The persistence of primary school students’ initial ideas about acids and bases in the mental models of adults†
Alena
Bučková
 and
Miroslav
Prokša
and
Miroslav
Prokša

Comenius University in Bratislava, Faculty of Natural Sciences, Department of Didactics in Science, Psychology and Pedagogy, Gymnázium Jura Hronca, Bratislava, Slovakia. E-mail: buckova64@uniba.sk
First published on 14th October 2020
Abstract
The paper describes our research aimed to verify the durability of pupils' initial ideas and concepts on the issue of acids and bases. First, we mapped their existence among primary school pupils and then compared them with the ideas of secondary and university educated adults. It has been confirmed that some initial ideas are very lasting and are also reflected in the understanding of acids and bases in adults. By comparing these results we identified which initial ideas were overcome by education and which persisted.
Introduction
The topic of acids and bases represents that part of chemistry that immediately concerns everyday life. Acids and bases are found in foodstuffs and detergents people use; they are components of a human body. Terms such as pH and neutralisation have become household words. In addition, people encounter the terms related to acids and bases in advertisements, patient information leaflets, various product characteristics, and in connection to a healthy life style and environmental protection on a daily basis.Therefore, it can be presumed that a primary school student is familiar with the topic of acids and bases and knows something to a certain degree before the topic is covered at school. They have acquired some knowledge through direct experience, from adults, or from media. The acquired information may or may not reflect current scientific concepts, yet it is determining as the nature of these concepts establishes what those students learn and how they learn it (Strike and Posner, 1985). Recent studies show that children and teenagers have a lot of ideas concerning the nature and their surroundings; these initial ideas tend to hinder the understanding of modern scientific concepts (Barke et al., 2009).
The research of students’ ideas in chemistry is based on a constructivist learning approach that states that students form their own cognitive structure that is based on their attitudes, skills, experience, etc., acquired before, during, and after the learning process. Their constructs differ from those that their teachers have and present when teaching. This fact has to be factored in when designing one's approach to teaching chemistry. If students really learn by progressive building of knowledge, teachers should modify curricula in a way that would reflect students’ prior knowledge and their interests as much as possible. Curriculum fulfils its purpose only when it pertains to activities and duties students will probably have later, after graduating (Dewey, 1998).
By applying the study's findings on current mental models of primary school students teachers could help their students integrate new acquired knowledge into their already existing conceptual framework in an easier way (Lin and Chiu, 2010). There have been several research studies conducted covering students’ understanding of acids and bases (Cros et al., 1986; Cros et al., 1988; Hand, 1989; Ross and Munby, 1991; Schmidt, 1997; Bradley and Mosimege, 1998; Ayas and Demircioğlu, 2002; Chiu, 2002; Pinarbasi, 2007; Kirbulut et al., 2010; Artdej et al., 2010; Muchtar, 2012; Pan and Henriques, 2015; Hoe and Subramaniam, 2016; Cooper et al., 2016; Demir Kacan and Celikler, 2016). These studies focus mainly on the occurrence of misconceptions among secondary school and university students.
The study of understanding of acids and bases is often part of a broader research in chemistry or natural sciences (Nakleh, 1992; Kind, 2004; Cetin-Dindar and Geban, 2017), as well as the application of the concept of acids and bases, for instance when performing a titration (Sheppard, 2006; Widarti et al., 2017). Moreover, there have been a number of studies covering the understanding of acids and bases in organic chemistry (Petterson et al., 2020).
The role of science teachers is not only helping students acquire correct scientific theories, but even helping them adjust or overcome their initial, less correct theories (Shtulman and Valcarcel, 2012). How to achieve this type of educational process has been the aim of many research studies for decades (Nadelson et al., 2018). There have been different strategies proposed sharing a graduate transformation of a child's knowledge of everyday phenomena, enriching of said knowledge, and its restructuralisation (Pine et al., 2001) – student-centred learning, inquiry-based learning, High Relevance Approach, Predict–Discuss–Explain–Observe–Discuss–Explain instruction strategy, to name a few. The analysis of the impact of those various methods is the aim of both earlier and recent research studies (Hand and Treagust, 1988; Nakhleh and Krajcik, 1994; Sisovic and Bojovic, 2000; Cetin et al., 2005; Demircioglu et al., 2005; Cetingul and Geban, 2005; Demircioglu, 2009; Cetingul and Geban, 2011; Sesen and Tarhan, 2011; Amry et al., 2017; Amalia et al., 2018). One of the most recent studies investigates the application of a computer simulation when teaching the topic of pH (Watson et al., 2020).
Among the researchers who have focused on students at a primary school level it is Rob Toplis who studied the ideas of 17 eighth-year students (between ages 12 and 13) before, during, and after the teaching sequence. Based on the observations in class and interviews before and after practical work done in groups he confirms that the preconceptions of students, acquired in their everyday lives, are so deeply rooted that they persist even after covering the particular topic at school (Toplis, 1998).
Recent studies include B. K. Bayrak's research which used a two-tier test to identify primary students’ conceptual understanding and alternative conceptions concerning the topic of acids and bases. The study was performed as a survey model (Bayrak, 2013).
