Open Access Article
Open Access ArticleHyperacmosins K–M, three new polycyclic polyprenylated acylphloroglucinols from Hypericum acmosepalum†
Mingxia Sun‡
a,
Xue Wang‡a,
Tingting Zhua,
Xinyue Suoa,
Jiajia Wanga,
Tengfei Ji§ *ab and
Bo Liu§
*ab and
Bo Liu§
 *c
*c
aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050, China
bKey Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, 810008, China
cSchool of Pharmacy, Shenyang Medical College, Shenyang, 110034, China
First published on 14th June 2021
Abstract
Hyperacmosins K–M (1–3), three new polycyclic polyprenylated acylphloroglucinol (PPAPs) derivatives, were isolated from the air-dried aerial parts of Hypericum asmosepalum. Compounds 1 and 2 both possessed a rare 5,5-spiroketal subunit with the loss of C-2′ carbonyl in the phloroglucinal ring, while compound 3 featured an unusual 1,2-seco-bicyclo[3.3.1] PPAP skeleton. Their structures were confirmed by NMR, HRESIMS, and CD spectra. The plausible biogenetic pathways of 1–3 were proposed, which gave an insight for future biomimetic synthesis of the novel compounds.
1. Introduction
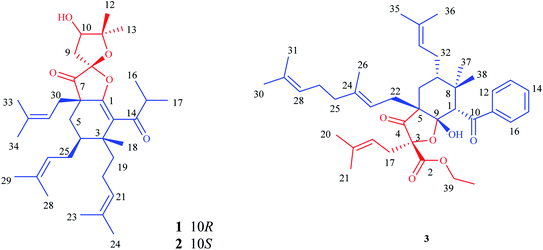
Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a group of structurally fascinating natural products, due to their highly oxygenated and various acylphloroglucinol-derived core structures that are decorated with prenyl substituents.1,2 To date, more than 800 natural occurring PPAPs with diverse carbon skeletons have been isolated. PPAPs exhibit a broad range of biological activities, such as antidepressant,3 antioxidant,3 anti-tumor,4 anti-inflammatory,5 antimicrobial,6 and anti-neurodegenerative,3,7,8 which have attracted great attention from the phytochemical and organic synthetic communities.9–13Hypericum acmosepalum is distributed in Guangxi, Yunnan, Sichuan and Guizhou provinces in China. As a kind of traditional chinese medicine, it has been used to treat hepatitis and relieve swelling and inflammation. Our previous phytochemical investigations on this plant have resulted in the isolation of many bioactive PPAPs with diverse carbon scaffolds.14–16 In our current study, three new PPAPs (1–3) (Fig. 1) were isolated from H. acmosepalum. Herein, we describe the isolation, structure and stereochemistry elucidation, as well as the plausible biosynthetic ways of the new compounds.
2. Results and discussion
Hyperacmosin K (1) was obtained as colorless oil. The molecular formula was established as C34H52O5 according to its HRESIMS data (m/z 541.3888, [M + H]+, for C34H53O5, calcd 541.3888), indicating 9 degrees of unsaturation. The IR spectrum displayed strong absorption bands due to carbonyls (1774 cm−1) and hydroxyl groups (3491 cm−1). The 1H NMR spectrum (CDCl3) exhibited two characteristic doublet methyls of isopropyl [δH 1.11 (d, J = 4.6 Hz, H3-17), 1.13 (d, J = 4.6 Hz, H3-16)], three singlet methyls [δH 1.42 (H3-12), 1.24 (H3-13) and 1.18 (H3-18)], six singlet isopentenyl methyls [δH 1.51 (H3-23), 1.62 (H3-24), 1.61 (H3-28), 1.72 (H3-29), 1.61 (H3-33), 1.72 (H3-34)], and three olefinic protons [δH 4.91 (t, J = 6.8 Hz, H-21), 5.06 (m, H-26), 5.02 (m, H-31)] (Table 1). The 13C NMR spectrum of compound 1 indicated a total of 34 carbon signals, including two carbonyl carbons [δC 206.2 (C-7), 207.6 (C-14)], eight olefinic carbons [δC 154.5 (C-1), 126.3 (C-2), 124.3 (C-21), 131.5 (C-22), 122.8 (C-26), 132.9 (C-27), 117.2 (C-31), 136.9 (C-32)], one ketal carbon [δC 110.2 (C-8)], two oxygenated carbons [δC 76.8 (C-10), 90.6 (C-11)]. In addition, the aforementioned functionalities accounted for 6 of 9 degrees of unsaturation, which implied the existence of three rings in the structure of 1 (Fig. 1).| No | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| a Recorded in CDCl3 (1H NMR 400 MHz, 13C NMR 125 MHz). | ||||||
| 1 | 154.5 | 156.6 | 56.7 | 3.98 s | ||
| 2 | 126.3 | 124.7 | 170.9 | |||
| 3 | 40.7 | 41.0 | 87.7 | |||
| 4 | 40.0 | 1.20 m | 39.9 | 1.23 m | 211.2 | |
| 5 | 27.9 | 1.32 d (8.2); | 28.0 | 1.33 m; | 55.6 | |
| 2.30 dd (14.4, 4.8) | 2.31 m | |||||
| 6 | 49.8 | 49.9 | 31.9 | 1.39 m; 1.70 m | ||
| 7 | 206.2 | 208.9 | 44.4 | 1.51 m | ||
| 8 | 110.2 | 109.2 | 38.7 | |||
| 9 | 41.4 | 2.15 dd (14.6, 1.4); | 42.4 | 2.26 dd (14.8, 2.0); | 105.7 | |
| 2.64 dd (14.6, 5.6) | 2.62 dd (14.8, 5.4) | |||||
| 10 | 76.8 | 4.05 dd (5.6, 1.4) | 77.4 | 4.03 brs | 200.3 | |
| 11 | 90.6 | 90.0 | 141.1 | |||
| 12 | 22.8 | 1.42 s | 26.8 | 1.35 s | 128.5 | 7.99 d (7.0) |
| 13 | 27.0 | 1.24 s | 22.2 | 1.31 s | 128.6 | 7.45 t (7.0) |
| 14 | 207.6 | 206.9 | 132.6 | 7.54 t (7.0) | ||
| 15 | 41.4 | 3.02 m | 41.7 | 3.11 m | 128.6 | 7.45 t (7.0) |
| 16 | 17.9 | 1.13 d (4.6) | 18.5 | 1.14 d (6.6) | 128.5 | 7.99 d (7.0) |
| 17 | 19.8 | 1.11 d (4.6) | 20.0 | 1.11 d (6.6) | 36.5 | 3.06 dd (14.6, 9.2); |
| 2.59 dd (14.6, 5.6) | ||||||
| 18 | 22.6 | 1.18 s | 22.7 | 1.17 s | 116.9 | 5.33 m |
| 19 | 36.5 | 1.67 m; 1.41 m | 36.0 | 1.84 m; 1.40 m | 136.4 | |
| 20 | 22.7 | 1.80 m; 1.45 m | 22.7 | 1.80 m; 1.45 m | 26.1 | 1.73 s |
| 21 | 124.3 | 4.91 t (6.8) | 124.4 | 4.93 m | 18.3 | 1.66 s |
| 22 | 131.5 | 131.4 | 29.5 | 2.45 m | ||
| 23 | 17.9 | 1.51 s | 17.9 | 1.52 s | 119.6 | 5.55 t (8.0) |
| 24 | 25.8 | 1.62 s | 25.8 | 1.63 s | 138.0 | |
| 25 | 28.0 | 2.15 m; 1.67 m | 28.0 | 2.16 m; 1.67 m | 40.4 | 2.09 m |
| 26 | 122.8 | 5.06 m | 122.8 | 5.06 t (7.0) | 16.6 | 1.71 s |
| 27 | 132.9 | 132.8 | 26.9 | 2.15 m | ||
| 28 | 18.1 | 1.61 s | 18.1 | 1.60 s | 124.2 | 5.14 t (6.8) |
| 29 | 26.2 | 1.72 s | 26.3 | 1.72 s | 131.8 | |
| 30 | 37.2 | 2.46 dd (14.4, 7.8); | 38.4 | 2.47 dd (14.4, 7.4); | 26.1 | 1.68 s |
| 2.59 dd (14.4, 7.8) | 2.76 dd (14.4, 7.4) | |||||
| 31 | 117.2 | 5.02 m | 116.8 | 5.00 t (7.4) | 17.9 | 1.61 s |
| 32 | 136.9 | 137.2 | 27.9 | 1.53 m | ||
| 33 | 18.3 | 1.61 s | 18.4 | 1.60 s | 123.2 | 5.01 t (6.0) |
| 34 | 26.2 | 1.72 s | 26.3 | 1.72 s | 133.1 | |
| 35 | 25.9 | 1.70 s | ||||
| 36 | 18.0 | 1.56 s | ||||
| 37 | 15.5 | 1.18 s | ||||
| 38 | 28.9 | 0.87 s | ||||
| 39 | 63.1 | 4.16 m | ||||
