Open Access Article
Open Access ArticleSynthesis and evaluation of ion-imprinted composite membranes of Cr(VI) based on β-diketone functional monomers†
Peng Li,
Xin Wang,
Guifang Wang,
Li Zhao,
Yuwen Hong,
Xianzhi Hu,
Futing Zi and
Huiling Cheng *
*
Faculty of Science, Kunming University of Science and Technology, Kunming, China. E-mail: ynchenghl@163.com
First published on 6th December 2021
Abstract
Using Cr(VI) as the imprinted ions and 2-allyl-1,3-diphenyl-1,3-propanedione (ADPD) (a compound synthesized by independent design) as the functional monomer, a series of chromium ion-imprinted composite membranes (Cr(VI)-IICMs) and corresponding non-imprinted composite membranes (NICMs) were synthesized and tested. The results showed that the Cr(VI)-IICM10 membrane prepared under optimal experimental conditions exhibited a high adsorption capacity towards Cr(VI) (Q = 30.35 mg g−1) and a high imprinting factor (α = 2.70). The structural characteristics of Cr(VI)-IICM10 and NICM10 were investigated using FE-SEM, ATR-FTIR, and BET techniques combined with UV-Vis photometry and inductively coupled plasma emission spectrometry (ICP-OES) to evaluate the adsorption performance and permeation selectivity, while the effect on adsorption permeance of varying the experimental conditions including the solvent type, pH, and temperature was also investigated. The results showed that Cr(VI)-IICM10 is a mesoporous material with excellent permeation selectivity, reusability, and favorable pH response, and that its adsorption behavior is in accordance with the Langmuir model and pseudo-first-order kinetics. Thus, Cr(VI)-IICM10 shows great potential towards utilization as a “smart membrane” to control the separation and removal of Cr(VI) in wastewater, and also proved a reasonable design of the new functional monomer ADPD.
1. Introduction
Statistics from the Ministry of Ecology and Environment of China show that as of 2019, wastewater emissions containing heavy metals (such as Cd, Pb, Cr, and Hg, etc.) totaled 120.7 tons. In addition, nearly 16% of soils were chronically polluted with heavy metals, resulting in almost 12 million tons of grain being contaminated every year.1,2 As a strongly toxic substance, the presence of Cr(VI) in the environment is problematic, as it can easily accumulate in the human body, resulting in liver and kidney damage, gastric ulcers, and cancer (mainly of the lungs and throat).3–6 At present, wastewater treatment measures for Cr(VI) primarily include chemical precipitation,7 electrochemical methods,8,9 ion exchange,10,11 and membrane separation.12,13 Although these methods are effective in their own right, each has its own drawbacks.14–16 For example, chemical precipitation methods may lead to secondary pollution, electrochemical methods tend to be expensive and consume lots of energy, and ion exchange methods often exhibit poor resin stability as these are easily oxidized which may lead to failure. Although membrane separation overcomes some of these drawbacks, offering excellent separation efficiency, environmental friendliness, and ease of operation, it is difficult to achieve the selective separation of metal ions using this method.To overcome some of these limitations, ion imprinting17,18 has emerged as a cutting-edge technology based on molecular imprinting techniques. This method adopts various metal ions as templates and binds functional monomers to them. The template ions are then removed by elution, resulting in systems with high selectivity towards these ions. In particular, the use of ion-imprinted composite membranes (IICMs)19,20 combines the benefits of both ion imprinting and membrane separation. Such membranes are formed by grafting an imprinted layer onto the membrane surface via in situ polymerization, allowing cavities of similar shape, size and function to the template ions to be formed at the membrane surface after elution and removal of the template. The resulting IICMs possess excellent separation efficiency, adsorption capacity, and selectivity. Among all ion-imprinted composite membranes (IICMs), the stimuli-responsive membrane is one of the most attractive materials.22,40 It has the potential to modify its permeation selectivity for metal ions by grafting pH-responsive or temperature-responsive polymers on the substrate membrane. However, the functional monomers currently available for use are almost limited to acrylic acid, methacrylic acid or N-isopropylacrylamide. Their enol/ketone group isomerization with changes in polarity or pH in solvents has attracted increasing interest from scientists and has been applied in the field of functional materials.22 It would be an interesting study to explore a novel stimuli-responsive smart membrane with predetermined affinity for Cr(VI) using β-diketone compounds as functional monomers.
