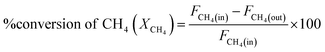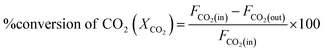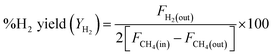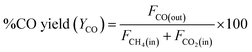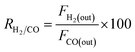Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solution combustion synthesis of Ni/La2O3 for dry reforming of methane: tuning the basicity via alkali and alkaline earth metal oxide promoters†
Yahia H. Ahmad a,
Assem T. Mohameda,
A. Kumar
a,
Assem T. Mohameda,
A. Kumar b and
Siham Y. Al-Qaradawi
b and
Siham Y. Al-Qaradawi *a
*a
aDepartment of Chemistry and Earth Sciences, College of Arts and Sciences, Qatar University, Doha 2713, Qatar. E-mail: siham@qu.edu.qa
bDepartment of Chemical Engineering, College of Engineering, Qatar University, Doha 2713, Qatar
First published on 15th October 2021
Abstract
The production of syngas via dry reforming of methane (DRM) has drawn tremendous research interest, ascribed to its remarkable economic and environmental impacts. Herein, we report the synthesis of K, Na, Cs, Li, and Mg-promoted Ni/La2O3 using solution combustion synthesis (SCS). The properties of the catalysts were determined by N2 physisorption experiments, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectrometry (XPS), and H2-TPR (temperature programmed reduction). In addition, their catalytic performance towards DRM was evaluated at 700 °C. The results demonstrated that all catalysts exhibited porous structures with high specific surface area, in particular, Mg-promoted Ni/La2O3 (Mg–Ni–La2O3) which depicted the highest surface area and highest pore volume (54.2 m2 g−1, 0.36 cm3 g−1). Furthermore, Mg–Ni–La2O3 exhibited outstanding catalytic performance in terms of activity and chemical stability compared to its counterparts. For instance, at a gas hourly space velocity (GHSV) of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1, it afforded 83.2% methane conversion and 90.8% CO2 conversion at 700 °C with no detectable carbon deposition over an operating period of 100 h. The superb DRM catalytic performance of Mg–Ni–La2O3 was attributed to the high specific surface area/porosity, strong metal-support interaction (MSI), and enhanced basicity, in particular the strong basic sites compared to other promoted catalysts. These factors remarkably enhance the catalytic performance and foster resistance to coke deposition.
000 mL g−1 h−1, it afforded 83.2% methane conversion and 90.8% CO2 conversion at 700 °C with no detectable carbon deposition over an operating period of 100 h. The superb DRM catalytic performance of Mg–Ni–La2O3 was attributed to the high specific surface area/porosity, strong metal-support interaction (MSI), and enhanced basicity, in particular the strong basic sites compared to other promoted catalysts. These factors remarkably enhance the catalytic performance and foster resistance to coke deposition.
1. Introduction
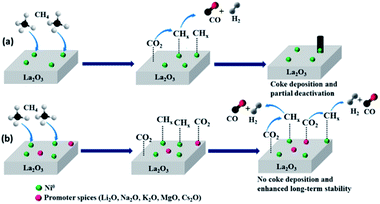
The dramatic increment in energy demand and rapid depletion of fossil fuels have spurred remarkable efforts towards green alternative energy sources. One of the dedicated approaches to address these issues is the dry reforming of methane (DRM) in which two greenhouse gases i.e. carbon dioxide and methane are converted to syngas (CO + H2), the feedstock for synthesis of methanol and other value-added chemicals. DRM is a highly endothermic reaction that becomes spontaneous at 640 °C.1 DRM can be catalysed by noble elements such as Rh, Ir, Pt, Pd, and Ru, which afford outstanding catalytic activity and stability.2–6 However, their high cost and scarcity prohibit wide-scale adoption.7 Several transition metals such as Co, Ni, and Fe have been suggested as substitutes for noble metals. Among them, Ni has drawn remarkable interest owing to its low cost and high catalytic activity. However, the deactivation of active sites induced by high temperature sintering and extensive deposition of coke are considered as the main stumbling blocks towards commercialization.8 Different strategies were employed to improve the performance of Ni-based catalysts towards DRM, such as alloying of Ni with other metals,9 addition of promoters,10 and confinement of Ni into mesoporous support.11According to previous reports, there is a consensus view that metal-support interaction (MSI) remarkably influence the catalytic performance of the DRM catalyst. Based on this, different metal oxides have been hired as supports for Ni-based DRM catalysts such as SiO2,12 Al2O3,13 TiO2,11 CeO2,14 ZrO2,15 and La2O3.12 Among them, La2O3 received great interest ascribed to its parallel function as support and promoter, where it is capable to afford basic sites that enhance the adsorption of CO2 via formation of La2O2CO3. This endows the removal of deposited coke through the following reaction: La2O2CO3 + C → La2O3 + 2CO.16,17 Concurrently, La2O3 enables strong interaction with Ni which promotes high dispersion of Ni particles, reduces the Ni particle size, and prevents the high temperature sintering.18
The addition of basic promoters, in particular, the alkaline earth metals oxides remarkably enhance the catalytic performance of Ni-based catalysts.19 Among these promoters, MgO received great interest attributed to its remarkable catalytic performance and enhanced coking resistance.10 For example, the catalytic performance of Ni-supported Mg–La mixed oxides (with different La3+/Mg2+ ratios) towards DRM was studied.20 Activity and stability measurements depicted that Ni/10MgO–La2O3 afforded the best performance and mainly forms monoclinic La2O2CO3, which enhanced the coke removal contrarily to the hexagonal phase, which has no impact on the catalytic activity.20 In another work, the DRM performance of MgO-promoted Ni/Al2O3 catalyst synthesized by loading of MgO on Ni/Al2O3 prepared by atomic layer deposition of Ni on Al2O3 nanoparticles was studied.21 It was found that the addition of MgO enhanced the amount and strength of the catalyst basic sites, and increase the intensity of surface oxygenated species that enhance the adsorption and activation of CO2. In addition, MgO promoted the resistance against coke formation, in particular, graphitic carbon, which is responsible for catalyst deactivation.21 Although the promotion of Ni/La2O3 with alkaline earth metals was previously investigated in the literature, however, the impact of alkali metals was not emphasized enough.
In terms of the synthesis approach, solution combustion synthesis (SCS) received increased interest as it permits the synthesis of wide range of materials in nanoscale dimensions such as metal oxides, sulfides, phosphates, metals and alloys.22 SCS allows the controlling of size, composition, and nanoarchitecture of materials through self-sustained exothermic reactions. This synthesis approach endows several merits such as simplicity, low cost, high porosity of synthesized materials, and small particle size.23 Nevertheless, several preparation routes were reported for the synthesis of Ni/La2O3 catalysts such as incipient wetness impregnation method24 and sol–gel approach,25 whereas its synthesis via solution combustion procedure was not previously investigated.
