Open Access Article
Open Access ArticleSynthesis of 9,9-bis(4-hydroxyphenyl) fluorene catalyzed by bifunctional ionic liquids†
Jialun Wei b,
Limei Yu
b,
Limei Yu *ab,
Lei Yanb,
Wei Bai
*ab,
Lei Yanb,
Wei Bai b,
Xinxin Lub and
Zhanxian Gaoab
b,
Xinxin Lub and
Zhanxian Gaoab
aState Key Lab of Fine Chemicals, Dalian University of Technology, Dalian, Liaoning 116024, China. E-mail: ochem@dlut.edu.cn; Tel: +86 13942698335
bSchool of Chemical Engineering, Dalian University of Technology, Dalian, Liaoning 116024, China
First published on 4th October 2021
Abstract
Through structural design, a series of bifunctional ionic liquids (BFILs) containing sulfonic acid (–SO3H) and sulfhydryl groups (–SH) were synthesized and characterized by NMR and MS. The acidity of these BFILs was measured by the Hammett acidity (H0) and the effective sulfhydryl molar content of BFILs was determined by Ellman's method. Moreover, BFIL's catalytic properties in the condensation reaction of 9-fluorenone and phenol were studied. BFIL catalyst 6c can achieve nearly 100% conversion of 9-fluorenone with a high selectivity of 9,9-bis(4-hydroxyphenyl) fluorene (95.2%).
Introduction
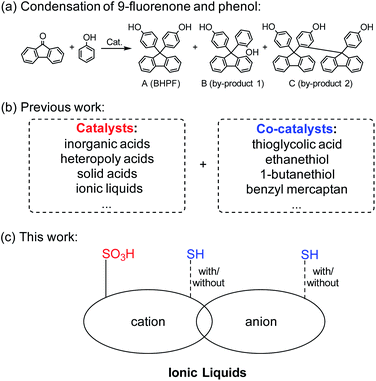
Bisphenol compounds1 are produced from the condensation reaction of aldehydes (or ketones) and phenol. 9,9-Bis(4-hydroxyphenyl) fluorene (BHPF) is a compound with a cardo-ring structure2 and it is an important raw material to produce epoxy resin,3,4 polycarbonate,5 acrylic resin6 and other materials7 with high thermal stability and good optical properties.8,9 BHPF can be obtained through the condensation reaction of 9-fluorenone and phenol under acidic catalysis (Scheme 1a). The reported catalysts include inorganic acids,10 organic acids,11 heteropoly acids,12 solid acids13 and acidic ionic liquids (ILs).14 To increase the reaction rate, it is necessary to add co-catalysts, such as thiol, thioglycolic acid15 etc. (Scheme 1b). The traditional catalysts and co-catalysts are difficult to recycle, and some of them like thiol compounds smell bad.ILs are considered as a new type of green solvent and catalyst, and have been widely used in separation,16 electrochemistry,17 and organic synthesis.18,19 Functionalized ILs20 take advantage of structure design, and can introduce specific functional groups into the cation and/or anion structure of the ILs for specific organic reactions. At present, many functionalized ILs are documented in the literature, and the functionalized groups include sulfonic acid groups,21,22 hydroxyl groups,23 alkenyl groups24 and amino groups25 etc.
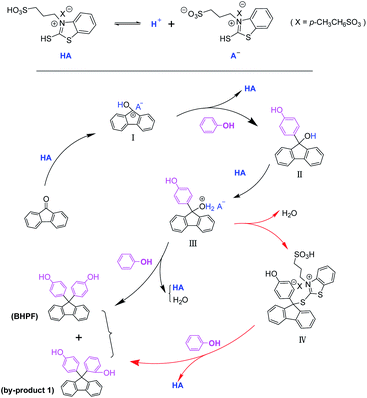
In this work, in view of the need for acid-thiol synergistic catalysis in the synthesis of BHPF, we introduced sulfonic acid groups (–SO3H) into the cationic structure of the ILs, and the sulfhydryl groups (–SH) were introduced into the cationic and/or anionic structure of ILs (Scheme 1c). The –SO3H and –SH bifunctional ILs were characterized by NMR and MS. Compared with the composite catalyst (Scheme 1b), this work designs bifunctional IL as a mono-catalyst for the condensation reaction between fluorenone and phenol, which could let us investigate thoroughly about the catalytic mechanism of –SO3H and –SH in the reaction.