The aim of this study was to synthesize a pH-responsive functional monomer with a β-diketone structure, namely 2-allyl-1,3-diphenylpropane-1,3-dione (ADPD). Its stimulatory responsiveness was verified by UV spectroscopic analysis in solvents of different pH values. A series of Cr(VI) imprinted composite membranes (Cr(VI)-IICM1–11) and the corresponding non-imprinted composite membranes (NICM1–11) were prepared with the support of commercial nylon filter membranes (Nylon). The optimized Cr(VI)-IICM10 was characterized and evaluated based on the results of field emission scanning electron microscopy (FE-SEM), attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR), nitrogen adsorption/desorption analysis and performance testing. Finally, the optimized Cr(VI)-IICM10 was successfully used to competitively transport Cr(VI) in a complex system and showed a pH-controlled effect, which is expected to achieve “controlled” separation for Cr(VI) ions in wastewater.
2. Experimental
2.1 Reagents
Nylon membranes (0.45 μm) were purchased from the Shanghai Xingya factory. Potassium chromate (K2CrO4), methanol, ethanol, acetic acid, isopropanol, acetonitrile, ethyl acetate and petroleum ether were supplied by Fengchuan Chemical Reagent Co. Ltd, China. Dibenzoylmethane (DBM), allyl bromide (AB), ethylene glycol dimethacrylate (EDMA), and 2,2-azobisisobutyronitrile (AIBN) were obtained from Aladdin Reagent Co. Ltd, China. All reagents were of analytical grade, and deionized (DI) water was used in the experiments.2.2 Synthesis of β-diketone functional monomers
Scheme 1 illustrates the procedure used for preparation of the β-diketone functional monomer 2-allyl-1,3-diphenyl-1,3-propanedione (ADPD). First, 20.0 mmol of dibenzoylmethane, 30.0 mmol of allyl bromide and 40 mmol of K2CO3 were added to a 100.0 mL round-bottom flask and dissolved in 60.0 mL of acetonitrile. The mixture was then added to a sealed reaction vial and allowed to react at room temperature for 36 h. Once the reaction was complete, as detected using thin layer chromatography (TLC), the mixture was transferred to a separating funnel and extracted using ethyl acetate (100 mL × 3). Following extraction, the mixture was washed in water (200 mL × 2) and dried under vacuum, and the organic phase was separated using silica gel column chromatography (VPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEA = 10
VEA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the volume ratio of petroleum ether to ethyl acetate) to obtain 4.28 g of yellowish target product with an 81% yield. (1H NMR (600 MHz, CDCl3) δ: 8.01–7.94 (m, 4H), 7.57 (ddt, 2H), 7.51–7.42 (m, 4H), 5.88 (ddt, 1H), 5.32 (t, 1H), 5.13–5.00 (m, 2H), 2.87 (tt, 2H); 13C NMR (151 MHz, CDCl3) δ: 195.29, 135.60, 134.79, 133.34, 128.64, 128.32, 117.00, 56.30, 33.28.)
1, the volume ratio of petroleum ether to ethyl acetate) to obtain 4.28 g of yellowish target product with an 81% yield. (1H NMR (600 MHz, CDCl3) δ: 8.01–7.94 (m, 4H), 7.57 (ddt, 2H), 7.51–7.42 (m, 4H), 5.88 (ddt, 1H), 5.32 (t, 1H), 5.13–5.00 (m, 2H), 2.87 (tt, 2H); 13C NMR (151 MHz, CDCl3) δ: 195.29, 135.60, 134.79, 133.34, 128.64, 128.32, 117.00, 56.30, 33.28.)
2.3 Synthesis of ion-imprinted and non-imprinted composite membranes
IICMs were synthesized based on previous studies,21,22 as follows: initially, appropriate quantities of K2CrO4 and ADPD (as listed in the ESI†) were dosed in conical flasks, and the corresponding solvents were added to react at 25 °C for 3 h, before adding the crosslinker (EDMA) and initiator (AIBN, 15.0 mg), and supplying nitrogen gas for 10 min to eliminate dissolved oxygen. The membranes were then immersed in the obtained pre-polymerization solution for 180 s, before being removed and sandwiched between two glass sheets for polymerization with thermal initiation at 60 °C for 24 h. Finally, Cr(VI) remaining in the imprinted composite membranes was removed using a mixture of methanol and acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The membranes were then further rinsed with methanol until they reached a pH of 7 and were finally vacuum dried to obtain the samples, labelled as Cr(VI)-IICM1–11. As a control, corresponding non-imprinted composite membranes (NICM1–11) were prepared and treated in a similar manner without the addition of Cr(VI).