| 40 | 14.1 | 1.22 t (7.0) | ||||
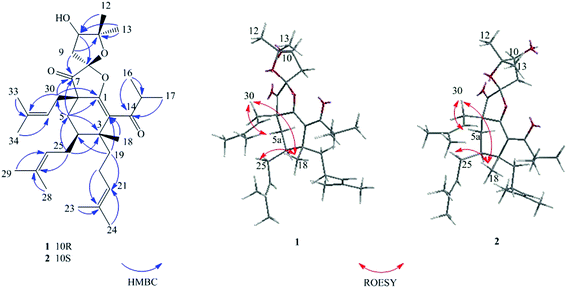
The structure of compound 1 was subsequently confirmed by inspecting its 2D NMR spectra. The HMBC correlations from H2-5 to C-1/C-3/C-4/C-6/C-7/C-30, from H2-30 to C-1/C-5/C-6/C-7/, from H2-25 to C-3/C-4/C-5, from H3-18/H2-19 to C-2/C-3/C-4 (Fig. 2), combining with the chemical shifts of C-1 and C-2, established the fragment of cyclohexene. Moreover, a 2,2-dimethyl-3-hydroxy-furan unit, was constructed by the HMBC correlations from H2-9 to C-7/C-8/C-10/C-11, from H-10 to C-8/C-9/C-11, from H3-12/H3-13 to C-10/C-11, as well as the presence of three oxygenated carbons C-8 (δC 110.2), C-10 (δC 76.8) and C-11 (δC 90.6). The HMBC cross-peaks from H2-5/H2-30/H2-9 to the carbonyl carbon C-7 (δC 206.2) indicated that C-7 was both connected to C-6 and C-8. Finally, considering one remaining unsaturation, as well as the chemical shifts of C-8 (δC 110.2) and C-1 (δC 154.5), it is speculated that C-8 should be connected with C-1 via an oxygen atom. Moreover, the HMBC correlations from H-15 to C-2/C-14/C-16/C-17 revealed that C-14 was attached to C-2. These data indicated 1 shared the same planar structure with Hyperscabin A as shown in Fig. 1.17
The relative configuration of 1 was confirmed by the key ROESY spectrum. The ROESY correlations of H2-30/H-5a, H2-30/H-18, and H3-18/H2-25 revealed that Me-18 and those two isopentenyl moieties attached to C-4/C-6 were in the same β-orientation (Fig. 2). The correlations of H-4/H-5b and H-4/H2-20 also indicated the H-4 and the isopentenyl linked to C-3 were α-orientation. The MM2-optimized structure for 1 revealed that those two five-member rings divided by C-8 were almost perpendicular to each other. By comparison with Hyperscabin A, the lack of ROESY correlation of H2-30/H2-9 indicated compound 1 possessed different stereogenic configuration of C-8 with the former as shown in Fig. 1. It could be further supported by some differences of their chemical shifts. [compound 1: H-9 (δH 2.15/2.64), C-7 (δC 206.2), C-8 (δC 110.2), C-9 (δC 41.4); Hyperscabin A: H-9 (δH 2.30/2.67), C-7 (δC 208.0), C-8 (δC 109.6), C-9 (δC 43.2)]. However, the relative configuration of C-10 could not be established by ROESY correlations.
Hyperacmosin L (2) displayed the same molecular formula of C34H52O5 as 1 based on the HRESIMS data. The UV and IR spectral data of 2 were identical to those of 1, as shown in the ESI.† Moreover, the NMR data of 2 was very close to that of 1 except for few slight shifts (1: δH 1.42, s, H3-12; 1.24, s, H3-13; 2: δH 1.35, s, H3-12; 1.31, s, H3-13) (Table 1). It was estimated that stereoisomerism of C-10 might cause these differences. Like 1, the relative configurations of C-3, 4, 6 and 8 in 2 could be determined via the ROESY correlations of H2-30/H-5a, H2-30/H-18, H3-18/H2-25, as well as the lack of ROESY correlation of H2-30/H2-9 (Fig. 2). And the relative configuration of C-10 could not be established by ROESY correlations.
