Data-driven activity reform: employing design research to improve scaffolding and concept development
Brianna L.
Minshall
and
Ellen J.
Yezierski
 *
*
Department of Chemistry & Biochemistry, Miami University, Oxford, Ohio 45056, USA. E-mail: yeziere@miamioh.edu
First published on 19th October 2020
Abstract
For six semesters, activities have been incorporated into first year general chemistry courses in an effort to build student conceptual chemistry knowledge. The activities follow a learning cycle pedagogy (similar to Process Oriented Guided Inquiry Learning or POGIL activities) and consist of guiding questions involving animations, models, simulations, or a data set and are completed by students working in groups. The efficacy of the learning cycle approach and learning outcomes from POGIL and other similar initiatives have been well studied; however, examining how scaffolding in chemistry learning cycles can improve learning outcomes has not been well studied. In Fall 2016, an activity was implemented in a first semester general chemistry course that focused on energy changes during bond breaking and bond making. The data showed that, even after working with the PhET Atomic Interactions simulation guided by the activity, about half of the students in the sample (N = 55) still thought bond-breaking was an exothermic process, even though they collected data from the simulation that indicated otherwise. After analyzing student answers, the activity was redesigned to increase scaffolding and improve concept development. Students’ performance improved greatly with the implementation of the second activity with 82% of students (N = 34) able to identify and distinguish between exothermic and endothermic processes. Results have implications for applying research-based techniques to activity development to improve students’ conceptual understanding in chemistry.
Introduction
In a previous survey of over 600 students, more than 85% of students selected an incorrect response when explaining the energetics of bond making and breaking associated with ATP hydrolysis (Galley, 2004). Across several studies, even after students had been taught that bond breaking was an endothermic process and bond making an exothermic process, about half of the students still believed it was vice versa (Cooper and Klymkowsky, 2013). Much literature describes students’ inaccurate ideas among general chemistry concepts; however, the energetics of bond breaking and making is a particularly important and foundational one as evidenced by the General Chemistry Anchoring Concepts Content Map from the ACS Exams Institute. The Anchoring Concept Map item titled, “Bonding: Atoms interact via electrostatic forces to form chemical bonds” (Holme et al., 2015), requires understanding what energy changes occur when a bond is formed and when a bond is broken. This is evidenced by the elaboration that follows, specifically “When chemical bonds form, the overall energy of the bonding atoms is lowered relative to free atoms, and therefore energy is released” and “To break a chemical bond requires an input of energy” (Holme et al., 2015). The aforementioned sub-statements demonstrate how essential electrostatic interactions are to the understanding of multiple general chemistry principles.Early work helps us to understand the nature of students’ difficulties and reveal that the challenge is more complex than students simply believing that bond breaking is an endothermic process. Boo (1998) concluded that students have no clear understanding of chemical bonds and the energetics involved in making and breaking these chemical bonds. Only 15% of students studied were able to correctly identify the five reactions presented as being exothermic. The majority of students had misconceptions about chemical bonds and thus were unable to describe overall energy changes that would occur during the reactions (Abell and Bretz, 2018). Some students believed bond making and bond breaking both require an input of energy while other students believed the input of energy is required to allow the bonds to break, but when the bond is actually broken, energy is released (Boo 1998). Recently, Abell and Bretz (2018) found 9 out of 26 students believed bond breaking could be an endothermic process or an exothermic process depending on which species were involved. Further, a different 9 out of 26 students believed bond breaking is endothermic and then exothermic. Only the remaining 8 out of 26 students correctly identified bond making as exothermic and bond breaking as endothermic (Abell and Bretz, 2018).
It is problematic to change a student's idea about a concept when they have been taught the opposite (or believe they were) in the past (Galley, 2004). When students were asked to identify where their ideas about chemical bonds and energy stemmed from, students commonly provided three sources: (1) how biologists talk about the conversion of ATP → ADP; (2) using macroscopic examples to describe a process at the molecular level; and (3) being told bond breaking was an exothermic process and bond making was an endothermic process in various levels of chemistry courses (Cooper and Klymkowsky, 2013). One can see how the first two sources may not specifically address what is going on at the molecular level and leave the student to fill in the gaps with inaccurate ideas that seemingly “work” for them. It is hard to imagine a teacher providing such inaccurate content as in source 3; however, if students believe it was, it could explain why the idea persists.
