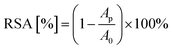Open Access Article
Open Access ArticleThe application of amino acid ionic liquids as additives in the ultrasound-assisted extraction of plant material†
Barbara Romana,
Anna Muzykiewicz-Szymańskab,
Paula Ossowicz-Rupniewskaa,
Adam Klimowicz b and
Ewa Janus
b and
Ewa Janus *a
*a
aWest Pomeranian University of Technology Szczecin, Faculty of Chemical Technology and Engineering, Department of Chemical Organic Technology and Polymeric Materials, Piastów Ave. 42, 71-065 Szczecin, Poland. E-mail: ejanus@zut.edu.pl
bPomeranian Medical University in Szczecin, Department of Cosmetic and Pharmaceutical Chemistry, Powstańców Wielkopolskich Ave. 72, 70-111 Szczecin, Poland
First published on 27th July 2021
Abstract
The aim of the present study was to determine the antioxidant activity of the aqueous extracts from Lycopodium clavatum, Cetraria islandica and Dipsacus fullonum obtained using aqueous solutions of ionic liquids by the ultrasound-assisted extraction (IL-UAE) method. Triethanolammonium salts [TEAH]+[AA]− of four amino acids of different hydrophobicity – isoleucine – Ile, methionine – Met, threonine – Thr and arginine – Arg, were chosen as ionic liquids, because they are based on natural, bio-renewable raw materials, such as amino acids and contain a pharmaceutically and cosmetically acceptable counterion of triethanolamine. Triethanolammonium salts were synthesized, identified by spectroscopic methods (NMR and FT-IR) and characterized by thermal methods (DSC and TGA). The 2.5% w/v aqueous solutions of triethanolammonium amino acid salts were used as the solvents in combination with ultrasound assisted extraction (UAE). The estimation of antioxidant properties was carried out using the DPPH, FRAP and CUPRAC assays. Total polyphenol content was measured using the reagent Folin–Ciocalteu. The results showed that the use of [TEAH]+[Thr]− or [TEAH]+[Met]− aqueous solutions increased the antioxidant activity of extracts in comparison to that achieved for extracts with pure water. The use of [TEAH]+[Thr]− as an additive for ultrasound-assisted extraction was characterized by obtaining plant extracts with the highest antioxidant potential, even 2.4-fold. The use of the AAIL-UAE method allowed obtaining higher amounts of polyphenols compared to pure water extracts, even 5.5-fold. The used method allowed the extraction of thermosensitive natural compounds, shortened the extraction time and lowered energy consumption.
Introduction
Antioxidants are a broad class of substances characterized by the ability to inhibit oxidation through various mechanisms of neutralizing or scavenging free radicals. As a result, cells of the living organism are protected against oxidative stress.1,2 Due to these properties, they exhibit anti-inflammatory properties, improve the sealing of blood vessels, and have anti-cancer and anti-mutagenic properties.2,3 The isolation of compounds with antioxidant properties includes commonly used methods such as Soxhlet extraction (SLE),4 solvent extraction,5 ultrasound-assisted extraction (UAE)6 or microwave extraction (MAE).7 The modern use of ultrasound-assisted extraction allows increasing the efficiency of the process while reducing energy and solvent consumption, shortening the extraction time and lowering temperatures.8 The use of ultrasound for the plant extraction causes disruption of cell walls, fragmentation of plant material and facilitates the migration of organic compounds to the solvent by rapid collapse of the bubbles formed in a variable pressure field under the influence of the cavitation phenomenon.9 Thus, this method is more environmentally friendly compared to the traditional methods using organic solvents and is classified as the so-called “green-extraction technique”.Ionic liquids are designable, low melting ionic compounds with unique properties such as low vapor pressure, high thermal and chemical stability and non-flammability, which can be appropriately modified by selecting the organic cation and organic or inorganic anion.10 In recent years, ionic liquids have been gaining recognition as substitutes for organic solvents, and their use is constantly expanding in organic synthesis, catalysis and separation processes as media and catalysts.11–13 Due to the wide and increasing use, a lot of attention is being paid to ionic liquids based on renewable raw materials, such as amino acids, proteins, fats, sugars and terpenes.13–16 Ionic liquids based on these raw materials are characterized by high biodegradability, low toxicity, easy access to reactants, and biocompatibility with living organisms.17 Due to the above properties, they can be promising additives for the separation of various substances from plant material.18–23
The combination of ultrasound-assisted extraction with ionic liquids instead of the commonly used organic solvents, e.g. methanol and chloroform allows obtaining extracts with higher antioxidant potential. Ionic liquids based on imidazolium cations are the most widely studied as solvents for extraction of natural products, also in combination with the UAE method.21 They provide good solubility of phenolic compounds. For example [BMIM][PF6] was effective for extraction of gallic acid, chlorogenic acid, rutin, psoralen, and bergapten from Ficus carica L.24 and magnolol and honokiol from cortex Magnoliae officinalis,25 whereas [BMIM][BF4] for chlorogenic acid from Lonicera japonica Thunb.26 Additionally, in the extraction of carnosic acid and rosmarinic acid from Rosmarinus officinalis the best yields were achieved with [OMIM][Br].27 Ionic liquids have also proved to be good solvents for alkaloids, for example [AMIM][Br] for the extraction of vindoline, catharanthine and vinblastine from Catharanthus roseus28 and [BMIM][BF4] for fangchinoline and tetrandrine from Stephaniae tetrandrae.29 Moreover, isoflavones were also extracted with imidazolium ionic liquids, and [EMIM][BF4] was efficiently used for tectorigenin, iristectorigenin A, irigenin, and irisflorentin extraction from Belamcanda chinensis,30 and [HMIM][Br] for genistin, genistein, daidzin, and daidzein from soy.31 Another interesting report revealed the combination of UAE with protic ionic liquids from the group of the ammonium salts, such as 2-hydroxyethylammonium acetate (2-HEAA) and 2-hydroxyethylammonium formate (2-HEAF) for extraction of the phycobiliproteins from the microalgae Spirulina (Arthrospira) platensis.32 The results were compared to that achieved with [BMIM][Cl] as the solvent and showed that the highest concentration of phycobiliproteins was achieved using the mixture 2-HEAA + 2-HEAF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) within 30 min of extraction. Other authors have shown that using the UAE method, the aqueous solution of ionic liquids exhibited higher efficiency of phenolics extraction than water or methanol as the solvent.24 Moreover, it was revealed that the shorter alkyl substituents in the cation of ILs provide a better miscibility with water and are the crucial factor for an increase in the extraction efficiency. Additionally, the ionic liquids based on small and hydrophilic anions, such as Cl−, Br−, BF4−, PF6−, CnCO2 and CnSO4, are more desired.21–23
1 v/v) within 30 min of extraction. Other authors have shown that using the UAE method, the aqueous solution of ionic liquids exhibited higher efficiency of phenolics extraction than water or methanol as the solvent.24 Moreover, it was revealed that the shorter alkyl substituents in the cation of ILs provide a better miscibility with water and are the crucial factor for an increase in the extraction efficiency. Additionally, the ionic liquids based on small and hydrophilic anions, such as Cl−, Br−, BF4−, PF6−, CnCO2 and CnSO4, are more desired.21–23
Among plants with antioxidant properties, we can distinguish the clubmoss (Lycopodium clavatum L.), the Icelandic moss (Cetraria islandica (L.) Ach.) and the wild teasel (Dipsacus fullonum L.). These plants are characterized by the content of antioxidant compounds such as polyphenols, terpenoids, phenolic acids, flavonoids, alkaloids and vitamins.33–37 The antioxidant activity of Lycopodium clavatum, Cetraria islandica and Dipsacus fullonum extracts has been confirmed in studies by different authors.36–47 Table 1 presents the results of the antioxidant potential of the extracts, for the preparation of which various extraction techniques were used. Generally conventional methods such as classical extraction under stirring under ambient conditions or at elevated temperatures with various organic solvents were used. Mainly ethanol (40–96%)36,41,45 and methanol (50–100%)35,38–40,42–44,46 were used to obtain extracts by maceration, and antioxidant potential was measured usually for dry extracts. Other solvents such as water, acetone, petroleum ether, chloroform and ethyl acetate were also used.35–38,42,43,47 Plant extracts of C. islandica and D. fullonum were obtained also using a Soxhlet extractor.40,42 In the case of D. fullonum, extraction was conducted under shaking sonication conditions.46 Moreover, different antioxidation activity assays were applied (Table 1). However, no reports were found about the antioxidant activity of extracts from these plants, prepared using aqueous solutions of ionic liquids.