1, v/v). The membranes were then further rinsed with methanol until they reached a pH of 7 and were finally vacuum dried to obtain the samples, labelled as Cr(VI)-IICM1–11. As a control, corresponding non-imprinted composite membranes (NICM1–11) were prepared and treated in a similar manner without the addition of Cr(VI).
2.4 Effect of synthesis ratio and solvents on adsorption capacity
The synthesis and performance of the imprinted system is affected by the type of solvent23 and the amount of functional monomers24 and crosslinkers25,26 used. In addition, the structure of ADPD may vary between keto and enol forms under conditions of altered solvent polarity, which can impact the interactions between it and the template ions.22,27 Therefore, in order to design IICMs with good recognition towards Cr(VI), a series of experiments were carried out using different types of solvents and varying ratios of Cr(VI) to ADPD and EDMA.2.5 Characterization
Absorption peaks of ADPD in various polar solvents and pH values were measured using a UV-visible (UV-Vis) spectrometer (UV 2550, Shimadzu, Japan). An attenuated total-reflective Fourier-transform infrared (ATR-FTIR) spectrometer (Nicolet 6700, Thermo Fisher, US) was used to characterize the chemical compositions of the membranes and record their absorption spectra within a wave number range of 4000–500 cm−1. The membrane morphology was studied at an accelerated voltage of 15.0 kV using a field emission scanning electron microscope (FE-SEM) (Nano 450, Nova, USA) at magnifications of 10k and 20k. Platinum was applied to the membranes prior to analysis to obtain a better resolution and signal mass. The pore structures of the membranes were determined via nitrogen adsorption/desorption (Sorp-mini II, Microtrac, Japan) in conjunction with BET theory, and the specific surface areas, pore volumes, and average apertures of the membranes were calculated.2.6 Adsorption experiments
 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution was used to remove the Cr(VI) ions from the membrane after each cycle, which were then dried before being recycled. Each experiment was repeated three times to ensure repeatability.
1, v/v) solution was used to remove the Cr(VI) ions from the membrane after each cycle, which were then dried before being recycled. Each experiment was repeated three times to ensure repeatability.3. Results and discussion
3.1 Effect of synthesis ratio and solvents on adsorption capacity
Table 1 shows that there are noticeable differences in the adsorbed amounts of Cr(VI) for the membranes synthesized using different component ratios, with the Cr(VI)-IICM10 membrane (Cr(VI)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ADPD
ADPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDMA ratio of 1
EDMA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) having the highest adsorption capacity of up to 30.35 mg g−1, as well as the highest imprinting factor, α, of 2.70. On the other hand, the solvent polarity32 also has an impact on adsorption of composite membranes, with the loading and selectivity towards Cr(VI) in the IICMs obtained using weakly polar solvents (e.g. ethanol, isopropanol) being significantly better than those of the membranes obtained using strongly polar solvents (e.g. methanol, acetonitrile). This may be attributed to ADPD undergoing structural interconversion in weakly polar solvents, and creating more coordination groups (such as –OH, C
30) having the highest adsorption capacity of up to 30.35 mg g−1, as well as the highest imprinting factor, α, of 2.70. On the other hand, the solvent polarity32 also has an impact on adsorption of composite membranes, with the loading and selectivity towards Cr(VI) in the IICMs obtained using weakly polar solvents (e.g. ethanol, isopropanol) being significantly better than those of the membranes obtained using strongly polar solvents (e.g. methanol, acetonitrile). This may be attributed to ADPD undergoing structural interconversion in weakly polar solvents, and creating more coordination groups (such as –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and α-H) in solution,33 which reinforced the imprinting of Cr(VI) during the preparation of the IICMs. These results are consistent with the UV-Vis absorption spectra of ADPD under different solvent polarity conditions (Fig. 1).