Triggered by the above discussions, herein, we introduce the synthesis of alkali metals oxides and MgO-promoted Ni/La2O3 using SCS for DRM. The freshly reduced samples as well as spent samples (after DRM operation) were investigated using different structural techniques. Besides, the catalytic activity and stability of studied catalysts was evaluated at 700 °C.
2. Experimental
2.1. Materials and reagents
Nickel nitrate hexahydrate (≥98.5%), sodium nitrate (≥98.5%), magnesium nitrate hexahydrate (≥99.0%), lithium nitrate, cesium nitrate (99.0%), potassium nitrate (99.0%), and glycine (≥98.5%) were purchased from Sigma–Aldrich. Lanthanum nitrate hexahydrate (99.99%) was purchased from Carl-Roth, Germany.2.2. Catalyst synthesis
2.3. Catalyst characterization
The catalysts morphology was investigated by field emission scanning electron microscopy (FESEM) using Philips XL-30 microscope. The morphology of reduced catalysts was investigated using bright field transmission electron microscopy (TEM) via FEI Tecnai G2 TF20 UT microscope at an accelerating voltage of 200 kV. The samples composition was investigated by inductively coupled plasma optical emission spectrometry (ICP-OES) via a spectrometer (PerkinElmer, Optima 5300 DV). The textural properties were examined via N2 sorption experiments at liquid nitrogen temperature (77 K) using the Brunauer–Emmett–Teller (BET) method. X-ray diffraction (XRD) spectra was recorded using X'Pert-Pro MPD diffractometer (PANalytical Co., Netherlands) using of Cu-Kα X-ray source (λ = 1.54059 Å) as a radiation source in the 2θ range (10–80). The chemical composition and the elemental oxidation states were investigated using XPS spectrophotometer Kratos Axis Ultra XPS equipped with a monochromatic Al-Kα radiation source (1486.6 eV) under ultra-high vacuum (UHV) (ca. 5 × 10−9 torr).The thermogravimetric analysis was executed using TGA 4000 analyzer (PerkinElmer, USA). Measurements were performed at the temperature range 25–850 °C at a heating rate of 10 °C min−1 under air flow. Samples reducibility was examined using temperature-programmed reduction of hydrogen (H2-TPR). Measurements were carried out with Micromeritics Autochem 2920 chemisorption analyzer with H2 uptake recorded via TCD detector. 50 mg of the supported catalyst was placed into a quartz U-shape tube and 10% H2 balanced in Ar was passed over the test sample in a flow rate of 10 mL min−1 until a stable baseline is attained. The sample was then heated in a ramp rate of 5 °C min−1 in the temperature range 30–850 °C. The basicity was examined by CO2-TPD. 50 mg of the sample was first reduced in a flow of 10% H2 at 800 °C for 2 h, then the ample was cooled down to room temperature. Then, the reduced sample was degassed at 300 °C in a flow of Ar for 1 h followed by cooling to 50 °C. Afterwards, 10% CO2 is injected at a rate of 30 mL min−1 for 1 h at 50 °C followed by purging of Ar to remove excess CO2, then the sample was heated under Ar flow at a ramp rate 10 °C min−1 to 900 °C and the signal of desorbed CO2 is detected by TCD.
2.4. DRM activity
DRM activity measurements were carried out in a fixed bed quartz tube reactor (d = 6 mm) at atmospheric pressure. 100 mg of the catalyst was loaded and fixed by quartz wool. Reactor temperature was recorded via K-type thermocouple located at the fixed bed. The catalyst was Pre-reduced with 10% H2 flow at 800 °C for 2 h. After which, N2 was purged to discard excess H2. The feed gas composition was (10% CH4 and 10% CO2 balanced with Ar) and was fed to the reactor in a rate of 50 mL min−1 giving rise to a weight hourly space velocity (WHSV) of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The composition of the outlet gas was investigated via a gas chromatograph (Agilent 7890B, Agilent Technologies, USA) coupled to a thermal conductivity detector (TCD). The catalytic performance was evaluated based on the change in the concentrations of CH4, CO2 in the inlet and outlet flow mixtures according to:
000 mL g−1 h−1. The composition of the outlet gas was investigated via a gas chromatograph (Agilent 7890B, Agilent Technologies, USA) coupled to a thermal conductivity detector (TCD). The catalytic performance was evaluated based on the change in the concentrations of CH4, CO2 in the inlet and outlet flow mixtures according to:where Fi(in) and Fi(out) are the molar flow rate of inlet and outlet of species i, respectively.
3. Results and discussions
Un-promoted and promoted Ni–La2O3 were prepared using one-pot solution combustion synthesis. This procedure has several structural merits such as simplicity, energy effectiveness, and uniform distribution of components into the material's matrix, facile control of composition via change of oxidizer-to-fuel ratio.26 The Ni content of as-prepared catalysts was investigated by ICP-OES. The wt% of Ni showed comparable values ranging between 6.63–7.14% (Table 1). The morphology of as-synthesized catalysts was explored by SEM (Fig. 1).| Catalyst | wt% of Ni | Specific surface area SBET (m2 g−1) | Pore volume (cm3 g−1) | Pore radius (nm) | Ni average particle sizea (nm) | Crystallite sizeb (nm) |
|---|---|---|---|---|---|---|
| a Calculated from TEM.b Calculated from XRD. | ||||||
| Ni–La2O3 | 7.14 | 37.3 | 0.18 | 3.7 | 10.3 | 10.8 |
| Cs–Ni–La2O3 | 6.94 | 32.9 | 0.15 | 2.1 | 13.4 | 14.0 |
| K–Ni–La2O3 | 6.69 | 29.7 | 0.16 | 2.1 | 11.6 | 12.1 |
| Li–Ni–La2O3 | 6.63 | 42.9 | 0.24 | 2.5 | 14.3 | 14.9 |
| Mg–Ni–La2O3 | 6.78 | 54.5 | 0.36 | 2.8 | 14.8 | 15.6 |
| Na–Ni–La2O3 | 6.95 | 26.8 | 0.13 | 2.2 | 13.0 | 13.6 |
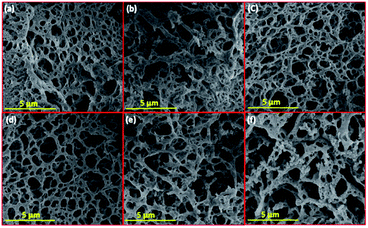 | ||
| Fig. 1 SEM images of fresh reduced samples; (a) Li–Ni–La2O3, (b) Na–Ni–La2O3, (c) K–Ni–La2O3, (d) Mg–Ni–La2O3, (e) Cs–Ni–La2O3, and (f) Ni–La2O3. | ||
All samples depict low-density spongy-like morphology with remarkable porous architecture containing large density of voids. This morphology can be attributed to the liberation of gases within short duration during the combustion process. Samples show similar morphology except Mg–Ni–La2O3, which exhibited higher density of voids with smaller size, which is an indication to higher porosity compared to other catalysts.