Visualization is an important anchoring concept within general chemistry (Holme et al., 2015). In chemistry courses, it is vital for students to conceptually understand energy at the atomic and molecular (or particulate) levels. In contrast, the concept of potential energy is often taught using macroscopic examples, usually with gravitational potential energy. The behaviors of objects at the macroscopic level versus at a particulate level are very different and can cause confusion among students (Tasker and Dalton, 2008). Students are unable to conceptually grasp what changes occur during chemical reactions because there is a knowledge gap being created by educators (Cooper and Klymkowsky, 2013) by likely not employing enough visualization opportunities to help students make macroscopic-particulate level connections regarding phenomena. Such connections are enabled by visualization and have been shown to help students close chemistry knowledge gaps (Tasker and Dalton, 2008).
In addition to visualization, other pedagogical approaches have been shown to narrow conceptual knowledge gaps. The POGIL (Process Oriented Guided Inquiry Learning) approach uses a learning cycle paradigm to help students build conceptual knowledge. The goal of the approach is to help students learn content through their own exploration and sense-making and improve various important learning skills (i.e., critical thinking, problem solving, etc.) (Moog and Spencer, 2008). This approach can be implemented in a variety of ways to align with the structure and environment of the classroom; however, there are a few key immutable features: (1) the lecture portion of class is removed and replaced with a POGIL session; (2) the activity is carefully designed to ensure it follows the learning cycle paradigm; and (3) the guiding questions should be ordered sequentially to help students approach the correct conclusion(s) and construct their knowledge along the way (Moog and Spencer, 2008). The session gives the instructor flexibility to walk around the classroom rather than standing at the front lecturing for the duration of the class. As the students are exploring the activity and generating knowledge of the concepts, the instructor has the ability to interact with the students and hear what they are discussing. As students are using higher order cognitive processes to construct knowledge, the feedback from the instructor can be beneficial to reinforcing this knowledge (Bailey et al., 2012). POGIL employs specific cooperative learning techniques including adherence to individual roles to encourage independence among group members. Becker et al. (2013) have shown how concept development can be traced through POGIL activities in a university-level physical chemistry class. Becker et al. used sociochemical norms as a lens to examine student discourse and reasoning. They assert that, in addition to building conceptual understanding, students “must be able to construct arguments using particulate-level ideas and representations.” Their findings amplify the roles that evidence and argumentation should play in classroom learning.
We sought, through a design experiment, to develop and test curricular materials that focused students on the use of particle-level evidence, dynamic visualization, and the learning cycle to stimulate concept development. For our purposes, students worked together in small groups, without roles, to complete the activity. The activity administered to students followed a learning cycle paradigm. During implementation of the activity, construction of knowledge is achieved through the students exploring the activity in their individual groups with minimal explanation from the instructor. The instructor walks around the classroom and listens for inaccurate ideas to address on a group by group basis and then address to the entire class (Yezierski et al., 2008; Bailey et al., 2012; Simonson, 2019).
For six semesters, activities have been incorporated into first year general chemistry courses to help students build conceptual knowledge about energy changes during bond making and breaking. The activities follow a learning cycle pedagogy, similar to POGIL activities. The activities in this study consisted of guiding questions involving animation, models, simulations, and a data set and are completed by students working in groups. Over several years, informal evaluations of student learning informed minor modifications to the activity. A more disciplined approach to evaluating the efficacy of the activity was warranted to ascertain the student learning outcomes and inform revisions to iteratively improve such learning outcomes.
The framework for improving the activity draws on the determination of how much and what type of scaffolding optimizes the learning outcomes without simply telling the students the answer. Scaffolding refers to the guidance provided to learners as they carry out an investigation and/or solve a problem. Scaffolding instruction draws on one of central points in Vygotsky's sociocultural theory – the zone of proximal development, or ZPD. Vygotsky characterizes the ZPD as the “distance” between what a learner can do by themselves and what they can do with assistance from a “competent assistant” (Vygotsky, 1978). Scaffolds can be designed to create smaller learning increments between the current knowledge state of the learner and the desired learning outcome. We assert that valuing students’ uncovering and use of evidence through a learning cycle approach can still benefit from scaffolding within the activity in the form of targeted questions and systematic prompting (Abels, 2014). The timing, wording, progression, and complexity of the questions warrants examination in light of the students’ achievement of the learning outcomes.
The efficacy of the learning cycle approach and learning outcomes from POGIL and other similar initiatives have been well studied; however, scholars have not yet examined the iterative process of curricular materials development of such activities to inform more and better activity generation by improving scaffolding.
The goal of this study is to explore how student concept development can be improved by better scaffolding student learning using iterations of a learning cycle activity to answer these research questions: (1) what conceptual understandings require more scaffolding to develop for an activity on the energy changes for bond making/breaking? (2) How can concept development be further scaffolded to increase the number of students holding accurate conceptual knowledge about bond breaking/making? (3) To what extent do answers to a revised activity demonstrate improvement in students’ conceptual understanding over the previous year's version?
