Open Access Article
Open Access ArticleEffect of free amino acids and peptide hydrolysates from sunflower seed protein on the formation of pyrazines under different heating conditions†
Furong Wang ab,
Hailiang Shencd,
Xi Yangab,
Ting Liuab,
Yali Yang*ab,
Xueru Zhouab,
Pengtao Zhaoab and
Yurong Guo
ab,
Hailiang Shencd,
Xi Yangab,
Ting Liuab,
Yali Yang*ab,
Xueru Zhouab,
Pengtao Zhaoab and
Yurong Guo ab
ab
aCollege of Food Engineering and Nutritional Science, Shaanxi Normal University, Campus Chang'an, No. 620, West Chang'an Avenue, Chang'an District, Xi'an 710119, PR China. E-mail: yangyali@snnu.edu.cn; Fax: +86 029 85310471; Tel: +86 029 85310471
bNational Research and Development Center of Apple Processing Technology, PR China
cCitrus Research Institute, Southwest University, Chongqing, PR China
dCitrus Research Institute, Chinese Academy of Agricultural Science, Chongqing, PR China
First published on 16th August 2021
Abstract
Most research concerning pyrazine formation in the Maillard reaction is mainly focused on free amino acids (FAAs), but limited information is available on the effect of peptides and proteins. In this study, three Maillard model systems (i.e., glucose and native sunflower seed protein, hydrolyzed peptides or FAAs, respectively) were prepared, and their effect on the formation of volatiles were further compared at different heating conditions by using of headspace solid-phase microextraction equipped with gas chromatography/mass spectrometry (HS-SPME-GC/MS). It was found that pyrazines were the characteristic volatile compounds in tested Maillard models, and with increasing heating temperature and time, the varieties of pyrazine formation significantly increased. The optimum reaction condition for pyrazine formation was at 140 °C for 90 min, which was subsequently applied to all sets of Maillard models. Further analysis showed that the short chain peptides generated by hydrolyzing sunflower seed protein (SSP), especially the molecular weight ranging from 1.2 to 3.0 kDa, significantly promoted the formation of pyrazines, which highlights the important role of peptides in the Maillard reaction models and is expected to intensify aroma promotion in sunflower seed oil.
1. Introduction
The Maillard reaction is a chemical reaction between a carbonyl compound and an amino compound under thermal conditions, which plays an essential role in formation of volatile compounds and confers foods with aromatic flavor.1–3 Pyrazines are characteristic products of the Maillard reaction (MRPs) and significantly contribute to the baked, roasted, meaty and popcorn-like aroma in heated foods.4,5 In some edible vegetable oil such as sunflower seed oil, peanut oil and canola oil, pyrazines are produced in heating processing and also identified as the typical volatile substances.6,7 Pyrazines, mainly 2,5-dimethypyrazine, are the key volatile components of baked sunflower seeds.Temperature and time are two key parameters that affect the formation of volatile compounds in MRPs. Several studies reported that formation of pyrazines increased with increased temperature.8,9 In glycine–glucose Maillard model system, more volatile compounds are produced at 180 °C than at 120 °C, which was probably because higher temperature is favorable for promoting the reaction rate between the sugar and amino groups.10,11 Zhang et al. showed that the system of xylose and soybean peptide exhibited a lower bitterness as temperature increased from 100 to 140 °C.12 Similar results were also reported by Lan et al., who found that high temperatures changed the pathway of the Maillard reaction and reduced the content of bitter amino acids.13 On the other hand, heating time has an important effect on MRPs. It was reported that the MRPs from Chinese shrimp waste hydrolysates (CSWHs) and xylose heated for 60 min exhibited a strong meaty aroma and umami taste as well as seafood aroma.14
So far, researchers have established numerous Maillard reaction models to study the pathways of pyrazines formation,6,15–18 where amino acids and carbonyl compounds were used as the main precursors. However, in the food systems, the content of FAAs is much lower than that of peptides or proteins, whereas the related reports regarding the formation of pyrazines in protein–carbohydrate and peptide–carbohydrate models are very limited. Previously, it was experimentally demonstrated that the peptides from whey protein hydrolysis played an important role in pyrazine formation.19–21
Although peptides are recognized as the precursors for MRPs, their contribution to formation of typical volatile compounds in sunflower seed oil processing has not been well understood.22–25 Therefore, this study was to explore the effect of heating temperature and time on the formation of pyrazines, with the aim to reveal the potential roles of FAAs and hydrolyzed sunflower seed peptides in the Maillard reaction model.
2. Materials and methods
2.1. Materials
Amino acids, including L-lysine, L-histidine, L-arginine, L-tyrosine, L-phenylalanine, L-isoleucine, L-leucine, L-serine, L-glutamate, L-valine, L-glycine, were purchased from Shanghai Yuanye Bio-Technology Co., Ltd, China. All of them have a purity higher than 98%. Sunflower seed protein isolates, trypsin from porcine pancreas were purchased from Shanghai Yuanye Bio-Technology Co., Ltd, China. 0.1 M potassium phosphate buffer (pH 7.8) and 0.2 M phosphate buffer (pH 8.0) were purchased from Xi'an Hat Biotechnology Co., Ltd, China. Glucose and absolute ethanol were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd, China. Edible solvent extraction sunflower seed oil was purchased from a local supermarket (Xi'an, China).2.2. Hydrolysis of sunflower seed protein (SSP)
SSP was dissolved in 0.1 M potassium phosphate buffer (pH 7.8) to achieve 5 mg mL−1 of concentration, followed by heating at 95 °C for 5 min and then cooling at room temperature. Trypsin was added to the solution at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w). The mixture was fully mixed and incubated in a water bath at room temperature for 20 h. Then, the solution was heated at 95 °C for 5 min to inactive the enzymes. The hydrolyzed samples were frozen at −18 °C for further use.
20 (w/w). The mixture was fully mixed and incubated in a water bath at room temperature for 20 h. Then, the solution was heated at 95 °C for 5 min to inactive the enzymes. The hydrolyzed samples were frozen at −18 °C for further use.
