Open Access Article
Open Access ArticleMesoporous silica shell-coated single gold nanorods as multifunctional orientation probes in dynamic biological environments†
Geun Wan Kima,
In-Seob Han*b and
Ji Won Ha *ac
*ac
aDepartment of Chemistry, University of Ulsan, 93 Daehak-ro, Nam-gu, Ulsan, 44610, Republic of Korea. E-mail: jwha77@ulsan.ac.kr; Fax: +82 52 712 8002; Fax: +82 52 259 1694; Tel: +82 52 712 8012 Tel: +82 52 259 2352
bSchool of Biological Sciences, University of Ulsan, 93 Daehak-ro, Nam-gu, Ulsan, 44610, Republic of Korea. E-mail: hanis@ulsan.ac.kr
cEnergy Harvest-Storage Research Center (EHSRC), University of Ulsan, 93 Daehak-ro, Nam-gu, Ulsan, 44610, Republic of Korea
First published on 1st December 2021
Abstract
Mesoporous silica shell-coated gold nanorods (AuNRs@mSiO2) can be employed as promising multifunctional orientation probes in biological studies owing to their anisotropic optical properties, enhanced stability, excellent biocompatibility, etc. In this study, the optical properties of single AuNRs@mSiO2 are characterized under dark-field and differential interference contrast (DIC) microscopy. Furthermore, we presented polarization-dependent, periodic DIC images and intensities of single AuNRs@mSiO2 at their localized surface plasmon resonance wavelength and investigated their use as multifunctional orientation probes in dynamic biological environments. Moreover, the real-time rotational motions of the AuNRs@mSiO2 on the HeLa cell membranes were tracked with millisecond temporal resolution. Overall, AuNRs@mSiO2 demonstrated their capacity to act as multifunctional optical probes owing to the combined effect of the Au core, which can serve as an orientation probe and a local heat generator for phototherapy, and the mesoporous silica shell, which can be used as a reservoir of chemotherapeutics owing to its excellent loading capacity.
Introduction
Plasmonic gold nanorods (AuNRs) have numerous biomedical applications because of their unique shape-induced optical properties which can be attributed to the localized surface plasmon resonance (LSPR) effect, facile surface modification, etc.1–4 For example, AuNRs have been used in phototherapy5–7 owing to their high efficiency in photothermal conversion and as local heat generators in chemotherapy treatments to activate and enhance photothermally induced drug release.8,9Despite their merits for biomedical applications, the presence of highly toxic cetyltrimethylammonium bromide (CTAB) bilayers used in the seed-mediated growth strategy limits the direct use of AuNRs.10 Thus, the surface functionalization of AuNRs is mandatory to improve their biocompatibility. Recently, mesoporous silica shell-coated AuNRs (AuNRs@mSiO2, core@shell) have exhibited excellent biocompatibility.11 Furthermore, they can be used for synergistic phototherapy and chemotherapy combination using the mesoporous silica pores as reservoirs for chemotherapeutics owing to their high loading capacity, while the AuNRs serve as a local heat generator to induce phototherapy and trigger drug release.11–16
AuNRs have also been effectively used as orientation probes with a few polarization-based optical techniques, including dark-field (DF) polarization microscopy,17 photothermal heterodyne imaging (PHI),18 differential interference contrast (DIC) polarization anisotropy,1,19 and total internal reflection (TIR) scattering microscopy.20–22 Therefore, AuNRs@mSiO2 can be used as multifunctional optical probes in biological studies. For example, the AuNR core can serve as an orientation probe and a heat generator in phototherapy, whereas the mesoporous silica shell can be used as a chemotherapeutics reservoir.11,13,15 However, our understanding of their use as multifunctional orientation probes is still limited in dynamic biological environments.
The scattering-based methods, such as DF microscopy, are challenging for identifying AuNRs from cellular components and other debris that scatter light. In this regard, interference-based DIC microscopy is more suitable for investigating rotational motions of AuNRs in biological environments.
In the present study, we aim to investigate the optical properties of single AuNRs@mSiO2 in DF and DIC microscopy. The effect of light polarization on the DIC image patterns and intensities of single AuNRs@mSiO2 at their LSPR wavelengths is observed, and their potential use for the development of multifunctional orientation probes in dynamic biological environments is examined under a DIC microscope.
