Beyond the beaker: students’ use of a scaffold to connect observations with the particle level in the organic chemistry laboratory
Liz
Keiner
 and
Nicole
Graulich
and
Nicole
Graulich
 *
*
Justus-Liebig-University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: Nicole.Graulich@didaktik.chemie.uni-giessen.de
First published on 2nd October 2020
Abstract
Understanding ongoing chemical processes in the laboratory requires constant shifting between different representational levels—the macroscopic, submicroscopic, and symbolic levels—and analysis of the various mechanistic features of each of these levels. Thus, the ability to explain observations of chemical phenomena with regard to their submicroscopic levels in the laboratory is a key requirement. Research shows that students have difficulty in discerning and comprehending the meaning and visualization of the submicroscopic level. Traditional laboratory instruction often fails to help students discern the relationship between their observations and the corresponding chemical processes. Consequently, there is a high demand for new teaching strategies which address these issues. Therefore, we developed and implemented a scaffold for the organic laboratory and tested it in a research study using qualitative methods. The scaffold encourages students to purposefully separate and connect the macroscopic and submicroscopic representational levels. The implementation of the scaffold was accompanied by semi-structured pre- and post-interviews with students (N = 22) and an analysis of students’ work with the scaffold in the laboratory. We analysed students’ sense-making approach while reflecting on organic syntheses before and after working with the scaffold, and characterized changes in their approach. The findings emphasize the need to develop further resources to support students’ understanding of the submicroscopic level. Implications of these findings for research and teaching to foster meaningful learning in the organic laboratory are discussed.
Introduction
Learning and teaching in the laboratory is a core component of chemistry education and has the potential to enrich the formation of science concepts (Hofstein and Lunetta, 1982; Johnstone, 1991; Bretz, 2019; Molvinger et al., 2020). Laboratory teaching aims at meeting numerous learning objectives required to successfully grasp practical syntheses, connect theory presented in lectures with laboratory observations, and understand the processes “beyond the beaker” (Bretz, 2019; Walker et al., 2019; Czysz et al., 2020).Most laboratory curricula still employ “cookbook” procedures that require students to follow the recipe, stepwise, in order to synthesize the desired product. Consequently, students acquire limited and superficial knowledge about procedures and chemical processes (Hofstein and Lunetta, 1982; Domin, 1999; Galloway and Bretz, 2015) and lose the connection between the theory taught in lectures and the practical work of the laboratory (Reid and Shah, 2007; Collison et al., 2012). While in the lab, the processes beyond the beaker may not be discussed thoroughly enough to help students develop robust understanding of processes at the macroscopic and submicroscopic levels and to translate between them (Gabel and Bunce, 1984; Johnstone, 2000; Gkitzia et al., 2019; Sumfleth and Nakoinz, 2019). Students’ difficulties in understanding and representing the nature of matter at the individual representational levels, and especially at the submicroscopic level, is a well-known challenge in chemistry education (Johnstone, 1982; Ben-Zvi et al., 1986; Griffiths and Preston, 1992; Kozma and Russell, 1997; Gilbert and Treagust, 2009a, 2009b; Cheng and Gilbert, 2017; Faulconer et al., 2018).
Besides the challenge of transitioning between representational levels, students also struggle to fully account for underlying mechanisms when analysing laboratory work-up procedures in organic chemistry. While they are able to identify the respective entities, they miss the properties and activities that produce a given phenomenon (Keiner and Graulich, 2020). Although research emphasizes the need to explicitly communicate each level and to connect the macroscopic and submicroscopic levels in a meaningful way, the potential for connecting macroscopic observations and analysing the submicroscopic level often goes untapped in traditional labs.
Increased awareness of this untapped potential has led to the implementation of various learning approaches to support students’ laboratory work in chemistry education. Researchers have developed additional instructional media to improve the teaching in organic laboratories. Pölloth et al. (2020), for example, created a library of online videos to prepare students for the upcoming laboratory techniques and syntheses in the laboratory. They could show that the videos have a positive impact on students’ self-concept of ability and that students show an increase of knowledge in a know-how test on laboratory techniques (Pölloth et al., 2020). Furthermore, there has been an increase in the use of digital badges (Hennah and Seery, 2017) and student-generated videos in chemistry laboratories (Schmidt-McCormack et al., 2017; Seery et al., 2017; Gallardo-Williams et al., 2020). In the organic teaching laboratory, for example, digital media applications, such as virtual reality, become more and more important (Ferrell et al., 2019). Virtual reality allows immersive interactions with a dynamic molecular world and engage students in exploring molecular structures, motions, and particle interactions. Such virtual reality applications could enable students to understand and visualize the invisible chemical processes beyond the beaker.
Other research groups focused on specific practical laboratory techniques and developed laboratory instructions for these techniques. For example, meaningful learning strategies for liquid–liquid extractions were developed and tested (Assadieskandar et al., 2020; Wu et al., 2020). Other approaches include restructuring the curriculum to foster students’ cognitive and practical skills (Seery et al., 2018; Lipton, 2020) and mixed approaches with tutorials and practical work (Molvinger et al., 2020). Supasorn et al. (2008) tested the impact of a pre-laboratory organic extraction simulation on comprehension and attitudes of undergraduate chemistry students. This instructional approach aimed at helping students to visualize extraction concepts at the molecular level, and afterwards to connect these concepts with the respective macroscopic observations. The pre-lab activity and the animations were limited to the extraction steps and did not presented further synthesis steps.
Other classroom interventions or initiatives, such as the Science Writing Heuristic (SWH) and Process-Oriented Guided Inquiry Learning (POGIL), focused on a well guided and structured prompting to support students in making sense of their laboratory work. The Science Writing Heuristic (SWH) (Schroeder and Greenbowe, 2008; Stephenson and Sadler-McKnight, 2016), a laboratory report format is based on a learning cycle whereby students explore concepts to look for trends or patterns, rather than to verify an expected outcome (Lawson, 1989; Keys et al., 1999; Lawson, 2001). Students who used the SWH format performed significantly better on an American Chemical Society (ACS) standardized exam (Hand et al., 2004), as well as on in-class lecture exams and quizzes (Burke et al., 2006; Greenbowe et al., 2007). Another widespread approach in chemistry education is the implementation of Process-Oriented Guided Inquiry Learning (POGIL) activities in the expository laboratory. The effectiveness of POGIL in general, and particularly in organic chemistry (Schroeder and Greenbowe, 2008; Vishnumolakala et al., 2017), was evidenced by improved student performance and a corresponding decrease in the number of students withdrawing from the course (Farrell et al., 1999; Spencer, 1999; Stegall et al., 2016).
A recent study emphasized the metacognitive aspect of using the representational levels. Thomas (2017) described a classroom intervention in which the teacher used the term ‘triangulation’ as an expression to stimulate metacognitive reflection in students to consider the importance and use of the three representational levels for learning chemistry. In this study, students improved their understanding due to this metacognitive reflection, however, students’ views of the importance and the cognitive processes associated with it varied across individuals.
The above approaches were successful in helping students understand and explain the underlying process of their lab procedures. However, the ability to reflect on and make connections between the macroscopic and the submicroscopic representational levels in a laboratory setting has not yet been explored as an in-lab means to support students’ understanding.
Based on the ongoing discussion of students’ difficulties in the organic laboratory, we designed and implemented a scaffold that aims at encouraging students to consciously separate and connect the macroscopic and submicroscopic representational levels during their practical lab work.
Theoretical framework
Explaining the interactions “beyond the beaker”
Chemists need to think “beyond the beaker” in order to understand the system that, for instance, extracts, purifies, and transports molecules in the flask (see Fig. 1). To explain the interactions beyond the beaker, chemists use models at the particle level. With regard to making inferences, one of the most powerful and productive ideas in chemistry education was the introduction of the “chemistry triplet”, also called the “Johnstone triangle” (Johnstone, 1982).Thanks to Johnstone's triangle, it is common practice to rationalize chemical phenomena at three levels of representation—the macroscopic (observable) level, the submicroscopic (particle) level, and the symbolic level (Gabel et al., 1992; Johnstone, 1993; Gabel, 1999; Gilbert and Treagust, 2009a, 2009b).
The macroscopic level describes tangible and visible observations of a phenomenon and is the most accessible level while conducting experiments in the laboratory. This includes observations, such as colour changes or the formation of new products, e.g. gases (Treagust et al., 2003).
The submicroscopic level describes the invisible processes of a phenomenon, the particle level (see Fig. 1), in terms of the movement, organization, or collision of particles, electrons, molecules, or atoms.
To communicate and visualize the observed processes, chemists commonly use the symbolic level, which includes pictorial, algebraic, physical, and computational forms (e.g. chemical equations, graphs, reaction mechanisms, analogies, and model kits) (Kozma and Russell, 1997; Russell et al., 1997; Treagust et al., 2003). The symbolic level can be seen as a mediator between the macroscopic and submicroscopic level. Often it cannot be clearly separated from the other representational levels because both the macroscopic and submicroscopic levels can be expressed symbolically (Taber, 2002).