2.3. Analysis of free amino acids (FAAs)
The hydrolyzed solution was filtered with Buchner funnel to obtain the supernatant, which was mixed with absolute ethanol at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v). Subsequently, the mixture was held for 30 min and centrifuged at 1000 rpm for 15 min. 10 mL supernatant was then evaporated in a water bath at 100 °C, after which 5 mL double-distilled water was added to dissolve the dry matter. The solution was filtered in a Millex-GP 0.22 μm filter (Merck Millipore LTD, Cork, Ireland) and then analyzed by using S-443D amino acid analyzer (Sakam, Germany).19
4 (v/v). Subsequently, the mixture was held for 30 min and centrifuged at 1000 rpm for 15 min. 10 mL supernatant was then evaporated in a water bath at 100 °C, after which 5 mL double-distilled water was added to dissolve the dry matter. The solution was filtered in a Millex-GP 0.22 μm filter (Merck Millipore LTD, Cork, Ireland) and then analyzed by using S-443D amino acid analyzer (Sakam, Germany).19
2.4. Gel permeation chromatography (GPC)
The dissolved sunflower seed protein and hydrolyzed sunflower seed protein were filtered through a 0.22 μm filter. Then, the samples were analyzed with a HELEOS system gel permeation chromatography (Wyatt, America) coupled with the Wyatt DAWN HELEOS-II laser detector and the Wyatt Optilab-rEX differential detector equipped with a Shodex OH-pak SB-806 column. The working conditions include ultra-pure water (0.2 mol L−1 potassium dihydrogen phosphate and 0.02% sodium azide) as the mobile phase, 0.5 mL min−1 of flow rate, 35 °C of column temperature and 500 μL of infusion volume.2.5. Maillard reaction models
Four groups of Maillard reaction model systems were prepared as follows: (1) native SSP model: 100 mg native SSP + 100 mg glucose; (2) native SSP & FAAs model: 100 mg native SSP, 100 mg glucose + FAAs (equivalent to the free amino acid content of trypsin hydrolysis of SSP), as shown in Table 1; (3) hydrolyzed SSP model: 100 mg hydrolyzed SSP products + 100 mg glucose. The mixtures of three groups were dissolved in 10 mL 0.2 M phosphate buffer (pH 8.0), and then transferred to a 20 mL SPME (Solid-Phase Microextraction) vials (Nanjing Daobang Biotechnology Co., Ltd, China) and heated in an oil bath at 100–140 °C range and 30–60 min of heating time, respectively. Subsequently, the mixtures were immediately cooled in a cold-water bath. The samples were analyzed using HS-SPME-GC/MS (Headspace Solid-Phase Microextraction equipped with Gas Chromatography/Mass Spectrometry).| Amino acid | Content (mg/100 mg of hydrolyzed SSP) |
|---|---|
| a Numbers are represented as the means ± standard deviations. Mean values with different superscript letters have significant differences (p < 0.05). | |
| Lysine | 0.2036 ± 0.0025a |
| Histidine | 0.2014 ± 0.0010a |
| Arginine | 0.2028 ± 0.0081a |
| Tyrosine | 0.1586 ± 0.0011b |
| Phenylalanine | 0.1188 ± 0.0023b |
| Isoleucine | 0.0054 ± 0.0006e |
| Leucine | 0.0328 ± 0.0007d |
| Serine | 0.0312 ± 0.0009d |
| Glutamic acid | 0.1202 ± 0.0097b |
| Valine | 0.0844 ± 0.0008c |
| Glycine | 0.0334 ± 0.0010d |
2.6. HS-SPME-GC/MS analysis
The samples were preheated in a water bath at 45 °C for 20 min. The volatile compounds with different experimental conditions were extracted at 50 °C for 30 min by HS-SPME with a DVD/Car/PDMS fiber (50/30 μm, Supelco, Bornem).GC-MS analyses were performed using an Agilent 8890 GC coupled with an Agilent 5977B quadrupole mass selective detector (MSD, Agilent Technologies, Diegem, Belgium) with a Varian DB-1701 capillary column (30 m length × 0.25 mm i.d.; 0.25 μm film thickness). The working conditions of GC-MS were as follows: the transfer line to MSD was maintained at 250 °C; the carrier gas (He) flow rate was 1.0 mL min−1; the electron ionization (EI) was 70 eV; the scanned acquisition parameter was ranged from 30 to 550 m/z; the initial oven temperature was 40 °C, held 2 min; the temperature increased from 40 to 100 °C at a rate of 10 °C min−1 and held for 5 min, and then raised to 220 °C at a rate of 10 °C min−1 and held for 15 min; the equilibrium time was 0.5 min; the injection port was in split mode and the split ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The volatile components were identified by comparison of the mass spectrum with mass spectral libraries (NIST 2017). The formation of pyrazines was calculated by the absolute peak area of each individual pyrazine in a semi-quantitative way.26
1. The volatile components were identified by comparison of the mass spectrum with mass spectral libraries (NIST 2017). The formation of pyrazines was calculated by the absolute peak area of each individual pyrazine in a semi-quantitative way.26
2.7. Statistical analysis
All experiments were carried out three times, and the data were analyzed by using SPSS Statistics version 22, at a 95% confidence interval (p = 0.050). The results are represented as the mean values ± standard deviations. Variations were evaluated using one-way analysis variance (ANOVA). Principal components analysis (PCA) was conducted to determine the relationships of pyrazines among different tested Maillard reaction models, which was analyzed using STAT-ITCF statistical software (ITCF, Bordeaux, France).3. Results and discussion
3.1. Analysis of FAAs and protein hydrolysates
As shown in Table 1, 11 FAAs were detected, where lysine, arginine and histidine were the most abundant, which was consistent with the FAAs measured in roasted sunflower seeds.27 It has been reported that amino acids are good nitrogen sources for formation of alkylpyrazines in Maillard reaction systems.28–30 Therefore, the FAAs detected in Table 1 were used as nitrogen sources to analyze the effects on volatile products, which was reflected in the establishment of native SSP & FAAs model.Table 2 presents the molecular weight distribution of SSP and hydrolyzed SSP products. The Mw (weight-average molecular weight) of SSP was 15.38 kDa, and 90% of Mw was larger than 3.0 kDa. However, the Mw of SSP hydrolysates was 2.47 kDa, mainly in the range of 1.2–3.0 kDa, which accounted for 88.9% of the hydrolyzed SSP, and the Mw below 2.0 kDa accounted for 42.1%. These results suggested that after trypsin hydrolysis, SSP was hydrolyzed into the short chain peptide fragments.