The findings evidence that students’ initial ideas are individual and are based on personal experience, skills, and interests. The student can also hold several concepts of the same phenomenon at the same time; concepts that could be indeed contradictory. Moreover, the initial ideas are very persistent and can ignore the obvious discord with the scientific theories (Driver et al., 1985). The understanding of a child's can accordingly include not only preconceptions – original, usually naïve ideas, concepts at diverse levels of the comprehension of the terms, misconceptions, i.e. wrong ideas, but also mental maps of an individual and their emotional functioning connected to a particular phenomenon (Doulík, 2004). The student's initial idea of the phenomenon is not isolated, for it is a part of their cognitive structure; hence the term instructuralisation – implementation – of the preconception (Škoda and Doulík, 2003).
The imperfect ideas of children's have three aspects: cognitive, affective, and conative (Čáp and Mareš, 2001). The cognitive aspect represents the knowledge of that specific area and is easy to ascertain even through testing. The affective aspect of a preconception starts to form immediately when the student encounters a particular phenomenon for the very first time. An emotional reaction that occurs can be positive, negative, or neutral, but has a strong impact on the student's eagerness to discover more about that specific phenomenon. The conative or behavioural aspect of a preconception is understood as a tendency to behave or do something in a certain way in relation to the phenomenon (Gavora, 1992).
This study explores the initial ideas that primary school students bring into the learning process in order to determine how to work with their ideas about acids and bases properly and facilitate their comprehensive and meaningful concept of understanding of the topic. To utilise findings on how the present learning process develops and changes students’ preconceptions of acids and bases, this study investigates to what degree the knowledge acquired at school has been implemented into the mental models of adults of different age groups and with different time spans since graduation. The data collection and their analysis provide groundwork for the didactic reconstruction of the curriculum of Chemistry for lower secondary school education in Slovakia.
Objectives of the study
The objectives of the exploratory part of the study were to identify the initial ideas of primary school students concerning the topic of acids and bases, compare those with the mental models of adults, and as a result, determine which ideas were persistent and which were overcome.Research methodology
A qualitative and quantitative non-experimental design was employed to investigate the initial ideas of primary school students and adults. The instrument for collecting data was a test with a set of 20 questions (3 multiple-choice questions, 17 open-ended questions). It had been composed using the authors’ pedagogical experience and reflecting the age category of students and their knowledge that should have been acquired in their previous school education. A specific and simple wording of the questions had been chosen in order to minimalise the chance of extrapolating answers based on the preceding questions as well as not to provide students with any hints for a particular question. Furthermore, some questions were seemingly repetitive, but by asking the same question differently it was feasible to ascertain the level of a respondent's understanding of the topic. All respondents were instructed to base their answers on their own concepts and understanding of the topic.Out of 20 questions, 7 were focused on acids, and 7 on bases. The majority of the questions investigated the cognitive aspect of preconceptions; two questions aimed directly at the affective aspect (see Table 1). There were multiple ways how to assess the answers given by the respondents. Some questions could be assessed as two-tier questions. Some of those questions even included a percentage scale for the respondents to grade their levels of certainty, and as such could be regarded as three-tier questions.
Participants of the study
The part of the research regarding the initial ideas of primary school students had two stages. The first stage, a pilot study, involved a sample of 49 students (Bučková and Prokša, 2017). The second stage, after editing a set of questions, involved a sample of 133 students, who had not participated in the first stage, from six eighth-year classes at three primary schools and one third-year class at an eighth-year secondary school (all between ages 12 and 13) in three different regions of Slovakia. Neither geographical, nor social aspect played any role in this research, though. The students were encouraged to provide any answer, even if not confident about its validity, and feedback if the questions were not clear to them. There was no time constraint; it usually took the students 20 minutes to complete the test.The part of the research focused on the mental models of adults involved a sample of 60 respondents. The respondents could be equally divided into three age groups: younger than 30 years, between 30 and 50 years, older than 50 years. All adult participants were secondary school or university educated, without any degree obtained in Chemistry. The identical test was given in person or sent via email with a further instruction that they were not allowed to consult their answers with anyone or use any literature.
Out of 193 participants’ responses, not one was invalidated and excluded from the final analysis. Given the size of the whole sample this research should be seen as the first probe producing vital information for future analyses of the initial ideas of primary school students regarding this topic in Slovakia.
Results and discussion
The test was prepared for a qualitative research of preconceptions of the students who have not encountered the topic of acids and bases at school yet. Their answers, as supported by the findings of the pilot study, varied to a great extent. Regarding the open-ended questions, the participants could respond in many ways with different parts of their answers having different level of correctness. As the intention was to do a qualitative research, the design of the Grounded Theory was applied (Corbin and Strauss, 2014). No correct answers had been previously established by the researchers in order to avoid any bias prior to the testing process. It was crucial to identify the answers that were significant through an analysis of the answers given by the respondents. Applying open coding, the answers were then added to the table in a shortened form without changing the original meaning. After that they were categorised based on their relevance; for example the answers that “an acid is a caustic”, “it is caustic”, “it burns”, “it has destructive effects” were put in the same category. Moreover, it was possible to categorise the answers further using other criteria; one option could be associated with their attributes (physical, chemical, health), another with the level of the understanding (macroscopic, submicroscopic, symbolic).This research paper operates with the categorisation of the answers based on their meaningfulness and relation to a scientific understanding of the particular topic This research paper operates with the categorisation of the answers based on their meaningfulness and relation to a scientific understanding of the particular topic. The current scientific understanding presented and taught at schools is the submicroscopic understanding of the Brønsted–Lowry theory of acids and bases that sees an acid as a particle that supplies a proton (H+ hydrogen ion) in a reaction, i.e. it is a proton donor, and a base as a particle that is able to accept a proton in a reaction, i.e. it is a proton acceptor (Barke and Harsch, 2014).