C and α-H) in solution,33 which reinforced the imprinting of Cr(VI) during the preparation of the IICMs. These results are consistent with the UV-Vis absorption spectra of ADPD under different solvent polarity conditions (Fig. 1).
| Solvent | QIIP (mg g−1) | QNIP (mg g−1) | α (QIIP/QNIP) | Cr(VI)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ADPD ADPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDMA EDMA |
QIIP (mg g−1) | QNIP (mg g−1) | α (QIIP/QNIP) |
|---|---|---|---|---|---|---|---|
| CH3OH | 13.54 | 8.13 | 1.66 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
12.64 | 10.87 | 1.16 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
21.54 | 12.91 | 1.67 | ||||
| CH3CN | 15.52 | 9.05 | 1.72 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
27.93 | 12.80 | 2.18 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
19.01 | 15.76 | 1.21 | ||||
| CH3CH2OH | 27.93 | 12.80 | 2.18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
18.18 | 16.05 | 1.13 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
22.44 | 14.57 | 1.54 | ||||
| (CH3)2CHOH | 19.61 | 9.75 | 2.01 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
30.35 | 11.23 | 2.70 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
17.69 | 13.38 | 1.32 |
3.2 Characteristics
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ∼280 nm) under more polar solvents. In contrast, enol structures (
O, ∼280 nm) under more polar solvents. In contrast, enol structures (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH, ∼245 nm) were noticeably increased in the lower polarity solvents.34 This is likely due to the formation of intramolecular hydrogen bonds from carbonyl groups within the diketone structures of ADPD in polar solvents, which increases their stability.35,36
C–OH, ∼245 nm) were noticeably increased in the lower polarity solvents.34 This is likely due to the formation of intramolecular hydrogen bonds from carbonyl groups within the diketone structures of ADPD in polar solvents, which increases their stability.35,36![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, while those observed from ∼1585–1440 cm−1 are associated with the skeletal vibrations of aromatic rings and the bending vibrations of –CH2. Additionally, the stretched vibrations of –CH2 and the
C, while those observed from ∼1585–1440 cm−1 are associated with the skeletal vibrations of aromatic rings and the bending vibrations of –CH2. Additionally, the stretched vibrations of –CH2 and the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretched vibrational peaks of aromatic rings (olefins) were observed at 2935 cm−1 and 3080 cm−1, respectively.
C–H stretched vibrational peaks of aromatic rings (olefins) were observed at 2935 cm−1 and 3080 cm−1, respectively.
 | ||
| Fig. 2 ATR-FTIR spectra of the functional monomer (ADPD) and the nylon, Cr(VI)-IICM10, and NICM10 membranes. | ||
As expected, all of characteristic peaks from ADPD were also observed in the Cr(VI)-IICM10 and NICM samples but not in the primary nylon membranes. In the spectra for Cr(VI)-IICM10 and NICM10, novel peaks located at 1460 cm−1 and 1545 cm−1 were attributed to the –CH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of the aromatic ring, respectively, while the absorption peaks at 2940 and 3075 cm−1 are attributed to the bending vibrations of methylene (–CH2) and the stretching vibrations of aromatic rings (olefins,
C groups of the aromatic ring, respectively, while the absorption peaks at 2940 and 3075 cm−1 are attributed to the bending vibrations of methylene (–CH2) and the stretching vibrations of aromatic rings (olefins, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), respectively. It is important to mention that a fresh absorption peak positioned at ∼1645 cm−1 in the Cr(VI)-IICM10 and NICM10 spectra was attributed to the stretching vibrations of the carbonyl group (C
C–H), respectively. It is important to mention that a fresh absorption peak positioned at ∼1645 cm−1 in the Cr(VI)-IICM10 and NICM10 spectra was attributed to the stretching vibrations of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). These results confirm that ADPD was successfully introduced to the nylon membrane substrate, and that ion-imprinted layers of Cr(VI) were subsequently formed on the membrane surface.
O). These results confirm that ADPD was successfully introduced to the nylon membrane substrate, and that ion-imprinted layers of Cr(VI) were subsequently formed on the membrane surface.
| Membranes | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| Nylon | 10.282 | 0.0373 | 14.525 |
| NICM10 | 9.233 | 0.0243 | 10.537 |
| Cr(VI)-IICM10 | 7.817 | 0.0168 | 8.618 |
However, in contrast with the nylon membranes, the specific surface area, hole volume, and aperture of the composite membranes was significantly reduced, and this reduction was significantly greater for Cr(VI)-IICM10 compared to NICM10. This may be due to the former having a large, layered imprint structure on its surface, while the latter only possesses a small polymer covering. These results are consistent with the FE-SEM images (Fig. 3), which indicates that a large number of identification site structures were successfully formed on the membrane surface.
