DOI:
10.1039/D1RA04910K
(Paper)
RSC Adv., 2021,
11, 27207-27214
Design, synthesis, and herbicidal activity of sec-p-menthane-7-amine derivatives as botanical herbicides†
Received
24th June 2021
, Accepted 2nd August 2021
First published on 9th August 2021
Abstract
In this study, a series of novel p-menthane type secondary amines (sec-p-menthane-7-amine derivatives 3a–3y) were synthesized and then characterized by FTIR, 1H NMR, 13C NMR, and HRMS. The post-emergence herbicidal activities of these amines against barnyard grass and rape were evaluated by the culture dish method. Most of the sec-p-menthane-7-amine derivatives showed excellent herbicidal activities equivalent to or even higher than either diuron or glyphosate. The alkyl-substituted derivatives were more active than the phenyl-substituted derivatives. The herbicidal activities of compounds 3p, 3r, 3u, and 3w against the root growth of barnyard grass were 404% higher, respectively, than those of glyphosate. The herbicidal activities of compounds 3q, 3v, 3w, and 3x against the root growth of rape were 561%, 494%, 491%, and 544% higher, respectively, than those of diuron, and 484%, 760%, 423%, and 665% higher respectively, than those of diuron against shoot growth of rape. In addition, compounds 3p, 3u, and 3v are almost harmless to rice, wheat, sorghum, maize, and peanuts at a concentration of 100 mg L−1. Most of the compounds are nontoxic to HUVEC-C and BALB/c 3T3 cells. It is indicated that the title compounds could be utilized as botanical herbicides for future weed control.
1 Introduction
With the increase in world population, it has become urgent to improve agricultural production to solve the problem of grain shortage.1 Weeds can compete with crops for nutrients, moisture, and light and bring pests and diseases, which cause the reduction of grain yields.2,3 In recent decades, herbicides have provided an effective approach for crop protection. However, traditional herbicides are chemically synthesized and their excessive and continual use can lead to drug residues, environmental pollution, herbicidal resistance, and other serious problems.4–6 Given the defects of synthetic herbicides, there is an urgent need to develop eco-friendly and sustainable herbicides with high activity and low toxicity such as botanical herbicides.7,8
Essential oils possess potential inhibitory properties against weed growth and seed germination. This phenomenon is called allelopathy.9 Many monoterpenes in the essential oils such as α-pinene, β-pinene, 1,8-cineole, carvacrol, thymol, and limonene exhibit allelopathic properties, which are widely distributed in many plants.9–15 Aside from their direct utilization, the chemically modified derivatives of essential oils including amides, amines, Schiff bases, thioureas, and esters have also attracted much attention due to their excellent herbicidal activities. The compounds derived from turpentine have been reported to exhibit a wide range of biological activities and have become the main source of botanical herbicides.16–20
In our previous studies, a series of herbicidal active substances with a p-menthane skeleton were synthesized from turpentine. Among them, p-menth-3-enylamine and cis-1,8-p-menthane-diamine type Schiff base compounds showed herbicidal activities against ryegrass and barnyard grass superior to those of commercial herbicide glyphosate.21–23 However, the imine group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of Schiff base compounds is usually unstable in storage and herbicidal assays because it can be easily oxidized by air, hydrolyzed in an acidic environment, and subjected to transamination by nucleophiles.24,25 Therefore, it is necessary to convert the Schiff bases into a more stable secondary amines using the reduction. It has been proven that secondary amines such as sec-p-menth-3-enylamines and cis-1,8-p-menthane-di-sec-amines exhibit higher herbicidal activities than that of Schiff bases, and they show low toxicity to common crops and mammal cells.26,27
N) of Schiff base compounds is usually unstable in storage and herbicidal assays because it can be easily oxidized by air, hydrolyzed in an acidic environment, and subjected to transamination by nucleophiles.24,25 Therefore, it is necessary to convert the Schiff bases into a more stable secondary amines using the reduction. It has been proven that secondary amines such as sec-p-menth-3-enylamines and cis-1,8-p-menthane-di-sec-amines exhibit higher herbicidal activities than that of Schiff bases, and they show low toxicity to common crops and mammal cells.26,27
Perillaldehyde is a natural monoterpene of the p-menthane group originating from plant Perilla frutescens. It was reported that perillaldehyde displayed various bioactivities such as anti-depression and antibacterial activity,28,29 but the herbicidal activity is rarely reported. In order to study the herbicidal activities of perillaldehyde-derived secondary amines with the p-menthane skeleton and find new herbicidal active substances, a variety of sec-p-menthane-7-amine derivatives were designed and synthesized using perillaldehyde as raw material. These derivatives were evaluated for their herbicidal activities against barnyard grass (Echinochloa crus-galli) and rape (Brassica napus) as well as with regard to crop safety and cytotoxicity to normal mammal cells.
2 Experimental methods
2.1 Materials
NMR spectra were recorded on an Avance III 500 MHz spectrometer (Bruker, Switzerland) using CDCl3 as solvent and TMS as the internal reference. FT-IR spectra were recorded using a Nicolet IS10 IR instrument (Thermo, USA) connected to an OMNIC operating system. HRMS analyses were performed using a XEVO G2-XS mass spectrometer (Waters, USA) under electron spray ionization. GC analysis was carried out using a GC-2014AF (Shimadzu, Japan) with a HP-5 quartz capillary column (30 m × 0.25 mm, df 0.25 μm). Herbicidal activities were carried out in a ZRG-1000B-L artificial climatic incubator from Shanghai Binglin Electronic Technology Co. Ltd (Shanghai, China). Cytotoxicity assays were completed in a Winooski EL-X800 microplate reader (BioTech Instrument, USA). Column chromatography was carried out on a 300 mm × 40 mm column of silica gel (200–300 meshes). Other reagents were analytically pure and obtained commercially from Shanghai Aladdin Chemistry Co. Ltd (Shanghai, China).