Reasoning about chemical phenomena
Analysing a given chemical phenomenon requires going one level below the visible surface to identify the entities and their interactions at play in the process (Hempel and Oppenheim, 1948; Salmon, 1984; Machamer et al., 2000; Talanquer, 2010; Braaten and Windschitl, 2011; Rottman and Keil, 2011; Yeo and Gilbert, 2014; Krist et al., 2018). In recent years, there has been increased interest in characterizing and fostering students’ ability to generate mechanistic explanations for phenomena in science education (Grotzer, 2003; Russ et al., 2008a; Russ et al., 2008b) and in chemistry education (Becker et al., 2016; Caspari et al., 2017; Caspari et al., 2018; Talanquer, 2018). A mechanistic explanation is described in the philosophy of science as a detailed description of the underlying process responsible for the phenomenon. Mechanistic explanations first identify the relevant entities involved in the process, their properties, the activities in which they are engaged, and their organization. The activities and the organization of core entities are responsible for the properties and behaviours of the system as a whole (Machamer et al., 2000). Usually, accounting for the underlying interactions of a mechanism refers to the invisible particulate or molecular level (Sevian and Talanquer, 2014; Becker et al., 2016). Thus, transitioning from the macroscopic to the submicroscopic level is a core competency needed to progress towards causal mechanistic reasoning (Crandell et al., 2019; Crandell et al., 2020).Typically, entities, properties, and activities are conceived as parts of the submicroscopic level, but each of these parts and its features are visible macroscopically. In a recent study, we investigated to what extent students transit between individual representational levels (macroscopic and submicroscopic) and characterized the activated mechanistic features at each of these levels while students were engaged in analysing laboratory work-up procedures in an organic chemistry lab (Keiner and Graulich, 2020).
Fig. 2 shows an overview of the features of the respective representational levels, based on our analysis in a previous study (Keiner and Graulich, 2020). The findings of this study revealed that students’ language is dominated by macroscopic terms and entities and tends not to consider submicroscopic activities. Only a few students could explain the given phenomenon at the submicroscopic level. It became apparent that students do not seem to know the level at which they are explaining a phenomenon or how to purposefully connect the representational levels to make sense of the phenomenon.
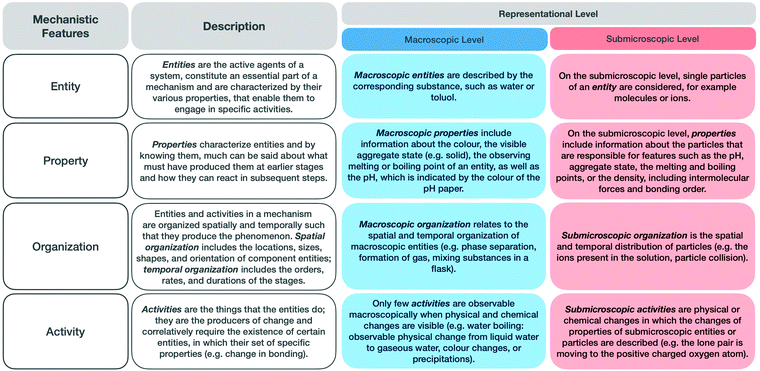 | ||
| Fig. 2 Description of the mechanistic features in general and at the macroscopic and submicroscopic representational levels. | ||
Our previous findings (2020) emphasized the need to create awareness in students that the macroscopic and submicroscopic levels both contribute to understanding the phenomenon; that students need opportunities to connect both levels; and that students should use their thought processes to evaluate the objectives of a synthesis step during laboratory work.
Scaffolding reasoning
Scaffolding is an instructional technique that, ideally, enables a novice to complete a task or problem that he or she could not have accomplished unassisted (Wood et al., 1976; Belland et al., 2011). Scaffolding students’ reasoning can be a powerful tool to help students activate and connect prior knowledge pieces. A scaffold could as well encourage students to reflect on how they approached a task in a specific context (Davis, 2000). Scaffolding can thus combine structuring the cognitive process of solving a problem with metacognitive and procedural prompting (Reiser, 2004). Scaffolding enables learners to internalise the guidance through distributed practice and, eventually, self-regulate their cognitive actions. Common features of a scaffold are: focusing learner's attention towards a goal, simplifying the task in a stepwise manner, modelling and demonstrating, prompting for ongoing diagnosis and assessment, or even prompting the eventual transfer of responsibility (Reiser, 2004; Puntambekar and Hubscher, 2005; Seel, 2011). Such prompts or questions could provide students with cues what knowledge to activate, connect, and include in their explanation (Kang et al., 2014; Kararo et al., 2019). Prompting students, for example, could direct student attention to facilitate awareness of potential knowledge gaps, help them organise their thoughts, and recognise a need to evaluate the validity of their solutions (Ge and Land, 2003).Learning or teaching with a scaffold is commonly a temporary support that is withdrawn when the learner progressively internalizes the scaffold. The cognitive load that a complex task, such as conducting an organic synthesis, may impose on the learner should be reduced by the scaffold (Sweller et al., 1998; Kirschner, 2002). Slowly students learn to independently solve the problem, building up the necessary reasoning skills and heuristics.
Goals and research questions
Based on our findings in a previous study (Keiner and Graulich, 2020), we developed a scaffold that purposefully prompts students to (a) carefully consider their macroscopic observations and submicroscopic explanations separately, and (b) connect both levels and judge if they reached their synthesis goal. In this study we attempt to elicit, qualitatively, whether the extent to which students’ make use of the scaffold changes the way they explain organic synthesis on both levels; and whether they make use of multiple mechanistic features when explaining organic syntheses. Our study is guided by the following research questions:1. To what extent does students’ activation of mechanistic features (at the macroscopic and submicroscopic levels) change after they have worked with the scaffold during two organic syntheses?
2. What kind of sense-making approaches (with regard to the representational levels) do students use while reasoning about an organic synthesis before and after working with the scaffold?
3. To what extent is each student's individual work with the scaffold related to a change in his or her sense-making approach?
The term “sense-making” is widely used in the educational literature (Fitzgerald and Palincsar, 2019) to express a learner's approach to make sense of phenomena. A sense-making process can be broadly understood as an expression of the learner's underlying mental models while being engaged to make a continuous effort to comprehend and explain, i.e. to make sense of a scientific phenomenon. Thus, in this study we use the term “sense-making approach” to capture students’ approaches to describe and explain chemical phenomena and the underlying molecular processes.
To answer these research questions, we performed a qualitative interview study with chemistry student teachers enrolled in an organic laboratory course.
Methods
Context and participants
The research study was conducted at a German university in July and August 2019. Students were recruited on a voluntary basis from the practical course “Organic Chemistry Laboratory” via in-class announcements. The course is part of the teacher training programme for student teachers and normally takes place during the summer break between the fifth and sixth semesters of their studies. The organic chemistry laboratory course covers a period of four weeks during which students attend a lecture from 8:00 am to 10:00 am and work in the lab from 10:00 am to 6:00 pm. Successful completion of the module “Organic Chemistry I” (OC I) is the prerequisite for participating in the practical “Organic Chemistry Laboratory” course. The OC I lecture provides basic knowledge of organic chemistry and discussions on typical reaction mechanisms (e.g. electrophilic addition reaction, nucleophilic substitution, radical polymerization, and esterification).During the laboratory course, students synthesize six given compounds through nucleophilic substitutions, electrophilic additions, or elimination reactions and report the synthesis in a traditional lab protocol that also requires information on the mechanism and the obtained yield. Students use typical laboratory procedures—isolation and purification (e.g. washing with sodium carbonate, fractional distillation)—for the work-up. These laboratory techniques are provided in advance in a written online manual. The protocol used in this laboratory course does not explicitly encourage students to connect the different representational levels to each other.
A total of 22 undergraduate chemistry student teachers (15 female and 7 male) agreed to participate in the study. They were between 22 and 47 years old (the average age was 24 years) and were invited via an announcement to students attending a class lecture or in the laboratory. All students who volunteered for this study were provided with information about their rights and the handling of the data; informed consent was obtained from all participants. Institutional Review Board approval is not required at German universities, but the recruitment process followed ethical guidelines and it was clarified to the students that they could opt out at any time.
All students provided written informed consent for (a) the use of transcripts of their interviews by the research team, (b) the analysis of their data by the research team, and (c) the use of their data, including their drawings and notes, for publication. In this study, participants were assigned pseudonyms; no identifying information was recorded or scanned that would allow the re-identification of participants’ data. The interviews were conducted in German, and students’ interview excerpts and written notes were translated from German to English.