Experimental
Materials
1-Methylimidazole (99%), 2-mercapto-1-methylimidazole (98%), benzothiazole (96%), 2-mercaptopyridine (98%), 2-mercaptobenzothiazole (98%), 2-mercapto-5-methyl-1,3,4-thiadiazole (98%), 2,5-dimercapto-1,3,4-thiadiazole (97%), 1,3-propanesulfonate (99%), trifluoromethanesulfonic acid (98%), sulfuric acid (98%), methanol (99.7%), ethyl acetate (99.7%), phosphoric acid (85%), pyridine (99.5%), phenol (99%), p-toluenesulfonic acid monohydrate (99%) were commercially available. 9-Fluorenone26 and 3-mercaptopropane sulfonate27 were self-made.Preparation of ionic liquids
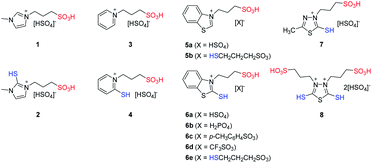
To find the relevance between the structure and catalytic effect of ILs in the synthetic reaction of BHPF, a series of IL catalysts were synthesized, the details on ILs' structure are shown in Fig. 1.A two-step method was employed to prepare the –SO3H and –SH bifunctional ILs. As shown in Scheme 2, taking IL 6c as an example, the synthetic procedure was as follows: firstly, 17.1 g 2-mercaptobenzothiazole (0.1 mol) and 100 ml ethyl acetate was added into a 250 ml round bottom flask, the solution was heated to 90 °C. Then 12.2 g 1,3-propanesulfonate was added slowly with vigorous stirring at 90 °C for 12 h. The final product was filtrated after cooled down, washed with 50 ml ethyl acetate for three times and dried at 50 °C under vacuum for 12 h to obtain zwitterion (Z). And the yields of zwitterions are over 80%. Secondly, stoichiometric amount of p-toluenesulfonic acid monohydrate was added slowly into the zwitterion aqueous solution. The mixture was stirred at 90 °C for 10 h, distilled by rotary distillation to remove water and dried under vacuum to form a yellow viscous ionic liquid (6c). The yield of IL 6c was nearly 98%. The other ILs, separately belong to different cationic series, such as 1-methylimidazole, 2-mercapto-1-methylimidazole, pyridine, 2-mercaptopyridine, benzothiazole, 2-mercaptobenzothiazole, 2-mercapto-5-methyl-1,3,4-thiadiazole and 2,5-dimercapto-1,3,4-thiadiazole, were synthesized by a similar procedure. And the total yield of all ILs is over 50%. Yields of zwitterions and ILs are shown in the ESI Part 1.†
Characterization of ionic liquids
1H and 13C NMR spectra were recorded in D2O using a Bruker Avance II 400 MHz NMR spectrometer. FT-IR spectra were recorded on a Nicolet 460 spectrometer (Nicolet, USA) in the region of 4000–400 cm−1. UV-vis spectra were accomplished on an UV (HP-8453) spectrophotometer at room temperature. MS was carried out on a Q-TOF Micro (Micromass UK Limited). The acidity of ILs (H0) was determined by the Hammett function method.28,29 The effective sulfhydryl –SH molar contents of ILs was analogically determined by the Ellman's method.30,31Results and discussion