2.2 Synthesis of p-menthane-7-aldehyde
To start, 50 g of perillaldehyde (1) and 2.5 g of Pd/C were added to a 100 mL micro autoclave with magnetic stirring. Then, the autoclave was filled with hydrogen for 5 MPa, and reacted at 120 °C for 15 h. After the reaction system was cooled to room temperature, the Pd/C was removed by filtration, and the crude product was recovered by rectification to obtain p-menthane-7-aldehyde (2, GC 98%, a mixture consisted of cis- and trans-isomer, ncis-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ntrans- = 1
ntrans- = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2.3 Synthesis of sec-p-menthane-7-amine derivatives
Firstly, 20 mmol p-menthane-7-aldehyde and 20.2 mmol amine were stirred in a 250 mL round-bottom flask containing 50 mL of methanol for 48 h. The system was cooled down to 0 °C in an ice bath, and then 60 mmol NaBH4 total was added three times over the course of 30 min and reacted at 0 °C for another 0.5–1 h. After stirring, 20 mL of cold distilled water was added to quench excess NaBH4. The mixture was extracted with CH2Cl2 and washed with distilled water three times. The organic phase was collected and dried over anhydrous Na2SO4 and then evaporated under reduced pressure to yield thick oil as a crude product. Finally, the product was purified by recrystallization or column chromatography eluted by petroleum ether and ethyl acetate (Scheme 1).
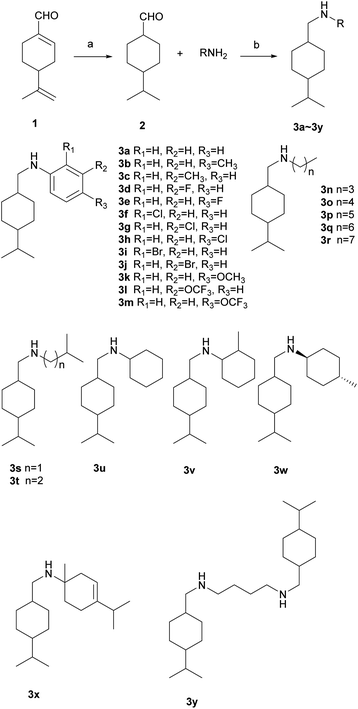 |
| | Scheme 1 Synthetic route for the sec-p-menthane-7-amine derivatives (a) substrate 50 g, Pd/C 2.5 g, 5 MPa, 120 °C, 15 h; (b) substrate 20 mmol, RNH2 20.2 mmol, methanol, room temperature (25 °C), 24 h, NaBH4 2 eq., ice-bath. | |
2.4 Herbicidal activity evaluation
The monocotyledon barnyard grass (Echinochloa crus-galli) and dicotyledon rape (Brassica napus) were chosen as the test plants for primary herbicide bioassay tests. First, 1 mmol of each compound was dissolved in 1 mL DMF in a 100 mL volumetric flask; then, 0.1 g Tween-80 was added as the emulsification reagent to the volume flask and diluted with distilled water to 10 mmol L−1. Then, the concentration was diluted with the control solution (1% DMF and 0.1% Tween-80 in distilled water) to 5, 2.5, 1.25, 0.625, 0.3125, 0.1563, 0.0781, 0.0391, 0.0195, 0.0098, and 0.0049 mmol L−1 in gradual succession. The seeds were soaked in warm water (28 °C) for 12 h, then filtered out with distilled water and incubated at 28 °C for another 24 h before use. Then, the test compound solution (10 mL for barnyard grass, 6 mL for rape) of each concentration and 10 mL of the control solution were added to the corresponding Petri dishes (9 cm in diameter) lined with two layers of filter paper (9 cm in diameter). Then, 10 seeds were added to each Petri dish (at each concentration and for the controls, with three replicates) and cultivated under artificial climate conditions: the seeds were incubated at 28 °C, illuminated at 5000 lx, photo-period 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (day/night), and kept at a relative humidity 70–80% for 96 h. The inhibition rate (%) of the root or shoot growth was calculated according to eqn (1).
8 (day/night), and kept at a relative humidity 70–80% for 96 h. The inhibition rate (%) of the root or shoot growth was calculated according to eqn (1).| |
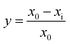 | (1) |
where y is the inhibition rate of the root or shoot growth, x0 is the root or shoot length of the blank control group, and xi is the root or shoot length of the group treated with the test compounds.
2.5 Crop safety
The crop safety assays for seven crops (rice, wheat, sorghum, maize, peanut, cucumber, and radish) were tested. To start, 100 mg of each compound was dissolved in 10 mL DMF in a 1 L volumetric flask; then 1 g Tween-80 was added as the emulsification reagent to the volumetric flask and diluted with distilled water to 100 mg L−1. Crop seeds were soaked in warm water for 15 h in a 28 °C incubator before use. Next, 8 mL of the test compound solution of 100 mg L−1 and 8 mL of the control solution were added to the corresponding Petri dishes (9 cm in diameter) lined with two layers of filter paper (9 cm in diameter) and 10 seeds of each crop were added to each Petri dish, with three replicates, which were then cultivated in the dark at 28 °C for 120 h. The crop safety was evaluated visually and rated with scores between 0 and 100, where complete control of the crop is 100 and no control is 0.
2.6 Cytotoxicity assays
The BALB/c 3T3 cell line, which is recommended for cytotoxicity evaluation tests by the European Union,30 was chosen as a target for cytotoxicity assay measurements by CCK-8 method.31 The HUVEC-C cell line was commonly used in pesticide safety analyses, so it was also selected.32 First, the test samples were dissolved in DMSO and diluted with the culture medium (DMEM containing 10% FBS and 1% mixture of penicillin and streptomycin for BALB/c 3T3 cells, and Kaighn's Modification of Ham's F-12 Medium containing 15% FBS for HUVEC-C cells) at 10 mmol L−1 concentration (DMSO was less than 0.1%). Next, a cryopreservation tube containing the BALB/c 3T3 or HUVEC-C cell line was removed from the liquid nitrogen tank and quickly unfrozen in a water bath at 37 °C. Then, the cellular suspension was transferred to a culture flask containing the corresponding complete medium. The cultures were incubated under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. After 1-2 generations of reproduction, the cells were cultivated in a 96-well plate at a density of 4 × 103 cells per 100 μL of the corresponding complete medium in each well for 24 h (culture conditions: 5% CO2 95% air at 37 °C). Then, 100 μL of each solution of the test compound (10 μmol L−1) and were added to a separate 96-well plate, respectively, and 100 μL of medium was added as a negative control. Then, the cultures were incubated at 37 °C. After 48 h of incubation, 10 μL of CCK-8 solution was added to each well and was allowed to incubate for 4 h. Later, the 96-well plate was shaken slightly to eliminate air bubbles; an absorbance at a wavelength of 450 nm was recorded. The inhibition rate (%) of cell growth was calculated according to eqn (2).| |
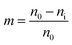 | (2) |
where m is the inhibition rate of cell growth, n0 is the OD value of the blank control group, and ni is the OD value of the experimental group.