TEM of freshly reduced samples was investigated to get more deep insights on the structural properties of catalysts. The TEM images of reduced samples are shown in Fig. 2. They demonstrate good dispersion of Ni nanoparticles in the matrix of support, whereas the promoter nanoparticles cannot be detected owing to high dispersion within the support. The particle size distribution of Ni (Fig. 2 inlets) was estimated in all cases and the average size is given in Table 1. Results depicted that the incorporation of promoter did not enhance the dispersion of Ni in the La2O3 matrix, so far, the particle size of Ni increases into the promoted samples compared to pristine Ni–La2O3. This may be attributed to partial weakening in metal-support interaction encountered by the insertion of promoter into the La2O3 matrix.27
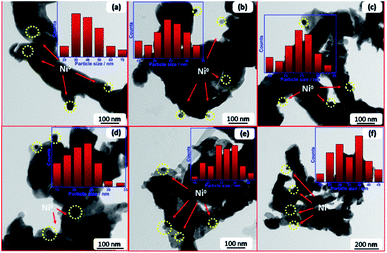 | ||
| Fig. 2 TEM images and particle size distribution of reduced samples; (a) Li–Ni–La2O3, (b) Na–Ni–La2O3, (c) K–Ni–La2O3, (d) Mg–Ni–La2O3, (e) Cs–Ni–La2O3, and (f) Ni–La2O3. | ||
Fig. 3 displayed N2 adsorption–desorption isotherms and pore size distributions of investigated Ni-based catalysts. According to the International Union of Pure and Applied Chemistry (IUPAC) classification, all catalysts exhibited type IV isotherms with H3 type hysteresis loop, which is characteristic feature for mesoporous materials.28 Pristine Ni–La2O3 and promoted-catalysts exhibited similar shape of the isotherm with a hysteresis loop extending from over a wide range of relative pressure P/P° of 0.2 to 1, which implies a non-uniformity of pore structure and a wide pore size distribution. The similarity in textural properties between Ni–La2O3 and X–Ni–La2O3 reflects that the addition of promoter has no remarkable impact of the porous structure.29 The specific surface area and pore dimensions (pore volume and pore diameter) were calculated using BJH (Barrett, Joyner, and Halenda) method and the data are given in Table 1. According to the obtained results, Mg–Ni–La2O3 exhibited the highest surface area and highest pore volume compared to other samples. This means that a greater number of accessible active sites available for the reactants, which can enhance the catalytic activity of Mg–Ni–La2O3 compared to other catalysts.
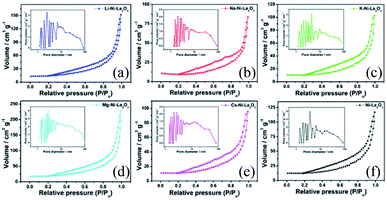 | ||
| Fig. 3 N2 adsorption/desorption isotherms and pore size distribution (inlet) of (a) Li–Ni–La2O3, (b) Na–Ni–La2O3, (c) K–Ni–La2O3, (d) Mg–Ni–La2O3, (e) Cs–Ni–La2O3, and (f) Ni–La2O3. | ||
Fig. 4a represents wide-angle XRD diffraction patterns of promoted and un-promoted Ni–La2O3 after reduction in H2 at 800 °C for 2 h. All samples revealed the presence of the diffractions at 2θ of 26.1, 29.1, 30.0, 39.5, 46.1, 52.2, 53.7, 55.4, 56.0, 60.4, 62.3, 66.8, 72.2, 73.5, 75.3, and 79.2, which are corresponding to (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), (202), (104), (203), (210), (211), and (114) which are the characteristic peaks of La2O3 (JCPDS card no. 05-0602), respectively. In addition, all samples exhibited a small diffraction peak at about 44.5°, corresponding to (111) diffraction of metallic Ni (JCPDS card no. 65-2865). No additional peaks were observed in the XRD patterns of promoted catalysts compared to un-promoted Ni–La2O3. This affirms that promoters have very small particle size and/or high dispersion in the La2O3 matrix, which is consistent with previous studies.30 The average crystallite size of different catalysts was estimated by Scherrer's equation using Ni(111) peak. The calculated values are given in Table 1. The results show that incorporation of promoters slightly increases Ni crystallite size, which is consistent with the data obtained from TEM.
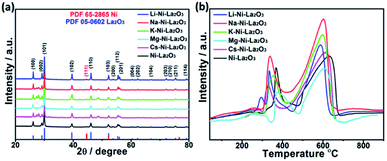 | ||
| Fig. 4 (a) XRD diffraction patterns of reduced catalysts and (b) H2-TPR profiles of as-prepared catalysts. | ||
Samples reducibility and redox properties of freshly prepared materials was probed by H2-TPR. Fig. 4b depicts the H2-TPR profiles of as-prepared Ni–La2O3 and promoted Ni–La2O3 samples. Similar reduction profiles were obtained for un-promoted and promoted samples (except Li–Ni–La2O3). Excluding Li-promoted catalyst, all other samples depicted two reduction peaks; the first peak in the temperature range of 320–410 °C, which is corresponding to weakly interacting NiO.31,32 Whereas, the second peak observed above 600 °C can be assigned to strongly interacting NiO in the form of perovskite.33,34 Li–Ni/La2O3 demonstrated three reduction peaks in the H2-TPR profile, the first peak can be attributed to the reduction of surface weakly interacting NiO species, the second peak can be assigned to bulk NiO, and the third peak corresponds to the reduction of strongly interacting NiO species in the form of perovskite. Furthermore, the lithium-promoted sample exhibits low reduction temperatures compared to other samples. The different behavior encountered by Li–Ni–La2O3 may arise from that Li2O remarkably weakens the interaction between Ni and La2O3, which is consistent to previous studies.35,36 Compared to un-promoted Ni–La2O3, all promoted samples reveal a negative shift in the reduction temperatures. This is consistent to the previous studies, which implies the same trend.37 This was attributed to competition between Ni and promoter species on the interaction with La2O3 support.37 This means that the insertion of promoter slightly decreases the interaction of Ni with La2O3 and shift the reduction of Ni species towards lower temperatures.38 Compared to other promoted catalysts, Mg–Ni–La2O3 demonstrated higher reduction temperatures, which affirms stronger MSI.