Research instrument
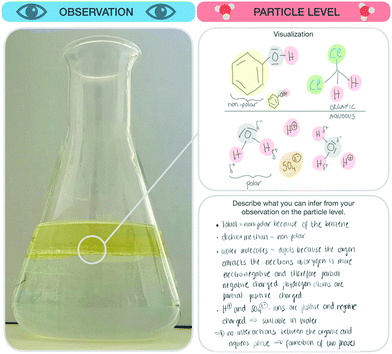
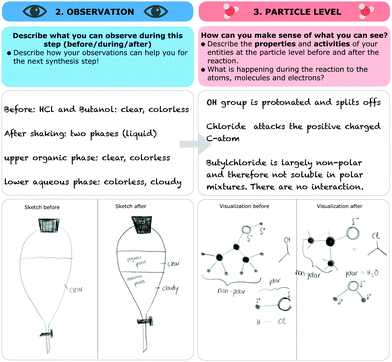
The research instrument consisted of a scaffold (see Fig. 3) designed to support students’ reflection on macroscopic observations, the respective submicroscopic level, and the goals of each individual step of the synthesis (e.g. isolation or purification of a given compound). Generally, students concentrate on the experiment as a whole and pay little attention to the individual steps (e.g. the purification steps). Since individual steps are important, we gave students explicit instructions to focus on these individual steps, collect their observations during the synthesis, and reflect on the ongoing chemical processes of each synthesis step at the particle level. As a first part, students were asked to note the name of the synthesis and describe the synthesis step they were working on currently. Second, students were asked to fill in four parts for each synthesis step: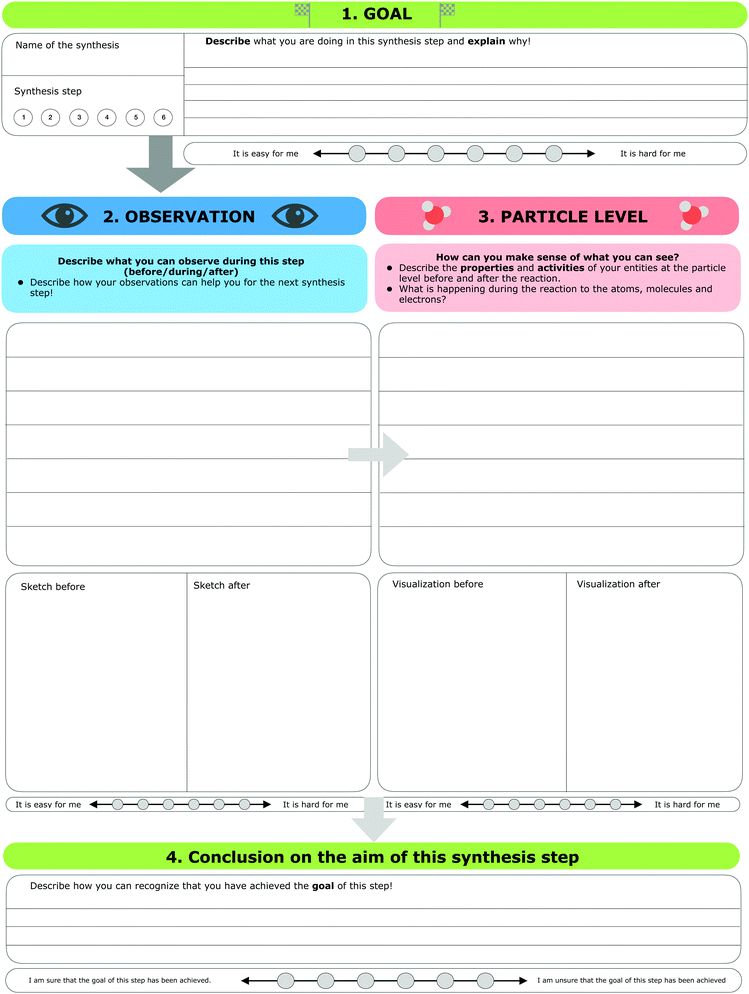 | ||
| Fig. 3 The scaffold used in the organic laboratory course for each synthesis step (translated from German). | ||
1. Goal. In the first part, students were instructed to describe the goal of this synthesis step and explain why they were working on this step.
2. Observation. After defining the goal of the synthesis step, students were prompted to describe and visualize their observations, before, during, and after this step. It should be noted that some observations did not always differ substantially. For example, if students mixed two colourless solutions for five hours and the solution remained colourless, they were not able to observe any visible changes. Furthermore, students were instructed to draw a sketch of their observations before and after the synthesis step, if possible.
3. Particle level. In the third part, students were prompted to describe what they had concluded after their observations (e.g. describe the underlying process of the mechanism of the reaction). Afterwards, students were prompted to visualize the process before and after this step at the submicroscopic level. Students were free to choose whether to draw the particles or use symbolic representations of the molecules.
4. Conclusion and aim. In the fourth part, students were prompted to decide, based on their observations and submicroscopic explanations, if they had achieved the goal of the synthesis step. Students could not always assess whether they had been successful or not because, as in the example with two colourless solutions, the results were hard to differentiate. After each of the first three parts, students were asked to rate the perceived difficulty of filling out these parts of the scaffold. Unlike the previous three parts, the last step required students to rate how sure they were that they had achieved the aim of this synthesis step.
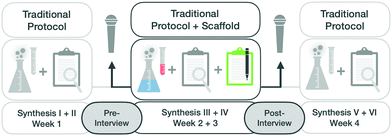
Data collection
This study aims to understand the extent to which a scaffold administered in an organic laboratory course can stimulate students to activate mechanistic features and influence their approach to making sense of synthesis steps. To compare students’ approaches before and after instruction, the first author conducted pre- and post-semi-structured interviews.During these semi-structured interviews, students were prompted to comment on their experiences in the lab and to explain, step-by-step, how they carried out one of the six organic syntheses. Additional questions based on their responses, were used to engage students in reflecting on the macroscopic or the submicroscopic level (see Fig. 4).
In our previous work, it became apparent that students could take shortcuts in explaining a given phenomenon. Thus, to ensure that we elicited the capabilities, we (a) prompted students to consider the submicroscopic level when they began to describe the macroscopic level of the synthesis and (b) prompted students to consider the macroscopic level when they began to describe the submicroscopic level. In the pre-interview, which took place after the second of six completed syntheses, students were asked to choose one synthesis and describe it step-by-step.
The decision to give the students the choice to choose which synthesis and, consequently, which mechanism they would like to discuss in the interview was made consciously. Giving students the opportunity to choose one of their two syntheses was intended to relieve their nervousness during the interview situation and allowed them to choose a familiar content area. The syntheses and related mechanisms are very similar. Almost every synthesis has certain preparatory steps, a main reaction, and ongoing purification steps. Due to these similarities of the respective syntheses, we considered it appropriate to allow the students choose which synthesis they want to explain in the interview. To answer our research questions, we were more interested in students’ sense-making approaches when they explained a synthesis of their choice, than to test them all on the same synthesis. We assume that this process helps them feel at ease with the task, so that the students feel more comfortable and are willing to share their thoughts.
The interview typically started with the sentence, “Please tell me in detail what you have done in your organic synthesis and why you have done the individual steps”. After the interview, students were introduced to the scaffold and told how to use it during the next two syntheses (see Fig. 4).
When filling out the worksheet following the interview, students were told there were multiple possible answers, and that we were mainly interested in their imagination and conceptions of levels, particularly the particle level. To show them how to use the scaffold in the laboratory, we demonstrated an exemplary solution to an organic synthesis that was not part of the course. After introducing the scaffold, the students used the scaffold for the next two syntheses.
During the lab activity, the first author played the role of a mediator. She interacted with the students, checked if they were comfortable with the instructional approach, answered questions, addressed any uncertainties and arranged the appointment for the second interview when students had completed their scaffold assignments. During the lab activity, a question most frequently asked by students was how detailed they should describe the individual parts of the scaffold and how they should depict the processes on the particle level. The first author advised the students to describe the chemical phenomena as detailed as possible and to choose which visualization (e.g. Lewis structures or particle drawings) they want to use to represent the synthesis step. Overall, students felt comfortable working with the scaffold and could always consult the first author or have a look at the provided sample worksheet.
The second interview, after working with the scaffold, had the same structure and prompts as the first one. In addition, it included students’ evaluation and perceptions of the scaffold. During the second interview students were not allowed to use their scaffold worksheets but could choose again one of the two syntheses for the interview. Giving students the opportunity to choose one of their two syntheses again, was intended to relieve their nervousness and allow them to choose a familiar content area.
The collected data included transcripts of the two semi-structured pre-and post-interviews with all students, and scans of their completed scaffold worksheets and field notes following the interviews. The interviews lasted on average 30 minutes.
Data analysis
To acquire deeper understanding of how students interacted with the scaffold, we conducted a qualitative data analysis. All interviews were transcribed verbatim and analysed using the coding software MAXQDA. To evaluate whether the scaffold led to changes in students’ approach and to answer our research questions, we analysed the data using the following steps:| Code | Student examples | Code description |
|---|---|---|
| EntityM | “I still have contaminations like water and sulphuric acid in my flask.” | Entities are described by using the name of the substance or naming a solution of a substance (e.g. reaction educts or products), without reference to the respective molecule or particle. |
| PropertyM | “Ethanol is liquid like sulphuric acid. Salicylic acid is solid. ” | Properties are described by reference to an observable physical or chemical property (e.g. the colour of a substance, the observable pH value, the aggregate state). |
| ActivityM | “Sodium chloride is formed , and we still have another chlorine whichsplits off.” | Activities are described by referring to an observable physical or chemical change of properties, with no reference to molecules or particles (e.g. an ongoing phase separation). |
| OrganizationM | “I could observe two phases ; in the aqueous phase there is water and sodium chloride, maybe sodium acetate…” | Organization of entities is described by referring to the temporal (e.g. stand overnight) and spatial organization (e.g. observation of two separate phases) of macroscopic entities. |
| EntityS | “… CaCl 2 which means, I have Ca 2+ and two Cl − ions.” | Entities are described by using the name of the particle or referring to the molecules of a substance (e.g. functional groups, ions, water molecules). |
| PropertyS | “…the partial positive charged carbon atom in acetic acid. ” | Properties are described by reference to an invisible physical or chemical property (e.g. deprotonated entity, aggregate state). |
| ActivityS | “The acetate molecule attacks the nucleophile.” | Activities are described by referring to an invisible physical or chemical transformation which results in a change of properties, with reference to molecules or particles. |
| OrganizationS | “…in the aqueous phase we have Na + and Cl − .” | Organization of entities is described by referring to the temporal and spatial organization (e.g. more H3O+ ions) of submicroscopic entities. |
The changes with regard to the activated mechanistic features from the pre- to the post-interview were explored for statistical significance using a paired-sample t-test.