Methods
Research design
This study investigated an approach aimed at remediating common student misconceptions centered on energy changes during bond making and breaking in a first semester general chemistry course by examining student answers to questions designed to help build accurate chemical knowledge. An activity was designed by one general chemistry professor and revised by another professor and the first author. The revision took place in Fall 2017 based on data obtained from an earlier implementation. The activity was designed to be used in conjunction with an interactive simulation and to be implemented during the thermochemistry unit. The goal of the activity was to help students develop a strong conceptual understanding of energy changes that occur during bond making and breaking, endothermic and exothermic processes, and the relationship between forces and potential energy in bond making and breaking.The implementation of the activity follows a design experiment (Cobb et al., 2003). The common features of a design experiment included herein are highly interventionist nature of the method and iterative design (Cobb et al., 2003). Each new version of the activity was tested in the course and data collected to evaluate its quality in building students’ conceptual understanding. Data drove subsequent versions of the instructions and questions in the activity. The structure of the activity allowed for students to work in groups on a guided inquiry activity with data being generated by a PhET simulation (“Atomic Interactions”, current Version 1.0.0, 2019) (Beale et al., 2019). Student answers to questions were collected in Fall 2016 to identify necessary revisions before implementation in Fall 2017. The intent was to identify patterns in student responses, whether correct or incorrect, and use the patterns to guide the crafting and/or revision of questions to more effectively support conceptual development.
Setting and sample
The activity was implemented in a first semester general chemistry course at a mid-size, public university. Each first semester general chemistry section consisted of approximately 150 students. The course is taken by mostly first- and second-year students with some third- and fourth-year students. The participants varied in race, ethnicity, and chemistry background. The activity was implemented during each of two in-class meetings known as “lecture” during the Fall 2016 and 2017 semesters. Part of the class period was dedicated to students working on the activity under study.Description of simulation and activity
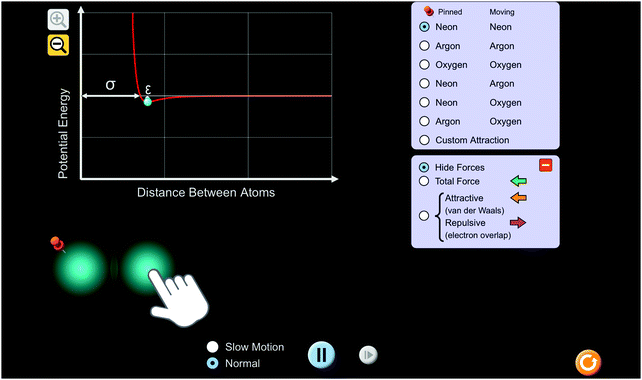
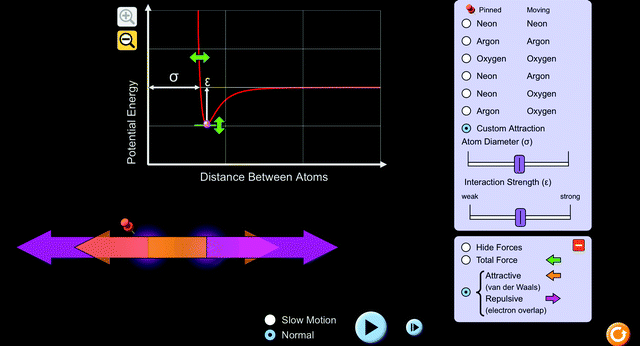
Students explored a PhET interactive simulation, “Atomic Interactions” (Beale et al., 2019) (Fig. 1). The activity that students used to guide their exploration was structured keeping common thermochemistry misconceptions in mind (Galley, 2004). To help students conceptually understand endothermic and exothermic processes, the activity provided directions for the students to use a potential energy versus distance between atoms graph and interactive atoms of various types within the simulation. As it is built into the simulation, students were able to move the atom on the right and observe resulting changes in potential energy on the graph. Using the “custom attraction” feature of the simulation, students were able to change conditions, namely, the diameter of the atom, attractive and repulsive forces, and the interaction strength (Fig. 2). Changing the conditions allowed students to vary factors and observe the way in which they affect potential energy between atoms. Students were able to view the magnitude and direction of the attractive and repulsive forces acting on each atom as a result of changing the conditions (Fig. 3).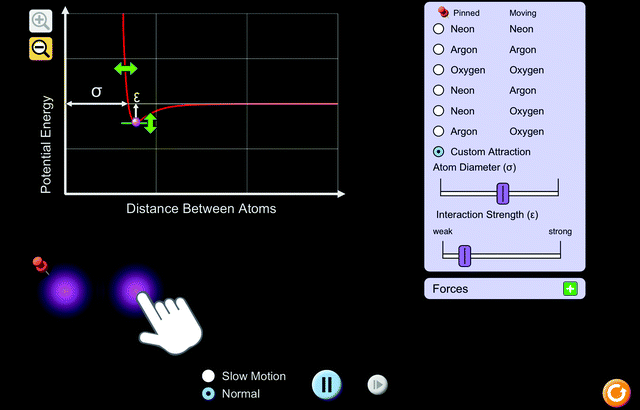 | ||
| Fig. 2 “Custom Attraction” screen of the simulation. Students were able to adjust the atom diameter and interaction strength used. | ||
In addition to directions that guided students to observe key phenomena, students were also presented with a number of questions to be answered during their exploration of the simulation. The activity was designed to build conceptual understanding of thermochemistry concepts as students experimented with the various features of the simulation. The complete and latest version of the activity may be found in the Appendix.