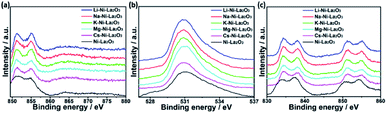
The surface composition and chemical entities of the freshly reduced catalysts were explored by XPS analysis. The XPS survey spectra of reduced catalysts depicted the presence of the promoter species in each case together with La, Ni, and O, which confirms their chemical composition (see Fig. 5 and S1†). The binding energies of Ni, La, and O were not significantly which confirms that their electronic environment was not affected by modification with the promoters (Fig. 5a–c).
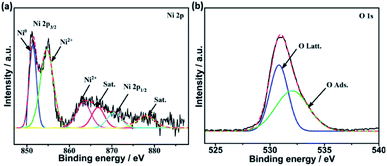
Fig. 6a demonstrates the high resolution spectra of Ni 2p in the reduced Mg–Ni–La2O3 catalyst. Deconvolution of Ni 2p region depicted two components. The low binding energy components at about 852.4 eV is assigned to metallic Ni and the other at higher binding energy 856.3 eV corresponds to NiO.39 The values of binding energies are relatively higher than normal values, which implies strong interaction between Ni and La2O3 support.40 Deconvoluted high resolution spectra of O 1 s region manifested two peaks. The first peak at 530.4–531 eV is attributed to lattice oxygen, whereas, the other peak at about 532.5 eV can be assigned to adsorbed oxygen species such as hydroxyls and carbonates.41
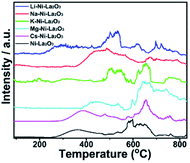
The basicity of samples was investigated by CO2-TPD. The desorption profiles are shown in Fig. 7. Three sets of peaks were observed. The desorption peaks below 400 °C can be attributed to weakly basic sites, the peaks 400–600 °C are assigned to basic sites of moderate strength, and the at temperatures higher than 600 °C corresponds to strong basic sites.42,43 The total amount of desorbed CO2 is larger in Mg–Ni–La2O3 compared to other catalysts, especially CO2 desorbed from strong basic sites. This implies greater amount and enhanced strength of basic sites which can facilitate the adsorption and activation of CO2 and enhance the DRM activity.
To investigate the effect of promoter type on the catalytic performance of Ni–La2O3, the DRM activity of investigated samples was examined at 700 °C for an operating time of 100 h using GHSV of 30 L g−1 h−1. Prior to the reaction, samples were reduced by 10% H2/Ar at 800 °C for 2 h to convert Ni species to active metallic Ni. After that, the gas mixture was purged through the fixed bed reactor and the catalytic activity was tested by evaluating the percentage of conversions of CO2 and CH4. Fig. 8 depicted the catalytic activity of promoted and un-promoted catalysts displayed in terms of variation of CO2 conversion, CH4 conversion, and H2/CO ratio with time-on-stream. The reaction was maintained for 100 h to explore long-term stability. Interestingly, all samples revealed slow increase in the conversion of CO2 and CH4 with time. According to previous reports, this increase in activity with time can be assigned to the incomplete reduction of Ni oxide species during the pre-treatment step.44 Other reports ascribed this enhancement during the initial conduction period to the slow establishment of equilibrium concentration of La2O2CO3, which is generated as an intermediate from the reaction of La2O3 with CO2.20,45 Intriguingly, all catalysts manifested low initial conversions for CH4 and CO2 followed by subsequent increase with time-on-stream until a steady state was attained which is consistent with previous studies.46 After this steady state CO2 and CH4 conversions remains constant until the end of operating time (100 h) with decay in the activity observed in CO2 and CH4 conversions in case of Ni–La2O3 and Li–Ni–La2O3. For all catalysts, CO2 conversions exhibited higher values than CH4 and the H2/CO ratio is less than unity. This can be attributed to the concurrent reverse water gas shift (RWGS), CO2 + H2 → CO + H2O.47
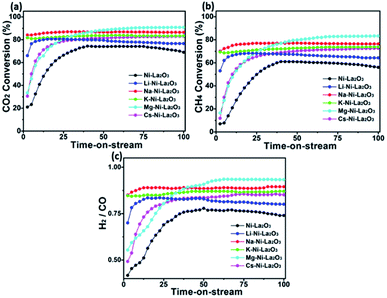 | ||
| Fig. 8 DRM catalytic performance measurements; (a) carbon dioxide conversion, (b) methane conversion, and (c) H2/CO ratio measured at 700 °C. | ||
It is well known that the ratio of H2/CO is influenced by DRM as well as the co-occurring side reactions (Boudouard, RWGS, and methane decomposition).12 The actual ratio of H2/CO is affected by coexisting side reactions such as RWGS, methane decomposition, and Boudouard reaction. All catalysts displayed a H2/CO ratio, which is fluctuating around almost constant value (Fig. 8c). This fluctuation is attributable to the concurrent carbon deposition and carbon gasification reactions.48 The value of H2/CO for all catalysts lies between 0.8 and 0.9, which affirms that RWGS is the dominating side reaction.
All promoted catalysts exhibited higher CO2 and methane conversions compared to Ni/La2O3, which provides a clear evidence on the role of promoter in enhancing the catalytic activity. The maximum CO2 conversion follows the order: Mg–Ni–La2O3 (90.8%) > Na–Ni/La2O3 (86.9%) > K–Ni–La2O3 (83.7%) > Cs–Ni–La2O3 (83.0%) > Li–Ni–La2O3 (80.6%) > Ni–La2O3 (74.4%). Similarly, the maximum CH4 conversion follows the same order with values of 83.2%, 76.3%, 73.7%, 72.5%, 64.3%, and 56.0%, in case of Mg–Ni–La2O3, Na–Ni–La2O3, K–Ni–La2O3, Cs–Ni–La2O3, Li–Ni–La2O3, and Ni–La2O3, respectively. After reaching the maximum conversion, all catalysts demonstrated negligible decay in the activity until the end of DRM operating time (100 h) except Li–Ni–La2O3 and un-promoted catalyst, which revealed a significant decay in the activity with time. Li–Ni–La2O3 demonstrated 5.0 and 5.7% decay in the CO2 and CH4 conversion, respectively, while Ni/La2O3 exhibited 7.3% and 21.3% in the conversions of CO2 and CH4, respectively. This provides a clear evidence for the stabilizing impact of promoting species on the long-term stability of catalysts compared to Li–Ni–La2O3 and Ni–La2O3.