Originally, Osuská and Pupala's categorisation of answers had been intended to be used. They introduce five types: structuralised, dogmatic, naïve, scientifically acceptable, and other (Osuská and Pupala, 1996). After analysing the students’ answers, this categorisation was modified. A coding system, based on the process described in the previous paragraphs, was developed from the data in conjunction with several educators.
Because no fully scientific answer had been given by any student, this type of an answer was removed. The answers were then coded as following (Table 2).
| Types of answers | Characteristics | Detailed explanation | Points |
|---|---|---|---|
| Acceptable | Scientific, or does not contradict a scientific concept; not complete | A student forms an answer in which they process scientific information that can even be simplified or incomplete. In addition, these answers can be pure reproductions of a learned fact, without any deeper understanding. | 2 |
| Naïve | Makes sense, but is not in accord with scientific concepts | A naïve answer is an answer with a concept that is comprehensible to the student that has produced it but it is not compatible with a scientific interpretation. The student finds it sufficiently explanatory and obviously believes it. | 1 |
| Unacceptable | Does not make sense, or is off-topic | The answer involves pieces of information that are incorrect, incorrectly combined or irrelevant to the contextual framework of the question. | 0 |
| Blank | If a respondent answered “I don’t know”, or did not answer at all | 0 |
The coding process was conducted by two authors and a collaborating educator independently. Such a triangulation of researchers allowed a diversification of assessment.
Assessment of answers provided by students
A fully scientific answer was not provided by any student at all, but those answers that could be deemed acceptable could be divided into several groups. The students answered that they would recognise an acid based on the fact that it was a caustic, it burnt, it had destructive effects (31% of students), it conducted electricity when in a liquid state (2%), it tasted sour (4%), or that it had a low pH (1%). The answer that an acid could be identified by the label or the term acid on the container was acceptable, too. (29%). An example of a naïve answer was describing an acid as a substance that boils, bubbles (3%), or stinks (26%). The unacceptable answers included identifications of an acid based on its density (3%), colour (8%), and the fact that it resembled water (1%). Naturally, the categorisation of answers relied on the level of specificity. Two students wrote that they would try to do an experiment. Because they did not specify what kind of an experiment, their answers were not accepted. The answer of one student that wrote, “I would pour something on it and see what happens. If it burnt a hole, it would be an acid” was regarded acceptable, yet not complete. Regarding this particular question, there were eleven groups of acceptable answers, two groups of naïve answers, and two groups of unacceptable answers. Every student could characterise an acid in more than one way. Therefore, there were 191 diverse answers produced by 133 students. Applying the coding system developed in the study, 53% of answers were acceptable, 20% naïve, 14% unacceptable, and 13% blank.When assessing the question “What does it mean when a substance is a base?” using the same method, the most common acceptable answer was, “[i]t is a hydroxide” (5% of students). Other students stated that it was a substance used for “defusing” the acid (3% of students). Some of the naïve answers contained a notion that bases had a negative pH. As far as wrong answers are concerned, the most common ones were that a base was a substance that could be considered main, the most important, necessary, and elementary (17% of students). As much as 57% of students answered this question with “I don’t know” (see Fig. 1). A lot of them added that they did not know because they had not covered it at school yet. The same applied to acids; they did not state this fact, though.
The preconceptions of primary school students discovered in the second stage of the research corresponded with the preconceptions in the first stage (Bučková and Prokša, 2017). After the initial test was administered and analysed (in the first stage), it became obvious that students were not familiar with the term base, and therefore it was imperative to adjust the wording of some questions. This lead to using the term hydroxide instead, as there was a higher chance that students could know this term. Students were then expected to provide examples of strong and weak hydroxides instead of strong and weak bases. As a consequence, even students that were not familiar with the term base were able to give sodium hydroxide, potassium hydroxide, or calcium hydroxide as answers (9%). This conclusion was derived from comparing answers for two different questions.
By examining other question pairs it was possible to achieve a deeper understanding of the students’ knowledge. For example, 31% of the students gave an acceptable answer when identifying strong and weak acids, and 43% of the students were successful when characterising the difference between a strong and weak acid. When, however, these two questions were assessed together as one two-tier question, in both parts simultaneously only 14% of students were successful. When a specific question was analysed as a three-tier question, i.e. considering even the level of certainty graded by the students, the most common misconceptions were apparent. If a student gave an incorrect answer to both the first and second tier, but was sure about their answers, it could be concluded that they had a wrong idea about that particular topic (Cetin-Dindar and Geban, 2011). If the value of the students’ certainty higher than 80% was considered sufficient enough, then out of 19 students certain of their answers 90% answered incorrectly. The most common misconception held was that a strong acid is more concentrated and a weak acid is more diluted, and that a strong acid could not be digested in comparison with a weak one. Applying the coding system, those answers were classified as naïve because the scientific model understands the strength of an acid as the ability of acids to protolyse; a strong acid ionises in a solution completely while a weak acid ionises only partially. A weak acid's ions and molecules in the solution realise equilibrium which lies strongly on the side of the molecules (Barke and Harsch, 2014).
Misconceptions related to the strength of acids belong to the most common ones and have been identified in many research studies (Pan and Henriques, 2015). Therefore, this study intended to map how students understand the term the strength of an acid intuitively, too. Merging the questions no. 3 and no. 4 into a two-tier question was done to establish if contingent knowledge of an example of a strong and weak acid represents a memorised fact or is based on a meaningful concept.