| Molar mass (kDa) | SSP | Hydrolyzed SSP |
|---|---|---|
| Mw (kDa) | 15.38 | 2.46 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| The range of molecular weights (%) | ||
| >26.15 | 5.3 | 0 |
| 14.2–26.15 | 22.4 | 0.3 |
| 8.0–14.2 | 42.2 | 0.8 |
| 3.0–8.0 | 26.9 | 9.5 |
| 1.8–3.0 | 2.4 | 46.8 |
| 1.2–1.8 | 0 | 42.1 |
3.2. Effect of heating conditions on formation of volatile compounds in Maillard model systems
| Substances | 30 min | 60 min | 90 min | 120 min | 150 min |
|---|---|---|---|---|---|
| a Numbers are represented as the means ± standard deviations. | |||||
| The model was heated at 100 °C | |||||
| Furan compounds | 5.94 ± 0.05 | 16.47 ± 0.11 | 12.63 ± 0.09 | 22.44 ± 0.23 | 28.79 ± 0.44 |
| Aldehydes and ketones | 9.41 ± 0.02 | 8.10 ± 0.08 | 6.53 ± 0.15 | 7.74 ± 0.07 | 5.83 ± 0.05 |
| Pyrazine compounds | 0.24 ± 0.01 | 0.77 ± 0.03 | 1.11 ± 0.02 | 1.86 ± 0.14 | 2.18 ± 0.08 |
| Pyrazines (% of total GC-MS peak area) | 2.00 | 3.00 | 5.00 | 6.00 | 6.00 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 110 °C | |||||
| Furan compounds | 5.39 ± 0.03 | 9.39 ± 0.06 | 11.89 ± 0.09 | 11.42 ± 0.56 | 12.15 ± 0.97 |
| Aldehydes and ketones | 4.54 ± 0.02 | 3.87 ± 0.10 | 3.29 ± 0.09 | 2.88 ± 0.22 | 2.89 ± 0.74 |
| Pyrazine compounds | 0.11 ± 0.005 | 0.46 ± 0.03 | 0.63 ± 0.04 | 0.81 ± 0.03 | 0.81 ± 0.05 |
| Pyrazines (% of total GC-MS peak area) | 1.00 | 3.00 | 4.00 | 5.00 | 5.00 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 120 °C | |||||
| Furan compounds | 22.06 ± 1.02 | 32.22 ± 2.34 | 34.49 ± 0.99 | 18.35 ± 0.86 | 32.49 ± 1.11 |
| Aldehydes and ketones | 6.59 ± 0.55 | 6.74 ± 0.25 | 6.43 ± 0.46 | 6.52 ± 0.05 | 8.92 ± 0.95 |
| Pyrazine compounds | 0.77 ± 0.03 | 1.32 ± 0.07 | 1.61 ± 0.03 | 2.30 ± 0.06 | 2.13 ± 0.06 |
| Pyrazines (% of total GC-MS peak area) | 2.60 | 3.30 | 3.80 | 8.50 | 4.40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 130 °C | |||||
| Furan compounds | 8.14 ± 0.34 | 10.67 ± 0.09 | 11.15 ± 0.97 | 15.25 ± 1.44 | 15.2 ± 0.85 |
| Aldehydes and ketones | 2.40 ± 0.03 | 2.88 ± 0.08 | 3.00 ± 0.32 | 4.46 ± 0.32 | 4.87 ± 0.53 |
| Pyrazine compounds | 0.32 ± 0.01 | 0.48 ± 0.08 | 0.87 ± 0.07 | 1.84 ± 0.02 | 1.01 ± 0.03 |
| Pyrazines (% of total GC-MS peak area) | 2.90 | 3.40 | 5.80 | 8.50 | 4.80 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 140 °C | |||||
| Furan compounds | 23.7 ± 0.34 | 24.62 ± 1.29 | 6.64 ± 0.06 | 6.85 ± 0.55 | 30.83 ± 1.87 |
| Aldehydes and ketones | 7.48 ± 0.45 | 10.08 ± 0.47 | 2.20 ± 0.01 | 2.24 ± 0.02 | 8.69 ± 0.94 |
| Pyrazine compounds | 1.74 ± 0.08 | 3.92 ± 0.07 | 0.55 ± 0.01 | 0.77 ± 0.04 | 3.76 ± 0.35 |
| Pyrazines (% of total GC-MS peak area) | 5.00 | 10.00 | 6.00 | 8.00 | 6.00 |
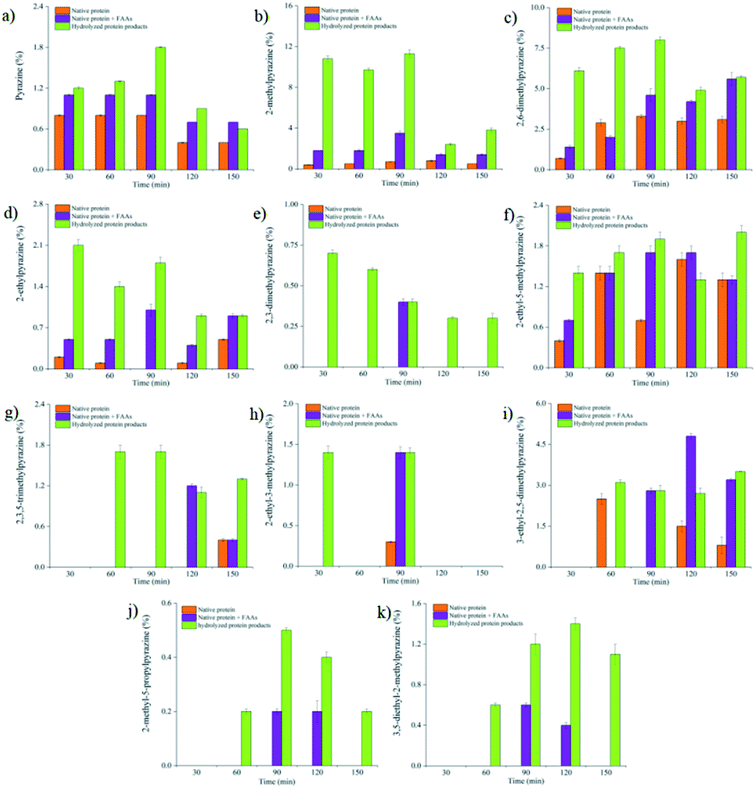
Among the pyrazine compounds generated, it should be noted that 2,5-dimethylpyrazine and 2,6-dimethylpyrazine were always eluted together at the selected chromatographic conditions and their peaks were linked together on the mass spectrometry. Thus, in our study, both were considered as 2,5(6)-dimethylpyrazine. In Table 3, although the peak area of pyrazines was relatively lower compared with other volatile compounds, their varieties were the greatest in all detected volatiles of native SSP model (Table S1†). The effect of heating conditions on substituted pyrazines and pyrazine was significant (p < 0.05). The increase in heating temperature could promote the formation of pyrazines. When the heating time was 150 min, 4 pyrazines were detected at 100 °C, while 9 pyrazines were detected at 140 °C in the native SSP model. Besides, the percentage of pyrazines in total GC-MS peak area increased significantly with heating temperature ranging from 100 to 140 °C, and the percentage of pyrazines were the highest at 140 °C (10%). Heating time could also affect the pyrazine formation. At the same temperature, the varieties of pyrazine formed increased with time. This may be because thermal degradation of the native SSP occurred, leading to generation of more amino compounds and promoting formation of pyrazines. The peak area of pyrazines showed an upward trend with the heating time increasing from 30 min to 120 min, whereas for a heating time of 150 min, the peak area of pyrazines decreased. This result indicated that the excessive heating time was not conducive to the formation of pyrazines.34