3.3 Adsorption experiments
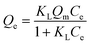 | (3) |
| Qe = KFC1/ne | (4) |
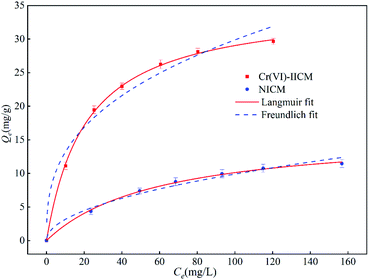 | ||
| Fig. 4 Adsorption isotherms for Cr(VI)-IICM10 and NICM10, along with Langmuir and Freundlich fitting curves for Cr(VI)-IICM10 and NICM10. | ||
The fitting parameters of the Langmuir and Freundlich isotherms are shown in Table 3, which reveals that the correlation coefficient of Cr(VI)-IICM10 in the Langmuir model (R2 = 0.9985) is better than that of the Freundlich model (R2 = 0.9562), indicating that distribution of adsorption sites is uniform, and that monolayer adsorption occurs.
| Isotherm model | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | KL | Qm (mg g−1) | R2 | KF | 1/n | R2 | |
| Cr(VI)-IICM10 | 29.66 | 0.0475 | 35.13 | 0.9985 | 5.813 | 0.3560 | 0.9562 |
| NICM10 | 11.47 | 0.0169 | 16.17 | 0.9959 | 1.012 | 0.4935 | 0.9815 |
Experimental data were fitted using the pseudo-first-order and pseudo-second-order adsorption kinetic models, as follows:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (5) |
 | (6) |
The adsorption of Cr(VI) on Cr(VI)-IICM10was analyzed, and the results are shown in Fig. 6. It is evident that the correlation coefficient of the pseudo first-order equation (R2 = 0.9960) was larger than that of the pseudo-second-order equation (R2 = 0.7544), indicating that the adsorption of Cr(VI) follows pseudo first-order kinetics.
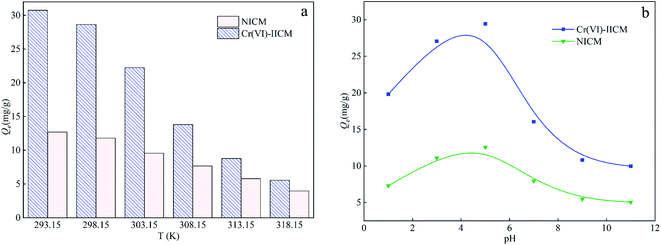
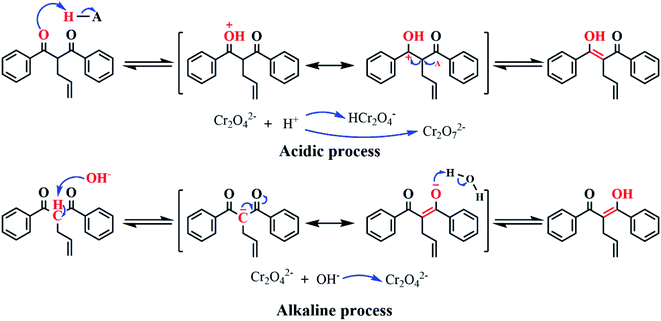
Fig. 7(b) shows that the adsorption capacity of both Cr(VI)-IICM10 and NICM10 towards Cr(VI) did vary modestly with the solution pH, with the former having a higher adsorption capacity at all pH values. In particular, the amount of Cr(VI) adsorbed on Cr(VI)-IICM10 was roughly twofold that on NICM10 at pH 5 owing to the imprinting of the Cr(VI) selective-recognition cavities. The Cr(VI) absorption capacity increased markedly from pH 1–5 and decreased significantly thereafter. A possible explanation for this is that ADPD produced enol anions (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–) under alkaline conditions, which is not conducive to the adsorption of chromate ions (CrO42−) on composite membranes (Fig. 8).22,39
C–O–) under alkaline conditions, which is not conducive to the adsorption of chromate ions (CrO42−) on composite membranes (Fig. 8).22,39
Under acidic conditions (pH < 7), ADPD is promoted to generate enol cations (C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH+),37 thus achieving greater adsorption capacity towards CrO42−. However, in strongly acidic solutions (pH < 3), the adsorption ability of composite membranes towards Cr(VI) was substantially reduced. It is believed that ADPD tends to form ketonic structures (C
OH+),37 thus achieving greater adsorption capacity towards CrO42−. However, in strongly acidic solutions (pH < 3), the adsorption ability of composite membranes towards Cr(VI) was substantially reduced. It is believed that ADPD tends to form ketonic structures (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in stronger acidic environments, which causes a reduction of enol cations (C–C
O) in stronger acidic environments, which causes a reduction of enol cations (C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH+) in the solution and eventually reduces the adsorption capacity of composite membranes (Fig. 8). These results demonstrate that Cr(VI)-IICM10 is pH-responsive and can potentially act as a “smart membrane” to control both the separation and enrichment of Cr(VI) from solutions.