Fig. 9 displays the TEM images and Ni particle size distributions of spent catalysts after running for 100 h at 700 °C. Some Ni particles were agglomerated owing to high temperature sintering. The average particle size of Ni in the spent samples is 23.1, 30.1, 26.3, 34.2, 29.4, and 28.6 nm in case of Mg–Ni–La2O3, Na–Ni–La2O3, K–Ni–La2O3, Li–Ni–La2O3, Cs–Ni–La2O3, and Ni–La2O3, respectively. Based on the ratio of change in the Ni particle size during the reaction, Li–Ni–La2O3 and Ni–La2O3 demonstrated the greatest increase in the Ni particle size, whereas, Mg–Ni–La2O3 and Na–Ni–La2O3 revealed the lowest ratio of increase in the Ni particle size (Fig. 9). This can explain the rapid decay in the DRM activity with time in case of un-promoted and Li-promoted samples compared to other catalysts. Owing to the increase in the particle size by sintering, the number of Ni active centers decreased by agglomeration. This decreased the catalytic activity and diminished the long-term stability. This was confirmed by TEM of spent samples, which manifested the formation of filamentous type carbon in the un-promoted catalyst in contrast to all promoted samples that reveal no observable coke formation (Fig. 9).
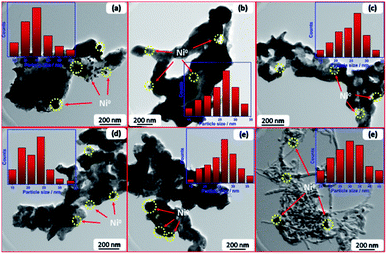 | ||
| Fig. 9 TEM images of spent samples; (a) Li–Ni–La2O3, (b) Na–Ni–La2O3, (c) K–Ni–La2O3, (d) Mg–Ni–La2O3, (e) Cs–Ni–La2O3, and (f) Ni–La2O3. | ||
Fig. 10a depicts XRD of spent samples after DRM operating time of 100 h at 700 °C. Similar diffraction patterns were obtained by promoted Ni–La2O3 and no observable peaks were obtained for the alkali metal oxides. New diffractions peaks were observed at 2θ of 15.6, 27.5, 28.0, 42.5, 47.4 48.6, 50.1, 69.9, 70.9, and 75.7° corresponding to La(OH)3 (JCPDS card no. 36-1481). La(OH)3 was formed as a result of reaction between La2O3 and H2O that produced by RWGS i.e. La2O3 + 3H2O → 2La(OH)3. In addition, new diffractions were observed at 14.2, 23.1, and 29.2° which can be indexed to La2O2CO3 (JCPDS card no. 48-1113). During the DRM reaction, La2O3 reacts with CO2 to form La2O2CO3.49 Unfortunately, we couldn't calculate the Ni crystallite size in the spent samples from XRD since the Ni(111) diffraction peak overlapped with the diffractions of La2O2CO3 at about 44.5°. Furthermore, the graphite peak at 2θ of 26° could not be identified owing to the interference with (100) diffraction peak of La2O3 (JCPDS card no. 05-0602).
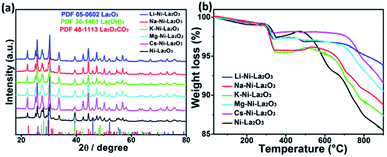 | ||
| Fig. 10 (a) XRD patterns and (b) TGA profiles of spent samples after 100 h operation time at 700 °C. | ||
Fig. 10b represents the TGA profiles of spent catalysts after DRM 100 h duration time at 700 °C. The slight weight loss before 200 °C can be assigned to the release of physisorbed water in La(OH)3.50 The slight weight gain in the temperature range 300–400 °C can be ascribed to the oxidation of metallic Ni to NiO.51
The weight decline in the temperature range 400–600 °C owing to oxidation of coke. Only un-promoted sample revealed a noticeable weight loss in 400–600 °C, which is attributed to the oxidation of filamentous carbon, whereas, no weight loss was observed for all promoted samples in the same temperature range owing to the absence of coke deposition, which is consistent to TEM results of the spent samples. On the other hand, the mass loss above 700 °C can be attributed to the decomposition of LaO2CO3.52
The XPS of spent samples was investigated to probe the change in the nature and the chemical states of different elements. High resolution spectra of C 1s can provide relevant information about the nature of carbon species, respectively at the utmost surface of the spent samples. Deconvolution of C 1s spectrum results in 3 peaks at 288.4 eV, 287.5 eV and 284.6 eV which can be assigned to CO32−, C–O, and C–C species, respectively.44 Whereas, the peak at lower binding energy (283.9 eV) can be assigned to Ni carbide species at the catalyst surface.53 The Ni 2p peak in spent samples was shifted in all catalysts towards higher binding energies assigned to NiO, implying partial decrease in the metallic character of Ni (Fig. S2a†).54 The differentiation between La3+ species in oxide and hydroxides is extremely difficult, thereon, this can be executed through the O 1s peaks.54 It is reported that the O 1s in La2O3 appear at lower binding energies than O 1s in La(OH)3. Based on this, it could be understood that La in the oxide has a greater tendency to donate electrons to the adjacent oxygen atoms compared to the La in the hydroxide. This shifted the binding energy of oxygen to lower values.20 This confirms that La2O3 was partially converted to La(OH)3 in spent samples which confirms the XRD analysis of spent catalysts (Fig. S2b†).
DRM requires bifunctional catalysts capable of promoting two simultaneous processes i.e. the cracking of methane and the oxidation of carbon species. It is well-known that deactivation of Ni-based catalysts during DRM is mainly assigned to the catalyst sintering as well as coke deposition.12 It is also noteworthy that, metal-support interaction (MSI) remarkably influence the catalytic activity and durability of the catalyst, where it enhances the stability catalyst against sintering and also against deactivation through carbon deposition. La2O3 has strong MSI with Ni catalyst, so far, it enhances Ni dispersion and stability against sintering at high reaction temperatures.55 It enhances the adsorption of CO2 to form La2O2CO3 which is capable of removal of deposited coke via the reaction: La2O2CO3 + 2C → La2O3 + 2CO.56 However, the role of MSI is not the prevailing factor affecting the catalytic performance of the investigated catalyst. This can be evident from the inferior catalytic activity and durability of un-promoted catalyst although it has higher MSI compared to promoted catalysts. Intriguingly, the particle size of Ni in fresh reduced Ni–La2O3 was smaller than all promoted samples, so far, it was expected to afford comparable catalytic performance towards DRM.57 However, Ni–La2O3 expressed the lowest activity and its spent catalyst showed the largest increase in the Ni particle size. Besides, only un-promoted sample manifested the formation of coke compared to all promoted samples. All these factors confirms imply that the catalyst basicity has the dominant role that affect the catalytic performance. Hence, Mg–Ni–La2O3 afforded the best catalytic activity and the highest stability.