| Substances | 30 min | 60 min | 90 min | 120 min | 150 min |
|---|---|---|---|---|---|
| a Numbers are represented as the means ± standard deviations. | |||||
| The model was heated at 100 °C | |||||
| Furan compounds | 1.96 ± 0.03 | 2.65 ± 0.08 | 6.22 ± 0.66 | 9.89 ± 0.34 | 10.12 ± 0.67 |
| Aldehydes and ketones | 2.59 ± 0.11 | 3.03 ± 0.24 | 2.89 ± 0.07 | 3.94 ± 0.08 | 4.07 ± 0.44 |
| Pyrazine compounds | 0 | 0.04 ± 0.003 | 0.18 ± 0.03 | 0.37 ± 0.01 | 0.54 ± 0.04 |
| Pyrazines (% of total GC-MS peak area) | 0 | 0.52 | 1.94 | 2.61 | 3.67 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 110 °C | |||||
| Furan compounds | 5.97 ± 0.27 | 10.89 ± 0.02 | 11.2 ± 0.44 | 12.45 ± 0.05 | 1.90 ± 0.13 |
| Aldehydes and ketones | 4.02 ± 0.32 | 4.11 ± 0.05 | 3.74 ± 0.06 | 4.42 ± 0.07 | 0.44 ± 0.03 |
| Pyrazine compounds | 0.09 ± 0.01 | 0.19 ± 0.01 | 0.25 ± 0.01 | 0.31 ± 0.07 | 0.09 ± 0.004 |
| Pyrazines (% of total GC-MS peak area) | 0.89 | 1.25 | 1.65 | 1.80 | 3.70 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 120 °C | |||||
| Furan compounds | 5.85 ± 0.09 | 9.34 ± 0.53 | 8.19 ± 0.16 | 10.62 ± 0.87 | 9.79 ± 0.34 |
| Aldehydes and ketones | 3.53 ± 0.09 | 4.01 ± 0.03 | 3.47 ± 0.22 | 5.55 ± 0.55 | 5.01 ± 0.04 |
| Pyrazine compounds | 0.08 ± 0.003 | 0.14 ± 0.01 | 0.25 ± 0.02 | 0.25 ± 0.12 | 0.32 ± 0.03 |
| Pyrazines (% of total GC-MS peak area) | 0.85 | 1.04 | 2.10 | 1.52 | 2.12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 130 °C | |||||
| Furan compounds | 7.65 ± 0.07 | 9.33 ± 0.77 | 10.22 ± 0.03 | 12.97 ± 0.96 | 10.5 ± 0.10 |
| Aldehydes and ketones | 2.57 ± 0.78 | 3.43 ± 0.45 | 5.55 ± 0.07 | 8.21 ± 0.34 | 7.10 ± 0.05 |
| Pyrazine compounds | 0.34 ± 0.04 | 0.34 ± 0.02 | 0.64 ± 0.10 | 0.65 ± 0.03 | 0.71 ± 0.03 |
| Pyrazines (% of total GC-MS peak area) | 3.22 | 2.60 | 3.90 | 2.98 | 3.88 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 140 °C | |||||
| Furan compounds | 7.83 ± 0.05 | 7.1 ± 0.10 | 17.15 ± 0.28 | 37.28 ± 3.44 | 9.91 ± 0.44 |
| Aldehydes and ketones | 1.64 ± 0.05 | 1.81 ± 0.03 | 8.49 ± 0.06 | 13.04 ± 1.12 | 5.36 ± 0.05 |
| Pyrazine compounds | 0.27 ± 0.06 | 0.55 ± 0.06 | 5.60 ± 0.03 | 9.09 ± 0.06 | 1.72 ± 0.13 |
| Pyrazines (% of total GC-MS peak area) | 2.77 | 5.81 | 17.93 | 15.3 | 10.12 |
In the native SSP & FAAs model, the varieties of detected pyrazines increased significantly with the increase of heating temperature. As shown in Table S2,† only two pyrazines were detected at 100 °C and three pyrazines at 110 °C, while ten pyrazines were detected at 140 °C. When the heating temperature was below 120 °C, the prolonged heating time has no significant effect on the formation of pyrazines (p > 0.05). However, when the temperature was higher than 120 °C, the influence of heating time on pyrazine formation was significant (p < 0.05). Five pyrazines were generated at 140 °C for 30 or 60 min, and eleven pyrazines were detected for 90 min or more time. These results may reflect that some pyrazines can only be generated at high heating temperature. For example, 2,3,5-trimethylpyrazine, 2-ethyl-3-methylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2-methyl-5-propylpyrazine and 3,5-diethyl-2-methylpyrazine were only detected at 140 °C for 90 min or more time in the native SSP & FAAs model, and these pyrazines were also detected at 140 °C for 150 min in native SSP model. These results were in agreement with the report by Misharina et al. who found that higher temperature could promote the formation of some specific pyrazines.34 Furthermore, the percentage of pyrazines in total GC-MS peak area increased with the increase of heating temperature and time in this model. The proportion of pyrazines in total volatiles increased from 2.77% to 17.93% at 140 °C with the heating time ranging from 30 to 90 min.
