science Foundation under grant numbers 1708589 and 1708664. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.References
- American Association for the Advancement of Science, (2011), Vision and Change in Undergraduate Biology Education: A Call to Action, http://visionandchange/org/files/2013/11/aaas-VISchange-web1113.pdf.
- Bacon D. R. and Stewart K. A., (2006), How fast do students forget what they learn in consumer behavior? A longitudinal study, J. Market. Educ., 28(3), 181–192.
- Benedek G. B. and Villars F. M. H., (2000), Physics with Illustrative Examples from Medicine and Biology: Mechanics, Springer Science & Business Media.
- Bialek W. and Botstein D., (2004), Introductory science and mathematics education for 21st-century biologists, Science, 303(5659), 788–790.
- Boix Mansilla V. and Duraising E. D., (2007), Targeted assessment of students' interdisciplinary work: An empirically grounded framework proposed, J. High. Educ., 78(2), 215–237.
- Brown T. E., LeMay H. E., Bursten B. E., Murphy C., Woodward P. and Stoltzfus M. E., (2018), Chemistry: The Central Science, 14th edn, Pearson.
- Bryfczynski S. P., (2012), BeSocratic: An Intelligent Tutoring System for the Recognition, Evaluation, and Analysis of Free-form Student Input [Unpublished doctoral dissertation], Clemson University.
- Cohen J., (2013), Statistical Power Analysis for the Behavioral Sciences, UK: Elsevier Science.
- Cooper M. M., (2020), The crosscutting concepts: Critical component or “third wheel” of three-dimensional learning? J. Chem. Educ., 97(4), 903–909.
- Cooper M. and Klymkowsky M., (2013), Chemistry, life, the universe, and everything: A new approach to general chemistry, and a model for curriculum reform, J. Chem. Educ., 90(9), 1116–1122.
- Cooper M. M., Caballero M. D., Ebert-May D., Fata-Hartley C. L., Jardeleza S. E., Krajcik J. S., et al., (2015), Challenge faculty to transform STEM learning, Science, 350(6258), 281–282.
- Cooper M. M., Posey L. A. and Underwood S. M., (2017), Core ideas and topics: Building up or drilling down? J. Chem. Educ., 94(5), 541–548.
- Corbin J. and Strauss A., (2015), Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 4th edn, Thousand Oaks, CA: SAGE Publications.
- Fisher K. M., Williams K. S. and Lineback J. E., (2011), Osmosis and diffusion conceptual assessment, CBE – Life Sci. Educ., 10(4), 418–429.
- Freeman S., Haak D. and Wenderoth M. P., (2011), Increased course structure improves performance in introductory biology, CBE – Life Sci. Educ., 10(2), 175–186.
- Friedler Y., Amir R. and Tamir P., (1987), High school students’ difficulties in understanding osmosis, Int. J. Sci. Educ., 9(5), 541–551.
- Geller B. D., Dreyfus B. W., Gouvea J., Sawtelle V., Turpen C. and Redish E. F., (2014), Entropy and spontaneity in an introductory physics course for life science students, Am. J. Phys., 82(5), 394–402.
- Gibbs J. W., (1897), Semi-permeable films and osmotic pressure, Nature, 55(1429), 461–462.
- Graham L. E., Graham J. M. and Wilcox L. W., (2003), Plant Biology, Prentice Hall.
- Green A. I., Parent K. N., Underwood S. M. and Matz R. L., (2021), Connecting Ideas Across Courses: Relating Energy, Bonds, and How ATP Hydrolysis Powers a Molecular Motor, The American Biology Teacher, 83(5), 291–298.
- Harris C. J., Krajcik J. S., Pellegrino J. W. and McElhaney K. W., (2016), Constructing Assessment Tasks that Blend Disciplinary Core Ideas, Crosscutting Concepts, and Science Practices for Classroom Formative Applications, Menlo Park, CA: SRI International.
- Haudek K. C., Prevost L. B., Moscarella R. A., Merrill J. and Urban-Lurain M., (2012), What are they thinking? Automated analysis of student writing about acid–base chemistry in introductory biology, CBE – Life Sci. Educ., 11(3), 283–293.
- Jones R. L., Ougham H., Thomas H. and Waaland S., (2012), The Molecular Life of Plants, UK: John Wiley & Sons.
- Joos G. and Freeman I. M., (1951), Theoretical Physics, London: Blackie & Son.
- Krajcik J. and Delen I., (2017), How to support learners in developing usable and lasting knowledge of STEM, Int. J. Educ. Math., Sci. Technol., 5(1), 21–28.