| Extraction method and conditionsa | Antioxidant capacity/activity assaysb | Antioxidant potential valuec | Ref. |
|---|---|---|---|
| a PEE – petroleum ether; s/l – solid/liquid; HRE -classical extraction under stirring under ambient conditions or at elevated temperature; UAE – ultrasound assisted extraction.b ABTS – 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) as a probe, DPPH – 2,2-diphenyl-1-picrylhydrazyl as a probe, FRAP – ferric reducing antioxidant power, ORAC – oxygen radical absorbance capacity.c PCE – pyrocatechol equivalent, TEAC – Trolox equivalent antioxidant capacity, GAE – gallic acid equivalents. | |||
| Lycopodium clavatum | |||
| HRE | DPPH | 11.3 ± 1.64% (PLE); no activity observed (CHCl3); 44.1 ± 5.94% (CHCl3 + alkaloid fraction); 26.6 ± 0.7% (EtOAc); 30.3 ± 0.15% (MeOH) | 38 |
PLE, CHCl3, CHCl3 + alkaloid fraction, EtOAc, MeOH, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.2 g ml−1 5.2 g ml−1 |
|||
| HRE | DPPH | 91.7 ± 0.6% (500 μg crude dry extract per ml); 85.6 ± 4.1% (250 μg crude dry extract per ml); 50.1 ± 3.2% (50 μg crude extract per ml) | 39 |
| Maceration (MeOH) after Soxhlet extraction (n-hexane) | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Cetraria islandica | |||
| HRE | DPPH | 40.68% (acetone); 18.62% (EtOH); ∼50.00% (rutin as standard) | 36 |
Acetone, EtOH, stirring 3 h, 50 °C, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 g ml−1 5 g ml−1 |
|||
| Soxhlet extraction | DPPH | IC50 = 678.38 μg of dry extract per ml; IC50 = 6.24 μg ascorbic acid as standard per ml water | 40 |
| MeOH | |||
| Total polyphenols | 38.08 μg PCE per mg of dry extract | ||
| HRE | DPPH | 5.61 ± 0.21 mg TEAC per g | 41 |
80% EtOH, 24 h, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g ml−1 200 g ml−1 |
|||
| Total polyphenols | 0.37 ± 0.01 mg GAE per g | ||
| Soxhlet extraction | DPPH | ∼36% (acetone); ∼47% (MeOH); ∼32% (aq) | 42 |
Acetone, MeOH, water, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 g ml−1 50 g ml−1 |
|||
| Total polyphenols | 25 ± 1.09 μg PCE (acetone); 38.083 ± 1.031 μg PCE (MeOH); 18.19 ± 1.018 μg PCE (water) | ||
| HRE | DPPH | From 17.5% (12.5 μg crude MeOH extract per ml) to 65.6% (200.0 μg crude MeOH extract per ml); IC50 = 75 μg crude MeOH extract per ml | 43 |
MeOH, 12 h, 45 °C, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g ml−1 20 g ml−1 |
Total polyphenols | 78.3 ± 0.00 mg GAE per g dried mass (MeOH); 45.3 ± 0.00 mg GAE per g dried mass (acetone) | |
Acetone, 12 h, 50 °C, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g ml−1 20 g ml−1 |
|||
| HRE | DPPH | IC50 = 1183.55 μg ml−1 | 44 |
MeOH, rt., 1 h, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66.7 g ml−1 66.7 g ml−1 |
Total polyphenols | 57.34 ± 3.30 μg GAE per mg dry extract | |
| HRE | DPPH | 25% (96% EtOH); 86% (70% EtOH); 69% (40% EtOH) | 45 |
96% EtOH, 70% EtOH, 40% EtOH; rt; 14 days; s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g ml−1 10 g ml−1 |
FRAP method | 486 μmol L−1 (96% EtOH); 135 μmol L−1 (70% EtOH); 158 μmol L−1 (40% EtOH)/ | |
| Total polyphenols | 0.137 mg GAE per g extract (96% EtOH); 0.586 mg GAE per g extract (70% EtOH); 0.328 mg GAE per g extract (40% EtOH) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Dipsacus fullonum | |||
| UAE | ORAC | Extract from leaves – 14.78 ± 0.94 mmol TEAC/100 g of dry weight | 46 |
| 50% MeOH, 20 min | Extract from roots – 10.87 ± 1.04 mmol TEAC/100 g of dry weight | ||
| HRE | DPPH | 4.01 ± 0.58 mmol TEAC/100 g dry weight of plant material | 47 |
Acetone/water ratio 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, shaking 60 min, s/l ratio 1 3 v/v, shaking 60 min, s/l ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 g ml−1 50 g ml−1 |
ORAC | 3.58 ± 0.35 mmol TEAC/100 g dry weight of plant material | |
| Total polyphenols | 19.52 ± 0.12 mg GAE per g dry weight of plant material | ||
The aim of the presented studies was to determine the antioxidant activity of the aqueous extracts from Lycopodium clavatum, Cetraria islandica and Dipsacus fullonum obtained using aqueous solutions of ionic liquids by the ultrasound-assisted extraction (IL-UAE) method. Among the many ionic liquids, we have chosen triethanolammonium salts of amino acids, because they are based on natural, bio-renewable raw materials, such as amino acids and contain a pharmaceutically and cosmetically acceptable counterion of triethanolamine. Moreover, the raw materials used to obtain these ionic liquids are inexpensive and easily available. Finally, due to their natural origin, they have improved properties from an environmental standpoint. Aqueous solutions of ionic liquids were selected for the extraction, due to the safety of water, but also the solubility of these amino acid ionic liquids (AAILs) only in water. Pure AAILs, i.e. without the water addition, were not used because of their high viscosity, which could increase even more during extraction and thus reduce the extraction efficiency due to the ability of some AAILs to dissolve the lignocellulose fraction.48–50 Moreover, the literature suggested a great advantage of using aqueous solutions of ILs over pure ILs.51–54 To measure the antioxidant potential of plant extracts, in vitro methods, based on the reduction properties of antioxidants, such as the DPPH, CUPRAC and FRAP assays were chosen. Additionally, the total polyphenol content was assessed using the Folin–Ciocalteu (F–C) method. Determination of biological activity by in vitro assays is easy to realize, less expensive and error-prone and also takes less time than traditional in vivo methods.55
Experimental
Materials
The following amino acids were used for the synthesis: L-threonine (Thr, >99 wt%, pure), L-methionine (Met, >99 wt%, pure), L-arginine (Arg, >99 wt%, pure), L-isoleucine (Ile, >99 wt%, pure), purchased from Roth, and triethanolamine (TEA) (>99 wt%, pure) from Acros Organics. DPPH (2,2-diphenyl-1-picrylhydrazyl), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) and Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) were purchased from Sigma-Aldrich (USA). Folin–Ciocalteu phenol reagent, iron(II) sulfate heptahydrate and gallic acid were obtained from Merck (Germany), whereas ethanol, 99.5% acetic acid, sodium acetate anhydrous, 36% hydrochloric acid, iron(III) chloride hexahydrate, copper(II) chloride dihydrate, and sodium carbonate anhydrous were obtained from Chempur (Poland). Neocuproine was delivered by J&K Scientific (Germany).The research plant material consisted of dry and cut L. clavatum herbs (Dary Natury, Poland), C. islandica (Kawon, Poland) and D. fullonum leaves (NatVita, Poland).
Methods
The 1H NMR and 13C NMR spectra were recorded using a BRUKER DPX-400 spectrometer (Billerica, MA, USA). The solvent for the compounds was D2O. The 1H NMR and 13C NMR spectra are found in the ESI.†FT-IR spectra were recorded using a Thermo Fisher Scientific Nicolet FT-IR 380 spectrometer (Waltham, MA, USA) equipped with an attenuated total reflectance (ATR) sample accessory (diamond plate). Spectra were recorded in a transmittance mode from 400 to 4000 cm−1 at a resolution of 4 cm−1.
The elemental analysis CHNS was performed using a Thermo Scientific™ FLASH 2000 CHNS/O analyzer (Waltham, MA, USA). Compounds were weighed with an accuracy of 0.000001 g in tin crucibles. 2,5-Bis(5-tert-butyl-2-benzo-oxazol-2-yl)thiophene (BBOT), sulphanilamide, L-cysteine and L-methionine were used as standards to calibrate the device in CHNS mode.
Specific rotation measurements [α]20D were carried out for aqueous solutions of triethanolammonium salts of amino acids using an AUTOPOL IV Rudolph Research Analytical automatic polarimeter.