According to the obtained results, the mechanism of DRM on un-promoted and promoted Ni–La2O3 can be represented by Scheme 1. Methane is firstly adsorbed at Ni sites and decomposed to CHx intermediate species according to eqn (1).58 Simultaneously, CO2 is adsorbed especially at the more basic sites on La2O3 or at the promoter oxide. CHx species are gasified according to eqn (2).58,59 However, the dissociation of methane on active Ni is usually a rapid process and the formed intermediate CHx fragments may not be adjacent to activated CO2 molecules, which results in further growth of carbon on the active Ni centers and deposition of coke.60 The presence of promoter species not only enhance the basicity of La2O3 but the promoter itself can act as active sites for adsorption and activation of CO2. The chemisorption of CO2 can generate CO and active oxygen species, which enhance the gasification of CHx species and prevent the coke deposition.60,61 This was affirmed by the results, which revealed coke deposition in case of un-promoted sample, whereas promoted samples do not exhibit observable deposited carbon after DRM durability tests. The MSI play an important role regarding to long-term stability, so far, Li–Ni–La2O3 exhibited weakened MSI encountered by Li2O, which results in sintering at high temperatures and diminishes the catalytic performance.
| xCH4 → CxH1−x + (5x − 1/2) H2 | (1) |
| CxH1−x + xCO2 → 2xCO + (1 − x/2) H2 | (2) |
To summarize, the addition of promoters enhances both the catalytic activity and durability of Ni–La2O3 through three different roles. Firstly, the addition of MgO or other alkali metals oxides to Ni–La2O3 not only enhance the basicity of the catalyst but also it can create surface oxygen ionic species that enhance the stability of La2O2CO3 phase which is liable for gasification of deposited coke, protecting Ni active centers from deactivation.33,61
The greater number of oxygen vacancies and the higher oxygen mobility enhances the resistance to coke formation.62 Furthermore, the incorporation of alkali metals and alkaline earth metals increase the density of lattice oxygen surface species which enhances the activation of methane C–H bond and thus foster the catalytic activity.21 Last but not least, the promoters enhance the surface alkalinity through increasing the number of basic sites which in turn enhance the activation of CO2 and facilitate the gasification of deposited carbon at the active sites.63 This was confirmed by the absence of carbon deposits in all spent catalysts except Ni–La2O3 owing to low basicity compared to Mg and other alkali metals.
4. Conclusions
Promoted and un-promoted Ni–La2O3 catalysts were successfully synthesized via one-step solution combustion route and their activity towards DRM was studied. All samples manifested high specific surface area and enhanced porosity owing to rapid ignition/combustion process and evolution of large amount of gases. In terms of DRM performance, all samples afforded high catalytic activity with CO2 and CH4 conversions higher than the equilibrium values at 700 °C. Promoted catalysts demonstrated enhanced catalytic activity and durability compared to un-promoted sample, which was ascribed to enhanced Lewis basicity. Particularly, Mg–Ni–La2O3 exhibited outstanding anti-coking capability compared to other samples. This can be assigned to two main factors. The first is the high surface area and high porosity compared to its counterparts, thereon, it can provide more active sites for DRM which enhances the catalytic activity. In addition, the incorporation of Mg afforded the increased number of basic sites while maintaining strong MSI compared to other promoters. This enhances the adsorption of CO2 and the supply of surface oxygen species, which inhibit the coke deposition and retard the catalyst deactivation via high temperature sintering. This is evidenced by comparing the percentages of adsorbed oxygen in promoted and unpromoted catalysts. In the unpromoted sample, the adsorbed oxygen constitutes 12.0% of the total oxygen, while in the promoted catalysts, the ratio of adsorbed oxygen ranges between 44–54% (see Fig. S3 and Table S1†). On the other hand, among all promoted catalysts, only Li–Ni–La2O3 revealed decay in long-term stability. This was assigned to the high temperature sintering endowed by weakened MSI.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication was supported by Qatar University, internal grant number QUCG-CENG-19/20-7. The findings achieved herein are solely the responsibility of the authors. The authors also acknowledge the technical support of Central Laboratories Unit (CLU), Center for Advanced Materials (CAM), and Gas Processing Center (GPC), Qatar University, Doha, Qatar.Notes and references
- S. Wang, G. Lu and G. J. Millar, Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art, Energy Fuels, 1996, 10, 896–904 CrossRef CAS.
- M. M. Souza, D. A. Aranda and M. Schmal, Reforming of methane with carbon dioxide over Pt/ZrO2/Al2O3 catalysts, J. Catal., 2001, 204, 498–511 CrossRef CAS.
- A. Erdohelyi, J. Cserényi and F. Solymosi, Activation of CH4 and its reaction with CO2 over supported Rh catalysts, J. Catal., 1993, 141, 287–299 CrossRef CAS.
- F. Wang, L. Xu, J. Yang, J. Zhang, L. Zhang, H. Li, Y. Zhao, H. X. Li, K. Wu and G. Q. Xu, Enhanced catalytic performance of Ir catalysts supported on ceria-based solid solutions for methane dry reforming reaction, Catal. Today, 2017, 281, 295–303 CrossRef CAS.
- M. Soria, C. Mateos-Pedrero, A. Guerrero-Ruiz and I. Rodríguez-Ramos, Thermodynamic and experimental study of combined dry and steam reforming of methane on Ru/ZrO2–La2O3 catalyst at low temperature, Int. J. Hydrogen Energy, 2011, 36, 15212–15220 CrossRef CAS.
- R. K. Singha, A. Yadav, A. Shukla, M. Kumar and R. Bal, Low temperature dry reforming of methane over Pd–CeO2 nanocatalyst, Catal. Commun., 2017, 92, 19–22 CrossRef CAS.
- G. P. Figueredo, R. L. Medeiros, H. P. Macedo, Â. A. de Oliveira, R. M. Braga, J. M. Mercury, M. A. Melo and D. M. Melo, A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3, Int. J. Hydrogen Energy, 2018, 43, 11022–11037 CrossRef CAS.
- T. Xie, L. Shi, J. Zhang and D. Zhang, Immobilizing Ni nanoparticles to mesoporous silica with size and location control via a polyol-assisted route for coking-and sintering-resistant dry reforming of methane, Chem. Commun., 2014, 50, 7250–7253 RSC.
- Z. Wu, B. Yang, S. Miao, W. Liu, J. Xie, S. Lee, M. J. Pellin, D. Xiao, D. Su and D. Ma, Lattice Strained Ni–Co alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane, ACS Catal., 2019, 9, 2693–2700 CrossRef CAS.
- Q. Zhang, X. Feng, J. Liu, L. Zhao, X. Song, P. Zhang and L. Gao, Hollow hierarchical Ni/MgO–SiO2 catalyst with high activity, thermal stability and coking resistance for catalytic dry reforming of methane, Int. J. Hydrogen Energy, 2018, 43, 11056–11068 CrossRef CAS.
- Y. Cao, M. Lu, J. Fang, L. Shi and D. Zhang, Hexagonal boron nitride supported meso SiO2-confined Ni catalysts for dry reforming of methane, Chem. Commun., 2017, 53, 7549–7552 RSC.