Likewise, when merging question no. 7, which deals with “defusing” an acid, and question no. 14, which deals with mixing an acid with a base, it was established that only seven students (5%) provided acceptable answers to both questions. Separately, the success rate of question no. 7 was 42%, and the success rate of question no. 14 was 11%. The students indicated a very low percentage of certainty in this three-tier question, so in that case it was not possible to talk about misconceptions. It was imperative to see their answers only as guesses; it was a lack of knowledge (Cetin-Dindar and Geban, 2011). Thus even questions no. 14 and no. 15 were not merged into a three-tier question and analysed as one, despite the fact that it would have been logical. To comprehend the students’ ideas better, other question pairs were compared as well. As an illustration, when a student answered question no. 12 that they would find a basic substance “at home in a sink”, they could refer to soap, which would mean an acceptable answer, or they could refer to water, which would be classified as an unacceptable answer. Consequently, by merging questions no. 12 and no. 11, where students were to provide an example of a basic substance, it was possible to distinguish such cases.
Two-tier questions, or alternatively three-tier questions, were assessed only for the needs of the qualitative research; they were not taken into account in the quantitative research.
The conative aspect of the students’ preconceptions was not assessed, but it was partially evident when the students described what action they would take, e.g. “…I’d pour it into the sink and open the tap,” or, “…I’d induce vomiting or pump the stomach.” Frequently, they answered that they would call someone more experienced or an adult.
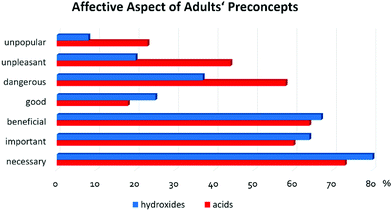
The affective aspect of the preconceptions was determined using a semantic differential scale. The results showed that the students considered acids dangerous (74%), unpleasant (67%), necessary (59%). When asked if acids were good or bad, 47% of students stayed neutral, i.e. opted for the middle alternative on the scale. The same occurred when asked if the acids were popular or unpopular. When asked about hydroxides, the majority of the students chose the neutral responses for all the items. At the same time, the number of those that refused to provide any answers concerning hydroxides increased (see Fig. 2).
The aim of this research was to gain an overview of what preconceptions primary school students have before they encounter this topic. Deriving from their most frequent answers (see Table 5), or from the fact that some answers were not given at all, an estimative model of the understanding of acids and bases was formed (Table 3) while examining any links and overlaps between not only the respondents, but even the answers of an individual.
| Acids | • Acids are dangerous, they burn materials, they stink |
| • Strong acids are more aggressive, they burn materials faster | |
| • Strong acids can kill, weak acids are not harmful | |
| • Strong and weak acids differ in concentration | |
| • The best-known acids are sulphuric acid, hydrochloric acid, citric acid, vinegar | |
| • Water is used for defusing an acid | |
| Bases | • Students do not know the chemical meaning of the term base |
| • Students can not provide an example of a basic substance | |
| • Bases are not dangerous, they are defused with water | |
| • Students know sodium hydroxide | |
| Common | • A neutral solution has no effects, it does not react, and it is harmless |
| • Students cannot provide an example of a neutral solution | |
| • Mixing an acid with a base leads to an explosion | |
| • The term salt means only kitchen salt | |
| • Students are not familiar with the term pH | |
| • Students know the pictogram depicting a caustic | |
Assessment of answers provided by adults
The responses provided by adult participants were assessed using the coding system the same way as the students’ responses; hence the outputs of their answers’ assessments were comparable. As an illustration, the ratio of acceptable, naïve, and unacceptable answers of the adults when identifying an acid and a base was different from the ratio of answers provided by the students (see Fig. 3). The adults gave more acceptable answers to those questions when characterising bases; nevertheless, 26% of their answers characterised a base only as the opposite to an acid, 8% saw a base only as a substance used for neutralising an acid, and 18% would identify a base if it had a high pH. Only one respondent gave a real scientific answer when stating that a base in accord with the Brønsted–Lowry theory “[a]ccepts H+ cation and has a pOH of less than 7.”Some differences occurred even between the age groups. To illustrate, no one in the younger than 30 age group used bicarbonate of soda as an example of a base; the older age groups used this particular example in 25% of their answers. The youngest age group knew how to use a pH scale, knew what an indicator and its function were, and recognised a pictogram for caustic/corrosive substances. The oldest age group saw the pictogram as a warning that it was “required to wash one's hands.” Regarding the bases, the oldest respondents often used a rather archaic Slovak term lúh (transl. lye). Some described a basic chemical as a chemical that was “right”, healthy, and vital for a human organism. Such answers are clearly products of the media's influence that keep mentioning the acidity of people's bodies and the need of consumption of alkaline foods.
Assessing the three-tier questions showed that misconceptions were held by 22% of all adult participants. Similarly to the students’ misconceptions, the most common one was the misconception about the relation between strength and concentration of an acid.
Regarding the affective aspect of the adult respondents’ ideas, it was evident that they considered acids equally important, crucial, and useful as bases (see Fig. 4).