Acidity analysis of ILs
Hammett acidity (H0) is widely used to evaluate the acidity of ILs. We measured the acidity of ILs by UV-Vis spectroscopy combined with Hammett function, and 4-nitroaniline was used as an indicator. The calculated H0 values of the ILs with different cationic and anionic structures were shown in Table 1.| Compoundsa | Amaxb | [B]b/% | [BH+]/% | H0c |
|---|---|---|---|---|
| a 20 mmol L−1 compounds and 0.16 mmol L−1 4-nitroaniline in water solution.b Maximum absorption wavelength of 4-nitroaniline(B), 382 nm.c H0 = pK(B)aq + log([B]/[BH+]), (pK(B)aq = 0.99). | ||||
| Bank | 2.56 | 100 | 0 | — |
| H2SO4 | 1.91 | 74.61 | 25.39 | 1.46 |
| 1 | 1.92 | 75.00 | 25.00 | 1.47 |
| 2 | 1.90 | 74.22 | 25.78 | 1.45 |
| 3 | 1.93 | 75.39 | 24.61 | 1.48 |
| 4 | 1.91 | 74.61 | 25.39 | 1.46 |
| 5a | 1.91 | 74.61 | 25.39 | 1.46 |
| 5b | 2.08 | 81.25 | 18.75 | 1.63 |
| 6a | 1.94 | 75.78 | 24.22 | 1.49 |
| 6b | 2.23 | 87.11 | 12.89 | 1.82 |
| 6c | 1.97 | 76.95 | 23.05 | 1.51 |
| 6d | 1.79 | 69.92 | 30.08 | 1.36 |
| 6e | 2.05 | 80.08 | 19.92 | 1.59 |
| 7 | 1.90 | 74.22 | 25.78 | 1.45 |
| 8 | 1.72 | 67.19 | 32.81 | 1.30 |
Comparing IL 1, 2, 3, 4, 5a, 6a and 7, the cationic structures of the synthesized –SO3H functionalized ILs have little effect on acidity, and the acidity of these ILs are equivalent to that of H2SO4. As to IL 6a, 6b, 6c, 6d and 6e, which have the same cationic and different anionic structures, the order of IL acidity (H0) representing by the IL anionic structure are as follows: CF3SO3− > HSO4− > p-CH3C6H4SO3− > HSCH2CH2CH2SO3− > H2PO4−. That means the acid strength of ILs is the same order as that of the maternal acid. IL 8 contains two –SO3H fragments, so it has the strongest acidity.
Sulfhydryl content analysis of ILs
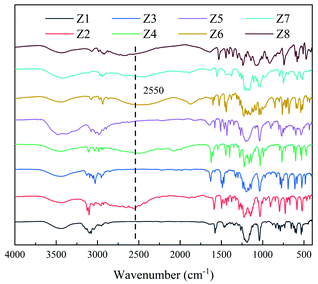
Zwitterions Z1, Z3, and Z5 don't contain sulfhydryl groups, and zwitterions Z2, Z4, Z6, Z7 and Z8 have sulfhydryl groups. Typical structural differences in the synthetic zwitterions were confirmed by infrared spectroscopy (IR), and the results are shown in Fig. 2. Peaks at 2550 cm−1 were ascribed to the –SH group in Z2, Z4, Z6, Z7 and Z8.At present, the methods for the determination of thiol and thiol derivatives include photometric analysis,30–34 electrochemical analysis35,36 and chromatography37,38 etc. The Ellman's method is the representative of the photometric analysis. As a qualitative and quantitative analysis of sulfhydryl compounds, the Ellman's method gives the sulfhydryl molar content of IL in solution thorough matching the UV absorbance at 412 nm that belong to 5-sulfhydryl-2-nitrobenzoic acid, which is the product from the reaction of the sulfhydryl compounds (ILs, thiol, thiol derivatives etc.) with the Ellman's reagent (5,5′-dithiobis (2-nitrobenzoic acid)).
