Open Access Article
Open Access ArticleVisible-light-driven cascade radical cyclization toward the synthesis of α-carbonyl alkyl-substituted benzimidazo[2,1-a]isoquinolin-6(5H)-one derivatives†
Jing Liu‡
ab,
Hong-Li Huang‡c,
Chen Wangb,
Yinghua Lib,
Huaqiang Lib,
Honggang Hub,
Shipeng Heb,
Hua Tang*b and
Fei Gao *b
*b
aDepartment of Chemistry, College of Sciences, Shanghai University, No. 99 Shangda Road, Shanghai 200444, China
bInstitute of Translation Medicine, Shanghai University, Shanghai, 200444, China
cCollege of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, Shandong 252059, China
First published on 1st September 2021
Abstract
A visible-light-driven cascade radical cyclization process of N-methacryloyl-2-phenylbenzimidazole has been established with α-carbonyl alkyl bromide. This protocol provides an efficient and practical method for the synthesis of various α-carbonyl alkyl-substituted benzimidazo[2,1-α]isoquinolin-6(5H)-ones in outstanding yields, mild reaction conditions and excellent functional group tolerance.
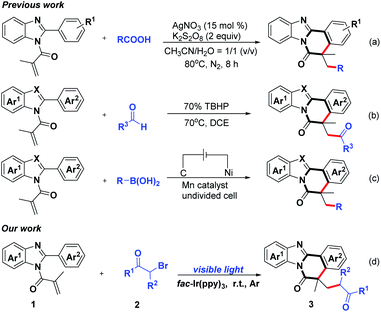
Benzimidazo-fused polycyclic motifs, especially benzimidazo[2,1-α]isoquinolin-6(5H)-one derivatives, have been frequently found in numerous pharmaceutical molecules, natural products and synthetic materials.1–7 As a consequence, considerable efforts have been made to develop efficient methods for the preparation of benzimidazo-fused polycyclic compounds. Among them, the cascade radical cyclization strategies have attracted special attention from chemists. In 2019, Yu and co-workers realized the construction of benzimidazo[2,1-α]isoquinolin-6(5H)-ones via silver-catalyzed decarboxylative radical cascade cyclization (Scheme 1a).8 A metal-free oxidative acylation/cyclization of N-methacryloyl-2-phenylbenzoimidazole with aryl aldehydes has also been found by Reddy's group (Scheme 1b).9 At the same time, Aiwen Lei's laboratory has devoted effort to the development of Mn-catalyzed electrochemical radical cascade cyclization reaction toward the preparation of benzimidazo[2,1-α]isoquinolin-6(5H)-ones (Scheme 1c).10 Despite important breakthroughs in the radical cascade cyclization, room still exists for developing a visible-light-driven pathway to acquire benzimidazo-fused polycyclic compounds under milder reaction conditions.
 | ||
| Scheme 1 Reported synthesis of benzo[4,5]imidazo[2,1-α]isoquinolin-6(5H)-one derivatives and our strategy. | ||
Recently, visible-light-mediated photoredox catalysis has arguably gained considerable momentum due to its inherent green, excellent functional group tolerance, safe and availability.11–17 α-Carbonyl alkyl bromide, which are readily available and inexpensive chemical reagents, could be successfully converted into α-carbonyl alkyl radicals for rapidly constructing C–C bonds under the irradiation of visible light.18–23 During the past decades, the applications on the visible-light-promoted cascade radical cyclization process for the synthesis of complex polycyclic hydrocarbons have been reported.24,25 In contrast to the previous achievements in the formation of benzimidazo-fused polycyclic compounds, research on visible-light-induced α-carbonyl alkylation to construct benzimidazo[2,1-α]isoquinolin-6(5H)-ones remains scarce. Inspired by these researches, we designed a novel visible-light-driven cascade radical cyclization for the synthesis of benzo[4,5]imidazo[2,1-α]isoquinoline tetracyclic skeleton, in which α-carbonyl alkyl radical generated from the corresponding bromide could undergo intermolecular radical addition to the C–C double bond, followed by the formation of intramolecular C–C bond, to synthesize the corresponding cyclization compounds (Scheme 1d).