The concepts identified among adults who encounter the topic of acids and bases only on the everyday life level are shown in Table 4. Correspondingly with the model of the students’ understanding, this model of the adults’ understanding of acids and bases has been constructed using the most frequent answers (Table 5), or based on the fact that the majority of respondents did not provide any answers to that particular question.
| Acids | • Acids taste sour, they stink, and they have a certain pH value |
| • A strong acid is more irritant, more effective, more harmful, and it has a lower pH value | |
| • Strong and weak acids differ in their concentrations | |
| • Adults know several commonly-used acids, they can classify some as strong or weak | |
| • Adults would use a base or water when defusing an acid | |
| Bases | • A base is the opposite to an acid, it has a different pH value |
| • A base is sodium hydroxide, bicarbonate of soda, lime, blood, saliva, beer, milk | |
| • A base is defused with an acid or with a neutral solution | |
| • Adults would use an indicator when identifying if a substance is a base | |
| Common | • A neutral solution is neither acidic, nor basic, and its pH is 7 |
| • An example of a neutral solution is water | |
| • Adults do not differentiate that there are some detergents containing acids and some containing bases | |
| • A reaction between an acid and a base is called neutralisation, i.e. the production of a neutral solution | |
| • An indicator shows acidity or basicity | |
| • pH indicates acidity or basicity | |
| • the term salt means cooking salt or a substance that has a structure of salt, a crystalline structure | |
| • Adults know intuitively what a pictogram depicting a caustic represents | |
Comparison of answers provided by primary school students and adults
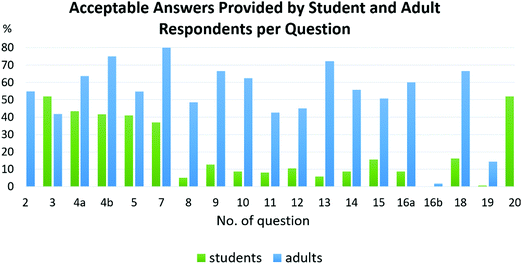
A very interesting finding is a comparison of the students and the adults’ ideas. Table 5 presents not only the most frequent answers, but also the percentages of those respondents that gave blank answers or stated “I do not know.” The rest of the answers, either acceptable, naïve, or unacceptable, are not shown in the table. Despite this fact it is possible to estimate what percentages of the respondents provided such answers.The biggest shift concerning overcoming erroneous ideas took place among the adults in the understanding of bases. The fact that they understand this term manifested itself when 48% of the adults answered question no. 7 on defusing an acid stating they would use a base to do so. Only 5% of the students answered the same way; the most common answer provided by the students was that they would use water (40%). Moreover, it was apparent even with the understanding of a mutual reaction between acids and bases as neutralisation when 50% of the respondents provided acceptable answers (Fig. 5). Nonetheless, the idea that a neutral solution was a product of such a mutual reaction persisted. Furthermore, the chemical term salt was also not comprehended.
The term pH was not commonly known to the students (21%), and in comparison to the adults’ responses it was connected only with indicating the level of acidity. The adults correctly defined this term in 48% of their answers, and could use pH or pOH scales when defining acids and bases (30%). The most frequent error was that they did not remember pH values, or that they switched the pH values of acids and bases.
The question where respondents had been asked about how they could tell if a substance was a base had the most distinctive difference in the ratio of the acceptable answers of the students and the adults. It was caused by the fact that the adults knew the term base and understood the function of an indicator as 34% of them wrote they would test basicity of a substance using an indicator. Only 2% of students answered this way. An interesting fact was that not one adult mentioned red cabbage as an indicator, yet the students did.
Another shift regarding the adult participants occurred in their understanding of acids as caustic substances; they were aware of the fact there were acids which were harmful and even acids that were not dangerous. The biggest issue, however, is perceiving bases still as totally harmless substances. Even among the adults’ responses there were speculations whether it was necessary to defuse a base at all.
There were two questions where the students scored more points than the adults (see Fig. 5). The first question dealt with a strong and a weak acid. The majority of the students answered that a strong acid is more caustic than a weak one – an answer that was acceptable taking into consideration their level of knowledge. The adults usually tried to answer this question with highlighting different pH levels or concentrations. The second question dealt with the pictogram depicting a caustic. The students did not have any problem answering, while the adults struggled.
Concentrating on preconceptions and their maintaining, or rather rediscovering by adults in their responses, it was observed that, for example, the idea that an acid stank was held by 26% of the students, but only 8% of the adults. But while in the younger than 30 age group 15% were of that opinion and in the 30 to 50 age group no one, in the older than 50 age group more than 35% of the adults were convinced it was so. Another preconception maintained was that all acids were caustic, and a similar trend was observed with the idea that a strong acid differed from a weak one in the fact that it was more concentrated (see Fig. 6).
 | ||
| Fig. 6 The comparison of the frequency of respondents who answered that the strong acid is different from the weak one by being concentrated. | ||
Comparing the answers indeed showed the adult participants had a higher percentage of acceptable answers (53%); but an adult participant provided 22 answers on average, whereas a student participant provided only 20.6 answers. The adults who could answer correctly created several different acceptable answers. The students compensated their lack of knowledge with creativity; thus, even though they generated fewer answers, there were a higher percentage of unacceptable answers.