Therefore, we divided the interview transcripts into smaller content-related “sections of meaning” (e.g. the description of a phase separation, the evolution of gas, or the main reaction step). The sections of meaning could be differentiated by an interview prompt or a content-related change by the student. Such a verbal section of meaning focuses on one aspect of the observed synthesis (e.g. a phase separation or a distillation process) and can occur at one or both representational levels. The subdivision into smaller sections of meaning was useful because the students in this study did not consistently use just one approach to describe their synthesis. Depending on the synthesis step, they may have reasoned on one of the two levels or changed the representational level in different ways. The subdivision into smaller sections of meaning provided the opportunity to better compare changes from the pre- to the post-interview (e.g. the explanation of a phase separation in both interviews). Consequently, we first separated or divided each section of meaning in students’ interview transcripts. The first part of analysis step II did not include any coding of the transcripts, but only the division of the interviews into content-related sections of meaning.
To better characterize students’ sense-making approaches, we were interested in the type of transition used to explain their synthesis steps in such a section of meaning. Therefore, we categorized, for example, whether a transition occurred from one representational level to another and whether this shift was caused by the interviewer's prompt or was made freely and deliberately by the student.
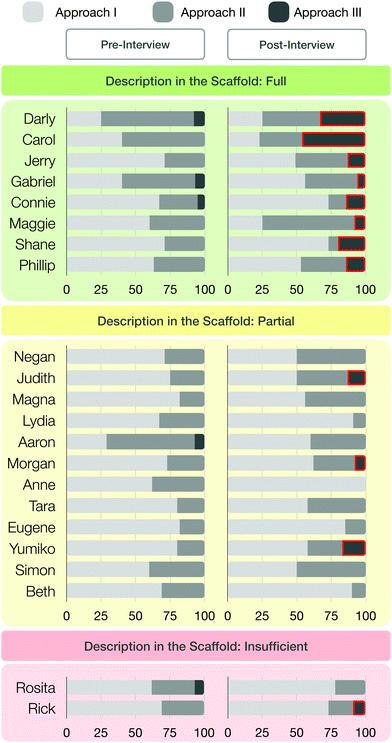
This process resulted in various combinations, such as from the macroscopic (M) to the submicroscopic (S) level without a prompt (M–S), from the macroscopic to the submicroscopic level after a prompt (M–P–S) or staying at the macroscopic level (MM). The individual sections of meaning were then coded with these three occurring combinations (MS; MM; MPS) between the macroscopic and submicroscopic representational level. In the next step of analysis, we determined the distribution of the respective transitions for each student in the pre- and post-interviews. Therefore, we counted how often students used which approach to explain the synthesis steps. For instance, we counted the amount of sections of meaning in which a student explained the chemical phenomena at the macroscopic level (sense-making approach I), in which the student transitioned after being prompted to the submicroscopic level (sense-making approach II), and in which the student switched to the submicroscopic level without a prompt (sense-making approach III). We, thus, generated a bar chart for each student, showing the respective percentages of each approach in the pre- and post-interview (cf.Fig. 12). The changes in students’ sense-making approaches from the pre- to the post-interview were explored for statistical significance using a paired-sample t-test.
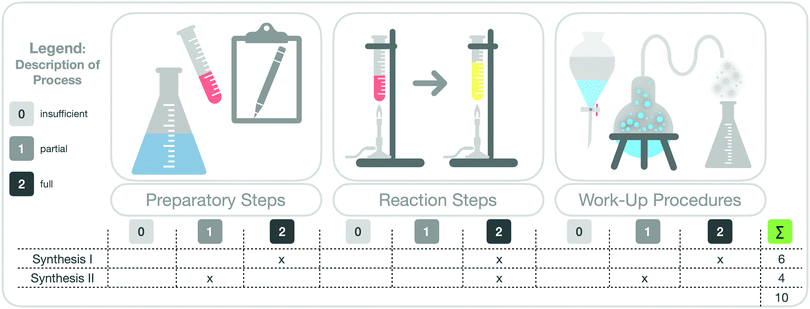
Furthermore, we divided a synthesis into three main parts (see Fig. 5). These were the preparatory steps, the main reaction step, and the work-up procedures (e.g. purification steps). For each of these three parts, we rated students’ description of the synthesis steps with the rubric by focusing on the completeness of the descriptions and on the correct separation of the macroscopic and submicroscopic levels. This rating indicates whether students made the correct observations of the respective step at the macroscopic level and described and visualized it at the particle level.
Unreadable or incomprehensible (insufficient) descriptions were awarded 0 points; partial descriptions of the individual synthesis step, with correct differentiations between the macroscopic and submicroscopic levels and with few aspects missing or incorrect, were awarded 1 point; full and correct descriptions of the synthesis step were awarded 2 points.
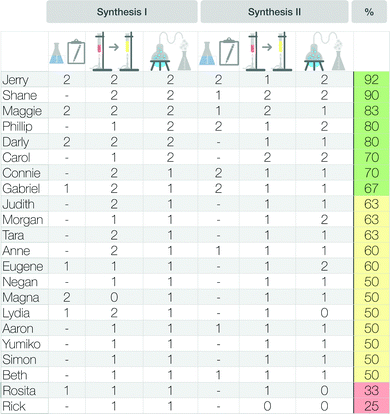
To assess how students worked with the scaffold overall, we summed up their points on both syntheses and calculated a total score. The total score may differ depending on the described organic synthesis because not every synthesis has a “preparatory step”. Some organic syntheses start directly with the “main reaction step”. We therefore calculated the percentage of available points achieved by each student. Based on the attained percentage, we divided the students into three “groups”, to indicate how students worked, in general, with the scaffold. Zero to 33% represented group I (mainly insufficient description); 34% to 66% represented group II (mainly partial description); and 67% to 100% represented group III (mainly full description). Fig. 5 gives an overview of this analysis.
The first author (PhD candidate with a master's degree in chemistry education) coded the entire data set from all rounds of coding and analysis. During the data analysis, the authors regularly discussed the coding scheme. Furthermore, constant discussions with the whole research group were conducted.
Results and discussions
We analysed students’ interview data and written work and characterized different sense-making approaches in students’ sections of meaning and the related changes with regard to the scaffold worksheet. Overall, students demonstrated increased understanding of organic synthesis in their post-scaffold interviews. To facilitate the interpretation and discussion of the results in accordance with our research questions, we divided the results into three parts. The first part describes students’ activation of mechanistic features while explaining their organic synthesis in the pre and post-scaffold interview. The second part illustrates students’ sense-making approach in the sections of meaning. The third part illustrates students’ work with the scaffold and the related changes in their sense-making approach.To what extent did students’ activation of mechanistic features (at the macroscopic and submicroscopic levels) change after they worked with the scaffold during a synthesis?
All features of a mechanism were activated at least once by each student during their explanation of the synthesis, but with varying frequencies. Because naming a property, organization, or activity is generally accompanied by a reference to an entity, it is unsurprising that entity was the mechanistic feature used most at both levels.
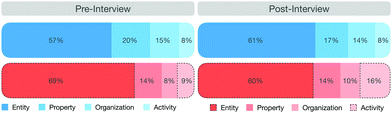
Our analysis revealed that students activate, on average, more macroscopic features than submicroscopic features. Close to equal percentages were obtained in the pre-interview (macroscopic features 75%, submicroscopic features 25%) and post-interview (macroscopic features 73%, submicroscopic features 27%). In the post-interview, students included more reasoning about the underlying chemical processes, as indicated by an increase of the feature activity. Fig. 6 shows an overview of the percentage of activated features in the pre- and post-interview. It is apparent that in both pre- and post-interviews, entities were activated most frequently (pre: EM: 57%; ES: 69%; post: EM: 61%; ES: 69%), followed by properties (pre: PM: 20%; PS: 14%; post: PM: 17%; PS: 14%), organization (pre: OM: 15%; OS: 8%; post: OM: 14%; OS: 10%), and activities (pre: AM: 8%; AS: 9%; post: AM: 8%; AS: 16%).
With regard to the activation of individual features at the representational levels, we did not observe any major differences except for two submicroscopic features—the entities and activities (see the dotted lines in Fig. 6). The submicroscopic activity was activated, on average, twice as often in the post-interview (16%) as in the pre-interview (8%). This increase is significant (p < 0.05) and has a large effect size (Cohen's d = 0.812). With the exception of two students, all students showed an increase in this mechanistic feature in the post-interview. The statistical analysis of the other features as well as the confidence intervals are presented in the appendix (cf.Table 3).