OH+) in the solution and eventually reduces the adsorption capacity of composite membranes (Fig. 8). These results demonstrate that Cr(VI)-IICM10 is pH-responsive and can potentially act as a “smart membrane” to control both the separation and enrichment of Cr(VI) from solutions.
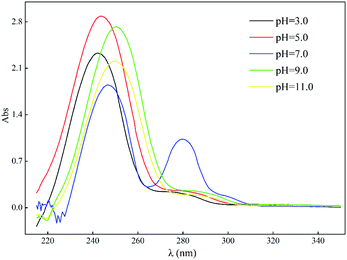
Fig. 9 shows that the changes of ADPD in solution with different pH. In a neutral medium, ADPD exhibited both ketone and enol structures and possesed two absorption peaks in the UV-Vis spectrogram; however, as the solution changed to basic or acidic, the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorption peak at 280 nm decreased substantially, which indicates that the diketone structures of ADPD were converted to enols.37 Further, as the acidity of the solution increased, the enolic structures of ADPD further decreased, which indicates that ADPD exists primarily as keto structures in more strongly acidic and basic solutions.38
O) absorption peak at 280 nm decreased substantially, which indicates that the diketone structures of ADPD were converted to enols.37 Further, as the acidity of the solution increased, the enolic structures of ADPD further decreased, which indicates that ADPD exists primarily as keto structures in more strongly acidic and basic solutions.38
These results indicate that the β-diketone functional monomer (ADPD) switches between ketone and enol in response to variations in the pH of the matrix solution. Hence, this monomer can be utilized for the generation of pH-responsive IICMs (e.g. Cr(VI)-IICM10) capable of achieving pH-controlled and selective separation of Cr(VI) in solution.
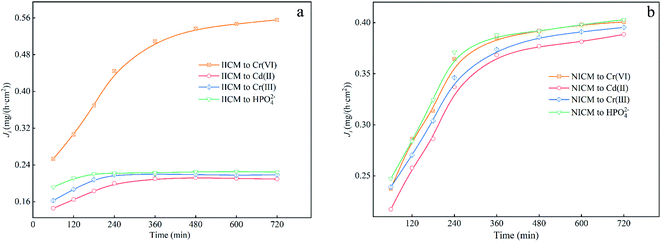 | ||
| Fig. 10 Selective permeation properties of (a) Cr(VI)-IICM10 and (b) NICM10 towards Cr(VI), Cd(II), Cr(III), and HPO42−. | ||
4. Conclusions
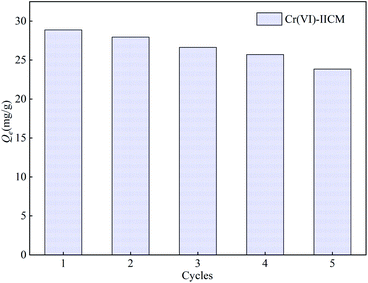
In this study, a novel ion-imprinted composite membrane (IICM) was successfully developed for the selective separation and rapid identification of Cr(VI) in solution. This was achieved using Cr(VI) as template ions alongside a customized β-diketone functional monomer (ADPD) in a combined ion surface imprinting and membrane separation technique. ATR-FTIR analysis revealed that ADPD was strongly grafted to the nylon membrane, while FE-SEM and BET further confirmed that an imprinted layer structure of Cr(VI) was formed on the Cr(VI)-IICM10 membrane. Further, UV-Vis and adsorption experiments indicated that Cr(VI)-IICM10 underwent structural changes in response to shifts in the pH, and also exhibited a high adsorption capacity towards Cr(VI) with a maximum value of 30.35 mg g−1. The adsorption rate was consistent with pseudo-first-order kinetics, and the equilibrium adsorption showed a good fit with Langmuir isotherms. Overall, Cr(VI)-IICM10 also exhibited remarkable osmotic selectivity, with permeation of Cr(VI) (Ji = 0.556) far higher than those of three competing ions (Ji ≈ 0.217), and showed excellent stability and reusability after five adsorption–desorption cycles. These results strongly suggest that the use of ADPD to fabricate IICMs is a cost-effective and convenient separation strategy, which holds promise as a reliable and controlled approach for the selective enrichment and separation of Cr(VI) from aqueous solution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (grant numbers 21764008).References
- X. H. Yuan, N. D. Xue and Z. G. Han, J. Environ. Sci., 2021, 101, 217–226 CrossRef PubMed.