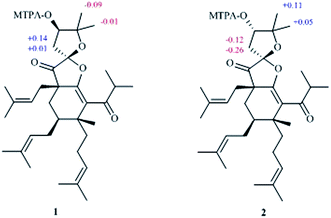
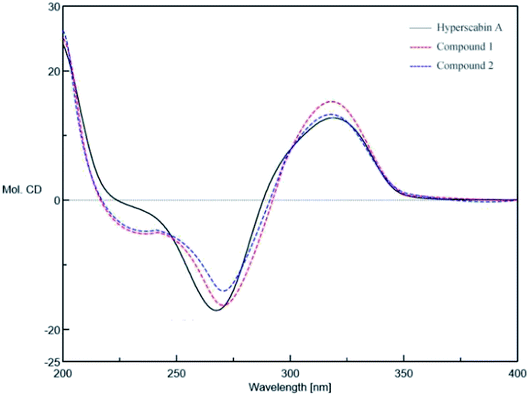
The absolute configuration of C-10 in 1 and 2 were validated by Mosher's experiment. The prepared (S)- and (R)-MTPA esters of 1 and 2 were subjected to 1H NMR analysis, and the distinct values of the 1H NMR chemical shifts (Δδ = δS-MTPA-ester − δR-MTPA-ester) were summarized for the proton signals adjacent to C-10, as shown in Fig. 3. According to these results, the absolute configurations of C-10 in 1 and 2 were confirmed to be R and S, respectively. The absolute configurations of 1 and 2 were further elucidated by the comparison of their CD spectra with Hyperscabin A (Fig. 4), whose absolute configuration had been determined as 3R,4S,6S,8S,10R by experimental and calculated ECD. Thus, the absolute configuration of 1 was further determined as 3R,4S,6S,8R,10R and 2 was determined as 3R,4S,6S,8R,10S.
Hyperacmosin M (3) was obtained as colorless oil. The molecular formula was established as C40H56O6 according to its HRESIMS data (m/z 655.3967, [M + Na]+, for C40H56O6Na, calcd 655.3969), indicating 13 degrees of unsaturation. The IR spectrum displayed strong absorption bands due to hydroxyl groups (3357 cm−1) and carbonyls (1760, 1717 cm−1). The 1H NMR spectrum (CDCl3) showed characteristic signals assignable to a benzoyl [δH 7.45 (2H, t, J = 7.0 Hz); 7.54 (1H, t, J = 7.0 Hz); 7.99 (2H, d, J = 7.0 Hz)], four olefinic protons [δH 5.01 (m, H-33), 5.14 (t, J = 6.8 Hz, H-28), 5.33 (m, H-18), 5.55 (t, J = 8.0 Hz, H-23)], nine singlet methyls [δH 0.87 (H3-38), 1.18 (H3-37), 1.56 (H3-36), 1.61 (H3-31), 1.66 (H3-21), 1.68 (H3-30), 1.70 (H3-35), 1.71 (H3-26), 1.73 (H3-20)], and one triplet methyl [δH 1.22 (t, J = 7.0 Hz, H3-40)] (Table 1). The 13C NMR spectrum of compound 3 indicated a total of 40 carbon signals, including three carbonyl carbons [δC 170.9 (C-2), 200.3 (C-10), 211.2 (C-4)], eight olefinic carbons [δC 116.9 (C-18), 136.4 (C-19), 119.6 (C-23), 138.0 (C-24), 124.2 (C-28), 131.8 (C-29), 123.2 (C-33), 132.6 (C-34)], one hemiketal carbon [δC 105.7 (C-9)], two oxygenated carbons [δC 87.7 (C-3), 63.1 (C-39)]. The aforementioned data suggested that 3 was also a PPAP derivative.
The planar structure of 3 was established by extensive analyses of its HMBC spectrum (Fig. 5). The cyclohexane ring was constructed by HMBC correlations from H-1 to C-5/C-7/C-8/C-9, from H3-37/H3-38 to C-1/C-7/C-8, from H-7 to C-6/C-8/C-32, and from H-22 to C-4/C-5/C-6/C-9. Moreover, the presence of a tetrahydrofuran ring between C-3 and the hemiketal carbon was elucidated based on their downfield shifted chemical shifts [δC 87.7 (C-3) and 105.7 (C-9)], as well as the HMBC correlations from H2-17 to C-2/C-3/C-4, and from H2-22 to C-4/C-5/C-6/C-9. Thus, the planar structure of 3 was confirmed as shown in Fig. 1.