3 Results and discussion
3.1 Synthesis and characterization of sec-p-menthane-7-amines derivatives
The sec-p-menthane-7-amine derivatives which were mixture of cis- and trans-isomers, were synthesized using perillaldehyde and amines as raw materials, NaBH4 as a reductant, were obtained in yields that ranged between 40–98%. The crude products were purified by silica gel column chromatography using petroleum ether and ethyl acetate as eluents. These compounds were confirmed by FTIR, 1H NMR, and 13C NMR spectroscopy as well as HRMS. In the FTIR spectra, the peak at 3436–3406 cm−1 was characteristic of the N–H stretching vibration band in secondary amines. The peaks in the ranges of 2960–2840 cm−1, 1650–1450 cm−1, and 1390–1300 cm−1 represented the stretching vibration band of C–H alkane groups, the stretching vibration band of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of an aromatic ring, and the stretching vibration band of C–N bonds, respectively. In the 1H NMR spectra, a singlet with δ 5.34 ppm belonged to the amino proton in compounds 3a–3m, and a singlet of δ 1.95 ppm belonged to the amino proton in compounds 3n–3y. Peaks ranging from δ 7.13–6.49 ppm belonged to the aromatic ring. A doublet with δ 3.15–2.38 ppm was assigned to the signals of H-7. Other peaks with δ 2.00–0.85 ppm belonged to the hydrogen proton of p-menthane. In the 13C NMR spectra, peaks ranging from δ 165.21–103.27 ppm were assigned to the phenyl. The peak with δ 50.50 ppm was attributed to C-7, and peaks with δ 44.18–19.80 ppm were assigned to the p-menthane. The total number of hydrogen and carbon atoms is consistent with that of the corresponding compounds, and it was further confirmed by HRMS that compounds 3a–3y are sec-p-menthane-7-amine derivatives.
C bonds of an aromatic ring, and the stretching vibration band of C–N bonds, respectively. In the 1H NMR spectra, a singlet with δ 5.34 ppm belonged to the amino proton in compounds 3a–3m, and a singlet of δ 1.95 ppm belonged to the amino proton in compounds 3n–3y. Peaks ranging from δ 7.13–6.49 ppm belonged to the aromatic ring. A doublet with δ 3.15–2.38 ppm was assigned to the signals of H-7. Other peaks with δ 2.00–0.85 ppm belonged to the hydrogen proton of p-menthane. In the 13C NMR spectra, peaks ranging from δ 165.21–103.27 ppm were assigned to the phenyl. The peak with δ 50.50 ppm was attributed to C-7, and peaks with δ 44.18–19.80 ppm were assigned to the p-menthane. The total number of hydrogen and carbon atoms is consistent with that of the corresponding compounds, and it was further confirmed by HRMS that compounds 3a–3y are sec-p-menthane-7-amine derivatives.
3.2 Herbicidal activities against barnyard grass and rape
The post-emergence herbicidal activities of newly synthesized compounds 3a–3y against barnyard grass and rape were evaluated. The toxicity regression equations were calculated by DPS v17.10 software. The commercial herbicides glyphosate and diuron were chosen as positive controls. The results were listed in Tables 1–4 and Fig. 1–2; the inhibition rates of compounds 3a–3m against barnyard grass growth can be seen in the ESI,† Tables S3 and S4,† and the toxicity regression equations, IC50 values and IC90 values were shown in Tables S5 and S6.† When treated with 3a–3y solutions, barnyard grass and rape exhibited different symptoms: the barnyard grass emerged with shoot and root flavescence, with some even nigrescence, and the rape seeds rotted and stunk in the solutions. As is demonstrated, most of the sec-p-menthane-7-amine derivatives showed remarkable herbicidal activities against barnyard grass and rape. For the barnyard grass, compounds 3a–3m showed very weak activity: the inhibition rates for root growth were less than 30% when treated at 5 mmol L−1, and even exhibited negative inhibition.