At the outset, we chose N-methacryloyl-2-phenylbenzimi-dazole (1a) and 2-bromoacetophenone (2a) as the model substrates. Optimization of the reaction conditions was explored by irradiating a mixture of model substrates, base and photocatalyst under Ar atmosphere using 5 W blue LEDs at room temperature (Table 1). In the presence of fac-Ir(ppy)3 (5% mol), K2HPO4 (2.0 equiv.), and CH3CN (1 mL), the target product 3aa was afforded in 60% isolated yield (entry 1). Next, the reaction was investigated using diverse photocatalysts. Unfortunately, some common photocatalysts, such as Ir[dF(CF3)ppy]2(dtbpy)PF6, Ru(ppy)3Cl2 and EosinY showed low catalytic activity (entries 2–4). Subsequently, further screening on the solvent revealed that DMF could provide a slightly improvement in reaction efficiency (entries 5–7). Gratifying, examination on the bases showed that 2,6-lutidine was the ideal choice and the desired product 3aa was obtained in 88% yield (entries 8–15). In addition, control experiments confirmed that the photocatalyst and visible light were essential for this transformation (entries 16–17).
| Entry | Photocatalyst | Base | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), fac-Ir(ppy)3 (0.005 mmol), base (0.2 mmol), solvent (anhydrous, 1 mL), Ar, 5 W blue LEDs, room temperature (r.t.), 16 h.b Isolated yield.c In the dark.d No photocatalyst. | ||||
| 1 | fac-Ir(ppy)3 | K2HPO4 | CH3CN | 60 |
| 2 | Ir[dF(CF3)ppy]2(dtbpy)PF6 | K2HPO4 | CH3CN | 15 |
| 3 | Ru(ppy)3Cl2 | K2HPO4 | CH3CN | Trace |
| 4 | EosinY | K2HPO4 | CH3CN | Trace |
| 5 | fac-Ir(ppy)3 | K2HPO4 | DMF | 70 |
| 6 | fac-Ir(ppy)3 | K2HPO4 | DMSO | 59 |
| 7 | fac-Ir(ppy)3 | K2HPO4 | DCM | 67 |
| 8 | fac-Ir(ppy)3 | NaHCO3 | DMF | 65 |
| 9 | fac-Ir(ppy)3 | Na2CO3 | DMF | <10 |
| 10 | fac-Ir(ppy)3 | Cs2CO3 | DMF | Trace |
| 11 | fac-Ir(ppy)3 | KH2PO4 | DMF | 50 |
| 12 | fac-Ir(ppy)3 | 2,6-Lutidine | DMF | 88 |
| 13 | fac-Ir(ppy)3 | Pyridine | DMF | 11 |
| 14 | fac-Ir(ppy)3 | DBU | DMF | 13 |
| 15 | fac-Ir(ppy)3 | DABCO | DMF | 41 |
| 16c | fac-Ir(ppy)3 | K2HPO4 | DMF | 0 |
| 17d | — | K2HPO4 | DMF | 0 |
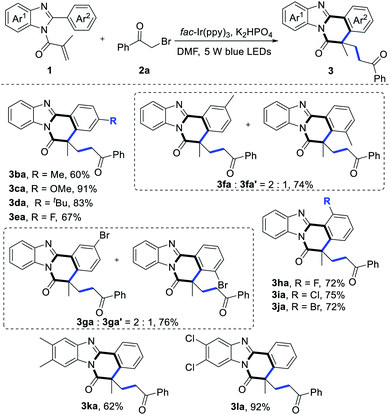
With the optimized reaction conditions in hand, we then surveyed the scope of substrate 1. As shown in Table 2, a series of N-methacryloyl-2-phenylbenzimidazoles could undergo cascade radical cyclization to synthesize the desired products successfully. Different substituents, including –F, –Cl, –Br, –Me, –OMe, and –tBu at the 2-benzene ring of benzimidazole were well tolerated (3ba–3ja), especially substrate 1c with strong electron-donating –OMe group transformed to the product 3ca in 91% yield. Notably, steric hindrance didn't affect the reaction efficiency, employing ortho-, meta- and para-substituted 2-arylbenzimidazoles as the starting materials, the cyclization products were obtained in good yields. When the meta-position of the 2-benzene ring of benzimidazole was substituted by a –Me or –Br group (1f and 1g), the regioisomers were afforded in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As expected, in the case of substrates 1k and 1l, good yields of the desired products were formed (3ka and 3la, 62% and 92%, respectively).
1. As expected, in the case of substrates 1k and 1l, good yields of the desired products were formed (3ka and 3la, 62% and 92%, respectively).