Experimental section
Materials and chemicals
AuNRs@mSiO2 were purchased from Nanopartz (Loveland, CO, USA). The cervical carcinoma cell line, HeLa-WT, was obtained from the Korean Cell Line Bank (Seoul, South Korea). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water (18.2 MΩ cm) was prepared using a Sartorius Arium®Pro Ultrapure water system.Sample preparation and characterization
The AuNRs@mSiO2 solution was diluted with 18.2 MΩ pure water to the proper concentration and then sonicated for 15 min at room temperature. The samples were prepared by spin-casting the solution on the precleaned glass slide and covered with a 22 mm × 22 mm No 1.5 coverslip. The concentration of AuNRs@mSiO2 on the glass surface was controlled to be ∼1 μm−2 to facilitate single-particle characterization and to minimize interparticle LSPR coupling. Structural characterization was performed using a transmission electron microscope (TEM; H-8100, HITACHI, JAPAN) and a scanning electron microscope (SEM; JSM-6500, JEOL, JAPAN).Cell culture
The HeLa cells were placed in a cell culture flask and grown in a cell culture medium supplemented with 10% FBS in a cell culture incubator (37 °C, 5% CO2) for 24 h. For sub-culturing, 200 μL of the cell suspension was transferred to a 22 × 22 mm2 No 1.5 poly-L-lysine-coated coverslip (Corning, NY) and stored in a Petri dish, which was then incubated for 1 h to allow the cells to attach to the coverslip. The coverslip was covered with 1.5 mL of the cell culture medium containing 10% FBS supplement, and the Petri dish was placed in the incubator for 24 h.DF microscopy and spectroscopy
DF microscopy was performed under a Nikon inverted microscope (ECLIPSE Ti-U). In DF mode, the microscope utilized a Nikon Plan Fluor 100 × 0.5–1.3 oil iris objective and a Nikon DF condenser. An Andor iXonEM + CCD camera (iXon Ultra 897) was employed to record DF images of AuNRs@mSiO2, which were analyzed using Image J. DF scattering spectra were measured and captured using an Andor spectrometer (SHAMROCK 303i, SR-303I-A) and an Andor CCD camera (Newton DU920P-OE). The scanning stage moved the sample to the desired location so that only the scattered light from the selected location was collected by the objective for spectral measurement. The scattered light was directed to the entrance of the spectrometer, dispersed by a grating (300 l mm−1), and detected by the Newton CCD camera. The background was measured at a region without any particles. Data analysis was performed with specially designed MATLAB programs.Differential interference contrast microscopy
DIC microscopy was performed using a Nikon inverted microscope (ECLIPSE Ti-U) comprising two Normarski prisms, two polarizers, and a quarter waveplate. The samples were illuminated through an oil immersion condenser (numerical aperture (NA) = 1.4). The signals that passed through the sample were collected using a Plan Apo oil-immersion objective (100×, NA = 1.4). A bandpass filter with a 720 nm central range (full width at half maximum, ±5 nm) was obtained from Thorlabs (Newton, NJ, USA) and inserted into the beam path of the microscope to illuminate the samples. A rotational study was conducted at 720 nm excitation by rotating the stage 10° per step for single AuNRs@mSiO2. The fixed AuNRs@mSiO2 were positioned in different orientations as the stage was rotated. The Andor iXonEM + CCD camera was used to record highly detailed DIC images of the AuNRs@mSiO2, which were analyzed using Image J.Results and discussion
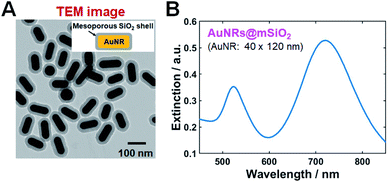
TEM and SEM images of single AuNRs@mSiO2 are shown in Fig. 1A and S1 (of the ESI†), respectively. The average width and length of AuNRs@mSiO2 were 40 nm and 120 nm, respectively. In the ultraviolet–visible light extinction spectrum of AuNRs@mSiO2 dispersed in water, shown in Fig. 1B, the transverse and longitudinal LSPR peaks were observed at 526 and 723 nm, respectively. The thickness of silica shell was approximately 18 nm.We performed single-particle measurements using the experimental setup shown in Fig. S2† to understand the scattering properties of AuNRs@mSiO2 and eliminated the effect of averaging under DF scattering microscopy and spectroscopy. The AuNRs@mSiO2 were illuminated with a randomly polarized white light tightly focused using a high NA oil condenser under DF microscopy. A DF scattering image of two AuNRs@mSiO2 (labeled as AuNR1@mSiO2 and AuNR2@mSiO2 and marked with yellow squares) deposited on a glass slide are shown in Fig. 2A; single-particle scattering spectra of the two highlighted nanorods are shown in Fig. 2B. The LSPR wavelength of the single AuNRs@mSiO2 in the single-particle spectra appeared at 690–760 nm, which can be ascribed to heterogeneity in particle size (see also Fig. S3†). This result from the single-particle measurements is consistent with the result from the ensemble experiments shown in Fig. 1B.