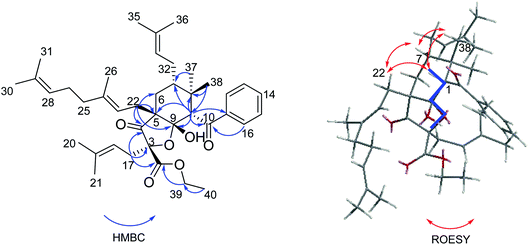 | ||
| Fig. 5 Key HMBC and ROESY correlations of 3 and molecular modeling showing the W-coupling from H-1 to C-3 of 3. | ||
The relative configuration of 3 was elucidated by ROESY spectrum (Fig. 5). The ROESY correlations from H-1 to H-7, H2-22, and H3-38 revealed that they possessed same orientation, which was supposed to be β. Due to the lack of valuable ROESY correlation, the orientation of the hydroxyl at C-9 could not be determined directly. However, the W-coupling of H-1 and C-3 could be observed in HMBC spectrum, because the presence of W-coupling is strictly depended on the specific confirmation. Only cis-fusion of the cyclohexane and the tetrahydrofuran rings could provide such a conformation with the five atoms of H-1, C-1, C-9, O-C-3, and C-3 in a plane and the four bonds between them to construct a “W” as shown in Fig. 5.18
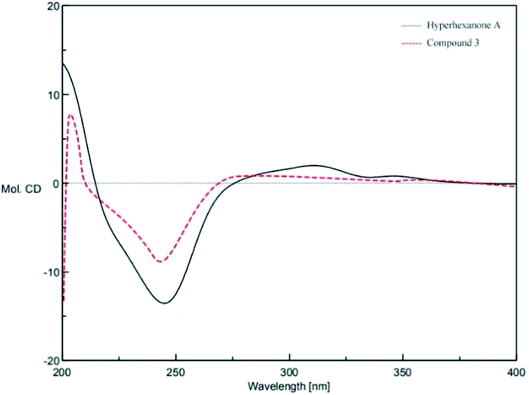
The absolute configuration of 3 was confirmed by comparing its CD spectrum with that of Hyperhexanone A, whose absolute configuration has been unambiguously determined as 1R,3R,5S,7S,9S by experimental and calculated ECD.18 The CD curve of 3 was in good agreement with that of Hyperhexanone A (Fig. 6). Therefore, the absolute configuration of 3 was determined as 1R,3R,5S,7S,9S.
3. Conclusion
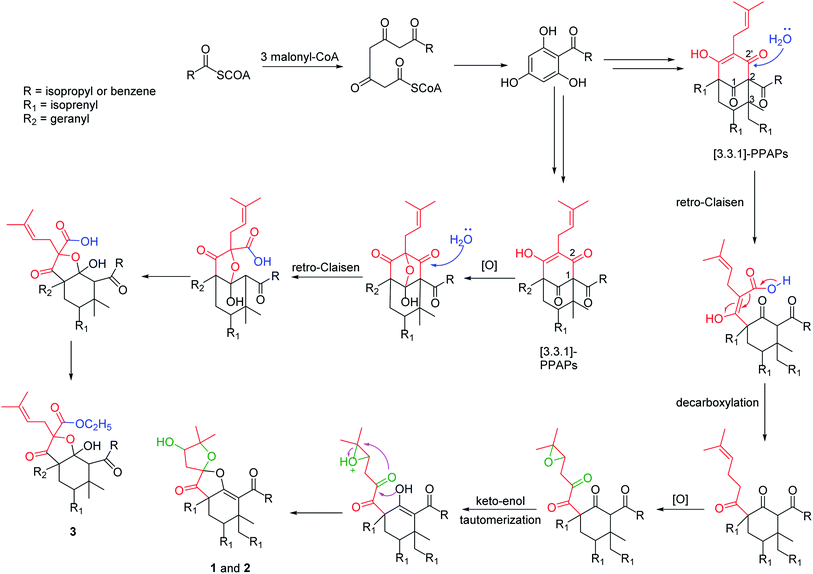
In summary, hyperacmosins K–M (1–3), three new polycyclic polyprenylated acylphloroglucinol derivatives were isolated from the air-dried aerial parts of H. asmosepalum. Particularly, compounds 1–2 shared the same planar constructure, which possessed a rare 5,5-spiroketal subunit with the loss of C-2′ carbonyl in the phloroglucinal ring, while compound 3 featured an unusual 1,2-seco-bicyclo[3.3.1] PPAP skeleton. In addition, the plausible biosynthetic pathways of compounds 1–3 was proposed in Scheme 1. Compounds 1–2 was considered to be generated from the representative [3.3.1]-type PPAPs via the retro-Claisen reaction, followed by decarboxylation, oxidation, keto–enol tautomerization, and intramolecular cyclization successively. Similarly, compound 3 could be formed from [3.3.1]-type PPAPs through oxidation, retro-Claisen reaction, and esterification.4. Experimental section
4.1 General experimental procedures
Optical rotations were measured with a JASCO P-2000 polarimeter (Jasco, Tokyo, Japan). UV spectra were recorded on a JASCO V-650 spectrophotometer (Jasco, Tokyo, Japan). ECD spectra were recorded on a JASCO J-810 spectrometer (Jasco, Tokyo, Japan). IR spectra recorded on a Nicolet 5700 IR spectrometer (Thermo Nicolet, Waltham, MA, USA). NMR data were measured on a Varian Inova-500 spectrometer (Varian Inc., Palo Alto, CA, USA) and a Mercury-400 spectrometer (Varian Inc., Palo Alto, CA, USA) using TMS as an internal standard. HRESIMS spectra were collected using an Agilent 1100 LC/MSD Trap-SL mass spectrometer (Agilent Technologies Ltd, Santa Clara, CA, USA). Column chromatography (CC) was carried out using silica gel (200–300 mesh), silica gel H (Qingdao Haiyang Chemistry Company, Qingdao, China), and MCI gel CHP20P (35–75 μm, Mitsubishi Chemical Corp., Tokyo, Japan). HPLC experiments were carried out on a preparative YMC-Pack ODS-A column (250 × 10 mm, 5 μm; YMC, Tokyo, Japan).4.2 Biological materials