Table 1 Inhibition rates of sec-p-menthane-7-amine derivatives against root growth of barnyard grass
| Compd |
Inhibition rates against root growth of barnyard grass |
| 2.5a |
1.25 |
0.625 |
0.313 |
0.156 |
0.078 |
0.039 |
0.0195 |
0.0098 |
0.0049 |
| The data in this line are the concentrations of different sec-p-menthane-7-amine derivatives solutions (mmol L−1). The inhibition rates of compounds 1 and 2 at 5 mmol L−1 against root growth of barnyard grass were 100% and 82.2%, against shoot growth were 100% and 77.6%. Have no inhibition activity at this concentration. |
| 1 |
81.2b |
54.3 |
53.4 |
48.5 |
21.5 |
20.2 |
c |
c |
c |
c |
| 2 |
45.4b |
3.3 |
5.0 |
9.6 |
7.2 |
0.1 |
c |
c |
c |
c |
| 3n |
100 |
100 |
98.7 |
88.5 |
66.9 |
59.6 |
54.0 |
49.6 |
42.1 |
32.1 |
| 3o |
100 |
100 |
100 |
95.2 |
74.7 |
67.1 |
59.3 |
55.3 |
47.9 |
37.6 |
| 3p |
100 |
100 |
100 |
97.2 |
88.5 |
79.1 |
68.7 |
60.8 |
55.0 |
46.0 |
| 3q |
100 |
100 |
100 |
97.5 |
88.4 |
75.1 |
66.5 |
57.8 |
49.0 |
40.8 |
| 3r |
100 |
100 |
100 |
95.6 |
91.0 |
83.1 |
72.7 |
66.9 |
60.2 |
52.3 |
| 3s |
100 |
100 |
99.7 |
91.7 |
74.0 |
67.3 |
58.4 |
53.2 |
47.8 |
22.4 |
| 3t |
100 |
100 |
99.6 |
92.5 |
79.4 |
69.4 |
62.4 |
50.3 |
46.4 |
36.5 |
| 3u |
100 |
100 |
100 |
95.4 |
81.5 |
72.6 |
66.5 |
60.3 |
53.6 |
46.9 |
| 3v |
100 |
100 |
100 |
98.3 |
90.0 |
70.3 |
64.2 |
56.8 |
50.6 |
44.6 |
| 3w |
100 |
100 |
100 |
95.2 |
86.6 |
75.7 |
68.7 |
60.5 |
53.9 |
45.2 |
| 3x |
100 |
100 |
99.8 |
96.1 |
87.7 |
82.0 |
73.1 |
62.2 |
52.6 |
33.5 |
| 3y |
100 |
100 |
100 |
95.9 |
88.8 |
57.1 |
34.8 |
16.4 |
c |
c |
| Diuron |
97.0 |
95.4 |
94.7 |
93.6 |
89.9 |
53.1 |
29.6 |
17.6 |
15.5 |
10.6 |
| Glyphosate |
100 |
99.8 |
92.3 |
83.9 |
79.6 |
69.5 |
43.9 |
24.9 |
16.6 |
8.9 |
Table 2 Inhibition rates of sec-p-menthane-7-amine derivatives against shoot growth of barnyard grass
| Compd |
2.5a |
1.25 |
0.625 |
0.313 |
0.156 |
0.078 |
0.039 |
0.0195 |
0.0098d |
| The data in this line are the concentrations of different sec-p-menthane-7-amine derivatives solutions (mmol L−1). The inhibition rates of compounds 1 and 2 at 5 mmol L−1 against root growth of barnyard grass were 100% and 82.2%, against shoot growth were 100% and 77.6%. Have no inhibition activity at this concentration. There were no inhibition activities of compounds against shoot growth of barnyard grass at 0.0049 mmol L−1. |
| 1 |
76.4b |
59.5 |
32.1 |
25.1 |
20.1 |
17.5 |
c |
c |
c |
| 2 |
38.8b |
13.6 |
6.8 |
1.7 |
2.7 |
3.3 |
c |
c |
c |
| 3n |
100 |
100 |
92.0 |
56.5 |
35.8 |
30.9 |
17.2 |
11.6 |
c |
| 3o |
100 |
100 |
99.5 |
70.9 |
45.7 |
33.7 |
24.7 |
17.1 |
c |
| 3p |
100 |
100 |
96.6 |
72.5 |
56.1 |
46.1 |
29.5 |
9.8 |
c |
| 3q |
100 |
100 |
96.5 |
66.9 |
52.3 |
34.9 |
28.6 |
12.4 |
c |
| 3r |
100 |
100 |
96.9 |
62.0 |
51.3 |
40.8 |
21.5 |
10.5 |
c |
| 3s |
100 |
100 |
97.5 |
72.4 |
45.9 |
30.4 |
21.2 |
15.2 |
c |
| 3t |
100 |
100 |
90.1 |
67.2 |
46.0 |
36.1 |
27.9 |
12.6 |
c |
| 3u |
100 |
100 |
95.5 |
76.4 |
54.1 |
50.3 |
35.5 |
26.2 |
19.3 |
| 3v |
100 |
100 |
100 |
87.3 |
71.0 |
50.7 |
40.5 |
34.6 |
23.4 |
| 3w |
100 |
100 |
93.6 |
77.2 |
59.0 |
46.3 |
30.7 |
23.2 |
10.3 |
| 3x |
100 |
100 |
91.5 |
68.4 |
58.7 |
52.0 |
39.9 |
26.9 |
19.0 |
| 3y |
100 |
81.4 |
43.2 |
26.7 |
15.6 |
c |
c |
c |
c |
| Diuron |
27.3 |
26.3 |
20.8 |
24.9 |
14.0 |
12.3 |
13.4 |
10.5 |
c |
| Glyphosate |
97.5 |
91.9 |
77.2 |
64.0 |
54.1 |
38.5 |
12.2 |
c |
c |
Table 3 Inhibition rates of sec-p-menthane-7-amine derivatives against root growth of rape
| Compd |
Inhibition rates against root growth of rape |
| 2.5a |
1.25 |
0.625 |
0.313 |
0.156 |
0.078 |
0.039 |
0.0195 |
0.0098b |
| The data in this line are the concentrations of different sec-p-menthane-7-amine derivatives solutions (mmol L−1). The inhibition rates at 0.0049 mmol L−1 to root and shoot growths of rape were not determined. Have no inhibition activity at this concentration. |
| 1 |
97.0 |
91.0 |
69.1 |
33.2 |
23.9 |
11.9 |
c |
c |
c |
| 2 |
98.4 |
81.2 |