- Q. Zhang, T. Zhang, Y. Shi, B. Zhao, M. Wang, Q. Liu, J. Wang, K. Long, Y. Duan and P. Ning, A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane, J. CO2 Util., 2017, 17, 10–19 CrossRef CAS.
- C. A. Schwengber, F. A. da Silva, R. A. Schaffner, N. R. C. Fernandes-Machado, R. J. Ferracin, V. R. Bach and H. J. Alves, Methane dry reforming using Ni/Al2O3 catalysts: evaluation of the effects of temperature, space velocity and reaction time, J. Environ. Chem. Eng., 2016, 4, 3688–3695 CrossRef CAS.
- M. Khajenoori, M. Rezaei and F. Meshkani, Dry reforming over CeO2-promoted Ni/MgO nano-catalyst: effect of Ni loading and CH4/CO2 molar ratio, J. Ind. Eng. Chem., 2015, 21, 717–722 CrossRef CAS.
- Y. Lou, M. Steib, Q. Zhang, K. Tiefenbacher, A. Horváth, A. Jentys, Y. Liu and J. A. Lercher, Design of stable Ni/ZrO2 catalysts for dry reforming of methane, J. Catal., 2017, 356, 147–156 CrossRef CAS.
- V. Tsipouriari and X. Verykios, Carbon and oxygen reaction pathways of CO2 reforming of methane over Ni/La2O3 and Ni/Al2O3 catalysts studied by isotopic tracing techniques, J. Catal., 1999, 187, 85–94 CrossRef CAS.
- X. E. Verykios, Catalytic dry reforming of natural gas for the production of chemicals and hydrogen, Int. J. Hydrogen Energy, 2003, 28, 1045–1063 CAS.
- L. Mo, X. Zheng, Q. Jing, H. Lou and J. Fei, Combined Carbon Dioxide Reforming and Partial Oxidation of Methane to Syngas over Ni–La2O3/SiO2 Catalysts in a Fluidized-Bed Reactor, Energy Fuels, 2005, 19, 49–53 CrossRef CAS.
- Z. Alipour, M. Rezaei and F. Meshkani, Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane, J. Ind. Eng. Chem., 2014, 20, 2858–2863 CrossRef CAS.
- J. Ni, L. Chen, J. Lin, M. K. Schreyer, Z. Wang and S. Kawi, High performance of Mg–La mixed oxides supported Ni catalysts for dry reforming of methane: the effect of crystal structure, Int. J. Hydrogen Energy, 2013, 38, 13631–13642 CrossRef CAS.
- B. Jin, S. Li and X. Liang, Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: role of MgO, Fuel, 2021, 284, 119082 CrossRef CAS.
- A. Varma, A. S. Mukasyan, A. S. Rogachev and K. V. Manukyan, Solution combustion synthesis of nanoscale materials, Chem. Rev., 2016, 116, 14493–14586 CrossRef CAS PubMed.
- V. Danghyan, A. Kumar, A. Mukasyan and E. Wolf, An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane, Appl. Catal., B, 2020, 273, 119056 CrossRef CAS.
- L. Mo, K. K. M. Leong and S. J. C. S. Kawi, A highly dispersed and anti-coking Ni–La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane, Catal. Sci. Technol., 2014, 4, 2107–2114 RSC.
- C. Chen, X. Wang, L. Zhang, X. Zou, W. Ding and X. Lu, Synthesis of mesoporous Ni–La2O3/SiO2 by poly(ethylene glycol)-assisted sol–gel route as highly efficient catalysts for dry reforming of methane with a H2/CO ratio of unity, Catal. Commun., 2017, 94, 38–41 CrossRef CAS.
- W. Wen and J.-M. Wu, Nanomaterials via solution combustion synthesis: a step nearer to controllability, RSC Adv., 2014, 4, 58090–58100 RSC.
- C. Montero, A. Ochoa, P. Castaño, J. Bilbao and A. G. Gayubo, Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming in a fluidized bed, J. Catal., 2015, 331, 181–192 CrossRef CAS.
- L. Xu, H. Song and L. Chou, Carbon dioxide reforming of methane over ordered mesoporous NiO–Al2O3 composite oxides, Catal. Sci. Technol., 2011, 1, 1032–1042 RSC.
- N. Habibi, Y. Wang, H. Arandiyan and M. Rezaei, Biogas Reforming for Hydrogen Production: A New Path to High-Performance Nickel Catalysts Supported on Magnesium Aluminate Spinel, ChemCatChem, 2016, 8, 3600–3610 CrossRef CAS.
- Z. Taherian, M. Yousefpour, M. Tajally and B. Khoshandam, A comparative study of ZrO2, Y2O3 and Sm2O3 promoted Ni/SBA-15 catalysts for evaluation of CO2/methane reforming performance, Int. J. Hydrogen Energy, 2017, 42, 16408–16420 CrossRef CAS.
- I. Rivas, J. Alvarez, E. Pietri, M. J. Pérez-Zurita and M. R. Goldwasser, Perovskite-type oxides in methane dry reforming: effect of their incorporation into a mesoporous SBA-15 silica–host, Catal. Today, 2010, 149, 388–393 CrossRef CAS.
- L. Mo, K. K. M. Leong and S. Kawi, A highly dispersed and anti-coking Ni–La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane, Catal. Sci. Technol., 2014, 4, 2107–2114 RSC.
- N. Wang, X. Yu, Y. Wang, W. Chu and M. Liu, A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier, Catal. Today, 2013, 212, 98–107 CrossRef CAS.
- G. Valderrama, M. R. Goldwasser, C. U. de Navarro, J. M. Tatibouet, J. Barrault, C. Batiot-Dupeyrat and F. Martínez, Dry reforming of methane over Ni perovskite type oxides, Catal. Today, 2005, 107, 785–791 CrossRef.
- S.-W. Park, D. Lee, S.-I. Kim, Y. J. Kim, J. H. Park, I. Heo, T. S. Chang and J. H. Lee, Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming, Catalysts, 2021, 11, 260 CrossRef CAS.
- F. Pompeo, N. N. Nichio, M. G. González and M. Montes, Characterization of Ni/SiO2 and Ni/Li–SiO2 catalysts for methane dry reforming, Catal. Today, 2005, 107, 856–862 CrossRef.
- L. Xu, H. Song and L. Chou, Carbon dioxide reforming of methane over ordered mesoporous NiO–MgO–Al2O3 composite oxides, Appl. Catal., B, 2011, 108, 177–190 CrossRef.
- J. Dias and J. Assaf, Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane, Catal. Today, 2003, 85, 59–68 CrossRef CAS.
- J. Requies, V. Barrio, J. Cambra, M. Güemez, P. Arias, V. La Parola, M. Pena and J. Fierro, Effect of redox additives over Ni/Al2O3 catalysts on syngas production via methane catalytic partial oxidation, Fuel, 2008, 87, 3223–3231 CrossRef CAS.
