Open Access Article
Open Access ArticleFacile synthesis and nematicidal activity evaluation of thiophosphinyl amide [(Pz)2P(S)NHR] and thiophosphonyl diamide [(Pz)P(S)(NHR)2] (Pz = 1,3,5-trimethylpyrazole, R = biphenyl derivatives)†
Xiao Chen ,
Xiaogang Lu
,
Xiaogang Lu ,
Haibo Liu
,
Haibo Liu ,
Hongmei Wang
,
Hongmei Wang * and
Chengxin Pei*
* and
Chengxin Pei*
State Key Laboratory of NBC Protection for Civilian, Beijing 102205, China. E-mail: wanghongmei@dicp.ac.cn; chengxin.ricd@yahoo.com.cn
First published on 10th November 2021
Abstract
A series of thiophosphinyl amide [(Pz)2P(S)NHR] and thiophosphonyl diamide [PzP(S)(NHR)2] compounds, where Pz = 1,3,5-trimethylpyrazole and N(H)R = derivatives of 2-aminobiphenyl, were synthesized via a facile two-step process. Reaction of pyrazolyl substituted bromophosphine with 2-aminobiphenyl derivatives and further reaction with elemental sulphur affords the corresponding thiophosphinyl amide and thiophosphonyl diamide. The intermediate species was used without prior purification for reaction with sulphur to yield the target compounds. The nematicidal activity evaluation suggests that some compounds could manifest moderate nematicidal activity towards Meloidogyne incogita, which is higher than that of their amide analogue bixafen.
Introduction
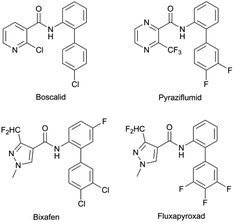
The biological activities of organophosphorus compounds are well documented and their action mode is in part related to the presence of the phosphate group in nucleotides and cell membranes.1–4 In the medical field, the phosphate containing drug remdesivir has been developed,5,6 while in the agricultural industry this approach has also garnered success, as exemplified by the pesticide Imicyafos.7–9 It should be noted that compared with the highly investigated small molecule organophosphorus compounds (like phosphate esters),4,10–13 there is comparatively little research on the synthesis and bio-activity of their pyrazole derivatives,14–20 an area of research that is particularly relevant since the bio-activity of such compounds is well documented.21–24 Indeed, some of the phosphonyl pyrazole derivatives have been shown to possess biological activity, like antimicrobial, anticancer and antioxidant activities.25–29Our primary interest in the bioactivity of organophosphorus prompted us to investigate agricultural pesticides, in particular those that act as a succinate dehydrogenase inhibitor (SDHI). To date, 23 SDHI compounds have been listed by the Fungicide Resistance Action Committee (FRAC) (selected SDHIs are listed in Fig. 1). Previous investigation suggests that fluopyram, one of these SDHI, could inhibit the growth of Meloidogyne incognita, with a 2 h EC50 value of 5.18 mg mL−1.30 This nematicide activity was higher than those of the other tested SDHIs, like boscalid, flutolanil, penthiopyrad and benzovindiflupyr.30 In this fertile area, a number of novel SDHI-based compounds and their nematicidal activity are currently under investigation, some of which will be released into the market soon, such as the new nematicide named cyclobutrifluram, developed by Syngenta.31 The promising nematicidal activity of fluopyram and cyclobutrifluram suggests that further research of SDHI-based compounds as effective nematicidal agents is warranted.32,33 Considering the potential bio-activity of organophosphorus compounds, we believe that a successful approach could involve the synergistic combination of the basic molecular scaffold of existing SDHIs with the biologically active organophosphorus moiety.
Herein, we report the facile synthesis of 16 novel thiophosphinyl amides and thiophosphonyl diamides, together with data on their nematicidal activity towards Meloidogyne incognita.
Results and discussion
Target compounds were designed by combination of specific structural features of certain SDHIs and the biologically active organophosphorus moiety, which should act synergistically to promote their biological activity. Among the 23 SDHIs listed by FRAC, 12 contain a 1-methylpyrazole group, which includes isoflucypram, penflufen, pydiflumetofen, fluxapyroxad, and sedaxane, underscoring the importance of pyrazole groups for biological activity. Furthermore, the SDHIs of acaricide pyflubumide similarly possess a 1,3,5-trimethylpyrazole group.34,35Based on these precedents, we decided to incorporate the 1,3,5-trimethylpyrazole moiety in our novel thiophosphine based nematicides. A C-5 methyl group should prevent migration of a phosphine group between C-2 and C-3, a process that has been widely observed for other pyrazole derivatives.36,37 In addition, some analogues of 2-aminobiphenyl have been employed as pesticidal agents.