| a Reaction conditions: 1 (0.1 mmol), 2a (0.2 mmol), fac-Ir(ppy)3 (0.005 mmol), K2HPO4 (0.2 mmol), dry DMF (1 mL), 5 W blue LEDs, r. t., Ar, 16 h. Isolated yield. |
|---|
 |
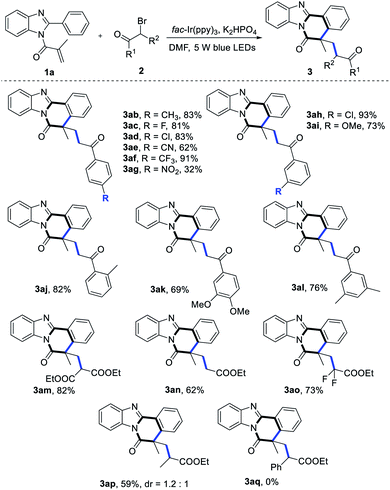
To further gain more information about this protocol, using 1a as the model substrate, we turned our attention to explore the scope of α-carbonyl alkyl bromide derivatives 2 (Table 3). First, a wide range of 2-bromoacetophenones were examined (2b–2l). To our delight, 2-bromoacetophenones with electron-donating substituents (–Me, –OMe) or electron-withdrawing groups (–F, –Cl, –CF3, –CN) at the ortho-position/meta-position/para-position of the benzene ring were all competent partners in this transformation (3ab–3aj), especially compound 2f and 2h successfully led to the desired products 3af and 3ah in 91% and 93% yields, respectively. By contrast, 2-bromoacetophenone bearing strong electron-drawing NO2 group at the para-position of the benzene ring afforded the expected product 3ag in 32% yield. Next, when 2-bromoacetophenones 2k or 2l was applied as the oxidant, this reaction proceeded smoothly and the corresponding cyclization products (3ak–3al) were obtained in good yields. In addition, α-bromoalkyl esters 2m–2p were also suitable reaction partners, delivering the corresponding products 3am–3ap in moderate to good yields. Finally, it seemed that this reaction conditions were not ideal for α-bromoalkyl esters 2q.
| a Reaction conditions: 1a (0.1 mmol), 2 (0.2 mmol), fac-Ir(ppy)3 (0.005 mmol), K2HPO4 (0.2 mmol), dry DMF (1 mL), 5 W blue LEDs, r. t., Ar, 16 h. Isolated yield. |
|---|
 |
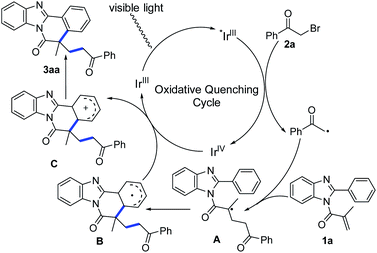
Based on the above experimental results and literature reports,26–28 a plausible reaction mechanism for this visible-light-induced cascade cyclization was described in Scheme 2. Upon irradiation with visible light, the ground-state [fac-IIIIr(ppy)3] was excited to the excited-state [fac-IIIIr(ppy)3]*, which was readily reduced by 2-bromoacetophenone (2a) to produce the corresponding α-carbonyl alkyl radical specie. Following intermolecular addition with the C–C double bond of the substrate 1a, the resulting radical intermediate A underwent radical addition cyclization to form the intermediate B. Subsequently, the intermediate B was oxidized by [fac-IVIr(ppy)3]* to produce the carbocation C, which finally transformed to the eventual product 3aa after rapid deprotonation.
Benzimidazo[2,1-α]isoquinolin-6(5H)-one derivatives have been reported as potential pharmaceutical molecules with antitumor activity.29,30 Then, we explored the cell cytotoxicity of several representative cyclization products against MDA-MB-231 cells (human breast cancer cells) and U87 cells (human glioblastoma cells). As shown in Fig. 1, these products almost showed definite antitumor activities, especially the IC50 of compound 3ab was 4.228 μM against U87 cells. It is very hopeful that these α-carbonyl alkyl-substituted benzimidazo[2,1-α]isoquinolin-6(5H)-ones show outstanding antitumor activity after further structural optimization.
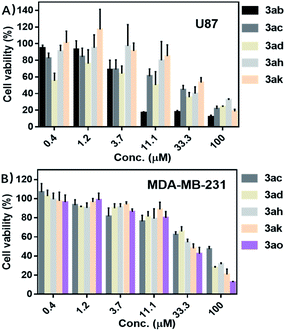 | ||
| Fig. 1 (A) Cytotoxicity of 3ab, 3ac, 3ad, 3ah and 3ak against U87 cells for 72 h. (B) Cytotoxicity of 3ac, 3ad, 3ah, 3ak and 3ao against MDA-MB-231 cells for 72 h. | ||
In conclusion, we have developed a simple and efficient photoredox-assisted cascade radical cyclization method to synthesize a series of α-carbonyl alkyl-containing benzimidazo[2,1-α]-isoquinolin-6(5H)-ones, some of which exhibited definite anticancer ability against U87 cells and MDA-MB-231 cells. Additional research on the improvement of biological activity is currently underway in our group. Moreover, this transformation shows the advantages of mild reaction conditions, good functional group compatibility and acceptable to excellent yields. We believe that this method will be widely used in organic synthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of Shanghai (No. 1ZR1422600), the Science and Technology Project of Nantong, China (No. MS22019026), and Shanghai Sailing Program (No. 20YF1414100).References
- C. Pan, C. Yuan and J.-T. Yu, Org. Biomol. Chem., 2021, 19, 619–626 RSC.