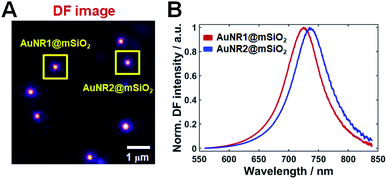 | ||
| Fig. 2 (A) DF image of single AuNRs@mSiO2 on a glass slide. (B) Single-particle scattering spectra of the two AuNRs@mSiO2 (AuNR1@mSiO2, AuNR2@mSiO2) marked with a yellow square in (A). | ||
DIC microscopy is better suited to probe orientation and rotational motion of nanoprobes in live cells.3,23–25 DIC microscopy resolves the optical path difference between two mutually orthogonally polarized beams separated by a shear distance along the optical axis of a Nomarski prism (see Fig. S4†), making it insensitive to scattered light from surrounding cellular components while maintaining its high-throughput capability. Therefore, the DIC microscopy-based single-particle rotational tracking technique is effectively used in studies of the rotational dynamics in live cells.
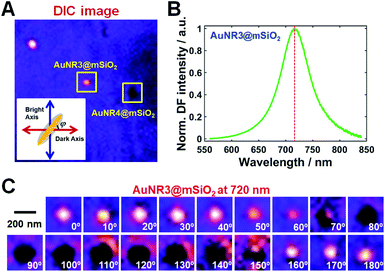
The optical properties of single AuNRs@mSiO2 fixed on a glass slide were then examined under DIC microscopy at 720 nm excitation close to their longitudinal LSPR wavelength; a DIC image of single AuNRs@mSiO2 on a glass slide at 720 nm is shown in Fig. 3A. The orientation of the AuNR core inside the silica shell was defined as the orientation angle φ between the longitudinal axis of AuNR and dark axis, as shown in the inset of Fig. 3A. The orientation angle increased in the counterclockwise direction toward the bright axis. Different DIC image patterns of single AuNRs@mSiO2 were observed owing to their spatial orientation on the glass slide; i.e., a bright image pattern was observed for AuNR3@mSiO2 since it was aligned parallel to the bright polarization axis (φ = 90°, blue arrow), whereas a dark image pattern was observed for AuNR4@mSiO2. Therefore, DIC microscopy facilitated orientation-dependent DIC image patterns of single AuNRs@mSiO2 to be observed with high contrast and sensitivity. The scattering spectrum of the AuNR3@mSiO2 highlighted by a yellow square in Fig. 3A was obtained to confirm that it was a single nanoparticle rather than an aggregate and is shown in Fig. 3B. The measured longitudinal LSPR peak of AuNR3@mSiO2 appeared at 720 nm, which is consistent with the single-particle spectra in Fig. 2B and S3.† Next, polarization-dependent DIC imaging was performed for the fixed AuNR3@mSiO2 at an excitation of 720 nm by rotating the stage 10° per step to position AuNR3@mSiO2 in different orientations. The complete set of obtained DIC images of AuNR3@mSiO2 from 0° to 180° at intervals of 10° is shown in Fig. 3C, where the image patterns periodically changed as a function of the rotation angle. Polarization-dependent DIC images of AuNR4@mSiO2 marked in Fig. 3A are also provided in Fig. S5.†
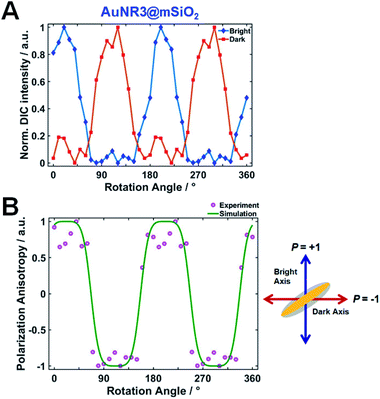
The normalized DIC intensities of AuNR3@mSiO2 for two bright (blue) and dark (red) polarization directions were then plotted as a function of rotation angle in Fig. 4A to gain a deeper insight into the polarization dependence. As the stage was rotated, the DIC bright (red) and dark (blue) intensity curves were anticorrelated, i.e., an increase in the bright intensity was accompanied by a decrease in the dark intensity, and vice versa. These observed polarization-sensitive, periodic DIC images and intensities of single AuNRs@mSiO2 indicate that the spatial orientation of single AuNRs@mSiO2 can be determined via DIC microscopy and polarization anisotropy (also see Fig. S5†).