As shown in Table 2, the molar content of sulfhydryl (–SH) for the traditional co-catalyst (thioglycolic acid) and some of the synthetic ILs were given by the Ellman's method. The sulfhydryl molar content of thioglycolic acid was 94.8% and close to the theoretical molar content. The measured molar content of sulfhydryl for IL 2 and IL 4 were zero, while IL 6a-6d, IL 7 and IL 8 contain little sulfhydryl molar content, less than 5.8%, which comes from the heterocyclic ring fragment in ILs. The sulfhydryl molar content of IL 5b and IL 6e were 96.1% and 98.6%, respectively, which are from the anionic structure of ILs contained sulfhydryl groups. It could be found that the sulfhydryl molar content measured by Ellman's reagent was related to the chemical environment where the –SH group was located. When the –SH group embeds in the heterocyclic fragment of ILs, the free and effective content of sulfhydryl groups was small. When it was located in the alkyl chain, the molar content of sulfhydryl groups was close to the theoretical value, so the cationic structure of ILs could influence the free –SH group in solution.31,39
| Compounds | Amaxc | SHd (%) |
|---|---|---|
| a 1 mmol L−1 compounds in potassium phosphate buffer solution (pH = 7.2).b 0.1 mmol HSCH2COOH, IL 5b and IL 6e.c Maximum absorption wavelength of 5-sulfhydryl-2-nitrobenzoic acid, 412 nm.d Sulfhydryl molar content of compounds. | ||
| HSCH2COOHb | 1.200 | 94.8 |
| 2 | 0 | 0 |
| 4 | 0 | 0 |
| 5bb | 1.216 | 96.1 |
| 6a | 0.734 | 5.8 |
| 6b | 0.674 | 5.3 |
| 6c | 0.698 | 5.5 |
| 6d | 0.722 | 5.7 |
| 6eb | 1.248 | 98.6 |
| 7 | 0.004 | 0.03 |
| 8 | 0.123 | 0.97 |
Optimizing reaction parameters on the condensation of 9-fluorenone and phenol
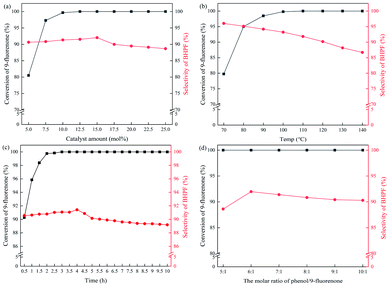
To screen BFIL catalysts and get the relationship among the structure of the BFILs with the conversion of fluorenone and the selectivity of BHPF, the reaction parameter on the condensation of fluorenone with phenol was optimized firstly. The crucial reaction parameters include catalyst amount, reaction temperature, reaction time and the molar ratio of phenol and 9-fluorenone. Using IL 6c ([SO3HC3MBT] [p-CH3C6H4SO3]) as catalyst, a series of univariate optimization experiments were carried out and the results are shown in Fig. 3.Fig. 3a shows that a conversion of 9-fluorenone was 80.5% under a low dosage (5 mol%) of IL 6c refer to the molar content of fluorenone. With the amount of IL 6c increasing from 5 mol% to 15 mol%, the conversion of 9-fluorenone increased from 80.6% to 100%, and the selectivity of BHPF changed from 90.7% to 92.0%. It shows that when the amount of IL 6c is increased, the activity of the condensation reaction is improved. When the amount of catalyst was increased to 25 mol%, the conversion of 9-fluorenone remained unchanged and the selectivity of BHPF changed from 92.0% to 88.7%. It illustrates that when the amount of IL catalyst increases and the reaction rates of the main and side (Scheme 1a, to produce compound B and C) reactions are both accelerated. Thereby the IL catalyst dosage was 15 mol% of fluorenone for further experiments.
As shown in Fig. 3b, reaction temperature was inspected from 70 °C to 140 °C. With 110 °C or higher temperature, 100% conversion of 9-fluorenone can be obtained, while the selectivity of BHPF became worse with increasing temperature. So 110 °C was chosen to be the optimised reaction temperature. In Fig. 3c, we can see that the conversion of 9-fluorenone was greater than 99% after 2 h with IL 6c. To compare the catalytic performance of BFILs with different structures (so as their acidity and electronic properties of –SH), the reaction time was 4 hours. At that point, not only condensation reactions with different ILs could reach the equilibrium state (usually within 0.5–1 h), but also the selectivity of BHPF was good.
In the reaction, phenol acts as both reactant and solvent. If the amount of phenol is small, it will be difficult for the reaction to proceed. Therefore in Fig. 3d, the molar ratio of phenol and 9-fluorenone was carried out from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
To conclude, the suitable condition for the synthesis of bisphenol fluorene catalysed by BFIL is as follows: N (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-fluorenone
9-fluorenone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL) = 6
IL) = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :0.15, reaction time 4 h, T (reaction temperature) = 110 °C.
:0.15, reaction time 4 h, T (reaction temperature) = 110 °C.