Consequently, they activated considerably fewer submicroscopic entities (−9%) in the post-interview. One can interpret this finding as an increase in process-oriented thinking in the post-interviews. This is a promising finding as we found in one of our previous studies (Keiner and Graulich, 2020) that students, when prompted to explain organic work-up procedures, tend to focus more on static entities and less on process-oriented features like the activity. Moreira et al. (2019) found that students tend to explain chemical phenomena without reference to the activity. They concluded that students’ explanations incorporate activities of one or more entities of the system only at a higher level of sophistication, indicating a change from a static to a more dynamic perspective of the phenomenon. In our previous study (Keiner and Graulich, 2020) we confirmed the observation that students often struggle to describe the activities that give rise to entities or properties that are responsible for earlier or subsequent activities. The following example illustrates students’ use of the feature activity.
Specifically, we compared student Darly's explanation from the pre- and post-interview in which he worked out the nucleophilic attack (the main reaction step) in two different organic syntheses. In the pre-interview, Darly listed many properties (e.g. “partially negative; partially positively charged; positively charged”) without reference to the formation (i.e. the respective activities). In the post-interview, he once again mentioned the comparable properties of entities (e.g. “negatively charged; positively polarized C-atom; oxygen is electronegative”). However, in contrast to the pre-interview, he included more descriptions about the formation of the properties (“attracts the electrons”) and the activity of the entity (“attacks as a nucleophile”).
To summarize our findings for the first research question, we noted that students activated all mechanistic features, but with different frequencies. They most frequently mentioned entities, followed by properties, organization, and, least frequently, activities. The high percentage of activated entities in both cases can be attributed to the fact that naming a property, organization, or activity is generally accompanied by a reference to an entity. Therefore, it is not surprising that the most used mechanistic feature at both levels was the entity. Although students activated, predominantly, the same features after working with the scaffold, we observed small qualitative differences in their explanations (see Fig. 7).
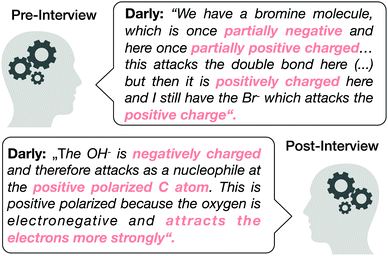 | ||
| Fig. 7 Illustrative quotes from Darly as he thought through a nucleophilic attack in the pre- and post-interviews. | ||
What kind of sense-making approaches (with regard to the representational levels) do students use while reasoning about an organic synthesis before and after working with the scaffold?
To answer this question, we examined how students made sense of organic synthesis steps with regard to their transition from the macroscopic level to the submicroscopic level, and analysed whether their sense-making changed after working with the scaffold. Depending on how students explained the reaction process in the section of meaning, different transitions were observable (see Fig. 8). The sections of meaning fell into one of three categories: students who transitioned from the macroscopic to the submicroscopic level independently, those who did so after a prompt by the interviewer, and those who remained at the macroscopic level even after a prompt. It is important to note that none of the students began their explanation of a phenomenon at the submicroscopic level. The sections of meaning categories are as follows:
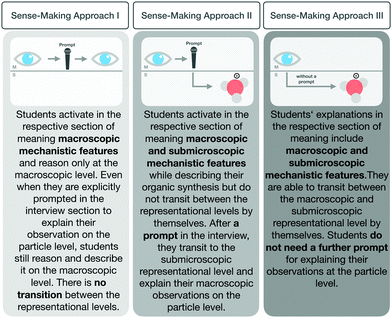 | ||
| Fig. 8 Three sense-making approaches which characterize students’ sections of meaning in explaining synthesis steps. | ||
Sense-making approach I. The first approach that characterized a section of meaning was a student explaining chemical synthesis macroscopically using macroscopic features. Even after a prompt to consider the particle level, the explanation remained at the macroscopic level and did not include submicroscopic features.
Sense-making approach II. This sense-making approach characterized those sections in which students analysed a synthesis step at the macroscopic level then, after a prompt from the interviewer, transitioned to the submicroscopic representational level and activated the submicroscopic features.
Sense-making approach III. The third sense-making approach characterized sections of meaning in which students described the chemical phenomenon at the macroscopic level and transitioned, without being prompted, to the submicroscopic level.
In the following section, we illustrate the sense-making approach with student examples. Fig. 9 shows student Carol's sense-making approach for the dissolving process of chloroctane with sodium acetate and methyltrioctylamine. First, he described the macroscopic appearance (aggregate states) of the substances (“liquid”; “colourless”; “solid”). Carol made no reference to the particle level of the different aggregate states (e.g. how the particles are arranged in the different aggregate states—closely packed or separated from each other).
 | ||
| Fig. 9 Carol's sense-making approach (I) to the aggregate states and the dissolving process in the pre-interview. | ||
Carol was first explicitly prompted to explain his observation (the dissolution process) at the particle level. However, he described his observations only at the macroscopic level (“it was relatively lumpy”; “it mixed better”), and did not mention the dissolving process at the particle level as a way to activate submicroscopic features, even after being prompted (“when you consider the particle level”) on the meaning of the different aggregate states at the particle level.
The following example of student Gabriel (Fig. 10) illustrates sense-making approach II. His sense-making approach dealt with the different aggregate states of the molecules involved in his organic synthesis. First, Gabriel named the different aggregate states (“solid“; “liquid”) without further explanations. After an interview prompt to consider the aggregate states at the particle level, Gabriel transitioned to the submicroscopic level, activated submicroscopic features (“particles”), and explained the different aggregate states with reference to the organization of the particles at the submicroscopic level (e.g. “particles are more distant from each other”; “particles are closer together”).
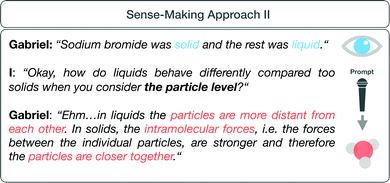 | ||
| Fig. 10 Gabriel's sense-making approach (Scalco et al., 2018) to the aggregate states in the pre-interview. | ||
Compared to sense-making approach I, Gabriel started to explain his observations at the particle level after the prompt. In sections of meaning that correspond to sense-making approach II, students tend to consider chemical phenomena at the macroscopic level and transition to the submicroscopic level after a prompt.
The third sense-making approach that emerged from students’ sections of meaning is illustrated by the example of student Darly (see Fig. 11). He described a phase separation and immediately started to make sense of his observation at the particle level, without an explicit prompt. He then transitioned from the macroscopic level (“I observe two phases”) to the submicroscopic level, activated submicroscopic mechanistic features, and explained his observation of organic synthesis at the particle level. Overall, sense-making approach I mostly characterized students’ explanations of their chosen organic synthesis in the pre- and post-interview (see Table 2), followed by sense-making approach II. Sense-making approach III was only present in a few sections of meaning. However, the occurrences of sense-making approach III increased slightly between the pre-scaffold and post-scaffold interviews—1% → 9%—after students had worked with the scaffold.
 | ||
| Fig. 11 Darly's sense-making approach (III) as he describes the separation of two phases in the pre-interview. | ||
| Approach I | Approach II | Approach III | |
|---|---|---|---|
| Pre-interview | 63% | 36% | 1% |
| Post-interview | 60% | 31% | 9% |
| t-Value | Cohen's d | 95% CI | |
|---|---|---|---|
| EM | 2.35* | 0.37 | [−0.23; 0.97] |
| PM | −0.66 | −0.14 | [−0.73; 0.45] |
| OM | 0.14 | 0.03 | [−0.56; 0.62] |
| AM | 0.48 | 0.12 | [−0.47; 0.71] |
| ES | 0.20 | 0.05 | [−0.54; 0.64] |
| PS | 1.21 | 0.24 | [−0.35; 0.84] |
| OS | 1.34 | 0.41 | [−0.19; 0.99] |
| AS | 4.61*** | 0.81 | [0.19; 1.42] |
To what extent is a student's individual work with the scaffold related to a change in the sense-making approach?
To better understand how much students' sense-making approach in the section of meaning had changed, we rated students’ description of the reaction process in the scaffold worksheet (cf. Appendix 2, Fig. 16), with reference to the rubric (see Fig. 5), and correlated their descriptions to any changes between the pre- and post-scaffold interviews. Fig. 12 shows an overview of the changes in each of the three sense-making groups, in combination with the ratings of students’ description in the scaffold worksheet.
We evaluated the increase of the sense-making approaches from the pre- to post-interview statistically. The data show a statistically significant increase (p < 0.01) and a large effect size (Cohen's d = 0.877) for sense-making approach III. The comparison of sense-making approach I and II as well as the confidence intervals are presented in the appendix (cf.Table 4).
| t-Value | Cohen's d | 95% CI | |
|---|---|---|---|
| SMA I | −0.58 | −0.11 | [−0.70; 0.48] |
| SMA II | −0.93 | −0.26 | [−0.85; 0.34] |
| SMA III | 2.92** | 0.88 | [0.25; 1.49] |
The eight students in group I (green box) described the synthesis steps in the scaffold more or less fully. The twelve students in group II (yellow box) described the synthesis steps in the scaffold only partially. Only the two students in group III (red box) described the steps insufficiently. Fig. 12 also shows the percentage of sense-making approaches I-III in the pre-scaffold interviews and the respective percentages in the post-scaffold interviews (visualized by different coloured bars, Fig. 12). We observed a relationship between the completeness of the description in the scaffold worksheet and the sense-making approaches in the pre- and post-scaffold interviews.