DIC polarization anisotropy using an intensity ratio instead of absolute intensities is less affected by intensity instabilities and can thus provide more accurate, reproducible, and reliable angle measurements.1,3,19 The DIC polarization anisotropy P can be obtained from the bright and dark intensities of a single DIC image of the AuNR@mSiO2, as19
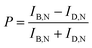 | (1) |
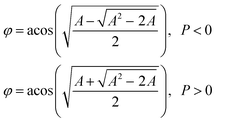 | (2) |
To compare with single AuNRs@mSiO2, we further obtained DIC images of an aggregate by rotating the stage with increments of 10° (Fig. S6†). Unlike single AuNRs@mSiO2, the DIC image patterns and intensities of the aggregate were not periodically changed as a function of rotational angle. Thus, we were not able to observe polarization-dependent DIC images and intensities in case of the aggregate.
Next, we tested if the DIC polarization anisotropy can be used to track fast rotational dynamics of single AuNRs@mSiO2 in real-time. Thus, we chose single AuNRs@mSiO2 rotating on a glass slide as a model system to verify. We observed that some AuNRs@mSiO2 were attached on a glass slide through non-specific binding (Fig. 5, Movies S1 and S2†) and showed fast rotational motions. As shown in Fig. 5C, the experimental P values were randomly changed over time in between −1 and +1 depending on the orientation of AuNR5@mSiO2. Therefore, we demonstrated that the DIC polarization anisotropy can be used to determine the orientation angles of single AuNRs@mSiO2 in fast rotational motions.
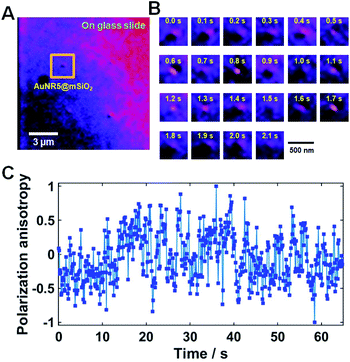 | ||
| Fig. 5 (A) DIC image of AuNR5@mSiO2 bound onto a glass slide. (B) 22 successive DIC images of AuNR5@mSiO2 chosen from Movie S2.† The temporal resolution was 100 ms. (C) DIC polarization anisotropy P for AuNR5@mSiO2 as a function of time for all consecutive frames in Movie S2.† | ||
The next crucial step was to verify that single AuNRs@mSiO2 could be used as high-contrast multifunctional orientation probes in dynamic biological environments under DIC microscopy. In recent studies, single AuNRs@mSiO2 have been employed as efficient drug delivery cargoes in biological applications.11,13–15,26 Tracking rotational and translational dynamics of individual AuNRs@mSiO2 on live cell membranes will provide a better understanding of drug delivery mechanisms and other cellular processes, such as endocytosis. AuNRs@mSiO2 rotating on live cell membranes were thus used as a model system to verify their use as multifunctional orientation probes in dynamic biological systems.