The air-dried aerial parts of H. acmosepalum were collected from Li jiang, Yunnan Province (100°11′ E; 26°11′ N), People's Republic of China, in July 2016. Associate Prof. Lin Ma was responsible for the identification of the plant. A voucher specimen (No ID-S-2764) was deposited in the Institute of Materia Medica, Chinese Academy of Medical Sciences.4.3 Extraction and isolation
The air-dried aerial parts of H. acmosepalum (15.0 kg) were extracted by 95% ethanol (150 L × 3 times) under reflux. The crude extract was suspended in H2O and partitioned with petroleum ether. The petroleum ether extract (562.0 g) was separated on a silica gel column (petroleum ether/EtOAc, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to gain five fractions (Fr.1–5). Fr.3 (95.2 g) was further purified by chromatography on a diol column, eluting with petroleum ether/EtOAc (100
100) to gain five fractions (Fr.1–5). Fr.3 (95.2 g) was further purified by chromatography on a diol column, eluting with petroleum ether/EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to yield fourteen fractions (Fr.3.1-Fr.3.14). Fr.3.11 (36.4 g) was separated over silica gel (petroleum ether/EtOAc, 9
100) to yield fourteen fractions (Fr.3.1-Fr.3.14). Fr.3.11 (36.4 g) was separated over silica gel (petroleum ether/EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to gain 10 fractions (Fr.3.11.1-Fr.3.11.10). Fr.3.11.5 (1.5 g) was purified over ODS (MeOH/H2O, 80
1) to gain 10 fractions (Fr.3.11.1-Fr.3.11.10). Fr.3.11.5 (1.5 g) was purified over ODS (MeOH/H2O, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to 100
20 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and finally by semi-preparative HPLC (MeOH/H2O 90
0) and finally by semi-preparative HPLC (MeOH/H2O 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 3 ml min−1, 254 nm) to yield 1 (8.4 mg). Fr.3.10 (11.5 g) was purified over ODS (MeOH/H2O, 70
10, 3 ml min−1, 254 nm) to yield 1 (8.4 mg). Fr.3.10 (11.5 g) was purified over ODS (MeOH/H2O, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 100
30 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to yield seven fractions (Fr.3.10.1-Fr.3.10.7). Fr.3.10.6 (667.0 mg) was purified by semi-preparative HPLC (MeOH/H2O 95
0) to yield seven fractions (Fr.3.10.1-Fr.3.10.7). Fr.3.10.6 (667.0 mg) was purified by semi-preparative HPLC (MeOH/H2O 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 ml min−1, 254 nm) to yield 2 (5.4 mg). Fr.3.10.1 (200.0 mg) was also purified by semi-preparative HPLC (MeOH/H2O 93
5, 3 ml min−1, 254 nm) to yield 2 (5.4 mg). Fr.3.10.1 (200.0 mg) was also purified by semi-preparative HPLC (MeOH/H2O 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 3 ml min−1, 254 nm) to yield 3 (6.4 mg).