- N. Wang, X. Yu, K. Shen, W. Chu and W. Qian, Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen, Int. J. Hydrogen Energy, 2013, 38, 9718–9731 CrossRef CAS.
- J. Requies, M. Cabrero, V. Barrio, M. Güemez, J. Cambra, P. Arias, F. Pérez-Alonso, M. Ojeda, M. Peña and J. Fierro, Partial oxidation of methane to syngas over Ni/MgO and Ni/La2O3 catalysts, Appl. Catal., A, 2005, 289, 214–223 CrossRef CAS.
- A. Nagu, K. Vasikerappa, P. Gidyonu, C. Prathap, M. V. Rao, K. S. R. Rao and B. D. Raju, Additive-free vapour-phase hydrogenation of benzonitrile over MgO-supported Ni catalysts, Res. Chem. Intermed., 2020, 46, 2669–2681 CrossRef CAS.
- L. Xu, W. Liu, X. Zhang, L. Tao, L. Xia, X. Xu, J. Song, W. Zhou, X. Fang and X. Wang, Ni/La2O3 Catalysts for Dry Reforming of Methane: Insights into the Factors Improving the Catalytic Performance, ChemCatChem, 2019, 11, 2887–2899 CrossRef CAS.
- M. Yu, K. Zhu, Z. Liu, H. Xiao, W. Deng and X. Zhou, Carbon dioxide reforming of methane over promoted NixMg1−xO (111) platelet catalyst derived from solvothermal synthesis, Appl. Catal., B, 2014, 148–149, 177–190 CrossRef CAS.
- Z. Zhang and X. E. Verykios, Carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 catalysts, Appl. Catal., A, 1996, 138, 109–133 CrossRef CAS.
- H. Zhou, T. Zhang, Z. Sui, Y.-A. Zhu, C. Han, K. Zhu and X. Zhou, A single source method to generate Ru–Ni–MgO catalysts for methane dry reforming and the kinetic effect of Ru on carbon deposition and gasification, Appl. Catal., B, 2018, 233, 143–159 CrossRef CAS.
- H. Wang, X. Dong, T. Zhao, H. Yu and M. Li, Dry reforming of methane over bimetallic Ni–Co catalyst prepared from La(CoxNi1−x)0.5Fe0.5O3 perovskite precursor: catalytic activity and coking resistance, Appl. Catal., B, 2019, 245, 302–313 CrossRef CAS.
- L. Xu, Z. Miao, H. Song and L. Chou, CO2 reforming of CH4 over rare earth elements functionalized mesoporous Ni–Ln (Ln = Ce, La, Sm, Pr)–Al–O composite oxides, Int. J. Hydrogen Energy, 2014, 39, 3253–3268 CrossRef CAS.
- E.-h. Yang and D. J. Moon, CO2 Reforming of Methane over Ni0/La2O3 Catalyst Without Reduction Step: Effect of Calcination Atmosphere, Top. Catal., 2017, 60, 697–705 CrossRef CAS.
- Q. Mu and Y. Wang, Synthesis, characterization, shape-preserved transformation, and optical properties of La(OH)3, La2O2CO3, and La2O3 nanorods, J. Alloys Compd., 2011, 509, 396–401 CrossRef CAS.
- Y. Chai, Y. Fu, H. Feng, W. Kong, C. Yuan, B. Pan, J. Zhang and Y. Sun, A Nickel-Based Perovskite Catalyst with a Bimodal Size Distribution of Nickel Particles for Dry Reforming of Methane, ChemCatChem, 2018, 10, 2078–2086 CrossRef CAS.
- X. Li, D. Li, H. Tian, L. Zeng, Z.-J. Zhao and J. Gong, Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles, Appl. Catal., B, 2017, 202, 683–694 CrossRef CAS.
- J. Shabaker, D. Simonetti, R. Cortright and J. Dumesic, Sn-modified Ni catalysts for aqueous-phase reforming: characterization and deactivation studies, J. Catal., 2005, 231, 67–76 CrossRef CAS.
- M. Akri, S. Pronier, T. Chafik, O. Achak, P. Granger, P. Simon, M. Trentesaux and C. Batiot-Dupeyrat, Development of nickel supported La and Ce-natural illite clay for autothermal dry reforming of methane: toward a better resistance to deactivation, Appl. Catal., B, 2017, 205, 519–531 CrossRef CAS.
- S. Li, H. Tang, D. Gong, Z. Ma and Y. Liu, Loading Ni/La2O3 on SiO2 for CO methanation from syngas, Catal. Today, 2017, 297, 298–307 CrossRef CAS.
- G. S. Gallego, F. Mondragón, J.-M. Tatibouët, J. Barrault and C. Batiot-Dupeyrat, Carbon dioxide reforming of methane over La2NiO4 as catalyst precursor—Characterization of carbon deposition, Catal. Today, 2008, 133, 200–209 CrossRef.
- J.-H. Kim, D. J. Suh, T.-J. Park and K.-L. Kim, Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts, Appl. Catal., A, 2000, 197, 191–200 CrossRef CAS.
- S. Y. Foo, C. K. Cheng, T.-H. Nguyen and A. A. Adesina, Kinetic study of methane CO2 reforming on Co–Ni/Al2O3 and Ce–Co–Ni/Al2O3 catalysts, Catal. Today, 2011, 164, 221–226 CrossRef CAS.
- C. C. Chong, H. D. Setiabudi and A. A. Jalil, Dendritic fibrous SBA-15 supported nickel (Ni/DFSBA-15): a sustainable catalyst for hydrogen production, Int. J. Hydrogen Energy, 2020, 45, 18533–18548 CrossRef CAS.
- X. Du, D. Zhang, R. Gao, L. Huang, L. Shi and J. Zhang, Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane, Chem. Commun., 2013, 49, 6770–6772 RSC.
- N. Wang, K. Shen, L. Huang, X. Yu, W. Qian and W. Chu, Facile Route for Synthesizing Ordered Mesoporous Ni–Ce–Al Oxide Materials and Their Catalytic Performance for Methane Dry Reforming to Hydrogen and Syngas, ACS Catal., 2013, 3, 1638–1651 CrossRef CAS.
- N. Wang, W. Qian, W. Chu and F. Wei, Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming, Catal. Sci. Technol., 2016, 6, 3594–3605 RSC.
- S. Podila, Y. A. Alhamed, A. A. AlZahrani and L. A. Petrov, Hydrogen production by ammonia decomposition using Co catalyst supported on Mg mixed oxide systems, Int. J. Hydrogen Energy, 2015, 40, 15411–15422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05511a |
| This journal is © The Royal Society of Chemistry 2021 |