Catalytic performance of BFILs in the synthesis of BHPF
Under above conditions, the conversion of 9-fluorene and the selectivity of bisphenol fluorenone using BFILs in Fig. 1. As catalyst were shown in Table 3. It is clear that IL 6c is the best catalyst among the thirteen BFILs for the condensation reaction, the conversion of 9-fluorenone is 100%, and the selectivity of BHPF is 91.8%.| Entry | ILs | Conversion of 9-fluorenone (%) | Selectivity (%) | H0c | SHd (%) | ||
|---|---|---|---|---|---|---|---|
| A (BHPF) | B | C | |||||
| a Reaction conditions:10 mmol 9-fluorenone, 60 mmol phenol, 15 mol% ILs, 110 °C for 4 h.b 60 °C for 8 h.c Hammett acidity (H0) of the ILs.d Sulfhydryl molar content of the ILs. | |||||||
| 1 | 1 | 50.6 | 87.1 | 9.8 | 3.1 | 1.47 | — |
| 2 | 2 | 53.0 | 87.0 | 9.6 | 3.4 | 1.45 | 0 |
| 3 | 3 | 48.2 | 87.4 | 9.2 | 3.4 | 1.48 | — |
| 4 | 4 | 49.1 | 87.2 | 9.3 | 3.5 | 1.46 | 0 |
| 5 | 5a | 52.0 | 87.1 | 9.6 | 3.3 | 1.46 | — |
| 6 | 5b | 100 | 92.8 | 3.8 | 3.4 | 1.63 | 96.1 |
| 7 | 6a | 93.4 | 90.3 | 6.9 | 2.8 | 1.49 | 5.8 |
| 8 | 6b | 79.7 | 94.8 | 4.4 | 0.8 | 1.82 | 5.3 |
| 9 | 6c | 100 | 91.8 | 6.0 | 2.2 | 1.51 | 5.5 |
| 10 | 6d | 100 | 90.0 | 7.3 | 2.7 | 1.36 | 5.7 |
| 11 | 6e | 100 | 91.4 | 5.3 | 3.3 | 1.59 | 98.6 |
| 12 | 7 | 99.5 | 91.8 | 5.8 | 2.4 | 1.45 | 0.03 |
| 13 | 8 | 93.6 | 90.0 | 7.9 | 2.2 | 1.30 | 0.97 |
| 14 | 6ab | 27.5 | 93.1 | 6.2 | 0.7 | 1.49 | 5.8 |
| 15 | 6cb | 100 | 95.2 | 3.0 | 1.8 | 1.51 | 5.5 |
| 16 | 6eb | 98.4 | 95.6 | 2.7 | 1.7 | 1.59 | 98.6 |
Bisphenols are synthesized by the condensation of phenol with a ketone or aldehyde, and catalysed by Brønsted acid catalysts and thiol derivatives co-catalysts. The catalysis in the reaction is intricate, but the rough mechanism process is widely accepted, and the details are as follow: (1) the Brønsted acid catalysts not only protonate the carbonyl of ketone to help monophenol addition, but also help the addition of the second phenol through protonating the hydroxyl of the intermediate produced from the nucleophilic addition of ketone with the first phenol; (2) thiols are typically added as a co-catalyst in the reaction, as they increase the reaction rate and the p, p′-regioselectivity through participating in the addition of the second phenol.40–42 According to the above views, let's sift the data in Table 3.
Firstly, the results of ILs 6a–6d (entry 7–10) which have the same cation (SO3HC3MBT) and different anion reveal that the ILs' anion correlate ILs' catalytic capability in the condensation through their effect on Hammett acidity (H0). When the anions are conjugated bases of inorganic acids, such as HSO4− (6a, entry7) and H2PO4− (6b, entry8), the conversion of 9-fluorenone are 93.4% and 79.7%, respectively. When the anions are conjugated bases of organic acids, such as p-CH3C6H4SO3− (6c, entry9) and CF3SO3− (6d, entry10), 9-fluorenone can be totally converted. IL 6b has lower conversion of 9-fluorenone because of its weak acidity, H0 equals to 1.82, but the selectivity of BHPF is the highest 94.8%. Using IL 6d (H0 = 1.36), 9-fluorene can be 100% converted, which may be derived from its higher acidity. Meanwhile IL 6c (H0 = 1.51) has similar acidity with IL 6a (H0 = 1.49), but they have different catalytic effect in the condensation, the reason may be that the anion of IL 6a (HSO4−) provides active proton.