59.7 |
44.9 |
38.7 |
23.0 |
12.9 |
c |
c |
| 3n |
100 |
100 |
98.9 |
95.6 |
82.6 |
60.3 |
53.4 |
33.9 |
23.4 |
| 3o |
100 |
100 |
100 |
98.0 |
87.0 |
56.6 |
40.1 |
28.1 |
17.6 |
| 3p |
100 |
100 |
99.8 |
97.9 |
92.4 |
65.5 |
50.2 |
29.5 |
15.8 |
| 3q |
100 |
100 |
100 |
98.5 |
94.4 |
79.5 |
50.2 |
46.4 |
33.4 |
| 3r |
100 |
100 |
100 |
98.2 |
85.0 |
55.9 |
38.5 |
20.6 |
15.0 |
| 3s |
100 |
100 |
93.8 |
89.5 |
68.3 |
48.9 |
35.2 |
13.4 |
c |
| 3t |
100 |
100 |
99.2 |
94.9 |
82.0 |
54.4 |
40.3 |
33.5 |
20.5 |
| 3u |
100 |
100 |
99.4 |
97.9 |
87.2 |
63.2 |
50.3 |
33.4 |
15.9 |
| 3v |
100 |
100 |
100 |
98.7 |
91.7 |
70.7 |
60.7 |
45.3 |
25.4 |
| 3w |
100 |
100 |
100 |
98.4 |
90.5 |
77.3 |
59.6 |
38.1 |
28.2 |
| 3x |
100 |
100 |
99.4 |
96.0 |
91.0 |
74.2 |
57.0 |
44.5 |
34.3 |
| 3y |
100 |
100 |
100 |
97.2 |
92.5 |
71.6 |
45.4 |
34.9 |
16.4 |
| Diuron |
85.0 |
80.4 |
78.1 |
69.5 |
52.8 |
36.2 |
26.4 |
16.4 |
c |
| Glyphosate |
97.9 |
90.9 |
88.5 |
81.4 |
77.7 |
69.7 |
62.8 |
52.0 |
28.1 |
Table 4 Inhibition rates of sec-p-menthane-7-amine derivatives against shoot growth of rape
| Compd |
Inhibition rates against shoot growth of rape |
| 2.5a |
1.25 |
0.625 |
0.313 |
0.156 |
0.078 |
0.039 |
0.0195 |
0.0098b |
| The data in this line are the concentrations of different sec-p-menthane-7-amine derivatives solutions (mmol L−1). The inhibition rates at 0.0049 mmol L−1 to root and shoot growths of rape were not determined. Have no inhibition activity at this concentration. |
| 1 |
99.4 |
71.6 |
45.2 |
29.0 |
14.1 |
c |
c |
c |
c |
| 2 |
87.1 |
53.3 |
41.7 |
30.9 |
31.0 |
22.5 |
14.5 |
c |
c |
| 3n |
100 |
100 |
95.5 |
67.0 |
47.0 |
38.6 |
25.1 |
17.0 |
c |
| 3o |
100 |
100 |
100 |
76.0 |
37.8 |
21.1 |
c |
c |
c |
| 3p |
100 |
100 |
96.1 |
72.4 |
38.4 |
25.0 |
13.9 |
c |
c |
| 3q |
100 |
100 |
91.0 |
76.1 |
42.7 |
34.2 |
22.6 |
14.9 |
c |
| 3r |
100 |
100 |
94.7 |
73.9 |
36.2 |
27.4 |
17.4 |
c |
c |
| 3s |
100 |
100 |
88.9 |
62.0 |
46.2 |
31.5 |
21.2 |
c |
c |
| 3t |
100 |
100 |
92.1 |
48.7 |
23.0 |
12.8 |
c |
c |
c |
| 3u |
100 |
100 |
96.1 |
76.0 |
48.2 |
31.3 |
22.3 |
12.2 |
c |
| 3v |
100 |
100 |
100 |
88.3 |
61.2 |
48.5 |
27.9 |
15.8 |
c |
| 3w |
100 |
100 |
98.7 |
77.5 |
35.3 |
26.1 |
14.2 |
c |
c |
| 3x |
98.6 |
96.2 |
87.7 |
73.2 |
51.8 |
36.3 |
30.2 |
21.6 |
11.9 |
| 3y |
100 |
98.5 |
93.8 |
60.4 |
42.9 |
22.1 |
15.2 |
c |
c |
| Diuron |
69.0 |
57.0 |
50.5 |
39.1 |
27.8 |
18.0 |
c |
c |
c |
| Glyphosate |
79.6 |
61.0 |
45.8 |
37.3 |
26.3 |
16.0 |
c |
c |
c |
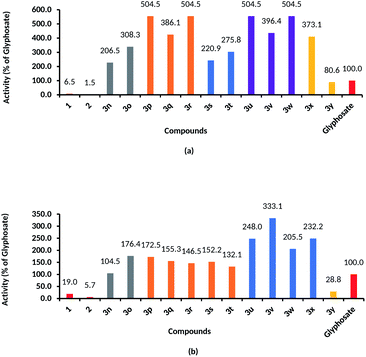 |
| | Fig. 1 Herbicidal activities of sec-p-menthane-7-amine derivatives against barnyard grass root growth (a) and shoot growth (b) comparing with glyphosate (IC50 of glyphosate/IC50 of test compounds). | |
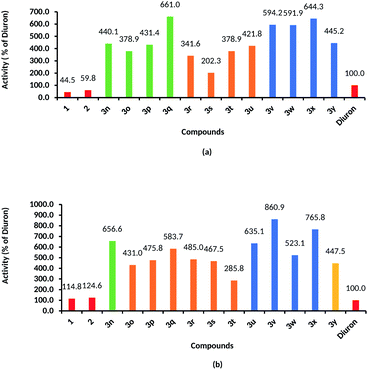 |
| | Fig. 2 Herbicidal activities of sec-p-menthane-7-amine derivatives against rape root growth (a) and shoot growth (b) comparing with diuron (IC50 of diuron/IC50 of test compounds). | |
Compounds 3n–3y displayed significant inhibition efficacy for both barnyard grass root and shoot. The inhibition rates of 3n–3y are higher than that of 1 and 2, indicating that the introduction of a secondary amino improves herbicidal activities. Root and shoot growth were completely inhibited at the concentrations of 2.5 mmol L−1 and 1.25 mmol L−1. When the treatment concentration was as low as 0.0049 mmol L−1, the inhibition rates of compounds 3p, 3r, 3u, 3v and 3w were 46.0%, 52.3%, 46.9%, 44.6%, 45.2%, respectively. The inhibition rates of 3n–3y for root growth were higher than that for shoot growth, at the concentration of 0.0098 mmol L−1, the inhibition rates against shoot growth of 3u, 3v and 3x were 19.3%, 23.4%, and 19.0%, respectively, but against root growth were higher than 50%. There are two N–H groups in the structure of compound 3y, but its inhibition rates are less than that of 3n–3x, indicating the increasing number of N–H groups cannot enhance the herbicidal activity.