The tests were assessed even with the point method. Every correct answer was assigned 2 points; a naïve answer was assigned 1 point. To reflect the fact that a respondent could provide more than one answer, we calculated the arithmetic mean of points given for each answer provided. That way every respondent could get 0 to 2 points per question regardless of how many answers provided. The students scored by this means an average of 12.14 points, while the adults scored an average of 22.5 points, confirming the original assumptions regarding the numbers of produced acceptable and unacceptable answers. The results were analysed even statistically (see Table 6).
| Students | Adults | |
|---|---|---|
| Count | 133 | 60 |
| Mean | 12.14 | 22.50 |
| Mode | 10 | 24 |
| Median | 11.50 | 23.75 |
| Standard deviation | 5.817 | 7.231 |
| Variance | 33.834 | 52.293 |
| Minimum | 0.00 | 4.5 |
| Maximum | 12.50 | 37 |
| Skewness | 0.57 | −0.423 |
| Kurtosis | 0.26 | −0.002 |
| Coefficient of variation | 0.48 | 0.321 |
Because the Shapiro–Wilk test denied the normal distribution at the level of 5% regarding the sample of students (W = 0.971; P-value = 0.006068), to confirm the H0 hypothesis that expected values of both samples were equal (specifically that the samples were drawn from the same distribution) the Wilcoxon (Mann–Whitney) test was applied. The H0 hypothesis was rejected (W = 6856; P = 1.454 × 10−15). As the data illustrate, the adult participants were more successful in answering provided questions. Accordingly, it is credible to state that the alternative hypothesis, the adults having statistically significantly better scores than the students, was valid.
It was also explored if the results of the oldest age group with the lowest average score (19.7 points) differ significantly from the results of the rest of adult groups. The Wilcoxon test proved the significance of this difference (W = 547; P = 0.01169). It also confirmed the oldest age group is still statistically more successful than the students’ group (W = 2011.5; P = 0.000226).
Limitations
There are a few limitations to this study inherent to the methodology used. The entire testing process was conducted with samples that were accessible to the researchers, so it is neither an intention, nor an option to generalise the findings to a larger population.Opinions on the proper size of the sample in a qualitative research presented in the scholarly literature are not united. Concerning the qualitative research, it is not the size of the sample itself that matters but rather the amount of information and profusion of detail obtained from each research subject (Agabrian, 2004). The sample should, however, be big enough to reach the saturation, i.e. the point when collecting new data does not yield new theoretical knowledge (Suter, 2011). A bigger sample of adult respondents could have provided a clearer picture of their concepts as it did with the student respondents. As far as the quantitative assessment goes, it would have been better if both samples had had a comparatively equal number of respondents.
Additionally, we did not investigate the background of adults, their education and professions, which, however, could provide a more comprehensive view of the evaluation of their answers.
As there were open-ended questions in the test, the processing of the respondents' answers was quite laborious and subjective. If circumstances permit, it is viable to use appropriate software when processing answers. However, a thorough analysis and assessment are still very time-consuming. To completely exclude bias in a qualitative research is neither conceivable, nor desired. According to W. N. Suter, the qualitative researcher often is the instrument, relying on his or her skills to receive information in natural contexts and uncover its meaning by descriptive, exploratory, or explanatory procedures.
Conclusion
These findings demonstrate that there is a common feature present in both groups of respondents – an excessive generalisation, i.e. respondents elevating the only piece of information held to a principle. In spite of the adults being considerably more successful than the students, the misconceptions present in their understanding of the topic are practically identical with the misconceptions of the students. The misconceptions held by the adults occurred just less frequently.To prevent such misconceptions from being constructed, students should be confronted with real compounds as often as possible. In addition, they should have an opportunity to compare various strong acids of the same concentration and to determine how the properties of a particular acid change in terms of its concentration. The same applies to bases and the mutual reactions between acids and bases. Notably, more attention should be paid to bases. Currently at primary schools hydroxides are only presented as the opposites to acids which is insufficient. Therefore, it is logical that to students the idea of a mutual reaction – neutralisation evokes the formation of neutral environment. It is also appropriate to introduce a simple particle understanding of acids and bases through images and computer simulations.
As this study illustrates, the deeply-rooted initial ideas persist in one's mind and after some time can overlay and thus replace the ideas acquired through the learning process unless those have been solidified properly. The role of school education is therefore to provide students with knowledge in the context of their everyday lives as much as possible.
A more detailed analysis of adults’ understanding of this area of chemistry could lead to a more extensive analysis of the acids and bases curriculum for the needs of a didactic reconstruction. In such case a multilevel test would be highly recommended as it could reveal the existing misconceptions in a better way, and, needless to say, the research would require a bigger sample of respondents.
Conflicts of interest
There are no conflicts to declare.References
- Agabrian M., (2004), Cercetarea calitativă a socialului: design si performare, [Qualitative research of the social: design and performance], Iasi: Institutul European.
- Amalia F. R., Ibnu S., Widarti H. R. and Wuni H., (2018), Students’ Mental Models of Acid and Base Concepts Taught Using the Cognitive Apprenticeship Learning Model, Jurnal Pendidikan IPA Indonesia, 7(2), 187–192 DOI:10.15294/jpii.v7i2.14264.
- Amry U. W., Rahayu S. and Yahmin, Y., (2017), Analisis Miskonsepsi Asam Basa pada Pembelajaran Konvensional dan Dual Situated Learning Model (Dslm) [Analysis of Acid-Base Misconceptions in Conventional Learning and Dual Situated Learning Model (Dslm)], Jurnal Pendidikan: Teori, Penelitian, dan Pengembangan, 2(3), 385–391 DOI:10.17977/jptpp.v2i3.8636.
- Artdej R., Ratanaroutai T., Coll R. K. and Thongpanchang T., (2010), Thai Grade 11 students’ alternative conceptions for acid–base chemistry, Res. Sci. Technol. Educ., 28(2), 167–183 DOI:10.1080/02635141003748382.