The 1,3,5-trimethylpyrazole substituted bromophosphine (1 and 2) were prepared following a previously published protocol.15 Due to the π-rich character of a pyrazol ring, PBr3 can directly react with 1,3,5-trimethylpyrazole in the presence of pyridine, which acts as both a solvent and an acid acceptor, leading to the corresponding phosphorylation products 1 and 2. After a standard reaction of 1 (or 2) with the corresponding analogues of 2-aminobiphenyl, in the presence of triethylamine, the resulting crude phosphinylamine (phosphonyldiamine) directly reacted with stoichiometric amount of S8, giving the desired compounds 1a–1h (or 2a–2h) (Fig. 2). Tedious purification procedures such as column chromatography could be avoided, and crystallization proceeded smoothly after a simple workup procedure. The crude products 1a–1h were directly isolated after the removal of a violet filtrate, followed by washing with petroleum ether. Spectroscopically pure compounds were obtained after crystallization from ethyl acetate/petroleum ether (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), affording products in moderate to high yields. To obtain the purified products 2a–2h, petroleum ether was added directly to a partially concentrated crude mixture, giving two separate layers, with the interface acting as a vehicle for slow crystallization of the products obtained in moderate yields.
1), affording products in moderate to high yields. To obtain the purified products 2a–2h, petroleum ether was added directly to a partially concentrated crude mixture, giving two separate layers, with the interface acting as a vehicle for slow crystallization of the products obtained in moderate yields.
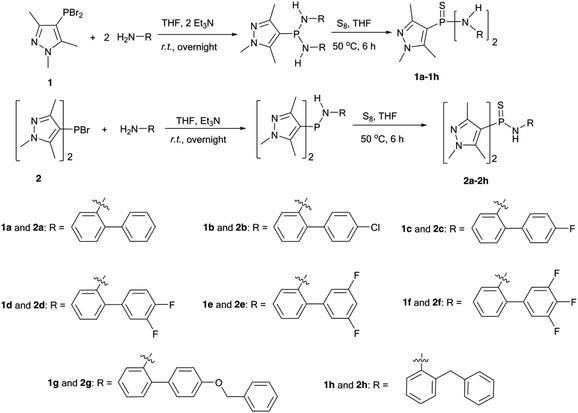 | ||
| Fig. 2 Synthesis and molecular structures of thiophosphinyl amide (1a–1h) and thiophosphonyl diamide (2a–2h). | ||
In the 1H NMR spectra, the –NH signals at around 5 ppm were observed as doublets in all compounds, which indicated the existence of 2JPH coupling and the formation of P–N bonds. IR spectroscopy analysis confirmed the presence of a secondary amine with a single sharp N–H stretch absorption band v(NH) observed at around 3380 cm−1. Taken together, the NMR and IR data both confirmed the presence of a secondary amide where the nitrogen was bound to a phosphorus atom. Meanwhile, the single doublet indicated the presence of symmetric –NH groups in 1a–1h in solution on the NMR timescale. Meanwhile, the P–C coupling phenomenon can be detected as well in the 13C NMR spectra and the coupling constants are highly consistent with that of reported bis(diethylamido)-1,3,5-trimethylpyrazol-4-ylthiophosphonate.15 In the 31P NMR spectra, a singlet was observed at around 41 ppm for compounds 1a–1h (Table 1).