The IC50 values of 3n–3x against barnyard grass root and shoot growth were 0.0088–0.0215 mmol L−1 and 0.0493–0.1571 mmol L−1, respectively, lower than that of diuron (IC50 values of root and shoot growth were 0.0529 mmol L−1 and >10 mmol L−1, respectively), were comparable to that of glyphosate (IC50 values of root and shoot growth were 0.0444 mmol L−1 and 0.1642 mmol L−1, respectively). By comparison of the IC50 values of glyphosate with the IC50 values of sec-p-menthane-7-amine derivatives in Fig. 1, it was found that the herbicidal activities of compounds 3p, 3r, 3u, and 3w against barnyard grass root growth were 404% higher than that of glyphosate, respectively. Compounds 3u, 3v, 3w, and 3x exhibited 148%, 233%, 105%, 132% higher herbicidal activities against shoot growth than those of glyphosate, respectively.
Compounds 3n–3y also exhibited good herbicidal activities against rape growth. The inhibition rates to rape root growth were higher than that of shoot growth. Most of the compounds showed over 50% control against the root growth of rape at a concentration of 0.078 mmol L−1: when the concentration was 0.0098 mmol L−1, many compounds still showed inhibition efficacy. The IC50 values of 3n–3y against root growth and shoot growth of rape were 0.0231–0.0755 mmol L−1 and 0.0813–0.2449 mmol L−1, respectively, lower than that of diuron (IC50 value of diuron against root and shoot growth of rape were 0.1527 mmol L−1 and 0.6999 mmol L−1, respectively), indicating that the herbicidal activities of sec-p-menthane-7-amines were higher than that of diuron. The IC50 values of 3q, 3v, 3w, and 3x against root growth of rape were 0.0231, 0.0257, 0.0258, 0.0237 mmol L−1, respectively, equivalent to that of glyphosate (IC50 value of glyphosate against root growth of rape was 0.0249 mmol L−1). The IC50 values of 3n–3y against shoot growth of rape were lower than that of glyphosate (IC50 value of glyphosate against root growth of rape was 0.6078 mmol L−1). It can be seen from Fig. 2 that the herbicidal activities of compounds 3q, 3v, 3w, and 3x against root growth of rape were 561%, 494%, 491%, 544% higher, respectively, than those of diuron, and 484%, 760%, 423%, 665%, respectively, higher than those of diuron against shoot growth of rape.
The structure–activity relationship was preliminarily obtained based on the herbicidal activities of sec-p-menthane-7-amine derivatives. The presence of electron-donating substituents on the phenyl ring was beneficial to herbicidal activities, compounds 3b, 3c (substituent CH3) and 3k (substituent OCH3) showed higher inhibition rates than compounds with electron-withdrawing substituents (halogen atom and OCF3 group). Compared with phenyl-substituted compounds, alkyl-substituted compounds showed better herbicidal activities, for example, compounds 3n-3y exhibited higher inhibition rates and lower IC50 values than those of compounds 3n–3y. Among the alkyl-substituted sec-p-menthane-7-amine derivatives, compounds with the alkyl-substituted part containing a cyclohexyl (3u, 3v, 3w) showed higher herbicidal activities than those containing a linear chain alkyl (3n, 3o, 3q, 3r), and compounds containing a branched alkyl (3s, 3t) showed weaker herbicidal activities. Compound 3x with two p-menthane skeletons in its structure showed great herbicidal activity, which meets the superposition principle. Compound 3y, with two secondary amino groups in the structure, showed weaker herbicidal activity than compounds with only one, indicating that the number of secondary amino groups key to herbicidal activities and that the increase of secondary amino groups may not necessarily enhance herbicidal activities. The possible reason is steric hindrance: 3y cannot easily to go through the cell wall to dock with the active site.
3.3 Crop safety
An eligible herbicide must be sensitive to weeds and highly safe to crops. Compounds 3p, 3u, and 3v were selected for the crop safety test targeting seven crops: rice, wheat, sorghum, maize, peanut, cucumber, and radish, to which glyphosate was used as a positive control. The results were listed in Table 5, and it can be found that these compounds were almost harmless (≤30%) to rice, wheat, sorghum, maize, peanut at the concentration of 100 mg L−1, while glyphosate caused over 80% injury to these crops. The cucumber and radish sustained critical injuries (>80%) with compounds 3p, 3u, and 3v, indicating that sec-p-menthane-7-amine derivatives could be developed as a potential herbicide for weed control in rice, wheat, sorghum, maize, and peanut fields.
Table 5 Crop safety of compounds 3p, 3u, 3v at 100 mg L−1
| Compd |
Injury rate (%) |
| Wheat |
Rice |
Sorghum |
Maize |
Peanut |
Cucumber |
Radish |
| 3p |
30 |
20 |
10 |
10 |
0 |
80 |
80 |
| 3u |
20 |
20 |
10 |
15 |
0 |
90 |
75 |
| 3v |
20 |
30 |
10 |
10 |
5 |
90 |
80 |
| Glyphosate |
90 |
40 |
90 |
95 |
95 |
90 |
60 |
3.4 Cytotoxicity analysis
Agrochemicals must be nontoxic or pose low toxicity to humans and other organisms. The cytotoxicity of compounds 3n–3y against BALB/c 3T3 and HUVEC-C cell lines were evaluated using the CCK-8 method. As showed in Table 6, when treated with a 10 μmol L−1 concentration of compounds 3n–3x, the inhibition rates against the growth of BALB/c 3T3 and HUVEC-C cells were 0.17% to 4.34% and −0.54% to 3.26%, respectively, meaning that these compounds exhibited nearly no toxicity to BALB/c 3T3 and HUVEC-C cells. Nevertheless, compound 3y showed severe toxicity to BALB/c 3T3 and HUVEC-C cells, where inhibition rates were 85.40% and 86.01%, respectively.