Thermogravimetric analysis (TG) was carried out using a thermomicrobalance TG 209 F1 Libra® from NETZSCH (Selb, Germany). Measurements were performed in the temperature range from 30 to 700 °C, at a heating rate of 10 °C min−1 with the following gas flows: nitrogen 25 ml min−1 and air 10 ml min−1. The samples (4–6 mg) were placed in a crucible of Al2O3. The TG spectra are found in the ESI.†
The phase transition temperatures were determined on the basis of Differential Scanning Calorimetry (DSC) analysis performed with a Q-100 TA Instruments (New Castle, DE, USA). The sample was loaded in an aluminum pan with a pierced lid. First, the sample was heated to 100 °C and kept at this temperature for about 5 min to remove any moisture. It was then cooled to −90 °C and reheated to 200 °C. The heating and cooling rate was 10 °C min−1. Measurements were performed under a nitrogen atmosphere. Indium and mercury were used as standards to calibrate the temperature. The DSC spectra are found in the ESI.†
The spectrophotometric measurements of antioxidant activity and total polyphenol content were performed using a Hitachi U-5100 (Japan) UV-VIS spectrophotometer in a 1 cm cuvette.
Synthesis of triethanolammonium salt of amino acids [TEAH]+[AA]−
To a round bottom flask with a capacity of 100 cm3, provided with a magnetic stirring bar, were weighed equimolar amounts of the L-amino acid and triethanolamine and dissolved in deionized water (30 cm3) for each 0.1 mol of amino acid. The solution was stirred for 12 h at ambient temperature and then the water was removed by rotary evaporation under reduced pressure. Then the obtained [TEAH]+[AA]− was dried for 12 h at 50 °C, 5 mbar in a vacuum dryer. The obtained compound was stored in a freezer.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 2.73 (t, J = 6 Hz, 6H, H+NC
3); 2.73 (t, J = 6 Hz, 6H, H+NC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2); 3.32 (d, J = 4.9 Hz, 1H, NH2C
2); 3.32 (d, J = 4.9 Hz, 1H, NH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ); 3.57 (t, J = 6 Hz, 6H, C
); 3.57 (t, J = 6 Hz, 6H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2OH); 4.02 (m, 1H, C
2OH); 4.02 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH3). 13C NMR(D2O), δ [ppm]: 19.33 (1C, CH
CH3). 13C NMR(D2O), δ [ppm]: 19.33 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3); 55.55 (3C, H+N
H3); 55.55 (3C, H+N![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2); 57.94 (3C,
H2); 57.94 (3C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2OH); 60.61 (1C,
H2OH); 60.61 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HNH2); 66.46 (1C,
HNH2); 66.46 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HOH); 174.12 (1C, CH
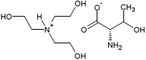
HOH); 174.12 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO−). FT-IR (ATR), ν [cm−1]: 420; 445; 489; 558; 699; 765; 871; 907; 929; 1028; 1091; 1105; 1182; 1246; 1314; 1343; 1382; 1414; 1450; 1474; 1620; 1635; 1653; 2500 ÷ 3500 (broad band with peaks at 2515; 2871; 2974; 3155). Elemental analysis calc. (%) for C10H24N2O6 (m.w.: 268.31u): C 44.77, H 9.02, N 10.44, O 37.75, found: C 44.59, H 8.95, N 10.35, O 37.66.
OO−). FT-IR (ATR), ν [cm−1]: 420; 445; 489; 558; 699; 765; 871; 907; 929; 1028; 1091; 1105; 1182; 1246; 1314; 1343; 1382; 1414; 1450; 1474; 1620; 1635; 1653; 2500 ÷ 3500 (broad band with peaks at 2515; 2871; 2974; 3155). Elemental analysis calc. (%) for C10H24N2O6 (m.w.: 268.31u): C 44.77, H 9.02, N 10.44, O 37.75, found: C 44.59, H 8.95, N 10.35, O 37.66.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2S) 1.96 (s, 3H, SC
2CH2S) 1.96 (s, 3H, SC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 2.46 (t, J = 7.5 Hz 2H, C
3); 2.46 (t, J = 7.5 Hz 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2S); 2.70 (t, J = 6.1 Hz, 6H, H+NC
2S); 2.70 (t, J = 6.1 Hz, 6H, H+NC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2); 3.57 (t, J = 6.1 Hz, 6H, C
2); 3.57 (t, J = 6.1 Hz, 6H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2OH); 3.59 (m, 1H, C
2OH); 3.59 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) NH2). 13C NMR(D2O), δ [ppm]: 13.87 (1C, S
NH2). 13C NMR(D2O), δ [ppm]: 13.87 (1C, S![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3); 28.88 (1C, CH2
H3); 28.88 (1C, CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2S); 30.30 (1C,
H2S); 30.30 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2CH2S); 54.00 (1C,
H2CH2S); 54.00 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HNH2); 55.49 (3C, H+N
HNH2); 55.49 (3C, H+N![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2); 58.10 (3C,
H2); 58.10 (3C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2OH); 175.51 (1C, CH
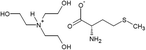
H2OH); 175.51 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO−). FT-IR (ATR), ν [cm−1]: 420; 543; 805; 874; 952; 980; 1118; 1150; 1185; 1243; 1275; 1316; 1330; 1352; 1405; 1456; 1507; 1560; 1607; 1635; 2400 ÷ 3300 (broad band with peaks at 2565; 2737; 2914; 3130). Elemental analysis calc. (%) for C11H26N2O5S (m.w.: 299.41): C 44.13, H 9.09, N 9.36, S 10.71, O 28.13, found: C 43.96, H 8.95, N 9.01, S 11.14, O 27.68.
OO−). FT-IR (ATR), ν [cm−1]: 420; 543; 805; 874; 952; 980; 1118; 1150; 1185; 1243; 1275; 1316; 1330; 1352; 1405; 1456; 1507; 1560; 1607; 1635; 2400 ÷ 3300 (broad band with peaks at 2565; 2737; 2914; 3130). Elemental analysis calc. (%) for C11H26N2O5S (m.w.: 299.41): C 44.13, H 9.09, N 9.36, S 10.71, O 28.13, found: C 43.96, H 8.95, N 9.01, S 11.14, O 27.68.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2C
2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2); 2.60 (t, J = 6.1 Hz, 6H, +NHC
2CH2); 2.60 (t, J = 6.1 Hz, 6H, +NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2); 3.06 (m, 2H, CH2CH2C
2); 3.06 (m, 2H, CH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2); 3.13 (m, 1H, C
2); 3.13 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) COO); 3.55 (t, J = 6.1 Hz, 6H, C
COO); 3.55 (t, J = 6.1 Hz, 6H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2OH). 13C NMR(D2O), δ [ppm]: 24.38 (1C,
2OH). 13C NMR(D2O), δ [ppm]: 24.38 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2CH2); 31.48 (1C, CH
H2CH2); 31.48 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2); 40.88 (1C,
H2); 40.88 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2NH); 55.45 (1C,
H2NH); 55.45 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HNH2); 55.56 (3C, H+N
HNH2); 55.56 (3C, H+N![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2); 58.78 (3C,
H2); 58.78 (3C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2OH); 156.65 (1C,
H2OH); 156.65 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) NH); 182.96 (1C, CH
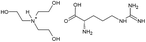
NH); 182.96 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO−). FT-IR (ATR), ν [cm−1]: 490; 548; 605; 699; 764; 846; 881; 913; 974; 1034; 1071; 1130; 1183; 1331; 1375; 1419; 1474; 1549; 1611; 1675; 1685; 1700; 1719; 2500 ÷ 3500 (broad band with peaks at 2861; 2943; 3046; 3297; 3358). Elemental analysis calc. (%) for C12H29N5O5 (m.w.: 323.4u): C 44.57, H 9.04, N 21.66, O 25.86, found: C 43.81, H 8.76, N 20.91, O 25.57.
OO−). FT-IR (ATR), ν [cm−1]: 490; 548; 605; 699; 764; 846; 881; 913; 974; 1034; 1071; 1130; 1183; 1331; 1375; 1419; 1474; 1549; 1611; 1675; 1685; 1700; 1719; 2500 ÷ 3500 (broad band with peaks at 2861; 2943; 3046; 3297; 3358). Elemental analysis calc. (%) for C12H29N5O5 (m.w.: 323.4u): C 44.57, H 9.04, N 21.66, O 25.86, found: C 43.81, H 8.76, N 20.91, O 25.57.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 0.83 (d, J = 7.1 Hz, 3H, CHC
3); 0.83 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 1.10 (m, 2H, C
3); 1.10 (m, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3); 1.79 (m, 1H, C
2CH3); 1.79 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2); 2.64 (t, J = 6.2 Hz, 6H, NHC
CH2); 2.64 (t, J = 6.2 Hz, 6H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2); 3.44 (d, J = 4.1 Hz, 1H, NH2C
2); 3.44 (d, J = 4.1 Hz, 1H, NH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ); 3.55 (t, J = 6.1 Hz, 6H, C
); 3.55 (t, J = 6.1 Hz, 6H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2OH). 13C NMR(D2O), δ [ppm]: 11.02 (1C, CH2
2OH). 13C NMR(D2O), δ [ppm]: 11.02 (1C, CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3); 14.69 (1C,
H3); 14.69 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2CH3); 24.32 (1C, CH
H2CH3); 24.32 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3); 36.06(1C,
H3); 36.06(1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HCH3); 55.51 (3C, H+N
HCH3); 55.51 (3C, H+N![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2); 58.42 (3C, CH2O
H2); 58.42 (3C, CH2O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ); 59.59 (1C,
); 59.59 (1C, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HNH2); 174.94 (1C, CH
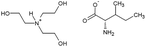
HNH2); 174.94 (1C, CH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OO−). FT-IR (ATR), ν [cm−1]: 536; 710; 749; 768; 801; 852; 872; 921; 963; 1034; 1085; 1126; 1186; 1248; 1271; 1307; 1327; 1349; 1381; 1394; 1417; 1436; 1461; 1496; 1507; 1512; 1539; 1557; 1559; 1572; 1604; 2500 ÷ 3400 (broad band with peaks at 2603; 2874; 2928; 2959). Elemental analysis calc. (%) for C12H28N2O5 (m.w.: 280.36u): C 51.41, H 10.07, N 9.99, O 30.04, found: C 51.51, H 10.25, N 10.10, O 29.33.
OO−). FT-IR (ATR), ν [cm−1]: 536; 710; 749; 768; 801; 852; 872; 921; 963; 1034; 1085; 1126; 1186; 1248; 1271; 1307; 1327; 1349; 1381; 1394; 1417; 1436; 1461; 1496; 1507; 1512; 1539; 1557; 1559; 1572; 1604; 2500 ÷ 3400 (broad band with peaks at 2603; 2874; 2928; 2959). Elemental analysis calc. (%) for C12H28N2O5 (m.w.: 280.36u): C 51.41, H 10.07, N 9.99, O 30.04, found: C 51.51, H 10.25, N 10.10, O 29.33.Ionic liquid-based ultrasound-assisted extraction (IL-UAE)
Dry plant raw material (0.50 g) was weighed with an accuracy of 0.01 into a glass vial and 10 cm3 of a 2.5% (w/v) aqueous solution of amino acid ionic liquid as an extraction solvent was added. Then it was shaken for 5 min. The obtained mixture was placed in an ultrasonic bath with a thermostat (Polsonic Sonic-2, Poland) under the following conditions: frequency of 40 kHz, temperature 35 °C, time 15, 30, 60, 120 or 240 min. After extraction, the extract was centrifuged and 2 cm3 of the supernatant was taken for UV-VIS spectrophotometric analysis.In vitro antioxidant activity and total polyphenol content determination
Evaluation of the antioxidant activity of extracts using the DPPH, FRAP, and Folin–Ciocalteu techniques was performed as described by Muzykiewicz et al.56 The CUPRAC evaluation was performed according to the technique described by Apak et al.57 In the DPPH method, antioxidant activities have been expressed as radical scavenging activity (RSA [%]) using the following equation:where Ap is the absorbance of the tested sample and A0 is the absorbance of the blank sample.
In the CUPRAC technique the results were expressed as Trolox equivalents [TEAC – mg Trolox per g raw material], whereas in the FRAP technique as FeSO4 equivalents [mg FeSO4 per g raw material]. The total polyphenol content was expressed as gallic acid equivalents [GAE – mg GA per g raw material]. The reference solution was an aqueous solution of an amino acid ionic liquid. The results are presented as arithmetic means ± standard deviation (mean ± SD).
To evaluate the antioxidant potential of the extracts with DPPH, the following procedure was used: a 0.3 mM ethanolic DPPH solution (the test reagent) was diluted with 96% (v/v) ethanol to obtain an absorbance of 1.000 ± 0.020 at 517 nm. 150 μl of the studied extract was added to 2850 μl of this solution, mixed and incubated at room temperature for 10 min. The absorbance measurement was taken at 517 nm.
To evaluate the ferric ion reducing power of the extracts, the FRAP method was applied. 1 volume of 10 mM TPTZ (in 40 mM HCl), 1 volume of 20 mM FeCl3 and 10 volumes of 0.3 M acetate buffer (pH 3.6) were mixed to obtain the working solution. 80 μl of the extract was added to 2320 μl of this solution and mixed vigorously. The absorbance was measured at 593 nm after 15 min of incubation at room temperature.
To determine the total polyphenol content, a 10% (v/v) aqueous solution of Folin–Ciocalteu reagent was prepared by tenfold dilution of the concentrated F–C solution and incubated in the dark at room temperature for 1 hour. 2700 μl of 5 mM Na2CO3 and 150 μl of the extract were mixed with 150 μl of diluted F–C reagent. The absorbance of the samples was measured at 750 nm after 15 min of incubation at room temperature.
To evaluate the reducing ability of cupric ions with the CUPRAC method 1000 μl of 0.01 M aqueous CuCl2 solution, 1000 μl of 7.5 mM neocuproine solution in 96% ethanol, 1000 μl of 1 M acetate buffer (pH 7), 600 μl of distilled water and 500 μl of the extract were thoroughly mixed. After 30 min of incubation at room temperature the absorbance was measured at 450 nm. In all methods the distilled water was used as a blank zero.
The presented results of the antioxidant activity of extracts prepared with the use of aqueous solutions of ionic liquids were reduced by the value of the antioxidant potential of the solvents, which remained at an insignificant, very low level.
Statistical analysis
Statistical analysis was performed with Statistica 12 software (Statsoft, Poland). The Wilcoxon signed-rank test (parameter z) was used to evaluate the statistical significance of differences between the antioxidant activity of extracts from the tested plants (obtained by the DPPH, FRAP, CUPRAC and F–C methods). Pearson's correlation coefficients (r) between the results determined with the different methods were also evaluated. The significance level was assumed as p < 0.05.Results and discussion
The amino acid ionic liquids were obtained in the direct reaction of triethanolamine (TEA) with L-amino acids – Ile, Met, Thr and Arg, representing different hydrophobicity, according to the amino acid hydrophobicity scales. Isoleucine is the most hydrophobic, methionine and threonine are characterized by middle hydrophobicity, and arginine is the most hydrophilic among all used amino acids. The structures of the synthesized compounds were confirmed by nuclear magnetic resonance (1H NMR and 13C NMR), FT-IR spectra and elemental analysis. Their physicochemical properties are given in Table 2. All synthesized triethanolammonium salts of amino acids were highly hygroscopic compounds, with a glass transition temperature, from −57.71 °C for [TEAH]+[Thr]− to −11.89 °C for [TEAH]+[Arg]−. Moreover, AAILs were optically active, with thermal stability to above 150 °C. Copies of NMR and FT-IR spectra, DSC and TGA thermograms were included in the ESI.† The investigated ionic liquids were hydrophilic and soluble in water.Ultrasound-assisted extraction of plant extracts with the addition of AAILs
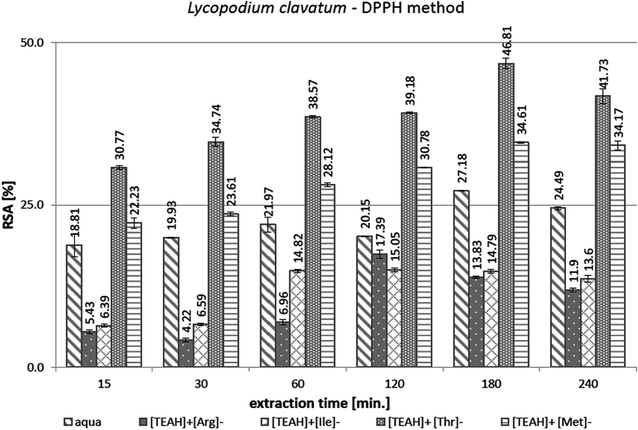
Traditional methods of obtaining plant extracts, rich in active compounds, require a long time. Therefore, methods to increase the efficiency of extraction of biologically active compounds from plants are desired. The use of ultrasound for the extraction of biomass brings about many benefits. It causes fragmentation of cell membranes and releases the cell contents into solution, which makes the extraction more efficient and significantly shortens the process time compared to the methods without sonication. Generally, UAE is the preferred method over MAE (Microwave Assisted Extraction) for the extraction of thermosensitive biomolecules, because it uses lower temperatures and does not cause overheating as in MAE. However, extraction time should be optimized, because longer time can result in a decrease of extraction efficiency due to degradation of target compounds, especially that susceptible to oxidation and thermal degradation. Combination of UAE with ILs can be a promising methodology of solid–liquid extraction for the recovery of useful compounds from natural sources.In preliminary studies, the effect of aqueous solutions of the four AAILs as solvents and extraction time on the antioxidant activity of extracts of L. clavatum by DPPH assay was determined (Fig. 1). The concentration of AAILs in aqueous solution was 2.5% and the ratio of dry plant to this solution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (g ml−1). The concentration of the ionic liquid in the extraction solution was not optimized, but established to test the effect of the same amount of various ionic liquids on the extraction efficiency.
20 (g ml−1). The concentration of the ionic liquid in the extraction solution was not optimized, but established to test the effect of the same amount of various ionic liquids on the extraction efficiency.
Extraction with the aqueous solution of [TEAH]+[Arg]− or [TEAH]+[Ile]− gave extracts of lower antioxidant activity (maximum 17.39% and 15.05% respectively, within 120 min) than achieved with pure water extraction (maximum 27.18% after 180 min). On the other hand, the values of antioxidant activity for extracts obtained with addition of [TEAH]+[Thr]− and [TEAH]+[Met]− were higher than that of pure water extracts, and achieved 46.81% and 34.61% respectively, within 180 minutes. Therefore these two latter compounds were chosen to prepare extracts from C. islandica and D. fullonum.
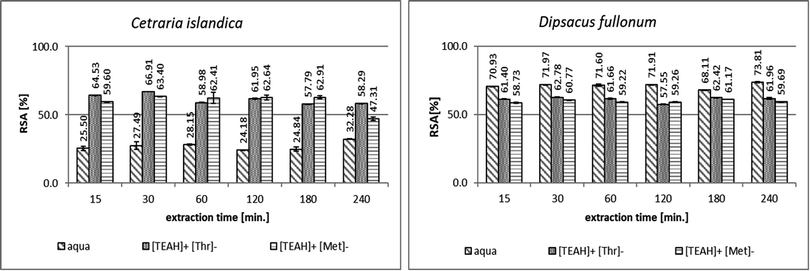
Fig. 2 shows the comparison of the results of the antioxidant activity measurements by DPPH assay for extracts obtained from C. islandica and D. fullonum by the ultrasound-assisted extraction with pure water and the aqueous solutions of AAILs. The highest antioxidant activity among the tested plants, 73.81 ± 0.59%, was determined for the extracts from D. fullonum (Fig. 2). For C. islandica extracts, the antioxidant potential reached values between 32.28% for pure water extracts after 240 min and 66.91% for extracts with [TEAH]+[Thr]− after 30 min. Extracts from C. islandica with AAILs achieved higher antioxidant activities than reported by Patriche et al.36 for extracts from C. islandica (Table 1) with acetone or 95% ethanol as a solvent – RSA% 40.68% or 18.62%, respectively.
The weakest antioxidant properties were reached for the extracts of L. clavatum (Fig. 1). Fig. 1 and 2 show that the use of the [TEAH]+[Thr]− and [TEAH]+[Met]− solutions in the extraction process resulted in an increase in the antioxidant activity of extracts from L. clavatum and C. islandica, compared to extracts of these plants obtained by UEA in pure water. Even two-fold higher antioxidant activity was achieved. Only in the case of D. fullonum plant (Fig. 2), the addition of AAILs to the extraction medium did not cause any improvement in the antioxidant activity, which remained at a similar level of about 60% RSA in all extraction times and was lower than for pure water extracts.
In the AAIL-assisted L. clavatum extraction (Fig. 1), the increased antioxidant activity of extracts in relation to the aqueous extracts obtained in the same time without ionic liquid addition was observed after any extraction time. In the case of extracts obtained with the use of [TEAH]+[Thr]− the time of 60 min was sufficient to double the antioxidant properties (RSA = 38.57%), compared to that obtained using water only (21.97% after 60 min). The maximum antioxidant activity of extracts obtained using [TEAH]+[Thr]− or [TEAH]+[Met]− aqueous solution was reached within 180 min.
In studies by Orhan et al.38 DPPH antioxidant activity was determined, expressed as the % inhibition of free radicals in L. clavatum extracts (petroleum ether, ethyl acetate, methanol, chloroform, and chloroform with alkaloid fraction). All obtained extracts showed antioxidant properties against DPPH radicals in the range of 11.3–44.1% (Table 1). The values were lower than that of the reference substance hydroxyanisole (BHA) used, the inhibition of which was 92.7%.38 The use of AAIL aqueous solutions in plant extracts resulted in higher antioxidant values ([TEAH]+[Thr]− 46.81%, [TEAH]+[Met]− 36.41%) compared to the organic solvents used (petroleum ether 11.3%, ethyl acetate 26.6%, methanol 30.3%, chloroform 0%, and chloroform with an alkaloid fraction of 44.1%).
In the extracts from C. islandica (Fig. 2) the highest antioxidant activity was obtained after 30 min for both ionic liquids. The use of [TEAH]+[Thr]− contributed to obtaining slightly higher RSA [%] values in the extract than that for [TEAH]+[Met]−. Longer ultrasound-assisted extraction time will not increase the antioxidant capacity.
Table 3 shows the total content of polyphenols in the extracts from L. clavatum, C. islandica and D. fullonum obtained with AAIL-assisted extraction, which ranged from 0.71 ± 0.01 to 8.16 ± 0.22 mg GA per g raw material. The use of [TEAH]+[Thr]− in the extraction medium for D. fullonum gave the highest concentration of polyphenols in extracts – 8.16 ± 0.22 mg GA per g of raw material within 120 min. An equally high content of polyphenols (7.38 ± 0.11 mg GA per g of raw material) was obtained after using [TEAH]+[Met]− in aqueous solution for extraction, within half the extraction time – 60 min. In the cases of plant extracts from C. islandica and L. clavatum with the use of [TEAH]+[Met]−, a higher content of polyphenols was obtained (5.31 ± 0.01 and 1.91 ± 0.08 mg GA per g raw material, respectively) compared to using [TEAH]+[Thr]− for extraction (4.10 ± 0.07 and 1.82 ± 0.10 mg GA per g raw material). When using aqueous solutions of ionic liquids for extraction, the amount of polyphenols was improved 10–15 times compared to the reported ethanol extracts41,45 (Table 1).
| Extraction time [min] | Aqua | [TEAH]+[Thr]− | [TEAH]+[Met]− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lycopodium clavatum | Cetraria islandica | Dipsacus fullonum | Lycopodium clavatum | Cetraria islandica | Dipsacus fullonum | Lycopodium clavatum | Cetraria islandica | Dipsacus fullonum | |
| 15 | 1.51 ± 0.04 | 0.74 ± 0.10 | 6.52 ± 0.08 | 0.98 ± 0.07 | 3.83 ± 0.03 | 7.44 ± 0.12 | 0.71 ± 0.01 | 3.85 ± 0.15 | 5.69 ± 0.20 |
| 30 | 1.06 ± 0.00 | 0.77 ± 0.04 | 6.94 ± 0.26 | 1.01 ± 0.01 | 4.10 ± 0.07 | 7.29 ± 0.16 | 0.83 ± 0.07 | 3.88 ± 0.01 | 6.73 ± 0.11 |
| 60 | 1.30 ± 0.05 | 0.60 ± 0.00 | 6.93 ± 0.12 | 1.32 ± 0.10 | 3.75 ± 0.16 | 7.10 ± 0.09 | 0.94 ± 0.00 | 4.60 ± 0.08 | 7.38 ± 0.12 |
| 120 | 1.45 ± 0.00 | 0.46 ± 0.01 | 6.96 ± 0.07 | 1.34 ± 0.13 | 3.73 ± 0.01 | 8.16 ± 0.22 | 1.44 ± 0.00 | 4.48 ± 0.17 | 7.30 ± 0.02 |
| 180 | 1.91 ± 0.01 | 0.45 ± 0.02 | 7.48 ± 0.02 | 1.53 ± 0.08 | 3.37 ± 0.12 | 7.57 ± 0.17 | 1.72 ± 0.13 | 4.52 ± 0.09 | 7.05 ± 0.10 |
| 240 | 1.66 ± 0.04 | 1.05 ± 0.14 | 7.63 ± 0.07 | 1.82 ± 0.12 | 4.14 ± 0.38 | 6.92 ± 0.11 | 1.91 ± 0.08 | 5.31 ± 0.01 | 6.98 ± 0.29 |
By comparing the results in terms of the extraction time, it was observed that the use of [TEAH]+[Thr]− shortened the extraction process to 30 min for C. islandica and to 60 min for L. clavatum, and provided relatively high amounts of polyphenols, 4.10 ± 0.07 mg GA per g and 1.82 ± 0.10 mg GA per g, respectively. The time for extraction using [TEAH]+[Met]− aqueous solution needed 240 min to obtain the maximum content of polyphenols in extracts from these two plants.
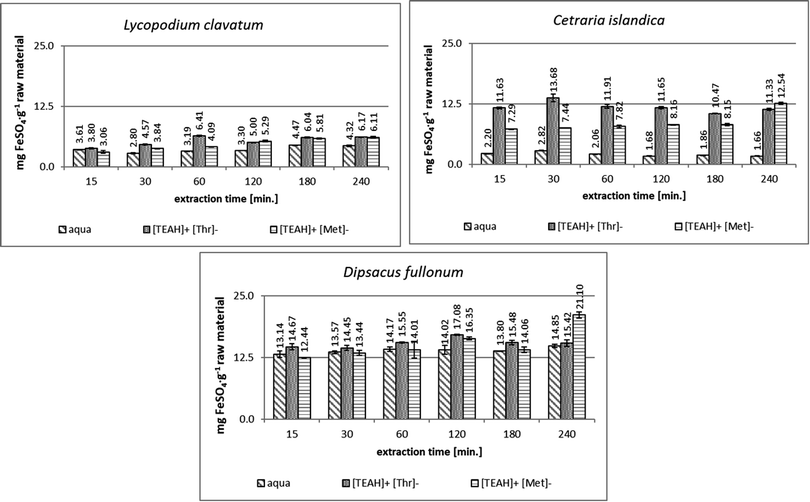
Determination of antioxidant activity for extracts by the FRAP method
The comparison of the antioxidant activity of the tested plant extracts using the FRAP method is shown in Fig. 3. The use of AAILs as additives for ultrasound-assisted extraction contributed to the increased antioxidant activity compared to aqueous plant extracts.The D. fullonum extracts (Fig. 3) showed the highest antioxidant capacity among the extracts of all plants tested. The highest activity was obtained in the extracts with [TEAH]+[Met]−-assisted extraction after 240 min (21.10 ± 0.59 mg FeSO4 per g raw material). The use of the [TEAH]+[Thr]− as the additive allows obtaining the maximum antioxidant activity of the extract (17.08 ± 0.11 mg FeSO4 per g raw material) in a twice shorter time – 120 min.
The addition of [TEAH]+[Thr]− to the extraction medium provided the extract from C. islandica (Fig. 3) with the highest antioxidant activity (13.68 ± 0.75 mg FeSO4 per g raw material), fivefold higher compared to that obtained with only water as the extractant and in the shortest extraction time i.e. 30 min, whereas the maximum antioxidant activity in the extract from C. islandica with the addition of [TEAH]+[Met]− was obtained after 240 min (12.54 ± 0.21 mg FeSO4 per g raw material). Also, in this extract the antioxidant activity was on average 4.5 times higher than in the aqueous extract of C. islandica.
The lowest antioxidant activity by the FRAP method was found in extracts from L. clavatum (Fig. 3). The use of [TEAH]+[Thr]− as the additive contributed to an increase in antioxidant activity. The best result was obtained after 60 min of extraction. Extraction with addition of [TEAH]+[Met]− provided after 240 min the extract of the highest antioxidant activity which was higher compared to the activity of the aqueous extract.
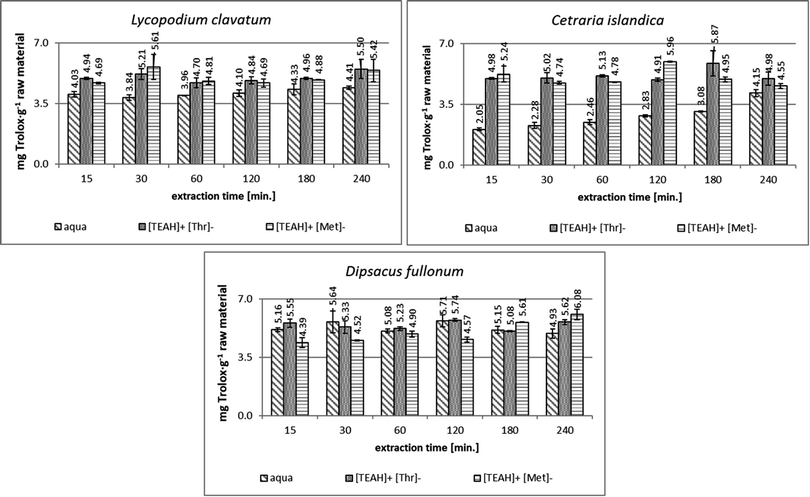
Determination of antioxidant activity for extracts by the CUPRAC method
In the analysis of the antioxidant potential using the CUPRAC method (Fig. 4), the extracts obtained from D. fullonum were characterized by the highest activity, as in the other methods used here. However, the extracts prepared with AAIL solutions as the extractant showed a potential similar to or lower than that of the corresponding water extracts. Only a slight increase in antioxidant activity of extracts with AAIL addition in comparison to aqueous extracts was achieved after 240 min of extraction – 5.62 mg TEAC per g raw material with addition of [TEAH]+[Thr]− and 6.08 mg TEAC per g raw material with [TEAH]+[Met]−. These values were 1.5-fold higher than that reported by Pilluza47 for extracts from D. fullonum with acetone/water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) as the solvent (Table 1).
3 v/v) as the solvent (Table 1).
In the extracts obtained from L. clavatum and C. islandica (Fig. 4), the addition of ionic liquids improved the antioxidant potential compared to pure water extracts. In the case of the extract from C. islandica even a 1.5-fold increase was observed. The [TEAH]+[Thr]− application seems to a little more improve the efficiency of the process than [TEAH]+[Met]−. The use of ionic liquid-based ultrasound-assisted extraction allowed for reduction of the process time compared to water. The antioxidant capacity of extracts from C. islandica was also confirmed by Ivanišová et al.41 In 80% ethanol extract from lichen, antioxidant activity was determined at the level of 5.61 ± 0.21 mg TEAC per g (Table 1). This value was comparable with our results for extracts with [TEAH]+[Thr]− addition.
Comparison and correlation of different methods of antioxidant activity determination
Table 4 shows the correlation coefficients (r) between the antioxidant activity and the total content of polyphenols in extracts from individual plants. In the case of C. islandica, the results obtained with all methods correlated with each other to a statistically significant degree (p < 0.05). In the group of results obtained for L. clavatum no statistically significant correlation was found between the total content of polyphenols and the antioxidant activity assessed using the DPPH and CUPRAC techniques. In the group of results obtained for D. fullonum only the results between the reduction abilities assessed with the FRAP and CUPRAC techniques were statistically significantly correlated. Moreover, the analysis of the statistical significance of the differences using the Wilcoxon signed-rank test showed that differences between the results obtained for all plants are statistically significant p < 0.001 (D. fullonum vs. L. clavatum: z = 7.233; D. fullonum vs. C. islandica: z = 5.780; L. clavatum vs. C. islandica: z = 5.062).| DPPH | FRAP | CUPRAC | Folin–Ciocalteu | |
|---|---|---|---|---|
| a p < 0.05.b NS – not statistically significant. | ||||
| Lycopodium clavatum | ||||
| DPPH | r = 1.000a | r = 0.873a | r = 0.619a | NS |
| FRAP | r = 1.000a | r = 0.582a | r = 0.674a | |
| CUPRAC | r = 1.000a | NS | ||
| Folin–Ciocalteu | r = 1.000a | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Cetraria islandica | ||||
| DPPH | r = 1.000a | r = 0.855a | r = 0.841a | r = 0.915a |
| FRAP | r = 1.000a | r = 0.733a | r = 0.875a | |
| CUPRAC | r = 1.000a | r = 0.787a | ||
| Folin–Ciocalteu | r = 1.000a | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Dipsacus fullonum | ||||
| DPPH | r = 1.000a | NS | NS | NS |
| FRAP | r = 1.000a | r = 0.513a | NS | |
| CUPRAC | r = 1.000a | NS | ||
| Folin–Ciocalteu | r = 1.000a | |||
High correlation coefficients between the antioxidant activity and the total content of polyphenols in extracts from C. islandica suggest that in the case of this plant, polyphenols may be responsible for its activity. Moreover the total polyphenol content could be an indicator of antioxidant properties of this plant.
Also the results of the total content of polyphenols in extracts from L. clavatum significantly correlated with the ability to reduce iron ions determined with the FRAP technique. It should be noted that statistically significant correlation coefficients were obtained between the antioxidant potential assessed by the DPPH technique and the reducing capacity (FRAP and CUPRAC techniques) of L. clavatum and C. islandica extracts. For all analyzed plants, the reduction abilities towards iron ions (FRAP method) and copper (CUPRAC method) also correlated to a statistically significant degree. High, statistically significant correlations between the results obtained by techniques using different mechanisms of action may indicate the different antioxidant nature of the plants studied. In some cases, in particular in the group of extracts from D. fullonum, no statistically significant correlations were found between the results obtained with different methods (except for the correlation between the FRAP and CUPRAC methods). This observation confirms the suggestions of Apak et al.58 on the validity of using at least two different methods, based on different mechanisms of action to assess antioxidant activity.
Our research shows that the analyzed plants, such as L. clavatum, C. islandica, and D. fullonum, can be a rich source of natural antioxidants, including phenolic compounds. The use of aqueous solutions of selected amino acid ionic liquids, as compared to distilled water, could have a positive effect on the efficiency of the ultrasound-assisted extraction process, including the reduction of the extraction time. The combination of the addition of the ionic liquids used with the “green extraction technique”, to which UAE could be included, seems to be the optimal solution to isolate natural antioxidants from plant material.
Conclusion
Plants are made up of plant cells that contain a rigid cell wall that prevents or hinders the extraction of certain components. Pre-treatment of the plant material increases the access to compounds inside the cells, thus increasing the efficiency of the extraction process. Ultrasound-assisted extraction of plant material with the addition of amino acid ionic liquids could enhance antioxidant activity with a shorter process time and lower energy consumption. The use of [TEAH]+[Thr]− as an additive for ultrasound-assisted extraction was characterized by obtaining plant extracts with the highest antioxidant potential. In addition, the presence of amino acids in the structure of ionic liquids makes these extraction solvents not only ecological, but also biocompatible, so their application in many industries, such as cosmetics, can be considered.Conflicts of interest
There are no conflicts of interest to declare.References
- M. P. Vinardell and M. Mitjans, Nanocarriers for Delivery of Antioxidants on the Skin, Cosmetics, 2015, 2, 342–354, DOI:10.3390/cosmetics2040342.
- S. B. Nimse and D. Pal, Free radicals, natural antioxidants, and their reaction mechanisms, RSC Adv., 2015, 5, 27986–28006, 10.1039/c4ra13315c.
- B. Roman, M. Retajczyk, Ł. Sałaciński and R. Pełech, Curcumin – Properties, Applications and Modification of Structure, Mini-Rev. Org. Chem., 2020, 17, 486–495, DOI:10.2174/1570193X16666190621110247.
- L. Wang and C. L. Weller, Recent advances in extraction of nutraceuticals from plants, Trends Food Sci. Technol., 2006, 17, 300–312, DOI:10.1016/j.tifs.2005.12.004.
- C. D. Stalikas, Extraction, separation, and detection methods for phenolic acids and flavonoids, J. Sep. Sci., 2007, 30, 3268–3295, DOI:10.1002/jssc.200700261.
- K. Vilkhu, R. Mawson, L. Simons and D. Bates, Applications and opportunities for ultrasound assisted extraction in the food industry — A review, Innovative Food Sci. Emerging Technol., 2008, 9, 161–169, DOI:10.1016/j.ifset.2007.04.014.
- W. Routray and V. Orsat, Microwave-Assisted Extraction of Flavonoids: A Review, Food Bioprocess Technol., 2012, 5, 409–424, DOI:10.1007/s11947-011-0573-z.
- Y. Jiao and Y. Zuo, Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of Polygoni multiflori, Phytochem. Anal., 2009, 20, 272–278, DOI:10.1002/pca.1124.
- F. Chemat, Z. e-Huma and M. K. Khan, Applications of ultrasound in food technology: Processing, preservation and extraction, Ultrason. Sonochem., 2011, 18, 813–835, DOI:10.1016/j.ultsonch.2010.11.023.
- P. Wasserscheid and W. Keim, Ionic liquids—new “solutions” for transition metal catalysis, Angew. Chem., Int. Ed., 2000, 39, 3772–3789, DOI:10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: solvents for synthesis and catalysis. 2, Chem. Rev., 2011, 111, 3508–3576, DOI:10.1021/cr1003248.
- J. C. Plaquevent, J. Levillain, F. Guillen, C. Malhiac and A. C. Gaumont, Ionic liquids: new targets and media for alpha-amino acid and peptide chemistry, Chem. Rev., 2011, 108, 5035–5060, DOI:10.1021/cr068218c.
- K. Fukumoto, M. Yoshizawa and H. Ohno, Room temperature ionic liquids from 20 natural amino acids, J. Am. Chem. Soc., 2005, 127, 2398–2399, DOI:10.1021/ja043451i.
- R. Gusain, S. Dhingra and O. P. Khatri, Fatty-Acid-Constituted Halogen-Free Ionic Liquids as Renewable, Environmentally Friendly, and High-Performance Lubricant Additives, Ind. Eng. Chem. Res., 2016, 55, 856–865, DOI:10.1021/acs.iecr.5b03347.
- E. B. Carter, S. L. Culver, P. A. Fox, R. D. Goode, I. Ntai, M. D. Tickell, R. K. Traylor, N. W. Hoffman and J. H. Davis, Sweet success: Ionic liquids derived from non-nutritive sweeteners, Chem. Commun., 2004, 6, 630–631, 10.1039/B313068A.
- F. Santamarta, M. Vilas, E. Tojo and Y. Fall, Synthesis and properties of novel chiral imidazolium-based ionic liquids derived from carvone, RSC Adv., 2016, 6, 31177–31180, 10.1039/c6ra00654j.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 2000 Search PubMed.
- H. Ullah, C. D. Wilfred and M. S. Shaharun, Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass, Sep. Sci. Technol., 2019, 54, 559–579, DOI:10.1080/01496395.2018.1505913.
- A. Berthod, M. J. Ruiz-Ángel and S. Carda-Broch, Recent advances on ionic liquid uses in separation techniques, J. Chromatogr. A, 2018, 1559, 2–16, DOI:10.1016/j.chroma.2017.09.044.
- E. V. Capela, J. A. P. Coutinho and M. G. Freire, Application of Ionic Liquids in Separation and Fractionation Processes, in Green Chemistry and Chemical Engineering, ed. B. Han and T. Wu, Springer, New York, 2019, pp. 637–665 Search PubMed.
- F. Chemat and M. A. Vian, Alternative Solvents for Natural Products Extraction, Springer-Verlag Berlin Heidelberg, Berlin, 2014, DOI:10.1007/978-3-662-43628-8.
- B. Tang, W. Bi, M. Tian and K. H. Row, Application of ionic liquid for extraction and separation of bioactive compounds from plants, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 904, 1–21, DOI:10.1016/j.jchromb.2012.07.020.
- S. P. M. Ventura, F. A. E Silva, M. V. Quental, D. Mondal, M. G. Freire and J. A. P. Coutinho, Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends, Chem. Rev., 2017, 117, 6984–7052, DOI:10.1021/acs.chemrev.6b00550.
- H. Qin, G. Zhou, G. Peng, J. Li and J. Chen, Application of Ionic Liquid-Based Ultrasound-Assisted Extraction of Five Phenolic Compounds from Fig (Ficus carica L.) for HPLC-UV, Food Anal. Methods, 2015, 8, 1673–1681, DOI:10.1007/s12161-014-0047-9.
- L. Zhang and X. Wang, Hydrophobic ionic liquid-based ultrasound-assisted extraction of magnolol and honokiol from cortex Magnoliae officinalis, J. Sep. Sci., 2010, 33, 2035–2038, DOI:10.1002/jssc.201000076.
- L. Zhang, J. Liu, P. Zhang, S. Yan, X. He and F. Chen, Ionic Liquid-Based Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica Thunb, Chromatographia, 2011, 73, 129–133, DOI:10.1007/s10337-010-1828-y.