- Ayas A. and Demircioğlu G., (2002), Student teachers’ understanding and misconceptions of acids, bases and salts in chemistry, First International Education Conference, Changing times, Changing Needs, Eastern Mediterranean University.
- Barke H-D. and Harsch N., (2014), Broensted Acids and Bases: They are not Substances but Molecules or Ions!, Afr. J. Chem. Educ., 4(4), 82–94.
- Barke H.-D., Hazari A. and Yitbarek S., (2009), Students’ Misconceptions and How to Overcome Them in Misconceptions in Chemistry, Berlin, Heidelberg: Springer DOI:10.1007/978-3-540-70989-3_3.
- Bayrak B. K., (2013), Using Two-Tier Test to Identify Primary Students’ Conceptual Understanding and Alternative Conceptions in Acid Base, Mevlana Int. J. Educ., 3(2), 19–26 DOI:10.13054/mije.13.21.3.2.
- Bradley M. D. and Mosimege J. D., (1998), Misconceptions in acids and bases: a comparative study of student teachers with different chemistry bakgrounds, S. Afr. J. Chem., 51(3), 137–145.
- Bučková A. and Prokša M., (2017), Chápanie problematiky kyselín a zásad u žiakov 8. a 9. ročníka ZŠ [Understanding the Issue of Acids and Bases in the 8th and 9th grade Elementary School Students], Biológia, ekológia, chémia, 21(1), http://bech.truni.sk/prilohy/BECH_1_2017.pdf.
- Cetin A., Kaya E. and Geban O., (2005), Facilitating conceptual change in acid–base concepts, Paper presented at the British Educational Research Association Annual Conference, Education Line.
- Cetin-Dindar A. and Geban O., (2011), Development of a three-tier test to assess high school students’ understanding of acids and bases, Procedia Soc. Behav. Sci., 15, 600–604 DOI:10.1016/j.sbspro.2011.03.147.
- Cetin-Dindar A. and Geban O., (2017), Conceptual understanding of acids and bases concepts and motivation to learn chemistry, J. Educ. Res., 110(1), 85–97 DOI:10.1080/00220671.2015.1039422.
- Cetingul P. I. and Geban O., (2005), Understanding of Acid-Base Concept by Using Conceptual Change Approach, Hacettepe Univ. J. Educ., 29, 69–74.
- Cetingul P. I. and Geban O., (2011), Using conceptual change texts with analogies for misconceptions in acids and bases, Hacettepe Univ. J. Educ., 41, 112–123.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating students’ reasoning about acid–base reactions, J. Chem. Educ., 93(10), 1703–1712 DOI:10.1021/acs.jchemed.6b00417.
- Corbin J. and Strauss A., (2014), Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 4th edn, Sage Publications.
- Cros D., Maurin M., Amouroux R., Chastrette M., Leber J. and Fayol M., (1986), Conceptions of first-year university students of the constituents of matter and the notions of acids and bases. Eur. J. Sci. Educ., 8(3), 305–313.
- Cros D., Chastrette M. and Fayol M., (1988), Conceptions of second year university students of some fundamental notions in chemistry, Int. J. Sci. Educ., 10(3), 331–336 DOI:10.1080/0950069880100308.
- Čáp J. and Mareš J., (2001), Psychologie pro učitele [Psychology for teachers], Praha: Portál.
- Demir Kacan S. and Celikler D., (2016), Evaluation of the Uses of Acids and Bases in Daily Life, J. Stud. Educ., 6(1), 89–95 DOI:10.5296/jse.v6i1.8649.
- Demircioglu G., Ayas A. and Demircioglu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6(1), 36–51.
- Demircioglu G., (2009). Comparison of the effects of conceptual change texts implemented after and before instruction on secondary school students’ understanding of acid-base concepts, Asia-Pac. Forum Sci. Learn. Teach., 10(2), 1–29.
- Dewey J., (1998), The Essential Dewey: Pragmatism, education, democracy, Bloomington: Indiana University Press, vol. 1.
- Doulík P., (2004), Dětská pojetí vybraných fenoménů z oblasti přírodovědného vzdělávání na základní škole [Children's Concepts of Selected Phenomena in the Field of Science Education at Primary school], Doctoral dissertation, Trnava: Trnava University.
- Driver R., Guesne E. and Tiberghien A., (1985), Children's ideas in science: Children's ideas and the learning of science, Philadelphia: Open University Press.
- Gavora P., (1992), Žiak a text [The Pupil and the Text], Bratislava: SPN.
- Hand B. M. and Treagust D. F., (1988), Application of a conceptual conflict teaching strategy to enhance student learning of acids and bases, Res. Sci. Educ., 18(1), 53–63 DOI:10.1007/BF02356580.
- Hand B., (1989), Students’ understanding of acids and bases: A two year study, Res. Sci. Educ., 19(1), 133–144 DOI:10.1007/BF02356853.
- Hoe K. Y. and Subramaniam R., (2016), On the prevalence of alternative conceptions on acid-base chemistry among secondary students: Insights from cognitive and confidence measures, Chem. Educ. Res. Pract., 17(2), 263–282 10.1039/C5RP00146C.
- Chiu M. H., (2002), Exploring mental models and causes of high school students’ misconceptions in acids-bases, particle theory and chemical equilibrium, Project Report in National Science Council, Taipei: National Science Council.