We noted that students who fully described the synthesis steps in the scaffold worksheet (green box) usually showed an increase in sense-making approach III in the post-scaffold interview; this result is highlighted with red boxes (see Fig. 12). This means that these students tended to describe their chosen synthesis in the post-scaffold interview at the macroscopic and submicroscopic levels without being explicitly prompted. Furthermore, we noticed a small increase in the number of students who explained the submicroscopic level after a prompt, whereas in the pre-scaffold interview they often remained at the macroscopic level even after being prompted. Students who partially described the synthesis steps in the scaffold (yellow box) showed few changes in their sense-making approach. During the pre-scaffold interviews, only one student was explaining a phenomenon using sense-making approach III. By contrast, during the post-scaffold interviews, at least three students used sense-making approach III. Further, some students made use of sense-making approach II, which indicates that they explained the synthesis steps at the submicroscopic level after being prompted.
Only two students insufficiently described the synthesis steps in the scaffold (red box), and we observed an increased use of sense-making approach III by one of them.
Based on the use of the scaffold (Fig. 2), students who described the reaction processes in detail tended to switch more often to the submicroscopic level, without prompts. Some of these students explained their syntheses more reflective and could better reactivate their knowledge from the work with the scaffold. Students who struggled to fully explain the reaction processes tended to switch less often to the submicroscopic level. This indicates that the scaffold may encourage students to consider the submicroscopic level.
Maggie's case illustrates these changes for group 1 in the post-scaffold interview. Fig. 13 and 15 show excerpts from Maggie's interview transcripts, and Fig. 14 shows parts of her scaffold worksheet.
 | ||
| Fig. 13 Maggie's sense-making approach (I) in the pre-scaffold interview where she reasoned over the formation of two phases. | ||
 | ||
| Fig. 14 An excerpt from Maggie's scaffold worksheet in which she describes the formation of two phases. Sketches and her description are translated from German into English. | ||
 | ||
| Fig. 15 Maggie's sense-making approach in the post-scaffold interview in which she reflected about the formation of two phases. | ||
Maggie fully described the synthesis steps in the scaffold worksheet but only started to use sense-making approach III in the post-scaffold interview (see Fig. 12). During her pre-interview, Maggie reasoned about the separation of two phases (synthesis—nucleophilic substitution of octyl acetate). She was then prompted by the interviewer to explain the formation of the two phases in more detail but her thought processes remained at the macroscopic level.
In her explanation Maggie named macroscopic properties (“hydrophobic and hydrophilic”) and said that the two properties are important for the separation of two phases “because of the characteristics of miscibility”. She did not explain what hydrophobic or hydrophilic meant at the particle level. Furthermore, she did not explain which functional groups were responsible for the formation of two phases at the submicroscopic level or how the functional groups relate to the formation of two phases. In her scaffold worksheet Maggie mentioned these missing properties and activities at the submicroscopic level (synthesis—nucleophilic substitution of tert-butyl chloride). She also described the macroscopic properties (“colourless”, “clear”, “liquid”, “observation of two phases after shaking”). With reference to the submicroscopic level, she alluded to the process of the formation of two phases by mentioning that the “OH group is protonated” (PS) and therefore “splits off” (AS). Next she described “the attack” (AS) of the chloride on the “positively charged” (PS) “C-atom” (ES). Maggie then considered the polarities of the resulting entities and the formation of the two phases. She said that “butyl chloride is largely non-polar and therefore not soluble in polar mixtures”. Additionally, she visualized her description in a drawing (see Fig. 14). She drew the partial positive and negative charges (see Maggie's sketch is on the right side of Fig. 14) and tagged the polar and non-polar parts of the molecules.
In her sketches of the particle level she used both symbolic representations (e.g. Lewis structures) and representations of particles (bubbles) to represent her thoughts. During the post-scaffold interview, Maggie again described the formation of two phases (synthesis—nucleophilic substitution of tert-butyl chloride). The following quotes are excerpts from Maggie's post-scaffold interview during which she activated submicroscopic features and described a submicroscopic property (“OH group is polar”) as well as the formation of that property (“because we have a delta plus on the hydrogen atom and a delta minus on the oxygen atom”).
Furthermore, she described properties and activities of the CC-bonds (“usually non-polar”; “cannot form hydrogen bonds”) and concluded by describing the non-existent interactions between the entities and the resulting formation of two phases. Thus, she was able explain the activities and properties that were missing in the pre-scaffold interview. Maggie's case is exemplary because it shows the connection between the scaffold worksheet and her thinking processes during the post-scaffold interview.
We observed various incidences of drawings or a description in the scaffold sheet seeming to encourage students to make more references to the submicroscopic level in the post-scaffold interview. As the work-up procedures are not normally explained in the laboratory report at the end of each synthesis (which Maggie's case demonstrates), we can assume that students’ shift towards the submicroscopic level was related to students’ work with the scaffold.
With regard to the changes effected by students’ use of the scaffold, students who described their reaction processes in detail switched more frequently to the submicroscopic level and of their own volition.
However, based on the exploratory and qualitative nature of the study, further research is needed to determine if these positive observations are replicable and how best to support those students who filled out the scaffold worksheet insufficiently and thus did not profit as much from the scaffold as other students.
Conclusions and implications
Successfully making sense of organic synthesis in the laboratory requires the ability to explain observations using multiple mechanistic features at the macroscopic and submicroscopic representational levels and to connect these levels in a meaningful way. We consciously do not propose “one” correct approach or a specific starting point to explain a chemical phenomenon in a meaningful way. It is, however, important to be aware of the coexistence and the role of the two representational levels to fully account for a chemical phenomenon. The order of use, i.e. whether a submicroscopic explanation is inferred from a macroscopic phenomenon or vice versa is secondary and depends on the context and the question asked.If a chemical phenomenon shows no obvious observations (e.g. a colourless solution reacts with a colourless solution), it is difficult to make sense of the observation. However, guiding students to attend to macroscopic changes, even if they are small or hardly visible is an essential skill in the inquiry process. Relating observable changes to the underlying molecular processes is as important as being aware, that a reaction may take place even when there is nothing to observe.
If students argue and explain only at one of the two representational levels and, for example, remain completely on the macroscopic level, they are missing the other half of the story. The same issue arises when students neglect macroscopic observations and only consider the submicroscopic level in their explanations. Thus, students need to be encouraged to consciously reflect on a chemical process macroscopically and submicroscopically (Taber, 2013). They also have to be able to connect both representational levels in a meaningful way (Anderson, 1978; Johnstone, 1991; Gabel et al., 1992; Treagust et al., 2003; Gilbert and Treagust, 2009a, 2009b; Krist et al., 2018; Keiner and Graulich, 2020). These abilities are addressed by the structure of the scaffold.
To reprise, the central goals of this study were as follows:
(1) Analyse to what extent students are able to activate mechanistic features (at the macroscopic and submicroscopic levels) during pre- and post-scaffold interviews.
(2) Characterize how students make sense of synthesis steps.
(3) Determine if there is a relationship between students’ sense-making approaches and their use of the scaffold.
Our analysis revealed that students use all mechanistic features at both representational levels to make sense of organic synthesis. In the pre- and post-scaffold interviews, students were able to activate entities and properties at both representational levels but used fewer activities and less organization. Analysing the use of these features in detail revealed that the causal link between the features (particularly during the pre-scaffold interview) was often missing and students’ explanations can be considered as static. This finding is in accordance with previous research (Grove et al., 2012; Caspari et al., 2018; Moreira et al., 2018; Keiner and Graulich, 2020) in which students’ explanations tend to include initial starting material and products but little description of the underlying reaction. During the post-scaffold interview, after students had completed two organic syntheses using the scaffold, we observed a small increase in the activation of process-related mechanistic features as students described activities more frequently. Thus, we can assume that students’ work with the scaffold encouraged them to reflect more deeply on the particle level of their synthesis steps. However, further research is needed to elucidate the effect of the scaffold using quantitative methods and a larger sample.
Our analysis also revealed that three main sense-making approaches characterize students’ explanations with regard to the transition between the macroscopic and submicroscopic representational levels:
(1) Sense-making approach I (i.e. macroscopic–prompt–macroscopic); (2) sense-making approach II (i.e. macroscopic–prompt–submicroscopic); (3) sense-making approach III (i.e. macroscopic–submicroscopic, without a prompt).
We observed that students who fully described their synthesis steps in the scaffold exhibited an improvement in their sense-making approach between the pre- and post-scaffold interviews: they transitioned more often, without prompts, from the macroscopic to the submicroscopic representational level (sense-making approach III). Although these observations are promising, it is apparent from the scaffold worksheets and interview transcripts that some students struggled to describe and visualize their observations at the particle level, even when explicitly prompted to think about the properties and activities of the entities while working with the scaffold. In this study, students often referred to the final product in their scaffold worksheet but did not explain how to get there (e.g. when they described a property, they did not mention how it is formed).