- Kramer P. J. and Boyer J. S., (1995), Water Relations of Plants and Soils, UK: Academic Press.
- Kramer E. M. and Myers D. R., (2012), Five popular misconceptions about osmosis, Am. J. Phys., 80(8), 694–699.
- Laverty J. T., Underwood S. M., Matz R. L., Posey L. A., Carmel J. H., Caballero M. D., et al., (2016), Characterizing college science assessments: The three-dimensional learning assessment protocol, PLoS One, 11(9), e0162333.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park, CA: SAGE.
- Mason K. A. and Mason, (2015), Understanding Biology, McGraw-Hill Education.
- Matz R. L., Underwood S. M. and Parent K. N., (2019), A three-dimensional approach to connecting biology & chemistry, Scientia, 127, 90–93.
- Momsen J. L., Long T. M., Wyse S. A. and Ebert-May D., (2010), Just the facts? Introductory undergraduate biology courses focus on low-level cognitive skills, CBE – Life Sci. Educ., 9(4), 435–440.
- Momsen J. L., Offerdahl E., Kryjevskaia M., Montplaisir L., Anderson E. and Grosz N., (2013), Using assessments to investigate and compare the nature of learning in undergraduate science courses, CBE – Life Sci. Educ., 12(2), 239–249.
- Moore J. W., Stanitski C. L. and Jurs P. C., (2009), Principles of Chemistry: The Molecular Science, 1st edn, Boston, MA: Cengage Learning.
- National Research Council, (2000), How People Learn: Brain, Mind, Experience, and School, Washington, DC: The National Academies Press.
- National Research Council, (2012a), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, DC: The National Academies Press.
- National Research Council, (2012b), Discipline-based Education Research: Understanding and Improving Learning in Undergraduate Science and Engineering, Washington, DC: The National Academies Press.
- Nelson P., (2003), Biological Physics (Updated Edition), W. H. Freeman.
- Nelson D. L. and Cox M. M., (2017), Lehninger Principles of Biochemistry, 7th edn, New York: W.H. Freeman.
- Odom A. L. and Barrow L. H., (1995), Development and application of a two-tier diagnostic test measuring college biology students’ understanding of diffusion and osmosis after a course of instruction, J. Res. Sci. Teach., 32(1), 45–61.
- Redish E. F., Bauer C., Carleton K. L., Cooke T. J., Cooper M., Crouch C. H., et al., (2014), NEXUS/physics: An interdisciplinary repurposing of physics for biologists, Am. J. Phys., 82(5), 368–377.
- Rivet A. E., Weiser G., Lyu X., Li Y. and Rojas-Perilla D., (2016), What Are Crosscutting Concepts in Science? Four Metaphorical Perspectives, 12th International Conference of the Learning Sciences, Singapore.
- Roche Allred Z. D., Farias A. J., Kararo A. T., Parent K. N., Matz R. L. and Underwood S. M., (2021), Students’ use of chemistry core ideas to explain the structure and stability of DNA, Biochem. Mol. Biol. Educ., 49(1), 55–68.
- Rubin D. C. and Wenzel A. E., (1996), One hundred years of forgetting: A quantitative description of retention, Psychol. Rev., 103(4), 734.
- Schwartz A. T. and Serie J., (2001), General chemistry and cell biology: An experiment in curricular symbiosis, J. Chem. Educ., 78(11), 1490.
- Shen J., Liu O. L. and Sung S., (2014), Designing interdisciplinary assessments in sciences for college students: An example on osmosis, Int. J. Sci. Educ., 36(11), 1773–1793.
- Sorensen K. H., (2000), Factors Influencing Retention in Introductory Biology Curriculum, National Association of Research in Science Teaching, Boston, MA.
- Stowe R. L. and Cooper M. M., (2019), Assessment in chemistry education, Isr. J. Chem., 59(6–7), 598–607.
- Strauss A. L., (1987), Qualitative Analysis for Social Scientists, Cambridge, UK: Cambridge University Press.
- Taiz L. and Zeiger E., (2010), Plant Physiology, Sinauer Associates.
- Tripp B. and Shortlidge E. E., (2019), A framework to guide undergraduate education in interdisciplinary science, CBE – Life Sci. Educ., 18(2), es3.
- Underwood S. M., Posey L. A., Herrington D. G., Carmel J. H. and Cooper M. M., (2018), Adapting assessment tasks to support three-dimensional learning, J. Chem. Educ., 95(2), 207–217.
Footnote |
| † Current affiliation: Center for Academic Innovation, University of Michigan, Ann Arbor, Michigan, USA. |
| This journal is © The Royal Society of Chemistry 2021 |