| Substances | 30 min | 60 min | 90 min | 120 min | 150 min |
|---|---|---|---|---|---|
| a Numbers are represented as the means ± standard deviations. | |||||
| The model was heated at 100 °C | |||||
| Furan compounds | 10.43 ± 0.56 | 15.84 ± 0.09 | 17.88 ± 1.23 | 30.00 ± 2.67 | 28.11 ± 0.45 |
| Aldehydes and ketones | 6.99 ± 0.32 | 7.60 ± 0.45 | 7.34 ± 0.88 | 5.5 ± 0.38 | 4.46 ± 0.12 |
| Pyrazine compounds | 1.54 ± 0.03 | 3.84 ± 0.04 | 5.36 ± 0.34 | 7.31 ± 0.11 | 11.21 ± 0.54 |
| Pyrazines (% of total GC-MS peak area) | 8.12 | 14.14 | 18.15 | 17.33 | 25.99 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 110 °C | |||||
| Furan compounds | 5.32 ± 0.78 | 9.57 ± 0.45 | 11.26 ± 0.22 | 11.62 ± 0.05 | 11.97 ± 0.09 |
| Aldehydes and ketones | 3.23 ± 0.21 | 2.29 ± 0.11 | 2.44 ± 0.31 | 2.15 ± 0.04 | 2.05 ± 0.02 |
| Pyrazine compounds | 0.89 ± 0.02 | 2.52 ± 0.34 | 3.34 ± 0.22 | 2.91 ± 0.06 | 3.88 ± 0.01 |
| Pyrazines (% of total GC-MS peak area) | 9.48 | 17.81 | 20.00 | 17.86 | 22.22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 120 °C | |||||
| Furan compounds | 22.83 ± 0.08 | 31.04 ± 1.66 | 35.83 ± 0.35 | 33.72 ± 0.98 | 62.65 ± 4.78 |
| Aldehydes and ketones | 5.15 ± 0.05 | 6.08 ± 0.87 | 5.44 ± 0.44 | 4.78 ± 0.09 | 8.35 ± 0.22 |
| Pyrazine compounds | 8.04 ± 0.02 | 11.01 ± 0.52 | 11.71 ± 0.21 | 14.24 ± 0.02 | 14.56 ± 0.99 |
| Pyrazines (% of total GC-MS peak area) | 22.85 | 23.38 | 22.59 | 27.55 | 17.21 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 130 °C | |||||
| Furan compounds | 9.45 ± 0.37 | 9.27 ± 0.03 | 6.93 ± 0.04 | 13.03 ± 0.07 | 14.3 ± 0.03 |
| Aldehydes and ketones | 2.20 ± 0.02 | 2.44 ± 0.05 | 1.79 ± 0.02 | 3.05 ± 0.08 | 3.58 ± 0.06 |
| Pyrazine compounds | 3.51 ± 0.07 | 2.51 ± 0.05 | 2.57 ± 0.01 | 3.38 ± 0.01 | 5.17 ± 0.09 |
| Pyrazines (% of total GC-MS peak area) | 23.86 | 18.20 | 23.43 | 17.66 | 22.71 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| The model was heated at 140 °C | |||||
| Furan compounds | 25.19 ± 1.93 | 24.23 ± 0.05 | 16.84 ± 0.07 | 41.33 ± 0.34 | 40.36 ± 3.54 |
| Aldehydes and ketones | 3.45 ± 0.05 | 6.26 ± 0.06 | 8.77 ± 0.08 | 14.43 ± 0.73 | 11.11 ± 0.93 |
| Pyrazine compounds | 9.63 ± 0.06 | 12.05 ± 0.77 | 12.70 ± 0.44 | 11.26 ± 0.31 | 12.82 ± 0.05 |
| Pyrazines (% of total GC-MS peak area) | 25.69 | 28.73 | 33.86 | 17.03 | 20.20 |
In Table S3,† 2-methylpyrazine and 2,5(6)-dimethylpyrazine were the main pyrazine compounds in hydrolyzed SSP model. In this model, the heating time and temperature have significant effect on pyrazine formation. When the heating temperature was 100 °C, two and six pyrazines were detected for 30 min and 150 min, respectively. As the heating temperature increased from 100 to 140 °C, more pyrazines were produced in this Maillard model, which was consistent with the results of other two models. It is worth noting that at 140 °C, when the heating time increased from 60 to 150 min, the varieties of pyrazines were similar and the changing of peak area of pyrazines was not significant (p > 0.05). This indicated that when the heating temperature was high enough, temperature exerted a major role in the kinetics of the Maillard reaction, and the heating time had little influence on the varieties of volatiles. Similar result was previously reported by Jousse et al.36 In addition, under the heating condition of 140 °C/90 min, eleven pyrazines were detected and the percentage of pyrazines in total volatiles was the highest: unsubstituted pyrazine (1.1%), 2-methylpyrazine (11.3%), 2,5(6)-dimethylpyrazine (8.0%), 2-ethylpyrazine (1.8%), 2-ethyl-5-dimethylpyrazine (1.9%), 2,3,5-trimethylpyrazine (1.7%), 2-ethyl-3-methylpyrazine (1.4%). Moreover, at the same heating temperature and time, the percentage of pyrazines was significantly higher in the hydrolyzed SSP model than that in the other tested model systems, especially at 140 °C/90 min, the percentage of pyrazines in the hydrolyzed SSP model was the most, accounted for 30% of total volatiles.
However, even if under longer heating time (150 min), only a few pyrazines were generated at 100 °C or 110 °C in all three Maillard model systems. This may be due to that the temperature was not high enough and restricted the production of pyrazine compounds. Compared with the other two model systems, the total percentage and varieties of pyrazines were higher in the hydrolyzed SSP model. This probably because the hydrolyzed SSP generated the short chain peptides fragments (Table 2) and the contribution of short chain peptides was more than that of FAAs for the pyrazines formation.