| 1H NMR (ppm) | 13C NMR (ppm) (JP–C, Hz) | 31P NMR (ppm) | IR (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-CH3 | 3-CH3 | 5-CH3 | –NH (2JPH, Hz) | 1-CH3 | 3-CH3 | 5-CH3 | C-3 | C-4 | C-5 | –NH | ||
| 1a | 3.63 | 2.18 | 1.94 | 5.42, 5.39 (8.3) | 36.36 | 13.78 | 11.27 | 144.08, 143.91 (25.5) | 109.56, 108.54 (153.0) | 148.73, 148.64 (13.5) | 40.40 | 3381 |
| 1b | 3.66 | 2.26 | 2.00 | 5.28, 5.25 (7.1) | 36.44 | 13.94 | 11.44 | 144.32, 144.14 (27.0) | 109.35, 108.32 (154.5) | 148.76, 148.68 (12.0) | 41.26 | 3385 |
| 1c | 3.66 | 2.31 | 1.94 | 5.67, 5.65 (8.2) | 36.43 | 13.93 | 11.41 | 144.17, 144.00 (25.5) | 109.47, 108.44 (154.5) | 148.71, 148.63 (12.0) | 42.20 | 3386 |
| 1d | 3.68 | 2.31 | 2.05 | 5.23, 5.21 (6.5) | 36.44 | 13.96 | 11.50 | 144.50, 144.33 (25.5) | 109.45, 108.42 (154.5) | 148.70, 148.61 (13.5) | 41.48 | 3388 |
| 1e | 3.67 | 2.34 | 2.04 | 5.25, 5.23 (6.7) | 36.44 | 14.00 | 11.51 | 144.53, 144.36 (25.5) | 109.16, 108.13 (154.5) | 148.78, 148.70 (12.0) | 41.90 | 3382 |
| 1f | 3.63 | 2.32 | 2.09 | 5.49, 5.47 (6.7) | 36.48 | 14.13 | 11.59 | 144.61, 144.44 (25.5) | 109.10, 108.07 (154.5) | 148.80, 148.71 (13.5) | 42.20 | 3387 |
| 1g | 3.64 | 2.22 | 2.00 | 5.45, 5.42 (8.9) | 36.41 | 13.89 | 11.36 | 144.09, 143.92 (25.5) | 109.65, 108.62 (154.5) | 148.76, 148.68 (12.0) | 40.53 | 3373 |
| 1h | 3.65 | 2.10 | 1.84 | 4.89, 4.86 (8.0) | 36.31 | 13.75 | 11.26 | 144.60, 144.43 (25.5) | 109.58, 108.56 (153.0) | 149.27, 149.18 (13.5) | 40.36 | 3385 |
| 2a | 3.70 | 2.29 | 1.97 | 5.26, 5.24 (7.7) | 36.36 | 14.00 | 11.44 | 144.65, 144.49 (24.0) | 108.79, 107.92 (130.5) | 149.36, 149.28 (12.0) | 29.61 | 3400 |
| 2b | 3.68 | 2.28 | 1.95 | 5.09, 5.07 (6.6) | 36.41 | 14.06 | 11.52 | 144.70, 144.54 (24.0) | 108.68, 107.82 (129.0) | 149.34, 149.26 (12.0) | 29.97 | 3402 |
| 2c | 3.68 | 2.28 | 1.95 | 5.09, 5.07 (5.3) | 36.40 | 14.04 | 11.51 | 144.69, 144.53 (24.0) | 108.72, 107.85 (130.5) | 149.31, 149.24 (12.0) | 29.86 | 3402 |
| 2d | 3.69 | 2.32 | 1.96 | 5.03, 5.01 (6.9) | 36.41 | 14.06 | 11.56 | 144.75, 144.59 (24.0) | 108.65, 107.79 (129.0) | 149.30, 149.22 (12.0) | 30.25 | 3395 |
| 2e | 3.70 | 2.31 | 1.96 | 5.02, 5.00 (7.3) | 36.39 | 14.02 | 11.56 | 144.78, 144.62 (24.0) | 108.64, 107.78 (129.0) | 149.31, 149.23 (12.0) | 30.31 | 3400 |
| 2f | 3.69 | 2.35 | 1.96 | 4.93, 4.91 (7.2) | 36.43 | 14.06 | 11.62 | 144.84, 144.68 (24.0) | 108.61, 107.75 (129.0) | 149.30, 149.23 (10.5) | 30.71 | 3401 |
| 2g | 3.67 | 2.28 | 1.95 | 5.24, 5.22 (6.8) | 36.38 | 14.04 | 11.49 | 144.65, 144.49 (24.0) | 108.79, 107.92 (130.5) | 149.36, 149.28 (12.0) | 29.61 | 3402 |
| 2h | 3.69 | 2.20 | 1.84 | 4.77, 4.75 (7.3) | 36.34 | 13.90 | 11.46 | 144.67, 144.51 (24.0) | 108.89, 108.03 (129.0) | 149.46, 149.39 (10.5) | 29.09 | 3402 |
The NMR spectra of 2a–2h mirrored those of 1a–1h. For example, the 1H NMR spectra showed that the –NH signals were also observed as doublets at similar ppm values. In addition, the methyl groups were observed as singlets indicating the presence of highly symmetric pyrazole groups in 2a–2h in solution on the NMR timescale. Compared with 1a–1h, the single sharp –NH stretch absorption bands v(NH) of 2a–2h were shifted to approximately 3400 cm−1, a slightly higher wavenumber. The coupling constants in 2a–2h are identify with that of in 1a–1h, except for a slightly lower constants for P–C4, which was influenced by the variation of substitutes on the P atom. The singlets in the 31P NMR spectra were shifted downfield to approximately 30 ppm in 2a–2h (Table 1).