Data collection
In both semesters, students were informed of the purpose and background of the Institutional Review Board-approved study and received a consent form. Multiple activities like the one under study were implemented during the Fall 2016 and 2017 semesters making them part of the classroom routine. Signed consent forms were submitted upon completion and collection of the first activity. On the day the activity under study was implemented in Fall 2016, every student placed a sheet of carbon paper attached to a plain sheet of paper under their activity sheet. Students were instructed to take notes on the activity sheet and turn in the carbon copy at the end of lecture. In Fall 2017, students were instructed to complete the activity, take a picture, and email the picture to researchers using an email account set up for the sole purpose of collecting data during the implementation of the activities in class.Data cleaning
In Fall 2016, of the 112 student activities submitted, 2 were excluded for illegible handwriting. Next, student responses were excluded if the student did not answer all examined questions, leaving 55 students’ activities in the sample.In Fall 2017, all 34 students who submitted an activity response consented to participate in the study. All submitted student responses were legible and complete. No activities submitted were excluded from the study.
Data analysis
In Fall 2016, the questions eliciting the most common misconceptions were analyzed (Galley, 2004) using descriptive qualitative analysis (Gall et al., 2007) and by carrying out the following steps. Student responses were recorded in an Excel spreadsheet using conditional formatting to sort through answers efficiently. Student responses were first deductively coded as “C” for correct or “I” for incorrect. Second, within each of these two codes, similar answers were grouped together to describe the features of the answers. Aligned with the overarching goal of the study to examine how conceptual understanding may be developed, the relationship between potential energy and forces was also examined by coding student answers to the questions: (1) what happens to the magnitude of the forces when the models of the atoms get closer? Why? (2) Why are there no arrows when the model of the right atom is further over on the right side? (3) Are the attractive and repulsive forces on each atom equal in magnitude and in opposite direction? If not, describe the cases when they are not equal and explain why. (4) What do you see? How would you explain this? Student responses were then grouped by similarity. In Fall 2017, the analysis was carried out using similar procedures as Fall 2016. The student answers to the questions involving distinguishing between endothermic and exothermic processes, magnitude of attractive and repulsive forces, and potential energy concepts were analyzed to observe if a there was a difference between the learning outcomes of the activities, respectively. For the relationship between potential energy and forces, students were asked, “Slide the atom on the right so the potential energy shown on the graph is at its minimum. How does the magnitude of the attractive and repulsive force vectors compare to each other?”Trustworthiness
We sought to use findings from two implementations of the activity to ensure the credibility and dependability of study findings. As the study is a design-based research one and early results reshaped the second version of the activity, there were some inconsistencies across the activities. However, the primary learning outcomes are the same. Additionally, the confirmability of findings is supported by an iterative coding process as described in the previous section. Codes and descriptions were negotiated multiple times until 100% agreement between the coders was reached. The transferability of findings is bolstered by the detailed description of the activities in the methods and student answers in the results and discussion sections.Results and discussion
Fall 2016
Students’ responses to the questions were evaluated and coded as being correct or incorrect. After looking at the correct and incorrect answers, we found that, although several questions elicited inaccurate ideas from many students, students’ ideas across terms were mostly consistent. Table 1 shows two related questions and the most representative correct and incorrect answers.
| Activity item | Correct example (C) | Incorrect answer (I) |
|---|---|---|
| What type of energy change would occur when a chemical bond is formed? | “Exothermic” | “Endothermic” |
| Explain. | “Potential energy decreases; energy is released.” | “Absorbs energy” |
| What type of energy change would occur when a chemical bond is broken? | “Endothermic” | “Exothermic” |
| Explain. | “Potential energy increases.” | “Energy increases and is released.” |
The good correspondence between the terms exothermic and endothermic and their respective definitions was important because it showed that, for example, when students characterized bond formation as exothermic, they knew that this meant energy being released. If there was evidence suggesting that they did not know what was meant by exothermic, we would be unable to draw conclusions about their ideas related to energy change when bonds form. We found that 41 out of 55 students evidenced a consistent use of terms and definitions for endothermic and exothermic.