The rotational motions of surface-bound AuNRs@mSiO2 were recorded at a temporal resolution of 100 ms under DIC microscopy, as shown in Movie S3.† The AuNR6@mSiO2 rotated quickly when it first fell on the HeLa cell membrane, as revealed by an image flicker. A captured DIC image of AuNR6@mSiO2 bound to a live cell membrane is shown in Fig. 6A. The DIC image patterns changed dynamically as a function of time, as demonstrated by the 20 consecutive frames from Movie S3† shown in Fig. 6B. The DIC polarization anisotropy P values for these 20 images were randomly distributed between −1 and +1, as demonstrated in Fig. 6C. More experimental data for AuNR7@mSiO2 and AuNR8@mSiO2 rotating on the live cell membrane are provided in Fig. S7 and S8.† The results support the utility of single AuNRs@mSiO2 as orientation probes under DIC microscopy and demonstrate that DIC polarization anisotropy allows precise determination of their real-time orientation during dynamic biological processes.
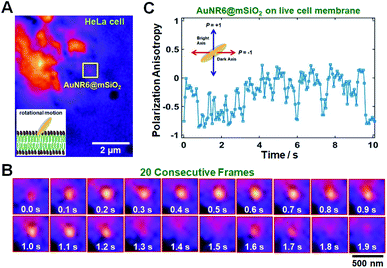 | ||
| Fig. 6 (A) DIC image of AuNR6@mSiO2 bound onto a live cell membrane. (B) 20 successive DIC images of AuNR6@mSiO2 chosen from Movie S3.† The temporal resolution was 100 ms. (C) DIC polarization anisotropy P for AuNR6@mSiO2 as a function of time for all consecutive frames in Movie S3.† The inset shows a schematic of P values for two special cases (φ = 0° and φ = 90°). | ||
Further, AuNRs@mSiO2 present two main advantages over bare AuNRs (no silica shell) that have been conventionally used as orientation probes in biological studies, whose orientation angles can both be obtained with high sensitivity under DIC microscopy. First, AuNRs@mSiO2 can be used as multifunctional orientation probes because of the added functions compared with the bare AuNR probe.27,28 For example, the mesoporous silica shell in a drug delivery system can provide excellent loading capacity, reduced toxicity, and enhanced stability. Second, as demonstrated in Fig. 5, the Au core inside the silica shell can be used as an orientation probe and a local heat generator to induce phototherapy in biological systems. Therefore, AuNRs@mSiO2 can be used as multifunctional orientation probes in various biomedical studies.
Conclusions
In summary, the optical properties of single AuNRs@mSiO2 at their LSPR wavelengths under DF and DIC microscopy and their use as orientation probes in dynamic biological systems were examined. Single AuNRs@mSiO2 showed polarization-sensitive, periodic DIC images and intensities at their LSPR wavelengths with high contrast and sensitivity. We for the first time described a real-time tracking of the rotational motions of AuNRs@mSiO2 on live cell membranes under DIC microscopy to verify the capability of single AuNRs@mSiO2 as multifunctional orientation probes. The results presented here support the potential use of single AuNRs@mSiO2 for the development of multifunctional orientation probes owing to the combined effect of the Au core, which can serve as an orientation probe and a local heat generator for phototherapy, and mesoporous silica shell, which can be used as efficient drug carriers due to its excellent loading capacity in many biological applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by two National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIP) (No 2018R1C1B3001154 and No 2019R1A6A1A11053838).Notes and references
- J. W. Ha, W. Sun, A. S. Stender and N. Fang, J. Phys. Chem. C, 2012, 116, 2766–2771 CrossRef CAS.
- Z. Ma, H. Xia, Y. Liu, B. Liu, W. Chen and Y. Zhao, Chin. Sci. Bull., 2013, 58, 2530–2536 CrossRef CAS.
- L. Xiao, J. W. Ha, L. Wei, G. Wang and N. Fang, Angew. Chem., Int. Ed., 2012, 51, 7734–7738 CrossRef CAS PubMed.
- R. A. Sperling, P. R. Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896–1908 RSC.
- M. R. K. Ali, Y. Wu and M. A. El-Sayed, J. Phys. Chem. C, 2019, 123, 15375–15393 CrossRef CAS.