If a mental model of how particles interact is missing in students’ reasoning processes, it is difficult for them to make a connection between the macroscopic and submicroscopic levels. Such mental models do not develop naturally in students (Deratzou, 2006). Thus, it is important to provide students with the opportunity to constantly reflect on and connect these representational levels. The scaffold sought to elicit this ability in students and it seems that reflective work with the scaffold had a positive effect on students’ ability to transition between the macroscopic and submicroscopic representational levels. This aspect of encouraging students to first think about how they can depict entities and chemical phenomena at the individual representational levels and how to combine them afterwards in a meaningful way has as well been illustrated by the study from Thomas (2017). The worksheet used in this study is to some extend similar to our scaffold and directed students’ metacognition in an explicit way, i.e. to attend to the separate nature of each of the representational levels and the relationships between them.
Conscious reflection on the macroscopic and submicroscopic representational levels does not happen automatically in the laboratory. This requires active engagement by students in the process such as scaffolding. For example, students who fully described their syntheses in the scaffold showed the most positive changes in their approach.
The structure of the scaffold worksheet could be applied to all occurring chemical processes where it is important that students’ observations are connected at the particle level and that students are able to transition between representational levels. For example, inorganic chemistry students could use the scaffold while analysing an unknown compound (analytical chemistry); or biology students could use the scaffold while testing various fertilizers in relation to plant growth and visual changes. This type of scaffolding can be smoothly integrated into existing teaching practices to expand upon what students already do and to help them connect their observations with the particle level.
Limitations
The conclusions drawn here should be considered with caution given some of the inherent limitations of this study. First, this was a qualitative study with volunteering students from a class of student teachers in an organic chemistry laboratory course. This student population may be driven by different motivations and interests with regard to chemistry than students majoring in chemistry. The implication drawn from this qualitative analysis are thus limited to this group. Second, primarily qualitative methods were used to describe the quality of students’ explanations in the pre- and post-scaffold interviews. The quantitative analysis should be interpreted with caution, as the sample is too small for a conclusive statistical statement. Due to the small number of students in the cohort, the confidence interval is large. Nevertheless, the large effect size for the increase of sense-making approach III indicates that the work with the scaffold seems to influence students’ reasoning. In order to generalize the results and to investigate whether this effect is sustainable or holds true for larger classes, further studies are necessary.Third, students were asked to explain their synthesis in an individual interview setting that was quite different from the environment in the laboratory. Students appeared to feel comfortable with the research team and the interview setting, which may have prompted them to pay closer attention to the work with the scaffold than they would have paid in a laboratory situation.
Conflicts of interest
There is no conflict of interest to declare.Appendix 1. Statistical analysis of the comparison of the pre- and post-interview data
The increase of the activated mechanistic features on the respective representational level was explored for statistical significance using a paired-sample t-test. The results are presented in Table 3. Students activated significantly more macroscopic entities and submicroscopic activities. The increase of the submicroscopic activity is coupled with a large effect size (Cohen's d = 0.81), whereas the macroscopic entity (Cohen's d = 0.37) and the submicroscopic organization (Cohen's d = 0.41) show a small effect. Due to the limited number of students in this study, it should be mentioned, that the confidence interval is very large and statistical statements are only possible to a limited extent.Furthermore, the sense-making approaches were explored for statistical significance using a paired-sample t-test. Table 4 shows the comparison of the sense-making approaches from the pre- to the post-interview. The data show that students used sense-making approach III significantly (p = 0.008) more often than sense-making approaches I or II. This increase is coupled with a large effect size (Cohen's d = 0.88). Changes in sense-making approach I and II are not statistically significant and show no effect size.
Appendix 2. Students’ evaluation of the scaffold
Fig. 16 shows the percentages achieved by students in the scaffold and the points achieved in the separate categories—preparatory steps, main reaction step, and work-up procedures. As not every synthesis has a preparatory step, some fields are left blank. The last column indicates the overall percentage of available points attained.Acknowledgements
This publication represents part of the first author's doctoral (Dr rer. nat.) thesis at the Faculty of Biology and Chemistry, Justus-Liebig-University Giessen, Germany. We thank all students who participated in the study. We also thank the Graulich research group for fruitful discussions about the research and Professor Richard Göttlich for his support.References
- Anderson G. W., (1978), The Playfair Collection and the teaching of chemistry at the University of Edinburgh, BRILL, pp. 1713–1858.
- Assadieskandar A., Rezende Miranda R. and Broyer R. M., (2020), Visually Tracking Acid–Base Extractions Using Colorful Compounds, J. Chem. Educ., 97, 1402–1405.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing Students’ Mechanistic Reasoning about London Dispersion Forces, J. Chem. Educ., 93, 1713–1724.
- Belland B. R., Glazewski K. D. and Richardson J. C., (2011), Problem-based learning and argumentation: testing a scaffolding framework to support middle school students’ creation of evidence-based arguments, Instru. Sci., 39, 667–694.
- Ben-Zvi R., Eylon B. and Silberstein J., (1986), Is an Atom of Copper Malleable? J. Chem. Educ., 63, 64.
- Braaten M. and Windschitl M., (2011), Working toward a stronger conceptualization of scientific explanation for science education, Sci. Educ., 95, 639–669.
- Bretz S. L., (2019), Evidence for the Importance of Laboratory Courses, J. Chem. Educ., 96, 193–195.
- Burke K., Greenbowe T. J. and Hand B. M., (2006), Implementing the science writing heuristic in the chemistry laboratory, J. Chem. Educ., 83, 1032.
- Caspari I., Weinrich M. L., Graulich N. and Sevian H., (2017), This mechanistic step is ‘‘productive’’: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19, 42–59.
- Caspari I., Graulich N. and Kranz D., (2018), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19, 1117–1141.
- Cheng M. M. and Gilbert J. K., (2017), Modelling students’ visualisation of chemical reaction, Int. J. Sci. Educ., 39, 1173–1193.
- Collison C. G., Cody J. and Stanford C., (2012), An SN1–SN2 Lesson in an Organic Chemistry Lab Using a Studio-Based Approach, J. Chem. Educ., 89, 750–754.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2019), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96, 213–226.
- Crandell O. M., Lockhart M. A. and Cooper M. M., (2020), Arrows on the Page Are Not a Good Gauge: Evidence for the Importance of Causal Mechanistic Explanations about Nucleophilic Substitution in Organic Chemistry, J. Chem. Educ., 97, 313–327.
- Czysz K., Schroeder L. and Clark G. A., (2020), Making Acids and Bases MORE Basic: Supporting Students’ Conceptualization of Acid−Base Chemistry through a Laboratory Exercise That Connects Molecular-Level Representations to Symbolic Representations and Experimentally Derived Evidence, J. Chem. Educ., 97, 484–489.
- Davis E. A., (2000), Scaffolding students' knowledge integration: prompts for reflection in KIE, Int. J. Sci. Educ., 22, 819–837.
- Deratzou S., (2006), A qualitative inquiry into the effects of visualization on high school chemistry students' learning process of molecular structure, Doctoral dissertation, Drexel University.
- Domin D. S., (1999), A review of laboratory instruction styles, J. Chem. Educ., 76, 543–547.
- Farrell J. J., Moog R. S. and Spencer J. N., (1999), A guided-inquiry general chemistry course, J. Chem. Educ., 76, 570.
- Faulconer E. K., Griffith J. C., Wood B. L., Acharyya S. and Roberts D. L., (2018), A comparison of online and traditional chemistry lecture and lab, Chem. Educ. Res. Pract., 19, 392–397.
- Ferrell J. B., Campbell J. P. and McCarthy D. R., (2019), Chemical Exploration with Virtual Reality in Organic Teaching Laboratories, J. Chem. Educ., 96, 1961–1966.
- Fitzgerald M. S. and Palincsar A. S., (2019), Teaching practices that support student sensemaking across grades and disciplines: a conceptual review, Rev. Res. Educ., 43, 227–248.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gabel D. L. and Bunce D. M., (1984), Research on problem solving: chemistry. Handbook of research on science teaching and learning, New York: Macmillan, vol. 11, pp. 301–326.
- Gabel D., Briner D. and Haines D., (1992), Modelling with magnets: a unified approach to chemistry problem solving, Sci. Teach., 59, 58–63.
- Gallardo-Williams M., Morsch L. A., Paye C. and Seery M. K., (2020), Student-generated video in chemistry education, Chem. Educ. Res. Pract., 21, 488–495.
- Galloway K. R. and Bretz S. L., (2015), Development of an Assessment Tool To Measure Students’ Meaningful Learning in the Undergraduate Chemistry Laboratory, J. Chem. Educ., 92, 1149–1158.
- Ge X. and Land S. M., (2003), Scaffolding students’ problem-solving processes in an ill-structured task using question prompts and peer interactions, Educ. Technol. Res. Dev., 51, 21–38.
- Gilbert J. K. and Treagust D. F., (2009a), Multiple representations in chemical education, Dordrecht: Springer, pp. 1–8.
- Gilbert J. K. and Treagust D. F., (2009b), Multiple representations in chemical education, ed. Gilbert J. K. and Treagust D. F., Dordrecht: Springer, vol. 4, pp. 333–350.
- Gkitzia V., Salta K. and Tzougraki C., (2019), Students’ Competence in Translating Between Different Types of Chemical Representations, Chem. Educ. Res. Pract., 21, 307–330.
- Greenbowe T. J., Poock J. R., Burke K. and Hand B. M., (2007), Using the science writing heuristic in the general chemistry laboratory to improve students' academic performance, J. Chem. Educ., 84, 1371.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Grotzer T. A., (2003), Learning to understand the forms of causality implicit in scientifically accepted explanations, Stud. Sci. Educ., 39, 74.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89, 850–853.