As shown in Table 1, students were asked to identify bond making and bond breaking as either being an endothermic process or an exothermic process. In addition, students were asked to provide reasoning for their choice between endothermic and exothermic. Of the 55 students, only 22 students correctly identified bond making as an exothermic process and bond breaking as an endothermic process. Of the 55, 19 students incorrectly identified both processes. The remaining 14 students had a combination of correct and incorrect responses. These students provided a correct characterization of the process or correct reasoning.
Since we are interested in understanding which students could describe the correct energy change during bond making and breaking, it is useful to focus on their results as they pertain to energy being released and absorbed. As such, we focused on their answers to the explain items. This means, at best, 27 students could properly describe and explain the energy changes during bond making and breaking.
To better understand how they were developing their ideas during the activity, the students were asked questions about the arrows (electrostatic force vectors) shown in the simulation when the atom pairs were at different distances from each other: (1) why are there no arrows when the model of the right atom is further over on the right side of the screen? (2) What happens to the magnitude of the forces when the models of the atoms get closer and why? (3) Are the attractive and repulsive forces on each atom equal in magnitude and in opposite direction? If not, describe the cases when they are not equal and explain why. Table 2 shows descriptions of the various groups of student answers with sample answers.
| Group | Description | Sample answer |
|---|---|---|
| 1 | Includes attraction and repulsion and mention of forces | “They are not always equal, once the atom reaches a certain proximity, the repulsive forces are greater than the attractive forces.” |
| 2 | Attraction and repulsion at PE minimum with no mention of forces | “No, they are not always equal. Closer together… bigger repulsions vs. far apart… bigger attractions.” |
| 3 | Forces are always equal | “They are always equal in magnitude.” |
| 4 | Relationship to equilibrium. | “The attractive and repulsive forces are not always equal because the forces of attraction and repulsion are equal and opposite.” |
| 5 | Not always equal in magnitude; pulse/oscillation movement | “They are not always equal in magnitude, one of the forces must be greater than another for them to pulse.” |
| 6 | Nucleus and subatomic particles | “No when the atoms are as close as possible, repulsiveness is greater in magnitude. The positive nuclei repel each other.” |
| 7 | No forces present | “When at the minimum, there is no force, below minimum there is attraction, and above there is repulsive.” |
| 8 | Did not specify difference in forces and their direction | “When they’re closer, the potential energy is greater than the kinetic energy. However, the potential and kinetic energy are always in proportion.” |
The results shown in Table 2 indicate there is a variable understanding of the relationship between electrostatic forces and potential energy. This was not surprising given the findings of Cooper and Klymkowsky (2013), who describe the sources for students’ consistent challenges with understanding the term, “chemical energy.” Additionally, not all students fit into one group or even a few. Students’ responses were categorized into several different groups suggesting that their ideas about the relationship between electrostatic forces and potential energy were not only inaccurate, but also inconsistent. Students still possess inaccurate ideas about forces at the conclusion of the simulation. Since students did not obtain a solid grasp of the relationship between electrostatic forces and potential energy during the simulation, this may explain why students were not able to correctly identify the energy changes that occur when bonds are made and broken.
After analyzing all responses and discovering which questions generated inaccurate or diverse responses, the data were used to determine how to improve question wording to better scaffold student learning. The same question types were used in the subsequent activity but were structured in a more guided way based on the literature (Moog and Spencer, 2008). The findings from Research question 1 demonstrated that students did not make connections among distance, electrostatic forces, and potential energy changes. As such, the activity design warranted more scaffolding with electrostatic forces (directing their attention to force vectors, net force vectors) and how they relate to potential energy (directing their attention to the values before and after changes in internuclear distance upon making and breaking bonds). In the revised activity, additional questions were designed to call students’ attention to more fine-grained changes within the simulation, as compared to the original activity which provided one open-ended question for multiple (but related) changes (What happens when…?). Since 60% of the sample was unable to characterize bond making and breaking as exothermic and endothermic, respectively, we identified ways to provide more guidance during the activity to stimulate more deliberate exploration of the relationship between electrostatic forces and potential energy.