- K. Turcheniuk, T. Dumych, R. Bilyy, V. Turcheniuk, J. Bouckaert, V. Vovk, V. Chopyak, V. Zaitsev, P. Mariot, N. Prevarskaya, R. Boukherroub and S. Szunerits, RSC Adv., 2016, 6, 1600–1610 RSC.
- X. Kang, X. Guo, X. Niu, W. An, S. Li, Z. Liu, Y. Yang, N. Wang, Q. Jiang, C. Yan, H. Wang and Q. Zhang, Sci. Rep., 2017, 7, 42069 CrossRef CAS.
- M. Zan, J. Li, M. Huang, S. Lin, D. Luo, S. Luo and Z. Ge, Biomater. Sci., 2015, 3, 1147–1156 RSC.
- X. Wu, J. Liu, L. Yang and F. Wang, Colloids Surf., B, 2019, 175, 239–247 CrossRef CAS.
- J. Wan, J.-H. Wang, T. Liu, Z. Xie, X.-F. Yu and W. Li, Sci. Rep., 2015, 5, 11398 CrossRef CAS.
- C. Li, K. Feng, N. Xie, W. Zhao, L. Ye, B. Chen, C.-H. Tung and L.-Z. Wu, ACS Appl. Nano Mater., 2020, 3, 5070–5078 CrossRef CAS.
- N. T. Ha Lien, A. D. Phan, B. T. Van Khanh, N. T. Thuy, N. Trong Nghia, H. T. My Nhung, T. Hong Nhung, D. Quang Hoa, V. Duong and N. Minh Hue, ACS Omega, 2020, 5, 20231–20237 CrossRef CAS PubMed.
- G. Liu, H. Liang, Y. He, L. Lu, L. Wang, P. Liu and K. Cai, J. Mater. Chem. B, 2020, 8, 9686–9696 RSC.
- J. Liu, C. Detrembleur, M.-C. De Pauw-Gillet, S. Mornet, C. Jérôme and E. Duguet, Small, 2015, 11, 2323–2332 CrossRef CAS PubMed.
- A. S. Monem, N. Elbialy and N. Mohamed, Int. J. Pharm., 2014, 470, 1–7 CrossRef CAS PubMed.
- X. Cui, W. Cheng and X. Han, J. Mater. Chem. B, 2018, 6, 8078–8084 RSC.
- C. Sönnichsen and A. P. Alivisatos, Nano Lett., 2004, 5, 301–304 CrossRef PubMed.
- W.-S. Chang, J. W. Ha, L. S. Slaughter and S. Link, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 2781–2786 CrossRef CAS.
- J. W. Ha, W. Sun, G. Wang and N. Fang, Chem. Commun., 2011, 47, 7743–7745 RSC.
- J. W. Ha, K. Marchuk and N. Fang, Nano Lett., 2012, 12, 4282–4288 CrossRef CAS.
- K. Marchuk and N. Fang, Nano Lett., 2013, 13, 5414–5419 CrossRef CAS.
- K. Marchuk, J. W. Ha and N. Fang, Nano Lett., 2013, 13, 1245–1250 CrossRef CAS PubMed.
- Y. Gu, J. W. Ha, A. E. Augspurger, K. Chen, S. Zhu and N. Fang, Nanoscale, 2013, 5, 10753–10764 RSC.
- Y. Gu, W. Sun, G. Wang and N. Fang, J. Am. Chem. Soc., 2011, 133, 5720–5723 CrossRef CAS.
- G. Wang, W. Sun, Y. Luo and N. Fang, J. Am. Chem. Soc., 2010, 132, 16417–16422 CrossRef CAS PubMed.
- Z. Song, Y. Liu, J. Shi, T. Ma, Z. Zhang, H. Ma and S. Cao, Mater. Sci. Eng. C, 2018, 83, 90–98 CrossRef CAS PubMed.
- G. W. Kim, S. Y. Lee and J. W. Ha, Analyst, 2017, 142, 899–903 RSC.
- S. Y. Lee and J. W. Ha, Phys. Chem. Chem. Phys., 2016, 18, 32682–32685 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06572f |
| This journal is © The Royal Society of Chemistry 2021 |