- Kind V., (2004), Beyond Appearances: Students’ misconceptions about basic chemical ideas, 2nd edn, http://www.rsc.org/learn-chemistry/resource/res00002202/beyond-appearances.
- Kirbulut D., Geban O. and Beeth M. E., (2010), Development of a Three-Tier Mulple-Choice Diagnostic Instrument to Evaluate Students’ Understanding of States of Matter, Paper presented at the European Conference on Research in Chemical Education (ECRICE), Krakow, Poland.
- Lin J. and Chiu M., (2010), The Mismatch between Students’ Mental Models of Acids/Bases and their Sources and their Teacher's Anticipations thereof, Int. J. Sci. Educ., 32(12), 1617–1646 DOI:10.1080/09500690903173643.
- Muchtar Z., (2012), Analyzing of Students’ Misconceptions on Acid-Base Chemistry at Senior High Schools in Medan, J. Educ. Pract., 3(15), 65–74.
- Nadelson L., Heddy B., Jones S., Taasoobshirazi G. and Johnson M., (2018), Conceptual Change in Science Teaching and Learning: Introducing the Dynamic Model of Conceptual Change, Int. J. Educ. Psychol., 7(2), 151–195 DOI:10.17583/ijep.2018.3349.
- Nakhleh M. B., (1992), Why some students don't learn chemistry: Chemical misconceptions, J. Chem. Educ., 69(3), 191 DOI:10.1021/ed069p191.
- Nakhleh M. B. and Krajcik J. S. (1994), Influence of levels of information as presented by different technologies on students' understanding of acid, base, and ph concepts, J. Res. Sci. Teach., 31(10), 1077–1096.
- Osuská Ľ. and Pupala B., (1996), “To je ako zázrak prírody”: fotosyntéza v žiakovom poňatí [“It's Like a Miracle of Nature”: Photosynthesis in the Student's Concept], Pedagogika, 56(3), 214–223.
- Pan H. and Henriques L., (2015), Students' Alternate Conceptions on Acids and Bases, Sch. Sci. Math., 115(5), 237–243 DOI:10.1111/ssm.12124.
- Petterson M. N., Watts F. M., Snyder-White E. P., Archer S. R., Shultz G. V. and Finkenstaedt-Quinn S. A., (2020), Eliciting student thinking about acid–base reactions via app and paper–pencil based problem solving, Chem. Educ. Res. Pract., 21(3), 878–892 10.1039/C9RP00260J.
- Pinarbasi T., (2007), Turkish Undergraduate Students' Misconceptions on Acids and Bases, J. Balt. Sci. Educ., 6(1), 23–34.
- Pine K., Messer D. and St. John K., (2001), Children's Misconceptions in Primary Science: A Survey of Teachers' Views, Res. Sci. Technol. Educ., 19(1), 79–96 DOI:10.1080/02635140120046240.
- Ross B. and Munby H., (1991), Concept mapping and misconceptions: A study of high-school students' understandings of acids and bases, J. Sci. Educ., 13(1), 11–23 DOI:10.1080/0950069910130102.
- Sesen B. A. and Tarhan L., (2011), Active-learning versus teacher-centered instruction for learning acids and bases, Res. Sci. Technol. Educ., 29(2), 205–226 DOI:10.1080/02635143.2011.581630.
- Sheppard K., (2006), High school students’ understanding of titrations and related acid-base phenomena, Chem. Educ. Res. Pract., 7(1), 32–45 10.1039/B5RP90014J.
- Shtulman A. and Valcarcel J., (2012), Scientific knowledge suppresses but does not supplant earlier intuitions, Cognition, 124(2), 209–215 DOI:10.1016/j.cognition.2012.04.005.
- Schmidt H.-J., (1997), Students’ Misconceptions - Looking for a Pattern, Sci. Educ., 81(2), 123–135.
- Sisovic D. and Bojovic S., (2000), Approaching the concepts of acids and bases by cooperative learning, Chem. Educ. Res. Pract., 1(2), 263–275 10.1039/A9RP90027F.
- Strike K. and Posner G., (1985), Conceptual change and science teaching, Eur. J. Sci. Educ., 4(3), 231–240 DOI:10.1080/0140528820040302.
- Suter W. N., (2011), Introduction to Educational Research: A Critical Thinking Approach, 2nd edn, SAGE Publication.
- Škoda J. and Doulík P., (2003), Tvorba a ověření nástrojů kvantitativní diagnostiky prekonceptů a možnosti jejího vyhodnocení [The Formation and Verification of Tools in Quantitative Diagnostics of Pre-Concepts and the Possibilities of its Evaluation], Pedagogika, 53(2), 177–189, https://pages.pedf.cuni.cz/pedagogika/?p=1915andlang=cs.
- Toplis R., (1998), Ideas about acids and alkalis, Sch. Sci. Rev., 80(291), 67–70.
- Watson S. W., Dubrovskiy A. V. and Peters M. L., (2020), Increasing Chemistry Students’ Knowledge, Confidence, and Conceptual Understanding of pH Using a Collaborative Computer pH Simulation, Chem. Educ. Res. Pract., 21(2), 528–535 10.1039/c9rp00235a.
- Widarti H., Permanasari A. and Mulyani S., (2017), Undergraduate students’ misconception on acidbase and argentometric titrations: A challenge to implement multiple representation learning model with cognitive dissonance strategy, Int. J. Educ., 9(2), 105–112 DOI:10.17509/ije.v9i2.5464.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0rp00156b |
| This journal is © The Royal Society of Chemistry 2021 |