Fall 2017
Before work with the simulation began, students were asked to indicate whether they believed bond making was an endothermic or exothermic process and whether bond breaking was an endothermic or exothermic process. Toward the end of the questions within the activity and simulation, students were asked again to indicate whether bond making was an endothermic or exothermic process and whether bond breaking was an endothermic or exothermic process based on data collected during the simulation. Fig. 4 shows how students’ thoughts changed or remained the same throughout the activity. No student who answered correctly before the activity answered incorrectly at the end. The 6 students that remained incorrect from pre- to post-activity were the same students.
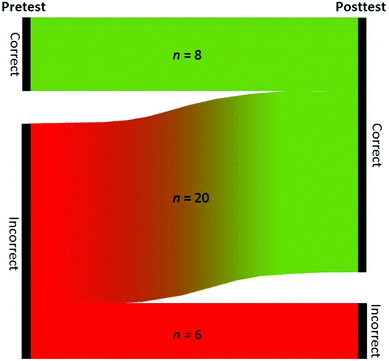 | ||
| Fig. 4 Pre-activity and post-activity data for students’ knowledge of bond making and bond breaking as either an endothermic or exothermic process. | ||
Before students completed the activity, only 8 of the 34 students correctly identified bond breaking as an endothermic process and bond making as an exothermic process. After completion of the activity, 28 of the 34 students correctly identified bond breaking as an endothermic process and bond making as an exothermic process. These results indicate that the redesigned activity was more effective in helping students make accurate conclusions and form correct conceptual ideas.
Compared to the previous implementation when only 40% of students were able to identify bond breaking as endothermic and bond making as exothermic, 82.4% of students in the later implementation with the revised activity correctly characterized the energy changes when bond making and breaking occurs.
From the results of the previous version of the activity, it was noted that students had a difficult time understanding the relationship between electrostatic forces and potential energy. This time, students were asked, “What is the magnitude of the attractive and repulsive forces when the potential energy is at a minimum?” Again, student responses were coded and grouped based on similarity. Descriptions of the student responses and sample answers are shown in Table 3.
| Group | What is the magnitude of the attractive and repulsive forces when the potential energy is at a minimum?” | Sample answer |
|---|---|---|
| 1 | Equal in magnitude, but opposite in direction. Equal or the same. | “Magnitudes are equal, but opposite in direction.” |
| 2 | Equal or the same. | “Total force shows no attraction or repulsion, so they are equal.” |
| 3 | Equal and opposite | “Equal, but opposite.” |
| 4 | Attractive force is greater than the repulsive force | “When they get closer, the attractive force is bigger than the repulsive force.” |
| 5 | Force equals zero | “The forces equal zero when the PE is at its lowest.” |
The results of the redesigned activity showed less variability in students’ responses as compared to 2016, as evidenced by fewer groups in Table 3 as compared to Table 2; however, many inaccurate ideas about electrostatic forces and potential energy were still apparent in the 2017 students’ answers. As such, even though the bond making/breaking ideas are much more frequently correctly evidenced in the 2017 activity, even more revisions are warranted. Such revisions are addressed in the Implications section.
After examining the results from the second implementation of the activity, students still struggled with the relationship between electrostatic forces and potential energy. Students believed that the forces cancel each other out, resulting in no forces present. Zohar and Levy (2017) also reported this finding related to a simulation similar to the PhET simulation. However, in our study, this inaccurate belief held by students did not seem to preclude them from building accurate conclusions and forming correct conceptual ideas about bond making and breaking.
Conclusions
Fall 2016
A mere 40% of students were correct in identifying bond making as an exothermic process and bond breaking as an endothermic process as well as providing correct reasoning for their answers. An additional 5.5% of students were correct in identifying bond making as an exothermic process and bond breaking as an endothermic process but did not provide correct explanations. Unfortunately, 34.5% of students were incorrect in identifying bond making as an exothermic process and bond breaking as an endothermic process as well as providing incorrect reasoning for their answers. Interestingly, 9.1% were incorrect in identifying bond making as an exothermic process and bond breaking as an endothermic process but provided explanations that coincided with the correct answers. The remaining 11.0% of students had inconsistent answers in terms of identifying bond making as an exothermic process and bond breaking as an endothermic process and/or identifying the reasoning for each process, respectively.
In response to the following items, students are not making critical connections between forces (i.e., attractive and repulsive) and potential energy as shown in Table 2: (1) why are there no arrows when the model of the right atom is further over on the right side of the screen? (2) What happens to the magnitude of the forces when the models of the atoms get closer and why? (3) Are the attractive and repulsive forces on each atom equal in magnitude and in opposite direction? If not, describe the cases when they are not equal and explain why. Students also provide inaccurate responses to questions requiring them to connect changes in potential energy to the release and absorption of heat. Lastly, students seem to be struggling to connect energy release and absorption to bond making and breaking. The lack of consistency in student answers here may be revealing a gap between forces of attraction and repulsion and how they relate to bond making as an exothermic process and bond breaking as an endothermic process.