3.3. Effect of Maillard model systems on the formation of pyrazines
In order to determine the relationship between the Maillard reaction precursors and MRPs, the effects of free amino acids and peptides on pyrazine formation were accessed. The native SSP & FAAs model, hydrolyzed SSP model were prepared with the native SSP model as the control. According to the results of the heating conditions above, the varieties and peak area of pyrazines were higher at 140 °C than that at other heating temperature. Therefore, we compared the effect of three model systems on formation of pyrazines at 140 °C. Limited pyrazines were detected during the Maillard reaction of native SSP and glucose. This may be due to the fact that under the heating conditions, a small amount of amino acids were released by the thermal degradation of SSP.As shown in Fig. 1a–d and f–i, the varieties of pyrazines in the native SSP & FAAs model was close to pyrazines in native SSP model. This result indicated that the FAAs produced by hydrolysis of native SSP had no significant effect on formation of pyrazines (p > 0.05). Additionally, the most varieties and percentage of pyrazines were detected in the hydrolyzed SSP model at 140 °C/90 min and the content of pyrazines was higher than that of the other two Maillard models. Eleven pyrazines were detected and 2-methylpyrazine and 2,5(6)-dimethylpyrazine were the main pyrazine compounds in the hydrolyzed SSP model at 140 °C/90 min (12% and 8%, respectively) (Fig. 1b and c). Compared with other tested Maillard model systems, the hydrolyzed SSP model could facilitate the formation of pyrazines, which was consistent with the previous researches.7,37 A possible explanation is that after trypsin hydrolysis, native SSP was broken into short chain peptides, resulting in more pyrazines formation.19 Therefore, the formation of pyrazines in three Maillard models revealed that peptides, especially the short chain peptides, play an important role in the production of pyrazine compounds.
In addition, the general mechanism of pyrazine formation by peptides and FAAs has been reported.26,38,39 The pyrazine formation by FAAs involves decarboxylation, while peptides are lack of free carboxyl groups at the α-carbon; thus peptides could not follow the typical Strecker degradation reaction to produce α-aminoketones. The mechanism between the reaction of dipeptides and α-dicarbonyl compounds has been proposed, where a complex α-ketoamide is produced by the reaction of dipeptides and α-dicarbonyl compounds and further reacts with the α-aminoketone to form pyrazines.26
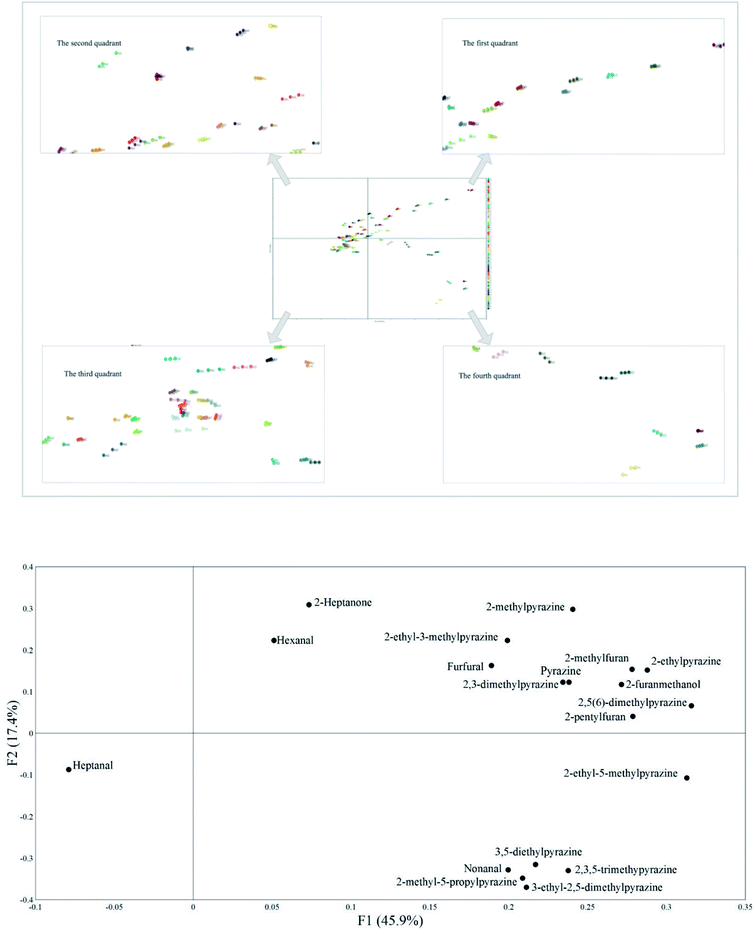
3.4. Principal component analysis (PCA)
Principal component analysis was performed on the data collected by HS-SPME-GC/MS. The distance of different points on the two principal components reflects the difference of tested Maillard models. The variance contribution rates of principal component 1 (F1) and principal component 2 (F2) were 45.9% and 17.4%, respectively, indicating that PCA could reflect most of information of samples and distinguish the results of different groups. According to the Fig. 2A, groups A (the Maillard models were heated at 100 °C), groups B (the Maillard models were heated at 110 °C), groups C (the Maillard models were heated at 120 °C) and groups D (the Maillard models were heated at 130 °C) were distributed in the negative half axis of F1. However, the groups E (the Maillard models were heated at 140 °C) were distributed in the positive half axis of F1. These results suggested that there was no significant difference between 100 °C, 110 °C, 120 °C and 130 °C on the principal component 1, while significant differences between the groups E and groups A & B & C & D were found on the principal component 1. In addition, the groups E were mainly distributed in the fourth quadrant, which proved that there was a significant difference between 140 °C and other heating temperature.Fig. 2B presents the distribution of volatile compounds on two principal components. Heptanal was listed in the negative half axis of F1, while other volatile compounds were listed in the positive half axis of F1. This may be because heptanal was only detected in the native SSP & FAAs model and the detected peak area was low. Furan compounds, unsubstituted pyrazine, 2-methylpyrazine, 2-ethylpyrazine, 2,5(6)-dimethylpyrazine, 2,3-dimethylpyrazine and 2-ethyl-3-methylpyrazine, hexanal and 2-heptanone were distributed in the positive half axis of F2. However, heptanal, nonanal, 2-ethyl-5-methylpyrazine, 3,5-diethylpyrazine, 2,3,5-trimethylpyrazine, 2-methyl-5-propylpyrazine and 3-ethyl-2,5-dimethylpyrazine were distributed in the negative half axis of F2. This probably because furan compounds, hexanal and 2-heptanone unsubstituted pyrazine, 2-methylpyrazine, 2-ethylpyrazine, 2,5(6)-dimethylpyrazine, 2,3-dimethylpyrazine and 2-ethyl-3-methylpyrazine were detected in all tested Maillard models with relatively higher peak area. However, pyrazine compounds distributed in the negative axis of F2 were only detected in the hydrolyzed SSP model at 140 °C/90 min and their peak areas were relatively low. Thus, these volatile compounds were classified into three categories. Heptanal was divided into the first category. Furan compounds, unsubstituted pyrazine, 2-methylpyrazine, 2-ethylpyrazine, 2,5(6)-dimethylpyrazine, 2,3-dimethylpyrazine and 2-ethyl-3-methylpyrazine, hexanal and 2-heptanone were classified into the second category. Nonanal, 2-ethyl-5-methylpyrazine, 3,5-diethylpyrazine, 2,3,5-trimethylpyrazine, 2-methyl-5-propylpyrazine and 3-ethyl-2,5-dimethylpyrazine were classified into the third category.