- Hand B., Wallace C. W. and Yang E. M., (2004), Using a Science Writing Heuristic to enhance learning outcomes from laboratory activities in seventh-grade science: quantitative and qualitative aspects, Int. J. Sci. Educ., 26, 131–149.
- Hempel C. G. and Oppenheim P., (1948), Studies in the logic of explanation, Philos. Sci., 15, 135–175.
- Hennah N. and Seery M. K., (2017), Using digital badges for developing high school chemistry laboratory skills, J. Chem. Educ., 94, 844–848.
- Hofstein A. and Lunetta V. N., (1982), The Role of the Laboratory in Science Teaching: Neglected Aspects of Research, Rev. Educ. Res., 52, 201–217.
- Johnstone A. H., (1982), Macro- and microchemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem., J. Comput. Assis. Learn., 7, 75–83.
- Johnstone A. H., (1993), The Development of Chemistry Teaching: a changing response to changing demand, J. Chem. Educ., 70, 701–705.
- Johnstone A. H., (2000), Teaching of chemistry- logical oder psychological? Chem. Educ. Res. Pract., 1, 9–15.
- Kang H., Thompson J. and Windschitl M., (2014), Creating opportunities for students to show what they know: the role of scaffolding in assessment tasks, Sci. Educ., 98, 674–704.
- Kararo A. T., Colvin R. A., Cooper M. M. and Underwood S. M., (2019), Predictions and constructing explanations: an investigation into introductory chemistry students’ understanding of structure–property relationships, Chem. Educ. Res. Pract., 20, 316–328.
- Keiner L. and Graulich N., (2020), Transitions between representational levels: characterization of organic chemistry students’ mechanistic features when reasoning about laboratory work-up procedures, Chem. Educ. Res. Pract., 21, 469–482.
- Keys C. W., Hand B., Prain V. and Collins S., (1999), Using the science writing heuristic as a tool for learning from laboratory investigations in secondary science, J. Res. Sci. Teach., 36, 1065–1084.
- Kirschner P. A., (2002), Cognitive load theory: implications of cognitive load theory on the design of learning, Learning and Instruction, 12, 1–10.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Krist C., Schwarz C. V. and Reiser B. J., (2018), Identifying Essential Epistemic Heuristics for Guiding Mechanistic Reasoning in Science Learning, J. Learn. Sci., 28, 160–205.
- Lawson A. E., (1989), A Theory of Instruction: Using the Learning Cycle To Teach Science Concepts and Thinking Skills. NARST Monograph, Number One, 1989.
- Lawson A. E., (2001), Using the learning cycle to teach biology concepts and reasoning patterns, J. Biol. Educ., 35, 165–169.
- Lipton M. A., (2020), Reorganization of the Organic Chemistry Curriculum to Improve Student Outcomes, J. Chem. Educ., 97, 960–964.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about mechanisms, Philos. Sci., 67, 1–25.
- Molvinger K., Ayral R.-M. and Filhol J.-S., (2020), Integrating Lecture and Laboratory Work for a Materials Chemistry Course to Engage and Motivate Students through Highly Visual and Intriguing Syntheses, J. Chem. Educ., 97, 866–872.
- Moreira P., Marzabal A. and Talanquer V., (2018), Using a mechanistic framework to characterise chemistry students’ reasoning in written explanations, Chem. Educ. Res. Pract., 20, 120–131.
- Moreira P., Marzabal A. and Talanquer V., (2019), Investigating the effect of teacher mediation on student expressed reasoning, Chem. Educ. Res. Pract., 20(3), 606–617.
- Pölloth B., Schwarzer S. and Zipse H., (2020), Student Individuality Impacts Use and Benefits of an Online Video Library for the Organic Chemistry Laboratory, J. Chem. Educ., 97, 328–337.
- Puntambekar S. and Hubscher R., (2005), Tools for scaffolding students in a complex learning environment: What have we gained and what have we missed? Educ. Psychol., 40, 1–12.
- Reid N. and Shah I., (2007), The role of laboratory work in university chemistry, Chem. Educ. Res. Pract., 8, 172–185.
- Reiser B. J., (2004), Scaffolding complex learning: the mechanisms of structuring and problematizing student work, J. Learn. Sci., 13, 273–304.
- Rottman B. M. and Keil F. C., (2011), What matters in scientific explanations: effects of elaboration and content, Int. J. Cog. Sci., 121, 324–337.
- Russ R. S., Coffey J. E., Hammer D. and Hutchison P., (2008a), Making Classroom Assessment More Accountable to Scientific Reasoning: A Case for Attending to Mechanistic Thinking, Sci. Educ., 93, 875–891.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008b), Recognizing Mechanistic Reasoning in Student Scientific Inquiry: A Framework for Discourse Analysis Developed From Philosophy of Science, Sci. Educ., 92, 499–525.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Salmon W. C., (1984), Scientific explanation and the causal structure of the world, Princeton: Princeton University Press.
- Scalco K. C., Talanquer V., Kiill K. B. and Cordeiro M. R., (2018), Making Sense of Phenomena from Sequential Images versus Illustrated Text, J. Chem. Educ., 95, 347–354.
- Schmidt-McCormack J. A., Muniz M. N., Keuter E. C., Shaw S. K. and Cole R. S., (2017), Design and implementation of instructional videos for upper-division undergraduate laboratory courses, Chem. Educ. Res. Pract., 18, 749–762.
- Schroeder J. D. and Greenbowe T. J., (2008), Implementing POGIL in the lecture and the Science Writing Heuristic in the laboratory—student perceptions and performance in undergraduate organic chemistry, Chem. Educ. Res. Pract., 9, 149–156.
- Seel N. M., (2011), Encyclopedia of the Sciences of Learning, Springer Science & Business Media.
- Seery M. K., Agustian H. Y., Doidge E. D., Kucharski M. M., O'Connor H. M. and Price A., (2017), Developing laboratory skills by incorporating peer-review and digital badges, Chem. Educ. Res. Pract., 18, 403–419.
- Seery M. K., Agustian H. Y. and Zhang X., (2018), A Framework for Learning in the Chemistry Laboratory, Isr. J. Chem., 59, 546–553.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Spencer J. N., (1999), New directions in teaching chemistry: a philosophical and pedagogical basis, J. Chem. Educ., 76, 566.
- Stegall S. L., Grushow A., Whitnell R. and Hunnicutt S. S., (2016), Evaluating the effectiveness of POGIL-PCL workshops, Chem. Educ. Res. Pract., 17, 407–416.
- Stephenson N. S. and Sadler-McKnight N. P., (2016), Developing critical thinking skills using the Science Writing Heuristic in the chemistry laboratory, Chem. Educ. Res. Pract., 17, 72–79.
- Sumfleth E. and Nakoinz S., (2019), Chemie verstehen–beobachtbare makroskopische Phänomene auf submikroskopischer Ebene modellbasiert interpretieren [Understand chemistry - interpret observable macroscopic phenomena at the submicroscopic level based on models], Z. Didakt. Naturwiss., 25, 231–243.
- Supasorn S., Suits J. P., Jones L. L. and Vibuljan S., (2008), Impact of a pre-laboratory organic-extraction simulation on comprehension and attitudes of undergraduate chemistry students, Chem. Educ. Res. Pract., 9, 169–181.
- Sweller J., Van Merrienboer J. J. and Paas F. G., (1998), Cognitive architecture and instructional design, Educ. Psychol. Rev., 10, 251–296.
- Taber K. S., (2002), Chemical misconceptions: prevention, diagnosis and cure, London: Royal Society of Chemistry.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2010), Exploring Dominant Types of Explanations Built by General Chemistry Students, Int. J. Sci. Educ., 32, 2393–2412.
- Talanquer V., (2018), Education Research and Practice in Asia-Pacific and Beyond, Singapore: Springer, pp. 39–52.
- Thomas G. P., (2017), ‘Triangulation:’an expression for stimulating metacognitive reflection regarding the use of ‘triplet’representations for chemistry learning, Chem. Educ. Res. Pract., 18, 533–548.
- Treagust D. F., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- Vishnumolakala V. R., Southam D. C., Treagust D. F., Mocerino M. and Qureshi S., (2017), Students’ attitudes, self-efficacy and experiences in a modified process-oriented guided inquiry learning undergraduate chemistry classroom, Chem. Educ. Res. Pract., 18, 340–352.
- Walker J. P., Van Duzor A. G. and Lower M. A., (2019), Facilitating Argumentation in the Laboratory: The Challenges of Claim Change and Justification by Theory, J. Chem. Educ., 96, 435–444.
- Wood D., Bruner J. S. and Ross G., (1976), The role of tutoring in problem solving, J. Child Psychol. Psychiatry, 17, 89–100.
- Wu N., Kubo T., Hall A. O., Zurcher D. M., Phadke S., Wallace R. L. and McNeil A. J., (2020), Adapting Meaningful Learning Strategies to Teach Liquid−Liquid Extractions, J. Chem. Educ., 97, 80–86.
- Yeo J. and Gilbert J. K., (2014), Constructing a scientific explanation—A narrative account, Int. J. Sci. Educ., 36, 1902–1935.
| This journal is © The Royal Society of Chemistry 2021 |