As to IL 5b (H0 = 1.63, entry6) and IL 6e (H0 = 1.59, entry11), their anion is HSCH2CH2CH2SO3−, and the molar content of –SH measured by the Ellman's method is 96.1% and 98.6% respectively. The high catalytic activity and excellent p, p′-regioselectivity of BHPF with IL 5b and IL 6e, illustrate that the protonic acid and –SH fraction have synergistic effect on the reaction of phenol with fluorenone.
Secondly, for IL 1–4, 5a, 6a, 7, 8 with the same anion (HSO4−) and different cations, the experimental results gave a clear scene on how the ILs' cationic structures affect the condensation. To discuss conveniently and efficiently, Fig. 4 takes the conversion of 9-fluorenone as X-axis and the H0 and SH% as Y-axis to export the relationship between ILs' structures and catalytic performance in the synthesis of BHPF.
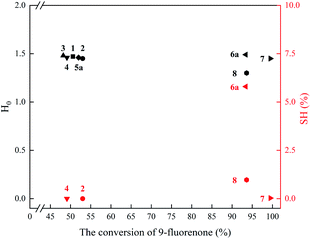 | ||
| Fig. 4 The relationship between the acidity and sulfhydryl content of ILs and the conversion of 9-fluorenone. Reaction conditions:10 mmol 9-fluorenone, 60 mmol phenol, 15 mol% ILs, 110 °C for 4 h. | ||
As shown in Fig. 4, IL 1–4, 5a have no effective sulfhydryl groups (SH% = 0) and the similar H0 (nearly 1.50), thus these five ILs have the similar conversion of 9-fluorenone (about 50%) and similar p, p′-regioselectivity of BHPF (about 87%). And it implies that –SH group in cation of IL 2 and IL 4 cannot “act” as a co-catalyst. In comparison, with IL 6a (H0 = 1.49, SH% = 5.8) or IL 8 (H0 = 1.30, SH% = 0.97) as the catalyst, the conversion of 9-fluorenone are 93.4% and 93.6%, respectively. That means that the active proton and the –SH fraction in IL 6a co-efficiently take part in the condensation reaction, so the same electron transferring procedure occurs with IL 8 as the catalyst. Compared with IL 2 and 4, IL 7 (H0 = 1.45, SH% = 0.03) has the similar –SH fraction in cation (N- and S-containing five-membered ring structure) with ILs 6 and 8, IL 7 can “act” as a proton catalyst and effective sulfhydryl co-catalyst, and achieve 99.5% conversion of 9-fluorenone, though its SH% is very low.
At last, when changing the reaction temperature to 60 °C and the reaction time to 8 h, the catalytic results of IL 6a, 6c and 6e (entry 14–16, Table 3) tell us again that IL 6c ([SO3HC3MBT] [p-CH3C6H4SO3]) is the best catalyst for the condensation reaction. And at lower reaction temperature, p, p′-selectivity of BHPF can be increased obviously. Using IL 6c (entry15) as catalyst at 60 °C, 9-fluorenone can be converted completely, and the selectivity of BFPF can reach 95.2%.
The mechanism discussion
According to the above results and related literature,14,42,43 a possible catalytic mechanism in the condensation of 9-fluorenone with phenol was proposed in Scheme 3. Firstly, the carbonyl of 9-fluorenone was activated by sulfonic acid group of IL 6c to form the intermediate I. Intermediate I could undergo nucleophilic addition with phenol to give Intermediate II, and then II reacted with IL 6c to obtain intermediate III. Without the participation of –SH group, intermediate III reacted with another molecule of phenol to obtain BHPF. While IL 6c contained sulfhydryl group and the length of HSO3CH2CH2CH2− group on N atom is suitable, so it may have the other route for the electron transfer. That is the proton exchange between –SH and –SO3− groups, then S atom nucleophilic attacked the C atom of carbonyl in 9-fluorenone to form intermediate IV. The part of intermediate IV coming from IL 6c is a large structural item, so it's the spatial effect that make the p-carbon in phenol rather than the o-carbon in phenol to attack the reactive center. So that the –SH group in some BFILs can influence the ration of BHPF and the by-product 1. Therefore, IL 6c increased the yield of BHPF and also the selectivity of BHPF.Recycling of ionic liquids