4. Conclusions
In this study, the volatile compounds were analyzed under different heating conditions in three Maillard model systems (i.e., native SSP model, native SSP & FAAs model and hydrolyzed SSP model). Pyrazines were the characteristic volatile compounds, and 2-methylpyrazine and 2,5(6)-dimethylpyrazine were the main pyrazine compounds in the tested Maillard models. 140 °C/90 min was considered to be the optimum heating condition for the formation of pyrazines in the Maillard reaction. The most varieties and percentage of pyrazines were detected in the hydrolyzed SSP model at 140 °C/90 min and the content of pyrazines was higher than that of the other two Maillard models (native SSP model and native SSP & FAAs model). Meanwhile, GPC analysis proved that the short chain peptides hydrolyzed by SSP, especially the molecular weight ranging from 1.2 to 3.0 kDa, dramatically promoted the formation of pyrazines. These results demonstrated that peptides were more effective in promoting the formation of pyrazines in the Maillard reaction than free amino acids. Thus, the production of flavor compounds from peptides should be paid more attention in heated-food processing.Author contributions
Yali Yang and Yurong Guo proposed and designed the experiment; Furong Wang wrote the paper; Ting Liu, Hailiang Shen, Xueru Zhou and Pengtao Zhao processed the data; Xi Yang corrected the language of the paper. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
No potential conflict of interest was reported by the authors.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31701563), opening topic project by Engineering Research Center of High Value Utilization of Western China Fruit Resources, Ministry of Education (XGZX2021G08), the Fundamental Research Funds for the Central Universities at Shaanxi Normal University (1301031057) and the Key Research and Development Program of Shaanxi province (No. 2019NY-124).References
- J. E. Hodge, Dehydrated Foods, Chemistry of Browning Reactions in Model Systems, J. Agric. Food Chem., 1953, 1(15), 625–651 CrossRef.
- F. Ledl and R. N. H. E. Schleicher, New Aspects of the Maillard Reaction in Foods and in the Human Body, Angew. Chem., Int. Ed., 1990, 29(6), 565–594 CrossRef.
- D. J. McWeeny, M. E. Knowles and J. F. Hearne, The chemistry of non-enzymic browning in foods and its control by sulphites, J. Sci. Food Agric., 1974, 25, 735–746 CrossRef CAS.
- J. A. Maga, Pyrazine update, Food Rev. Int., 1992, 8(4), 479–558 CrossRef CAS.
- A. Adams and N. De Kimpe, Chemistry of 2-acetyl-1-pyrroline, 6-acetyl-1,2,3,4-tetrahydropyridine, 2-acetyl-2-thiazoline, and 5-acetyl-2,3-dihydro-4H-thiazine: extraordinary Maillard flavor compounds, Chem. Rev., 2006, 106(6), 2299 CrossRef CAS PubMed.
- G. L. Baker, J. A. Cornell, D. W. Gorbet, S. F. O'Keefe, C. A. Sims and S. T. Talcott, Determination of Pyrazine and Flavor Variations in Peanut Genotypes During Roasting, J. Food Sci., 2003, 68(1), 394–400 CrossRef CAS.
- S. M. V. Ruth and J. P. Roozen, Aroma compounds of oxidized sunflower oil and its oil-in-water emulsion: volatility and release under mouth conditions, Eur. Food Res. Technol., 2000, 210(4), 258–262 CrossRef.
- A. N. Yu and A. D. Zhang, The effect of pH on the formation of aroma compounds produced by heating a model system containing L-ascorbic acid with L-threonine/L-serine, Food Chem., 2010, 119(1), 214–219 CrossRef CAS.
- J. M. Ames, A. B. Defaye and L. Bates, The effect of pH on the volatiles formed in an extruded starch-glucose-lysine model system, Food Chem., 1997, 58(4), 323–327 CrossRef CAS.
- J. M. Ames, R. C. E. Guy and G. J. Kipping, Effect of pH, Temperature, and Moisture on the Formation of Volatile Compounds in Glycine/Glucose Model Systems, J. Agric. Food Chem., 2001, 49(9), 4315–4323 CrossRef CAS PubMed.
- H. Xu, W. He, X. Liu and Y. Gao, Impact of High Temperature on the Maillard Reaction between Ribose and Cysteine in Supercritical Carbon Dioxide, Food Sci. Biotechnol., 2009, 18(1), 66–72 CAS.
- Z. Zhang, W. Elfalleh, S. He, M. Tang, J. Zhao, Z. Wu, J. Wang and H. Sun, Heating and cysteine effect on physicochemical and flavor properties of soybean peptide Maillard reaction products, Int. J. Biol. Macromol., 2018, 120, 2137–2146 CrossRef CAS PubMed.
- X. Lan, P. Liu, S. Xia, C. Jia, D. Mukunzi, X. Zhang, W. Xia, H. Tian and Z. Xiao, Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose–soybean peptide system: Further insights into thermal degradation and cross-linking, Food Chem., 2010, 120(4), 967–972 CrossRef CAS.
- L. Cai, D. Li, Z. Dong, A. Cao and J. Li, Change regularity of the characteristics of Maillard reaction products derived from xylose and Chinese shrimp waste hydrolysates, LWT--Food Sci. Technol., 2016, 65, 908–916 CrossRef CAS.
- A. N. Yu, Z. W. Tan and F. S. Wang, Mechanistic studies on the formation of pyrazines by Maillard reaction between L-ascorbic acid and L-glutamic acid, LWT--Food Sci. Technol., 2013, 50(1), 64–71 CrossRef CAS.