The in vitro nematicidal activity was evaluated according to the classic protocol reported by Faske and Hurd.30 After exploring a concentration of 40 mg L−1 for 24 h, no obvious inhibitory activity could be detected for the lead compound bixafen towards M. incognita. In contrast, moderate nematicidal activities were observed for compounds 1a–1d, 2a, 2b and 2e, and particularly for 1c, which had the highest inhibitory rate at 62.1%. Moreover, after treatment with 1c, the activity of the surviving nematodes was highly inhibited. We thus demonstrated that modifying the basic structure scaffold present in the SDHI bixafen by incorporating an organophosphorus moiety was a successful approach for screening compounds with encouraging nematicidal activity. Motivated by the above encouraging results, studies involving further modification of phosphorylated pyrazole derivatives and the evaluation of their nematicidal activity are currently underway.
Experimental
Materials and methods
All manipulations of air-sensitive materials were performed under rigorous exclusion of oxygen and moisture in Schlenk-type glassware or on a dual manifold Schlenk line, interfaced with a high vacuum line. Tetrahydrofuran, toluene and triethylamine were predried using an MBraun solvent purification system (SPS-800) and then they were degassed, dried and stored over 4 Å molecular sieves. Starting materials 1 and 2 were synthesized following the previously published procedure.15 The other chemicals were purchased from commercial source and used without further purification. The 1H, and 31P NMR spectra were recorded on a Bruker Avance II 300 MHz NMR spectrometer. 13C spectra were obtained from a Bruker Avance II 600 MHz NMR spectrometer. Chemical shifts for 1H and 13C spectra are reported as parts per million (ppm) relative to tetramethylsilane and referenced to the residual 1H or 13C resonances of the deuterated solvents. The 31P NMR data was referenced to H3PO4. IR spectra, from 4000 to 400 cm−1, were obtained using a Nicolet IS10 FT-IR spectrometer equipped with a room temperature DLaTGS detector and a diamond ATR unit. Mass spectra (ESI) were recorded on a Bruker ultraflextreme MALDI-TOF instrument in positive ionization mode.Synthesis of compounds 1a–1h
The corresponding primary amine (18.0 mmol) and Et3N (1.822 g, 18.0 mmol) was dissolved in 100 mL dry THF. Then, a THF solution of 1 (2.699 g, 9.0 mmol, in 20 mL dry THF) was added to the mixture previously cooled to 0 °C. After addition, the solution was slowly warmed to room temperature and was stirred overnight. Filtration was used to remove the formed white solid Et3N·HBr. Then, S8 (0.288 g, 9.0 mmol) was added to the mixture and the mixture was further stirred for 6.0 h at 50 °C. After filtration, the violet filtrate was removed under vacuum and the residue was washed with 30 mL of petroleum ether, giving the crude product. The target compound could be further purified by crystallization using ethyl acetate/petroleum ether(v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| Compound | Inhibition (%) | Compound | Inhibition (%) |
|---|---|---|---|
| 1a | 34.2 ± 1.2 | 2a | 26.7 ± 1.0 |
| 1b | 23.7 ± 1.6 | 2b | 31.5 ± 1.8 |
| 1c | 62.1 ± 0.8 | 2c | — |
| 1d | 24.8 ± 1.7 | 2d | — |
| 1e | — | 2e | 37.3 ± 2.2 |
| 1f | — | 2f | — |
| 1g | — | 2g | — |
| 1h | — | 2h | — |
| Bixafen | — |
Conclusions
A series of thiophosphinyl amide and thiophosphonyl diamide compounds were synthesized and purified by facile purification methods and characterized by standard spectroscopic/analytic techniques. Our preliminary nematicidal activity investigation shows that some compounds manifested higher nematicidal activity than the lead compound bixafen. Hence, the modification of SDHI by the incorporation of an organothiophosphorus group could be a successful approach for the synthesis of nematicidal compounds with improved activities.Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
We acknowledge China Agricultural University for the nematicidal activity determination of our compounds.References
- S. Demkowicz, J. Rachon, M. Daśko and W. Kozak, RSC Adv., 2016, 6, 7101–7112 RSC.