- G. W. Qin, Z. D. Niu, J. D. Yu, Z. H. Li, J. Y. Ma and P. Xiang, Chemosphere, 2021, 267, 129205 CrossRef CAS PubMed.
- K. H. Cheung and J. D. Gu, Int. Biodeterior. Biodegrad., 2007, 59, 8–15 CrossRef CAS.
- T. Pavesi and J. C. Moreira, J. Appl. Toxicol., 2020, 40, 1183–1197 CrossRef CAS PubMed.
- T. L. DesMarais and M. Costa, Curr. Opin. Toxicol., 2019, 14, 1–7 CrossRef PubMed.
- S. Y. Guo, C. Q. Xiao, N. Zhou and R. Chi, Environ. Chem. Lett., 2021, 19, 1413–1431 CrossRef CAS.
- B. Verma and C. Balomajumder, Bull. Chem. Soc. Ethiop., 2020, 34, 67–74 CrossRef CAS.
- Y. A. El-Taweel, E. M. Nassef, I. Elkheriany and D. Sayed, Egypt. J. Pet., 2015, 24, 183–192 CrossRef.
- C. Pan, L. D. Troyer, J. G. Catalano and D. E. Giammar, Environ. Sci. Technol., 2016, 50, 13502–13510 CrossRef CAS PubMed.
- Y. Q. Xing, X. M. Chen and D. H. Wang, Environ. Sci. Technol., 2007, 41, 1439–1443 CrossRef CAS PubMed.
- A. S. Dharnaik and P. K. Ghosh, Environ. Technol., 2014, 35, 2272–2279 CrossRef CAS PubMed.
- J. T. Liu, W. D. Zhang, Z. Q. Ren and J. A. Ma, Ind. Eng. Chem. Res., 2009, 48, 4500–4506 CrossRef CAS.
- S. Caprarescu, R. G. Zgarian, G. T. Tihan, V. Purcar, E. E. Totu, C. Modrogan, A. L. Chiriac and C. A. Nicolae, Polymers, 2020, 12, 1792 CrossRef CAS PubMed.
- M. Bodzek, Ecol. Chem. Eng. S, 2013, 20, 633–658 CAS.
- S. Sharma and A. Bhattacharya, Appl. Water Sci., 2017, 7, 1043–1067 CrossRef CAS.
- S. S. Kerur, S. Bandekar, M. S. Hanagadakar, S. S. Nandi, G. M. Ratnamala and P. G. Hegde, Mater. Today, 2021, 42, 1112–1121 CAS.
- J. J. Wang, Y. J. Han, J. Li and J. Wei, Sep. Purif. Technol., 2017, 177, 62–70 CrossRef CAS.
- Z. Q. Ren, X. Y. Zhu, J. Du, D. L. Kong, N. Wang, Z. Wang, Q. Wang, W. Liu, Q. S. Li and Z. Y. Zhou, Appl. Surf. Sci., 2018, 435, 574–584 CrossRef CAS.
- D. S. Sun, M. J. Meng, Y. Qiao, Y. H. Zhao, Y. S. Yan and C. X. Li, Sep. Purif. Technol., 2018, 194, 64–72 CrossRef CAS.
- J. Zhang, R. Wang, X. Ou, X. Zhang, P. Liu, Z. Chen, B. Zhang, C. Liu, S. Zhao, Z. Chen, J. Zhu, S. Lu and P. Zhang, Sep. Purif. Technol., 2021, 259, 118165 CrossRef CAS.
- Y. M. Liu, D. Q. Hu, X. Z. Hu, S. L. Chen, L. Zhao, Y. L. Chen, P. Yang, X. C. Qin, H. L. Cheng and F. T. Zi, Anal. Lett., 2020, 53, 1113–1139 CrossRef CAS.
- H. L. Cheng, X. F. Zhu, S. X. Yang, Y. X. Wu, Q. Cao and Z. T. Ding, J. Appl. Polym. Sci., 2013, 128, 363–370 CrossRef CAS.
- W. Meouche, K. Laatikainen, A. Margaillan, T. Silvonen, H. Siren, T. Sainio, I. Beurroies, R. Denoyel and C. Branger, Eur. Polym. J., 2017, 87, 124–135 CrossRef CAS.