To evaluate the recyclability of IL, a series of recycling experiments using IL 6c were carried out. After the completion of the first reaction, deionized water was added to the reaction mixture to extract IL 6c, which was washed with diethyl ether for three times and then dried under vacuum at 100 °C for 12 h. The recovered IL was reused for five times under the same conditions. As shown in Fig. 5, the conversion of 9-fluorenone decreased by 1.4% and the selectivity of BHPF reduces by only 1.0% after the first loop. This indicated that the IL was stable and could be reused five times finally.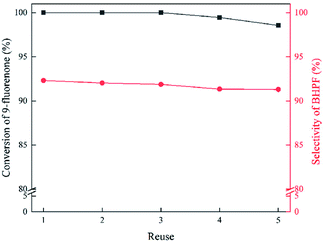 | ||
| Fig. 5 Reuse of 6c in the condensation reaction. Reaction conditions: 20 mmol 9-fluorenone, 120 mmol phenol, 15 mol% 6c, 110 °C for 4 h. | ||
Conclusions
In this work, a series of BFILs containing sulfonic acid and sulfhydryl groups were synthesized, and how the structure of BFILs to affect its catalytic capability in the condensation of 9-fluorenone with phenols, are also discussed. Generally speaking, the synthesis of biphenols need protonic acid as catalyst to gain a high conversion of ketone and thiol derivatives as co-catalyst to obtain a well p, p′-regioselectivity of biphenols. Thus, it is important to qualify the BFILs' catalytic characters, and the total acidity of ILs were characterized with Hammett acidity (H0). Moreover, we resourcefully use the Ellman's method to get the molar content of free and catalytic active sulfhydryl in the ILs. The results indicate that IL 6c ([HSO3C3MBT] [p-CH3C6H4SO3]) has the best catalytic activity, the conversion of 9-fluorenone was 100%, and the selectivity of BHPF could reach 95.2% at 60 °C for 8 h and 91.8% at 110 for 4 h. This work is the base that designing the better IL catalysts in the future.Conflicts of interest
There are no conflicts to declare.References
- M. Thoene, L. Rytel, N. Nowicka and J. Wojtkiewicz, Toxicol. Res., 2018, 7, 371–380 CrossRef CAS PubMed.
- Z. Q. Hu, S. J. Li and C. H. Zhang, J. Appl. Polym. Sci., 2007, 106, 2494–2501 CrossRef CAS.
- W. X. Deng, Y. W. Zhong, Q. Jie, X. B. Huang and J. W. Peng, Adv. Mater. Res., 2014, 936, 28–33 Search PubMed.
- W. Z. Li, X. G. Yuan, J. Huang, B. A. Peng, F. Zhou, J. Ma and X. D. Jia, Polymer, 2017, 109, 126–136 CrossRef CAS.
- N. Kato, S. Ikeda, M. Hirakawa and H. Ito, J. Appl. Polym. Sci., 2017, 134, 45042–45047 CrossRef.
- G. Q. Song, X. Chen, L. Shen and C. Q. Hu, Xiandai Huagong, 2012, 32, 77–80 CAS.
- F. Bao, L. H. Zong, N. Li, Y. Y. Song, Y. X. Pan, J. Y. Wang and X. G. Jian, Thermochim. Acta, 2020, 683, 178184–178194 CrossRef CAS.
- N. P. Godman, D. B. Barbee, F. B. Carty, C. D. McMillen, C. A. Corley, E. Shurdha and S. T. Iacono, Polym. Chem., 2016, 7, 5799–5804 RSC.
- Z. Hu, C. Zhang and S. Li, J. Macromol. Sci., Part B: Phys., 2016, 55, 272–284 CrossRef CAS.
- A. E. Bal'tser, D. A. Zaitsev, N. G. Zubritskaya, A. G. Bazanov and N. N. Machalaba, Fibre Chem., 2015, 47, 8–13 CrossRef.
- J. Zhang and S. Liu, Jingxi Huagong Zhongjianti, 2008, 38, 47–49 CAS.
- Q. P. Gao and W. Z. Wang, Huaxue Gongchengshi, 2009, 23, 12–13 CAS.
- W. Liu, J. Wang, Q. H. Qiu, L. Ji, C. Y. Wang and M. L. Zhang, Pigm. Resin Technol., 2008, 37, 9–15 CrossRef CAS.
- L. Yan, L. M. Yu, M. C. Shen, S. K. Luo and Z. X. Gao, New J. Chem., 2019, 43, 15700–15705 RSC.