- H. Yu, H. Yang, E. Shi and W. Tang, Med. Drug Discovery, 2020, 8, 100063 CrossRef CAS PubMed.
- J. B. Rodriguez and C. Gallo-Rodriguez, ChemMedChem, 2019, 14, 190–216 CAS.
- C. Schultz, Bioorg. Med. Chem., 2003, 11, 885–898 CrossRef CAS PubMed.
- J. Grein, N. Ohmagari, D. Shin, G. Diaz, E. Asperges, A. Castagna, T. Feldt, G. Green, M. L. Green, F.-X. Lescure, E. Nicastri, R. Oda, K. Yo, E. Quiros-Roldan, A. Studemeister, J. Redinski, S. Ahmed, J. Bernett, D. Chelliah, D. Chen, S. Chihara, S. H. Cohen, J. Cunningham, A. D'Arminio Monforte, S. Ismail, H. Kato, G. Lapadula, E. L'Her, T. Maeno, S. Majumder, M. Massari, M. Mora-Rillo, Y. Mutoh, D. Nguyen, E. Verweij, A. Zoufaly, A. O. Osinusi, A. DeZure, Y. Zhao, L. Zhong, A. Chokkalingam, E. Elboudwarej, L. Telep, L. Timbs, I. Henne, S. Sellers, H. Cao, S. K. Tan, L. Winterbourne, P. Desai, R. Mera, A. Gaggar, R. P. Myers, D. M. Brainard, R. Childs and T. Flanigan, N. Engl. J. Med., 2020, 382, 2327–2336 CrossRef CAS PubMed.
- M. Wang, R. Cao, L. Zhang, X. Yang, J. Liu, M. Xu, Z. Shi, Z. Hu, W. Zhong and G. Xiao, Cell Res., 2020, 30, 269–271 CrossRef CAS PubMed.
- L. G. Costa, Toxicol. Sci., 2017, 162, 24–35 CrossRef PubMed.
- S. Wada, K. Toyota and A. Takada, J. Nematol., 2011, 43, 1–6 Search PubMed.
- S. Wada and K. Toyota, Microbes Environ., 2008, 23, 331–336 CrossRef PubMed.
- G. Georgiadis, C. Mavridis, C. Belantis, I. E. Zisis, I. Skamagkas, I. Fragkiadoulaki, I. Heretis, V. Tzortzis, K. Psathakis, A. Tsatsakis and C. Mamoulakis, Toxicology, 2018, 406–407, 129–136 CrossRef CAS PubMed.
- M. Eddleston, N. A. Buckley, P. Eyer and A. H. Dawson, Lancet, 2008, 371, 597–607 CrossRef CAS.
- M. Jokanovic, Toxicology, 2001, 166, 139–160 CrossRef CAS PubMed.
- F. Worek, H. Thiermann and T. Wille, Arch. Toxicol., 2020, 94, 2275–2292 CrossRef CAS PubMed.
- A. Ben Akacha, N. Ayed, B. Baccar and C. Charrier, Phosphorus, Sulfur Silicon Relat. Elem., 1988, 40, 63–68 CrossRef CAS.
- A. A. Tolmachev, A. I. Sviridon, A. N. Kostyuk and A. M. Pinchuk, Heteroat. Chem., 1995, 6, 449–459 CrossRef CAS.
- A. A. Tolmachev, S. P. Ivonin and A. M. Pinchuk, Heteroat. Chem., 1995, 6, 407–412 CrossRef CAS.
- N. S. Goulioukina, N. N. Makukhin, E. D. Shinkarev, Y. K. Grishin, V. A. Roznyatovsky and I. P. Beletskaya, Org. Biomol. Chem., 2016, 14, 10000–10010 RSC.