Fall 2017
In Fall 2016, 40% of participants demonstrated scientifically accurate ideas about bond making and breaking. In 2016, inconsistencies in definitions of endothermic and exothermic were observed as well as inconsistencies in reasoning for why a process is endothermic or exothermic. In 2017, 23.5% of students initially demonstrated an understanding of bond making and breaking before the activity. Following completion of the activity, 82.4% of students demonstrated an understanding of bond making and breaking. The redesigned activity helped students to better identify whether bond making and breaking, respectively, was endothermic or exothermic compared to the activity implemented in Fall 2016. The extra scaffolding informed by the challenges noted in 2016 seemed to help students make sounder connections among forces, potential energy, and energy changes.
Limitations
Based on student answers, it is not obvious which side of the “well” they are looking at when commenting on the Morse potential diagram. As such, it was difficult to interpret some of their statements about potential energy and conclude if they were referring to bonded or unbonded atoms. Further, we offered students a 50/50 selection when asking whether a reaction was endothermic or exothermic and whether that means energy is released or absorbed. It would have been better to offer an open-ended question to get a better reflection of their understanding, or lack thereof. There were several incomplete answers in the data set, not all students completed the activity, and not every student who completed the activity sent their activity to researchers. The data collection method used in each semester, respectively, had their own advantages and disadvantages. In Fall 2016, the carbon paper made it difficult to read some of the responses as the students did not write hard enough for their answer to transfer and it would smear very easily; however, the response rate was much higher than the following year. In Fall 2017, having students email a picture of their activity made it easier to read their responses, but many students forgot to email their completed activity at the end of lecture. The pictures were very clear; however, the response rate was lower than the previous year and did not yield a large enough sample size for statistical tests. We cannot do a direct comparison of the activities implemented in 2016 and 2017 because the same questions were not asked; however, the nature of each question set within the activity was the same.Future directions
If more class time for picture data collection was allocated, we could improve the response rate. In addition, the activity could be modified to help students make a stronger connection between electrostatic forces and potential energy. Future work could explore how the meaning of balanced forces at the atomic level influences students’ understanding of energy changes during bond making and breaking. Further, it would be beneficial to analyze student progress throughout the general chemistry course sequence. Activities could be implemented in the second semester course to identify knowledge gaps students are bringing with them from the previous semester. In addition, these activities should aim to let the student communicate what they are thinking and how they are approaching the activity. Acknowledging gaps at the beginning of the semester may be beneficial to struggling students. Current work is underway to implement the activity herein in addition to other simulation-based learning cycle activities in a larger scale classroom with the aim of broadly disseminating tested activities to the chemistry education community.Conflicts of interest
There are no conflicts to declare.Appendix
Name (please print) _________________________________
1. When a chemical bond is broken, energy is absorbed/released. (circle one)
2. When a chemical bond is formed, energy is absorbed/released. (circle one)
Go to http://phet.colorado.edu/en/simulation/atomic-interactions
3. Select “Custom Attraction.” Drag the right atom from side to side. Observe the changes in the graph.
a. Drag the “atom diameter” slider to the far right. Drag the “interaction strength” slider to the middle. Move the right atom at the bottom of the screen from side to side. Watch what happens to the point on the graph as the atoms move closer together. Record your observations.
___________________________________________________________________________
___________________________________________________________________________
b. With the same atom diameter and interaction strength, watch what happens as the atoms move farther apart. Record your observations.
______________________________________________________________________________________________________________________________________________________
4. Click the green “ +” next to “Forces.” Select “Attractive/Repulsive Forces.”
a. The color of the attractive force vector (arrow) is ______________.
b. The color of the repulsive force vector (arrow) is ______________.
5. Click the pause button at the bottom middle of the screen.
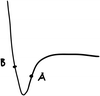
a. Drag the point on the graph to Point A as shown here. The orange/pink (circle one) arrows are larger in magnitude. At this point, the attractive/repulsive (circle one) forces between the atoms are greater. (Hint: Click “total force” to check your answer).
b. Drag the point on the graph to Point B. The orange/pink (circle one) arrows are larger in magnitude. At this point, the attractive/repulsive (circle one) forces between the atoms are greater. (Hint: Click “total force” to check your answer).
6. Slide the atom on the right so the potential energy shown on the graph is at its minimum.
a. How does the magnitude of the attractive and repulsive force vectors compare to each other?
________________________________________________________________________
b. Select “total force.” What do you see? Does this support your answer to 6a?