Table 6 Cytotoxicity of sec-p-menthane-7-amine derivatives at 10 μmol L−1
| Compd |
Inhibition rate (%) to BALB/c 3T3 |
Inhibition rate (%) to HUVEC-C |
| When the growth inhibition rate of the compound against the cells is negative (the absolute value is higher than 10%), it is generally considered that the compound promotes the proliferation of the cells. |
| 2 |
1.58 |
1.64 |
| 3n |
2.00 |
−0.41a |
| 3o |
2.61 |
2.85 |
| 3p |
0.17 |
−0.14a |
| 3q |
1.56 |
−0.54a |
| 3r |
2.35 |
−0.14 |
| 3s |
2.52 |
1.90 |
| 3t |
4.34 |
2.31 |
| 3u |
2.09 |
3.40 |
| 3v |
2.09 |
1.09 |
| 3w |
0.35 |
3.26 |
| 3x |
1.13 |
2.05 |
| 3y |
85.40 |
86.01 |
4 Conclusions
In summary, a series of novel sec-p-menthane-7-amine derivatives were designed and synthesized using perillaldehyde as raw material. The results indicated that some of these compounds exhibited better herbicidal activities than commercial herbicides diuron and glyphosate. The IC50 values of 3n–3y against root and shoot growth of barnyard grass were 0.0088–0.0551 mmol L−1 and 0.0493–0.5711 mmol L−1, respectively. The herbicidal activities of compounds 3p, 3r, 3u and 3w against root growth of barnyard grass were 404% higher, respectively, than those of glyphosate. The herbicidal activities of compounds 3u, 3v, 3w, and 3x against shoot growth of barnyard grass were 148%, 233%, 105%, 132% higher, respectively, than those of glyphosate. The IC50 values of 3n–3y against root and shoot growth of rape were 0.0231–0.0755 mmol L−1 and 0.0813–0.2449 mmol L−1, respectively. The herbicidal activities of sec-p-menthane-7-amine derivatives against root and shoot growth of rape were higher than those of diuron. The structure–activity relationship studies showed that the alkyl-substituted sec-p-menthane-7-amine derivatives exhibited higher herbicidal activities than those of phenyl-substituted derivatives, and compound 3u with an alkyl-substituted part containing a cyclohexyl, showed the best herbicidal activities: the IC50 value against barnyard grass root growth was 0.0088 mmol L−1. Furthermore, compounds 3p, 3u, and 3v were found to show good safety to rice, wheat, sorghum, maize and peanut. Compounds 3n–3x have been verified to be nontoxic for BALB/c 3T3 and HUVEC-C cells. In conclusion, compound 3u was expected to be the most promising botanical herbicide for future weed control.
Author contributions
Synthesis and characterization, writing original draft, H. M. Z.; writing, review, and editing, Y. X. C., S. C. X., J. W., H. H. D., Z. D. Z. and J. X. J. All authors have read and agree to the published version of the manuscript.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 31870557). The cytotoxicity of the synthesized compounds was evaluated by Jiangsu KeyGEN BioTECH Co. Ltd.
References
- H. Chen, X. Liu, H. Wang, S. Wu, J. Li, C. Jin and H. Xu, J. Hazard. Mater., 2021, 402, 123744 CrossRef CAS PubMed.
- Z. Wang, Z. Sun, L. Xiao, Y. Zhou and F. Du, J. Agric. Food Chem., 2019, 67, 14102–14109 CrossRef CAS PubMed.
- D. Huang, S. Zhu, H. Lan, Z. Lin and X. Wang, Ind. Crops Prod., 2019, 129, 24–34 CrossRef CAS.
- D. Zhao, X. Han, M. Wang, Y. Zeng, Y. Li, G. Ma, J. Liu, C. Zheng, M. Wen, Z. Zhang, P. Zhang and C. Zhang, J. Agric. Food Chem., 2020, 68, 11207–11214 CrossRef CAS.
- J. Li, Y. Wang, Y. Wu, R. Li, S. Liang, J. Zhang, Y. Zhu and B. Xie, Pestic. Biochem. Physiol., 2021, 172, 104766 CrossRef CAS.
- A. Guan, C. Liu, X. Yang and M. Dekeyser, Chem. Rev., 2014, 114, 7079–7107 CrossRef CAS PubMed.
- F. Dayan and S. Duke, Plant Physiol., 2014, 166, 1090–1105 CrossRef.
- G. Feng, M. Chen, H. Ye, Z. Zhang, H. Li, L. Chen, X. Chen, C. Yan and J. Zhang, Ind. Crops Prod., 2019, 132, 41–47 CrossRef CAS.
- I. Amri, L. Hamrouni, M. Hanana and B. Jamoussi, Int. J. Appl. Biol. Pharm. Technol., 2013, 4, 96–114 Search PubMed.
- S. Kordali, A. Cakir, H. Ozer, R. Cakmakci, M. Kesdek and E. Mete, Bioresour. Technol., 2008, 99, 8788–8795 CrossRef CAS.
- A. Kashkooli and M. Saharkhiz, J. Essent. Oil-Bear. Plants., 2014, 17, 859–874 CrossRef CAS.
- M. Verdeguer, M. Blázquez and H. Boira, Nat. Prod. Res., 2012, 26, 1602–1609 CrossRef CAS.
- C. Laosinwattana, P. Wichittrakarn and M. Teerarak, Ind. Crops Prod., 2018, 126, 129–134 CrossRef CAS.
- M. Ootani, M. Rodrigues, A. Sander, A. Capone, R. Fidelis, W. Oliveira, H. Barros, A. Portella, R. Aguiarc and W. Santos, Biocatal. Agric. Biotechnol., 2017, 12, 59–65 CrossRef.
- M. Verdeguer, M. Sánchez and F. Araniti, Plants, 2020, 9, 1571–1622 CrossRef CAS.
- L. Liu, J. Liao, W. Duan and F. Lei, Lett. Org. Chem., 2015, 12, 283–289 CrossRef CAS.
- Q. Hu, G. Lin, W. Duan, M. Huang and F. Lei, Molecules, 2017, 22, 1678–1693 CrossRef.
- A. Barton, B. Dell and A. Knight, J. Agric. Food Chem., 2010, 58, 10147–10155 CrossRef CAS.
- J. Rui, Q. Zhang, X. Wang, X. Xu, H. Xu, W. Rao and S. Wang, Chin. J. Org. Chem., 2017, 37, 218–225 CrossRef CAS.
- Y. Ma, W. Duan, G. Lin, L. Liu, Z. Huang and F. Lei, Chem. Ind. For. Prod., 2017, 37, 54–62 CAS.