- M. Negroni, A. D'Agostina and A. Arnoldi, Effects of Olive, Canola, and Sunflower Oils on the Formation of Volatiles from the Maillard Reaction of Lysine with Xylose and Glucose, J. Agric. Food Chem., 2001, 49(1), 439–445 CrossRef CAS PubMed.
- F. Van Lancker, A. Adams and N. De Kimpe, Impact of the N-terminal amino acid on the formation of pyrazines from peptides in Maillard model systems, J. Agric. Food Chem., 2012, 60(18), 4697–4708 CrossRef CAS PubMed.
- J. S. Kim and Y. S. Lee, Study of Maillard reaction products derived from aqueous model systems with different peptide chain lengths, Food Chem., 2009, 116(4), 846–853 CrossRef CAS.
- G. L. L. Scalone, T. Cucu, N. De Kimpe and B. De Meulenaer, Influence of Free Amino Acids, Oligopeptides, and Polypeptides on the Formation of Pyrazines in Maillard Model Systems, J. Agric. Food Chem., 2015, 63(22), 5364–5372 CrossRef CAS PubMed.
- G. L. L. Scalone, P. Lamichhane, T. Cucu, N. De Kimpe and B. De Meulenaer, Impact of different enzymatic hydrolysates of whey protein on the formation of pyrazines in Maillard model systems, Food Chem., 2019, 278(25), 533–544 CrossRef CAS PubMed.
- G. L. L. Scalone, A. G. Ioannidis and P. Lamichhane, et al., Impact of whey protein hydrolysates on the formation of 2,5-dimethylpyrazine in baked food products, Food Res. Int., 2020, 132, 109089 CrossRef CAS PubMed.
- C. H. Schlichtherle and R. Amadò, Analysis of Taste-Active Compounds in an Enzymatic Hydrolysate of Deamidated Wheat Gluten, J. Agric. Food Chem., 2002, 50(6), 1515–1522 CrossRef PubMed.
- H. Hashiba, Glucose–diglycine condensation product participating in oxygen-dependent browning, J. Agric. Food Chem., 1975, 23(3), 539–542 CrossRef CAS.
- Z. Jiang and A. Brodkorb, Structure and antioxidant activity of Maillard reaction products from α-lactalbumin and β-lactoglobulin with ribose in an aqueous model system, Food Chem., 2012, 133(3), 960–968 CrossRef CAS.
- F. V. Lancker, A. Adams and N. De Kimpe, Chemical modifications of peptides and their impact on food properties, Chem. Rev., 2011, 111(12), 7876–7903 CrossRef PubMed.
- F. Van Lancker, A. Adams and N. De Kimpe, Formation of pyrazines in Maillard model systems of lysine-containing dipeptides, J. Agric. Food Chem., 2010, 58(4), 2470–2478 CrossRef CAS PubMed.
- F. D. Gunstone, Sunflower seeds and sunflower seed oil, Lipid Technol., 2010, 19(10), 240 CrossRef.
- A. Arnoldi, C. Arnoldi, O. Baldi and A. Griffini, Flavor components in the Maillard reaction of different amino acids with fructose in cocoa butter–water. Qualitative and quantitative analysis of pyrazines, J. Agric. Food Chem., 1988, 36(5), 988–992 CrossRef CAS.
- P. E. Koehler and G. V. Odell, Factors affecting the formation of pyrazine compounds in sugar-amine reactions, J. Agric. Food Chem., 1970, 18(6), 895–898 CrossRef CAS.
- M. A. Hemaimi, C. Cerny and L. B. Fay, Mechanisms of Formation of Alkylpyrazines in the Maillard, J. Agric. Food Chem., 1995, 43(11), 2818–2822 CrossRef.
- A. Limacher, J. Kerler, T. Davidek, F. Schmalzried and I. Blank, Formation of furan and methylfuran by Maillard-type reactions in model systems and food, J. Agric. Food Chem., 2008, 56(10), 3639–3647 CrossRef CAS PubMed.
- A. Adams, C. Bouckaert, F. V. Lancker, B. D. Meulenaer and N. De Kimpe, Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes, J. Agric. Food Chem., 2011, 59(20), 11058–11062 CrossRef CAS PubMed.
- S. Hong Mei, L. I. Xia, Z. Chun Hui, D. Xian Bing, L. I. Yin and J. Wei, Comparison of volatile compounds in different Maillard reaction productions from chicken bone extract and its enzymatic hydrolysate, J. Instrum. Anal., 2013, 32(6), 661–667 Search PubMed.
- T. A. Misharina, R. V. Golovnya and V. N. Yakovleva, Effect of time and temperature on the preparation of pyrazines in model reactions of the synthesis of aroma-forming substances, Russ. Chem. Bull., 1992, 41(7), 1258–1263 CrossRef.
- I. Blank and L. B. Fay, Formation of 4-Hydroxy-2,5-dimethyl-3(2H)-furanone and 4-Hydroxy-2(5)-ethyl-5(2)-methyl-3(2H)-furanone through Maillard Reaction Based on Pentose Sugars, J. Agric. Food Chem., 1996, 44(2), 531–536 CrossRef CAS.
- F. Jousse, T. Jongen, W. Agterof, S. Russell and P. Braat, Simplified Kinetic Scheme of Flavor Formation by the Maillard Reaction, J. Food Sci., 2010, 67(7), 2534–2542 CrossRef.
- F. Wang, H. Shen, T. Liu, X. Yang, Y. Yang and Y. Guo, Formation of pyrazines in Maillard model systems: effects of structures of lysine-containing dipeptides/tripeptides, Foods, 2021, 10(2), 273 CrossRef PubMed.
- A. Adams, V. Polizzi, M. van Boekel and N. De Kimpe, Formation of pyrazines and a novel pyrrole in Maillard model systems of 1,3-dihydroxyacetone and 2-oxopropanal, J. Agric. Food Chem., 2008, 56(6), 2147–2153 CrossRef CAS PubMed.
- F. Van Lancker, A. Adams, F. A. Owczarek, B. De Meulenaer and N. De Kimpe, Mechanistic Insights into Furan Formation in Maillard Model Systems, J. Agric. Food Chem., 2011, 59(1), 229–235 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1–S3 were presented in the supplementary materials. See DOI: 10.1039/d1ra05140g |
| This journal is © The Royal Society of Chemistry 2021 |