________________________________________________________________________
7. Click the orange circle to reset the simulation. Select the argon/argon interaction in the PhET simulation. A chemical bond is an attraction between atoms that occurs when the potential energy of the system is at the minimum.
a. Start with the atoms far apart (unbonded) and move the right atom closer until you reach minimum potential energy (bonded).
b. In the process of forming this bond, did the potential energy of the system increase or decrease? ___________________
c. Was energy released or absorbed when the bond formed? ___________________
d. Considering this change in energy, is the formation of a chemical bond an exothermic or endothermic process? _________________
8. Reminder: A chemical bond is an attraction between atoms that occurs when the potential energy of the system is at the minimum.
a. Start with the atoms at the potential energy minimum (bonded) and move the right atom farther away from the left atom (unbonded).
b. In the process of breaking this bond, did the potential energy of the system increase or decrease? ________________
c. Was energy released or absorbed when the bond was broken? _________________
d. Considering this change in energy, is the breaking of a chemical bond an exothermic or endothermic process? ___________________
9. Compare your answers to 1 and 8c.
My answers are the same/different. (Circle one)
10. Compare your answers to 2 and 7c.
My answers are the same/different. (Circle one)
11. Select “Custom Attraction.” Experiment with interaction strength, which varies the potential energy change when a bond is formed or broken. How does the energy change that occurs when a bond is broken relate to the strength of the bond? ______________________________________________________________________________________________________________________________________________________
Authored by Brianna Minshall and Ellen Yezierski, Miami University 2017
Acknowledgements
We thank Dr Stacey Lowery Bretz for allowing us to conduct research in her student-centered classroom and her students enrolled in first semester general chemistry over the two years for their participation. We also thank the Yezierski research group for their feedback on the revised activity and proposed data collection and analysis methods.References
- Abell T. N. and Bretz S. L., (2018), Dissolving salts in water: students’ particulate explanations of temperature changes, J. Chem. Educ., 95, 504–511.
- Abels S., (2014), Inquiry-based science education and special needs—teachers’ reflection on an inclusive setting, Science Education in the 21st Century: Challenges and Concerns, vol. 2, issue 2, pp. 124–154.
- Bailey C. P., Minderhout V. and Loertscher J., (2012), Learning transferable skills in large lecture halls: implementing a POGIL approach in biochemistry, Biochem. Mol. Biol. Educ., 40(1), 1–7.
- Beale P., Carpenter Y.-y., McKagan S., Moore E., Podolefsky N. and Rouinfair A., (2019), Atomic Interactions, available at: https://phet.colorado.edu/sims/html/atomic-interactions/latest/atomic-interactions_en.html.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using particulate nature of matter: an example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 1(14), 81–94.
- Boo H. K., (1998), Students’ understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Cobb P., Confrey J., diSessa A., Lehrer R. and Schauble L., (2003), Design experiments in educational research, Educ. Res., 32(1), 9–13.
- Cooper M. M. and Klymkowsky M. W., (2013), The trouble with chemical energy: why understanding bond energies requires an interdisciplinary systems approach, CBE: Life Sci. Educ., 12, 306–312.
- Gall M. D., Gall J. P. and Borg W. R., (2007), Education research: An introduction, 8th edn, Boston: Pearson.
- Galley W. C., (2004). Exothermic bond breaking: a persistent misconception, J. Chem. Educ., 81(4), 523–525.
- Holme T., Luxford C. and Murphy K., (2015), Updating the general chemistry anchoring concepts content map, J. Chem. Educ., 92(6), 1115–1116.
- Moog R. S. and Spencer J. N., (2008), POGIL: An Overview, ACS Symposium Series Process Oriented Guided Inquiry Learning (POGIL), pp. 1–13.
- Simonson S. R., (2019), POGIL: An introduction to process oriented guided inquiry learning for those who wish to empower learners, Sterling, VA: Stylus Publishing.
- Tasker R. and Dalton R., (2008), Visualizing the molecular world–Design, evaluation, and use of animations, Dordrecht: Springer, pp. 103–131.
- Vygotsky L. S., (1978), Mind in society: The development of higher psychological processes, Cambridge, MA: Harvard University Press.
- Yezierski E. J., Bauer C. F., Hunnicutt S. S., Hanson D. M., Amaral K. E. and Schneider J. P., (2008), POGIL implementation in large classes: strategies for planning, teaching, and management, Process Oriented Guided Inquiry Learning (POGIL), pp. 60–71.
- Zohar A. and Levy S. T., (2017), Attraction vs. Repulsion–learning about chemical bonding with the ELI-chem simulation’, Chais conference on instructional technologies research, Israel: Open University, pp. 76–83.
| This journal is © The Royal Society of Chemistry 2021 |