- C. E. Kast and A. Bernkop-Schnurch, Biomaterials, 2001, 22, 2345–2352 CrossRef CAS.
- S. Raeissi and C. J. Peters, Green Chem., 2009, 11, 185–192 RSC.
- S. K. Tang, G. A. Baker and H. Zhao, Chem. Soc. Rev., 2012, 41, 4030–4066 RSC.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS.
- X. M. Liu, J. X. Zhou, X. W. Guo, M. Liu, X. L. Ma, C. S. Song and C. Wang, Ind. Eng. Chem. Res., 2008, 47, 5298–5303 CrossRef CAS.
- S. G. Lee, Chem. Commun., 2006, 10, 1049–1063 RSC.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed.
- R. Kore and R. Srivastava, Catal. Commun., 2011, 12, 1420–1424 CrossRef CAS.
- J. D. Holbrey, M. B. Turner, W. M. Reichert and R. D. Rogers, Green Chem., 2003, 5, 731–736 RSC.
- Q. H. Zhang, X. Y. Ma, S. M. Liu, B. Q. Yang, L. J. Lu, Y. D. He and Y. Q. Deng, J. Mater. Chem., 2011, 21, 6864–6868 RSC.
- J. P. Shang, Z. P. Li, C. N. Su, Y. Guo and Y. Q. Deng, RSC Adv., 2015, 5, 71765–71769 RSC.
- U. Garazi, M. Ainhoa, S. Raul, H. Maria Teresa and D. Esther, RSC Adv., 2015, 5, 103210–103217 RSC.
- H. F. Liu, F. X. Zeng, L. Deng, B. Liao, H. Pang and Q. X. Guo, Green Chem., 2013, 15, 81–84 RSC.
- C. Thomazeau, H. Olivier-Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264–5265 CrossRef CAS PubMed.
- Z. Y. Duan, Y. L. Gu, J. Zhang, L. Y. Zhu and Y. Q. Deng, J. Mol. Catal. A: Chem., 2006, 250, 163–168 CrossRef CAS.
- G. D. Ellman, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS.
- K. R. Christian, K. Gerald and J. Hermann, Anal. Bioanal. Chem., 2002, 373, 266–276 CrossRef PubMed.
- O. Eral and S. Neseligolu, Clin. Biochem., 2014, 47, 326–332 CrossRef PubMed.
- J. Kuligowski, M. R. El-Zahry, A. Sanchez-Illana, G. Quintas, M. Vento and B. Lendl, Analyst, 2016, 141, 2165–2174 RSC.
- S. Y. Lim, K. H. Hong, D. I. Kim, H. Kwon and H. J. Kim, J. Am. Chem. Soc., 2014, 136, 7018–7025 CrossRef.
- P. T. Lee, D. Lowinsohn and R. G. Compton, Analyst, 2014, 139, 3755–3762 RSC.
- S. Sornambikai, M. R. Abdul Kadir, A. S. Kumar, N. Ponpandian and C. Viswanathan, Anal. Methods, 2017, 9, 6791–6800 RSC.
- B. Benkova, V. Lozanov, I. P. Ivanov, A. Todorova, I. Milanov and V. Mitev, J. Chromatogr. B: Biomed. Sci. Appl., 2008, 870, 103–108 CrossRef CAS.
- M. F. Mora, A. M. Stockton and P. A. Willis, Electrophoresis, 2013, 34, 309–316 CrossRef CAS PubMed.
- H. Faulstich, P. Tews and D. Heintz, Anal. Biochem., 1993, 208, 357–362 CrossRef CAS PubMed.
- E. L. Margelefsky, R. K. Zeidan, V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2007, 129, 13691–13697 CrossRef CAS PubMed.
- V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2003, 125, 9403–9413 CrossRef CAS PubMed.
- R. K. Zeidan, V. Dufaud and M. E. Davis, J. Catal., 2006, 239, 299–306 CrossRef CAS.
- V. V. Stijn, H. Sasja, G. Jan, Y. Feng, T. Joice, S. Mario, D. Wim, R. L. Yuriy, H. Ive and S. Bert F, ACS Catal., 2012, 2, 2700–2704 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05967j |
| This journal is © The Royal Society of Chemistry 2021 |