- S. Xu, S. Zhu, J. Wang, L. Bi, Y. Chen, J. Liu, Y. Gu and Z. Zhao, Chin. Chem. Lett., 2017, 28, 1509–1513 CrossRef CAS.
- S. Zhu, S. Xu, J. Wang, Z. Zhao and J. Jiang, J. Agric. Food Chem., 2016, 64, 9702–9707 CrossRef CAS.
- S. Zhu, S. Xu, X. Yi, J. Wang, Z. Zhao and J. Jiang, Ind. Crops Prod., 2018, 115, 111–116 CrossRef CAS.
- M. Durgun, H. Turkmen, M. Ceruso and C. Supuran, Bioorg. Med. Chem. Lett., 2015, 25, 2377–2381 CrossRef CAS.
- M. Bharara, S. Tonks and A. Gorden, Chem. Commun., 2007, 39, 4006–4008 RSC.
- H. Dong, S. Xu, J. Wang, H. Zhang, Y. Chen, L. Bi and Z. Zhao, New J. Chem., 2020, 44, 8280–8288 RSC.
- S. Xu, X. Zeng, S. Dai, J. Wang, Y. Chen, J. Song, Y. Shi, X. Chen, S. Liao and Z. Zhao, J. Agric. Food Chem., 2020, 68, 11829–11838 CrossRef CAS.
- J. Tian, Y. Wang, Z. Lu, C. Sun, M. Zhang, A. Zhu and X. Peng, J. Agric. Food Chem., 2016, 64, 7404–7413 CrossRef CAS.
- J. Xu, W. Hu, S. Dong, L. Yi, J. Zeng and M. Li, Pharmacol. Rep., 2019, 71, 430–437 CrossRef.
- S. Terpiłowska and A. Siwicki, Cent. Eur. J. Immunol., 2010, 35, 58–62 Search PubMed.
- J. Bi, Y. Liu, X. Liu, S. Lei and X. Chen, J. Endodont., 2020, 46, 1–7 Search PubMed.
- S. Li, D. Xu, J. Guo and Y. Sun, Environ. Toxicol., 2016, 31, 1785–1795 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data and spectra for sec-p-menthane-7-amine derivatives 3a–3y, inhibition rates of compounds 3a–3m, toxicity regression equations and the IC50 and IC90 values. See DOI: 10.1039/d1ra04910k |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ab,
Yuxiang Chena,
Shichao Xua,
Jing Wangab,
Huanhuan Dong
ab,
Yuxiang Chena,
Shichao Xua,
Jing Wangab,
Huanhuan Dong a,
Zhendong Zhao
a,
Zhendong Zhao *a and
Jianxin Jiang*b
*a and
Jianxin Jiang*b
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of Schiff base compounds is usually unstable in storage and herbicidal assays because it can be easily oxidized by air, hydrolyzed in an acidic environment, and subjected to transamination by nucleophiles.24,25 Therefore, it is necessary to convert the Schiff bases into a more stable secondary amines using the reduction. It has been proven that secondary amines such as sec-p-menth-3-enylamines and cis-1,8-p-menthane-di-sec-amines exhibit higher herbicidal activities than that of Schiff bases, and they show low toxicity to common crops and mammal cells.26,27
N) of Schiff base compounds is usually unstable in storage and herbicidal assays because it can be easily oxidized by air, hydrolyzed in an acidic environment, and subjected to transamination by nucleophiles.24,25 Therefore, it is necessary to convert the Schiff bases into a more stable secondary amines using the reduction. It has been proven that secondary amines such as sec-p-menth-3-enylamines and cis-1,8-p-menthane-di-sec-amines exhibit higher herbicidal activities than that of Schiff bases, and they show low toxicity to common crops and mammal cells.26,27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ntrans- = 1
ntrans- = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (day/night), and kept at a relative humidity 70–80% for 96 h. The inhibition rate (%) of the root or shoot growth was calculated according to eqn (1).
8 (day/night), and kept at a relative humidity 70–80% for 96 h. The inhibition rate (%) of the root or shoot growth was calculated according to eqn (1).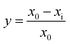
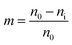
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of an aromatic ring, and the stretching vibration band of C–N bonds, respectively. In the 1H NMR spectra, a singlet with δ 5.34 ppm belonged to the amino proton in compounds 3a–3m, and a singlet of δ 1.95 ppm belonged to the amino proton in compounds 3n–3y. Peaks ranging from δ 7.13–6.49 ppm belonged to the aromatic ring. A doublet with δ 3.15–2.38 ppm was assigned to the signals of H-7. Other peaks with δ 2.00–0.85 ppm belonged to the hydrogen proton of p-menthane. In the 13C NMR spectra, peaks ranging from δ 165.21–103.27 ppm were assigned to the phenyl. The peak with δ 50.50 ppm was attributed to C-7, and peaks with δ 44.18–19.80 ppm were assigned to the p-menthane. The total number of hydrogen and carbon atoms is consistent with that of the corresponding compounds, and it was further confirmed by HRMS that compounds 3a–3y are sec-p-menthane-7-amine derivatives.
C bonds of an aromatic ring, and the stretching vibration band of C–N bonds, respectively. In the 1H NMR spectra, a singlet with δ 5.34 ppm belonged to the amino proton in compounds 3a–3m, and a singlet of δ 1.95 ppm belonged to the amino proton in compounds 3n–3y. Peaks ranging from δ 7.13–6.49 ppm belonged to the aromatic ring. A doublet with δ 3.15–2.38 ppm was assigned to the signals of H-7. Other peaks with δ 2.00–0.85 ppm belonged to the hydrogen proton of p-menthane. In the 13C NMR spectra, peaks ranging from δ 165.21–103.27 ppm were assigned to the phenyl. The peak with δ 50.50 ppm was attributed to C-7, and peaks with δ 44.18–19.80 ppm were assigned to the p-menthane. The total number of hydrogen and carbon atoms is consistent with that of the corresponding compounds, and it was further confirmed by HRMS that compounds 3a–3y are sec-p-menthane-7-amine derivatives.