7, 3 ml min−1, 254 nm) to yield 3 (6.4 mg).
Hyperacmosin K (1). Colorless oil; [α]D20 +109.4 (c 0.1115, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 203 (4.22) and 268 (3.90) nm; CD (MeOH) λmax (Δε) 272 (−14.96), 320 (13.50) nm; IR υmax 3491, 2971, 2925, 1774, 1640, 1448, 1379 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 541.3888 [M + H]+ (calcd for C34H53O5, 541.3888). Hyperacmosin L (2). Colorless oil; [α]D20 +133.8 (c 0.068, MeOH); UV (MeOH) λmax (log
ε) 203 (4.22) and 268 (3.90) nm; CD (MeOH) λmax (Δε) 272 (−14.96), 320 (13.50) nm; IR υmax 3491, 2971, 2925, 1774, 1640, 1448, 1379 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 541.3888 [M + H]+ (calcd for C34H53O5, 541.3888). Hyperacmosin L (2). Colorless oil; [α]D20 +133.8 (c 0.068, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 203 (4.35), 268 (3.99) nm; CD (MeOH) λmax (Δε) 271 (−15.15), 319 (14.55) nm; IR υmax 3480, 2971, 2930, 1774, 1640, 1447, 1378 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 541.3888 [M + H]+ (calcd for C34H53O5, 541.3888).
ε) 203 (4.35), 268 (3.99) nm; CD (MeOH) λmax (Δε) 271 (−15.15), 319 (14.55) nm; IR υmax 3480, 2971, 2930, 1774, 1640, 1447, 1378 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 541.3888 [M + H]+ (calcd for C34H53O5, 541.3888).
Hyperacmosin M (3). Colorless oil; [α]D20 −54.5 (c 0.044, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 204 (4.74), 245 (4.35) nm; CD (MeOH) λmax (Δε) 245 (−13.56), 311 (1.96), 346 (0.78) nm; IR υmax 3357, 2967, 2922, 1760, 1717, 1689, 1447, 1374 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 655.3967 [M + Na]+ (calcd for C40H56O6Na, 655.3969).
ε) 204 (4.74), 245 (4.35) nm; CD (MeOH) λmax (Δε) 245 (−13.56), 311 (1.96), 346 (0.78) nm; IR υmax 3357, 2967, 2922, 1760, 1717, 1689, 1447, 1374 cm−1; 1H NMR (400 MHz) and 13C NMR (125 MHz) data see Table 1; HRESIMS m/z 655.3967 [M + Na]+ (calcd for C40H56O6Na, 655.3969).
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by Projects of International Cooperation and Exchanges NSFC (NSFC-VR, No 81361138020); National Science and Technology Major Projects for “Major New Drugs Innovation and Development”, Research and Development of New Drug Varieties from Natural Product Sources and Their Key Innovative Technological Systems (No 2018ZX09711001-001-001, 2018ZX09711001-001-003 and 2018ZX09711001-008-009); the CAMS Innovation Fund for Medical Sciences (CIFMS); and the CAMS Initiative for Innovative Medicine (No CAMS-I2M 2019-I2M-3-001).References
- I. P. Singh and S. B. Bharate, Phloroglucinol compounds of natural origin, Nat. Prod. Rep., 2006, 23, 558–591 RSC.
- R. Ciochina and R. B. Grossman, Polycyclic Polyprenylated Acylphloroglucinols, Chem. Rev., 2006, 106, 3963–3986 CrossRef CAS PubMed.
- L. Verotta, Are acylphloroglucinols lead structures for the treatment of degenerative diseases?, Phytochem. Rev., 2002, 1, 389–407 CrossRef CAS.
- D. S. Tian, P. Yi, L. Xia, X. Xiao, Y. M. Fan, W. Gu, L. J. Huang, Y. B. David, Y. T. Di, C. M. Yuan, X. J. Hao and A.-G. Garmultins, Biogenetically Related Polycyclic Acylphloroglucinols from Garcinia multiflora, Org. Lett., 2016, 18, 5904–5907 CrossRef CAS PubMed.
- G. M. Raso, M. Pacilio, G. D. Carlo, E. Esposito, L. Pinto and R. Meli, In vivo and in vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum, J. Pharm. Pharmacol., 2002, 54, 1379–1383 CrossRef CAS PubMed.
- Z. Saddiqe, I. Naeem and A. Maimoona, A review of the antibacterial activity of Hypericum perforatum L, J. Ethnopharmacol., 2010, 131, 511–521 CrossRef CAS PubMed.