- X.-X. Guo, D.-W. Gu, Z. Wu and W. Zhang, Chem. Rev., 2015, 115, 1622–1651 CrossRef CAS PubMed.
- G. Yadav and S. Ganguly, Eur. J. Med. Chem., 2015, 97, 419–443 CrossRef CAS PubMed.
- L.-Y. Xie, J. Qu, S. Peng, K.-J. Liu, Z. Wang, M.-H. Ding, Y. Wang, Z. Cao and W.-M. He, Green Chem., 2018, 20, 760–764 RSC.
- Y. Li, J. Zhu, H. Xie, S. Li, D. Peng, Z. Li, Y. Wu and Y. Gong, Chem. Commun., 2012, 48, 3136–3138 RSC.
- G. Meng, H.-Y. Niu, G.-R. Qu, J. S. Fossey, J.-P. Li and H.-M. Guo, Chem. Commun., 2012, 48, 9601–9603 RSC.
- E. Moriarty, M. Carr, S. Bonham, M. P. Carty and F. Aldabbagh, Eur. J. Med. Chem., 2010, 45, 3762–3769 CrossRef CAS PubMed.
- K. Sun, S.-J. Li, X.-L. Chen and Y. Liu, Chem. Commun., 2019, 55, 2861–2864 RSC.
- R. Boora, G. Ravi kumar and B. V. Subba Reddy, Org. Biomol. Chem., 2019, 17, 9627–9630 RSC.
- Y. Yuan, Y. Zheng, B. Xu, J. Liao, F. Bu, S. Wang, J. Hu and A. Lei, ACS Catal., 2020, 10, 6676–6681 CrossRef CAS.
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- C. Wang, P. H. Dixneuf and J. F. Soulé, Chem. Rev., 2018, 118, 7532–7585 CrossRef CAS PubMed.
- L. Zou, P. Li, B. Wang and L. Wang, Chem. Commun., 2019, 55, 3737–3740 RSC.
- Y.-P. Wu, M. Yan, Z.-Z. Gao, J.-L. Hou, H. Wang, D.-W. Zhang, J. Zhang and Z.-T. Zhang, Chin. Chem. Lett., 2019, 30, 1383–1386 CrossRef CAS.
- Y. Kong, W. Xu, X. Liu and J. Weng, Chin. Chem. Lett., 2020, 31, 3245–3249 CrossRef CAS.
- W. Ou, R. Zou, M. Han, L. Yu and C. Su, Chin. Chem. Lett., 2020, 31, 1899–1902 CrossRef CAS.
- X. Yong, Y.-F. Han, Y. Li, R.-J. Song and J.-H. Li, Chem. Commun., 2018, 54, 12816–12819 RSC.
- W. Dong, Y. Yuan, X. Gao, M. Keranmu and W. Li, J. Org. Chem., 2019, 84, 1461–1467 CrossRef CAS PubMed.
- Q. Li, Y. Yin, Y. Li, J. Zhang, M. Huang, J. K. Kim and Y. Wu, Org. Chem. Front., 2019, 6, 3238–3243 RSC.
- P. Gandeepan, J. Koeller, K. Korvorapun, J. Mohr and L. Ackermann, Angew. Chem., Int. Ed., 2019, 58, 9820–9825 CrossRef CAS PubMed.
- B. Zhao, Z. Li, Y. Wu, Y. Wang, J. Qian, Y. Yuan and Z. Shi, Angew. Chem., Int. Ed., 2019, 131, 9548–9552 CrossRef.
- H.-L. Huang, J.-Y. Du, Q.-L. Li, F. Gao and C.-L. Ma, J. Org. Chem., 2020, 85, 3963–3972 CrossRef CAS PubMed.
- F.-L. Zeng, K. Sun, X.-L. Chen, X.-Y. Yuan, S.-Q. He, Y. Liu, Y.-Y. Peng, L.-B. Qu, Q.-Y. Lv and B. Yu, Adv. Synth. Catal., 2019, 361, 5176–5181 CrossRef CAS.
- B. Wang, L. Zou, L. Wang, M. Sun and P. Li, Chin. Chem. Lett., 2021, 32, 1229–1232 CrossRef CAS.
- D. Lu, Y. Wan, L. Kong and G. Zhu, Org. Lett., 2017, 19, 2929–2932 CrossRef CAS PubMed.
- L.-L. Mao, D.-G. Zheng, X.-H. Zhu, A.-X. Zhou and S.-D. Yang, Org. Chem. Front., 2018, 5, 232–236 RSC.
- Y. Yuan, W. Dong, X. Gao, X. Xie and Z. Zhang, Org. Lett., 2019, 21, 469–472 CrossRef CAS PubMed.
- L. W. Deady and T. Rodemann, Aust. J. Chem., 2001, 54, 529–534 CrossRef CAS.
- X. Wang and A. B. Fulp, WO2005002503A2, 2005.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05936j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |