Open Access Article
Open Access ArticleSynthesis of photothermal antimicrobial cotton gauze using AuNPs as photothermal transduction agents†
Fengyi Cao *,
Changmin Wei,
Gangqing Ma,
Like Hou,
Rencong Zhang,
Lin Mei
*,
Changmin Wei,
Gangqing Ma,
Like Hou,
Rencong Zhang,
Lin Mei and
Qi Qin*
and
Qi Qin*
School of Materials and Chemical Engineering, Zhongyuan University of Technology, Zhengzhou 450007, P. R. China. E-mail: caofengyi0513@126.com; qq@zut.edu.cn
First published on 28th July 2021
Abstract
Cotton gauze has been used as a wound dressing since the 19th century, and still plays an important role in current clinical therapies. However, the antimicrobial ability of cotton gauze is limited. In this work, gold nanoparticles (AuNPs) were used as photothermal transduction agents to synthesize modified photothermal antimicrobial cotton gauze. The modified cotton gauze was synthesized by immersing and heating the clinical cotton gauze with AuNPs solution. XPS, ICP-OES, FTIR, XRD and SEM characterizations confirmed that AuNPs were successfully decorated on the surface of cotton gauzes. Besides, the mechanical properties, air and water vapour permeability performance of cotton gauze were not changed after modification. Photothermal antimicrobial experiments confirmed that AuNPs modified on the cotton gauze could convert light to heat, inducing rapid temperature increase of the cotton gauze. And the heat could kill microbial cells permeated in the modified cotton gauze, giving it the potential of being used for photothermal antimicrobial therapy.
1. Introduction
Cotton gauze has been used as a wound dressing to manage surgical, traffic and burn wounds since the 19th century.1 It could help absorb blood, plasma and other fluids from the wound,2,3 and its softness makes it possible to be cut and folded as needed. Besides, cotton gauze has an extremely low price compared with other kinds of wound dressings. But one significant drawback of cotton gauze is that when the wound is infected, it could be permeated by the microbial fluids, and its porosity structure and large surface area could support the microbial growth, causing serious wound infection and hindering the wound healing process.4,5 To overcome it, significant efforts have been made to develop antimicrobial cotton gauze.6–10There are two basic methods to enhance the antimicrobial ability of cotton gauze: one is the chemical method, and the other is the physical method. The chemical method is incorporating antimicrobial agents, such as an antimicrobial drug,11 metal component,12,13 antimicrobial peptide,8 polycation,14 or ionic liquid15 to enhance the antimicrobial ability. Xiang et al.12 used mussel-inspired catechol/amino chemistry to immobilize zwitterionic AgNPs on cotton gauze, and the sustained release of Ag+ could give the modified cotton gauze excellent bactericidal activity. Gomes et al.8 incorporated different kinds of antimicrobial peptides into polyelectrolyte multilayer films over cotton gauzes, and the antimicrobial peptides could provide higher antimicrobial effects in comparison with the controls. The physical method is using the photothermal effect or nano-daggers to produce antimicrobial ability.10,16 Li et al.10 decorated polydopamine, perfluorocarbon and silver nanoparticles on cotton gauze to obtain a lotus leaf-mimic surface structure, and the antibacterial ability could be enhanced by the photothermal effect of polydopamine. Yuan et al.16 coated ZIF-L nano-daggers on cotton gauze, and the nano-dagger arrays could use the sharp tips to rupture the adhered microbial cells. Compared with chemical methods, research based on the physical method is still limited and needs to be further studied.
Photothermal therapy (PTT) is based on the photothermal effect of photothermal transduction agents (PTAs),17,18 which could transform light energy into heat, causing cell membrane rupture of microbial cells.19 Compared with traditional antimicrobial therapy methods, PTT exhibits many advantages such as short treatment time, broad spectrum antibacterial ability and negligible resistance effect.20,21 To date, several species of PTAs have been discovered including noble metal materials,22–24 graphene and graphene analogue-based materials,25,26 mental sulfide/oxide materials,27–29 and small organic molecules.30–32 The PTAs could be used for antimicrobial therapies whether as nanomaterials or composite materials. Li et al.33 synthesized gold nanoclusters decorated amine-functionalized graphene oxide nanosheets as efficient visible light active antibacterial agent, and the complexed nanosheets appeared enhanced antibacterial activity. Cai et al.34 developed a multi-functional hydrogel chelated with Cu nanoparticles, finding that the hydrogel could kill bacteria through photothermal therapy.
In this work, gold nanoparticles (AuNPs) were modified on cotton gauze to synthesize photothermal antimicrobial cotton gauze. As shown in Scheme 1, under xenon lamp irradiation the AuNPs decorated on the cotton gauze could be used as PTAs, adsorb white light and convert light to heat. The heat could induce rapid temperature increase of the modified cotton gauze, and kill the microbial cells permeated in it.
2. Experimental
2.1. Reagents and materials
Absorbent cotton gauze was purchased form Kangyuan medical and health materials company (Heze, China). Chloroauric acid (HAuCl4) was purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Citrate sodium was purchased from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). Sabouraud dextrose broth (SDB) and casein soya bean digest broth (TSB) powders were purchased from Guangdong Huankai Microbial Sci. & Tech. Co., Ltd (Guangzhou, China). Second-generation microbial strains of Candida albicans (ATCC10231) and Escherichia coli (ATCC25922) were purchased from Shanghai Luwei Technology Co., Ltd (Shanghai, China). HSF culture medium was purchased from iCell Bioscience Inc. (Shanghai, China). PBS was purchased from Procell Life Science & Technology Co., Ltd (Wuhan, China). CCK-8 was purchased from Invigentech Inc. (USA).2.2. Synthesis of gold nanoparticles (AuNPs)
Citrate protected AuNPs were synthesized following the reported procedure.35 Briefly, 300 mL distilled water were poured into a round-bottom flask. After being boiled, 30 mL 10 mM HAuCl4 solution were added, then 34.8 mL 38.8 mM sodium citrate solution were rapidly added in the flask under vigorous stirring. The mixture was boiled for 10 min, then transferred to room temperature and stirred till cooled to room temperature.2.3. Characterization of synthesized AuNPs
The morphology of AuNPs was observed under transmission electron microscope (FEI Tecnai G2 F20, USA) and the ultraviolet-visible absorbance spectroscopy was measured by an ultraviolet-visible spectrophotometer (UV1800PC, China). In addition, the photothermal performance of AuNPs at different concentrations (0.2, 0.4 and 0.8 mM) were investigated by tracing the temperature increases under xenon lamp (PLS-SXE300, China) irradiation. The current of xenon lamp was set as 21 A, and the irradiation distance was set as 8.9 cm.2.4. Synthesis of AuNPs modified cotton gauze
To decorate AuNPs on the cotton gauze, cotton gauzes were immersed in 0.8 mM AuNPs solution for 1 h, then taken out and heated in a 180 °C oven for 7 min.36 The attained modified cotton gauzes immersed and heated once were marked as Au-1. Then Au-1 cotton gauzes were immersed and heated again, the attained modified cotton gauzes were marked as Au-2. In the end, the Au-1 and Au-2 cotton gauzes were washed with distilled water for 3 times to remove the non-attached AuNPs, and dried at 70 °C to gain AuNPs modified cotton gauzes.2.5. X-ray photoelectron spectroscopy (XPS)
To evaluate the introduction of Au atoms on the AuNPs modified cotton gauzes, the original, Au-1 and Au-2 cotton gauzes were cut into 0.4 cm × 0.4 cm pieces, and measured on an X-ray photoelectron spectrometer (ESCALAB 250Xi, USA) with binding energy from 0 eV to 600 eV.2.6. Inductively coupled plasma-optical emission spectroscopy (ICP-OES)
To measure the weight ratios of Au atoms on the AuNPs modified cotton gauzes, 6 cm × 6 cm pieces of Au-1 and Au-2 cotton gauzes were weighed, digested with 0.5 mL aqua regia, and diluted with 8 mL distilled water. Then the digested solutions were measured using an inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Agilent 5110, USA).2.7. Fourier transform infrared spectroscopy (FTIR)
To evaluate the chemical composition changes before and after modification, the original, Au-1 and Au-2 cotton gauzes were cut into small scraps, mixed with 200 mg KBr, ground and repressed into pieces. Then the samples were measured on a spectrophotometer (Nicolet iS10, USA) with wavelength from 500 cm−1 to 3750 cm−1.2.8. X-ray diffraction (XRD)
To evaluate the crystal structures of AuNPs on the AuNPs modified cotton gauzes, the original, Au-1 and Au-2 cotton gauzes were cut into 2.8 cm × 2.8 cm pieces, fixed on XRD specimen holders, and analysed on a diffractometer (Rigaku Dmax 2200, Japan) in a continuous mode with 2θ scanned from 30° to 90°.2.9. Scanning electron microscope (SEM)
To observe the distribution of AuNPs on the AuNPs modified cotton gauzes, the original, Au-1 and Au-2 cotton gauzes were cut into 0.4 cm × 0.4 cm pieces, and treated with spray-gold. Then the cotton gauzes were observed on a scanning electron microscope (FEI Quanta 250 FEG, USA).2.10. Mechanical property
Mechanical property is an important factor of cotton gauze, as the AuNPs modified cotton gauzes were synthesized under a relative high temperature, their mechanical properties were measured to investigate the effect of modification process on the mechanical properties. Firstly, the original, Au-1 and Au-2 cotton gauzes were cut into 4 cm × 0.7 cm rectangle pieces, then the tensile strength and tensile elongation at break were measured on an electronic universal testing machine for non-metallic material (SUST, china). The stretching speed was set as 20 mm min−1.2.11. Air permeability
The air permeability of the original, Au-1 and Au-2 cotton gauzes were measured according to ASTM D737 method using a YG461E air permeability tester (Ningbo Textile Instruments, China) at the standard condition of 65% RH and 20 °C. The differential pressure was set as 100 Pa and the test area was set as 38.3 cm2.2.12. Water vapour permeability
The water vapour permeability of the original, Au-1 and Au-2 cotton gauzes were measured according to ASTM E96 method using YG(B)216T water vapour permeability tester (Wenzhou Jigao Testing Instrument Co. Ltd, China), the atmosphere was controlled as 65% RH and 20 °C. The rate of water vapour permeation through each sample was determined by 24 h testing.2.13. Photothermal performance of AuNPs modified cotton gauze
For photothermal performance, the original, Au-1 and Au-2 cotton gauzes were cut into 2.8 cm × 2.8 cm pieces, fixed to the center of 6 cm round glass dishes, and put under xenon lamp irradiation. The current of xenon lamp was set as 21 A, and the irradiation distance was set as 8.9 cm. The thermal photos were recorded at an interval of 10 s by a thermal imager (Testo 868, Germany).2.14. Cytotoxicity evaluation of AuNPs modified cotton gauze
To evaluate the cytotoxicity of the AuNPs modified cotton gauzes, cell viabilities of human skin fibroblasts (HSF) were detected by CCK-8 method. Briefly, the original, Au-1 and Au-2 cotton gauzes were cut into 0.7 cm × 0.7 cm pieces. After sterilization, they were placed in a 24-well plate, then 1 mL HSF cell suspension (5 × 104 cells per mL) was seeded in each well and cultured at 37 °C in 5% CO2. After further culture for 24 h, the culture medium was removed and each well was washed with PBS twice, then 500 μL CCK-8 solution (10%) was added and further incubated for 2 h. The absorbance of the solution at 450 nm was detected by a microplate reader (Spark 10M, Switzerland). Furthermore, cells without cotton gauze samples were used as the control. The relative cell viability (%) was calculated as ODSample/ODControl.2.15. Antimicrobial experiments of AuNPs modified cotton gauze
To evaluate the photothermal antimicrobial activity of modified cotton gauzes, Candida albicans (fungus) and Escherichia coli (bacterium) were used. Candida albicans was cultured with SDB culture medium and Escherichia coli was cultured with TSB culture medium. Firstly, the second-generation microbial strains of Candida albicans and Escherichia coli were recovered with culture media overnight, then the microbial solutions were subcultured using fresh culture media. When the optical density of microbial solutions at 600 nm reached 0.1, antimicrobial experiments were conducted as following.For antimicrobial test, the original, Au-1 and Au-2 cotton gauzes were cut into 0.7 cm × 0.7 cm pieces. Then each sample was put into the centre of a 5 mL transparent glass bottle and sterilized under UV light for 1 h. Subsequently, 10 μL microbial solution was added on each sample to attain microbial cells permeated cotton gauze. Then the glass bottle was put under xenon lamp irradiation. The current of xenon lamp was set as 21 A, and the irradiation distance was set as 8.9 cm. To investigate the effect of irradiation time on the antimicrobial ability, the irradiation time was set as 0 s, 10 s, 20 s and 40 s. After irradiation, 500 μL fresh culture medium was added into the glass bottle and further cultured for 24 h. Then 200 μL culture medium was suctioned into a 96-well plate, and its optical density at 600 nm was measured using a microplate reader (DNM-9602, China).
3. Results and discussion
3.1. Characterization of the synthesized AuNPs
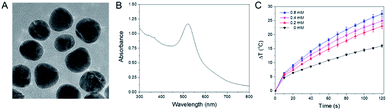
As shown in Fig. 1A, the synthesized citrate protected AuNPs were polydisperse with an average diameter of about 11 nm. Ultraviolet-visible spectra (Fig. 1B) showed that the AuNPs had a broad absorbance from 300 to 800 nm with absorbance peak at 520 nm. Under xenon lamp irradiation for 120 s (Fig. 1C), the temperature of distilled water (0 mM) increased 16.0 °C, and for AuNPs solutions at 0.2, 0.4 and 0.8 mM, the temperatures increased 23.0, 24.9 and 27.4 °C, respectively. Besides, the temperatures of the AuNPs solutions were increased with the increasing irradiation time. These results confirmed that AuNPs could adsorb light, and convert light to heat, resulting in temperature increases of the AuNPs solutions.3.2. Characterization of AuNPs modified cotton gauze
After modified with AuNPs, the colours of cotton gauzes turned from white to grape, and the woven structures were not changed (Fig. 2A). Besides, the colour of Au-2 cotton gauze was darker than that of the Au-1 cotton gauze.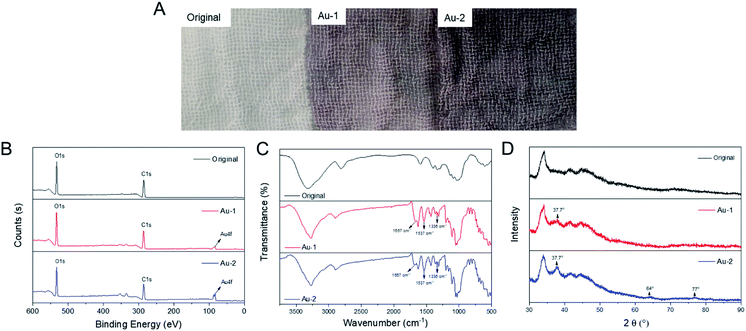 | ||
| Fig. 2 Characterization of the original, Au-1 and Au-2 cotton gauzes: (A) photos, (B) XPS spectra, (C) FTIR spectra, (D) XRD patterns. | ||
To verify whether AuNPs were successfully decorated on the cotton gauzes, XPS, ICP-OES, FTIR, XRD and SEM characterizations were carried out. In the XPS spectra (Fig. 2B), the Au 4f peak was not observed on the original cotton gauze, but observed at 84 eV on the Au-1 and Au-2 cotton gauzes, confirming the existing of Au atoms on AuNPs modified cotton gauze. Besides, ICP-OES results demonstrated that the weight ratios of Au atoms on Au-1 and Au-2 cotton gauzes were 0.11% and 0.24% respectively.
In the FTIR spectra (Fig. 2C), for Au-1 and Au-2 cotton gauzes, after modified with citrate protected AuNPs, asymmetric and symmetric stretching vibrations of carboxylate groups were observed at 1538 cm−1 and 1335 cm−1. In addition, the peak at 1667 cm−1 was probably related to hydrogen bond configurations of COO−Na+⋯Na+−OOC interactions.37 These characteristic absorptions of citrate sodium showed that AuNPs were successfully decorated on the cotton gauzes.
In the XRD patterns (Fig. 2D), the Bragg's reflection of AuNPs at 37.7° was seen on both Au-1 and Au-2 cotton gauzes, and the intensity of Au-1 cotton gauze was much lower than that of Au-2 cotton gauze, demonstrating that the density of AuNPs on Au-1 cotton gauze was much lower than that of Au-2 cotton gauze. Besides, other Bragg's reflections of AuNPs at 64° and 77° were seen on Au-2 cotton gauze, while they were not seen on Au-1 cotton gauze due to the lower content of AuNPs.
The SEM images were taken to observe the distribution of AuNPs on the modified cotton gauzes. As seen in Fig. 3A, before modification the fibers of original cotton gauze were smooth. After modification (Fig. 3B and C), there were nanoparticles on the fibers of Au-1 and Au-2 cotton gauzes. And compared with Au-1 cotton gauze, more nanoparticles were seen on Au-2 cotton gauze, confirming that more AuNPs were decorated on the Au-2 cotton gauze than that on the Au-1 cotton gauze.
3.3. Mechanical and permeable properties of AuNPs modified cotton gauze
The tensile strength and tensile elongation at break were measured to investigate the effect of modification process on the mechanical properties of cotton gauzes. Before modification, the tensile strength of original cotton gauze was 13.58 ± 0.88 MPa, and the tensile elongation at break was 26.04 ± 3.82%. After modification, the tensile strengths of Au-1 and Au-2 cotton gauzes were 14.50 ± 0.64 MPa and 13.79 ± 0.45 MPa; the tensile elongations at break of Au-1 and Au-2 cotton gauzes were 31.75 ± 3.28% and 34.10 ± 1.73%. These results showed that the tensile strength and tensile elongation at break were seldom changed after modification, confirming that the modification process had no obvious negative effects on the mechanical properties of cotton gauzes.Air and water vapour permeability abilities were important to gauzes used as wound dressings. Therefore, the air and water vapour permeability performance of original, Au-1 and Au-2 cotton gauzes were studied. As shown in Table 1, the air permeability of original cotton gauze was 473.4 ± 8.3 cm3 cm−2 s−1, and for Au-1, Au-2 cotton gauzes, the values were 473.4 ± 6.6 and 493.4 ± 3.1 cm3 cm−2 s−1 respectively, showing that the AuNPs modified cotton gauzes had similar air permeability performance to the original cotton gauze. In addition, the same as the air permeability results, water vapour permeability results showed that the water vapour permeability performance of the AuNPs modified cotton gauzes were similar to that of the original cotton gauze, demonstrating that the AuNPs modified cotton gauzes retained the good air and water vapour permeability of the original cotton gauze.
| Samples | Air permeability (cm3 cm−2 s−1) | Water vapour permeability (g per m2 per 24 h) |
|---|---|---|
| Original | 473.4 ± 8.3 | 1476.1 ± 36.0 |
| Au-1 | 473.4 ± 6.6 | 1496.9 ± 62.4 |
| Au-2 | 493.4 ± 3.1 | 1538.5 ± 36.0 |
3.4. Photothermal performance of AuNPs modified cotton gauze
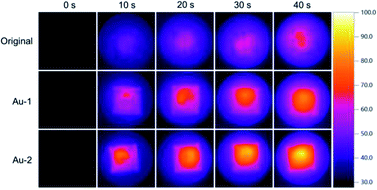
With AuNPs decorated on the surface, AuNPs modified cotton gauzes were supposed to have photothermal abilities. Therefore, the thermal images of original, Au-1 and Au-2 cotton gauzes under xenon lamp irradiation were taken.As shown in Fig. 4, under irradiation all the cotton gauzes had temperature increases with increasing irradiation time, but the increasing speed differed. Before irradiation, the temperatures of all the cotton gauzes were about 30 °C; after 10 s irradiation, the highest temperature of original cotton gauze was increased to 44.6 °C, while for Au-1 and Au-2 cotton gauzes, the temperatures were increased to 62.1 and 72.8 °C. After 40 s irradiation, the highest temperature of original cotton gauze was increased to 62.8 °C, while for Au-1 and Au-2 cotton gauzes, the temperatures were increased to 77.2 and 90.5 °C. These data demonstrated that AuNPs on the AuNPs modified cotton gauzes could effectively convert light to heat, inducing the rapid temperature increases of cotton gauzes. Moreover, the temperature increasing speeds were related to the weight ratios of AuNPs on the AuNPs modified cotton gauzes.
3.5. Cytotoxicity evaluation of AuNPs modified cotton gauze
The cytotoxicity of original, Au-1 and Au-2 cotton gauzes were evaluated using human skin fibroblasts (HSF). As shown in Fig. 5, the relative cell viability of original cotton gauze was 72.30%, which was much lower than the control (without cotton gauzes). This was because the adhesion area of cotton gauze sample was much lower than that of the control due to the porosity structure of the cotton gauze. Besides, the relative cell viabilities of Au-1 and Au-2 cotton gauzes were 72.4% and 75.4% respectively, showing that Au-1 and Au-2 cotton gauzes were biocompatibility to HSF cells. This could be understood from the gold release behaviours of Au-1 and Au-2 cotton gauzes (Table S1†) that even soaked in water for 72 h, the release of gold from Au-1 and Au-2 cotton gauzes was 0.00% and 1.65% respectively. Therefore, the modification of AuNPs on the cotton gauzes could not induce cytotoxicity to human skin fibroblasts.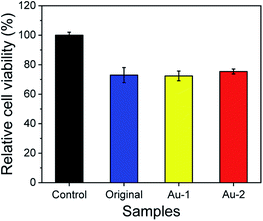 | ||
| Fig. 5 Relative cell viability of the original, Au-1 and Au-2 cotton gauzes via human skin fibroblasts using cells without cotton gauze as the control. | ||
3.6. Antimicrobial ability of AuNPs modified cotton gauze
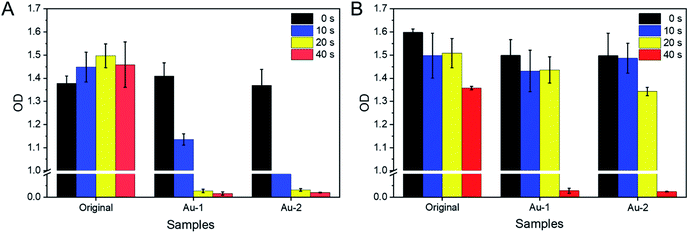
The photothermal antimicrobial ability of original, Au-1 and Au-2 cotton gauzes under different irradiation time were investigated by detecting the OD values of sample solutions after culture for 24 h, and the OD values could reflect the cell numbers of microbes. It was seen (Fig. 6) that without irradiation, when cultured with original or AuNPs modified cotton gauzes, the microbes could grow fast, confirming that all the cotton gauzes were biocompatible for the microbes could grow fast, confirming that all the cotton gauzes were biocompatible for Candida albicans and Escherichia coli growth. While under irradiation, the numbers of microbes cultured with original and AuNPs modified cotton gauzes differed.For Candida albicans (Fig. 6A), after 40 s irradiation the cell number of the original cotton gauze was not affected by irradiation. While on the Au-2 cotton gauze, 10 s irradiation could kill the most of Candida albicans cells, and induced big decrease of the cell number. In addition, 20 s irradiation could kill all the Candida albicans cells on Au-1 and Au-2 cotton gauzes, confirming that the AuNPs modified cotton gauzes could be used for Candida albicans-related photothermal antimicrobial therapy. For Escherichia coli (Fig. 6B), the same as Candida albicans, 40 s irradiation on the original cotton gauze had no effect on the cell number. 20 s irradiation on Au-1 and Au-2 cotton gauzes had no significant effects on the cell numbers of Escherichia coli, and 40 s irradiation on Au-1 and Au-2 cotton gauzes was needed to kill all the Escherichia coli cells. It was supposed that this difference was induced by the differences of microbial species. And these results supported that with AuNPs modified on the cotton gauzes, xenon lamp irradiation could kill the microbes permeated in the cotton gauzes, confirming that the AuNPs modified cotton gauze could be used for photothermal antimicrobial therapy.
Cotton gauzes have promising prospect in wound dressings due to their air permeability and absorbency abilities, but lack antimicrobial abilities when the wound is infected. In this work, we were trying to synthesis cotton gauzes with photothermal potential, and AuNPs were chosen as photothermal transduction agents. Compared with other PTAs, AuNPs are easy to prepare,38 and the light sources of AuNPs used for PTT could be visible light, which are easier to attain. In addition, compared with other methods reported to synthesis antimicrobial cotton gauzes,11–16 the immersing and heating procedure used to synthesis AuNPs modified cotton gauzes is easy to operate. What's more, only a short treatment time of 40 s was needed to fully kill the microbial cells permeated in the cotton gauze, making the therapy extremely efficient.
4. Conclusions
In this work, AuNPs were used as photothermal transduction agents, and modified on the clinical cotton gauzes by a simple immersing and heating process. XPS, ICP-OES, FTIR, XRD and SEM results demonstrated that AuNPs were successfully decorated on the cotton gauzes. And the mechanical and permeable performance were not affected by the modification process. In addition, after being modified on the cotton gauzes, the photothermal ability of AuNPs were retained, and under xenon lamp irradiation, the photothermal effects could kill microbes permeated in the AuNPs modified cotton gauzes. This work gives us a new strategy to synthesize photothermal antimicrobial cotton gauze.Author contributions
Fengyi Cao: conceptualization, methodology, data curation, writing-original draft, writing-review & editing. Changmin Wei: methodology, investigation, formal analysis. Gangqing Ma: methodology, investigation. Like Hou: methodology, formal analysis. Renchong Zhang: methodology, formal analysis. Lin Mei: conceptualization, methodology, validation. Qi Qin: supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Science and Technology Guiding Project of China Textile Industry Federation (No. 2018073), the National Natural Science Foundation of China (No. 51703260, 51703259, 51603236) and Program for Science and Technology of Henan Province (No. 182102310635).Notes and references
- A. Lumbreras-Aguayo, H. I. Meléndez-Ortiz, B. Puente-Urbina, C. Alvarado-Canché, A. Ledezma, J. Romero-García and R. Betancourt-Galindo, Carbohydr. Polym., 2019, 205, 203 CrossRef CAS PubMed.
- T. S. Kim, J. R. Cha and M. S. Gong, Fibers Polym., 2017, 18, 453 CrossRef CAS.
- S. Kittinaovarat, N. Hengprapakron and W. Janvikul, Carbohydr. Polym., 2012, 87, 16 CrossRef CAS.
- E. Pinho and G. Soares, J. Mater. Chem. B, 2018, 6, 1887 RSC.
- M. Rehan, S. Zaghloul, F. A. Mahmoud, A. S. Montaser and A. Hebeish, Mater. Sci. Eng., C, 2017, 80, 29 CrossRef CAS PubMed.
- A. S. Montaser, M. Rehan, W. M. El-Senousy and S. Zaghloul, Carbohydr. Polym., 2020, 244, 116479 CrossRef CAS PubMed.
- M. E. El-Naggar, A. M. Abdelgawad, D. A. Elsherbiny, W. A. El-shazly, S. Ghazanfari, M. S. Abdel-Aziz and Y. K. Abd-Elmoneam, J. Cluster Sci., 2020, 31, 1349 CrossRef CAS.
- A. P. Gomes, J. F. Mano, J. A. Queiroz and I. C. Gouveia, Carbohydr. Polym., 2015, 127, 451 CrossRef CAS PubMed.
- S. Panawes, P. Ekabutr, P. Niamlang, P. Pavasant, P. Chuysinuan and P. Supaphol, J. Drug Delivery Sci. Technol., 2017, 41, 182 CrossRef CAS.
- Y. Yuan, H. Wu, H. Lu, Y. Zheng, J. Y. Ying and Y. Zhang, Chem. Commun., 2019, 55, 699 RSC.
- S. Panawes, P. Ekabutr, P. Niamlang, P. Pavasant, P. Chuysinuan and P. Supaphol, J. Drug Delivery Sci. Technol., 2017, 41, 182 CrossRef CAS.
- J. Xiang, R. Zhu, S. Lang, H. Yan, G. Liu and B. Peng, Chem. Eng. J., 2021, 409, 128291 CrossRef CAS.
- A. S. Montaser, K. Jlassi, M. A. Ramadan, A. A. Sleem and M. F. Attia, Int. J. Biol. Macromol., 2021, 173, 203 CrossRef CAS PubMed.
- J. M. Souza, M. Henriques, P. Teixeira, M. M. Fernandes, R. Fangueiro and A. Zille, Fibers Polym., 2019, 20, 922 CrossRef CAS.
- D. Bains, G. Singh, N. Kaur and N. Singh, ACS Sustainable Chem. Eng., 2018, 7, 969 CrossRef.
- S. Li, A. Chen, Y. Chen, Y. Yang, Q. Zhang, S. Luo, M. Ye, Y. Zhou, Y. An, W. Huang, T. Xuan, Y. Pan, X. Xuan, H. He and J. Wu, Chem. Eng. J., 2020, 402, 126202 CrossRef CAS.
- Y. Chen, Y. Gao, Y. Chen, L. Liu, A. Mo and Q. Peng, J. Controlled Release, 2020, 328, 251 CrossRef CAS PubMed.
- J. Shi, J. Li, Y. Wang, J. Cheng and C. Y. Zhang, J. Mater. Chem. B, 2020, 8, 5793 RSC.
- J. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Liang, Z. Li, S. Zhu, Y. Zheng, K. Yeung, X. Wang and S. Wu, Nat. Commun., 2019, 10, 4490 CrossRef PubMed.
- Y. Yang, L. Ma, C. Cheng, Y. Deng, J. Huang, X. Fan, C. Nie, W. Zhao and C. Zhao, Adv. Funct. Mater., 2018, 28, 1705708 CrossRef.
- Z. Yu, X. Li, F. Xu, X. Hu, J. Yan, N. Kwon, G. Chen, T. Tang, X. Dong, Y. Mai, D. Chen, J. Yoon, X. He and H. Tian, Angew. Chem., 2020, 132, 3687 CrossRef.
- C. Bermúdez-Jiménez, M. G. Romney, S. A. Roa-Flores, G. Martínez-Castañón and H. Bach, Nanomedicine, 2019, 22, 102093 CrossRef PubMed.
- Y. Qiao, F. Ma, C. Liu, B. Zhou, Q. Wei, W. Li, D. Zhong, Y. Li and M. Zhou, ACS Appl. Mater. Interfaces, 2018, 10, 193 CrossRef CAS PubMed.
- T. Deng, H. Zhao, M. Shi, Y. Qiu, S. Jiang, X. Yang, Y. Zhao and Y. Zhang, Small, 2019, 15, 1902647 CrossRef CAS PubMed.
- L. Mei, X. Gao, Y. Shi, C. Cheng, Z. Shi, M. Jiao, F. Cao, Z. Xu, X. Li and J. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 40153 CrossRef CAS PubMed.
- R. Kurapati, M. Vaidyanathan and A. M. Raichur, RSC Adv., 2016, 6, 39852 RSC.
- Y. Yu, M. Song, C. Chen, Y. Du, C. Li, Y. Han, F. Yan, Z. Shi and S. Feng, ACS Nano, 2020, 14, 10688 CrossRef CAS PubMed.
- X. Wang, K. Su, L. Tan, X. Liu, Z. Cui, D. Jing, X. Yang, Y. Liang, Z. Li, S. Zhu, K. W. K. Yeung, D. Zheng and S. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 15014 CrossRef CAS PubMed.
- Z. Feng, X. Liu, L. Tan, Z. Cui, X. Yang, Z. Li, Y. Zheng, K. W. K. Yeung and S. Wu, Small, 2018, 14, 1704347 CrossRef PubMed.
- H. Jin, G. Zhao, J. Hu, Q. Ren, K. Yang, C. Wan, A. Huang, P. Li, J. Feng, J. Chen and Z. Zou, ACS Appl. Mater. Interfaces, 2017, 9, 25755 CrossRef CAS PubMed.
- H. Maaoui, R. Jijie, G. Pan, D. Drider, D. Caly, J. Bouckaert, N. Dumitrascu, R. Chtourou, S. Szunerits and R. Boukherroub, J. Colloid Interface Sci., 2016, 480, 63 CrossRef CAS PubMed.
- B. Zheng, Q. He, X. Li, J. Yoon and J. Huang, Coord. Chem. Rev., 2021, 426, 213548 CrossRef CAS.
- X. Li, S. Li, Q. Bai, N. Sui and Z. Zhu, Colloids Surf., B, 2020, 196, 111313 CrossRef CAS PubMed.
- B. Tao, C. Lin, Y. Deng, Z. Yuan, X. Shen, M. Chen, Y. He, Z. Peng, Y. Hu and K. Cai, J. Mater. Chem. B, 2019, 7, 2534 RSC.
- C. Katherine, R. Griffith, B. Michael and J. Michael, Anal. Chem., 1995, 67, 735 CrossRef.
- Q. Xu, X. Ke, L. Shen, N. Ge, Y. Zhang, F. Fu and X. Liu, Int. J. Biol. Macromol., 2018, 111, 796 CrossRef CAS PubMed.
- J. W. Park and J. S. Shumaker-Parry, J. Am. Chem. Soc., 2014, 136, 1907 CrossRef CAS PubMed.
- M. Khafaji, M. Zamani, M. Golizadeh and O. Bavi, Biophys. Rev., 2019, 11, 335 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Contact angle, water absorbency, gold release, microbial cell adhesion morphology. See DOI: 10.1039/d1ra01597d |
| This journal is © The Royal Society of Chemistry 2021 |