- Y. Guo, N. Zhang, C. M. Chen, J. F. Huang, X. N. Li, J. J. Liu, H. C. Zhu, Q. Y. Tong, J. W. Zhang, Z. W. Luo, Y. B. Xue and Y. H. Zhang, Tricyclic Polyprenylated Acylphloroglucinols from St John's Wort, Hypericum perforatum, J. Nat. Prod., 2017, 80, 1493–1504 CrossRef CAS PubMed.
- J. A. Richard, R. H. Pouwer and D. Y. K. Chen, The Chemistry of the Polycyclic Polyprenylated Acylphloroglucinols, Angew. Chem., Int. Ed., 2012, 51, 4536–4561 CrossRef CAS PubMed.
- H. P. Pepper, S. J. Tulip, Y. Nakano and J. H. George, Biomimetic Total Synthesis of (±)-Doitunggarcinone A and (+)-Garcibracteatone, J. Org. Chem., 2014, 79, 2564–2573 CrossRef CAS PubMed.
- X. W. Yang, R. B. Grossman and G. Xu, Research Progress of Polycyclic Polyprenylated Acylphloroglucinols, Chem. Rev., 2018, 118, 3508–3558 CrossRef CAS PubMed.
- J. B. Yang, R. D. Liu, J. Ren, Q. Wei, A. G. Wang and Y. L. Su, Two new prenylated phloroglucinol derivatives from Hypericum scabrum, J. Asian Nat. Prod. Res., 2016, 5, 436–442 CrossRef PubMed.
- R. D. Liu, J. Ma, J. B. Yang, A. G. Wang and Y. L. Su, Two new polyprenylated acylphloroglucinols from Hypericum scabrum, J. Asian Nat. Prod. Res., 2014, 7, 717–723 CrossRef PubMed.
- W. Gao, J. W. Hu, W. Z. Hou, F. Xu, J. Zhao, F. Xu, H. Sun, J. G. Xing, Y. Peng, X. L. Wang, T. F. Ji, L. Li and Z. Y. Gu, Four new prenylated phloroglucinol derivatives from Hypericum scabrum, Tetrahedron Lett., 2016, 57, 2244–2248 CrossRef CAS.
- X. Wang, J. J. Wang, X. Y. Suo, H. R. Sun, B. Zhen, H. Sun, J. G. Li and T. F. Ji, Hyperacmosins H-J, three new polycyclic polyprenylated acylphloroglucinol derivatives from Hypericum acmosepalum, J. Asian Nat. Prod. Res., 2020, 22, 521–530 CrossRef CAS PubMed.
- X. Wang, M. J. Shi, J. J. Wang, X. Y. Suo, H. R. Sun, B. Zhen, H. Sun, J. G. Li and T. F. Ji, Hyperacmosins E-G, three new homoadamantane-type polyprenylated acylphloroglucinols from Hypericum acmosepalum, Fitoterapia, 2020, 142, 104535 CrossRef CAS PubMed.
- X. Y. Suo, M. J. Shi, J. Dang, H. L. Yue, Y. D. Tao, B. Zhen, J. J. Wang, X. Wang, H. R. Sun, H. Sun, G. F. Qiang, T. F. Ji and B. Liu, Two new polycyclic polyprenylated acylphloroglucinol derivatives from Hypericum acmosepalum, J. Asian Nat. Prod. Res. DOI:10.1080/10286020.2021.1880395.
- J. Ma, G. Y. Xia, Y. D. Zang, C. J. Li, J. B. Yang, J. W. Huang, J. J. Zhang, Y. L. Su, A. G. Wang and D. M. Zhang, Three new decarbonyl prenylphloroglucinols bearing unusual spirost subunits from Hypericum scabrum and their neuronal activities, Chin. Chem. Lett., 2021, 32, 1173–1176 CrossRef CAS.
- H. C. Zhu, C. M. Chen, J. Yang, D. Y. Li, J. W. Zhang, Y. Guo, J. P. Wang, Z. W. Luo, Y. B. Xue and Y. H. Zhang, Hyperhexanone A, a crucial intermediate from bicyclo[3.3.1]- to cyclohexanone monocyclic-polycyclic polyprenylated acylphloroglucinols, Tetrahedron, 2016, 72, 4655–4659 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03533a |
| ‡ M. S. and X. W. contributed equally. |
| § Corresponding author E-mail: liubopaul@163.com (Bo Liu); jitf@imm.ac.cn (Tengfei Ji) |
| This journal is © The Royal Society of Chemistry 2021 |