- G. Zu, R. Zhang, L. Yang, C. Ma, Y. Zu, W. Wang and C. Zao, Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from Rosmarinus officinalis, Int. J. Mol. Sci., 2012, 13, 11027–11043, DOI:10.3390/ijms130911027.
- L. Yang, H. Wang, Y. Zu, C. Zhao, L. Zhang, X. Chen and Z. Zhang, Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions, Chem. Eng. J., 2011, 172, 705–712, DOI:10.1016/j.cej.2011.06.039.
- L. Zhang, Y. Geng, W. Duan, D. Wang, M. Fu and X. Wang, Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae, J. Sep. Sci., 2009, 32, 3550–3554, DOI:10.1002/jssc.200900413.
- S. Li, S. Li, Y. Huang, C. Liu, L. Chen and Y. Zhang, Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential α-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography, J. Sep. Sci., 2017, 40, 2565–2574, DOI:10.1002/jssc.201700258.
- S. Magiera and A. Sobik, Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods, J. Food Compos. Anal., 2017, 57, 94–101, DOI:10.1016/j.jfca.2016.12.016.
- R. D. P. Rodrigues, F. C. de Castro, R. S. de Santiago-Aguiar and M. V. P. Rocha, Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent, Algal Res., 2018, 31, 454–462, DOI:10.1016/j.algal.2018.02.021.
- J. Kukuła and E. Witkowska-Banaszczak, Medicinal plants of the Dipsacaceae, Post. Fitoter., 2014, 15, 232–238 Search PubMed.
- N. N. Trofimova, A. Gromova and A. A. Semenov, Serratene triterpenoids from Lycopodium clavatum L. (Lycopodiaceae), Russ. Chem. Bull., 1996, 45, 961–963 CrossRef.
- I. Orhan, E. Küpeli, B. Sener and E. Yesilada, Appraisal of anti-inflammatory potential of the clubmoss, Lycopodium clavatum L, J. Ethnopharmacol., 2007, 109, 146–150, DOI:10.1016/j.jep.2006.07.018.
- S. Patriche, I. O. Ghinea, G. Adam, G. Gurau, B. Furdui, R. M. Dinica, L. F. Rebegea and M. Lupoae, Characterization of Bioactive Compounds from Romanian Cetraria islandica (L) Ach., Rev. Chim., 2019, 70, 2186–2191, DOI:10.37358/RC.19.6.7302.
- İ. Gülçin, M. Oktay, Ö. Küfrevioğlu and A. Aslan, Determination of antioxidant activity of lichen Cetraria islandica (L) Ach, J. Ethnopharmacol., 2002, 79, 325–329, DOI:10.1016/s0378-8741(01)00396-8.
- I. Orhan, B. Özçelik, S. Aslan, M. Kartal, T. Karaoglu, B. ŞeneR, S. Terzioglu and M. I. Choudhary, Antioxidant and antimicrobial actions of the clubmoss Lycopodium clavatum L, Phytochem. Rev., 2007, 6, 189–196, DOI:10.1007/s11101-006-9053-x.
- E. L. Konrath, B. M. Neves, P. S. Lunardi, C. dos Santos Passos, A. Simões-Pires, M. G. Ortega, C. A. Goncalves, J. L. Cabrera, J. C. Fonseca Moreira and A. T. Henriques, Investigation of the in vitro and ex vivo acetylcholinesterase and antioxidantactivities of traditionally used Lycopodium species from South America on alkaloid extracts, J. Ethnopharmacol., 2012, 139, 58–67, DOI:10.1016/j.jep.2011.10.042.
- D. Grujičić, I. Stošić, M. Kosanić, T. Stanojković, B. Ranković and O. Milošević-Djordjević, Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria islandica, Cytotechnology, 2014, 66, 803–813, DOI:10.1007/s10616-013-9629-4.
- E. Ivanišová, M. Kačániová, J. Petrová, H. Frančáková and M. Tokár, The Evaluation of Antioxidant and Antimicrobial Effect of Tussilago farfara L. and Cetraria islandica L., J. Anim. Sci. Biotechnol., 2016, 49, 46–51 Search PubMed.
- M. Kosanić and B. R. Ranković, Lichen as possible sources of antioxidants, Pak. J. Pharm. Sci., 2011, 24, 165–170 Search PubMed , PMID: 21454165..
- I. Tas, A. B. Yildirim, G. C. Ozyigitoglu, M. Z. Yavuz and A. U. Turker, Determination of biological activities (antibacterial, antioxidant and antiproliferative) and metabolite analysis of some lichen species from Turkey, EJBPS, 2017, 4(4), 13–20 CAS.
- C. Fernández-Moriano, P. K. Divakar, A. Crespo and M. P. Gómez-Serranillos, Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: identification of active compounds, Phytomedicine, 2015, 22, 847–855, DOI:10.1016/j.phymed.2015.06.005.
- N. Stadnytska, I. Fito, V. Novikov, I. Jasicka-Misiak and P. P. Wieczorek, Effect of extraction solvent on total phenolic content, total flavonoid content and antioxidant activity of Cetraria islandica, Int. J. PharmTech Res., 2020, 13(3), 198–205, DOI:10.20902/IJPTR.2019.130310.
- J. Oszmiański, A. Wojdyło, P. Juszczyk and P. Nowicka, Roots and Leaf Extracts of Dipsacus fullonum L. and Their Biological Activities, Plants, 2020, 9(1), 78, DOI:10.3390/plants9010078.
- G. Piluzza and S. Bullitta, Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area, Pharm. Biol., 2011, 49(3), 240–247, DOI:10.3109/13880209.2010.501083.
- Y. An, M. Zong, H. Wu and N. Li, Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: Biomass fractionation, enzymatic digestion and ionic liquid reuse, Bioresour. Technol., 2015, 192, 165–171, DOI:10.1016/j.biortech.2015.05.064.
- H. Hou, J. Xu, N. Li and M. Zong, Effect of anion structures on cholinium ionic liquids pretreatment of rice straw and the subsequent enzymatic hydrolysis, Biotechnol. Bioeng., 2015, 112, 1, DOI:10.1002/bit.25335.
- H. Ren, M. Zong, H. Wu and N. Li, Efficient pretreatment of wheat straw using novel renewable cholinium ionic liquids to improve enzymatic saccharification, Ind. Eng. Chem. Res., 2016, 55, 1788–1795, DOI:10.1021/acs.iecr.5b03729.
- M. G. Bogdanov, I. Svinyarov, R. Keremedchieva and A. Sidjimov, Ionic liquid-supported solid– liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glaucium flavum Cr. (Papaveraceae), Sep. Purif. Technol., 2012, 97, 221–227, DOI:10.1016/j.seppur.2012.02.001.
- A. F. M. Cláudio, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Enhanced extraction of caffeine from Guaraná seeds using aqueous solutions of ionic liquids, Green Chem., 2013, 15, 2002–2010, 10.1039/C3GC40437D.
- B. D. Ribeiro, M. A. Z. Coelho, L. P. N. Rebelo and I. M. Marrucho, Ionic liquids as additives for extraction of saponins and polyphenols from mate (Ilex paraguariensis) and tea (Camellia sinensis), Ind. Eng. Chem. Res., 2013, 52, 12146–12153, DOI:10.1021/ie400529h.
- M. G. Bogdanov and I. Svinyarov, Ionic liquid-supported solid–liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr. (Papaveraceae), Sep. Purif. Technol., 2013, 103, 279–288, DOI:10.1016/j.seppur.2012.10.035.
- L. D. Mello, S. Hernandez, G. Marrazza, M. Mascini and L. T. Kubota, Investigations of the antioxidant properties of plant extracts using a DNA-electrochemical biosensor, Biosens. Bioelectron., 2006, 21, 1374–1382, DOI:10.1016/j.bios.2005.05.012.
- A. Muzykiewicz, J. Zielonka-Brzezicka and A. Klimowicz, The antioxidant potential of flesh, albedo and flavedo extracts from different varieties of grapefruits, Acta Sci. Pol., Technol. Aliment., 2019, 18, 453–462, DOI:10.17306/J.AFS.2019.0731.
- R. Apak, K. Güçlü, M. Ozyürek and S. E. Karademir, Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method, J. Agric. Food Chem., 2004, 52, 7970–7981, DOI:10.1021/jf048741x.
- R. Apak, S. Gorinstein, V. Böhm, K. M. Schaich, M. Özyürek and K. Güçlü, Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report), Pure Appl. Chem., 2013, 85, 957–998, DOI:10.1351/PAC-REP-12-07-15.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03840k |
| This journal is © The Royal Society of Chemistry 2021 |