- J. L. Urraca, M. C. Carbajo, M. J. Torralvo, J. Gonzalez-Vazquez, G. Orellana and M. C. Moreno-Bondi, Biosens. Bioelectron., 2008, 24, 155–161 CrossRef CAS PubMed.
- K. Yoshimatsu, T. Yamazaki, I. S. Chronakis and L. Ye, J. Appl. Polym. Sci., 2012, 124, 1249–1255 CrossRef CAS.
- H. Q. Hu, Z. Ren, Y. Xi, L. L. Fang, D. F. Fang, L. M. Yang, P. H. Shao, H. Shi, K. Yu and X. B. Luo, Chem. Eng. J., 2021, 420, 129611 CrossRef CAS.
- E. V. Sergeeva, L. N. Puntus, F. Kajzar, I. Rau, B. Sahraoui, I. S. Pekareva, K. Y. Suponitsky, I. S. Bushmarinov and K. A. Lyssenko, Opt. Mater., 2013, 36, 47–52 CrossRef CAS.
- J. Li, F. Ji, D. H. L. Ng, J. Liu, X. M. Bing and P. Wang, Chem. Eng. J., 2019, 369, 611–620 CrossRef CAS.
- J. S. Cao, X. D. Wu, L. T. Wang, G. S. Shao, B. Y. Qin, Z. H. Wang, T. Wang and Y. J. Fu, Int. J. Biol. Macromol., 2021, 181, 1231–1242 CrossRef CAS PubMed.
- L. P. Zhao, L. S. Li, C. Zhu, M. Ghulam and F. Qu, Anal. Chim. Acta, 2020, 1097, 161–168 CrossRef CAS PubMed.
- D. Wu, X. Fang, J. Song, L. Qu, X. Zhou, H. Xiang, J. Wang and J. Liu, Dyes Pigm., 2021, 184, 108851 CrossRef CAS.
- I. E. Charif, S. M. Mekelleche and D. Villemin, J. Theor. Comput. Chem., 2010, 9, 1021–1032 CrossRef CAS.
- S. K. Wu, G. S. Dai, L. S. Liu and J. K. Chang, Polym. Degrad. Stab., 1986, 16, 169–186 CrossRef CAS.
- J. Zawadiak and M. Mrzyczek, Spectrochim. Acta, Part A, 2010, 75, 925–929 CrossRef PubMed.
- K. R. Wursthorn and E. H. Sund, J. Heterocycl. Chem., 1972, 9, 25–29 CrossRef CAS.
- J. Zhao, J. Zhao, X. He, Z. Tan, X. Cheng, Q. Han and C. Zhou, J. Mater. Chem. C, 2021, 9, 1000–1007 RSC.
- N. D. Tivendale, N. W. Davies, J. Horne, J. J. Ross and J. A. Smith, Aust. J. Chem., 2015, 68, 345–348 CrossRef CAS.
- L. Zhang, J. Zhang, Z. J. Li, Y. Y. Qin, Q. P. Lin and Y. G. Yao, Chem.–Eur. J., 2009, 15, 989–1000 CrossRef CAS PubMed.
- A. A. Adeniyi and J. Conradie, Electrochim. Acta, 2019, 297, 947–960 CrossRef CAS.
- J. Q. Fu, L. X. Chen, J. H. Li and Z. Zhang, J. Mater. Chem. A, 2015, 3, 13598–13627 RSC.
- X. Q. Cai, J. H. Li, Z. Zhang, F. F. Yang, R. C. Dong and L. X. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 305–313 CrossRef CAS PubMed.
- S. F. Xu, L. X. Chen, J. H. Li, Y. F. Guan and H. Z. Lu, J. Hazard. Mater., 2012, 237, 347–354 CrossRef PubMed.
- L. Y. Wang, J. H. Li, J. N. Wang, X. T. Guo, X. Y. Wang, J. Choo and L. X. Chen, J. Colloid Interface Sci., 2019, 541, 376–386 CrossRef CAS PubMed.
- J. Lu, Y. Y. Qin, Y. L. Wu, M. J. Meng, Y. S. Yan and C. X. Li, Environ. Sci. Water Resour., 2019, 5, 1626–1653 RSC.
- Y. A. B. Neolaka, Y. Lawa, J. N. Naat, A. A. P. Riwu, H. Darmokoesoemo, G. Supriyanto, C. I. Holdsworth, A. N. Amenaghawon and H. S. Kusuma, React. Funct. Polym., 2020, 147, 104451 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07678g |
| This journal is © The Royal Society of Chemistry 2021 |