- S. Fischer, J. Hoyano and L. K. Peterson, Can. J. Chem., 1976, 54, 2710–2714 CrossRef CAS.
- J. Zhang, M. Wenzel, K. Schnaars, F. Hennersdorf, K. Schwedtmann, J. Maerz, A. Rossberg, P. Kaden, F. Kraus, T. Stumpf and J. J. Weigand, Dalton Trans., 2021, 50, 3550–3558 RSC.
- M. Eddleston, Annu. Rev. Pharmacol. Toxicol., 2019, 59, 341–360 CrossRef CAS PubMed.
- S. G. Kucukguzel and S. Senkardes, Eur. J. Med. Chem., 2015, 97, 786–815 CrossRef CAS PubMed.
- J. Elguero, P. Goya, N. Jagerovic and A. M. S. Silva, Targets Heterocycl. Syst., 2002, 6, 52–98 CAS.
- A. Ansari, A. Ali, M. Asif and Shamsuzzaman, New J. Chem., 2017, 41, 16–41 RSC.
- M. F. Khan, M. M. Alam, G. Verma, W. Akhtar, M. Akhter and M. Shaquiquzzaman, Eur. J. Med. Chem., 2016, 120, 170–201 CrossRef CAS PubMed.
- N. Sudhapriya, C. Balachandran, S. Awale and P. T. Perumal, New J. Chem., 2017, 41, 5582–5594 RSC.
- J.-M. Kee, R. C. Oslund, A. D. Couvillon and T. W. Muir, Org. Lett., 2015, 17, 187–189 CrossRef CAS PubMed.
- S. A.-G. Abdel-Aziz, T. El-Sayed Ali, K. M. El-Mahdy and S. M. Abdel-Karim, Eur. J. Chem., 2011, 2, 25–35 CrossRef CAS.
- S. K. Nayab Rasool, C. Subramanyam, D. B. Janakiramudu, P. Supraja, R. Usha and C. N. Raju, Phosphorus, Sulfur Silicon Relat. Elem., 2018, 193, 470–474 CrossRef CAS.
- S. K. Thaslim Basha, D. Subba Rao, G. Madhava, S. T. Basha, M. N. Devamma, M. S. Saddala, U. R. Asupatri and C. N. Raju, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1064–1074 CrossRef CAS.
- T. R. Faske and K. Hurd, J. Nematol., 2015, 47, 316–321 CAS.
- N. Umetsu and Y. Shirai, J. Pestic. Sci., 2020, 45, 54–74 CrossRef CAS PubMed.
- G. Li, K. Zhang, J. Xu, J. Dong and Y. Liu, Recent Pat. Biotechnol., 2007, 1, 212–233 CrossRef CAS PubMed.
- J. Chen, Q. X. Li and B. Song, J. Agric. Food Chem., 2020, 68, 12175–12188 CrossRef CAS PubMed.
- N. Sugimoto, A. Takahashi, R. Ihara, Y. Itoh, A. Jouraku, T. Van Leeuwen and M. Osakabe, Insect Biochem. Mol. Biol., 2020, 123, 103410 CrossRef CAS PubMed.
- T. Furuya, K. Machiya, S. Fujioka, M. Nakano and K. Inagaki, J. Pestic. Sci., 2017, 42, 132–136 CrossRef CAS PubMed.
- S. P. Ivonin, T. E. Terikovska, A. A. Chaikovskaya, A. P. Marchenko, G. N. Koydan, A. M. Pinchuk and A. A. Tolmachev, Heteroat. Chem., 1999, 10, 213–221 CrossRef CAS.
- A. A. Chaikovskaya, Y. V. Dmitriv, S. P. Ivonin, A. M. Pinchuk and A. A. Tolmachev, Heteroat. Chem., 2005, 16, 599–604 CrossRef CAS.
- X. Ji, J. Li, B. Dong, H. Zhang, S. Zhang and K. Qiao, Crop Protect., 2019, 122, 84–89 CrossRef CAS.
- R. S. Hussey and K. R. Barker, Plant Dis. Rep., 1973, 57, 1025–1028 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C and 31P NMR spectra, IR spectra and MS spectra. See DOI: 10.1039/d1ra06232h |
| This journal is © The Royal Society of Chemistry 2021 |